Abstract
Heterodimeric transcription factor hypoxia inducible factor-1 (HIF-1) functions as a master regulator of oxygen homeostasis in almost all nucleated mammalian cells. The fundamental process adapted to cellular oxygen alteration largely depends on the refined regulation on its alpha subunit, HIF-1α. Recent studies have unraveled expanding and critical roles of HIF-1α, involving in a multitude of developmental, physiological, and pathophysiological processes. This review will focus on the current knowledge of HIF-1α-targeting genes and its interacting proteins, as well as the concomitant functional relationships between them.
Keywords: Hypoxia inducible factor-1alpha, targeting gene, interacting protein
Introduction
Cellular and systemic oxygen homeostasis is a finely regulated process which is essential for energy metabolism as well as the survival of mammalian cells. It has well established that hypoxia inducible factor-1 (HIF-1), a heterodimeric transcriptional factor composed of the constitutively expressed HIF-1β (also named aryl-hydrocarbon receptor nuclear transporter, ARNT) subunit and the highly regulated HIF-1α subunit, plays a critical role in cellular adaptation to changes in oxygen availability [1,2]. Both HIF-1α and HIF-1β subunits are members of a subfamily of basic helix-loop-helix (bHLH) transcription factors containing periodic-aryl hydrocarbon receptor nuclear translocator-single-minded (Per-Arnt-Sim, PAS) domain, which required for heterodimerization and the binding of the subunits to DNA. The HIF-1α subunit also contains an oxygen-dependent degradation (ODD) domain and two transactivation domains (TAD), rendering it to proteasomal degradation and target gene regulation respectively.
Since HIF-1 was first identified as a key regulator required for hypoxia-induced transcription of the human erythropoietin (EPO) gene [3], the transcription factor has been attracting tremendous interests owing to its rapidly expanding and critical role involving in a multitude of developmental, physiological, and pathophysiological processes [4]. This review attempts to outline the current knowledge of HIF-1α-targeting genes and its interacting proteins, the functional relationships between them will also be discussed.
The target genes of HIF-1α
It has been unraveled that, HIF-1α is hydroxylated by oxygen-activated prolyl hydroxylases (PHDs) and rapidly degraded via ubiquitin-proteasome pathway under normoxic conditions. But under hypoxic or hypoxic-mimic conditions, PHDs activity is inhibited and HIF-1α accumulates, translocates to nucleus, and heterodimerize with HIF-1β [5-8]. As a transcriptional factor, the heterodimer HIF-1 recognizes and binds to the consensus sequence 5’-(A/G) CGTG-3’ named hypoxia-responsive elements (HREs) to activate the transcriptional activity of target genes [9].
For the purpose of understanding the regulatory network of HIF-1, a range of methods and techniques, such as luciferase reporter assay, electrophoretic mobility shift assay (EMSA) and chromatin immunoprecitation (ChIP), have been widely used to identify the downstream-target genes of HIF-1. Recently, Benita et al. [10] reported a new strategy to find HIF-1-target genes based on integrative genomic approach and computational strategies. Using this strategy and microarray analysis, they recovered 41% of the previously confirmed HIF-1-target genes and successfully identified ANKRD37 as a novel target gene of HIF-1α. Meanwhile, Barahona et al. described a powerful computational strategy based on the combination of phylogenetic footprinting and transcription profiling meta-analysis for the identification of HIF-target genes, which was validated by ChIP assay and disclosed several novel HIF targets, including RE1-silencing transcription factor co-repressor (RCOR2) [11]. Besides genome-wide analyses, proteomics strategies have also been applied to identify the direct targets of HIF-1. Our group performed two-dimensional electrophoresis (2DE) analysis to compare the protein expression profiles within hypoxia-treated human acute promonocytic leukemic U937 cells and untreated U937 cells. The differential expression proteins were identified by mass spectrometry, and among them, several potential target genes of hypoxia/HIF-1, such as N-myc downstream regulated gene 1 (NDRG1), were identified [12]. Moreover, by using differential gel electrophoresis (DIGE) based proteomics analysis, we further identified three novel direct targets of HIF-1α, which were confirmed by luciferase and ChIP assay [13]. To date, more than 100 direct target genes of HIF-1 have been uncovered, which have been shown to be functionally involved in tumor metastasis, angiogenesis, energy metabolism, cell differentiation and apoptosis [13,14]
