Abstract
Propionibacterium acnes (P. acnes) bacteria play a key role in the pathogenesis of acne vulgaris. Although our previous studies have demonstrated that vaccines targeting a surface sialidase or bacterial particles exhibit a preventive effect against P. acnes, the lack of therapeutic activities and incapability of neutralizing secretory virulence factors motivate us to generate novel immunotherapeutics. In this study, we develop an immunotherapeutic antibody to secretory Christie-Atkins-Munch-Peterson (CAMP) factor of P. acnes. Via agroinfiltration, P. acnes CAMP factor was encapsulated into the leaves of radishes. ICR mice intranasally immunized with whole leaves expressing CAMP factor successfully produced neutralizing antibodies that efficiently attenuated P. acnes-induced ear swelling and production of macrophage-inflammatory protein-2. Passive neutralization of CAMP factor enhanced immunity to eradicate P. acnes at the infection site without influencing bacterial growth elsewhere. We propose that CAMP factor is a novel therapeutic target for the treatment of various P. acnes-associated diseases and highlight the concept of neutralizing P. acnes virulence without disturbing the bacterial commensalism in human micorbiome.
Keywords: Acne vulgaris, Agroinfiltration, Passive immunization, Propionibacterium acnes, Radish leaves
1. Introduction
Propionibacterium acnes (P. acnes) is a Gram-positive, anaerobic, ubiquitous commensal, and opportunistic pathogen [1, 2]. Nearly everyone hosts P. acnes [3, 4], which accounts for approximately half of the total skin microbiome [5], with an estimated density of 102–105–6 cm2 [6, 7]. P. acnes predominates (more than 46% of total bacteria) in facial skin [8]; however, it can be found almost everywhere on the body [9, 10]. P. acnes colonizes the sebaceous follicles [6] and is one of the pathogens involved in the progression of inflammation in acne vulgaris [11, 12] and tissue damage by releasing various virulence factors [13, 14]. The inflammatory reaction is marked initially by suppuration, followed by granulomatous inflammation, and, over time, by fibrosis and scarring. Once the hair follicle wall has ruptured, P. acnes escapes from the damaged follicles and then enters the dermis in most cases of late-stage and/or severe acne vulgaris [15]. Aside from acne vulgaris, many human diseases such as implant infections, pulmonary sarcoidosis, osteomyelitis and endocarditis have been linked to P. acnes infections [9, 16, 17].
Examination of the genome of P. acnes has revealed that Christie-Atkins-Munch-Peterson (CAMP) factor is a potential secretory virulence factor [18]. The bacterium carries five genes with sequence homology (approximately 32%) to the co-hemolytic CAMP factor of Streptococcus agalactiae (S. agalactiae) [19, 20]. CAMP factor of S. agalactiae potentially can bind to the Fc fragment of immunoglobulins of the Immunoglobulin G (IgG) and Immunoglobulin M (IgM) classes [19]. In addition, it has been reported that CAMP factor of S. agalactiae acts as a pore-forming toxin [20]. Although it is unclear if P. acnes CAMP factor exhibits a similar co-hemolytic activity as that of S. agalactiae, it has been reported that when P. acnes was grown on a sheep blood agar plate in close proximity to β-hemolytic microorganisms [21], it synergistically enhances hemolysis similar to the classical CAMP reaction first described by Charlistie and co-authors [22]. Moreover, we have recently demonstrated that P. acnes CAMP factor enhances hemolysis and cytolysis by Staphylococcus aureus (S. aureus) β-hemolysin, suggesting that S. aureus may shrewdly utilize the secreted P. acnes CAMP factor to intensify its virulence [23].
There are many challenges in treating acne vulgaris. Current treatments using anti-acne agents including antibiotics lack bacterial specificity, imbalance human microbiome homeostasis, and have a risk of generating drug-resistant bacteria [24]. Benzoyl peroxide, an agent for mild acne, releases oxygen free radicals that oxidizes bacterial proteins in the sebaceous follicles to decrease the number of anaerobic bacteria and irritating-type free fatty acids [25]. Although its use does not predispose to skin infection and develop bacterial resistance [26], it has some adverse effects on the skin that may include stinging, dryness, and peeling [27]. The increased oxygen free-radical by benzoyl peroxide could even increase the risk of skin cancer [25, 28]. Importantly, most antibiotics targeting bacterial particles are incapable of inactivating the secretory toxins [29]. Alternatively, isotretinoin is a powerful and effective medication derived from vitamin A [30], often prescribed by doctors to treat severe acne only after other treatments have failed. However, isotretinoin is strictly regulated due to the induction of serious side effects. As little as one dose of isotretinoin can cause severe birth defects in pregnant woman taking this medicine [31]. P. acnes has been recognized as an ubiquitous commensal on the human body [32, 33] and only becomes pathogenic in some diseases [13, 34]. Systemic treatment of P. acnes infection using anti-acne agents or antibiotics may carry risks of disrupting the commensalisms of P. acnes and have incapacity to naturalizing secretory toxins of P. acnes.
In our previous efforts, we have generated anti-P. acnes vaccines using a surface sialidase [35] and killed P. acnes [12] as antigens. Although we have demonstrated that these anti-P. acnes vaccines decrease P. acnes-induced inflammation [35], they may not have the capability to neutralize the virulence factors secreted from P. acnes. In addition, these vaccines designed as preventive modalities may lack the therapeutic effects. Notably, to achieve preventive effects, these anti-P. acnes vaccines have to be administrated in the early childhood. Many people may be reluctant to receive these vaccines since they cannot predict if they will suffer from acne vulgaris. Thus, there is an urgent need for the development of immunotherapeutics for acne vulgaris. It has been documented that inhibition of secreted virulence factors may present less selective pressure for the generation of microbial resistance [36]. Inhibition of secreted virulence factors may not directly influence the growth of commensal P. acnes [37], minimizing the risk of altering the homeostasis of resident human microbes. Accordingly, neutralization of bacteria-induced virulence and inflammation without directly killing bacteria would be an excellent immunotherapeutic for the treatment of acne vulgaris. After neutralization of secreted virulence factors, the “disarmed” bacteria in local lesions could be eliminated naturally by immune systems. Therefore, passive transfer of antibodies against toxins would complement other treatments, as it would be able to neutralize circulating P. acnes toxins while keeping the P. acne at an optimal balance. Thus, passive immunization to toxins of P. acnes in place of commonly used therapy such as anti-acne agents and antibiotics would have benefit for certain condition of skin inflammation.
In this study, we employ a passive immunization approach to attenuate the virulence of secretory CAMP factor of P. acnes. The factor was expressed in plant leaves using agroinfiltration. There are several advantages to expressing the proteins in the plants, such as low cost and high yield [38, 39]. Plants can also be grown on site, reducing the need for costly refrigerated transport and storage [40, 41]. Furthermore, the main advantages associated with plants include posttranslational modifications (PTMs) and production of correctly folded and assembled multimeric proteins, low risk of contamination with pathogens and endotoxins such as those occurring in mammalian and bacterial systems, and the avoidance of ethical problems associated with transgenic animals and animal materials [42].
Overall, this study provides a novel therapeutic target (CAMP factor) for treatment of acne vulgaris and presents a concept of suppressing P. acnes-induced local lesions without disturbing the commensalisms of P. acnes. The concept may be able to be broadly applied for treating human diseases caused by commensal microbes that become pathogens in local lesions [43].
2. Materials and methods
2.1. Molecular cloning and expression of recombinant green fluorescence protein (GFP) and CAMP factors
A polymerase chain reaction (PCR) product encoding a putative mature protein (29–267 amino acid residues) of CAMP factor (accession number: gi/50842175) was generated using gene-specific primers based on the complete genome of P. acnes [13]. The forward PCR primer (5’-TAAGGCCTCTGTCGACGTCGAGCCGACGACGACCATCTCG-3’) consisted of nucleotides containing a SalI site (GTCGAC) and the reverse PCR primer (5’-CAGAATTCGCAAGCTTGGCAGCCTTCTTGACATCGGGGGAG-3’) consisted of nucleotides containing a HindIII site (AAGCTT). PCR was performed by using P. acnes genomic DNA as a template. The amplified DNA products were inserted at the restriction enzyme sites into an In-Fusion™ Ready pEcoli-6×HN-GFPuv expression vector and transformed into competent cells [Escherichia coli (E. coli), BL21 (DE3), Invitrogen, Carlsbad, CA], which were subsequently selected on Luria-Bertani (LB) plates containing ampicillin (50 µg/ml) and cultured overnight at 37°C. To express the GFP and CAMP factor, a pEcoli-6×HN-GFPuv and pEcoli-6×HN-CAMP factor plasmids were transformed into E. coli, BL21 (DE3) competent cells. A 2 ml aliquot of the overnight culture was added in 200 ml LB medium (1: 100 dilution) and incubated at 37°C until reaching optical density at 600 nm (OD600) of 0.6. Subsequently, Isopropyl-β D-thiogalactoside (IPTG) (Sigma-Aldrich, St. Louis, MO) was added into the culture to a final concentration of 1 mM for 4 h. After centrifugation at 3,000 × g at 4°C for 5 min, bacterial pellets were resuspended with 6 M urea. The supernatant was collected by centrifugation at 13,000 × g for 20 min then loaded onto a column with 2 ml Ni-NTA agarose (QIAGEN, Valencia, CA), which had been equilibrated previously with Buffer A (20 mM Tris-HCl, 0.5 M NaCl, 1 mM 2-mercaptoethanol, pH 8.0) containing 6 M urea. The column was washed sequentially with 5 ml aliquots of Buffer A containing 6-0 M urea gradient. The bound fraction was then eluted with 5 ml of Buffer A containing 500 mM Imidazole. The purified and refolded proteins were dialyzed overnight at 4°C in 5 liters of phosphate-buffered saline (PBS) by using Spectra/Por molecular porous membrane tubing [molecular weight cut off (MWCO): 3,500] (Spectrum Laboratories Inc., Rancho Dominguez, CA) and then concentrated by lyophilization. A 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and coomassie blue staining were used to determine the protein expression.
