Abstract
OBJECTIVE:
We compared associations of epilepsy remission status and severity as well as psychiatric and other comorbidities with child and parent-proxy reports of health-related quality of life (HRQoL) in adolescents previously diagnosed with epilepsy.
METHODS:
In a prospective, community-based study of newly diagnosed childhood epilepsy, HRQoL of 277 children was assessed 8 to 9 years after diagnosis by using child and parent-proxy versions of the Child Health Questionnaire (CHQ). Multiple linear regression models adjusted for age and gender were used to compare associations of epilepsy remission and “complicated” epilepsy (secondary to an underlying neurologic insult or epileptic encephalopathy) status and psychiatric and other comorbidities with HRQoL.
RESULTS:
Mean age of epilepsy onset was 4.4 years (SD: 2.6). At the 9-year reassessment, children were, on average, 13.0 years old (SD: 2.6); 64% were seizure-free for 5 years, 31% were taking antiepileptic drugs, and 19% had a complicated epilepsy. Prevalence of comorbidities at follow-up were 26% psychiatric diagnosis; 39% neurodevelopmental spectrum disorder (NDSD); 24% chronic medical illness; and 15% migraine. In multivariable analysis, having a psychiatric disorder was broadly associated with child (6 of 11 scales) and parent-proxy (7 of 12 scales) HRQoL (P ≤ .0125). Five-year remission and complicated epilepsy status had few or no associations with HRQoL. Although parent-proxy HRQoL was strongly associated with NDSD (6 of 11 scales), child-reported HRQoL was not (2 of 11 scales).
CONCLUSIONS:
Psychiatric comorbidities are strongly associated with long-term HRQoL in childhood-onset epilepsy, which suggests that comprehensive epilepsy care must include screening and treatment for these conditions, even if seizures remit.
Keywords: epilepsy, psychiatric comorbidity, quality of life, Child Health Questionnaire, child and adolescent health
WHAT'S KNOWN ON THIS SUBJECT:
Children with epilepsy have a high burden of psychiatric and neurodevelopmental comorbidity, and they experience poor long-term psychosocial outcomes even after remission of their epilepsy. Little is known about how such comorbidities are associated with quality of life in adolescence.
WHAT THIS STUDY ADDS:
At long-term follow-up, psychiatric comorbidity has a greater impact on quality of life than does epilepsy remission status, which raises concerns about screening and treating mental health disorders in adolescents with childhood-onset epilepsy as they transition to adult care settings.
Epilepsy is a common neurologic disease with a lifetime cumulative risk of 1% to 2%; approximately one-half of epilepsy in the population first occurs during childhood.1 Given the broad physical and psychosocial impacts of childhood-onset epilepsy, measurement of health-related quality of life (HRQoL) is a critical patient-reported outcome measure.2 Beyond seizures, children with epilepsy (CWE) face numerous challenges including a high burden of psychiatric and behavioral comorbidities that often go untreated or even unrecognized.3–10 Such comorbidity might predate the onset of epilepsy or persist in the setting of seizure remission, thereby suggesting that epilepsy is a pervasive condition encompassing more than seizures.11,12 The observation that CWE, many of whom are in remission, have poor psychosocial outcomes as adults with respect to employment, educational attainment, socioeconomic status, and quality of life, highlights the pervasiveness of epilepsy's health impacts and suggests that factors not related to seizures might have an impact on psychosocial outcomes, including HRQoL.13–18 These factors include more than simply having a chronic disease; worse psychosocial outcomes have been observed in children with absence epilepsy compared with children with juvenile rheumatoid arthritis.19
Psychiatric comorbidity might negatively impact long-term HRQoL outcomes in CWE.20 Among adults with epilepsy, comorbid psychiatric conditions, particularly depression and anxiety, have been shown to adversely impact HRQoL; their influence might be even stronger than epilepsy-related factors.21–29 Although several studies have examined factors associated with poor HRQoL in CWE, in these studies investigators have typically focused on the role of epilepsy-related factors like seizure severity and frequency, syndromes, number of antiepileptic drugs (AEDs) and toxicity, and sociodemographic variables.30–42 Although a limited number of studies have revealed a negative association of comorbidity with HRQoL in CWE, no studies have been performed to examine the independent effects of chronic comorbidities, including psychiatric and neurodevelopmental disorders, on HRQoL in CWE nearly a decade after childhood-onset epilepsy.30,35–37,40,43–46
In a community-based study of children with newly diagnosed epilepsy followed prospectively, we examined the relative impacts of 5-year remission and “complicated” epilepsy (secondary to an underlying neurologic insult or epileptic encephalopathy) status, in addition to chronic comorbidity (eg, psychiatric diagnosis, neurodevelopmental disorders, migraine, chronic medical conditions) to determine if they were differentially associated with HRQoL at follow-up during adolescence. Given evidence of persistent poor long-term psychosocial outcomes among children with childhood-onset epilepsy, even in the setting of seizure remission, in conjunction with evidence that psychiatric comorbidity is more strongly associated with HRQoL than epilepsy-related factors among adults with epilepsy, we hypothesized that psychiatric or neurodevelopmental comorbidity would be more strongly associated with worse long-term HRQoL than 5-year remission status. Because previous studies have revealed that CWE and their parents have varying perspectives about the overall impact of epilepsy on HRQoL, a secondary aim of our study was to determine if parents and children report these associations with the child's HRQoL differently; we hypothesized that parent-child differences would exist.47–48
METHODS
Sample
The Connecticut Study of Epilepsy is a prospective, community-based cohort study with ongoing prospective follow-up, for which 613 children with newly diagnosed epilepsy were recruited (1993–1997). Details of methods, recruitment, and follow-up were published previously.49 Inclusion criteria were: initial diagnosis of epilepsy by participating physicians (or diagnosis by a nonparticipating physician with referral to a participating physician within 3 months of diagnosis) during the recruitment period and first of 2 or more unprovoked seizures having occurred between the ages of 28 days and 15 years.
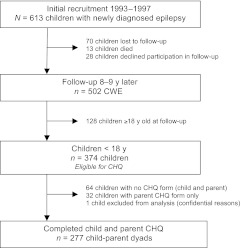
Nine years after enrollment (2002–2006), a time interval felt to be sufficient to evaluate long-term outcomes in CWE, children and family members from the original cohort participated in a reassessment protocol that included evaluation of HRQoL of the child with epilepsy by both the child with epilepsy and the child's parent.12 The Child Health Questionnaire (CHQ) was used to assess HRQoL in CWE younger than 18 years; 278 child-parent dyads completed the CHQ. One child-parent dyad was subsequently excluded from this analysis for confidential reasons (n = 277) (Fig 1). Details regarding recruitment, retention, and sample selection are in Appendix 1.
FIGURE 1.
Recruitment, follow-up, and sample selection.
Institutional review board approval was obtained at all sites. Written informed consent was obtained from the parent and written assent from the child at the time of initial enrollment and at the 9-year assessment.
Data Collection
Clinical and demographic data of children and parents were obtained via a structured in-person interview with the parent by trained research associates during initial enrollment and at the 9-year assessment, in conjunction with ongoing review of neurologic medical records and telephone interviews.12,49–51 Cognitive function was assessed with information from neurologic medical records, parent interviews, school records, and standardized neuropsychological testing using the Wechsler Intelligence Scale for Children (Appendix 2).52–54
At the 9-year assessment children were classified as having ever had (or not) 1 of several chronic comorbidities: (1) psychiatric disorder; (2) neurodevelopmental spectrum disorder (NDSD); (3) migraine; or (4) chronic medical condition. Chronic comorbidities were not mutually exclusive. We categorized children as having a psychiatric comorbidity if they had ever been given a diagnosis of depression, anxiety disorder, bipolar disorder, obsessive-compulsive disorder (OCD), oppositional defiant disorder (ODD), attention-deficit/hyperactivity disorder (ADHD), or conduct disorder as recorded in parental interviews and medical chart review. We further classified psychiatric disorders into internalizing (depression, anxiety disorder, bipolar disorder, OCD) and externalizing disorders (ODD, ADHD, conduct disorder).55 Children were classified with NDSD from information obtained during the structured parent interview and from medical record abstraction. Parents were asked if their child had ever been diagnosed or described as having: (1) developmental delay, (2) language delay, (3) language problems, and (4) dyslexia. NDSD also included children with a diagnosis of autism spectrum disorder, the diagnosis of which was based on parental interview, medical chart review, and expert review of medical records by a child psychiatrist using diagnostic criteria of the Diagnostic and Statistical Manual of Mental Disorders, Third Edition.56 Children were classified as having ever been diagnosed with migraine by use of parental interview with corroboration from medical records. Migraine was analyzed as a separate comorbidity category because it is a paroxysmal neurologic comorbidity, common in CWE, and often treated with AEDs. Chronic medical comorbidity was categorized by using information from parent interview only as the study obtained only neurologic medical records. Specifically, parents reported if their child had any of the following conditions: (1) diabetes; (2) asthma; (3), cancer; (4) arthritis; or (5) other chronic disease requiring continued treatment or monitoring. Because of the high number of reports of “allergy” in the “other” disease category, an “allergy” subcategory was delineated among those children with a chronic medical condition.