Tumor metastasis
Metastasis is a critical step in tumor progression and causes high mortality in human deaths. Increasing evidences implicate HIF-1 function in tumor cell metastasis. Immunohistochemistry analysis showed that HIF-1α was overexpressed in tumor types compared with the respective normal tissues, including colon, breast, gastric, lung, skin, ovarian, pancreatic, prostate, and renal carcinomas [15]. Target genes of HIF-1α have been further identified and suggested to be involved in tumor metastasis in several aspects. Among them, TWIST, a most important and crucial transcription factor directly regulated by HIF-1, is implicated to be essential to hypoxia mediated epithelial-mesenchymal transition (EMT) and cancer metastasis [16]. HIF-1α- and TWIST-null mice show similarities in their phenotypes and siRNA-mediated repression of TWIST in HIF-1α- overexpressing or hypoxic cells reversed metastastic phenotypes. Further reports showed that HIF-1α can regulate intercellular adhersion molecules, such as α5β3, α5β5, and β1 intergrins, expression in tumor matrix [17-19]. On the other hand, E-cadherin, a calcium-dependent cell adhesion protein, is also down-regulated by HIF-1α via its direct regulation of TCF3, ZFHX1A, ZFHX1B, which repress E-cadherin gene transcription [20]. Matrixmetalloproteinases, MMP2 and MMP9 have also been reported to be regulated by HIF-1α [21,22]. The impact of hypoxia on MMP-9 expression as well as keratinocyte migration could be attenuated by inhibitors of protein kinase C (PKC), which indicated hypoxia-induced tumor cell migration is mediated by increased MMP-9 via the PKC pathway. Besides, urokinase-type plasminogen activator receptor (uPAR) and plasminogen activator inhibitor-1 (PAI-1), two major components of fibrinolysis system, have also been shown to be the target of HIF-1α and that is important for hypoxia-induced metastasis [23,24]. Another mechanism of metastatic spread in various cancer types involves in chemokine/cytokine receptors. It has been reported that HIF-1α could regulate the expression of CXC chemokine receptor-4 (CXCR4), c-Met and CC chemokine receptor 7 (CCR7), which in turn play critical roles in cell chemotaxis, homing as well as hypoxia-induced invasive growth [25-27].
Tumor angiogenesis
Angiogenesis plays an important role in the pathogenesis of tumor progressing. HIF-1 is known to stimulate angiogenic response by activating transcription of the genes encoding several growth factors, including vascular endothelial growth factor (VEGF). The HIF-1α-knockout mice mainly showed abnormal vascular development [28]. In further research , Tsuzuki Y et al. reported that HRE-/- ES tumors produced the same level of VEGF as the VEGF-/- ES tumors indicating the role of HIF-1α/HRE in transcription regulation of VEGF production in tumor cell [29]. Recently,calcitonin receptor-like receptor (CRLR), a G-protein coupled receptor shows a remarkable range of effects on promotion of angiogenesis, has been reported to be transcriptionally upregulated by HIF-1α [30]. It has also been showed that, the expression of Semaphorin 4D (Sema4D), one member of 20 semaphorins, which promotes angiogenesis, has a correlation with HIF-1 activity in HNSCC specimens [31]. In addition, other factors such as stem cell factor (SCF) and angiopoietin 2 (ANGPT2), which promote angiogenesis by binding to their cognating receptors, were further identified as the targets of HIF-1α [32,33].
SUMO-specific protease 1 (SENP1) has been shown to be essential for the stability and activity of HIF-1α under hypoxia conditions [34]. Recently, the same group also reported that HIF-1α could direct regulate the transcriptional activity of SENP-1, which indicates a positive feedback loop mediated by SENP1 within hypoxic endothelial cells that is important for VEGF production and angiogenesis [35].