2.2. CAMP factor-induced inflammation
An amount of 50 µg purified CAMP factor in 25 µl PBS was intradermally injected in the central portion of the right ear. As a control, purified GFP was injected into the left ear of the same mice. To prevent leakage, proteins were gradually injected into mouse ears using a 28-gauge needle followed by a slow withdrawal of the needle. For histological observation, the ear was cross-sectioned, stained with hematoxylin and eosin (H&E) (Sigma diagnostics, St Louis, MO), and viewed on a Zeiss Axioskop2 plus microscope (Carl Zeiss, Thornwood, NY).
2.3. Plant materials
Japanese radish sprouts (Kaiware-daikon) (Raphanus sativus L.) obtained from a commercial supplier (ICREST International, JCP, Carson, CA) were 9 cm in length with two leaflets. These sprouts were grown at room temperature under a 23 Watt fluorescent bulb (Philips, Portland, OR) and sprayed with water daily.
2.4. Vector construction
The binary vector pBI121 (provided by Professor Nigel Crawford) harboring the reporter β-glucuronidase (GUS) driven by the cauliflower mosaic virus 35S promoter was used for gene construction [44]. The open reading frames of CAMP factor cDNA cloned in a pEcoli-6×HN-GFPuv vector as described in “Molecular cloning and expression of recombinant GFP and CAMP factors” section was amplified by PCR using a forward primer (5′-CCTTCTAGAGGAGATATACCATGGGTCATAATCAT-3’) and a reverse primer (5′-TCCCCCGGGTTAATTAATTAAGCGGCCGCC-3’). The spore coat-associated protein (SCAP) cDNA cloned in a pIVEX- maltose binding protein (MBP) vector [45, 46] was amplified using a forward primer (5′-AGATCTAGAATGTCTGGTTCTCATCATCATCATC-3’) and a reverse primer (5′-GCCCCCGGGTTAGCCTTCGATCCCGAGGTT-3’). The primers were designed to add restriction sites to the ends of PCR products. Specifically, the restriction sites XbaI and SmaI were encoded into the forward and reverse primers, respectively. PCR products were treated with XbaI and SmaI then cloned into polylinker sites of pBI121 vectors to generate 35S::CAMP factor-Histidine (His) and 35S::SCAP-MBP-His constructs.
2.5. Agrobacterium tumefaciens transformation
All constructs were transformed into an Agrobacterium tumefaciens strain LBA4404 using a liquid nitrogen freeze-thaw method [47]. A single colony of LBA4404 cells was inoculated in 5 ml of YEP medium [10 mg/ml bacto-trypton (DIFCO, Detroit, MI), 10 mg/ml yeast extract (DIFCO, Detroit, MI), and 5 mg/ml NaCl (Sigma, St. Louis, MO; pH 7.5)] with 250 rpm shaking at 28°C overnight. Subsequently, 50 ml of fresh YEP was inoculated with 2 ml of liquid culture and incubated with 250 rpm shaking at 28°C until the OD600 reached 0.8. The bacteria were centrifuged at 3,000 × g for 5 min at 4°C and the pellet was resuspended in 1 ml of 20 mM calcium chloride. The bacteria (0.2 ml) were transferred to a 1.5 ml microfuge tube and 1 µg of gene constructs was added. The mixture was frozen in liquid nitrogen for 5 min then thawed in a 37°C water bath for 5 min. One ml of YEP medium was added to the mixture and incubated with 250 rpm shaking at 28°C for 2 to 4 h. The bacteria were centrifuged at 3,000 × g for 5 min then resuspended in 0.1 ml of YEP. Transformants were incubated at 28°C for 2 to 3 days and selected by plating bacteria on YEP-agar medium (YEP medium containing 1.5% agar) containing antibiotics (50 µg/ml kanamycin and 50 µg/ml streptomycin).
2.6. Agroinfiltration of gene constructs into leaves and protein extraction
A single colony of A. tumefaciens transformants was cultured in 2 ml of YEP medium containing 50 µg/ml kanamycin and 50 µg/ml streptomycin with 250 rpm shaking at 28°C until OD600 reached approximately 0.5. Afterward, the bacteria were collected by centrifugation at 3000 ×g for 5 min, and resuspended in 2 ml sterile didistilled water (ddH2O). All bacterial suspensions were maintained at room temperature for 30 min until agroinfiltration. Non-transformed Agrobacterium served as a negative control and was cultured under the same conditions as the transformants without adding kanamycin in the medium. For syringe infiltration, the central lower epidermises (i.e., the centermost 25 mm2 area) of potted seedlings leaves were wounded with a sterile scalpel (number 15, Feather Safety Razor Co., Osaka, Japan) and 0.1 ml of Agrobacterium bacterial suspension (5×107 CFU) was injected into the wound site, which was positioned between a finger and a 1 ml syringe (BD, Bioscience, San Diego, CA). Infiltration was confirmed by visually monitoring the diffusion of bacterial suspension toward the leaf margin [48]. Agroinfiltrated leaves were grown for five days before GUS assays and immunization was performed. Agroinfiltrated leaves were stained using a histochemical GUS assay solution consisting of 0.1 M NaPO4 (pH 7.0), 0.5 mM K3Fe(CN)6, 0.5 mM K4Fe(CN)6, 0.1% (v/v) Triton X-100, and 0.05% (w/v) 5-bromo-4-chloro-3-indolyl-β-D-glucuronic acid, cyclohexylammonium salt (Sigma, St. Louis, MO). Leaves were submerged in the staining solution and incubated at 37°C in the dark overnight. After incubation, leaves were removed from the staining solution and immersed in a stop solution containing 42.5% (v/v) ethanol, 10% (v/v) formaldehyde, and 5% (v/v) acetic acid [44]. Quantitative determination of GUS activity was accomplished by the fluorometric assay. Whole leaves were grounded with 200 µl of 1 × CCLR [100 mM K-phosphate (pH 7.8), 1 mM EDTA, 10% (v/v) glycerol, 1% (v/v) Triton X-100 and 7 mM β-mercaptoethanol]. The mixture was centrifuged at 13,000 × g for 5 min at 4°C and 200 µl supernatant was removed to a new microtube on ice followed by mixing with 1 mM 4-Methylumbelliferyl-D-glucuronide buffer at 37°C for 1 h [44]. The enzymatic reaction was measured spectrofluorometrically with excitation at OD365 and emission at OD455 by SpectraMAX GeminiEM spectrofluorometer (Molecular Devices, Sunnyvale, CA). To investigate the dynamic expression of antigen in radish leaves, leaves were removed at 0, 1, 3, and 5 days to quantify the level of GUS.
Purification of CAMP factor and SCAP from leaf tissues were carried out by affinity chromatography on a Ni–NTA agarose column (Qiagen, Valencia, CA) with certain modifications. The column was washed with water and equilibrated with buffer A (8 M guanidine, 100 mM NaH2PO4, 10 mM Tris-HCl, pH 8.0). Leaf material (1 g) was ground under liquid nitrogen using mortar and pestle in 15 ml ice-cold extraction buffer A. Guanidine-solubilized proteins were centrifuged at 13,000 × g for 20 min to remove the debris and insoluble material and the supernatant was gently stirred with 1.6 ml Ni–NTA agarose resin for 1 h at room temperature. The mixture was loaded onto a column previously equilibrated with buffer A. Briefly, the column was washed with buffer B (8 M urea, 100 mM NaH2PO4, 10 mM Tris-HCl, pH 6.8). Finally, proteins were eluted with buffer C (8 M urea, 100 mM NaH2PO4, 10 mM Tris-HCl, pH 6.3), buffer D (8 M urea, 100 mM NaH2PO4, 10 mM Tris-HCl, pH 5.9), and buffer E (8 M urea, 100 mM NaH2PO4, 10 mM Tris-HCl, pH 4.5).
