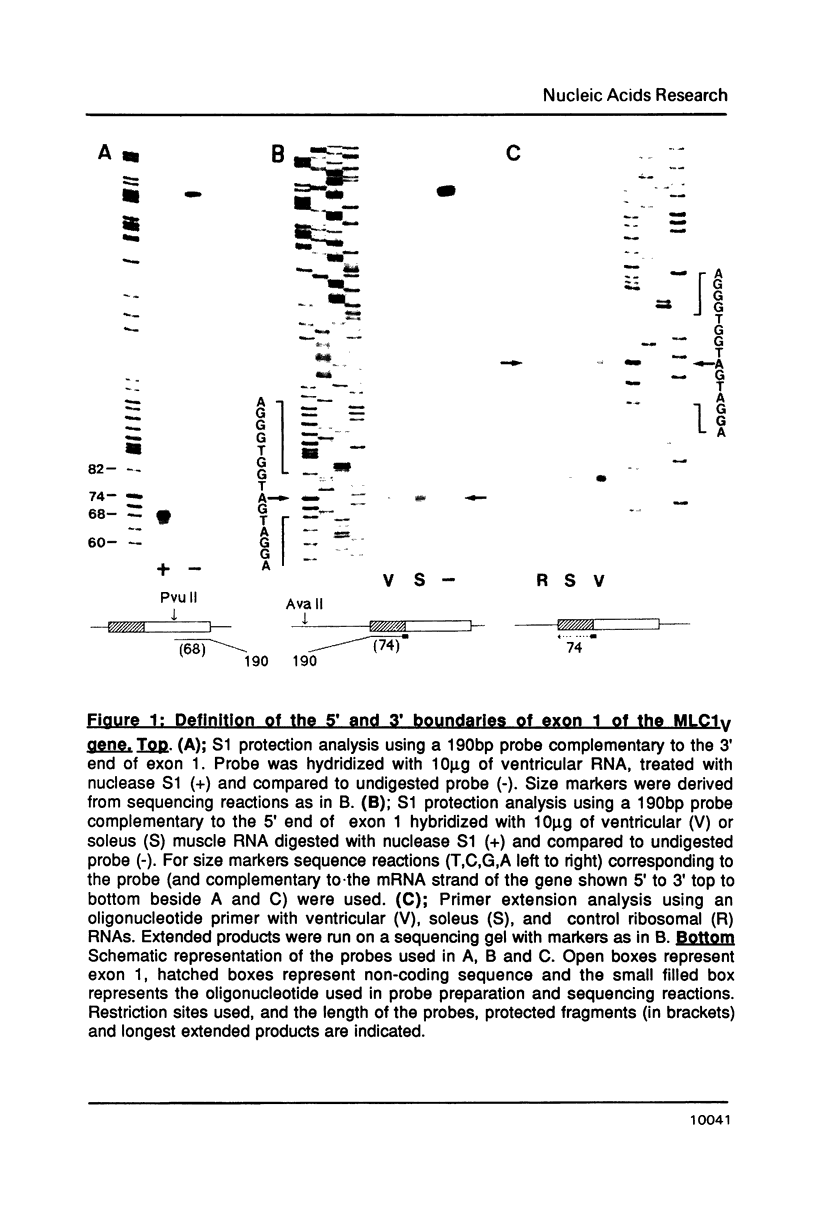
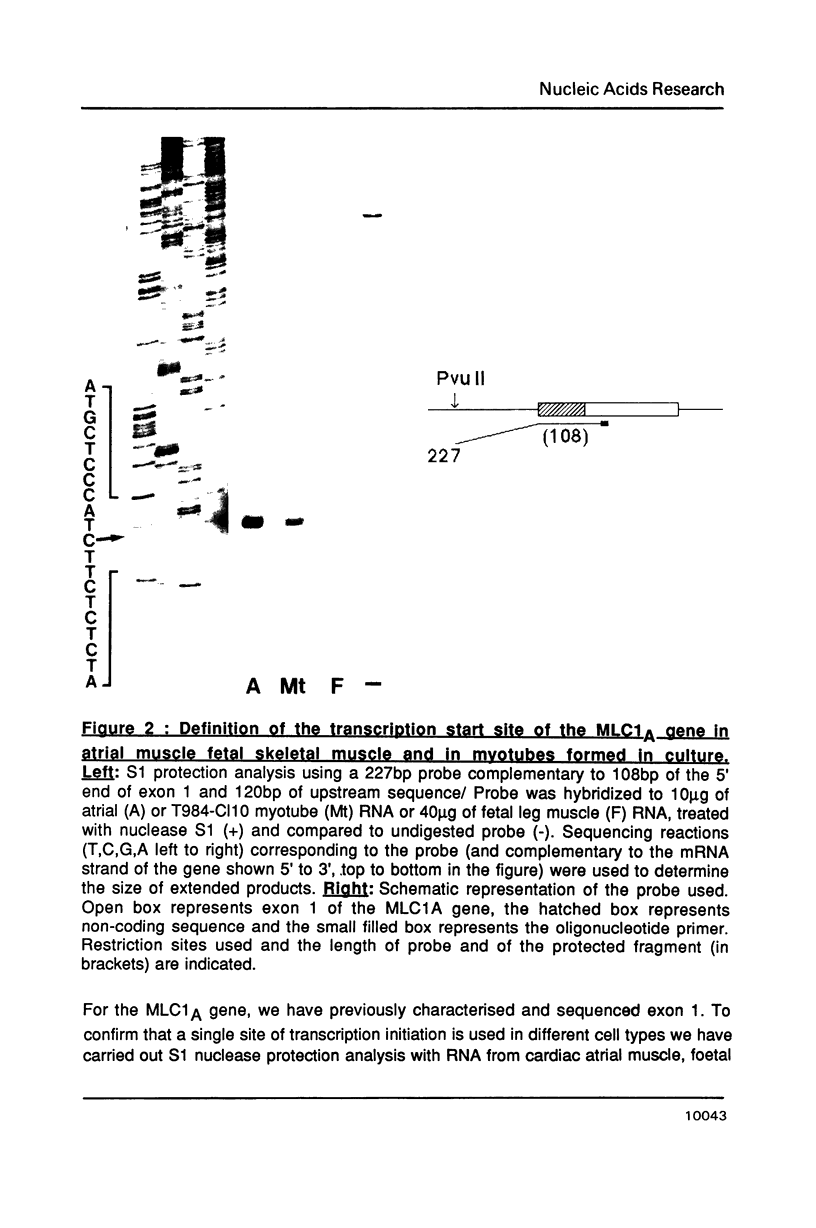
Abstract
There are three principal myosin alkali light chain (MLC) genes expressed in mouse striated muscle. The skeletal muscle gene MLC1F/MLC3F, the ventricular muscle/slow skeletal muscle gene MLC1V(MLC1S), and the atrial muscle/foetal striated muscle gene MLC1A(MLC1emb). MLC1V and MLC1A are expressed in both cardiac and skeletal muscle, and we show here that these genes use a single site of initiation of transcription, and therefore the same proximal promoter region, in both muscle types, and in myogenic cell lines in culture. We have previously shown that for the MLC1F/MLC3F gene, 1200bp of upstream sequence from the MLC1F promoter is sufficient to allow tissue specific and developmentally regulated expression. We have therefore isolated, characterised, and sequenced over 1200bp upstream of each of the three MLC genes in order to look for elements which may be involved in their regulation. Detailed comparison of their promoter sequences, as well as those of the cardiac and skeletal muscle alpha-actin genes, reveals a number of common elements. Among these is an "MLC-sequence" (CCTTTTATAG) common to all MLC genes, including those of chick and rat, and a "cardiac sequence" common to the mouse MLC1A, MLC1V and alpha-cardiac actin genes expressed in the heart.