Tumor energy metabolism
The energy metabolism process in tumor presents a shift from glucose metabolism coupled with mitochondrial oxidative to anaerobic glycolysis, which is known as the Warburg effect and shows decreased mitochondrial respiration versus increased lactate production [36]. Glucose transporters GLUT1 and GLUT3, responsible for constitutive or basal glucose uptake, and 6-Phosphofructo-2-kinase (pfkfb3), a key regulator of fructose-2,6-bisphosphate (Fru-2,6-P2) which regulates glycolytic flux, have been confirmed as the targets of HIF-1α [37-40]. In this process, the transactivation of these genes regulated by HIF-1α reprograms the intracellular fate of glucose, resulting in increased flux of reducing equivalents from glucose to lactate [41]. Two glycolytic enzymes, namely phosphoglycerate kinase 1 (PGK 1) and pyruvate kinase M2 (PKM2) have been shown to be transcriptionally activated by HIF-1 [42,43]. The monocarboxylate transporter MCT4, which mediates lactic acid efflux from most tissues, is up-regulated by hypoxia through a HIF-1α -dependent mechanism [44]. HIF-1 also actively suppresses metabolism by directly transactivating the gene encoding pyruvate dehydrogenase kinase 1 (PDK1) [45]. As a kinase of pyruvate dehydrogenase (PDH), PDK1 prevents the pyruvate oxidative decarboxylation to acetyl-CoA and reduce pyruvate entry into the tricarboxylic acid (TCA) cycle. Forced PDK1 expression could rescue hypoxic HIF-1α null cells from apoptosis accompanied with increased ATP level and decreased ROS generation [46].
MXI1, a C-Myc antagonist, which protects against C-Myc-dependent sensitization to hypoxia-induced apoptosis, has been revealed as a transcriptional target of the HIF-1 [47,48]. Furthermore, study shows that dysregulated activity of HIF-1 in VHL-deficient renal carcinoma cells leads to the inhibition of C-Myc transcriptional activity by MXI-1 expression and increased C-Myc degradation by the proteasome, then the resulting loss of C-Myc-dependent PGC-1β expression is responsible for the reduction in mitochondrial biogenesis and energy metabolism reprogramming [47,48].
Cell differentiation
Self-renewal and the capacity to differentiate into specialized cell types are two essential properties of stem cells. It is well documented that hypoxia and HIF-1α can promote the undifferentiated state of various stem and precursor cell populations. Recently, it is reported that normal hematopoietic stem cells (HSCs) maintain intracellular hypoxia and stabilize HIF-1α protein, and HSCs keep cell cycle quiescence through the precise regulation of HIF-1α level [49]. Hypoxic culture appears to be necessary to maintain full pluripotency of human embryonic stem (hES) cells, as the appearance of differentiated regions of cultured hES cells, such as the production of human chorionic gonadotropin and progesterone, the loss of stage-specific embryonic antigen-4 and Oct-4 in early-stage mammalian embryos, is markedly reduced under hypoxic conditions [50]. Further study showed that HIF-2α but not HIF-1α regulated Oct-4 by binding its promoter and inducing expression and transcriptional activity of Oct-4 [51]. Besides, evidence suggests that hypoxia can prevent the differentiation of progenitor cells as well as promote de-differentiation state of cancer cells [52-54]. It has been shown that hypoxia blocks differentiation of neuronal and myogenic progenitors via HIF-1-Notch signaling, whereby HIF-1α interacts with Notch intracellular domain, be recruited to Notch-responsive promoter, and elevate expression of Notch downstream genes which involve in stem cell maintenance [52-54]. Erythropoietin (EPO), a glycoprotein hormone that is secreted into the blood and binds to its cognate receptor on erythroid progenitor cells, stimulates red blood cells survival and differentiation has been identified as a direct target of HIF-1α in the past few years [55]. More interestingly, HIF-1α-stabilized insults such as hypoxia, cobalt chloride, desferrioxamine and tiron, 4,5-dihydroxy-1,3-benzene disulfonic acid can induce differentiation of acute myeloid leukemia [8,56,57]. Also, all-trans retinoic acid (ATAR), a clinically effective differentiation-inducing drug for acute promyelocytic leukemia (APL) rapidly increases endogenous and inducible expressed HIF-1α protein in leukemic cells [58]. Especially, intermittent hypoxia induces tumor arrest and differentiation with prolonged survival in a mouse model of acute myeloid leukemia [59]. Our results suggest that hypoxia-induced AML cell differentiation involves in interaction of HIF-1α with C/EBPα and Runx 1 proteins (see below) [60]. In addition, galectin-1, a member of the galectin family and has a high affinity for galactose and N-acetylglucosamine moieties of glycoproteins, is shown to be direct target of HIF-1 and C/EBP, and its synergistic induction by these two proteins partially contributes to AML cell differentiation [61,62].
By the way, HIF-1α plays an important role in the genetic program that regulates chondrogenesis. In hypoxic prechondrogenic cells, the expression of Sox9, a DNA-binding protein of the high mobility group (HMG) family as well as a key regulator of chondrocyte differentiation, is regulated and increased by HIF-1α which has been shown can directly bind to the promoter to Sox9 gene, in turn, resulting in the changing cellular landscape in endochondrial bone formation [63].