At the 9-year assessment we classified children using several epilepsy indicators. Remission status was defined as being seizure-free for 5 years or not, a definition consistent with previous literature.15–18,57–58 Medication status was classified as either currently taking or not taking AEDs. Children were designated as having a “complicated” epilepsy, a proxy for epilepsy etiology and syndrome, if their epilepsy was secondary to an underlying neurologic insult or an electroclinical syndrome considered to be an epileptic encephalopathy.59
Measures
HRQoL was assessed by using the CHQ, a self-administered, generic HRQoL measure with child (CHQ-CF87) and parent-proxy (“parent-reported”) (CHQ-PF50) versions.60 The CHQ-CF87 includes 87 items in 11 scales (physical function, role/social limitations–physical, role/social limitations–behavioral, role/social limitations–emotional, mental health, self-esteem, behavior, bodily pain/discomfort, general health perceptions, family activities) and 2 global items (behavior, general health). The CHQ-PF50 includes 50 items in 12 scales (physical function, role/social limitations–physical, role/social limitations–emotional/behavioral, mental health, self-esteem, behavior, bodily pain/discomfort, general health perceptions, family activities, parent impact on time, parent emotional impact), 2 global items (behavior, general health), and 2 summary scores (physical, psychosocial). Each score is transformed to a 0-to-100 scale (100 indicates best health). Summary scores (CHQ-PF50 only) are transformed to T scores (mean: 50; SD: 10) calculated against a reference general US population.
Analysis
We used 4 multiple linear regression models to assess unique associations of chronic comorbidities and epilepsy indicators with child-reported and parent-reported HRQoL. For all models, dependent variables were each scale, item, or summary score of the CHQ-CF87 or CHQ-PF50. All models were adjusted for age and gender. In model 1 the independent variables included 4 dichotomized comorbidity (psychiatric, NDSD, migraine, and chronic medical condition) indicators and 2 epilepsy (5-year remission and complicated epilepsy status) indicators. Current AED status was not included in model 1 because it was highly correlated with 5-year seizure-free status (r = −0.70). (In a sensitivity analysis including current AED status in lieu of 5-year remission status (model 2), results were similar.) All other covariates had correlations of r ≤ 0.50 with each other. Multicollinearity was assessed by using variance inflation factor (vif) testing (vif ≤ 1.48).61 Adjusted predicted means were calculated for having a given comorbidity compared with not having the comorbidity, in addition to being seizure-free for 5 years versus not in model 1. To rule out an interaction between psychiatric comorbidity and 5-year remission status, we analyzed child-reported and parent-reported HRQoL by using the same variables as in model 1, except that the analysis was stratified by 5-year remission status (model 3). We also determined if internalizing and externalizing psychiatric disorders had different associations with child-reported or parent-reported HRQoL with a multiple linear regression model (model 4) that included dichotomous internalizing and externalizing comorbidity categories in lieu of 1 psychiatric comorbidity category, but which was otherwise similar to model 1.
All analyses were performed by using Stata (11.0) (Stata Corp, College Station, TX) and SAS 9.2 (SAS Institute, Inc, Cary, NC) and a conservative a priori P value of ≤.0125 for statistical significance (with adjustment for multiple comparisons using 4 multiple linear regression models). This study was powered at >90% to detect between-group differences of ≥0.5 SD.
RESULTS
Sample Characteristics
Characteristics of the study participants are presented in Tables 1 and 2. Thirty-percent of the sample (n = 83) had 1 chronic comorbidity (NDSD, psychiatric, migraine, or chronic medical condition), and 31.0% (n = 86) had 2 or more comorbidities; 39.4% (n = 109) had no comorbidity. Internal consistency reliability was high (α ≥ .75 for all scales).
TABLE 1.
Demographic and Clinical Characteristics for CWE and Their Parents (N = 277 Dyads)
| Children demographics and clinical characteristics | |
| Female, n (%) | 129 (46.6) |
| Age of epilepsy onset, mean (SD); range, y | 4.4 (2.6); <1–9 |
| Age of epilepsy diagnosis, mean (SD); range, y | 5.1 (2.5); <1–11 |
| Age at CHQ administration, mean (SD); range, y | 13.0 (2.6); 8–17 |
| Education level at CHQ administration, n (%) | |
| Preschool to 5th grade | 67 (24.5) |
| 6th–8th grade | 107 (39.2) |
| 9th–12th grade | 99 (36.3) |
| Seizure-free for ≥5 y, n (%) | 177 (63.9) |
| Currently taking AEDs, n (%) | 87 (31.4) |
| Complicated epilepsy, n (%)a | 53 (19.1) |
| Full-scale IQ ≥ 80, n (%)b | 230 (83.0) |
| Normal neurologic examination, n (%)c | 232 (83.8) |
| Normal brain MRI, n (%)c | 232 (89.2) |
| Parent-respondent demographics | |
| Female, n (%) | 252 (91.0) |
| Age, mean (SD); range, y | 42.5 (5.4); 27–58 |
| Race, n (%) | |
| White | 222 (80.4) |
| Black | 33 (12.0) |
| Otherd | 21 (7.6) |
| College education or higher, n (%) | 119 (43.1) |
| Employed full- or part-time, n (%) | 216 (78.3) |
| Married, n (%) | 198 (71.7) |
| Biological parent, n (%) | 265 (95.7) |
Missing data from child education (2 children graduated high school and 2 children were in vocational school), 1 missing from parent race, parent education, parent employment, and parent marital status.
“Complicated” was defined by history of remote symptomatic epilepsy or epileptic encephalopathy (Dulac59).
At the 8- to 9-year follow-up.
At baseline or 8- to 9-year follow-up.
Includes Hispanic, Asian, and other.
TABLE 2.
Chronic Comorbidities in CWE (N = 277)
| Comorbid Condition | n (%) |
|---|---|
| NDSD (any) | 108 (39.0) |
| Developmental delaya | 83 (30.0) |
| Language delaya | 66 (23.8) |
| Language problema | 54 (19.5) |
| Dyslexiaa | 10 (3.6) |
| Autismb | 6 (2.2) |
| Psychiatric disorder (any)c | 71 (25.6) |
| Internalizing psychiatric disorders (any) | 28 (10.1) |
| Depression | 23 (8.3) |
| Anxiety disorder | 10 (3.6) |
| Bipolar disorder | 5 (1.8) |
| Obsessive-compulsive disorder | 7 (2.5) |
| Externalizing psychiatric disorders (any) | 58 (20.9) |
| Oppositional defiant disorder | 4 (1.4) |
| Attention deficit hyperactivity disorder | 55 (19.9) |
| Conduct disorder | 12 (4.3) |
| Migrainec | 41 (14.8) |
| Chronic medical condition (any)a | 66 (23.8) |
| Asthma | 48 (17.3) |
| Diabetes | 4 (1.4) |
| Cancer | 1 (0.4) |
| Arthritis | 2 (0.7) |
| Allergies | 15 (5.4) |
| Other | 12 (4.3) |
Parent interview only.
Parent interview, medical chart review and expert review of medical records by child psychiatrist.
Medical chart review and parent interview.
