Abstract
OBJECTIVE:
To compare school- versus provider-based approaches to improving influenza vaccination coverage among adolescents in rural Georgia.
METHODS:
We used a nonrandomized, 3-armed design: (1) a middle- and high school-based influenza vaccination intervention in 1 county; (2) a provider-based influenza vaccination intervention in a second county; and (3) a standard-of-care condition in a third county. Interventions also included distribution of an educational brochure, school presentations, and community-based outreach to enhance vaccine knowledge and awareness among adolescents and their parents.
RESULTS:
During the 2008–2009 influenza season, 70 (19%) of 370 students were vaccinated in the school-based county and 110 (15%) of 736 students were vaccinated in the provider-based county, compared with 71 (8%) of 889 students in the standard-of-care county (risk ratio [RR]school: 2.4 [95% confidence interval (CI): 1.7–3.2]; RRprovider: 1.9 [95% CI: 1.4–2.5]). During 2009–2010, seasonal influenza vaccination coverage was 114 (30.4%) of 375 of students in the school-based county, 122 (16.9%) of 663 of students in the provider-based county, and 131 (15.2%) of 861 students in the standard-of-care county (RRschool: 2.3 [95% CI: 1.9–2.9]; RRprovider: 1.2 [95% CI: 0.97–1.5]).
CONCLUSIONS:
Special efforts to promote influenza vaccination among rural, predominantly black students were associated with increased vaccination coverage. The school-based influenza vaccination intervention was associated with the highest levels of vaccination coverage. This study revealed the efficacy of school-based influenza education to improve vaccination rates among adolescents.
Keywords: influenza vaccine, school-based clinics, adolescents, pandemic preparedness
WHAT'S KNOWN ON THIS SUBJECT:
Vaccination of adolescents is important to reduce the transmission of influenza in households and communities. Vaccination in schools is 1 strategy to reach this population. School-based influenza vaccination intervention studies have been predominantly conducted in elementary schools comprising nonminority populations.
WHAT THIS STUDY ADDS:
In this study the efficacy of school-based influenza education to improve vaccination rates among adolescents regardless of location of vaccination is demonstrated. School-based influenza vaccination intervention was associated with higher levels of vaccination coverage compared with provider-based influenza vaccination intervention.
Despite efforts to enhance vaccination among persons at highest risk of complications, influenza remains a significant health burden in the United States.1 Research indicates that vaccinating school-aged children against influenza not only protects them directly, but may also reduce transmission to persons at high risk for complications such as infants and the elderly.2,3 Despite these findings, estimates of vaccination coverage remain low for school-aged children, with only 15.3% of those aged 13 to 18 years vaccinated against seasonal influenza in 2009–2010.4
School-based vaccination clinics have the potential to efficiently vaccinate large numbers of children at lower total cost than primary care physicians or public clinics.5–7 Previous studies have shown that school-based vaccination clinics have vaccinated 40% to 50% of targeted school-aged children for influenza,3,5,8–10 reduced influenza illness directly among the students and indirectly in the community,3,10–12 and have reduced costs from a societal perspective by decreasing school and work absenteeism.2,5
Although demonstrating effectiveness, school-based vaccination interventions have been predominantly conducted with younger students and nonminority populations. Whether this vaccine delivery strategy would be comparably efficacious for adolescent and minority populations is unknown. Given the Advisory Committee on Immunization Practices recently expanded recommendations to vaccinate all persons older than 6 months of age, including school-aged children aged 5 to 18 years,13 it is important to develop strategies for reaching these traditionally underserved populations.14–22 Multicomponent interventions, which include providing education about immunizations to a target population and improving access to vaccination, may be an effective delivery strategy.23 The purpose of this study was to determine the efficacy of 2 multicomponent interventions designed to enhance influenza vaccination coverage among a predominantly black adolescent population attending public middle and high-schools in 3 counties in rural Georgia.