Cell apoptosis
It has been shown that hypoxia, the most physiologically relevant stress, induces apoptosis by leading to DNA damage at a significant high level [64]. Graeber TG et al. reported that hypoxia induced apoptosis in oncogenically transformed cells, whereas loss of p53 tumor-suppressor gene reduced hypoxia-induced cell death. Accordingly, highly apoptotic regions strongly correlate with hypoxic regions in transplanted tumors expressing wild-type p53 but not p53-deficient tumors [65]. p53 has been suggested to regulate cell programmed death and growth arrest through transcriptional activation of its downstream molecules, like Puma, Bax and p21 [66-68]. Studies also indicate that wild-type p53 can be stabilized by HIF-1α, and the interaction between HIF-1 and p53 can be either direct [69]or indirect [70], the later is probably mediated by binding of HIF-1α to p53 ubiquitin ligase Mdm2 [71]. Further results show that another direct target of HIF-1α, named NPM, a multifunctional protein which is overexpressed in actively proliferating cells and cancer cells, is associated with P53. Suppression of NPM expression by shRNA increases hypoxia-induced apoptosis [72]. In addition, HIF-1α has been shown to induce the expression of human urocortin 2 (hUcn2) via a specific HRE in the 3'FLR of hUcn2 gene, which may help to preserve cardiac function and prevent apoptosis in ischaemic conditions in the heart [73].
BNIP3, a member of the Bcl-2 family that is expressed in mitochondria and induces apoptosis, has been reported directly regulated by HIF-1α, play a dedicated role in the pathological progression of hypoxia-mediated apoptosis, necrosis and autophagy [74,75]. Besides, BH3-only Bcl-2 family protein Noxa, a molecule mediating p53-induced apoptosis, has also been identified as a target of HIF-1α. Noxa-mediated hypoxic cell death relates to reactive oxygen species and resultant cytochrome c release [76].
The interacting proteins of HIF-1α
The mechanism of signal transduction by HIF-1α is a complex multistep process, while the ultimate goal is to execute its role as a transcription factor. After synthesis in the cytosol, HIF-1α needs to undergo nuclear translocation to access target promoters and to become transcriptionally active. The whole process is tightly regulated by direct interaction with other proteins, which have been identified in large numbers of studies by employing Co-Immunoprecipitation (Co-IP), glutathione S-transferase (GST)-pull down, immuno-colocalization and yeast two-hybrid assays. Functionally, HIF-1α interacting proteins can mainly be categorized into following classes.
Interacting proteins regulating HIF-1α stability
As documented that HIF-1α protein is rapidly degraded by the proteasome in normoxia, but stabilized under hypoxia. The most recognized HIF-1α degradation pathway is mediated by its direct binding with Hippel-Lindau (VHL) tumor suppressor protein, pVHL. pVHL is part of a multiprotein complex that includes elongin C, Rbx1 and Cul2 [77-79]. This complex functions as an E3 ubiquitin ligase that directly binds to and targets HIF-1α for ubiquitination and proteasomal degradation.
The physical interaction of HIF-1α and pVHL is critically controlled by prolyl hydroxylation and acetylation of HIF-1α protein [6,80,81]. O2-dependent hydroxylation of two proline residues (P402 and P564) in ODD domain of HIF-1α by PHDs is required for binding of pVHL [82,83]. In mammalian cells, three isoforms of PHDs, PHD1, PHD2 and PHD3, have been identified and shown to hydroxylate HIF-1α in vitro [84,85]. However, only PHD2 was proved to control the steady-state levels of HIF-1α by experiments carried out in human cells [86]. Although HIF-1α and PHD2 bind to each other, efficient PHD2 activity is dependent on OS-9, a protein that binds to both HIF-1α and PHD2, thereby ensuring stable complex formation [87]. In the same degradation domain of HIF-1α protein, acetylation of Lys532 by ARD1 also enhances interaction of HIF-1α with pVHL and subsequent HIF-1α degradation [81]. Metastasis-associated protein 1 (MTA1) binds to HIF-1α and counteracts the activity of ARD1 [88]. Interestingly, most of these proteins can be regulated by hypoxia. PHD2 and MTA1 are hypoxia inducible while OS-9 and ARD1 expression decrease under hypoxic conditions.