Full text
PDF





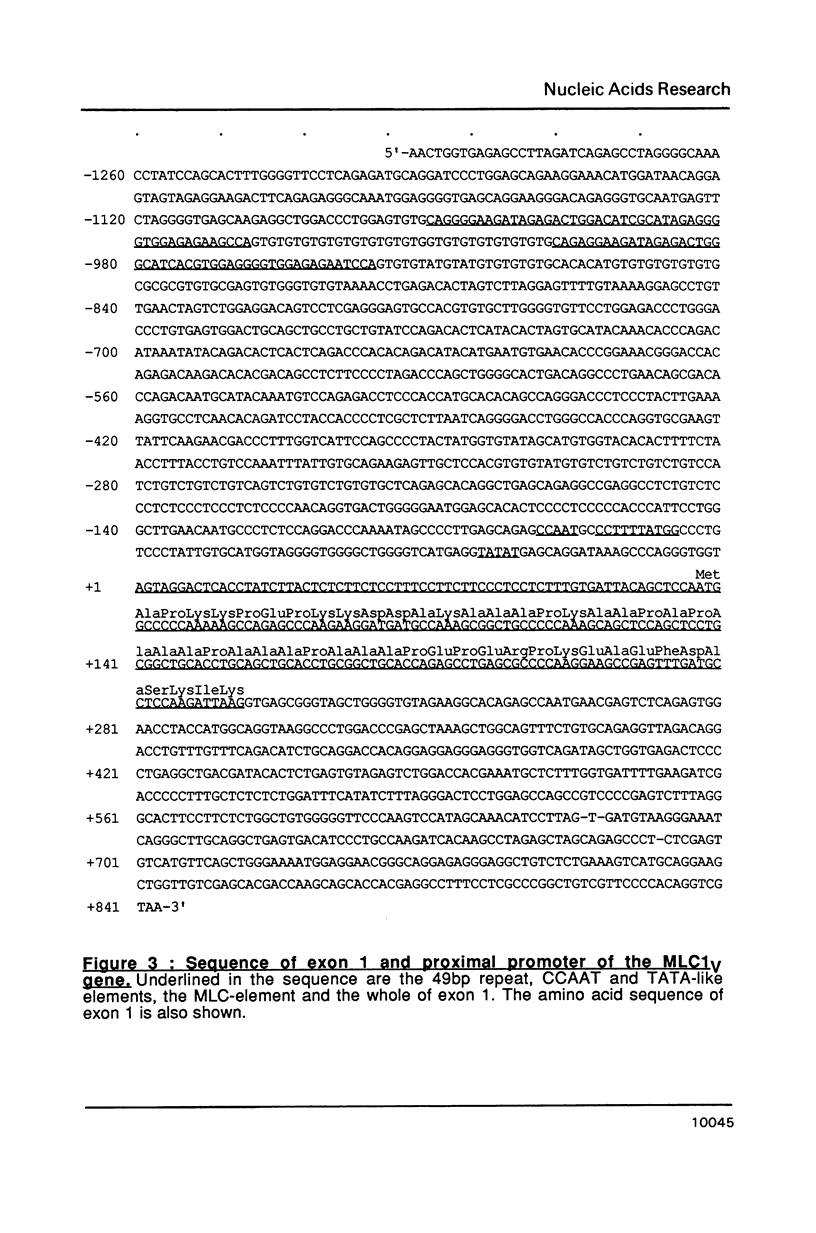







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton P. J., Buckingham M. E. The myosin alkali light chain proteins and their genes. Biochem J. 1985 Oct 15;231(2):249–261. doi: 10.1042/bj2310249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton P. J., Cohen A., Robert B., Fiszman M. Y., Bonhomme F., Guénet J. L., Leader D. P., Buckingham M. E. The myosin alkali light chains of mouse ventricular and slow skeletal muscle are indistinguishable and are encoded by the same gene. J Biol Chem. 1985 Jul 15;260(14):8578–8584. [PubMed] [Google Scholar]
- Barton P. J., Robert B., Cohen A., Garner I., Sassoon D., Weydert A., Buckingham M. E. Structure and sequence of the myosin alkali light chain gene expressed in adult cardiac atria and fetal striated muscle. J Biol Chem. 1988 Sep 5;263(25):12669–12676. [PubMed] [Google Scholar]
- Barton P. J., Robert B., Fiszman M. Y., Leader D. P., Buckingham M. E. The same myosin alkali light chain gene is expressed in adult cardiac atria and in fetal skeletal muscle. J Muscle Res Cell Motil. 1985 Aug;6(4):461–475. doi: 10.1007/BF00712583. [DOI] [PubMed] [Google Scholar]
- Billeter R., Quitschke W., Paterson B. M. Approximately 1 kilobase of sequence 5' to the two myosin light-chain 1f/3f gene cap sites is sufficient for differentiation-dependent expression. Mol Cell Biol. 1988 Mar;8(3):1361–1365. doi: 10.1128/mcb.8.3.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cross S. L., Feinberg M. B., Wolf J. B., Holbrook N. J., Wong-Staal F., Leonard W. J. Regulation of the human interleukin-2 receptor alpha chain promoter: activation of a nonfunctional promoter by the transactivator gene of HTLV-I. Cell. 1987 Apr 10;49(1):47–56. doi: 10.1016/0092-8674(87)90754-9. [DOI] [PubMed] [Google Scholar]
- Daubas P., Klarsfeld A., Garner I., Pinset C., Cox R., Buckingham M. Functional activity of the two promoters of the myosin alkali light chain gene in primary muscle cell cultures: comparison with other muscle gene promoters and other culture systems. Nucleic Acids Res. 1988 Feb 25;16(4):1251–1271. doi: 10.1093/nar/16.4.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubas P., Robert B., Garner I., Buckingham M. A comparison between mammalian and avian fast skeletal muscle alkali myosin light chain genes: regulatory implications. Nucleic Acids Res. 1985 Jul 11;13(13):4623–4643. doi: 10.1093/nar/13.13.4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkner F. G., Zachau H. G. Correct transcription of an immunoglobulin kappa gene requires an upstream fragment containing conserved sequence elements. Nature. 1984 Jul 5;310(5972):71–74. doi: 10.1038/310071a0. [DOI] [PubMed] [Google Scholar]
- Felsani A., Maione R., Ricci L., Amati P. Coordinate expression of myogenic functions and polyoma virus replication. Cold Spring Harb Symp Quant Biol. 1985;50:753–757. doi: 10.1101/sqb.1985.050.01.093. [DOI] [PubMed] [Google Scholar]