METHODS
Study Design
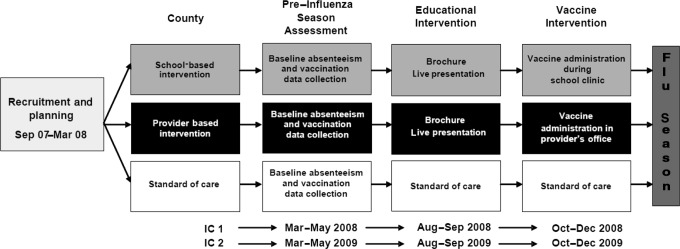
This study employed a nonrandomized, 3-armed controlled design (Fig 1). Three counties in rural Georgia were each assigned to 1 of the following intervention arms: (1) a multicomponent school-based influenza vaccination intervention condition; (2) a multicomponent provider-based influenza vaccination condition; and (3) a standard-of-care condition. In the school-based county, the multicomponent intervention consisted of an educational component and a structural component (provision of free influenza vaccination via school-based influenza vaccine clinics). In the provider-based county, the multicomponent intervention included the same educational component but a different structural component (provision of free influenza vaccination by local health care providers). The trial was conducted in 2 intervention cycles (IC1 and IC2) during 2 consecutive influenza seasons (2008–2009 and 2009–2010).
FIGURE 1.
Study design.
Study Setting and Population
Three counties within the same public health district in eastern Georgia were selected for this study. The 3 participating counties were selected because they are relatively small (with 1 public middle and high school), rural and have substantial low income and black populations (Table 1).24
TABLE 1.
Demographic Information of Study Counties
| School-Based | Provider-Based | Standard of Care | |
|---|---|---|---|
| Total county population (2006), n | 5949 | 8257 | 10 468 |
| County, % black students | 57 | 32 | 53 |
| Total No. of students (middle and high, 2007–2008) | 418 | 757 | 853 |
| Students, % black | 96a,b | 38c | 53 |
| Students eligible for free or reduced lunch, % | 82d | 65 | 69 |
School-based versus provider-based: P < .001; school-based versus standard of care: P < .001.
The percentage of students who were black was higher than the population percent because a large proportion of white students attend private rather than public schools.
Provider-based versus standard of care: P < .001.
School-based versus provider-based: P < .001; school-based versus standard of care: P < .001.
Educational Intervention
The educational intervention was implemented in both multicomponent intervention counties at the beginning of each intervention cycle, before implementation of the structural interventions. Both intervention counties received the same educational intervention25 consisting of (1) a brochure mailed home through the school (targeted toward adolescents and their parents) and (2) a school presentation (targeted toward adolescents). The educational intervention in both multicomponent intervention counties was based on the health belief model,26 and the theory of reasoned action.27 The school presentation included a skit presented by a volunteer group of students, question-and-answer session facilitated by study staff. The skit addressed health belief model and theory of reasoned action constructs, including self-efficacy, social norms, perceived benefits, perceived barriers, perceived susceptibility, perceived severity, and students' sense of invincibility. The skit itself served as a cue to action. Both the brochures and school skits were developed collaboratively by trained study staff and participants from the community.
Vaccine Eligibility
To be eligible to receive the vaccine for this study, (1) adolescents must have been enrolled at a target school in a participating county, (2) families resided in the target counties, (3) parents provided written informed permission for their adolescent to be vaccinated, and (4) parents completed a brief medical history form documenting adolescents' medical conditions that might contraindicate influenza vaccination. The primary vaccine was a live-attenuated influenza vaccine (LAIV). Trivalent inactivated vaccine was made available to students with contraindications to LAIV. Both vaccines were offered free in both intervention counties. Study protocols were approved by Emory University, Medical College of Georgia, and the Georgia Department of Community Health institutional review boards.
