Abstract
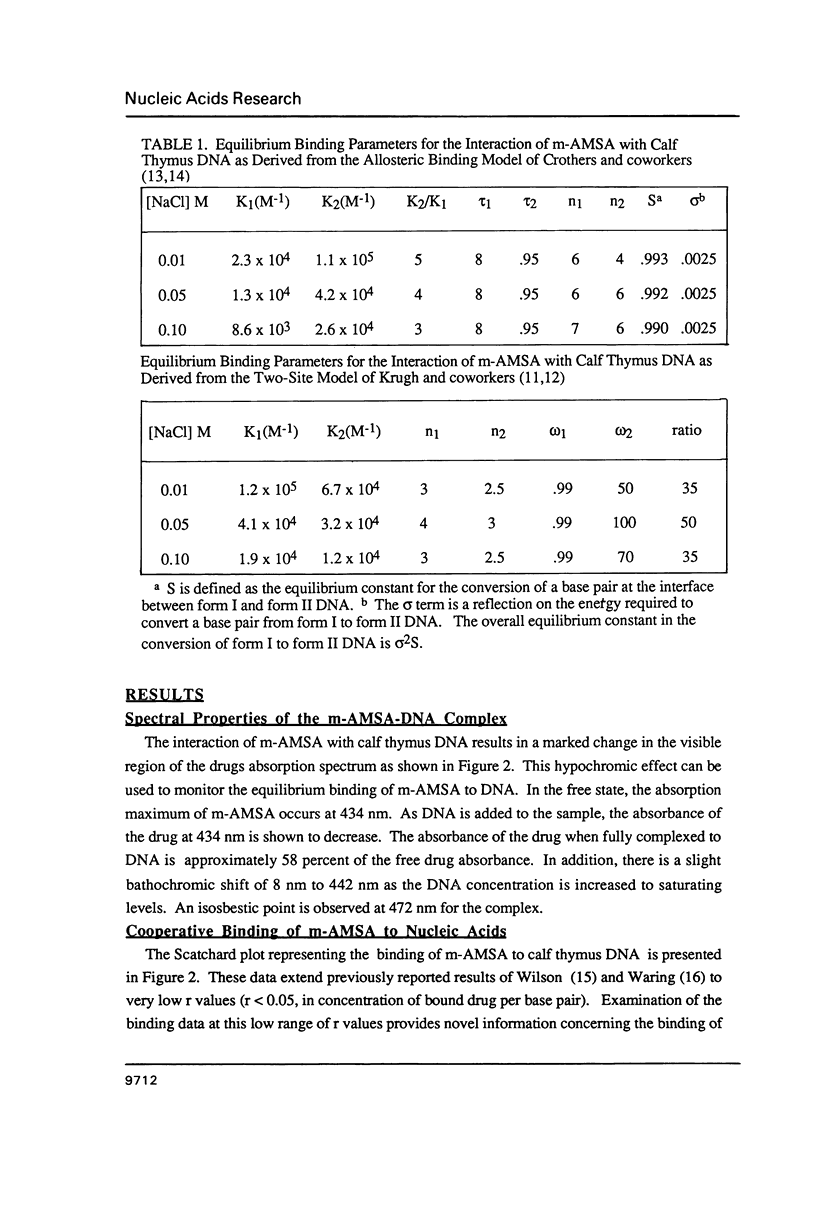
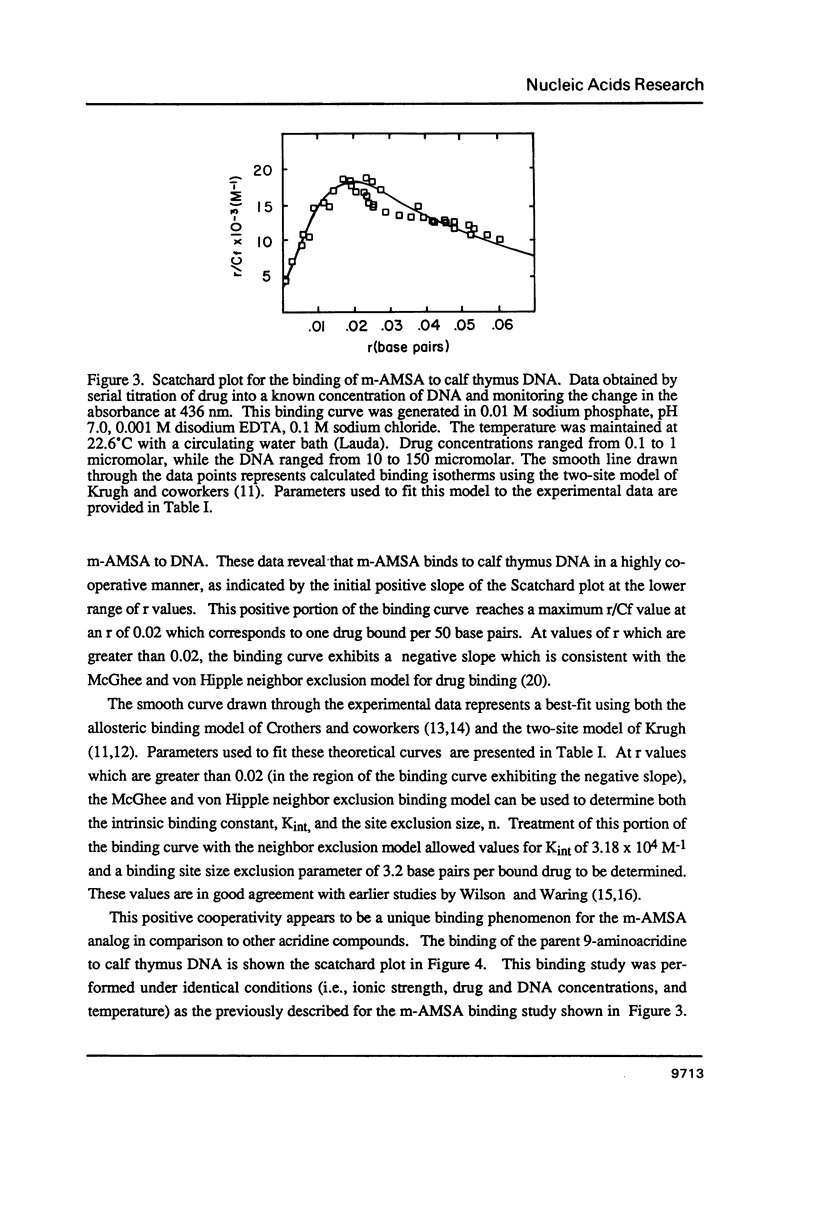
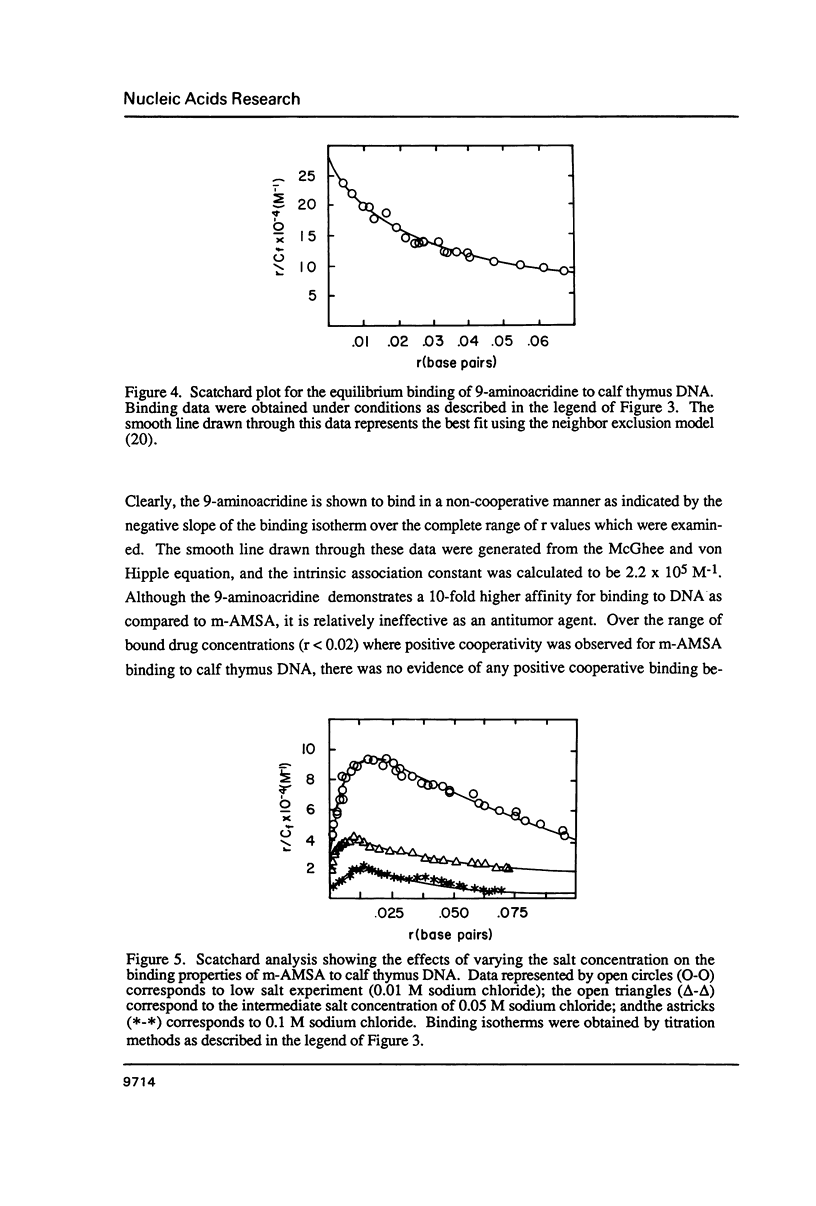
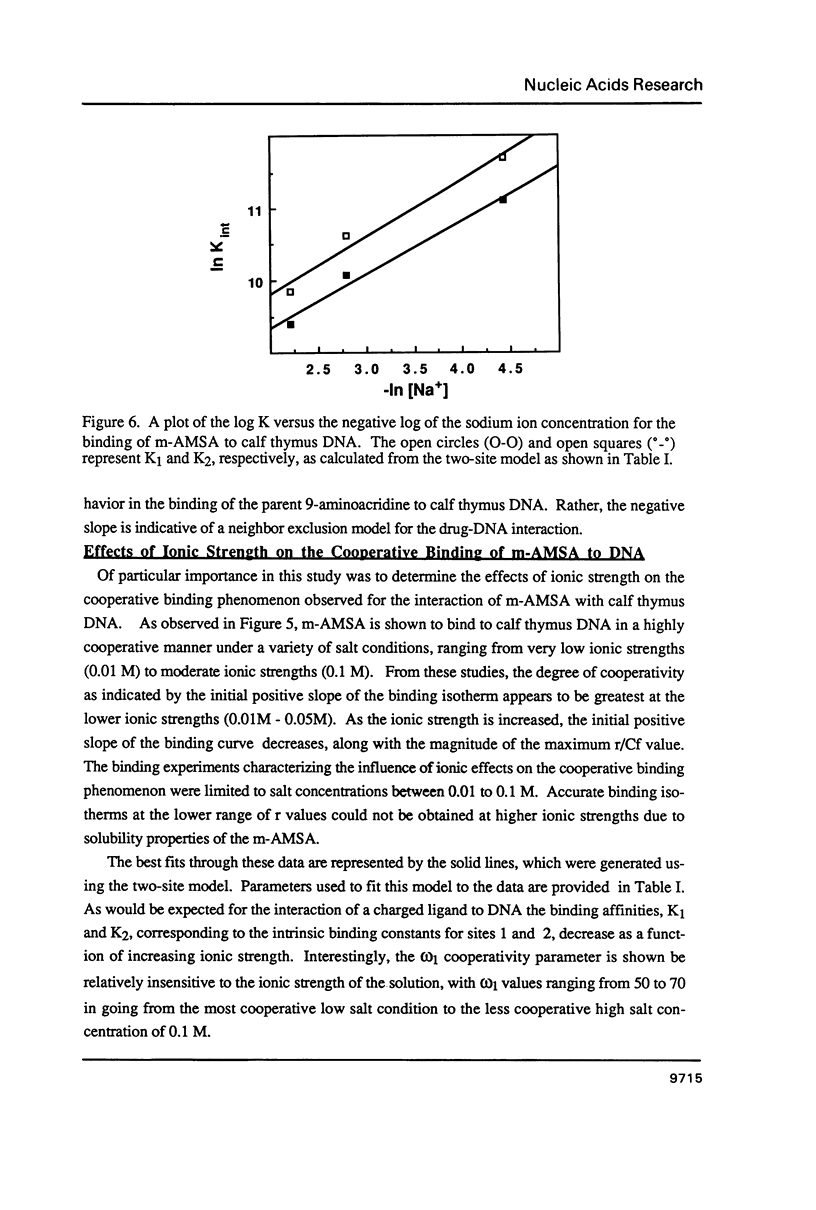
The equilibrium binding of the antitumor agent m-AMSA (4'-(9-acridinylamino) methane-sulfon-m-ansidide) has been examined by optical methods. These studies which have focused on the low bound drug concentrations (r values less than 0.02, base pairs) reveal m-AMSA to bind calf thymus DNA in a highly cooperative manner as indicated by the initial positive slope of the Scatchard plot. In contrast, the studies on the parent 9-aminoacridine under identical conditions demonstrate that this compound binds DNA in a noncooperative (neighbor exclusion) manner. The positive cooperative binding phenomenon of m-AMSA is probed as a function of ionic concentration and shown to exist over the range of salt concentrations examined (0.01 to 0.1 M); however, the magnitude of the cooperative binding is altered. This observation of cooperativity is consistent with earlier studies on biologically active compounds and may be related to such binding parameters as binding sequence selectivity and/or structural perturbations to the DNA structure.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cain B. F., Atwell G. J., Denny W. A. Potential antitumor agents. 16.4'-(Acridin-9-ylamino)methanesulfonanilides. J Med Chem. 1975 Nov;18(11):1110–1117. doi: 10.1021/jm00245a013. [DOI] [PubMed] [Google Scholar]
