Abstract
BACKGROUND:
Tests are available to measure children's exposure to tobacco smoke. One potential barrier to testing children for tobacco-smoke exposure is the belief that parents who smoke would not want their child tested. No previous surveys have assessed whether testing children for exposure to tobacco smoke in the context of their child's primary care visit is acceptable to parents.
OBJECTIVE:
To assess whether testing children for tobacco-smoke exposure is acceptable to parents.
DESIGN AND METHODS:
We conducted a national random-digit-dial telephone survey of households from September to November 2006. The sample was weighted by race and gender, based on the 2005 US Census, to be representative of the US population.
RESULTS:
Of 2070 eligible respondents contacted, 1803 (87.1%) completed the surveys. Among 477 parents in the sample, 60.1% thought that children should be tested for tobacco-smoke exposure at their child's doctor visit. Among the parental smokers sampled, 62.0% thought that children should be tested for tobacco-smoke exposure at the child's doctor visit. In bivariate analysis, lower parental education level, allowing smoking in the home, nonwhite race, and female gender were each associated (P < .05) with wanting the child tested for tobacco-smoke exposure.
CONCLUSIONS:
The majority of nonsmoking and smoking parents want their children tested for tobacco-smoke exposure during the child's health care visit.
Keywords: smoking, tobacco, pediatrics, family practice, parent, smoking cessation, secondhand smoke, environmental tobacco smoke, tobacco control, testing
WHAT'S KNOWN ON THIS SUBJECT:
Tests to measure children's exposure to tobacco smoke exist but are not currently used in the child health care setting. No previous national studies have assessed whether parents would accept testing of their children in this context.
WHAT THIS STUDY ADDS:
This study shows that the majority of parents in general, and even parents who smoke, want their children tested for tobacco-smoke exposure in the context of the child's health care setting.
According to parent self-reports, over 30% of children are exposed to tobacco smoke in their own homes.1–3 However, biochemical measures of exposure reveal that over two-thirds of children actually are exposed to tobacco smoke.4 The difference between self-reported and biochemically measured exposure may be reflective of social desirability bias or exposure that occurs from other units in multiunit housing,5 outside of the home, in the car, in day care, in restaurants, on public sidewalks, in doorways, or from other venues.
In 2006, the Surgeon General concluded that there is no safe level of exposure to tobacco smoke.6 The vast majority of parents, regardless of smoking status, understand that visible secondhand smoke is harmful to children.7 Knowing that secondhand smoke is harmful, parents usually try to minimize child exposure by opening windows, turning on fans, or smoking outside.7,8 These measures are of variable success because tobacco smoke can drift and contaminate surfaces and people with residual tobacco-smoke poisons,9 also known as third-hand smoke.7 Sometimes this contamination has a characteristic odor, but the extent to which people notice it may vary as a result of decreased olfaction in people who smoke, the presence of other masking odors, and the intensity of the tobacco contamination.
An existing test for tobacco-smoke exposure that measures cotinine, the principle metabolite of nicotine, has good sensitivity for detecting exposure that has occurred in the past 3 days.10 Such a test might be important for explaining the concept that invisible tobacco contamination still can be absorbed into children despite the protections that parents put in place to mitigate visible tobacco-smoke exposure. Previous studies have shown that beliefs about this residual contamination, or third-hand smoke, are strongly associated with strict rules prohibiting smoking in the home.7
One potential barrier to testing children for tobacco-smoke exposure is the belief that parents who smoke may not want their child tested. No previous surveys have assessed parental acceptance of testing for tobacco-smoke exposure in the context of the child's health care visit. We hypothesized that compared with parents who do not smoke, parents who smoke would have lower rates of wanting their child tested for tobacco-smoke exposure.
METHODS
Respondents
The Social Climate Survey of Tobacco Control was administered to a representative sample of US adults from September to November of 2006. Households were selected using random-digit dialing procedures. Once a household was reached, the adult to be interviewed was selected by asking to speak with the person in the household who is 18 years of age or older and who will have the next birthday. The survey frame is limited to households with landline telephones and does not include households that are wireless only. Educational attainment is higher among this sample than in the US population. The underrepresentation of adults with lower levels of education may be attributed to wireless substitution. Wireless substitution continues to increase and reduces the validity of surveys that omit wireless-only households from the sample frame. However, the wireless substitution rate was substantially lower when we collected these data in 2006 (12.8%) than the most recent estimate from the last 6 months of 2009 (24.5%).11 The sample was weighted by race and gender within each census region, based on current US Census estimates. The institutional review board at Mississippi State University reviewed and approved this project on June 8, 2006. Informed consent was obtained orally as part of the introduction to the telephone interview by trained interviewers.