The interaction between HIF-1α and pVHL is also regulated by other HIF-1α interacting proteins. The protein SSAT2 interacts with HIF-1α, pVHL and elongin C, stabilizes the interaction of pVHL and elongin C, and promotes ubiquitination of HIF-1α [89]. Ubiquitination of HIF-1α can also be reversed by its interaction with pVHL-interacting deubiquitinating enzyme 2 (VDU2), which stabilizes HIF-1α through deubiquitination [90,91]. ATP6V0C competes with pVHL in HIF-1α binding by directly interacting with HIF-1α under the stimulation of bafilomycin [92]. Hepatitis B virus X protein (HBx), which is closely involved in the development of hepatocellular carcinoma, directly interacts with the bHLH/PAS domain of HIF-1α and decreases the binding of pVHL to HIF-1α [93].
Despite the pivotal role of pVHL complex in cellular oxygen homeostasis, it is not the only E3 ligase that mediates HIF-1a degradation. In renal carcinoma cell lines that lack functional pVHL and express stable HIF-1α protein in normoxia, the HSP90 antagonist geldanamycin promotes ubiquitination and proteasomal degradation of HIF-1α by disrupting its association with HSP90, indicating that HIF-1α/ HSP90 association protects HIF-1α from degradation via an O2/PHD2/pVHL-independent pathway [94-96]. The receptor of activated protein kinase C (RACK1) can compete with HSP90 for binding to HIF-1α and subsequently subject HIF-1α for O2/PHD2/pVHL-independent proteasomal degradation by recruiting elongin C, the same E3 ubiquitin ligase component that is bound by pVHL [97,98]. SSAT1, a protein bears 46% amino acid identity to SSAT2, also binds to HIF-1α and triggers its ubiquitination and degradation [99]. However, in contrast to SSAT2, which plays a role in the O2/PHD2/pVHL-dependent degradation of HIF-1α, SSAT1 executes its role by stabilizing the interaction of HIF-1α with RACK1 [99]. Thus, the paralogs SSAT1 and SSAT2 play complementary roles in promoting O2-independent and O2-dependent degradation of HIF-1α. A mammalian septin family member, SEPT9_v1, interacts with HIF-1α and protects it from O2-independent proteasomal degradation by preventing the interaction of HIF-1α with RACK1 [100]. It is also reported that upon HSP90 inhibition, a protein complex consisting of HSP70 and HIF-1α is formed to facilitate 20S proteasomal degradation in an ubiquitin-independent manner [101-103]. The copper metabolism MURR1 domain containing 1 protein (COMMD1) has been shown can compete with HSP90 and form a tripartite complex with HSP70/ HIF-1α, thus facilitates HIF-1α proteolysis [104]. Hypoxia-associated factor (HAF) has also been identified as an E3 ligase for HIF-1α that mediates the degradation of HIF-1α through a novel pVHL and O2-independent pathway [105,106]. As reported by Ravi R et al., Mdm2 is another E3 ligase that is suggested to bind HIF-1α and mediate its ubiquitination/proteasomal degradation in the presence of p53 which acts as a molecular chaperone [107]. However, contradictory results suggest that HIF-1α degradation is not affected by Mdm2 or p53 status of the cells in hypoxia, Mdm2 promotes HIF-1α activation rather than its degradation [108].
Jun activation domain-binding protein-1 (Jab1), a coactivator of AP-1 transcription factor, has been reported to directly bind to HIF-1α, interfere with HIF-1α-p53 interaction, and lead to stabilization of HIF-1α protein under hypoxia [109].
HIF-1α stability is also modulated by the covalent attachment of small ubiquitin-related modifier (SUMO). Hypoxia induces SUMOylation of HIF-1α, which promotes its binding to pVHL-E3 complex for degradation [34]. PIASy has been identified as the E3 ligase for hypoxia-induced HIF-1α SUMOylation. As a result, PIASy negatively regulates HIF-1α stability and activity [110]. However, different SUMOylation inducers may have distinct efficiencies, as HIF-1α has also been reported to be stabilized with increased SUMOylation by RSUME, an enhancer of SUMO conjugation, during hypoxia [111].
Other proteins, such as MSF-A, PSMA7 and LANA were also reported to interact with HIF-1α and regulate its degradation and activation, but the molecular mechanisms are relatively obscure [112-114].