Recruitment
In both intervention counties, informational packets were mailed to the home of each adolescent enrolled in middle and high-school. The packets contained (1) a cover letter describing the project, co-signed by the school principal and the county health officer, (2) a parental consent form for influenza vaccination, (3) a brief medical history form, (4) an educational brochure designed to enhance influenza vaccine acceptance, (5) Centers for Disease Control and Prevention–approved vaccine information statements for LAIV28 and trivalent inactivated vaccine,29 and (6) an envelope for parents' convenience and confidentiality in returning forms to the school. A second information packet was mailed approximately 1 month after the initial mailing to increase the number of participants. To further increase enrollment, study staff attended school open house events and advertisements were placed in local newspapers to alert parents about the dates that influenza vaccine would be available and when packets would be mailed home or available for pick-up at school.
Study Procedures
School-Based Intervention
We met with school officials to establish tailored implementation plans for the educational and school-based components. The school-based intervention activities were implemented from October to November for both intervention years. On days in which the school-based vaccinations were administered, the research project coordinator registered nurse provided a list of students eligible to receive influenza vaccination to the on-site screener. The screener checked the list of eligible students to ensure that each student could receive vaccination and informed the nurse (from the local health department) which vaccine they should receive. One copy of the vaccination record was retained for the study and another copy was given to the student for their personal records. Staff recorded required information regarding each vaccination in the Georgia Registry of Immunization Transactions and Services (GRITS), Georgia's immunization information system. All students vaccinated at the clinic were observed for potential adverse events for at least 20 minutes before returning to class. One or 2 clinic days per intervention cycle were conducted, and the vaccine was made available at the county health department for those students unable to attend a school clinic. Vaccine was provided to students and school staff free.
Provider-Based Intervention
In the provider-based county, a list of eligible students was given to 3 participating health care providers (2 private physicians and the county health department). Starting each October, vaccine was shipped to the participating providers. As the health department and private physicians administered the vaccine, they were asked to submit the completed list of children vaccinated, and were reimbursed for administering the vaccine at the Medicare rate. Vaccine was provided to students and school staff free. Also, clinic staff documented demographic information and type of vaccine received. Both intervention counties had an established plan for reporting adverse events, although no severe reactions were reported in either intervention year.
Data Collection
Vaccination Rates
Receipt of influenza vaccination was the primary outcome. Baseline influenza immunization data for all 3 counties was obtained by using GRITS. During IC1 and IC2, information regarding vaccine coverage levels and student demographics was collected for the intervention counties by the health department or physician's offices. Vaccination rates were determined by the number of adolescents vaccinated divided by the total number eligible for vaccination.
Absenteeism
To assess the potential effect of influenza vaccination on school attendance, each school provided anonymous all cause absentee data for the academic year for baseline and both intervention years for all 3 study conditions.
Data Analyses
Effect of the interventions on vaccination coverage was assessed by comparing the percentage of vaccination coverage in each county's middle and high schools during baseline, IC1, and IC2. A Bonferroni adjustment was used to adjust the significance level and provide a set of 95% confidence intervals comparing the 3 counties' schools (middle and high school) within a school year. All data were analyzed by using SAS 9.2 (SAS Institute, Inc, Cary, NC).
RESULTS
Demographics
School sizes varied, with the standard-of-care county being the largest and the school-based intervention county the smallest. The school-based intervention county had a significantly higher percentage of black students, followed by the provider-based county, and students eligible for free or reduced lunches (a proxy for family income) compared with the provider-based intervention and standard-of-care counties (Table 1).
Vaccination Coverage
Influenza vaccination coverage for each of the counties during baseline, IC1, and IC2 are shown in Table 2. On the basis of data from GRITS, background vaccination rates of students in each county at baseline were comparable between the school-based and standard-of-care counties. At baseline, adolescents in the provider-based county were ∼50% more likely to receive the influenza vaccine than were adolescents in the standard-of-care county (risk ratio [RR]: 1.5; P = .008) and adolescents in the school-based county were half as likely to receive an influenza vaccine as adolescents in the provider-based county (RR: 0.5; P = .002) (Table 3). For both intervention years we continued to use GRITS data to assess vaccination coverage in the standard-of-care county.
TABLE 2.