Social Climate Survey of Tobacco Control
The Social Climate Survey of Tobacco Control is an annual cross-sectional survey designed to operationalize the concept of the social climate of tobacco into a comprehensive set of quantifiable social and environmental indicators across the social institutions that characterize society. These social institutions include (1) family and friendship groups; (2) education; (3) workplace; (4) government and political order; (5) health and medical care; (6) recreation, leisure, and sports; and (7) mass culture and communication. Survey items were developed and selected on the basis of an extensive review of extant tobacco-control surveys and then reviewed by a panel of tobacco-control researchers.
Measures
Two questions from the Behavior Risk Factor Surveillance System and the National Health Interview Survey were used to assess the current smoking status of respondents. Respondents were asked, “Have you smoked at least 100 cigarettes in your entire life?” Respondents who reported that they had were then asked, “Do you now smoke cigarettes every day, some days, or not at all?” Respondents who reported that they now smoke every day or some days were categorized as current smokers.
To determine whether a child resided in the household, respondents were asked, “How many children under 18 years of age currently live in your household?” Respondents who reported that at least 1 child lived in their household were asked, “How old are each of your children?” Parent status was defined as having at least 1 of their children under the age of 18 living in their household at the time of the survey.
To assess the general rate of accepting a test for tobacco-smoke exposure of their child, parents were asked, “If a test were available from your child's doctor that would show whether or not your child has been exposed to secondhand smoke, would you get your children tested?” The response categories were “yes,” “no,” and “don't know.” We also asked whether health insurance should pay for this test, with the same response categories.
Specific questions about the acceptability of a blood test were asked because blood draws are recommended at 1 and 2 years of age, and this would potentially be a feasible way to incorporate such testing without increasing practice burden. All respondents were asked, “If this test were a blood test, would you get your children tested in any case or only if they were already getting blood drawn for another reason?” The response categories were “yes,” “yes, but only if my child were already getting blood drawn for another reason,” “no,” and “don't know.” Levels of acceptability for the blood test were categorized as “would accept test,” “would accept test only if blood were being drawn for another reason,” “would not accept test,” and “don't know,” which was categorized as “would not accept test.” The acceptability of a urine-based test was not specifically assessed.
Rules about smoking in the home were tested with 2 separate questions. Half the sample was asked the standard question, “Please tell me which best describes how cigarette smoking is handled in your home,” with the following 4 response categories: (1) “No one is allowed to smoke in my home;” (2) “Only special guests are allowed to smoke in my home;” (3) “People are allowed to smoke only in certain areas of my home;” and (4) “People are allowed to smoke anywhere in my home.”12,13 Half the sample was asked a simplified version of the question to improve future administration of this item: “Which statement best describes the rules about smoking in your home?” with the following 3 response categories: (1) “No one is allowed to smoke anywhere;” (2) “Smoking is permitted in some places or at some times;” or (3) “Smoking is permitted anywhere.” Because the questions about strict rules and permissive categories were nearly identical, with the 3-category answer picking up slightly more permissive policies, we combined the data into the following 3 categories: no one allowed to smoke; smoking allowed some places or times; and smoking permitted anywhere. Additional validated questions were drawn from previous surveys and have been described elsewhere.14,15
Analysis
In exploratory analyses, we used χ2 procedures to compare differences between region, gender, race (white versus nonwhite), age, education, residence (rural versus urban), physician type (pediatrician versus family practitioner), having a home smoking ban, household member smoking status, and known tobacco-smoke exposure of the child in the past week and differences in parent attitudes about testing. Associations were considered significant at the P < .05 level. In our multivariate analyses, we treated “don't know” and “refused to answer” as missing data. The percentage of questions answered in each question set is reported in the footnotes of each data table.
