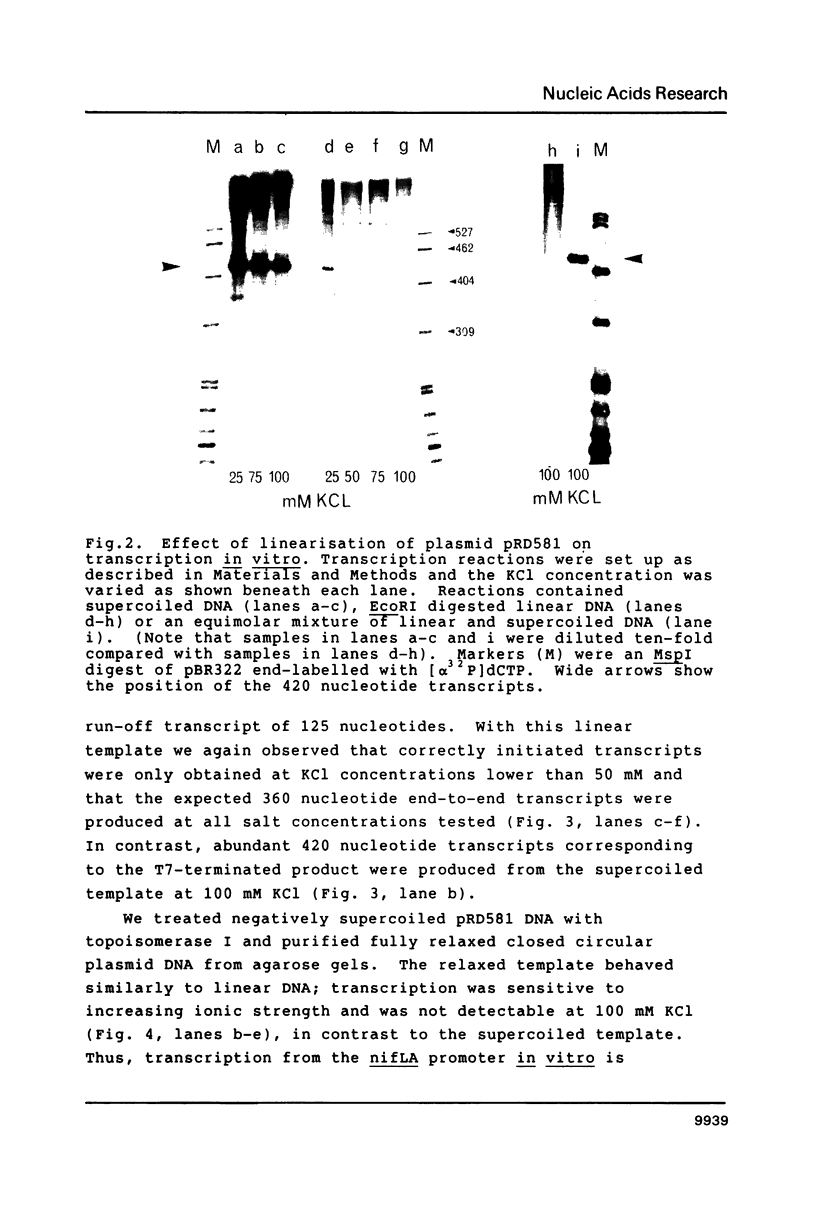
Abstract
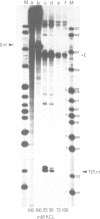
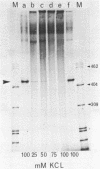
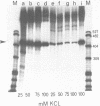
Expression from the K. pneumoniae nifLA promoter is oxygen sensitive and is also inhibited by the DNA gyrase inhibitor coumermycin A1 under anaerobic growth conditions. The activity of this promoter was found to be highly sensitive to changes in DNA topology in vitro. Transcription was completely dependent on negative supercoiling at physiological salt concentrations although transcription from linear or fully relaxed closed circular templates was detectable at KCl concentrations lower than 50 mM. These observations suggest that aerobic regulation of nif transcription may be mediated through the level of DNA supercoiling.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aliabadi Z., Warren F., Mya S., Foster J. W. Oxygen-regulated stimulons of Salmonella typhimurium identified by Mu d(Ap lac) operon fusions. J Bacteriol. 1986 Mar;165(3):780–786. doi: 10.1128/jb.165.3.780-786.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin S., Henderson N., Dixon R. Requirements for transcriptional activation in vitro of the nitrogen-regulated glnA and nifLA promoters from Klebsiella pneumoniae: dependence on activator concentration. Mol Microbiol. 1987 Jul;1(1):92–100. doi: 10.1111/j.1365-2958.1987.tb00532.x. [DOI] [PubMed] [Google Scholar]
- Balke V. L., Gralla J. D. Changes in the linking number of supercoiled DNA accompany growth transitions in Escherichia coli. J Bacteriol. 1987 Oct;169(10):4499–4506. doi: 10.1128/jb.169.10.4499-4506.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowiec J. A., Zhang L., Sasse-Dwight S., Gralla J. D. DNA supercoiling promotes formation of a bent repression loop in lac DNA. J Mol Biol. 1987 Jul 5;196(1):101–111. doi: 10.1016/0022-2836(87)90513-4. [DOI] [PubMed] [Google Scholar]
- Buchanan-Wollaston V., Cannon M. C., Beynon J. L., Cannon F. C. Role of the nifA gene product in the regulation of nif expression in Klebsiella pneumoniae. Nature. 1981 Dec 24;294(5843):776–778. doi: 10.1038/294776a0. [DOI] [PubMed] [Google Scholar]
- Cannon M., Hill S., Kavanaugh E., Cannon F. A molecular genetic study of nif expression in Klebsiella pneumoniae at the level of transcription, translation and nitrogenase activity. Mol Gen Genet. 1985;198(2):198–206. doi: 10.1007/BF00382996. [DOI] [PubMed] [Google Scholar]
- Collins J. J., Brill W. J. Control of Klebsiella pneumoniae nif mRNA synthesis. J Bacteriol. 1985 Jun;162(3):1186–1190. doi: 10.1128/jb.162.3.1186-1190.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R., Eady R. R., Espin G., Hill S., Iaccarino M., Kahn D., Merrick M. Analysis of regulation of Klebsiella pneumoniae nitrogen fixation (nif) gene cluster with gene fusions. Nature. 1980 Jul 10;286(5769):128–132. doi: 10.1038/286128a0. [DOI] [PubMed] [Google Scholar]
- Dixon R., Kennedy C., Kondorosi A., Krishnapillai V., Merrick M. Complementation analysis of Klebsiella pneumoniae mutants defective in nitrogen fixation. Mol Gen Genet. 1977 Nov 29;157(2):189–198. doi: 10.1007/BF00267397. [DOI] [PubMed] [Google Scholar]