Influenza Vaccination Coverage
| Baseline (2007–2008) |
IC1 (2008–2009) |
IC2 (2009–2010) |
||||
|---|---|---|---|---|---|---|
| Total No. of Students Enrolled | No. Vaccinated (%) | Total No. of Students Enrolled | No. Vaccinated (%) | Total No. of Students Enrolled | No. Vaccinated (%) | |
| School-based county | 418 | 22 (5.2) | 370 | 70 (18.9)a | 375 | 114 (30.4)b |
| Provider-based county | 650 | 65 (10.0) | 736 | 110 (14.9)a | 663 | 122 (18.4) |
| Standard-of-care county | 853 | 56 (6.6) | 889 | 71 (8.0) | 861 | 131 (15.2)b |
Increase from baseline: P < .001 and P = .006, respectively.
Increase from IC1: P < .0005.
TABLE 3.
Comparison of Influenza Vaccination Rates
| Baseline RR (95% CI) | IC1 RR (95% CI) | IC2 RR (95% CI) | |
|---|---|---|---|
| School-based county vs standard-of-care county | 0.8 (0.50–1.29) | 2.4 (1.74–3.22)c | 2.3 (1.90–2.87)c |
| Provider-based county vs standard-of-care county | 1.5 (1.08–2.15)a | 1.9 (1.41–2.48)c | 1.2 (0.97–1.51) |
| School-based county vs provider-based county | 0.5 (0.33–0.84)b | 1.3 (0.96–1.66) | 1.7 (1.32–2.06)c |
CI indicates confidence interval.
P < .05.
P < .005.
P < .0001.
In IC1, in the school-based county, 87 (24%) of 370 students returned a consent form. Of those returning consents, 70 of 87 students were vaccinated (81%). This represents 19% of the total school population and reflects a 72% increase in influenza vaccination coverage from baseline to IC1 (P < .001) (Table 2). In the provider-based county, 20% (145/736) of students returned consents. Of those returning consents, 110 were vaccinated (76%). This represents 15% of student population and reflects a 33% increase from baseline to IC1 (P = .006) (Table 2). Of students vaccinated, 83% received LAIV in the school-based county and 87% received LAIV in the provider-based county. When compared with the standard-of-care county (6.6% from GRITS), both the school- and provider-based intervention counties demonstrated significantly higher rates of vaccination. The students in the school-based county were more than twice as likely to be vaccinated compared with students in the standard-of-care county (RR: 2.4; P < .0001) (Table 3). The students in the provider-based county were 1.9 times more likely to be vaccinated compared with the students in the standard-of-care county (RR: 1.9; P < .0001) (Table 3). However, no significant difference between the school- and provider-based interventions was observed (RR: 1.3; P = .09) (Table 3).
In IC2, 127 (34%) of 375 students in the school-based county returned a consent form; 114 (90%) students were vaccinated, representing 30% of the total school population, reflecting a 61% increase in coverage from IC1 to IC2 (P = .0003). In the provider-based county, 28% (183/663) of students returned a consent form; 122 (67%) students were vaccinated, totaling 18% of the student population; this was a 19% increase from IC1 to IC2 (P = .08) (Table 2). Of those vaccinated, 72% received LAIV in the school-based county and 89% received LAIV in the provider-based county. For the second intervention year, when we compared the intervention counties to the standard-of-care county (15.2% from GRITS), only the school-based intervention demonstrated significantly higher vaccination prevalence among adolescents. Students were more than twice as likely to be vaccinated in the school-based county compared with students in the standard-of-care county (RR: 2.3; P < .0001). The school-based intervention also demonstrated significantly higher vaccination prevalence than the provider-based intervention (RR: 1.9; P < .0001) (Table 3).
Subsequently, we examined a subset of the data to assess the effect of the intervention on black adolescents in our 2 intervention counties. In IC1, 18% of black students were vaccinated in the school-based county (64/355) compared with 16% of black students in the provider-based county (45/283) (RR: 1.1; P = .48). In IC2, black students were twice as likely to be vaccinated in the school-based county (31%; 110/360) than in the provider-based county (16%; 40/256) (RR: 2.0; P < .0001). Black adolescents in the school-based county were 70% more likely to receive influenza vaccine than those in the provider-based county (Table 4). No significant differences in absenteeism were observed between the 2 intervention counties and standard-of-care county.