Multivariate logistic regression models controlling for gender, race, age, education, and rural versus urban residence were used to examine whether smoking status was independently associated with acceptability of testing. A more extensive final model included key variables thought to be related to the outcome variable: physician type (pediatrician versus family practitioner), having a home smoking ban, household member smoking status, and known tobacco exposure of the child in the past week.
RESULTS
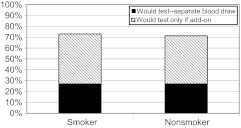
Of 2070 eligible respondents contacted, 1803 (87.1%) completed the surveys and 18.6% were smokers. The study sample included 477 parents, of which 19.2% smoked. Table 1 gives the basic frequency characteristics of the survey sample. One-third of all parents believed that their child was exposed to tobacco smoke in the past week. Overall, 60.1% of the sample would accept testing their child for tobacco-smoke exposure. However, when a blood test was specifically queried and included a choice for testing when blood was already being drawn for another reason, 70.1% of the nonsmoking parents endorsed testing compared with 73.6% of the parents who smoke (Fig 1). A similarly large majority (73%) believed insurance should pay for tobacco-smoke exposure testing.
TABLE 1.
Sample Characteristics
| Characteristicsa | Percentage of Total, n = 477 |
|---|---|
| Gender | |
| Male | 45.5 |
| Female | 54.5 |
| Self-reported race | |
| White | 64.9 |
| Nonwhite | 35.1 |
| Age, y | |
| 18–24 | 24.3 |
| 25–44 | 56.5 |
| ≥45 | 19.2 |
| Education | |
| Less than high school | 6.4 |
| High school graduate | 29.1 |
| Some college | 24.0 |
| College degree | 40.5 |
| Geographic region | |
| Northeast | 18.6 |
| Midwest | 22.6 |
| South | 37.1 |
| West | 21.7 |
| Community description | |
| Rural | 25.3 |
| Urban | 74.7 |
| Child's primary care provider | |
| Pediatrician | 71.4 |
| Family practitioner | 26.9 |
| Smoking status | |
| Smoker | 19.2 |
| Nonsmoker | 80.8 |
| Other adult smoker in home | |
| No | 82.6 |
| Yes | 17.4 |
| Child exposed to secondhand smoke in past week | |
| Yes | 38.0 |
| No | 62.0 |
| Home smoking rule | |
| Smoking allowed | 20.1 |
| Smoking not allowed | 79.9 |
| Would accept test | |
| Yes | 60.1 |
| No | 30.3 |
| Do not know | 9.1 |
Items in this question set were answered by 99% of respondents, except for education 94.2% and smoking status 97.2%.
FIGURE 1.
Acceptability of blood test.
In bivariate analysis, lower parent education, smoking allowed in the home, nonwhite race, and female gender were each associated with wanting one's child tested for tobacco-smoke exposure (P < .05 for each). (Table 2). Small cell size did not permit detailed comparison of our findings by race. In a final multivariate logistic regression model controlling for gender, race, age, education, practice type, geographic location, rural urban residence, known exposure of the child in the past 7 days, and home smoking rules, we found no significant differences by parent smoking status in attitudes about testing children (odds ratio: 0.703 [95% confidence interval: 0.328–1.53]). Other simpler models with sociodemographic variables also did not yield any independent associations with parent smoking status or wanting the child tested. However, in our final model, compared with those who graduated college, those who had only a high school education (odds ratio: 4.91 [95% confidence interval: 1.09–22.1]) or some college (odds ratio: 5.29 [95% confidence interval: 1.19–23.5]) were more likely to want testing. Compared with white race, nonwhite race remained independently associated with wanting testing (odds ratio: 3.43 [95% confidence interval: 1.718–6.85]). Low cell size may have limited our ability to detect other independent associations.
TABLE 2.