- Garner I., Minty A. J., Alonso S., Barton P. J., Buckingham M. E. A 5' duplication of the alpha-cardiac actin gene in BALB/c mice is associated with abnormal levels of alpha-cardiac and alpha-skeletal actin mRNAs in adult cardiac tissue. EMBO J. 1986 Oct;5(10):2559–2567. doi: 10.1002/j.1460-2075.1986.tb04535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard J. M., Herbomel P., Ott M. O., Mottura-Rollier A., Weiss M., Yaniv M. Determinants of rat albumin promoter tissue specificity analyzed by an improved transient expression system. Mol Cell Biol. 1987 Jul;7(7):2425–2434. doi: 10.1128/mcb.7.7.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing P., Shenk T. The adenovirus type 5 E1A transcriptional control region contains a duplicated enhancer element. Cell. 1983 Jul;33(3):695–703. doi: 10.1016/0092-8674(83)90012-0. [DOI] [PubMed] [Google Scholar]
- Hu M. C., Sharp S. B., Davidson N. The complete sequence of the mouse skeletal alpha-actin gene reveals several conserved and inverted repeat sequences outside of the protein-coding region. Mol Cell Biol. 1986 Jan;6(1):15–25. doi: 10.1128/mcb.6.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakob H., Buckingham M. E., Cohen A., Dupont L., Fiszman M., Jacob F. A skeletal muscle cell line isolated from a mouse teratocarcinoma undergoes apparently normal terminal differentiation in vitro. Exp Cell Res. 1978 Jul;114(2):403–408. doi: 10.1016/0014-4827(78)90499-8. [DOI] [PubMed] [Google Scholar]
- Jaynes J. B., Johnson J. E., Buskin J. N., Gartside C. L., Hauschka S. D. The muscle creatine kinase gene is regulated by multiple upstream elements, including a muscle-specific enhancer. Mol Cell Biol. 1988 Jan;8(1):62–70. doi: 10.1128/mcb.8.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto T., Makino K., Niwa H., Sugiyama H., Kimura S., Amemura M., Nakata A., Kakunaga T. Identification of the human beta-actin enhancer and its binding factor. Mol Cell Biol. 1988 Jan;8(1):267–272. doi: 10.1128/mcb.8.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmiec E. B., Angelides K. J., Holloman W. K. Left-handed DNA and the synaptic pairing reaction promoted by Ustilago rec1 protein. Cell. 1985 Jan;40(1):139–145. doi: 10.1016/0092-8674(85)90317-4. [DOI] [PubMed] [Google Scholar]
- Minty A., Kedes L. Upstream regions of the human cardiac actin gene that modulate its transcription in muscle cells: presence of an evolutionarily conserved repeated motif. Mol Cell Biol. 1986 Jun;6(6):2125–2136. doi: 10.1128/mcb.6.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa T., Boxer L. M., Kedes L. CArG boxes in the human cardiac alpha-actin gene are core binding sites for positive trans-acting regulatory factors. