Abstract
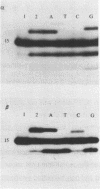
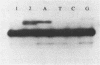
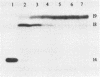
DNA polymerases catalyze the addition of deoxyribonucleotides onto DNA primers in a template-directed manner. The requirement for template instruction distinguishes these enzymes from other nucleotidyl transferases, such as terminal deoxynucleotidyl transferase, that do not utilize a template. An oligonucleotide substrate was used to characterize a novel, non-templated nucleotide addition reaction carried out by DNA polymerases from a variety of procaryotic and eucaryotic sources. The products of the reaction, in which a deoxyribonucleotide was added to the 3' hydroxyl terminus of a blunt-ended DNA substrate, were analyzed by electrophoresis on high resolution, denaturing polyacrylamide gels. DNA polymerase from Thermus aquaticus, polymerase alpha from chick embryo, rat polymerase beta, reverse transcriptase from avian myeloblastosis virus, and DNA polymerase I from Saccharomyces cerevisiae all carried out the blunt-end addition reaction. The reaction required a duplex DNA substrate but did not require coding information from the template strand. These results demonstrate that template instruction is not an absolute requirement for the catalysis of nucleotidyl transfer reactions by DNA polymerases.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbotts J., SenGupta D. N., Zmudzka B., Widen S. G., Notario V., Wilson S. H. Expression of human DNA polymerase beta in Escherichia coli and characterization of the recombinant enzyme. Biochemistry. 1988 Feb 9;27(3):901–909. doi: 10.1021/bi00403a010. [DOI] [PubMed] [Google Scholar]
- Alt F. W., Baltimore D. Joining of immunoglobulin heavy chain gene segments: implications from a chromosome with evidence of three D-JH fusions. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4118–4122. doi: 10.1073/pnas.79.13.4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. S., Lawrence C. B., Wilson S. H., Beattie K. L. Genetic relatedness of human DNA polymerase beta and terminal deoxynucleotidyltransferase. Gene. 1987;60(2-3):163–173. doi: 10.1016/0378-1119(87)90224-1. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H. The molecular structure of centromeres and telomeres. Annu Rev Biochem. 1984;53:163–194. doi: 10.1146/annurev.bi.53.070184.001115. [DOI] [PubMed] [Google Scholar]
- Chang L. M. DNA polymerases from bakers' yeast. J Biol Chem. 1977 Mar 25;252(6):1873–1880. [PubMed] [Google Scholar]
- Chien A., Edgar D. B., Trela J. M. Deoxyribonucleic acid polymerase from the extreme thermophile Thermus aquaticus. J Bacteriol. 1976 Sep;127(3):1550–1557. doi: 10.1128/jb.127.3.1550-1557.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. M., Beardsley G. P. Functional effects of cis-thymine glycol lesions on DNA synthesis in vitro. Biochemistry. 1987 Aug 25;26(17):5398–5403. doi: 10.1021/bi00391a027. [DOI] [PubMed] [Google Scholar]
- Clark J. M., Beardsley G. P. Thymine glycol lesions terminate chain elongation by DNA polymerase I in vitro. Nucleic Acids Res. 1986 Jan 24;14(2):737–749. doi: 10.1093/nar/14.2.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. M., Joyce C. M., Beardsley G. P. Novel blunt-end addition reactions catalyzed by DNA polymerase I of Escherichia coli. J Mol Biol. 1987 Nov 5;198(1):123–127. doi: 10.1016/0022-2836(87)90462-1. [DOI] [PubMed] [Google Scholar]
- Cox E. C. Bacterial mutator genes and the control of spontaneous mutation. Annu Rev Genet. 1976;10:135–156. doi: 10.1146/annurev.ge.10.120176.001031. [DOI] [PubMed] [Google Scholar]
- Drake J. W. Comparative rates of spontaneous mutation. Nature. 1969 Mar 22;221(5186):1132–1132. doi: 10.1038/2211132a0. [DOI] [PubMed] [Google Scholar]
- Greider C. W., Blackburn E. H. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985 Dec;43(2 Pt 1):405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Schaaper R. M., Loeb L. A. Depurination-induced infidelity of deoxyribonucleic acid synthesis with purified deoxyribonucleic acid replication proteins in vitro. Biochemistry. 1983 May 10;22(10):2378–2384. doi: 10.1021/bi00279a012. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. The mutational specificity of DNA polymerase-beta during in vitro DNA synthesis. Production of frameshift, base substitution, and deletion mutations. J Biol Chem. 1985 May 10;260(9):5787–5796. [PubMed] [Google Scholar]
- Kunkel T. A. The mutational specificity of DNA polymerases-alpha and -gamma during in vitro DNA synthesis. J Biol Chem. 1985 Oct 15;260(23):12866–12874. [PubMed] [Google Scholar]
- Loeb L. A., Kunkel T. A. Fidelity of DNA synthesis. Annu Rev Biochem. 1982;51:429–457. doi: 10.1146/annurev.bi.51.070182.002241. [DOI] [PubMed] [Google Scholar]
- Matsukage A., Nishikawa K., Ooi T., Seto Y., Yamaguchi M. Homology between mammalian DNA polymerase beta and terminal deoxynucleotidyltransferase. J Biol Chem. 1987 Jul 5;262(19):8960–8962. [PubMed] [Google Scholar]
- Randall S. K., Eritja R., Kaplan B. E., Petruska J., Goodman M. F. Nucleotide insertion kinetics opposite abasic lesions in DNA. J Biol Chem. 1987 May 15;262(14):6864–6870. [PubMed] [Google Scholar]
- Sagher D., Strauss B. Insertion of nucleotides opposite apurinic/apyrimidinic sites in deoxyribonucleic acid during in vitro synthesis: uniqueness of adenine nucleotides. Biochemistry. 1983 Sep 13;22(19):4518–4526. doi: 10.1021/bi00288a026. [DOI] [PubMed] [Google Scholar]
- Takeshita M., Chang C. N., Johnson F., Will S., Grollman A. P. Oligodeoxynucleotides containing synthetic abasic sites. Model substrates for DNA polymerases and apurinic/apyrimidinic endonucleases. J Biol Chem. 1987 Jul 25;262(21):10171–10179. [PubMed] [Google Scholar]
- Walmsley R. M. Yeast telomeres: the end of the chromosome story? Yeast. 1987 Sep;3(3):139–148. doi: 10.1002/yea.320030302. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Tanabe K., Takahashi T., Matsukage A. Chick embryo DNA polymerase alpha. Polypeptide components and their microheterogeneity. J Biol Chem. 1982 Apr 25;257(8):4484–4489. [PubMed] [Google Scholar]