Acceptability of Testing Among Parents
| Would Test, n = 281 | Would Not Test, n = 149 | Do Not Know, n = 46 | Pa | |
|---|---|---|---|---|
| Smoking status of respondent | ||||
| Nonsmoker | 60.6 | 29.9 | 9.4 | |
| Smoker | 62.0 | 28.9 | 9.1 | .963 |
| Other adult smoker in house | ||||
| Yes | 63.7 | 29.2 | 7.1 | |
| No | 59.7 | 30.8 | 9.6 | .618 |
| Gender | ||||
| Male | 56.5 | 36.0 | 7.5 | |
| Female | 64.0 | 25.4 | 10.7 | .011 |
| Race | ||||
| White | 52.7 | 36.6 | 10.7 | |
| Nonwhite | 74.7 | 19.1 | 6.2 | <.001 |
| Age, y | ||||
| 18–24 | 65.4 | 26.8 | 7.8 | |
| 25–44 | 61.4 | 28.7 | 9.9 | |
| 45–64 | 52.8 | 39.0 | 8.1 | .163 |
| Education | ||||
| <12 y | 82.1 | 15.4 | 2.6 | |
| High school graduate | 68.0 | 25.8 | 6.2 | |
| Some college | 55.1 | 33.3 | 11.6 | |
| College graduate | 54.0 | 33.9 | 12.1 | .004 |
| Region | ||||
| Northeast | 63.6 | 28.9 | 7.4 | |
| Midwest | 54.9 | 34.0 | 11.1 | |
| South | 63.9 | 29.0 | 7.1 | |
| West | 56.7 | 30.5 | 12.8 | .350 |
| Residence | ||||
| Urban | 58.8 | 31.8 | 9.4 | |
| Rural | 65.2 | 26.2 | 8.5 | .760 |
| Child's physician | ||||
| Family practitioner | 56.9 | 33.0 | 10.1 | |
| Pediatrician | 66.1 | 25.3 | 8.7 | .225 |
| Child exposed to secondhand smoke in the past 7 daysb | ||||
| Yes | 63.0 | 28.0 | 9.1 | |
| No | 59.0 | 31.8 | 9.2 | .563 |
| Home smoking rule | ||||
| Smoking is not allowed | 58.5 | 31.0 | 10.5 | |
| Smoking is allowed | 67.7 | 28.5 | 3.8 | .036 |
Items in this question set answered by more than 97% of respondents, except for education 94.2% and physician type 61.3%.
Any “yes” to “during the past 7 days, in which of the following places: home, car, someone else's car, daycare, indoor public place, relative's house, friends house, some other place, have your children been exposed to secondhand smoke?”
DISCUSSION
We found that the majority of parents wanted their child tested for tobacco-smoke exposure in the context of their pediatric visit. Somewhat surprisingly, a similar majority of parents who smoke wanted their child tested. Majority support for testing persisted across all sociodemographic, geographic, and practitioner categories surveyed. Parents with lower education, women, nonwhites, and those who lived in homes where smoking was allowed were all more likely to want their child tested for tobacco-smoke exposure. Favorable parental attitudes toward testing in some of these groups may be reflective of greater tobacco exposure of their children, making the test more likely to show a positive exposure result.
Strong majorities of parents who smoke and those who do not would accept testing if it were an add-on to an existing blood test. This additional information about blood-test acceptability could be important in deciding how to operationalize a test for tobacco-smoke exposure in the context of busy office practices.
The study results are more generalizable because of the nationally representative sample; however, detailed racial and ethnic comparisons were limited by small cell size. Understanding more about how testing children for tobacco smoke might be perceived by the respondents, beyond simple approval of the test, was not possible in this survey.
Currently, there is not 1 “ideal” test for tobacco-smoke exposure, so the current gold standard is probably a combination of survey measures and cotinine testing.16,17 Having a biological test may decrease the underreporting of tobacco use and exposure that normally occurs as a result of the social stigma associated with tobacco use.18–20
Getting test results of exposure may help put some parents at ease, knowing that their children are unexposed. However, more likely, it will confirm a suspicion of exposure. In this case, the results might help nonsmokers advocate for safer environments for themselves and their children at home, in the car, at day care, or in other places children spend time. Parental smokers themselves may be genuinely curious to know whether their mitigation efforts are working. Some smokers may believe it is safe to smoke inside when children are not present. However, recent studies indicate that house dust and surfaces become contaminated when cigarettes are smoked indoors.9 When faced with data showing that their children still are exposed, they may enforce stricter smoking bans, have increased motivation to quit smoking, or be more likely to use nicotine-replacement therapy.