TABLE 4.
Effects of Intervention on Influenza Vaccination Rates Among Black Students
| Intervention Type | IC1, n/N (%) | RR (95% CI) | IC2, n/N (%) | RR (95% CI) |
|---|---|---|---|---|
| School-based county | 64/355 (18) | 1.1 (0.80–1.61) | 110/360 (31) | 2.0 (1.41–2.71)a |
| Provider-based county | 45/283 (16) | 40/256 (16) |
CI indicates confidence interval.
P < .0001.
DISCUSSION
The aim of this study was to compare the efficacy of 2 multicomponent influenza vaccination interventions (1 school-based and 1 provider-based intervention) to a standard-of-care approach and to each other. In the first year, vaccination coverage was higher in both intervention counties than in the standard-of-care county. In the second year, the school-based intervention county had significantly higher coverage than the provider-based intervention county and the standard-of-care county, as well as a 61% increase in vaccination coverage from the first year. The provider-based intervention county also showed an increase in vaccination coverage over the 2 intervention years compared with baseline. The substantial increase in vaccination coverage in IC2 in the school-based county compared with the minimal increase seen in the provider-based county may be attributed to the difficulty that family-practice physicians face in these rural counties of sustaining vaccination compliance because of the overwhelming demands on their time and competing priorities. Overall, the findings suggest that the educational intervention helped improve influenza vaccination coverage among adolescents regardless of venue of vaccination; however, the school-based influenza vaccination program was associated with greater vaccine acceptance than the provider-based intervention.
The effect of the educational intervention may be attributed to several factors. First, both parents and students were targeted. Educating students may have motivated them to protect themselves,30 whereas educating parents may have influenced their decision to allow vaccination, as well as influencing adolescents' acceptance of their parents' decision.31 Second, the interventions were developed in collaboration with local community members, which may have increased their salience and effect in the target population.25 Finally, the study team was available in person (during open houses and the school presentation) to answer parents' and students' questions and address concerns.
We observed lower influenza vaccination coverage rates than previously published; however, this may be partly attributable to our sample: middle and high school students consisting of a rural, low-income, predominantly black population.3,5,8–10 Previous studies have evaluated influenza vaccination interventions in either elementary schools or day care programs. These studies observed a trend for lower participation rates with lower socioeconomic status and black race.3,5,8–10 We found that the school-based intervention was associated with higher vaccination levels among black adolescents compared with the provider-based intervention.
The educational intervention was tailored for students in the school presentation and was well received, possibly increasing adolescents' vaccination intention.32 Educating students about influenza vaccine and the adverse health consequences of influenza that can be prevented with vaccination may provide motivation for students to get vaccinated.30
In this study, the major obstacle to vaccination was obtaining parental consent for children, although there was an increase in returned consents from the first year to the second. This improvement could be attributed to the familiarity of parents with our program or the educational brochures mailed to the parents, but we cannot rule out an effect of the media coverage of the 2009 H1N1 influenza pandemic. Despite the 2009 H1N1 influenza pandemic, planning and implementation of our education and vaccination clinics were consistent in both intervention years. Another barrier to obtaining parental consent may have been the complexity of the consent forms. We used several methods to improve parental consent, including participating in school open houses, placing a cover sheet with instructions in the packets, using different colored stickers to indicate what lines should be signed, and including the telephone number of the research project coordinator registered nurse so parents could call and ask questions.
Although the ensuing media spotlight on the 2009 H1N1 influenza pandemic may have played some role in increasing vaccination coverage, the school-based intervention county still showed significantly higher vaccination coverage than either the provider-based intervention or standard-of-care counties, which suggests that increases in coverage from IC1 to IC2 in the school-based county could not be attributed solely to the 2009 H1N1 influenza pandemic.