Association of Psychiatric Comorbidity With HRQoL
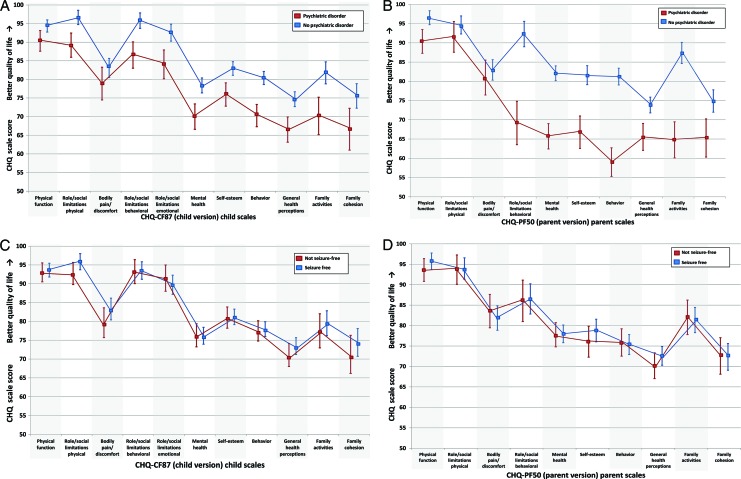
Psychiatric comorbidity was robustly associated with both child-reported and parent-reported HRQoL. In multivariable models adjusted for age and gender, having a psychiatric disorder was associated with worse HRQoL across the majority of the child-reported HRQoL scales (6 scales, 1 global item) (Fig 2A; Table 3). This strong association was also seen in parent-reported HRQoL (7 scales, 1 global item, and the psychosocial summary score) (Fig 2B; Table 3). Although the associations with psychiatric comorbidity and HRQoL were seen across multiple scales, they were not seen in scales related to physical function and role limitations–physical and bodily pain/discomfort. In contrast, both child-reported and parent-reported HRQoL were minimally if at all associated with indicators of epilepsy severity. Not being seizure-free for 5 years was unrelated to worse child-reported HRQoL across all scales and items, and was associated with only 1 scale (parent emotional impact) for parent-reported HRQoL (Fig 2 C and D; Table 3). Complicated epilepsy status was not associated with child-reported HRQoL, and was associated only with worse HRQoL on the physical function scale and the physical summary score for parent-reported HRQoL (Table 3). When the above model was stratified according to 5-year remission status the same pattern of results persisted; psychiatric comorbidity was associated with lower magnitude HRQoL scores in both child and parent reports.
FIGURE 2.
Adjusted predicted HRQoL mean scale scores (± confidence intervals) calculated from multiple linear regression models for having versus not having a psychiatric comorbidity (A and B) and being seizure-free for 5 years or not (C and D). A, Child report: with psychiatric comorbidity versus without psychiatric comorbidity; B, parent-proxy report: with psychiatric comorbidity versus without psychiatric comorbidity; C, child report: seizure-free for 5 years versus not seizure-free for 5 years; D, parent-proxy report: seizure-free for 5 years versus not seizure-free for 5 years.
TABLE 3.
Impact of Chronic Comorbidity and Epilepsy Severity on HRQoL in CWE
| Chronic Comorbidity |
Epilepsy Indicators |
|||||
|---|---|---|---|---|---|---|
| Psychiatric Disorder, β (95% CI) | NDSD, β (95% CI) | Chronic Medical Condition, β (95% CI) | Migraine, β (95% CI) | Complicated Epilepsy, β (95% CI) | Not Seizure-Free (5 y), β (95% CI) | |
| Child report (CHQ-CF87) | ||||||
| Physical function | −1.5 (−5.0 to 2.1) | −3.2 (−6.7 to 0.4) | −1.8 (−5.2 to 1.5) | −1.9 (−6.1 to 2.3) | −4.4 (−8.3 to −0.6)a | −0.6 (−3.8 to 2.7) |
| Role/social limitations–physical | −5.2 (−9.3 to −1.1)a | −2.7 (−6.8 to 1.4) | −1.7 (−5.6 to 2.3) | −2.7 (−7.6 to 2.1) | −3.2 (−7.7 to 1.3) | −3.1 (−6.9 to 0.7) |
| Role/social limitations–behavioral | −6.8 (−11.3 to −2.3) | −2.9 (−7.4 to 1.5) | −1.4 (−5.7 to 2.9) | −2.9 (−8.2 to 2.4) | −4.7 (−9.6 to 0.2) | −0.3 (−4.4 to 3.8) |
| Role/social limitations–emotional | −5.2 (−10.1 to −0.3)a | −8.7 (−13.6 to −3.8) | −3.1 (−7.8 to 1.6) | −2.2 (−8.0 to 3.6) | 1.1 (−4.2 to 6.5) | 1.8 (−2.7 to 6.3) |
| Mental health | −7.7 (−12.0 to −3.4) | −0.5 (−4.8 to 3.8) | −2.5 (−6.6 to 1.6) | −4.4 (−9.5 to 0.7) | 0.9 (−3.8 to 5.6) | 0.1 (−3.8 to 4.1) |
| Self-esteem | −7.1 (−11.1 to −3.1) | 2.0 (−1.9 to 5.9) | −0.6 (−4.4 to 3.2) | −4.1 (−8.8 to 0.3) | −0.2 (−4.6 to 4.1) | −0.2 (−3.8 to 3.5) |
| Behavior | −7.1 (−10.9 to −3.3) | −5.4 (−9.2 to −1.6) | −0.4 (−4.0 to 3.3) | −2.5 (−7.0 to 2.0) | −1.8 (−6.0 to 2.3) | −0.5 (−4.0 to 3.0) |
| Bodily pain/discomfort | −5.0 (−10.5 to 0.6) | 6.0 (0.5 to 11.6)a | −0.4 (−5.7 to 4.9) | −6.9 (−13.5 to −0.4)a | −1.4 (−7.5 to 4.7) | −3.6 (−8.8 to 1.5) |
| General health perceptions | −7.6 (−11.9 to −3.2) | 0.5 (−3.8 to 4.8) | −2.8 (−6.9 to 1.3) | −5.9 (−10.9 to −0.8)a | 1.5 (−3.2 to 6.2) | −2.4 (−6.3 to 1.6) |
| Family activities | −9.8 (−16.2 to −3.4) | −5.5 (−11.9 to 0.8) | 0.4 (−5.7 to 6.4) | −4.2 (−11.7 to 3.4) | 3.6 (−3.3 to 10.6) | −2.0 (−7.9 to 3.8) |
| Family cohesion | −7.2 (−14.4 to −0.1)a | −1.9 (−9.0 to 5.2) | −3.2 (−10.0 to 3.6) | −2.0 (−10.4 to 6.4) | 1.6 (−6.2 to 9.3) | −3.2 (−9.7 to 3.4) |
| Global behavior | −10.0 (−15.6 to −4.5) | −4.2 (−9.7 to 1.3) | −0.3 (−5.6 to 5.0) | 1.5 (−5.0 to 8.1) | −2.4 (−8.5 to 3.6) | −1.0 (−6.0 to 4.1) |
| Global general health | −6.3 (−11.7 to −1.0)a | 0.4 (−4.9 to 5.6) | −2.1 (−7.1 to 3.0) | −1.1 (−7.3 to 5.2) | 0.4 (−5.4 to 6.2) | −3.1 (−7.9 to 1.8) |
| Parent report (CHQ-PF50) | ||||||
| Physical function | −2.9 (−6.8 to 1.1) | −4.7 (−8.6 to −0.8)a | −2.3 (−6.0 to 1.5) | −0.8 (−5.4 to 3.8) | −6.9 (−11.1 to −2.6) | −2.1 (−5.7 to 1.5) |
| Role/social limitations–physical | 0.5 (−4.6 to 5.6) | −5.6 (−10.6 to −0.6)a | −4.7 (−9.5 to 0.1) | 2.0 (−4.0 to 7.9) | −7.1 (−12.6 to −1.6)a | −0.3 (−4.9 to 4.4) |
| Role/social limitations–emotional/ behavioral | −16.4 (−23.6 to −9.2) | −16.6 (−23.7 to −9.5) | −7.1 (−13.9 to −0.3)a | 1.0 (−7.4 to 9.4) | 1.0 (−6.8 to 8.8) | −0.5 (−7.0 to 6.1) |
| Mental health | −14.2 (−18.4 to −10.0) | −4.6 (−8.7 to −0.4)a | −4.1 (−8.1 to −0.1) | −4.2 (−9.1 to 0.7) | 2.5 (−2.1 to 7.0) | −0.2 (−4.1 to 3.6) |
| Self-esteem | −10.7 (−16.1 to −5.4) | −6.0 (−11.3 to −0.7)a | −3.9 (−9.0 to 1.1) | −5.4 (−11.7 to 0.9) | 2.8 (−3.0 to 8.6) | −2.7 (−7.6 to 2.2) |
| Behavior | −17.1 (−21.9 to −12.4) | −13.7 (−18.4 to −9.0) | −3.0 (−7.5 to 1.5) | −3.7 (−9.3 to 1.8) | 4.7 (−0.4 to 9.9) | 0.5 (−3.8 to 4.8) |
| Bodily pain/discomfort | −1.4 (−4.4 to −7.2) | −6.5 (−12.3 to −0.8)a | −7.0 (−12.4 to −1.5)a | −8.5 (−15.3 to −1.8)a | 2.2 (−4.1 to 8.5) | 1.7 (−3.6 to 7.0) |
| General health perceptions | −2.6 (−7.1 to 1.8) | −9.6 (−14.0 to −5.2) | −5.7 (−9.9 to −1.5) | −9.4 (−14.6 to −4.2) | −0.6 (−5.4 to 4.3) | −2.4 (−6.5 to 1.7) |