2.7. Intranasal immunization with whole leaves containing recombinant CAMP factors
Female Institute of Cancer Research (ICR) mice (3 to 6 weeks old; Harlan, Indianapolis, IN) were utilized for intranasal immunization [49]. Mice were maintained in accordance to institutional guidelines. The central areas (25 mm2) of five radish leaves expressing GUS or CAMP factors alone were excised using a sterile scalpel. To avoid Agrobacterium transgene introgression, leaf sections were pooled and ground in 700 µl ddH2O and then sterilized by an ultraviolet (UV) crosslinker (Spectronics, Westbury, NY) at 7,000 J/m2 for 30 min. Inactivation of sterilized Agrobacterium was confirmed by their inability to form colonies on YEP agar plates (data not shown). It has been indicated that plant-based vaccines administered via intranasal delivery offer many advantages with respect of antigen levels and dosage control compared to those administered via oral delivery [50, 51]. Whole leaves containing either CAMP factor or GUS alone (as a negative control) without adjuvants were then intranasally inoculated into the nasal cavities of ICR mice (25 µl of whole leaves/mouse). Three boosts at the same dose were performed at 1, 2, and 4 weeks after the first immunization.
2.8. Western blotting
To detect antigen expression, 15 µg recombinant GUS and 20 µg of whole leaves expressing CAMP factors or SCAP alone were separated using 10% SDS-PAGE. Bands were electrophoretically transferred to nitrocellulose membranes [52]. Membranes were probed with anti-CAMP factor serum obtained from mice immunized with UV-irradiated E. coli, BL21 (DE3) [45, 46, 53] over-expressing P. acnes CAMP factors. To confirm antibody production in the immunized mice, purified CAMP factor (65 µg) was loaded into a 10% SDS-PAGE and transferred to a nitrocellulose membrane. The blot was immuno-reacted to serum (1:500 dilution) obtained from mice immunized for four weeks with whole leaves containing CAMP factor. Antibodies IgG were detected with anti-mouse horseradish peroxidase-conjugated IgG (1:5,000 dilution, Promega, Madison, WI). Peroxidase activity was visualized with a Western lighting chemiluminescence kit (PerkinElmer, Boston, MA).
2.9. Culture of P. acnes
P. acnes ATCC 6919 (American Type Culture Collection, Manassas, VA) was cultured on Reinforced Clostridium Medium (RCM, Oxford, Hampshire, England) under anaerobic conditions using Gas-Pak (BD, Sparks, MD) at 37°C until reaching OD600 = 1.0–3.0 (logarithmic growth phase). Bacteria were harvested by centrifugation at 13,000 × g for 10 min, washed with PBS three times, and suspended to appropriate amount of PBS for experiments.
2.10. Passive immunization of anti-CAMP factor serum against P. acnes-induced inflammation
Complements in the serum were inactivated by heating at 56°C for 30 min. P. acnes was pre-treated with 5 % (v/v) inactivated anti-GUS serum or anti-CAMP factor serum in the medium at 37°C for 2 h. The 2 h incubation of anti-GUS serum (3.63 ± 1.47 × 108 CFU) and anti-CAMP factor serum (3.3 ± 1.2 × 108 CFU), respectively, did not significantly influence the growth of P. acnes. ICR mice were injected intradermally with an amount of 25 µl aliquots of anti-GUS or anti-CAMP neutralized P. acnes (1 × 107 CFU) suspended in PBS overnight. As a control, 25 µl of PBS was injected into the right ear of the same mice. The increase in ear thickness was measured using a micro caliper (Mitutoyo, Japan) after the bacterial injection, the increase in ear thickness of P. acnes injected ear was calculated as % of a PBS-injected control. For histological observation, 3 days after injection the ear was excised, cross-sectioned, stained with H&E, and viewed on a Zeiss Axioskop2 plus microscope. To count the bacterial colonization, the bacteria-injected ears were homogenized in 1 ml of sterile PBS for 1 min on a vibrating homogenizer (mini-beadbeater, Biospec Products, Bartlesville, OK) in the presence of 0.5 ml of 2.0 mm zirconia beads (Biospec Products, Bartlesville, OK). The bacterial number in homogenates was quantified by serially diluting the bacteria and plating them on a RCM plate. After centrifugation at 1,3000 × g, macrophage-inflammatory protein-2 (MIP-2) in supernatants was measured by an enzyme-linked immunosorbent assay (ELISA) kit as directed by the manufacturer (BD Biosciences, San Diego, CA).
To investigate whether passive administration of neutralizing antiserum influences the survival of P. acnes at other sites, the left ears of ICR mice were injected intradermally with an amount of 25 µl aliquots of anti-GUS serum or anti-CAMP factor serum neutralized P. acnes (1 × 107 CFU). The same amount of live P. acnes (1 × 107 CFU) alone was injected into the right ears of the same mice. After overnight incubation, the bacteria number was calculated by counting colonies on RCM plates.
3. Results
3.1. P. acnes CAMP factor is a virulence factor
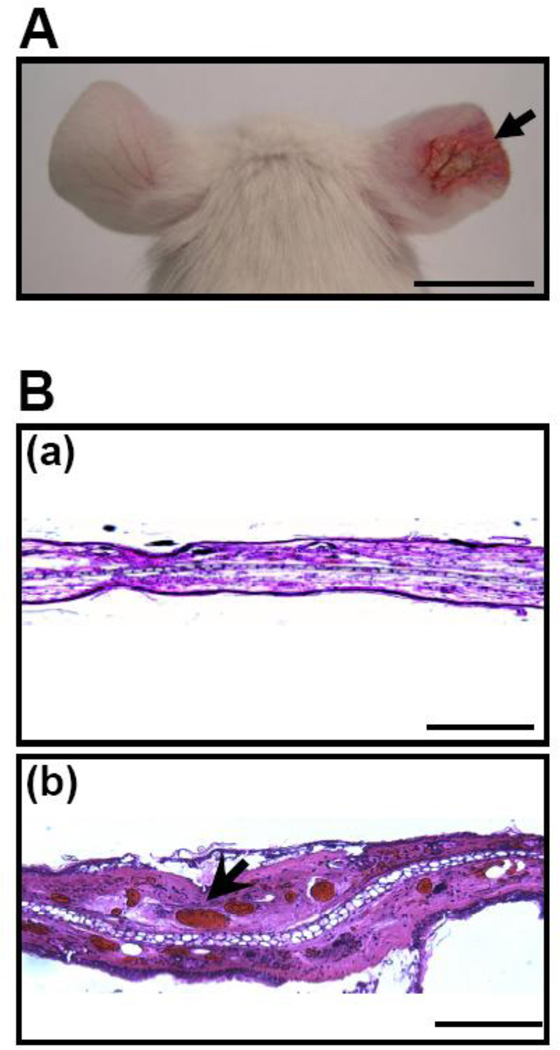
To examine the toxicity of P. acnes CAMP factor, recombinant CAMP factor was (50 µg in 25 µl PBS) intradermally injected into mouse ears of ICR mice. Twenty hours after injection, swelling with severe cutaneous erythema (Fig. 1A, right ear) was observed in ears injected with a CAMP factor, but not in ears injected with a GFP (Fig. 1A, left ear). Injection of P. acnes in mouse ear induced skin inflammation [35], and the secreted CAMP factor was detectable in the lesion (Fig. S1). In addition, we observed that CAMP factor caused erythrocytes hemolysis (Fig. S2), suggesting that P. acnes CAMP factor is a hemolytic factor. Noticeably, in H&E staining histology, injection of CAMP factor caused tremendous red deposits derived from ruptured erythrocytes (Fig. 1B, b) compared to GFP-injected ear skin (Fig. 1B, a). These observations reveal that P. acnes CAMP factor, a reported hemolytic pore-forming toxin [20], is a virulence factor.
Fig. 1.
P. acnes CAMP factor exerted virulence activity. Ears of ICR mice were injected intradermally with recombinant GFP (left ear) and CAMP factors (right ear). (A) Inflammation-induced ear redness (arrow) was visualized 24 hours after injection. (B) Ear swelling was observed in an H&E-stained frozen tissue section of GFP- (a) or CAMP factor- (b) injected ear. The magnification 4× images indicated the dilated veins filled with erythrocytes (arrowheads). Bars (A)= 1 cm. Bars [B(a)]= 2 mm. Bars [B(b)]= 0.5 mm.