- Chaires J. B., Dattagupta N., Crothers D. M. Studies on interaction of anthracycline antibiotics and deoxyribonucleic acid: equilibrium binding studies on interaction of daunomycin with deoxyribonucleic acid. Biochemistry. 1982 Aug 17;21(17):3933–3940. doi: 10.1021/bi00260a005. [DOI] [PubMed] [Google Scholar]
- Dattagupta N., Hogan M., Crothers D. M. Interaction of netropsin and distamycin with deoxyribonucleic acid: electric dichroism study. Biochemistry. 1980 Dec 23;19(26):5998–6005. doi: 10.1021/bi00567a009. [DOI] [PubMed] [Google Scholar]
- Feigon J., Denny W. A., Leupin W., Kearns D. R. Interactions of antitumor drugs with natural DNA: 1H NMR study of binding mode and kinetics. J Med Chem. 1984 Apr;27(4):450–465. doi: 10.1021/jm00370a007. [DOI] [PubMed] [Google Scholar]
- Graves D. E., Krugh T. R. Adriamycin and daunorubicin bind in a cooperative manner to deoxyribonucleic acid. Biochemistry. 1983 Aug 2;22(16):3941–3947. doi: 10.1021/bi00285a033. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Mandelkern M., Crothers D. M. Solution structural studies of chromatin fibers. Biochemistry. 1981 Mar 17;20(6):1438–1445. doi: 10.1021/bi00509a006. [DOI] [PubMed] [Google Scholar]
- Legha S. S., Gutterman J. U., Hall S. W., Benjamin R. S., Burgess M. A., Valdivieso M., Bodey G. P. Phase 1 clinical investigation of 4'-(9-acridinylamino)methanesulfon-m-anisidide (NSC 249992), a new acridine derivative. Cancer Res. 1978 Nov;38(11 Pt 1):3712–3716. [PubMed] [Google Scholar]