Interacting proteins regulating transcriptional activity of HIF-1α
To exert its role as transcription factor, stabilized HIF-1α need to form a heterodimeric complex with HIF-1β and bind to HREs located within the promoters of target genes [9]. Besides its role in HIF-1α degradation, recent study has shown that COMMD1 can also prevent the dimerization of HIF-1α and HIF-1β and subsequently inhibit HIF-1-mediated gene expression [115]. Fatyol K et al. reported that the p14ARF tumor suppressor protein could directly inhibit the transcriptional activity of HIF-1 by sequenstering HIF-1α from nucleoplasm to the nucleolus [116]. In spermatozoa, the testis specific gene antigen 10 (TSGA10) co-localizes with HIF-1α and consequently prevents the nuclear localization and transcriptional activity of HIF-1α [117].
Besides HIF-1β, HIF-1α can also dimerize with other PAS family members. HIF-1α has been shown to interact and form transcriptionally active heterodimers with MOP3 and MOP9 respectively, although their targets remain unclear [118-120]. The circadian factor PER2, another PAS protein, has been reported to bind to HIF-1α, prevent HIF-1α/HIF-1β mediated transcription, and regulate the circadian rhythm of VEGF production in hypoxic tumor cells [121]. However, the interaction of HIF-1α with PER1 is thought to alter the stability of PER1 by protecting it from proteolytical degradation [122].
Although PHD2-mediated hydroxylation within ODD domain of HIF-1α has been well documented, it is unraveled that HIF-1α can also be hydroxylated in its C-terminal TAD domain (N803 in human HIF-1α) by factor inhibiting HIF-1 (FIH-1) under normoxic conditions [123,124]. The hydroxylation results in suppression of HIF-1α transcriptional activity via blocking the association of HIF-1α with its coactivators [123]. These coactivators, including p300/CBP, SRC-1 and TIF2, play two critical roles: bind to and stabilize the transcription initiation complex containing RNA polymerase II and also possess histone acetyltransferase activity that is required for the polymerase to access DNA within chromatin and transcribe it into RNA [125-128]. Recruitment of coactivators to HIF-1α is regulated by other interacting proteins. Thiol-redox regulator Ref-1 interacts with HIF-1α and modifies its C-terminal TAD domain, which facilitates the binding of coactivators [125,126]. Histone deacetylase 7 (HDAC7) co-translocates to the nucleus with HIF-1α under hypoxic conditions and increases the transcriptional activity of HIF-1α through the formation of a complex with HIF-1α and p300 [129]. Regulatory associated protein of mTOR (Raptor) interacts with HIF-1α and promotes its activity by enhancing its association with cofactors p300/ CBP, which may be a link between the mTOR and HIF-1α signaling [130]. The orphan nuclear receptor estrogen-related receptors (ERRs) physically interact with HIF-1α and also serve as essential cofactors of HIF-1α in mediating hypoxic responses [131]. Recently, the SIRT1 deacetylase was found to bind and deacetylate HIF-1α at Lys674, which is acetylated by PCAF [132]. Thereby, SIRT1 inactivated HIF-1α by blocking p300 recruitment and consequently repressed HIF-1 target genes [132]. Moon HE et al. found that Necdin, a growth suppressor, directly associates with HIF-1α at ODD domain and inhibits the transcriptional activity of HIF-1 as well as angiogenic activity under hypoxia [133]. Budde A et al. applied T7 phage display system and identified a domain inherent in the retinoblastoma protein (pRB). Further study confirmed the physical and functional interaction between HIF-1α and pRB, as a result, causes HIF-1α transcriptional activation and reverses the transcriptional repressor function of pRB [134].
HIF-1α interacting transcription factors
As a master transcription factor responsible for cellular oxygen homeostasis, HIF-1 regulates the expression of dozens of genes containing HRE motif. Nevertheless, HIF-1α has been found to interact with a variety of transcription factors and interfere with their transcriptional activities. Since these HIF-1α-interacting transcription factors are modulators for various cellular activities, the functional range of HIF-1α has been greatly extended.