3.2. Expression of CAMP factor in plant leaves
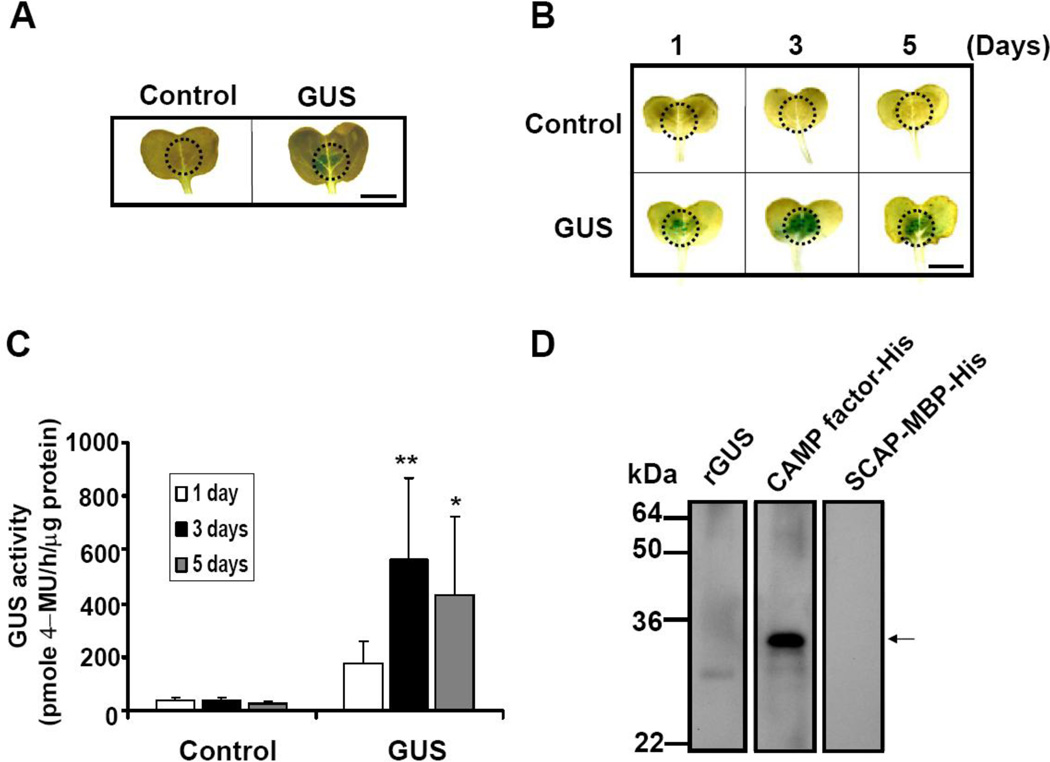
To test if radish is a good platform for protein expression, GUS was transiently expressed in the radish leaves by bombarding an A. tumefaciens harboring a 35S::GUS construct via a pressure infiltration. After five days of post-infiltration, expression of GUS within the leaves was detected by histochemical GUS staining (Fig. 2A). Control infiltrations, in which A. tumefaciens lacking 35S::GUS constructs were used, did not yield detectable GUS expression (Fig. 2A). A time-course study was performed to examine the yields of GUS production as a function of time. Histochemical staining and GUS activity assay illustrated the highest yield of GUS production on day 3 post-infiltration (Fig. 2B and 2C).
Fig. 2.
CAMP factors and GUS transiently expressed in radish leaves. (A) Leaves of radish (Raphanw sativus L.) were infiltrated with A. tumefaciens (LBA4404 strains) transforming a 35S::GUS construct (right). Leaves infiltrated with non-transformed LBA4404 cells (left) served as negative controls. Dotted circles indicate locations of syringe infiltration with A. tumefaciens. Blue stained areas indicate the GUS expression. The dynamic pattern of GUS expression in radish leaves from 1 to 5 days after infiltration was analyzed by (B) histochemical and (C) GUS activity assays. (*p<0.05 and **p<0.005, by Student’s t-test). (D) Detection of CAMP factor expression by Western blot analysis. Ground radish leaves (20 µg) infiltrated with A. tumefaciens carrying a 35S::CAMP factor-His (CAMP factor-His), a 35S::SCAP-MBP-His (SCAP-MBP-His) or 15 µg recombinant GUS (rGUS) were run on a 10% (w/v) SDS-PAGE and blotted onto a nitrocellulose membrane. The membranes were then probed with anti-CAMP factor serum produced by mice immunized with UV-irradiated E. coli, BL21 (DE3) over-expressing CAMP factor. An arrow indicates CAMP factor appearing at a molecular weight of 29 kDa. Bar = 6 mm.
To express the P. acnes CAMP factor in radish leaves, radish leaves were infiltrated with an A. tumefaciens containing a 35S::CAMP factor-His construct to encapsulate CAMP factor within leaves. The expression of CAMP factor was detected by a Western blot analysis. Twenty µg of recombinant GUS, purified CAMP factor with a His tag as well as SCAP, a surface protein of Bacillus anthracis [45, 46, 53], with a His tag and a MBP tag were separated in a 10% SDS-PAGE and immune-reacted with mouse anti-CAMP factor serum produced by mice immunized with UV-irradiated E.coli, BL21 (DE3) [45, 46, 53] over-expressing P. acnes CAMP factor. A band at 29 kDa corresponding to the expression of a CAMP factor-His fusion protein appeared in leave samples that were infiltrated with an A. tumefaciens containing a 35S::CAMP factor-His construct (Fig. 2D), indicating that P. acnes CAMP factor was successfully expressed in radish leaves. The data demonstrate that agroinfiltrated leaves can act as a bioreactor for the production of CAMP factor.
3.3. Immunization of whole leaves expressing CAMP factors
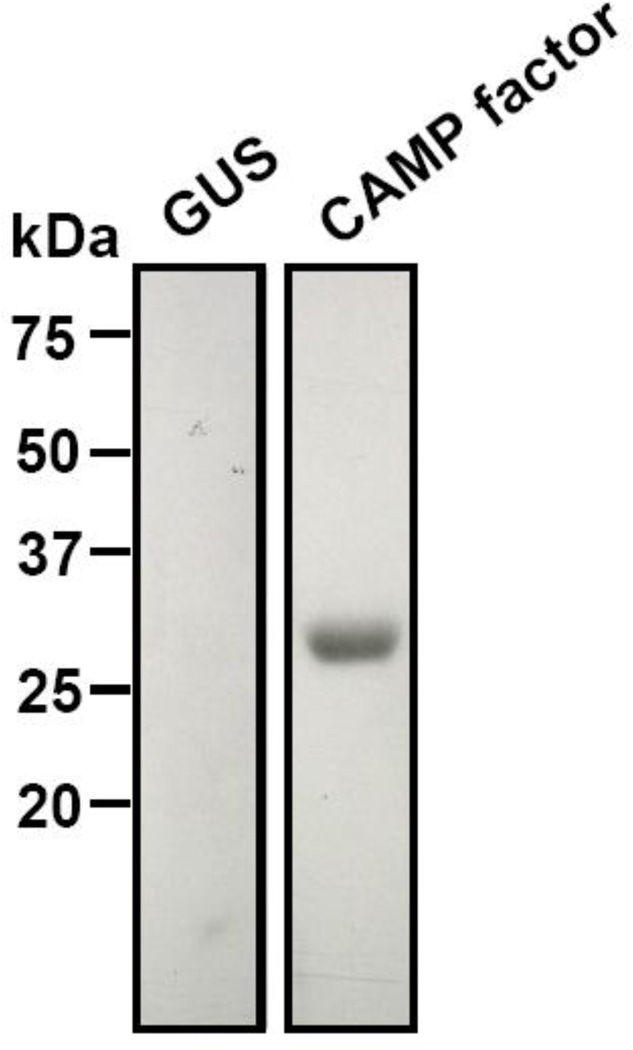
To test if CAMP factor encapsulated radish leaves can function as vaccines, whole leaves expressing P. acnes CAMP factor were ground in sterile ddH2O, UV-inactivated, and then inoculated intranasally into ICR mice (25 µl of whole leaves/mouse) for immunization. No exogenous adjuvants were used for immunization. Via a Western blot assay, the anti-CAMP factor antibody in mouse serum was detectable four weeks after immunization (Fig. 3, CAMP factor). In contrast, no antibodies against CAMP factor were detected in the mice immunized with 25 µl of whole leaves containing GUS (Fig. 3, GUS). This result clearly demonstrates that the mice successfully produce antibodies to P. acnes CAMP factor after immunization with antigen encapsulated radish leaves.
Fig. 3.
Mice Immunized with CAMP factor-encapsulated whole leaves producing CAMP factor specific antibodies. Purified CAMP factor from leaf extracts (65 µg) run on a 10% (w/v) SDS-PAGE was blotted onto a nitrocellulose membrane and immuno-reacted to sera obtained from mice immunized with 25 µl of whole leaves containing GUS (left) or CAMP factors (right). A single band with 29 kDa indicates the purified CAMP factor reactive to serum from CAMP factor-immunized mice, verifying the immunogenicity of CAMP factor.