| Family activities | −17.9 (−23.8 to −11.9) | −11.0 (−16.9 to −5.1) | −6.9 (−12.5 to −1.2)a | −1.6 (−8.6 to 5.4) | 1.8 (−4.7 to 8.3) | 0.7 (−4.8 to 6.1) |
| Family cohesion | −8.1 (−14.4 to −1.8)a | −3.8 (−10.1 to 2.4) | −5.0 (−11.0 to 1.0) | −9.6 (−17.0 to −2.2)a | 8.4 (1.5 to 15.2)a | 0.2 (−5.5 to 6.0) |
| Global behavior | −22.2 (−28.7 to −15.8) | −11.6 (−18.0 to −5.2) | −0.5 (−6.7 to 5.6) | −3.2 (−10.8 to 4.4) | −0.6 (−7.6 to 6.4) | 0.0 (−5.9 to 5.9) |
| Global general health | −1.8 (−6.4 to 2.8) | −7.6 (−12.1 to −3.0) | −6.1 (−10.4 to −1.7) | −12.6 (−18.0 to −7.2) | −0.3 (−5.3 to 4.7) | −2.9 (−7.1 to 1.4) |
| Parent impact on time | −11.4 (−17.3 to −5.4) | −12.8 (−18.7 to −6.9) | −4.1 (−9.8 to 1.5) | −6.6 (−13.6 to 0.3) | −2.9 (−9.4 to 3.6) | −1.9 (−7.4 to 3.6) |
| Parent emotional impact | −13.3 (19.7 to −7.0) | −19.9 (−26.2 to −13.6) | −3.8 (−9.8 to 2.3) | −3.3 (−10.7 to 4.2) | 2.7 (−4.2 to 9.7) | −9.8 (−15.6 to −4.0) |
| Summary (T) scores | ||||||
| Physical summary score | 1.1 (−1.0 to 3.3) | −3.8 (−6.0 to −1.7) | −2.8 (−4.8 to −0.7) | −2.0 (−4.5 to 0.6) | −3.3 (−5.7 to −1.0) | −0.9 (−2.9 to 1.1) |
| Psychosocial summary score | −10.7 (−13.6 to −7.8) | −7.7 (−10.6 to −4.8) | −2.6 (−5.4 to 0.1) | −2.4 (−5.8 to 1.0) | 2.3 (−0.8 to 5.5) | −1.3 (−4.0 to 1.4) |
N = 277 child-parent dyads. Results are from multiple linear regression models. Dependent variables included each respective CHQ-CF87 and CHQ-PF50 scale, item, or summary score, in separate regression models. Independent variables for all models included dichotomous epilepsy (5-year seizure-free and complicated epilepsy status) and chronic comorbidity indicators (psychiatric disorders, NDSD, chronic medical conditions, migraine) in addition to age and gender. CI indicates confidence interval.
P ≤ .0125.
Association of Internalizing Compared With Externalizing Psychiatric Disorders With HRQoL
Of those children with any psychiatric disorder (n = 71), 39.4% (n = 28) had an internalizing disorder and 81.7% (n = 58) had an externalizing disorder; 21% (n = 15) of children with a psychiatric disorder had both at least 1 internalizing and externalizing disorder. In multivariable model 4 the presence of an internalizing psychiatric disorder was significantly associated with worse child-reported and parent-reported HRQoL (10 scales, 1 global item in child-reported; 9 scales, 2 global items, and psychosocial summary score in parent-reported) (Table 4). In contrast, the presence of an externalizing psychiatric disorder was minimally associated with either child or parent-reported HRQoL.
TABLE 4.
Impact of Internalizing Versus Externalizing Psychiatric Disorders on HRQoL in CWE
| Externalizing Psychiatric Disorders, β (95% CI) | Internalizing Psychiatric Disorders, β (95% CI) | |
|---|---|---|
| Child report (CHQ-CF87) | ||
| Physical function | 3.6 (−0.2 to 7.4) | −10.3 (−15.2 to −5.5)a |
| Role/social limitations–physical | −6.0 (−10.6 to −1.5) | −4.5 (−10.3 to 1.3) |
| Role/social limitations–behavioral | −3.5 (−8.5 to 1.5) | −8.5 (−14.9 to −2.1)a |
| Role/social limitations–emotional | −1.2 (−6.6 to 4.3) | −10.3 (−17.2 to −3.4)a |
| Mental health | −1.2 (−5.9 to 3.6) | −13.0 (−19.1 to −7.0)a |
| Self-esteem | −0.9 (−5.3 to 3.4) | −12.6 (−18.2 to −7.1)a |
| Behavior | −4.0 (−8.2 to 0.3) | −8.7 (−14.1 to −3.3) |
| Bodily pain/discomfort | 0.8 (−5.3 to 6.9) | −14.6 (−22.4 to −6.8)a |
| General health perceptions | −2.5 (−7.2 to 2.2) | −14.2 (−20.1 to −8.2)a |
| Family activities | −4.7 (−11.7 to 2.4) | −14.3 (−23.3 to −5.3)a |
| Family cohesion | −1.4 (−9.3 to 6.5) | −13.4 (−23.5 to −3.4)a |
| Global behavior | −11.0 (−17.2 to −4.9)a | −5.0 (−12.8 to 2.9) |
| Global general health | −3.8 (−9.6 to 2.1) | −11.5 (−18.9 to −4.0)a |
| Parent report (CHQ-PF50) | ||
| Physical function | 0.5 (−3.9 to 4.8) | −5.3 (−10.9 to 0.3) |
| Role/social limitations–physical | 1.2 (−4.4 to 6.9) | −4.8 (−12.0 to 2.4) |
| Role/social limitations–emotional/behavioral | −4.6 (−12.3 to 3.0) | −31.5 (−41.2 to −21.8)a |
| Mental health | −3.6 (−7.8 to 0.7) | −26.6 (−32.0 to −21.2)a |
| Self-esteem | 1.6 (−4.0 to 7.2) | −25.6 (−32.8 to −18.5)a |
| Behavior | −11.9 (−16.9 to −6.9)a | −21.2 (−27.6 to −14.8)a |
| Bodily pain/discomfort | 7.9 (1.6 to 14.2) | −13.0 (−21.0 to −5.0) |
| General health perceptions | −2.3 (−7.2 to 2.6) | −4.4 (−10.7 to 1.9) |
| Family activities | −10.4 (−16.6 to −4.2)a | −29.1 (−36.9 to −21.2)a |
| Family cohesion | −4.7 (−11.6 to 2.2) | −14.6 (−23.4 to −5.8) |
| Global behavior | −13.3 (−20.1 to −6.6)a | −31.4 (−40.1 to −22.8)a |
| Global general health | 3.0 (−2.1 to 8.0) | −12.0 (−18.4 to −5.7)a |
| Parent impact on time | −0.1 (−6.3 to 6.0) | −29.8 (−37.7 to −22.0)a |
| Parent emotional impact | −6.0 (−12.9 to 0.9) | −21.1 (−29.9 to −12.3)a |
| Summary (T) scores | ||
| Physical summary score | 1.9 (−0.5 to 4.3) | −1.7 (−4.8 to 1.4) |
| Psychosocial summary score | −3.7 (−6.6 to −0.8)a | −18.8 (−22.6 to −15.1)a |
N = 277 child-parent dyads. Age- and gender-adjusted multiple linear regression models. Dependent variables included each respective CHQ-CF87 and CHQ-PF50 scale, item, or summary score, in separate regression models. Independent variables for all models included dichotomous indicators of internalizing and externalizing psychiatric disorders (coefficients shown above), in addition to dichotomous indicators for NDSD, chronic medical conditions, migraine, complicated epilepsy, and 5-year seizure-free status. Internalizing and externalizing psychiatric disorders were not mutually exclusive. Of those children with any psychiatric disorder (n = 71), 39.4% (n = 28) had an internalizing disorder, 81.7% (n = 58) had an externalizing disorder, and 21% (n = 15) had both at least 1 internalizing and externalizing disorder. CI indicates confidence interval.