- Manning G. S. The molecular theory of polyelectrolyte solutions with applications to the electrostatic properties of polynucleotides. Q Rev Biophys. 1978 May;11(2):179–246. doi: 10.1017/s0033583500002031. [DOI] [PubMed] [Google Scholar]
- Marsoni S., Wittes R. Clinical development of anticancer agents--a National Cancer Institute perspective. Cancer Treat Rep. 1984 Jan;68(1):77–85. [PubMed] [Google Scholar]
- McGhee J. D., von Hippel P. H. Theoretical aspects of DNA-protein interactions: co-operative and non-co-operative binding of large ligands to a one-dimensional homogeneous lattice. J Mol Biol. 1974 Jun 25;86(2):469–489. doi: 10.1016/0022-2836(74)90031-x. [DOI] [PubMed] [Google Scholar]
- Minford J., Pommier Y., Filipski J., Kohn K. W., Kerrigan D., Mattern M., Michaels S., Schwartz R., Zwelling L. A. Isolation of intercalator-dependent protein-linked DNA strand cleavage activity from cell nuclei and identification as topoisomerase II. Biochemistry. 1986 Jan 14;25(1):9–16. doi: 10.1021/bi00349a002. [DOI] [PubMed] [Google Scholar]
- Pommier Y., Covey J. M., Kerrigan D., Markovits J., Pham R. DNA unwinding and inhibition of mouse leukemia L1210 DNA topoisomerase I by intercalators. Nucleic Acids Res. 1987 Aug 25;15(16):6713–6731. doi: 10.1093/nar/15.16.6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier Y., Mattern M. R., Schwartz R. E., Zwelling L. A., Kohn K. W. Changes in deoxyribonucleic acid linking number due to treatment of mammalian cells with the intercalating agent 4'-(9-acridinylamino)methanesulfon-m-anisidide. Biochemistry. 