3.4. Passive neutralization of CAMP factor abrogated P. acnes-induced inflammation and bacteria colonization, without affecting P. acnes commensalisms
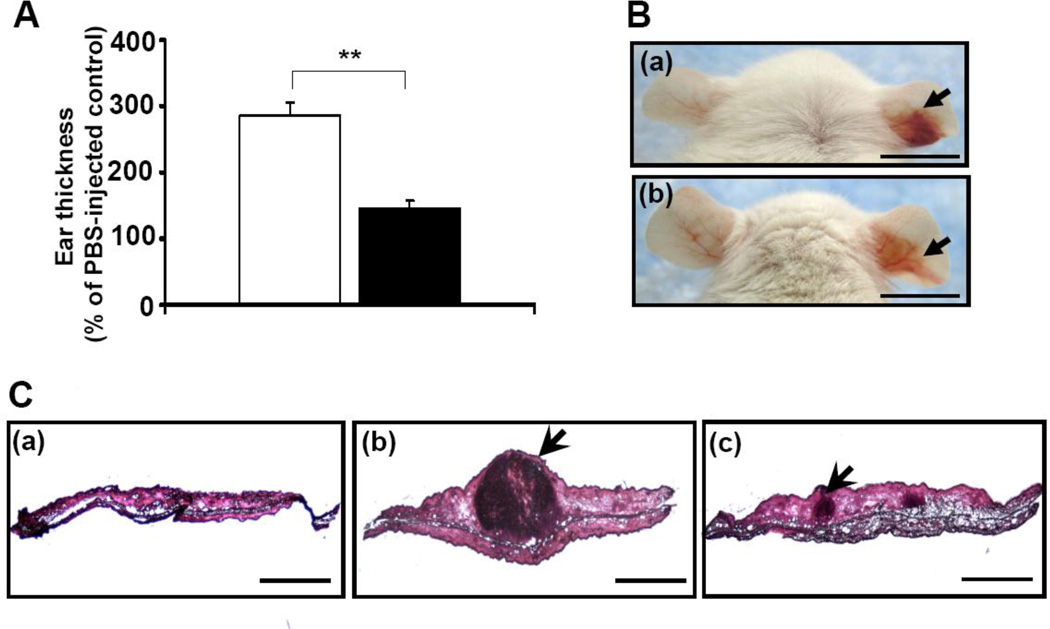
Passive immunization that targets secretory virulence factor is a means that can neutralize bacterial virulence in local lesions without systemically disrupting the bacterial commensalisms. To test whether passive immunization with antibodies to CAMP factors can effectively protect mice from P. acnes infection, P. acnes bacteria were first incubated with 5 % (v/v) serum obtained from mice immunized with 25 µl of CAMP factor-expressed whole leaves. As a control, bacteria were incubated with serum harvested from mice immunized with 25 µl of GUS-expressed whole leaves. Then ears of naïve ICR mice were injected intradermally with serum neutralized P. acnes and bacteria-induced inflammation was measured subsequently. Three days after injection, ear thickness in the mice injected with anti-CAMP factor serum neutralized P. acnes was twofold lower than that in the control mice (Fig. 4A). Furthermore, compared to control mice injected with PBS (Fig. 4C, a) or P. acnes/anti-GUS serum (Fig. 4B, a right ear; Fig. 4C, b), the cutaneous erythema (Fig. 4B b, right ear) and granulomatous responses (Fig. 4C, c) were considerably suppressed when mice were injected with anti-CAMP factor serum neutralized P. acnes.
Fig. 4.
Passive immunization of mice with neutralizing antibody to CAMP factor diminished P. acnes-induced inflammation. (A) 5 % (v/v) anti-GUS (open bar) or anti-CAMP factor (solid bar) serum-treated P. acnes (1 × 107 CFU) was inoculated into the right ears of ICR mice to induce an increase in ear thickness as described in the “Materials and Methods”. As a control, an equal volume of PBS was injected into the left ears of the same mice. Ear thickness was measured with a micro-caliper at the indicated times after bacteria injection. The ear thickness of P. acnes-injected ear was calculated as % of a PBS-injected control. Error bars represented mean ± SE of four mice (**p<0.005, by Student’s t-test). (B) Ear redness (arrows) was visualized 3 days after injection with anti-GUS serum (a) or anti-CAMP factor (b) serum treated-P. acnes (1 × 107 CFU). Bar = 1 cm. (C) Ear inflammation was observed in an H&E-stained frozen tissue section of ear injected with PBS alone (a) or P. acnes treated with anti-GUS (b) or anti-CAMP factor (c) serum. The granulamatous reactions (arrowheads) were visualized under magnification 4× (a, b, c; bars= 2 mm).
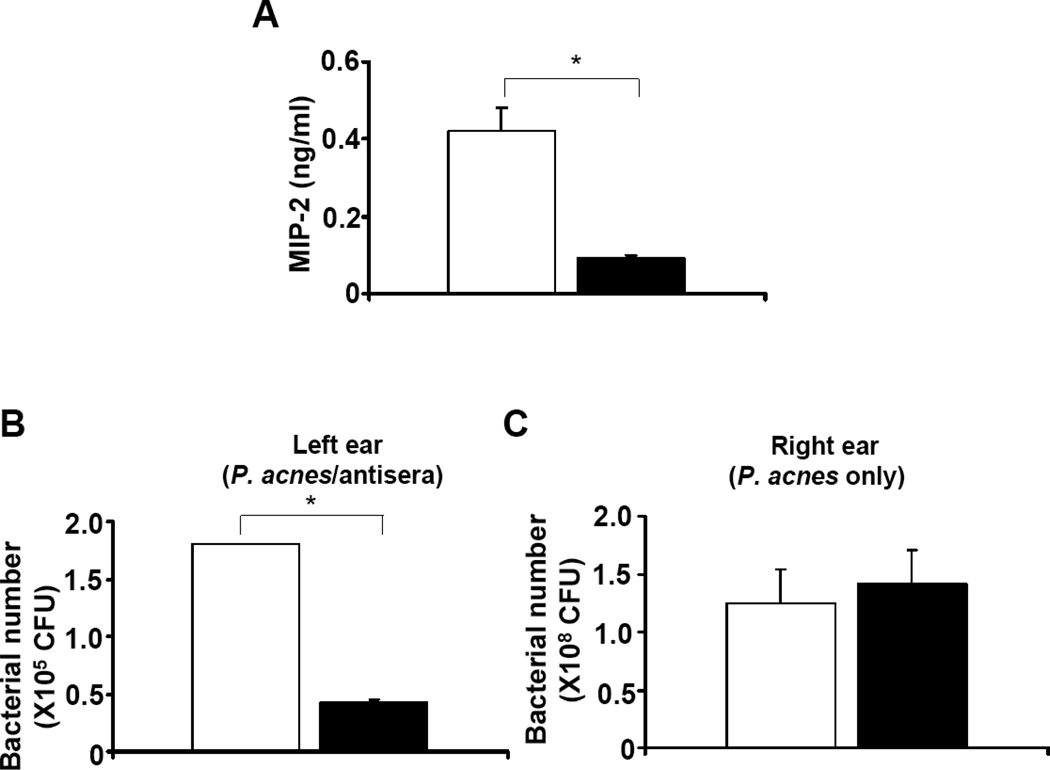
It has been known that P. acnes can induce the production of several pro-inflammatory cytokines, including interleukin (IL)-1β, -8, -12, and tumor necrosis factor-α (TNF-α), via toll-like receptor 2 [54–56]. In addition, a significant increase in the level of the pro-inflammatory cytokine MIP-2, a murine counterpart of IL-8, was detected when P. acnes was administrated into mice [35, 57]. To determine whether passive immunization of CAMP factor can reduce the production of P. acnes-induced pro-inflammatory cytokines, mouse ears were excised and homogenized three days after injection with serum neutralized P. acnes. The level of MIP-2 was measured by an ELISA. MIP-2 production in the ear injected with anti-CAMP factor serum neutralized P. acnes was approximately 80 % (Fig. 5A solid bar; 0.09 ± 0.01 ng/ml) less then that detected in the ear injected with anti-GUS serum treated P. acnes (Fig. 5A open bar; 0.42 ± 0.05 ng/ml). Above results demonstrate that a neutralizing antibody to the CAMP factor was produced in mice immunized with CAMP factor encapsulated whole leaves. In addition, passive administration of neutralizing antibodies to CAMP factors confers protection against P. acnes-induced inflammation.
Fig. 5.
Passive neutralization of P. acnes CAMP factor reduced the production of pro-inflammatory MIP-2 cytokine and bacterial colonization without altering P. acnes survival at other body site. (A) Measurement of pro-inflammatory MIP-2 cytokine was carried out by a sandwich ELISA using a Quantikine M mouse MIP-2 set. Compared to the neutralization with anti-GUS serum (open bar), passive neutralization with anti-CAMP factor serum (solid bar) markedly suppressed the P. acnes-induced increase in MIP-2. (B) The left ears of mice were injected with P. acnes (1 × 107 CFU) in the presence of anti-GUS serum (open bar) or anti-CAMP factor serum (solid bar). (C) The right ears of both mice from (B) were injected with live P. acnes (1 × 107 CFU) alone. Bacterial colonization (CFU) was quantified in agar plates as described in “Materials and Methods. Error bars represent mean ± SE of four mice (*p<0.05, by Student’s t-test).
To explore if passive neutralization of CAMP factor can enhance the clearance of “disarmed” P. acnes by immune cells at an infected site, the left ears of mice injected with anti-CAMP factor or anti-GUS serum treated P. acnes were excised and homogenized. As shown in Fig. 5B, the number of P. acnes recovered from mouse ears administered passively with anti-CAMP factor serum (Fig. 5B solid bar; 0.42 ± 0.02×105 CFU) was much lower than that recovered from ears administered with anti-GUS serum (Fig. 5B open bar; 1.80 ± 0.01 × 105 CFU), suggesting that inactivation of P. acnes virulence by neutralizing CAMP factor enhanced the capability of immune systems in eradicating infected bacteria.
To prove the passive neutralization of CAMP factor carries no risk of destroying the commensal P. acnes, live P. acnes was injected (without serum treatment) into the right ear of a mouse that was injected with serum neutralized P. acnes on the left ear. Bacterial colonization in right ears was enumerated overnight after injection (Fig. 5C). The numbers of P. acnes in that right ears of mice injected with anti-GUS serum- (Fig. 5C open bar; 1.25 ± 0.28 × 108 CFU) or anti-CAMP factor serum- (Fig. 5C solid bar; 1.41 ± 0.28 × 108 CFU) treated P. acnes on their left ears are not significant different. Furthermore, injection of right ears with live P. acnes alone resulted in a similar inflammatory response to that of left ears with live P. acnes plus anti-GUS serum (Fig. S3). These results indicate that passive neutralization of CAMP factor can naturally eliminate P. acnes in the infected lesions without influencing the colonization of P. acnes at other sites.