- Dorman C. J., Barr G. C., Ni Bhriain N., Higgins C. F. DNA supercoiling and the anaerobic and growth phase regulation of tonB gene expression. J Bacteriol. 1988 Jun;170(6):2816–2826. doi: 10.1128/jb.170.6.2816-2826.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott T., Geiduschek E. P. Defining a bacteriophage T4 late promoter: absence of a "-35" region. Cell. 1984 Jan;36(1):211–219. doi: 10.1016/0092-8674(84)90091-6. [DOI] [PubMed] [Google Scholar]
- Hahn S., Hendrickson W., Schleif R. Transcription of Escherichia coli ara in vitro. The cyclic AMP receptor protein requirement for PBAD induction that depends on the presence and orientation of the araO2 site. J Mol Biol. 1986 Apr 5;188(3):355–367. doi: 10.1016/0022-2836(86)90160-9. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., Dorman C. J., Stirling D. A., Waddell L., Booth I. R., May G., Bremer E. A physiological role for DNA supercoiling in the osmotic regulation of gene expression in S. typhimurium and E. coli. Cell. 1988 Feb 26;52(4):569–584. doi: 10.1016/0092-8674(88)90470-9. [DOI] [PubMed] [Google Scholar]
- Hill S. How is nitrogenase regulated by oxygen? FEMS Microbiol Rev. 1988 Apr-Jun;4(2):111–129. doi: 10.1111/j.1574-6968.1988.tb02738.x. [DOI] [PubMed] [Google Scholar]
- Hill S., Kennedy C., Kavanagh E., Goldberg R. B., Hanau R. Nitrogen fixation gene (nifL) involved in oxygen regulation of nitrogenase synthesis in K. pneumoniae. Nature. 1981 Apr 2;290(5805):424–426. doi: 10.1038/290424a0. [DOI] [PubMed] [Google Scholar]
- Hunt T. P., Magasanik B. Transcription of glnA by purified Escherichia coli components: core RNA polymerase and the products of glnF, glnG, and glnL. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8453–8457. doi: 10.1073/pnas.82.24.8453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson D. J., Higgins C. F. Two genetically distinct pathways for transcriptional regulation of anaerobic gene expression in Salmonella typhimurium. J Bacteriol. 1986 Oct;168(1):389–397. doi: 10.1128/jb.168.1.389-397.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Q. T., Wu Q. L., Ma Z. F., Shen S. C. Oxygen sensitivity of the nifLA promoter of Klebsiella pneumoniae. J Bacteriol. 1986 Apr;166(1):353–356. doi: 10.1128/jb.166.1.353-356.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz R. G., Haselkorn R. Anaerobic regulation of nitrogen-fixation genes in Rhodopseudomonas capsulata. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6805–6809. doi: 10.1073/pnas.83.18.6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer H., Amouyal M., Nordheim A., Müller-Hill B. DNA supercoiling changes the spacing requirement of two lac operators for DNA loop formation with lac repressor. EMBO J. 1988 Feb;7(2):547–556. doi: 10.1002/j.1460-2075.1988.tb02844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambden P. R., Guest J. R. Mutants of Escherichia coli K12 unable to use fumarate as an anaerobic electron acceptor. J Gen Microbiol. 1976 Dec;97(2):145–160. doi: 10.1099/00221287-97-2-145. [DOI] [PubMed] [Google Scholar]
- Lamond A. I. Supercoiling response of a bacterial tRNA gene. EMBO J. 1985 Feb;4(2):501–507. doi: 10.1002/j.1460-2075.1985.tb03656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane S. A., Merrick M. J. Analysis of the Klebsiella pneumoniae ntrB gene by site-directed in vitro mutagenesis. Mol Microbiol. 1987 Sep;1(2):133–142. doi: 10.1111/j.1365-2958.1987.tb00505.x. [DOI] [PubMed] [Google Scholar]