As discussed above, direct association of HIF-1α and p53 not only induces HIF-1α degradation via recruiting the mdm2 E3 Ubiquitin ligase, but may be also necessary for stabilization-dependent accumulation of wild-type p53 [71,107]. HIF-1α competes with T-cell factor-4 (TCF-4) for direct binding to β-catenin, thereby inhibits β-catenin-TCF-4 transcriptional activity but enhances HIF-1α-mediated transcription [135]. The dynamic role of β-catenin is thought to be a way of tumors’ adaptation to hypoxia by constraining cell growth [135]. Study also shows that HIF-1α and Smad3 associate with each other and cooperatively activate VEGF promoter, suggesting a synergic activity between hypoxia and TGF-β [136]. HIF-1α has been shown to form a weak complex with Myc in vivo and functionally antagonizes its activity, resulting in derepression of p21cip1 along with cell cycle arrest [137]. Tendler DS et al. suggested that the direct interaction of HIF-1α and IFN regulatory factor-1 (IRF-1) might be the underlying mechanism by which macrophages infiltrating into tumors, being activated to express nitric oxide synthase (NOS2) and to produce NO, a mediator of tumor apoptosis [138]. Under hypoxia, Sp1 serves as a molecular switch by recruiting HIF-1α to the promoter of MSH2 and MSH6 genes, thereby decreases levels of MutSα, a complex of MSH2 and MSH6 protein which recognizes base mismatches, leading to hypoxia-induced genetic instability [139]. The EPO 3'-enhancer contains both HIF-1α and HNF-4 binding sites, hypoxia induces the interaction between HNF-4 and HIF-1α, which results in augmentation of gene expression by the EPO enhancer [140].
Results from our group have revealed that HIF-1α contributes to the differentiation of acute myeloid leukemia cells via transcriptional activity-independent mechanisms [7]. HIF-1α can interact with hematopoiesis-related transcription factors C/EBPα and Runx1 so as to increase their transcriptional activities [56,141,142]. Meanwhile, the dimerization of HIF-1α and HIF-β as well as HIF-1 transcriptional activity is inhibited [141,142]. The functional switch of HIF-1α may present dual effects. On the one hand, inhibition of HIF-1 transcriptional activity by C/EBPα and Runx1 helps to produce low-oxygen microenvironment of the bone marrow due to inhibition of angiogenesis. On the other hand, increased transcriptional activity of C/EBPα and Runx1 mediated by HIF-1α promotes differentiation of leukemic cells [141].
Perspective
Functional activities of HIF-1α along with underlying mechanisms have been greatly expanded in the past decades (Figure 1). It has been revealed that HIF-1α participates in a variety of physiological and pathophysiological processes, including but not limited in tumor metastasis, angiogenesis, energy metabolism, cell differentiation and apoptosis, which could be either dependent or independent on its transcriptional activity. The discoveries of HIF-1α target genes as well as its interacting proteins not only extend our knowledge for hypoxia adaptation, but also provide potential drug targets that are more controlled and/or specific, and eventually offer the opportunities for therapeutic intervention.
Figure 1.
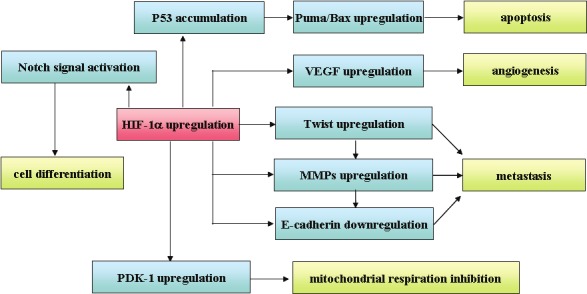
Representative target genes of HIF-1α and their functions.
References
- 1.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Semenza GL. Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr Opin Genet Dev. 1998;8:588–594. doi: 10.1016/s0959-437x(98)80016-6. [DOI] [PubMed] [Google Scholar]
- 3.Wang GL, Semenza GL. Molecular basis of hypoxia-induced erythropoietin expression. Curr Opin Hematol. 1996;3:156–162. doi: 10.1097/00062752-199603020-00009. [DOI] [PubMed] [Google Scholar]
- 4.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 5.Semenza GL. Oxygen homeostasis. Wiley Interdiscip Rev Syst Biol Med. 2010;2:336–361. doi: 10.1002/wsbm.69. [DOI] [PubMed] [Google Scholar]