1984 Jun 19;23(13):2927–2932. doi: 10.1021/bi00308a012. [DOI] [PubMed] [Google Scholar]
- Record M. T., Jr, Anderson C. F., Lohman T. M. Thermodynamic analysis of ion effects on the binding and conformational equilibria of proteins and nucleic acids: the roles of ion association or release, screening, and ion effects on water activity. Q Rev Biophys. 1978 May;11(2):103–178. doi: 10.1017/s003358350000202x. [DOI] [PubMed] [Google Scholar]
- Rosenberg L. S., Carvlin M. J., Krugh T. R. The antitumor agent mitoxantrone binds cooperatively to DNA: evidence for heterogeneity in DNA conformation. Biochemistry. 1986 Mar 11;25(5):1002–1008. doi: 10.1021/bi00353a008. [DOI] [PubMed] [Google Scholar]
- Ross W. E., Glaubiger D., Kohn K. W. Qualitative and quantitative aspects of intercalator-induced DNA strand breaks. Biochim Biophys Acta. 1979 Mar 28;562(1):41–50. doi: 10.1016/0005-2787(79)90124-2. [DOI] [PubMed] [Google Scholar]
- Stone M. P., Gopalakrishnan S., Harris T. M., Graves D. E. Carcinogen-nucleic acid interactions: equilibrium binding studies of aflatoxins B1 and B2 with DNA and the oligodeoxynucleotide d(ATGCAT)2. J Biomol Struct Dyn. 1988 Apr;5(5):1025–1041. doi: 10.1080/07391102.1988.10506447. [DOI] [PubMed] [Google Scholar]