- MacNeil D., Zhu J., Brill W. J. Regulation of nitrogen fixation in Klebsiella pneumoniae: isolation and characterization of strains with nif-lac fusions. J Bacteriol. 1981 Jan;145(1):348–357. doi: 10.1128/jb.145.1.348-357.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick M. J. Nitrogen control of the nif regulon in Klebsiella pneumoniae: involvement of the ntrA gene and analogies between ntrC and nifA. EMBO J. 1983;2(1):39–44. doi: 10.1002/j.1460-2075.1983.tb01377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minchin S. D., Austin S., Dixon R. A. The role of activator binding sites in transcriptional control of the divergently transcribed nifF and nifLA promoters from Klebsiella pneumoniae. Mol Microbiol. 1988 Jul;2(4):433–442. doi: 10.1111/j.1365-2958.1988.tb00049.x. [DOI] [PubMed] [Google Scholar]
- Newman B. M., Cole J. A. The chromosomal location and pleiotropic effects of mutations of the nirA+ gene of Escherichia coli K12: the essential role of nirA+ in nitrite reduction and in other anaerobic redox reactions. J Gen Microbiol. 1978 May;106(1):1–12. doi: 10.1099/00221287-106-1-1. [DOI] [PubMed] [Google Scholar]
- Ninfa A. J., Magasanik B. Covalent modification of the glnG product, NRI, by the glnL product, NRII, regulates the transcription of the glnALG operon in Escherichia coli. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5909–5913. doi: 10.1073/pnas.83.16.5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasse-Dwight S., Gralla J. D. Probing co-operative DNA-binding in vivo. The lac O1:O3 interaction. J Mol Biol. 1988 Jul 5;202(1):107–119. doi: 10.1016/0022-2836(88)90523-2. [DOI] [PubMed] [Google Scholar]
- Sinden R. R., Pettijohn D. E. Chromosomes in living Escherichia coli cells are segregated into domains of supercoiling. Proc Natl Acad Sci U S A. 1981 Jan;78(1):224–228. doi: 10.1073/pnas.78.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. W., Neidhardt F. C. Proteins induced by anaerobiosis in Escherichia coli. J Bacteriol. 1983 Apr;154(1):336–343. doi: 10.1128/jb.154.1.336-343.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G., Hedge P. J., te Heesen S., Edelman A., Broome-Smith J. K. Kanamycin-resistant vectors that are analogues of plasmids pUC8, pUC9, pEMBL8 and pEMBL9. Gene. 1986;41(2-3):337–342. doi: 10.1016/0378-1119(86)90117-4. [DOI] [PubMed] [Google Scholar]
- Strauch K. L., Lenk J. B., Gamble B. L., Miller C. G. Oxygen regulation in Salmonella typhimurium. J Bacteriol. 1985 Feb;161(2):673–680. doi: 10.1128/jb.161.2.673-680.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitson P. A., Hsieh W. T., Wells R. D., Matthews K. S. Supercoiling facilitates lac operator-repressor-pseudooperator interactions. J Biol Chem. 1987 Apr 15;262(11):4943–4946. [PubMed] [Google Scholar]
- Wong P. K., Popham D., Keener J., Kustu S. In vitro transcription of the nitrogen fixation regulatory operon nifLA of Klebsiella pneumoniae. J Bacteriol. 1987 Jun;169(6):2876–2880. doi: 10.1128/jb.169.6.2876-2880.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N., Droffner M. L. Mechanisms determining aerobic or anaerobic growth in the facultative anaerobe Salmonella typhimurium. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2077–2081. doi: 10.1073/pnas.82.7.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]