This campaign required extensive resource commitment by both the health departments and the school systems. The demands on health departments in small rural counties were substantial, including the need to close the health department during school clinic hours. Leadership of health department supervisors and support of school administrators were key to the success of this study.
Several factors might have contributed to the lack of observed differences in absenteeism among the 3 counties. Herd immunity effects would not be likely with 18% or 30% vaccination rates. The sample size was small and reduced the power of our analysis and the ability to detect small changes.
This study had several limitations. First, although the study was implemented in 3 rural counties, the number of adolescents and numbers of schools was limited, reducing the precision of the effect estimates. Second, results may not be generalizable to other rural or urban geographic locations. Third, influenza vaccination data for the standard-of-care county was available only on a countywide level, whereas our intervention counties had vaccination data within our study schools. Fourth, the GRITS data may be incomplete. Fifth, during our second intervention year, the 2009 H1N1 influenza pandemic occurred, which may have played a role in increasing vaccination coverage.
CONCLUSIONS
Improving vaccination of children as a strategy for community control of influenza continues to be a topic of substantial interest, particularly after the 2009 H1N1 influenza pandemic and recent expansion of Advisory Committee on Immunization Practices recommendations. In this study we demonstrate the efficacy of a brief, tailored influenza education program delivered in schools that improved influenza vaccine uptake, regardless of whether vaccine was provided at the school or local physicians' offices. This study also demonstrated acceptance of a school-based influenza immunization program by parents and adolescents in a rural, predominantly minority population. This evaluation provides important information indicating the feasibility of implementing school-based influenza education and vaccination programs in traditionally hard-to-reach populations. Study findings demonstrate potential implications for improving coverage of other vaccines recommended for adolescents. Expansion of the definition of the medical home to include schools may also help to reach adolescents and improve vaccination compliance.
ACKNOWLEDGMENTS
This work was supported by Centers for Disease Control and Prevention grant 5 R18 IP000166 and NIH grant 5T32AI074492-02 (to Dr Painter). LAIV used in this campaign was from an in-kind donation by MedImmune Inc.
We thank Dianne Miller and Ashley Freeman for administrative support; the superintendents, principals, teachers and staff, parents, and students in our participating counties for participation and support; Melba McNorrill, Carol Usry, Amy Jenkins, and Dr Ketty M. Gonzalez, district health director for the East Central Health District, for support; Tracy Culbreath (GRITS); county health departments in the East Central Health District; private providers in our provider-based county; and our community liaisons in our intervention counties.
Dr Gargano made substantial contributions to the conception and design of the study, acquisition of data, analysis and interpretation of the data, and drafting the article; Dr Pazol made substantial contributions to the conception and design of the study, acquisition of data, and analysis and interpretation of the data; Dr Sales made substantial contributions to the conception and design of the study and analysis and interpretation of the data; Dr Painter made substantial contributions to the conception and design of the study, acquisition of data, and analysis and interpretation of the data; Mr Morfaw and Ms Jones assisted with acquisition of the data; Mr Weiss made substantial contributions to the analysis and interpretation of the data; Dr Murray made substantial contributions to the analysis and interpretation of the data; Drs Buehler, Wingood, Orenstein, and DiClemente made substantial contributions to the conception and design of the study; Dr Hughes made substantial contributions to the conception and design of the study, and analysis and interpretation of the data; and all authors critically reviewed and revised the article for important intellectual content and gave final approval of the version to be published.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
Funded by the Centers for Disease Control and Prevention and National Institutes of Health (NIH) (Painter).