At present, the marker that is best suited to detect an individual's tobacco use and exposure is cotinine, a primary metabolite of nicotine and thus specific for tobacco smoke.21 Cotinine can be measured in a variety of tissues and body fluids, including blood, saliva, urine, hair, nails, and teeth. The levels of cotinine in each of these fluids and tissues vary on the basis of the type of sample, the intensity of the exposure, the time elapsed since exposure, and the metabolism of the individual. At present, there are no commercially available assays for cotinine assessment other than the urine dipsticks that are designed for qualitative assessment of active smoking.22 Low-level cotinine assessment is currently only accomplished by specific research laboratories and thus is not reimbursable by insurance. In addition, these research assays all require a substantial amount of processing, thus results are not available for immediate feedback and more often take weeks for reporting. Regarding the type of sample best suited for use in children, serum seems to be the most reliable.10 Given that many young children are screened for lead exposure and iron deficiency by a blood test, an additional aliquot of serum for cotinine assay at these testing points would not alter current pediatric practice.
Saliva collection may be problematic because of the need for the child to hold a swab in his or her mouth for a sufficient time to collect an adequate sample. Collection of hair samples, indicating a longer period of tobacco-smoke exposure, also can be challenging because they need to be cut closely to the scalp, and the direction of hair growth must be indicated on the sample. Collection of a sufficient quantity of hair may be objectionable for parents. Similarly, collection of a sufficient quantity of nail clippings is difficult in a young child.
Urine is arguably one of the easiest samples to obtain, either by a toilet-trained child voiding into a cup or a diapered child voiding onto cotton batting from which the urine can be collected. The hydration status of the child may alter the levels of cotinine substantially, however, requiring a correction for urinary creatinine,6 which can vary substantially with the age of the child. In addition, there is a great deal of variability between subjects and within subjects for urine, saliva, and serum cotinine, such that a similar cotinine level from 2 different subjects may represent differing levels of tobacco-smoke exposure. In addition, within an individual child, a single urine cotinine is only accurate for very recent (2–3 days) exposure, with a high level of variability such that an individual measure is very unlikely to represent an average tobacco-smoke exposure over time.23 As such, single measures of urinary cotinine cannot reliably ascertain changes in exposure to tobacco smoke.
Single measures of cotinine can be used qualitatively, however, to demonstrate exposure to tobacco smoke. In several studies24–27, of variable success, that have sought to use cotinine feedback to intervene with parents about their own smoking as it impacts on their child's cotinine levels, none were able to provide rapid feedback of tobacco-smoke exposure levels because of the testing procedure, thus personal correlation by the parent or caregiver of times of exposure would have been difficult.24–27 These studies attempted to use cotinine as a biomarker for tobacco-smoke exposure reduction and did not focus on the delivery of state-of-the-art tobacco dependence treatment for parents. In addition, the overall source of exposure to tobacco smoke is likely more complex than has often been considered, and a cotinine level is likely to be a blend of actual smoke exposure plus that of nicotine contamination, or third-hand smoke. Components of tobacco smoke, including nicotine, can be absorbed into surfaces such as furniture, walls, carpets, and clothing or deposit as house dust and then be reemitted as volatile toxic compounds over a period of days to months.9,28 Use of a single cotinine measure as a marker of exposure to home tobacco smoke is likely to be confounded by this tobacco-smoke contamination and its persistence. Therefore, future interventions that include biofeedback of child exposure may need to focus on using exposure as a teachable moment for delivery of cessation medications and quitline enrollment coupled with longer-term retesting for child exposure.
CONCLUSIONS
By itself, knowledge about the child being exposed to tobacco may not motivate many people to quit smoking. However, future interventions might use testing as adjunctive support for cessation and setting strict no-smoking policies in the home and car. In cases where the parent does not know of any exposure, a child's positive test may lead to the discovery of an unknown source, such as a previously contaminated home, a caregiver's secret smoking, or seepage of tobacco smoke into the home, such as from another unit in multiunit housing.5,9,29 In the case of multiunit housing, such information might help landlords set no-smoking policies for the entire building affected or help clinicians support parents seeking smoke-free environments in the context of the unequal tenant-landlord dynamic. In many jurisdictions, the public supports outdoor smoke-free legislation, especially in areas frequented by children.30 Nonsmoking families who discovered positive tests in their children would likely advocate for stricter outdoor smoke-free legislation. The findings in this study support ongoing efforts to document the tobacco-smoke exposure status of children in the context of child health care settings, so that appropriate tobacco-control interventions can occur.