P ≤ .0125.
Comparison of Child and Parent-Reported HRQoL Associations With Different Comorbidities
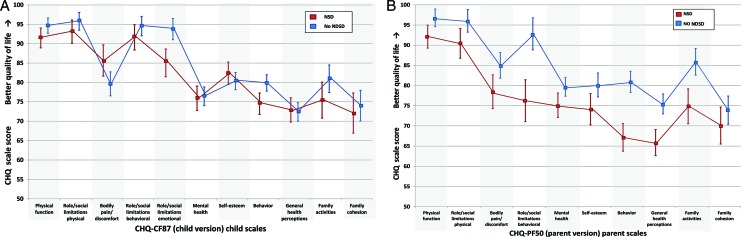
In multivariable analysis, psychiatric comorbidity was significantly associated with both worse child-reported and worse parent-reported HRQoL (Table 3; Fig 2). Associations of comorbid migraine and chronic medical conditions with HRQoL were similar between child and parent reports; comorbid migraine and chronic medication condition were minimally predictive of worse child-reported or parent-reported HRQoL (Table 3). In contrast, such similarities between child and parent reports were not observed for NDSD; having an NDSD was significantly associated with worse child-reported HRQoL on only 2 scales, although it was strongly associated with worse parent-reported HRQoL across the majority of scales (6 scales, 2 global items, both summary scores) (Table 3; Fig 3).
FIGURE 3.
The association of NDSD with HRQoL in CWE (N = 277): differences in child and parent-proxy reports. Adjusted predicted HRQoL mean scale scores (± confidence intervals) were calculated from multiple linear regression models for having versus not having an NDSD: A, child-reported; B, parent-proxy report.
DISCUSSION
In this 8- to 9-year follow-up of adolescents with newly diagnosed epilepsy in childhood, we found that chronic comorbidities, particularly psychiatric disorders, are more strongly associated with worse HRQoL than 5-year seizure remission. Having a psychiatric disorder, particularly an internalizing disorder, was significantly associated with worse child-reported and parent-reported HRQoL across the majority of CHQ scales, with differences seen predominantly in scales related to psychological and family health, but not those related to physical function. The presence of NDSD comorbidity was also associated with worse parent-reported HRQoL, although such an association was not seen in child-reported HRQoL. In contrast, 5-year seizure remission and complicated epilepsy status were minimally, if ever, associated with child-reported or parent-reported HRQoL, which suggests that chronic comorbidities, particularly psychiatric disorders, but not seizure-freedom or complicated epilepsy status, have the more important impact on worse HRQoL in adolescents with childhood-onset epilepsy.
To our knowledge, the independent effects of chronic comorbidities, including psychiatric diagnosis and NDSDs, on HRQoL nearly a decade after childhood-onset epilepsy have not previously been reported. Sillanpää et al found that subjects on AEDs, whether in remission or not, had worse quality of life compared with controls or subjects in remission off AEDs in a long-term population-based cohort study of HRQoL outcomes of adults with childhood-onset epilepsy; the potential impact of comorbid conditions on HRQoL was not analyzed.16 Although several cross-sectional studies have revealed a negative association for comorbid conditions with HRQoL in CWE, these reports differ from the results of our analysis in that they used a parent proxy-report of HRQoL only, included children with predominantly active epilepsy, or analyzed a limited or unspecified number of chronic comorbidities.30,35–37,40,43–46 Psychiatric comorbidity is significantly associated with worse HRQoL among adults with epilepsy, and congruent with our results in children, this association is stronger than seizure control status.21–26 Our results are, therefore, concordant with the notion that factors beyond seizures and epilepsy might be driving poor quality-of-life outcomes in CWE.37
Although children and parents both reported a significant association of psychiatric comorbidity with HRQoL, the perspective of children and parents varied with respect to the magnitude of this association, in addition to the impact of NDSD. Parents reported that NDSD was significantly associated with worse HRQoL across multiple scales, whereas children did not. Such parent-child differences might be due in part to the observation that children may have different perspectives of HRQoL from that of their adult proxies.62 From this cohort, we previously found that CWE self-reported HRQoL that was comparable to healthy sibling controls, whereas parents' rating of the same children indicated worse HRQoL for CWE compared with sibling controls.47 Verhey et al similarly reported parent-child differences in their report of HRQoL among CWE, and studies of children with other chronic health conditions, including cancer, asthma, diabetes, inflammatory bowel disease, and attention-deficit/hyperactivity disorder have also demonstrated that parents tend to proxy-report the child's HRQoL as worse than the child's self-report of their own HRQoL.47–48,63–68 Although child-reported HRQoL might have been strongly influenced by mood state, thereby contributing to the observed strong association between child-reported HRQoL and psychiatric comorbidity, parent-reported HRQoL might have been driven by factors beyond the child's “mood.”26 For example, parental anxiety and depression, which have been negatively associated with worse HRQoL in CWE, could influence parental concerns about chronic comorbidities, such as NDSD, thereby contributing to observed child-parent differences.40,69
The CHQ, a generic instrument, might not be sensitive to the nuances of epilepsy. This lack of sensitivity might have contributed to our finding that 5-year remission and complicated epilepsy status were minimally associated with HRQoL. Epilepsy-targeted HRQoL measures are preferable to generic ones in longitudinal studies owing to better ability to detect change.70 Our analysis, however, was restricted to the 9-year follow-up; at enrollment, children were on average only 5 years old and unable to complete an HRQoL measure. Differences in magnitude of HRQoL between child and parent reports represent general trends only because the CHQ-CF87 and the CHQ-PF50 have varied item wording and scale length, which make direct comparisons limited, and we have previously shown that parent-child agreement is low.47 We did not define chronic comorbidities by using screening instruments or on the basis of symptomology; thus, the rates of chronic comorbidities in this cohort might be an underestimate. Because chronic comorbidities were categorized by using heterogeneous sources of data (parental interview, medical chart review, and expert review of records by a child psychiatrist for autism spectrum disorder) and were not mutually exclusive, comorbidities were analyzed by using a dichotomous approach that might not capture the full context of the data. The generalizability of our findings might be limited because our sample population was relatively homogeneous (>80% white), although representative of the state of Connecticut in the early 1990s.
Psychiatric comorbidity in children with childhood-onset epilepsy seems to have a greater impact on HRQoL at the 9-year follow-up than do indicators of epilepsy severity, most notably 5-year seizure-free remission status. High-quality pediatric epilepsy care must include the recognition and treatment of chronic comorbid conditions, notably psychiatric disorders. Comprehensive epilepsy treatment and education programs require child and parent perspectives, particularly as children transition through adolescence into adulthood.71 Whether such interventions can improve long-term psychosocial outcomes into adulthood is a question that needs further investigation.