4. Discussion
Analysis of the genome of P. acnes reveals that CAMP factor is a potential virulence factor [18] that is cytotoxic, which leads to inflammation and tissue injury [13, 14]. It has been reported that P. acnes encodes five different CAMP factor (CAMP factors 1 to 5) genes [58]. The CAMP factor (accession number: gi/50842175) we have cloned in this study is the CAMP factor 2. Recently, it has been demonstrated that only CAMP factor 2 and 4 are detectable in the secretion of P. acnes (KPA171202) [44]. In addition, CAMP factor 2, but not CAMP factor 4, is a major active co-hemolytic factor of P. acnes [44]. Our data (Fig. 4 and 5A) shows that neutralization of P. acnes CAMP factor using anti-serum competently attenuates P. acnes-induced inflammation in vivo, suggesting that CAMP factor 2 significantly contributes to the virulence of P. acnes. On the other hand, previous data indicated that recombinant CAMP factor of S. agalactiae was toxic when administered to mice [20]. Moreover, differential production of CAMP factors was found in various P. acnes isolates [59]. Our recent data demonstrated that CAMP factor 2 was secreted into a granulomatous inflammatory microenvironment when P. acnes was injected intradermally into mouse ears (unpublished data). Furthermore, results of proteomics analysis showed that CAMP factor 2 was significantly up-regulated under anaerobic conditions (unpublished data), suggesting that P. acnes may become pathogenic and produce CAMP factor 2 in an anaerobic microenvironment of acne lesions although it is unclear whether P. acnes, under commensal conditions, constantly secretes CAMP factors or not. Thus, it is worth investigating whether the secreted CAMP factors are detectable in acne lesions and healthy skins in humans.
The lack of an excellent acne animal model has been hindering the development of vaccines and drugs targeting P. acnes infection [60], because most animals (rabbits, mice, and hamsters) never have acne lesions due to insufficient triglycerides to harbor P. acnes. Rhino mice, a genetic mutant mouse with utricles that create larger follicles, have been utilized to determine compound comedogenicity [61]. However, Rhino mice cannot produce antibodies against thymus-dependent antigens [62], and thus may not be an appropriate model for vaccination. Remarkably, there are no animal models for investigating the severe acne lesion, occurring when P. acnes enters dermis and interacts with phagocytes to cause tissue injury. An infectious granulomatous reaction is a form of cytolysis/cell death at the centre of a granuloma. Our results indicate that intradermal injection of P. acnes into mouse ears induced a remarkable granulomatous response (Fig. 4C), which is characterized as a lesion of epithelioid macrophages and often surrounded by a lymphocyte cuff [63]. In the case of acne vulgaris, P. acnes could enter the dermis once the follicular wall was ruptured [64]. Injection of P. acnes into mouse ears may represent an animal model for the granulomatous type of acne inflammation that follows follicular rupture. The granuloma creates a microenvironment where P. acnes and immune cells counteract each other to gain maximum survivals. It has been reported that P. acnes can induce tumor necrosis factor (TNF)-α mediated cell death, which subsequently progress to a T-cell-mediated granulomatosis [65].
Through advances in molecular and genetic techniques, protein expression in plants has been optimized for high-level production [66]. Recently, synthesis of codon-optimized bacteria gene in plants is powerful and common [67]. It is conceivable that P. acnes and radish sprouts have very different tRNA pools. Thus, synthesis of a codon-optimized CAMP factor gene ought to enhance the production of CAMP factor in plant cells [68]. On the other hand, intranasal immunization of mice with ground leaves expressing CAMP factor elicits detectable antibodies to P. acnes CAMP factor, indicating that intranasal administration of whole plant leaves may be a new regimen for vaccination. However, it had been reported that vaccination via an intranasal route can cause facial nerve paralysis [69]. Therefore, the safety of intranasal administration is worthy to be investigated since the human respiratory tract is not exposed to plant leaves on a routine basis.
Passive immunization is the induction of immunity acquired by the transfer of antibodies from another individual [70]. There are many advantages of passive immunization. (a) High specific activity [71] (b) Unlike active immunization (vaccines), biological effects of passive immunization are immediate and can be of value where symptoms have already occurred. Thus, the modality using passive neutralization of P. acnes CAMP factor may benefit patients who have already developed acne. (c) The lack of cell-mediated immunity and direct bactericidal effect will have low impact on microbe commensalisms. (d) No adjuvant-derived side effects are induced. (e) The administered dose can be adjusted based on the severity of disease. (f) It can be easily combined with other therapies. Additionally, unlike active immunization, which requires time to induce protective immunity and depends on the host’s ability to mount an immune response, passive antibody can theoretically confer protection regardless of the immune status of the host [72]. Moreover, P. acnes is a major inhabitant of adult human skin, where it resides within sebaceous follicles, usually as a harmless commensal [6]. In this study, we emphasize that passive immunization that targets secretory CAMP factors instead of bacterial surface proteins can neutralize the P. acnes virulence without directly killing bacteria, lowering the risk of creating drug-resistant P. acnes and altering the commensalisms of P. acnes. Compared to active immunization of a CAMP factor-targeted vaccine (Fig. S4), passive neutralization of CAMP factor (Fig. 4A) displays roughly equal potency with respect to the suppression of P. acnes-induced ear inflammation. The therapeutic antibodies to CAMP factors developed in this study can be extended for the treatment of various P. acnes-associated human diseases including implant infections, pulmonary sarcoidosis, osteomyelitis, and endocarditis [9, 16, 17]. Our future studies will include generating the therapeutic monoclonal antibodies to P. acnes CAMP factor. A variety of techniques such as microneedles for transdermal delivery of therapeutic antibodies have been developed [73]. Epicutaneous application of a human monoclonal antibody to CAMP factor onto the skins of patients with severe acne with the help of microneedles may locally eradicate P. acnes without interrupting the residence of P. acnes and other commensals in other locations of our body. Future studies will also include establishing a CAMP factor-specific pro-inflammatory profile and comparing the efficacy and safety of CAMP factor-targeted vaccines with surface sialidase- [35] and killed P. acnes-based vaccines [12]. Moreover, further clinical observations for the use of passive immunization to P. acnes as a therapeutic treatment reducing virulence without disturbing the commensal relationship of the host with the organism will be required.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants (R01-AI067395-01, R21-R022754-01, R21-I58002-01 and 1R41AR056169). We thank for Drs. Y.T. Liu and K Yamasaki for their valuable comments. We appreciate Dr. Christina Niemeyer and Dan MacLeod for their critical reviews. Also, we are grateful to C.W. Lo for his technique assistance.