- IC
- intervention cycle
- LAIV
- live-attenuated influenza vaccine
- GRITS
- Georgia Registry of Immunization Transactions and Services
- RR
- risk ratio
REFERENCES
- 1. Advisory Committee on Immunization Practices; Smith NM, Bresee JS, Shay DK, Uyeki TM, Cox NJ, Strikas RA. Prevention and Control of Influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006;55(RR-10):1–42 [PubMed] [Google Scholar]
- 2. Davis MM, King JC, Jr, Moag L, Cummings G, Magder LS. Countywide school-based influenza immunization: direct and indirect impact on student absenteeism. Pediatrics. 2008;122(1). Available at: www.pediatrics.org/cgi/content/full/122/1/e260 [DOI] [PubMed] [Google Scholar]
- 3. King JC, Jr, Cummings GE, Stoddard J, et al. A pilot study of the effectiveness of a school-based influenza vaccination program. Pediatrics. 2005;116(6). Available at: www.pediatrics.org/cgi/content/full/116/6/e863 [DOI] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention Seasonal influenza vaccination coverage among children aged 6 months-18 years: eight immunization information system sentinel sites, United States, 2009–10 influenza season. MMWR Morb Mortal Wkly Rep. 2010;59(39):1266–1269 [PubMed] [Google Scholar]
- 5. Schmier J, Li S, King JC, Jr, Nichol K, Mahadevia PJ. Benefits and costs of immunizing children against influenza at school: an economic analysis based on a large-cluster controlled clinical trial. Health Aff (Millwood). 2008;27(2):w96–w104 [DOI] [PubMed] [Google Scholar]
- 6. White T, Lavoie S, Nettleman MD. Potential cost savings attributable to influenza vaccination of school-aged children. Pediatrics. 1999;103(6). Available at: www.pediatrics.org/cgi/content/full/103/6/e73 [DOI] [PubMed] [Google Scholar]
- 7. Wilson T. Economic evaluation of a metropolitan-wide, school-based hepatitis B vaccination program. Public Health Nurs. 2000;17(3):222–227 [DOI] [PubMed] [Google Scholar]
- 8. Carpenter LR, Lott J, Lawson BM, et al. Mass distribution of free, intranasally administered influenza vaccine in a public school system. Pediatrics. 2007;120(1). Available at: www.pediatrics.org/cgi/content/full/120/1/e172 [DOI] [PubMed] [Google Scholar]
- 9. Effler PV, Chu C, He H, et al. Statewide school-located influenza vaccination program for children 5–13 years of age, Hawaii, USA. Emerg Infect Dis. 2010;16(2):244–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. King JC, Jr, Stoddard JJ, Gaglani MJ, et al. Effectiveness of school-based influenza vaccination. N Engl J Med. 2006;355(24):2523–2532 [DOI] [PubMed] [Google Scholar]
- 11. Monto AS, Davenport FM, Napier JA, Francis T., Jr Effect of vaccination of a school-age population upon the course of an A2-Hong Kong influenza epidemic. Bull World Health Organ. 1969;41(3):537–542 [PMC free article] [PubMed] [Google Scholar]
- 12. Monto AS, Davenport FM, Napier JA, Francis T., Jr Modification of an outbreak of influenza in Tecumseh, Michigan by vaccination of schoolchildren. J Infect Dis. 1970;122(1):16–25 [DOI] [PubMed] [Google Scholar]
- 13. Fiore AE, Uyeki TM, Broder K, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59(RR-8):1–62 [PubMed] [Google Scholar]
- 14. Bryant WK, Ompad DC, Sisco S, et al. Determinants of influenza vaccination in hard-to-reach urban populations. Prev Med. 2006;43(1):60–70 [DOI] [PubMed] [Google Scholar]
- 15. Figaro MK, Belue R. Prevalence of influenza vaccination in a high-risk population: impact of age and race. J Ambul Care Manage. 2005;28(1):24–29 [DOI] [PubMed] [Google Scholar]
- 16. Harper SA, Fukuda K, Uyeki TM, Cox NJ, Bridges CB. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2005;54(RR-8):1–40 [PubMed] [Google Scholar]