CONCLUSIONS
Resolution of seizures does not necessarily mean that chronic comorbidities, such as psychiatric disorders, stop posing challenges in patients with epilepsy, thereby highlighting the need for future research directed toward “preventing, limiting, and reversing the comorbidities associated with epilepsy and its treatment,” a current epilepsy research benchmark of the National Institute of Neurological Disorders and Stroke.72 High quality, comprehensive epilepsy care must focus not only on the effective treatment of seizures, but must also include the recognition and treatment of chronic comorbid conditions, given their potential association with poor long-term outcomes, particularly as CWE transition into adult care settings.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health, National Institute of Neurological Disorders and Stroke grant R37-NS31146 (principal investigator Dr Berg). Dr Baca was supported by Robert Wood Johnson Foundation Clinical Scholars Program grant 59982.
We thank Drs Susan R. Levy and Francine M. Testa for initial recruitment of subjects; France Nguyen-Grozavu, PhD, MPH, MA, and Aaron Cook, MPH, for technical support; and Dr Honghu Liu for statistical consulting assistance.
APPENDIX 1: RECRUITMENT, FOLLOW-UP, AND SAMPLE SELECTION
The Connecticut cohort (n = 613) was initially recruited from 16 of the 17 pediatric neurologists practicing in the state of Connecticut during the recruitment period 1993–1997. The epilepsies and underlying causes were classified by 3 pediatric epileptologists. Although our study was not strictly speaking a population-based study, the characteristics of the study cohort are highly comparable to those of a Canadian study that is generally accepted as population based with respect to participant age at onset and gender and the proportion of participants with epilepsy with certain well-recognized forms, intellectual disability, and mortality.49,52,73–76 In our investigation, retention of participants in follow-up at the 9-year mark was excellent, with only 83 (13%) having been lost before the 9-year reassessment (Fig 1). The 13% included 13 children who had died and 70 children who could not be located at the time of the reassessment and thus were lost to follow-up. Of those followed at 9 years (n = 502), only 28 (5%) did not participate at some level in the reassessment protocol.
The CHQ was administered to participants younger than 18 years. In addition, children had to be able to understand the questionnaire well enough to fill it out by themselves in a meaningful fashion. Of the 502 children who participated at any level in the 9-year assessment protocol, 374 were younger than 18 years. Of the young adults aged 18 years or older (n = 128), 104 completed the Quality of Life in Epilepsy-89 (QoLIE-89) and thus were not included in this analysis. Of the 374 children eligible to complete the CHQ, 278 (74.1%) completed parent and child forms of the CHQ. We had to exclude another case for confidential reasons, so we had 277 for analysis. Thirty-two children younger than 18 years had a parent complete the parent form (CHQ-PF50), but the child did not complete the child form. Of those 32 children, 24 had low cognitive function consistent with mild to severe intellectual disability (IQ < 70). Sixty-four children younger than 18 years did not have a parent or child CHQ form. Of those 64 children, 29 had low cognitive function consistent with mild to severe intellectual disability (IQ < 70).
APPENDIX 2: COGNITIVE FUNCTION ASSESSMENT
Comprehensive cognitive function assessment was a component of the comprehensive 9-year reassessment protocol in children and has been the focus of separate analyses and articles.52,78 Classification of global cognitive function was conducted by using available information from neurologic medical records and parent interviews at study entry, and at 5 and 9 years later, school records and formal testing done as part of a school evaluation or our own standardized neuropsychological evaluation. Specifically, standardized neuropsychological evaluation included interviews with parents as well as a neuropsychological test battery that was administered by a licensed psychologist or trained psychometrician using the Wechsler Intelligence Scale for Children.54 If research-standardized results were not available, we used information from school performance, the neurologists' developmental assessment, special education programs, and school records and performance (eg, early graduation, advanced placement courses, entrance to college), in addition to parental report. These data sources also provided information about severe intellectual impairment that would preclude formal testing. As such, formal research cognitive function testing data were available for 335 members of the original cohort, and data from cognitive assessments performed by the schools were available for an additional 55 children. On the basis of this accumulated information, estimates of cognitive function were made in conjunction with a neuropsychologist. Level of global cognitive function was then characterized as within normal range (“normal” consistent with an IQ score of ≥80), borderline (consistent with IQ scores of 70–79), mildly retarded (consistent with IQ scores of 60–69), moderately or severely retarded (consistent with IQ scores of <60), and neurologically devastated–not testable. Overall, approximately three-quarters of the entire cohort (n = 613) were considered to be in the normal intellectual range, and ∼25% had levels of function consistent with borderline to severe intellectual disabilities. These findings are highly comparable to those of the Canadian population-based study and the estimate from the National Collaborative Perinatal Study.74,75,77 Among the sample of 277 children who completed the CHQ, however, more than 80% (n = 230) were considered to be in the normal intellectual range (IQ ≥ 80).
All authors participated in the concept and design of the study, analysis and interpretation of the data, and drafting or revision of the manuscript and have approved the manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
Funded by the National Institutes of Health (NIH).
- HRQoL
- health-related quality of life
- CWE
- children with epilepsy
- AED
- antiepileptic drug
- CHQ
- Child Health Questionnaire
- NDSD
- neurodevelopmental spectrum disorder
REFERENCES
- 1. Linehan C, Berg A. Epidemiologic aspects of epilepsy. In: Wyllie E, Cascino GD, Gidal BE, Goodkin HP. eds. Wyllie's Treatment of Epilepsy: Principles and Practice. Philadelphia, PA: Lippincott, Williams & Wilkins; 2011:2–10 [Google Scholar]
- 2. Ronen GM, Streiner DL, Rosenbaum P. Health-related quality of life in childhood epilepsy: moving beyond “seizure control with minimal adverse effects.” Health Qual Life Outcomes. 2003;1:36–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alfstad KA, Clench-Aas J, Van Roy B, Mowinckel P, Gjerstad L, Lossius MI. Psychiatric symptoms in Norwegian children with epilepsy aged 8–13 years: effects of age and gender? Epilepsia. 2011;52(7):1231–1238 [DOI] [PubMed] [Google Scholar]
- 4. Caplan R, Siddarth P, Gurbani S, Hanson R, Sankar R, Shields WD. Depression and anxiety disorders in pediatric epilepsy. Epilepsia. 2005;46(5):720–730 [DOI] [PubMed] [Google Scholar]
- 5. Clarke DF, Roberts W, Daraksan M, et al. The prevalence of autistic spectrum disorder in children surveyed in a tertiary care epilepsy clinic. Epilepsia. 2005;46(12):1970–1977 [DOI] [PubMed] [Google Scholar]