- Walker G. T., Stone M. P., Krugh T. R. Ethidium binding to left-handed (Z) DNAs results in regions of right-handed DNA at the intercalation site. Biochemistry. 1985 Dec 3;24(25):7462–7471. doi: 10.1021/bi00346a065. [DOI] [PubMed] [Google Scholar]
- Waring M. J. DNA-binding characteristics of acridinylmethanesulphonanilide drugs: comparison with antitumour properties. Eur J Cancer. 1976 Dec;12(12):995–1001. doi: 10.1016/0014-2964(76)90066-9. [DOI] [PubMed] [Google Scholar]
- Wilson W. D., Lopp I. G. Analysis of cooperativity and ion effects in the interaction of quinacrine with DNA. Biopolymers. 1979 Dec;18(12):3025–3041. doi: 10.1002/bip.1979.360181210. [DOI] [PubMed] [Google Scholar]
- Wilson W. R., Baguley B. C., Wakelin L. P., Waring M. J. Interaction of the antitumor drug 4'-(9-acridinylamino)methanesulfon-m-anisidide and related acridines with nucleic acids. Mol Pharmacol. 1981 Sep;20(2):404–414. [PubMed] [Google Scholar]
- Winkle S. A., Krugh T. R. Equilibrium binding of carcinogens and antitumor antibiotics to DNA: site selectivity, cooperativity, allosterism. Nucleic Acids Res. 1981 Jul 10;9(13):3175–3186. doi: 10.1093/nar/9.13.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winton E. F., Hearn E. B., Vogler W. R., Johnson L., Logan T., Raney M. Amsacrine in refractory adult acute leukemia: a pilot study of the Southeastern Cancer Study Group. Cancer Treat Rep. 1983 Nov;67(11):977–980. [PubMed] [Google Scholar]