Abbreviations
- ATCC
American Type Culture Collection
- CAMP factor
Christie, Atkins, Munch-Peterson factor
- CFU
Colony forming unit
- ddH2O
Didistilled water
- E. coli
Escherichia coli
- ELISA
Enzyme-linked immunosorbent assay
- GFP
Green fluorescence protein
- GUS
β-Glucuronidase
- His
Histidine
- H&E
Hematoxylin and eosin
- ICR
Institute of Cancer Research
- IgG
Immunoglobulin G
- IgM
Immunoglobulin M
- IL
Interleukin
- IPTG
Isopropyl-β-D-thiogalactoside
- LB
Luria-Bertani
- MBP
Maltose binding protein
- MIP-2
Macrophage-inflammatory protein-2
- OD
Optical density
- PBS
Phosphate-buffered saline
- P. acnes
Propionibacterium acnes
- PCR
Polymerase chain reaction
- PTMs
posttranslational modifications
- RCM
Reinforced clostridium medium
- SCAP
Spore coat-associated protein
- SDS-PAGE
Sodium dodecyl sulfate-polyacrylamide gel
- S. agalactiae
Streptococcus agalactiae
- S. aureus
Staphylococcus aureus
- TNF-α
tumor necrosis factor-α
- UV
Ultraviolet
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gao Z, Tseng CH, Pei Z, Blaser MJ. Molecular analysis of human forearm superficial skin bacterial biota. Proc Natl Acad Sci U S A. 2007;104(8):2927–2932. doi: 10.1073/pnas.0607077104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dekio I, Sakamoto M, Hayashi H, Amagai M, Suematsu M, Benno Y. Characterization of skin microbiota in patients with atopic dermatitis and in normal subjects using 16S rRNA gene-based comprehensive analysis. J Med Microbiol. 2007;56(Pt 12):1675–1683. doi: 10.1099/jmm.0.47268-0. [DOI] [PubMed] [Google Scholar]
- 3.Brook I, Frazier EH. Infections caused by Propionibacterium species. Rev Infect Dis. 1991;13(5):819–822. doi: 10.1093/clinids/13.5.819. [DOI] [PubMed] [Google Scholar]
- 4.Ahn CY, Ko CY, Wagar EA, Wong RS, Shaw WW. Microbial evaluation: 139 implants removed from symptomatic patients. Plast Reconstr Surg. 1996;98(7):1225–1229. doi: 10.1097/00006534-199612000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Tancrede C. Role of human microflora in health and disease. Eur J Clin Microbiol Infect Dis. 1992;11(11):1012–1015. doi: 10.1007/BF01967791. [DOI] [PubMed] [Google Scholar]
- 6.Leyden JJ, McGinley KJ, Vowels B. Propionibacterium acnes colonization in acne and nonacne. Dermatology. 1998;196(1):55–58. doi: 10.1159/000017868. [DOI] [PubMed] [Google Scholar]
- 7.McGinley KJ, Webster GF, Leyden JJ. Regional variations of cutaneous propionibacteria. Appl Environ Microbiol. 1978;35(1):62–66. doi: 10.1128/aem.35.1.62-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, et al. Topographical and temporal diversity of the human skin microbiome. Science. 2009;324(5931):1190–1192. doi: 10.1126/science.1171700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishiwaki T, Yoneyama H, Eishi Y, Matsuo N, Tatsumi K, Kimura H, et al. Indigenous pulmonary Propionibacterium acnesprimes the host in the development of sarcoid-like pulmonary granulomatosis in mice. Am J Pathol. 2004;165(2):631–639. doi: 10.1016/S0002-9440(10)63327-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamada T, Eishi Y, Ikeda S, Ishige I, Suzuki T, Takemura T, et al. In situ localization of Propionibacterium acnesDNA in lymph nodes from sarcoidosis patients by signal amplification with catalysed reporter deposition. J Pathol. 2002;198(4):541–547. doi: 10.1002/path.1243. [DOI] [PubMed] [Google Scholar]
- 11.Williams RE, Doherty VR, Perkins W, Aitchison TC, Mackie RM. Staphylococcus aureusand intra-nasal mupirocin in patients receiving isotretinoin for acne. Br J Dermatol. 1992;126(4):362–366. doi: 10.1111/j.1365-2133.1992.tb00679.x. [DOI] [PubMed] [Google Scholar]
- 12.Nakatsuji T, Liu YT, Huang CP, Zoubouis CC, Gallo RL, Huang CM. Antibodies elicited by inactivated propionibacterium acnes-based vaccines exert protective immunity and attenuate the IL-8 production in human sebocytes: relevance to therapy for acne vulgaris. J Invest Dermatol. 2008;128(10):2451–2457. doi: 10.1038/jid.2008.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruggemann H, Henne A, Hoster F, Liesegang H, Wiezer A, Strittmatter A, et al. The complete genome sequence of Propionibacterium acnes, a commensal of human skin. Science. 2004;305(5684):671–673. doi: 10.1126/science.1100330. [DOI] [PubMed] [Google Scholar]
- 14.Kim J. Acne vaccines: therapeutic option for the treatment of acne vulgaris? J Invest Dermatol. 2008;128(10):2353–2354. doi: 10.1038/jid.2008.221. [DOI] [PubMed] [Google Scholar]
- 15.Toyoda M, Morohashi M. Pathogenesis of acne. Med Electron Microsc. 2001;34(1):29–40. doi: 10.1007/s007950100002. [DOI] [PubMed] [Google Scholar]
- 16.Nakatsuji T, Rasochova L, Huang CM. Vaccine therapy for P. acnes-associated diseases. Infect Disord Drug Targets. 2008;8(3):160–165. doi: 10.2174/1871526510808030160. [DOI] [PubMed] [Google Scholar]
- 17.Zouboulis CC. Acne and sebaceous gland function. Clin Dermatol. 2004;22(5):360–366. doi: 10.1016/j.clindermatol.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Bruggemann H. Insights in the pathogenic potential of Propionibacterium acnes from its complete genome. Semin Cutan Med Surg. 2005;24(2):67–72. doi: 10.1016/j.sder.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Jurgens D, Sterzik B, Fehrenbach FJ. Unspecific binding of group B streptococcal cocytolysin (CAMP factor) to immunoglobulins and its possible role in pathogenicity. J Exp Med. 1987;165(3):720–732. doi: 10.1084/jem.165.3.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lang S, Palmer M. Characterization of Streptococcus agalactiae CAMP factor as a pore-forming toxin. J Biol Chem. 2003;278(40):38167–38173. doi: 10.1074/jbc.M303544200. [DOI] [PubMed] [Google Scholar]
- 21.Choudhury TK. Synergistic lysis of erythrocytes by Propionibacterium acnes. J Clin Microbiol. 1978;8(2):238–241. doi: 10.1128/jcm.8.2.238-241.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christie R, Atkins NE, Munch-Petersen E. A note on a lytic phenomenon shown by group B streptococci. Aust J Exp Biol Med Sci. 1944;22:197–200. doi: 10.1038/icb.1945.30. [DOI] [PubMed] [Google Scholar]
- 23.Lo CW, Lai YK, Liu YT, Gallo RL, Huang CM. Staphylococcus aureus hijacks a skin commensal to intensify its virulence: immunization targeting beta-hemolysin and CAMP factor. J Invest Dermatol. 2011;131(2):401–409. doi: 10.1038/jid.2010.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ochsendorf F. Systemic antibiotic therapy of acne vulgaris. J Dtsch Dermatol Ges. 2006;4(10):828–841. doi: 10.1111/j.1610-0387.2006.06053.x. [DOI] [PubMed] [Google Scholar]
- 25.Slaga TJ, Klein-Szanto AJ, Triplett LL, Yotti LP, Trosko KE. Skin tumor-promoting activity of benzoyl peroxide, a widely used free radical-generating compound. Science. 1981;213(4511):1023–1025. doi: 10.1126/science.6791284. [DOI] [PubMed] [Google Scholar]
- 26.Weinberg JM. The utility of benzoyl peroxide in hydrophase base (Brevoxyl) in the treatment of acne vulgaris. J Drugs Dermatol. 2006;5(4):344–349. [PubMed] [Google Scholar]
- 27.Tanghetti EA, Popp KF. A current review of topical benzoyl peroxide: new perspectives on formulation and utilization. Dermatol Clin. 2009;27(1):17–24. doi: 10.1016/j.det.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Zhao J, Lahiri-Chatterjee M, Sharma Y, Agarwal R. Inhibitory effect of a flavonoid antioxidant silymarin on benzoyl peroxide-induced tumor promotion, oxidative stress and inflammatory responses in SENCAR mouse skin. Carcinogenesis. 2000;21(4):811–816. doi: 10.1093/carcin/21.4.811. [DOI] [PubMed] [Google Scholar]
- 29.Simpson LS, Burdine L, Dutta AK, Feranchak AP, Kodadek T. Selective toxin sequestrants for the treatment of bacterial infections. J Am Chem Soc. 2009;131(16):5760–5762. doi: 10.1021/ja900852k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Layton AM, Dreno B, Gollnick HP, Zouboulis CC. A review of the european directive for prescribing systemic isotretinoin for acne vulgaris. J Eur Acad Dermatol Venereol. 2006;20(7):773–776. doi: 10.1111/j.1468-3083.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- 31.Haider A, Shaw JC. Treatment of acne vulgaris. Jama. 2004;292(6):726–735. doi: 10.1001/jama.292.6.726. [DOI] [PubMed] [Google Scholar]
- 32.McGinley KJ, Webster GF, Leyden JJ. Facial follicular porphyrin fluorescence: correlation with age and density of Propionibacterium acnes. Br J Dermatol. 1980;102(4):437–441. doi: 10.1111/j.1365-2133.1980.tb06557.x. [DOI] [PubMed] [Google Scholar]
- 33.Mourelatos K, Eady EA, Cunliffe WJ, Clark SM, Cove JH. Temporal changes in sebum excretion and propionibacterial colonization in preadolescent children with and without acne. Br J Dermatol. 2007;156(1):22–31. doi: 10.1111/j.1365-2133.2006.07517.x. [DOI] [PubMed] [Google Scholar]
- 34.Perry AL, Lambert PA. Propionibacterium acnes. Lett Appl Microbiol. 2006;42(3):185–188. doi: 10.1111/j.1472-765X.2006.01866.x. [DOI] [PubMed] [Google Scholar]