- 6. Davies S, Heyman I, Goodman R. A population survey of mental health problems in children with epilepsy. Dev Med Child Neurol. 2003;45(5):292–295 [DOI] [PubMed] [Google Scholar]
- 7. Dunn DW, Austin JK, Harezlak J, Ambrosius WT. ADHD and epilepsy in childhood. Dev Med Child Neurol. 2003;45(1):50–54 [PubMed] [Google Scholar]
- 8. Ettinger AB, Weisbrot DM, Nolan EE, et al. Symptoms of depression and anxiety in pediatric epilepsy patients. Epilepsia. 1998;39(6):595–599 [DOI] [PubMed] [Google Scholar]
- 9. Ott D, Siddarth P, Gurbani S, et al. Behavioral disorders in pediatric epilepsy: unmet psychiatric need. Epilepsia. 2003;44(4):591–597 [DOI] [PubMed] [Google Scholar]
- 10. Sillanpää M. Learning disability: occurrence and long-term consequences in childhood-onset epilepsy. Epilepsy Behav. 2004;5(6):937–944 [DOI] [PubMed] [Google Scholar]
- 11. Austin JK, Harezlak J, Dunn DW, Huster GA, Rose DF, Ambrosius WT. Behavior problems in children before first recognized seizures. Pediatrics. 2001;107(1):115–122 [DOI] [PubMed] [Google Scholar]
- 12. Berg AT, Vickrey BG, Testa FM, Levy SR, Shinnar S, DiMario F. Behavior and social competency in idiopathic and cryptogenic childhood epilepsy. Dev Med Child Neurol. 2007;49(7):487–492 [DOI] [PubMed] [Google Scholar]
- 13. Camfield C, Camfield P, Smith B, Gordon K, Dooley J. Biologic factors as predictors of social outcome of epilepsy in intellectually normal children: a population-based study. J Pediatr. 1993;122(6):869–873 [DOI] [PubMed] [Google Scholar]
- 14. Kokkonen J, Kokkonen ER, Saukkonen AL, Pennanen P. Psychosocial outcome of young adults with epilepsy in childhood. J Neurol Neurosurg Psychiatry. 1997;62(3):265–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shackleton DP, Kasteleijn-Nolst Trenite DG, de Craen AJ, Vandenbroucke JP, Westendorp RG. Living with epilepsy: long-term prognosis and psychosocial outcomes. Neurology. 2003;61(1):64–70 [DOI] [PubMed] [Google Scholar]
- 16. Sillanpää M, Haataja L, Shinnar S. Perceived impact of childhood-onset epilepsy on quality of life as an adult. Epilepsia. 2004;45(8):971–977 [DOI] [PubMed] [Google Scholar]
- 17. Sillanpää M, Jalava M, Kaleva O, Shinnar S. Long-term prognosis of seizures with onset in childhood. N Engl J Med. 1998;338(24):1715–1722 [DOI] [PubMed] [Google Scholar]
- 18. Wakamoto H, Nagao H, Hayashi M, Morimoto T. Long-term medical, educational, and social prognoses of childhood-onset epilepsy: a population-based study in a rural district of Japan. Brain Dev. 2000;22(4):246–255 [DOI] [PubMed] [Google Scholar]
- 19. Wirrell EC, Camfield CS, Camfield PR, Dooley JM, Gordon KE, Smith B. Long-term psychosocial outcome in typical absence epilepsy. Sometimes a wolf in sheeps' clothing. Arch Pediatr Adolesc Med. 1997;151(2):152–158 [DOI] [PubMed] [Google Scholar]
- 20. Lach LM, Ronen GM, Rosenbaum PL, et al. Health-related quality of life in youth with epilepsy: theoretical model for clinicians and researchers. Part I: the role of epilepsy and co-morbidity. Qual Life Res. 2006;15(7):1161–1171 [DOI] [PubMed] [Google Scholar]
- 21. Boylan LS, Flint LA, Labovitz DL, Jackson SC, Starner K, Devinsky O. Depression but not seizure frequency predicts quality of life in treatment-resistant epilepsy. Neurology. 2004;62(2):258–261 [DOI] [PubMed] [Google Scholar]
- 22. Gilliam F, Hecimovic H, Sheline Y. Psychiatric comorbidity, health, and function in epilepsy. Epilepsy Behav. 2003;4(suppl 4):S26–S30 [DOI] [PubMed] [Google Scholar]
- 23. Johnson EK, Jones JE, Seidenberg M, Hermann BP. The relative impact of anxiety, depression, and clinical seizure features on health-related quality of life in epilepsy. Epilepsia. 2004;45(5):544–550 [DOI] [PubMed] [Google Scholar]
- 24. Kwan P, Yu E, Leung H, Leon T, Mychaskiw MA. Association of subjective anxiety, depression, and sleep disturbance with quality-of-life ratings in adults with epilepsy. Epilepsia. 2009;50(5):1059–1066 [DOI] [PubMed] [Google Scholar]
- 25. Loring DW, Meador KJ, Lee GP. Determinants of quality of life in epilepsy. Epilepsy Behav. 2004;5(6):976–980 [DOI] [PubMed] [Google Scholar]
- 26. Tracy JI, Dechant V, Sperling MR, Cho R, Glosser D. The association of mood with quality of life ratings in epilepsy. Neurology. 2007;68(14):1101–1107 [DOI] [PubMed] [Google Scholar]
- 27. Park SP, Song HS, Hwang YH, Lee HW, Suh CK, Kwon SH. Differential effects of seizure control and affective symptoms on quality of life in people with epilepsy. Epilepsy Behav. 2010;18(4):455–459 [DOI] [PubMed] [Google Scholar]
- 28. Canuet L, Ishii R, Iwase M, et al. Factors associated with impaired quality of life in younger and older adults with epilepsy. Epilepsy Res. 2009;83(1):58–65 [DOI] [PubMed] [Google Scholar]
- 29. Kwon OY, Park SP. What is the role of depressive symptoms among other predictors of quality of life in people with well-controlled epilepsy on monotherapy? Epilepsy Behav. 2011;20(3):528–532 [DOI] [PubMed] [Google Scholar]
- 30. Adewuya AO, Oseni SB. Impact of psychiatric morbidity on parent-rated quality of life in Nigerian adolescents with epilepsy. Epilepsy Behav. 2005;7(3):497–501 [DOI] [PubMed] [Google Scholar]
- 31. Austin JK, Huster GA, Dunn DW, Risinger MW. Adolescents with active or inactive epilepsy or asthma: a comparison of quality of life. Epilepsia. 1996;37(12):1228–1238 [DOI] [PubMed] [Google Scholar]
- 32. Benavente-Aguilar I, Morales-Blanquez C, Rubio EA, Rey JM. Quality of life of adolescents suffering from epilepsy living in the community. J Paediatr Child Health. 2004;40(3):110–113 [DOI] [PubMed] [Google Scholar]
- 33. Devinsky O, Westbrook L, Cramer J, Glassman M, Perrine K, Camfield C. Risk factors for poor health-related quality of life in adolescents with epilepsy. Epilepsia. 1999;40(12):1715–1720 [DOI] [PubMed] [Google Scholar]
- 34. Hamiwka L, Singh N, Niosi J, Wirrell E. Perceived health in children presenting with a “first seizure.” Epilepsy Behav. 2008;13(3):485–488 [DOI] [PubMed] [Google Scholar]
- 35. Hoare P. The quality of life of children with chronic epilepsy and their families. Seizure. 1993;2(4):269–275 [DOI] [PubMed] [Google Scholar]
- 36. Miller V, Palermo TM, Grewe SD. Quality of life in pediatric epilepsy: demographic and disease-related predictors and comparison with healthy controls. Epilepsy Behav. 2003;4(1):36–42 [DOI] [PubMed] [Google Scholar]
- 37. Modi AC, King AS, Monahan SR, Koumoutsos JE, Morita DA, Glauser TA. Even a single seizure negatively impacts pediatric health-related quality of life. Epilepsia. 2009;50(9):2110–2116 [DOI] [PubMed] [Google Scholar]
- 38. Sabaz M, Cairns DR, Bleasel AF, et al. The health-related quality of life of childhood epilepsy syndromes. J Paediatr Child Health. 2003;39(9):690–696 [DOI] [PubMed] [Google Scholar]
- 39. Stevanovic D. Health-related quality of life in adolescents with well-controlled epilepsy. Epilepsy Behav. 2007;10(4):571–575 [DOI] [PubMed] [Google Scholar]
- 40. Williams J, Steel C, Sharp GB, et al. Parental anxiety and quality of life in children with epilepsy. Epilepsy Behav. 2003;4(5):483–486 [DOI] [PubMed] [Google Scholar]
- 41. Wu DY, Ding D, Wang Y, Hong Z. Quality of life and related factors in Chinese adolescents with active epilepsy. Epilepsy Res. 2010;90(1–2):16–20 [DOI] [PubMed] [Google Scholar]
- 42. Sabaz M, Cairns DR, Lawson JA, Bleasel AF, Bye AM. The health-related quality of life of children with refractory epilepsy: a comparison of those with and without intellectual disability. Epilepsia. 2001;42(5):621–628 [DOI] [PubMed] [Google Scholar]