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6702–6706. doi: 10.1073/pnas.84.19.6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscat G. E., Kedes L. Multiple 5'-flanking regions of the human alpha-skeletal actin gene synergistically modulate muscle-specific expression. Mol Cell Biol. 1987 Nov;7(11):4089–4099. doi: 10.1128/mcb.7.11.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabeshima Y., Fujii-Kuriyama Y., Muramatsu M., Ogata K. Alternative transcription and two modes of splicing results in two myosin light chains from one gene. Nature. 1984 Mar 22;308(5957):333–338. doi: 10.1038/308333a0. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. A regulatory upstream promoter element in the Drosophila hsp 70 heat-shock gene. Cell. 1982 Sep;30(2):517–528. doi: 10.1016/0092-8674(82)90249-5. [DOI] [PubMed] [Google Scholar]
- Periasamy M., Strehler E. E., Garfinkel L. I., Gubits R. M., Ruiz-Opazo N., Nadal-Ginard B. Fast skeletal muscle myosin light chains 1 and 3 are produced from a single gene by a combined process of differential RNA transcription and splicing. J Biol Chem. 1984 Nov 10;259(21):13595–13604. [PubMed] [Google Scholar]
- Phan-Dinh-Tuy F., Tuil D., Schweighoffer F., Pinset C., Kahn A., Minty A. The 'CC.Ar.GG' box. A protein-binding site common to transcription-regulatory regions of the cardiac actin, c-fos and interleukin-2 receptor genes. Eur J Biochem. 1988 May 2;173(3):507–515. doi: 10.1111/j.1432-1033.1988.tb14027.x. [DOI] [PubMed] [Google Scholar]
- Robert B., Daubas P., Akimenko M. A., Cohen A., Garner I., Guenet J. L., Buckingham M. A single locus in the mouse encodes both myosin light chains 1 and 3, a second locus corresponds to a related pseudogene. Cell. 1984 Nov;39(1):129–140. doi: 10.1016/0092-8674(84)90198-3. [DOI] [PubMed] [Google Scholar]
- Shirakata M., Nabeshima Y., Konishi K., Fujii-Kuriyama Y. Upstream regulatory region for inducible expression of the chicken skeletal myosin alkali light-chain gene. Mol Cell Biol. 1988 Jun;8(6):2581–2588. doi: 10.1128/mcb.8.6.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strehler E. E., Periasamy M., Strehler-Page M. A., Nadal-Ginard B. Myosin light-chain 1 and 3 gene has two structurally distinct and differentially regulated promoters evolving at different rates. Mol Cell Biol. 1985 Nov;5(11):3168–3182. doi: 10.1128/mcb.5.11.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R. Transient accumulation of c-fos RNA following serum stimulation requires a conserved 5' element and c-fos 3' sequences. Cell. 1985 Oct;42(3):889–902. doi: 10.1016/0092-8674(85)90285-5. [DOI] [PubMed] [Google Scholar]
- Walsh K., Schimmel P. Two nuclear factors compete for the skeletal muscle actin promoter. J Biol Chem. 1987 Jul 15;262(20):9429–9432. [PubMed] [Google Scholar]
- Whalen R. G., Butler-Browne G. S., Gros F. Identification of a novel form of myosin light chain present in embryonic muscle tissue and cultured muscle cells. J Mol Biol. 1978 Dec 15;126(3):415–431. doi: 10.1016/0022-2836(78)90049-9. [DOI] [PubMed] [Google Scholar]