ACKNOWLEDGMENTS
This publication was made possible by grant 4 D1A RH 00005-01-01 from the Office of Rural Health Policy of the Department of Health and Human Services through the Rural Health Safety and Security Institute, Social Science Research Center, Mississippi State University; and by a grant from the Flight Attendant Medical Research Institute to the American Academy of Pediatrics Julius B Richmond Center of Excellence.
Jonathan P Winickoff, MD, MPH, was supported by grants from the Flight Attendant Medical Research Institute (024032), the National Cancer Institute (NCI) (K07 CA100213 A 01), and the NCI/National Institute on Drug Abuse/Agency for Healthcare Research and Quality (R01 CA127127-01).
Footnotes
This work was presented in part at the Annual Meetings of the Pediatric Academic Societies, Toronto, Ontario, Canada, May 5–8, 2007.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the Office of Rural Health Policy or the American Academy of Pediatrics.
FINANCIAL DISCLOSURE: The authors have indicated that they have no personal financial relationships relevant to this article to disclose.
REFERENCES
- 1. Schuster MA, Franke T, Pham CB. Smoking patterns of household members and visitors in homes with children in the United States. Arch Pediatr Adolesc Med. 2002;156(11):1094–1100 [DOI] [PubMed] [Google Scholar]
- 2. Soliman S, Pollack HA, Warner KE. Decrease in the prevalence of environmental tobacco smoke exposure in the home during the 1990s in families with children. Am J Public Health. 2004;94:314–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. King K, Martynenko M, Bergman MH, Liu YH, Winickoff JP, Weitzman M. Family composition and children's exposure to adult smokers in their homes. Pediatrics. 2009;123(4). Available at: www.pediatrics.org/cgi/content/full/123/4/e559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moritsugu KP. The 2006 Report of the Surgeon General: the health consequences of involuntary exposure to tobacco smoke. Am J Prev Med. 2007;32(6):542–543 [DOI] [PubMed] [Google Scholar]
- 5. Kraev TA, Adamkiewicz G, Hammond SK, Spengler JD. Indoor concentrations of nicotine in low-income, multi-unit housing: associations with smoking behaviours and housing characteristics. Tob Control. 2009;18(6):438–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. US Department of Health and Human Services The Health Consequences of Involuntary Tobacco Smoke: A Report of the Surgeon General. Washington, DC: US Department of Health and Human Services; 2006 [Google Scholar]
- 7. Winickoff JP, Friebely J, Tanski SE, et al. Beliefs about the health effects of “thirdhand” smoke and home smoking bans. Pediatrics. 2009;123(1). Available at: www.pediatrics.org/cgi/content/full/123/1/e74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johansson A, Hermansson G, Ludvigsson J. How should parents protect their children from environmental tobacco-smoke exposure in the home? Pediatrics. 2004;113(4). Available at: www.pediatrics.org/cgi/content/full/113/1/e291 [DOI] [PubMed] [Google Scholar]
- 9. Matt GE, Quintana PJ, Hovell MF, et al. Households contaminated by environmental tobacco smoke: sources of infant exposures. Tob Control. 2004;13(1):29–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matt GE, Bernert JT, Hovell MF. Measuring secondhand smoke exposure in children: an ecological measurement approach. J Pediatr Psychol. 2008;33(2):156–175 [DOI] [PubMed] [Google Scholar]
- 11. Blumberg SJ, Luke JV. National Center for Health Statistics. Wireless substitution: early release of estimates from the National Health Interview Survey, July–December 2009 [article online], 2010. Available at: www.cdc.gov/nchs/data/nhis/earlyrelease/wireless201005.htm Accessed Nov 22, 2010
- 12. Winickoff JP, Hillis VJ, Palfrey JS, Perrin JM, Rigotti NA. A smoking cessation intervention for parents of children who are hospitalized for respiratory illness: the stop tobacco outreach program. Pediatrics. 2003;111(1):140–145 [DOI] [PubMed] [Google Scholar]