- 43. Camfield C, Breau L, Camfield P. Assessing the impact of pediatric epilepsy and concomitant behavioral, cognitive, and physical/neurologic disability: Impact of Childhood Neurologic Disability Scale. Dev Med Child Neurol. 2003;45(3):152–159 [DOI] [PubMed] [Google Scholar]
- 44. Sherman EM, Griffiths SY, Akdag S, Connolly MB, Slick DJ, Wiebe S. Sociodemographic correlates of health-related quality of life in pediatric epilepsy. Epilepsy Behav. 2008;12(1):96–101 [DOI] [PubMed] [Google Scholar]
- 45. Sherman EM, Slick DJ, Connolly MB, Eyrl KL. ADHD, neurological correlates and health-related quality of life in severe pediatric epilepsy. Epilepsia. 2007;48(6):1083–1091 [DOI] [PubMed] [Google Scholar]
- 46. Taylor J, Jacoby A, Baker GA, Marson AG. Self-reported and parent-reported quality of life of children and adolescents with new-onset epilepsy. Epilepsia. 2011;52(8):1489–1498 [DOI] [PubMed] [Google Scholar]
- 47. Baca CB, Vickrey BG, Hays RD, Vassar SD, Berg AT. Differences in child versus parent reports of the child's health-related quality of life in children with epilepsy and healthy siblings. Value Health. 2010;13(6):778–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Verhey LH, Kulik DM, Ronen GM, et al. Quality of life in childhood epilepsy: what is the level of agreement between youth and their parents? Epilepsy Behav. 2009;14(2):407–410 [DOI] [PubMed] [Google Scholar]
- 49. Berg AT, Shinnar S, Levy SR, Testa FM. Newly diagnosed epilepsy in children: presentation at diagnosis. Epilepsia. 1999;40(4):445–452 [DOI] [PubMed] [Google Scholar]
- 50. Berg AT, Mathern GW, Bronen RA, et al. Frequency, prognosis and surgical treatment of structural abnormalities seen with magnetic resonance imaging in childhood epilepsy. Brain. 2009;132(pt 10):2785–2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Berg AT, Testa FM, Levy SR, Shinnar S. Neuroimaging in children with newly diagnosed epilepsy: a community-based study. Pediatrics. 2000;106(3):527–532 [DOI] [PubMed] [Google Scholar]
- 52. Berg AT, Langfitt JT, Testa FM, et al. Global cognitive function in children with epilepsy: a community-based study. Epilepsia. 2008;49(4):608–614 [DOI] [PubMed] [Google Scholar]
- 53. Berg AT, Langfitt JT, Testa FM, et al. Residual cognitive effects of uncomplicated idiopathic and cryptogenic epilepsy. Epilepsy Behav. 2008;13(4):614–619 [DOI] [PubMed] [Google Scholar]
- 54. Wechsler D. Wechsler Intelligence Scale for Children. 3rd ed San Antonio, TX: Psychological Corp; 1991 [Google Scholar]
- 55. Austin JK, Caplan R. Behavioral and psychiatric comorbidities in pediatric epilepsy: toward an integrative model. Epilepsia. 2007;48(9):1639–1651 [DOI] [PubMed] [Google Scholar]
- 56. Berg AT, Plioplys S, Tuchman R. Risk and correlates of autism spectrum disorder in children with epilepsy: a community-based study. J Child Neurol. 2011:26(5);540–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Geerts A, Arts WF, Stroink H, et al. Course and outcome of childhood epilepsy: a 15-year follow-up of the Dutch Study of Epilepsy in Childhood. Epilepsia. 2010;51(7):1189–1197 [DOI] [PubMed] [Google Scholar]
- 58. Commission on Epidemiology and Prognosis, International League Against Epilepsy Guidelines for epidemiologic studies on epilepsy. Epilepsia. 1993;34(4):592–596 [DOI] [PubMed] [Google Scholar]
- 59. Dulac O. Epileptic encephalopathy. Epilepsia. 2001;42(suppl 3):23–26 [DOI] [PubMed] [Google Scholar]
- 60. Landgraf JM, Abetz L, Ware JA. The CHQ User's Manual. Boston, MA: Health Institute, New England Medical Center; 1996 [Google Scholar]
- 61. Chatterjee S, Hadi AS. Influential observations, high leverage points, and outliers in linear regression. Statist Sci. 1986;1(3):379–393 [Google Scholar]
- 62. Upton P, Lawford J, Eiser C. Parent-child agreement across child health-related quality of life instruments: a review of the literature. Qual Life Res. 2008;17(6):895–913 [DOI] [PubMed] [Google Scholar]
- 63. Eiser C, Vance YH, Horne B, Glaser A, Galvin H. The value of the PedsQLTM in assessing quality of life in survivors of childhood cancer. Child Care Health Dev. 2003;29(2):95–102 [DOI] [PubMed] [Google Scholar]
- 64. Felder-Puig R, Frey E, Proksch K, Varni JW, Gadner H, Topf R. Validation of the German version of the Pediatric Quality of Life Inventory (PedsQL) in childhood cancer patients off treatment and children with epilepsy. Qual Life Res. 2004;13(1):223–234 [DOI] [PubMed] [Google Scholar]
- 65. Upton P, Eiser C, Cheung I, et al. Measurement properties of the UK-English version of the Pediatric Quality of Life Inventory 4.0 (PedsQL) generic core scales. Health Qual Life Outcomes. 2005;3:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Varni JW, Burwinkle TM. The PedsQL as a patient-reported outcome in children and adolescents with Attention-Deficit/Hyperactivity Disorder: a population-based study. Health Qual Life Outcomes. 2006;4:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Varni JW, Burwinkle TM, Jacobs JR, Gottschalk M, Kaufman F, Jones KL. The PedsQL in type 1 and type 2 diabetes: reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales and type 1 diabetes module. Diabetes Care. 2003;26(3):631–637 [DOI] [PubMed] [Google Scholar]
- 68. Varni JW, Burwinkle TM, Rapoff MA, Kamps JL, Olson N. The PedsQL in pediatric asthma: reliability and validity of the Pediatric Quality of Life Inventory generic core scales and asthma module. J Behav Med. 2004;27(3):297–318 [DOI] [PubMed] [Google Scholar]
- 69. Ferro MA, Avison WR, Campbell MK, Speechley KN. The impact of maternal depressive symptoms on health-related quality of life in children with epilepsy: a prospective study of family environment as mediators and moderators. Epilepsia. 2011;52(2):316–325 [DOI] [PubMed] [Google Scholar]
- 70. Birbeck GL, Kim S, Hays RD, Vickrey BG. Quality of life measures in epilepsy: how well can they detect change over time? Neurology. 2000;54(9):1822–1827 [DOI] [PubMed] [Google Scholar]
- 71. Camfield P, Camfield C. Transition to adult care for children with chronic neurological disorders. Ann Neurol. 2011;69(3):437–444 [DOI] [PubMed] [Google Scholar]
- 72. Kelley MS, Jacobs MP, Lowenstein DH. The NINDS epilepsy research benchmarks. Epilepsia. 2009;50(3):579–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Berg AT, Shinnar S, Testa FM, Levy SR, Smith SN, Beckerman B. Mortality in childhood-onset epilepsy. Arch Pediatr Adolesc Med. 2004;158(12):1147–1152 [DOI] [PubMed] [Google Scholar]
- 74. Camfield C, Camfield P. Preventable and unpreventable causes of childhood-onset epilepsy plus mental retardation. Pediatrics. 2007;120(1):52–55 [DOI] [PubMed] [Google Scholar]
- 75. Camfield CS, Camfield PR, Gordon K, Wirrell E, Dooley JM. Incidence of epilepsy in childhood and adolescence: a population-based study in Nova Scotia from 1977 to 1985. Epilepsia. 1996;37(1):19–23 [DOI] [PubMed] [Google Scholar]
- 76. Camfield CS, Camfield PR, Veugelers PJ. Death in children with epilepsy: a population-based study. Lancet. 2002;359(9321):1891–1895 [DOI] [PubMed] [Google Scholar]
- 77. Ellenberg JH, Hirtz DG, Nelson KB. Age at onset of seizures in young children. Ann Neurol. 1984;15(2):127–134 [DOI] [PubMed] [Google Scholar]
- 78. Berg AT, Langfitt JT, Testa FM, et al. Global cognitive function in children with epilepsy: a community-based study. Epilepsia. 2008;49(4):608–614 [DOI] [PubMed] [Google Scholar]