- 17. Lashuay N, Tjoa T, Zuniga de Nuncio ML, Franklin M, Elder J, Jones M. Exposure to immunization media messages among African American parents. Prev Med. 2000;31(5):522–528 [DOI] [PubMed] [Google Scholar]
- 18. O'Malley AS, Forrest CB. Immunization disparities in older Americans: determinants and future research needs. Am J Prev Med. 2006;31(2):150–158 [DOI] [PubMed] [Google Scholar]
- 19. Ostbye T, Taylor DH, Lee AM, Greenberg G, van Scoyoc L. Racial differences in influenza vaccination among older Americans 1996–2000: longitudinal analysis of the Health and Retirement Study (HRS) and the Asset and Health Dynamics Among the Oldest Old (AHEAD) survey. BMC Public Health. 2003;3:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rangel MC, Shoenbach VJ, Weigle KA, Hogan VK, Strauss RP, Bangdiwala SI. Racial and ethnic disparities in influenza vaccination among elderly adults. J Gen Intern Med. 2005;20(5):426–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Winston CA, Wortley PM, Lees KA. Factors associated with vaccination of medicare beneficiaries in five U.S. communities: results from the racial and ethnic adult disparities in immunization initiative survey, 2003. J Am Geriatr Soc. 2006;54(2):303–310 [DOI] [PubMed] [Google Scholar]
- 22. Setse RW, Euler GL, Gonzalez-Feliciano AG, et al. Influenza vaccination coverage: United States, 2000–2010. MMWR Surveill Summ. 2011;60 (suppl):38–41 [PubMed] [Google Scholar]
- 23. Shefer A, Briss P, Rodewald L, et al. Improving immunization coverage rates: an evidence-based review of the literature. Epidemiol Rev. 1999;21(1):96–142 [DOI] [PubMed] [Google Scholar]
- 24. Georgia Department of Education Enrollment by race/ethnicity, gender and grade level (PK-12). Available at: http://app3.doe.k12.ga.us/ows-bin/owa/fte_pack_ethnicsex.entry_form Accessed October 28, 2010
- 25. Painter JE, Sales JM, Pazol K, Grimes T, Wingood GM, DiClemente RJ. Development, theoretical framework, and lessons learned from implementation of a school-based influenza vaccination intervention. Health Promot Pract. 2010;11(suppl 3):42S–52S [DOI] [PubMed] [Google Scholar]
- 26. Rosenstock I. Historical origins of the health belief model. Health Educ Monogr. 1974;2(4):328–335 [DOI] [PubMed] [Google Scholar]
- 27. Montano DE. Predicting and understanding influenza vaccination behavior. Alternatives to the health belief model. Med Care. 1986;24(5):438–453 [DOI] [PubMed] [Google Scholar]
- 28. US Department of Health and Human Services Vaccine Information Statement: Live, Intranasal Influenza Vaccine, 2010–2011. Atlanta, GA: US Department of Health and Human Services; 2010 [Google Scholar]
- 29. US Department of Health and Human Services Vaccine Information Statement: Inactivated Influenza Vaccine, 2010–2011. Atlanta, GA: US Department of Health and Human Services; 2010 [Google Scholar]
- 30. Unti LM, Coyle KK, Woodruff BA, Boyer-Chuanroong L. Incentives and motivators in school-based hepatitis B vaccination programs. J Sch Health. 1997;67(7):265–268 [DOI] [PubMed] [Google Scholar]
- 31. Rosenthal SL, Kottenhahn RK, Biro FM, Succop PA. Hepatitis B vaccine acceptance among adolescents and their parents. J Adolesc Health. 1995;17(4):248–254 [DOI] [PubMed] [Google Scholar]
- 32. Painter JE, Sales JM, Pazol K, et al. Psychosocial correlates of intention to receive an influenza vaccination among rural adolescents. Health Educ Res. 2010;25(5):853–864 [DOI] [PubMed] [Google Scholar]
- 33. Coleman MS, Washington ML, Orenstein WA, Gazmararian JA, Prill MM. Interdisciplinary epidemiologic and economic research needed to support a universal childhood influenza vaccination policy. Epidemiol Rev. 2006;28:41–46 [DOI] [PubMed] [Google Scholar]