- 13. Abrams DB, Boutwell WB, Grizzle J, Heimendinger J, Sorensen G, Varnes J. Cancer control at the workplace: the Working Well Trial. Prev Med. 1994;23(1):15–27 [DOI] [PubMed] [Google Scholar]
- 14. Winickoff JP, Tanski SE, McMillen RC, Hipple BJ, Friebely J, Healey EA. A national survey of the acceptability of quitlines to help parents quit smoking. Pediatrics. 2006;117(4). Available at: www.pediatrics.org/cgi/content/full/117/4/e695 [DOI] [PubMed] [Google Scholar]
- 15. American Academy of Pediatrics Julius B Richmond Center of Excellence The Social Climate of Tobacco Control [Web site]. Available at: www.socialclimate.org/ Accessed Nov 22, 2010
- 16. Hovell MF, Meltzer SB, Wahlgren DR, et al. Asthma management and environmental tobacco smoke exposure reduction in Latino children: a controlled trial. Pediatrics. 2002;110(5):946–956 [DOI] [PubMed] [Google Scholar]
- 17. Hovell MF, Zakarian JM, Wahlgren DR, Matt GE, Emmons KM. Reported measures of environmental tobacco smoke exposure: trials and tribulations. Tob Control. 2000;9(Suppl 3):III22–III28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Callais F, Momas I, Roche D, Gauvin S, Reungoat P, Zmirou D. Questionnaire or objective assessment for studying exposure to tobacco smoke among asthmatic and healthy children: the French VESTA Study. Prev Med. 2003;36(1):108–113 [DOI] [PubMed] [Google Scholar]
- 19. Seifert JA, Ross CA, Norris JM. Validation of a five-question survey to assess a child's exposure to environmental tobacco smoke. Ann Epidemiol. 2002;12(4):273–277 [DOI] [PubMed] [Google Scholar]
- 20. Wells AJ, English PB, Posner SF, Wagenknecht LE, Perez-Stable EJ. Misclassification rates for current smokers misclassified as nonsmokers. Am J Public Health. 1998;88(10):1503–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev. 1996;18(2):188–204 [DOI] [PubMed] [Google Scholar]
- 22. Best D, Green EM, Smith JH, Perry DC. Dipstick tests for secondhand smoke exposure. Nicotine Tob Res. 2010;12(6):551–556 [DOI] [PubMed] [Google Scholar]
- 23. Matt GE, Hovell MF, Quintana PJ, et al. The variability of urinary cotinine levels in young children: implications for measuring ETS exposure. Nicotine Tob Res. 2007;9(1):83–92 [DOI] [PubMed] [Google Scholar]
- 24. Chilmonczyk BA, Palomaki GE, Knight GJ, Williams J, Haddow JE. An unsuccessful cotinine-assisted intervention strategy to reduce environmental tobacco smoke exposure during infancy. Am J Dis Child. 1992;146(3):357–360 [DOI] [PubMed] [Google Scholar]
- 25. McIntosh NA, Clark NM, Howatt WF. Reducing tobacco smoke in the environment of the child with asthma: a cotinine-assisted, minimal-contact intervention. J Asthma. 1994;31(6):453–462 [DOI] [PubMed] [Google Scholar]
- 26. Wakefield M, Banham D, McCaul K, et al. Effect of feedback regarding urinary cotinine and brief tailored advice on home smoking restrictions among low-income parents of children with asthma: a controlled trial. Prev Med. 2002;34(1):58–65 [DOI] [PubMed] [Google Scholar]
- 27. Wilson SR, Yamada EG, Sudhakar R, et al. A controlled trial of an environmental tobacco smoke reduction intervention in low-income children with asthma. Chest. 2001;120(5):1709–1722 [DOI] [PubMed] [Google Scholar]
- 28. Singer BC, Hodgson AT, Guevarra KS, Hawley EL, Nazaroff WW. Gas-phase organics in environmental tobacco smoke: 1. effects of smoking rate, ventilation, and furnishing level on emission factors. Environ Sci Technol. 2002;36(5):846–853 [DOI] [PubMed] [Google Scholar]
- 29. Winickoff JP, Gottlieb M, Mello MM. Regulation of smoking in public housing. N Engl J Med. 2010;362(24):2319–2325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thomson G, Wilson N, Edwards R. At the frontier of tobacco control: a brief review of public attitudes toward smoke-free outdoor places. Nicotine Tob Res. 2009;11(6):584–590 [DOI] [PubMed] [Google Scholar]