- 35.Nakatsuji T, Liu YT, Huang CP, Zouboulis CC, Gallo RL, Huang CM. Vaccination targeting a surface sialidase of P. acnes: implication for new treatment of acne vulgaris. PLoS One. 2008;3(2) doi: 10.1371/journal.pone.0001551. e1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rasko DA, Moreira CG, Li de R, Reading NC, Ritchie JM, Waldor MK, et al. Targeting QseC signaling and virulence for antibiotic development. Science. 2008;321(5892):1078–1080. doi: 10.1126/science.1160354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cogen AL, Nizet V, Gallo RL. Skin microbiota: a source of disease or defence? Br J Dermatol. 2008;158(3):442–455. doi: 10.1111/j.1365-2133.2008.08437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma JK. Genes, greens, and vaccines. Nat Biotechnol. 2000;18(11):1141–1142. doi: 10.1038/81113. [DOI] [PubMed] [Google Scholar]
- 39.Tregoning J, Maliga P, Dougan G, Nixon PJ. New advances in the production of edible plant vaccines: chloroplast expression of a tetanus vaccine antigen, TetC. Phytochemistry. 2004;65(8):989–994. doi: 10.1016/j.phytochem.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Chargelegue D, Obregon P, Drake PM. Transgenic plants for vaccine production: expectations and limitations. Trends Plant Sci. 2001;6(11):495–496. doi: 10.1016/s1360-1385(01)02123-9. [DOI] [PubMed] [Google Scholar]
- 41.Streatfield SJ, Jilka JM, Hood EE, Turner DD, Bailey MR, Mayor JM, et al. Plant-based vaccines: unique advantages. Vaccine. 2001;19(17–19):2742–2748. doi: 10.1016/S0264-410X(00)00512-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shih SM, Doran PM. Foreign protein production using plant cell and organ cultures: Advantages and limitations. Biotechnol Adv. 2009;27(6):1036–1042. doi: 10.1016/j.biotechadv.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 43.Soderquist B, Holmberg A, Unemo M. Propionibacterium acnes as an etiological agent of arthroplastic and osteosynthetic infections--two cases with specific clinical presentation including formation of draining fistulae. Anaerobe. 2010;16(3):304–306. doi: 10.1016/j.anaerobe.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 44.Jefferson RA. Assaying chimeric genes in plants: the GUS gene fusion. Plant Mol Biol Rep. 1987;5(4):387–405. [Google Scholar]
- 45.Liu YT, Lin SB, Huang CP, Huang CM. A novel immunogenic spore coat-associated protein in Bacillus anthracis: characterization via proteomics approaches and a vector-based vaccine system. Protein Expr Purif. 2008;57(1):72–80. doi: 10.1016/j.pep.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu PF, Shi W, Zhu W, Smith JW, Hsieh SL, Gallo RL, et al. Vaccination targeting surface FomA of Fusobacterium nucleatum against bacterial co-aggregation: Implication for treatment of periodontal infection and halitosis. Vaccine. 2010;28(19):3496–3505. doi: 10.1016/j.vaccine.2010.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.An GPR, Ebert A, Mitra SBH. Plant molecular biology manual. 1988 [Google Scholar]
- 48.Schob H, Kunz C, Meins F., Jr Silencing of transgenes introduced into leaves by agroinfiltration: a simple, rapid method for investigating sequence requirements for gene silencing. Mol Gen Genet. 1997;256(5):581–585. doi: 10.1007/s004380050604. [DOI] [PubMed] [Google Scholar]
- 49.Mantis NJ. Vaccines against the category B toxins: Staphylococcal enterotoxin B, epsilon toxin and ricin. Adv Drug Deliv Rev. 2005;57(9):1424–1439. doi: 10.1016/j.addr.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 50.Medina-Bolivar F, Wright R, Funk V, Sentz D, Barroso L, Wilkins TD, et al. A non-toxic lectin for antigen delivery of plant-based mucosal vaccines. Vaccine. 2003;21(9–10):997–1005. doi: 10.1016/s0264-410x(02)00551-0. [DOI] [PubMed] [Google Scholar]
- 51.Davis SS. Nasal vaccines. Adv Drug Deliv Rev. 2001;51(1–3):21–42. doi: 10.1016/s0169-409x(01)00162-4. [DOI] [PubMed] [Google Scholar]
- 52.Gil F, Titarenko E, Terrada E, Arcalis E, Escribano JM. Successful oral prime-immunization with VP60 from rabbit haemorrhagic disease virus produced in transgenic plants using different fusion strategies. Plant Biotechnol J. 2006;4(1):135–143. doi: 10.1111/j.1467-7652.2005.00172.x. [DOI] [PubMed] [Google Scholar]
- 53.Liu PF, Haake SK, Gallo RL, Huang CM. A novel vaccine targeting Fusobacterium nucleatum against abscesses and halitosis. Vaccine. 2009;27(10):1589–1595. doi: 10.1016/j.vaccine.2008.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim J. Review of the innate immune response in acne vulgaris: activation of Toll-like receptor 2 in acne triggers inflammatory cytokine responses. Dermatology. 2005;211(3):193–198. doi: 10.1159/000087011. [DOI] [PubMed] [Google Scholar]
- 55.Kim J, Ochoa MT, Krutzik SR, Takeuchi O, Uematsu S, Legaspi AJ, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169(3):1535–1541. doi: 10.4049/jimmunol.169.3.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nagy I, Pivarcsi A, Kis K, Koreck A, Bodai L, McDowell A, et al. Propionibacterium acnes and lipopolysaccharide induce the expression of antimicrobial peptides and proinflammatory cytokines/chemokines in human sebocytes. Microbes Infect. 2006;8(8):2195–2205. doi: 10.1016/j.micinf.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 57.Itakura M, Tokuda A, Kimura H, Nagai S, Yoneyama H, Onai N, et al. Blockade of secondary lymphoid tissue chemokine exacerbates Propionibacterium acnes-induced acute lung inflammation. J Immunol. 2001;166(3):2071–2079. doi: 10.4049/jimmunol.166.3.2071. [DOI] [PubMed] [Google Scholar]
- 58.Valanne S, McDowell A, Ramage G, Tunney MM, Einarsson GG, O'Hagan S, et al. CAMP factor homologues in Propionibacterium acnes: a new protein family differentially expressed by types I and II. Microbiology. 2005;151(Pt 5):1369–1379. doi: 10.1099/mic.0.27788-0. [DOI] [PubMed] [Google Scholar]
- 59.Holland C, Mak TN, Zimny-Arndt U, Schmid M, Meyer TF, Jungblut PR, et al. Proteomic identification of secreted proteins of Propionibacterium acnes. BMC Microbiol. 2010;10:230. doi: 10.1186/1471-2180-10-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Webster GF, Ruggieri MR, McGinley KJ. Correlation of Propionibacterium acnes populations with the presence of triglycerides on nonhuman skin. Appl Environ Microbiol. 1981;41(5):1269–1270. doi: 10.1128/aem.41.5.1269-1270.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nakano K, Kiyokane K, Benvenuto-Andrade C, Gonzalez S. Real-timereflectance confocal microscopy, a noninvasive tool for in vivo quantitative evaluation of comedolysis in the rhino mouse model. Skin Pharmacol Physiol. 2007;20(1):29–36. doi: 10.1159/000096169. [DOI] [PubMed] [Google Scholar]
- 62.Takaoki M, Kawaji H. Impaired antibody response against T-dependent antigens in rhino mice. Immunology. 1980;40(1):27–32. [PMC free article] [PubMed] [Google Scholar]
- 63.Semel J, Bowe B, Guo A, Lee L, Rife L, Polikoff D, et al. Propionibacterium acnes-enhanced lens-induced granulomatous uveitis in the rat. Invest Ophthalmol Vis Sci. 1992;33(5):1766–1770. [PubMed] [Google Scholar]
- 64.Kligman AM. An overview of acne. J Invest Dermatol. 1974;62(3):268–287. doi: 10.1111/1523-1747.ep12676801. [DOI] [PubMed] [Google Scholar]
- 65.Chen YL, Yu CK, Lei HY. Propionibacterium acnes induces acute TNFalpha-mediated apoptosis of hepatocytes followed by inflammatory T-cell-mediated granulomatous hepatitis in mice. J Biomed Sci. 1999;6(5):349–356. doi: 10.1007/BF02253524. [DOI] [PubMed] [Google Scholar]
- 66.Fletcher SP, Muto M, Mayfield SP. Optimization of recombinant protein expression in the chloroplasts of green algae. Adv Exp Med Biol. 2007;616:90–98. doi: 10.1007/978-0-387-75532-8_8. [DOI] [PubMed] [Google Scholar]
- 67.Jabeen R, Khan MS, Zafar Y, Anjum T. Codon optimization of cry1Ab gene for hyper expression in plant organelles. Mol Biol Rep. 2010;37(2):1011–1017. doi: 10.1007/s11033-009-9802-1. [DOI] [PubMed] [Google Scholar]
- 68.Zhang J, Shi Z, Kong FK, Jex E, Huang Z, Watt JM, et al. Topical application of Escherichia coli-vectored vaccine as a simple method for eliciting protective immunity. Infect Immun. 2006;74(6):3607–3617. doi: 10.1128/IAI.01836-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sendi P, Locher R, Bucheli B, Battegay M. Intranasal influenza vaccine in a working population. Clin Infect Dis. 2004;38(7):974–980. doi: 10.1086/386330. [DOI] [PubMed] [Google Scholar]
- 70.A Keller M, Stiehm ER. Passive immunity in prevention and treatment of infectious diseases. Clinical Microbiology Reviews. 2000;13(4):602–614. doi: 10.1128/cmr.13.4.602-614.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hu WG, Nagata LP. Antibody gene-based prophylaxis and therapy for biodefence. Hum Vaccin. 2008;4(1):74–78. doi: 10.4161/hv.4.1.4778. [DOI] [PubMed] [Google Scholar]
- 72.Casadevall A. Passive antibody administration (immediate immunity) as a specific defense against biological weapons. Emerg Infect Dis. 2002;8(8):833–841. doi: 10.3201/eid0808.010516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li G, Badkar A, Nema S, Kolli CS, Banga AK. In vitro transdermal delivery of therapeutic antibodies using maltose microneedles. Int J Pharm. 2009;368(1–2):109–115. doi: 10.1016/j.ijpharm.2008.10.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.