- 6.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 7.Song LP, Zhang J, Wu SF, Huang Y, Zhao Q, Cao JP, Wu YL, Wang LS, Chen GQ. Hypoxia-inducible factor-1alpha-induced differentiation of myeloid leukemic cells is its transcriptional activity independent. Oncogene. 2008;27:519–527. doi: 10.1038/sj.onc.1210670. [DOI] [PubMed] [Google Scholar]
- 8.Huang Y, Du KM, Xue ZH, Yan H, Li D, Liu W, Chen Z, Zhao Q, Tong JH, Zhu YS, Chen GQ. Cobalt chloride and low oxygen tension trigger differentiation of acute myeloid leukemic cells: possible mediation of hypoxia-inducible factor-1alpha. Leukemia. 2003;17:2065–2073. doi: 10.1038/sj.leu.2403141. [DOI] [PubMed] [Google Scholar]
- 9.Wenger RH, Gassmann M. Oxygen(es) and the hypoxia-inducible factor-1. Biol Chem. 1997;378:609–616. [PubMed] [Google Scholar]
- 10.Benita Y, Kikuchi H, Smith AD, Zhang MQ, Chung DC, Xavier RJ. An integrative genomics approach identifies Hypoxia Inducible Factor-1 (HIF-1)-target genes that form the core response to hypoxia. Nucleic Acids Res. 2009;37:4587–4602. doi: 10.1093/nar/gkp425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ortiz-Barahona A, Villar D, Pescador N, Amigo J, del Peso L. Genome-wide identification of hypoxia-inducible factor binding sites and target genes by a probabilistic model integrating transcription-profiling data and in silico binding site prediction. Nucleic Acids Res. 2010;38:2332–2345. doi: 10.1093/nar/gkp1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han YH, Xia L, Song LP, Zheng Y, Chen WL, Zhang L, Huang Y, Chen GQ, Wang LS. Comparative proteomic analysis of hypoxia-treated and untreated human leukemic U937 cells. Proteomics. 2006;6:3262–3274. doi: 10.1002/pmic.200500754. [DOI] [PubMed] [Google Scholar]
- 13.Liao SH, Zhao XY, Han YH, Zhang J, Wang LS, Xia L, Zhao KW, Zheng Y, Guo M, Chen GQ. Proteomics-based identification of two novel direct targets of hypoxia-inducible factor-1 and their potential roles in migration/invasion of cancer cells. Proteomics. 2009;9:3901–3912. doi: 10.1002/pmic.200800922. [DOI] [PubMed] [Google Scholar]
- 14.Wenger RH, Stiehl DP, Camenisch G. Integration of oxygen signaling at the consensus HRE. Sci STKE. 2005;2005:re12. doi: 10.1126/stke.3062005re12. [DOI] [PubMed] [Google Scholar]
- 15.Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830–5835. [PubMed] [Google Scholar]
- 16.Yang MH, Wu MZ, Chiou SH, Chen PM, Chang SY, Liu CJ, Teng SC, Wu KJ. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell Biol. 2008;10:295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- 17.Lee SH, Lee YJ, Han HJ. Role of hypoxia-induced fibronectin-integrin beta1 expression in embryonic stem cell proliferation and migration: Involvement of PI3K/Akt and FAK. J Cell Physiol. 2011;226:484–493. doi: 10.1002/jcp.22358. [DOI] [PubMed] [Google Scholar]
- 18.Cowden Dahl KD, Robertson SE, Weaver VM, Simon MC. Hypoxia-inducible factor regulates alphavbeta3 integrin cell surface expression. Mol Biol Cell. 2005;16:1901–1912. doi: 10.1091/mbc.E04-12-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryu MH, Park HM, Chung J, Lee CH, Park HR. Hypoxia-inducible factor-1alpha mediates oral squamous cell carcinoma invasion via upregulation of alpha5 integrin and fibronectin. Biochem Biophys Res Commun. 2010;393:11–15. doi: 10.1016/j.bbrc.2010.01.060. [DOI] [PubMed] [Google Scholar]
- 20.Krishnamachary B, Zagzag D, Nagasawa H, Rainey K, Okuyama H, Baek JH, Semenza GL. Hypoxia-inducible factor-1-dependent repression of E-cadherin in von Hippel-Lindau tumor suppressor-null renal cell carcinoma mediated by TCF3, ZFHX1A, and ZFHX1B. Cancer Res. 2006;66:2725–2731. doi: 10.1158/0008-5472.CAN-05-3719. [DOI] [PubMed] [Google Scholar]
- 21.Luo Y, He DL, Ning L, Shen SL, Li L, Li X, Zhau HE, Chung LW. Over-expression of hypoxia-inducible factor-1alpha increases the invasive potency of LNCaP cells in vitro. BJU Int. 2006;98:1315–1319. doi: 10.1111/j.1464-410X.2006.06480.x. [DOI] [PubMed] [Google Scholar]
- 22.O'Toole EA, van Koningsveld R, Chen M, Woodley DT. Hypoxia induces epidermal keratinocyte matrix metalloproteinase-9 secretion via the protein kinase C pathway. J Cell Physiol. 2008;214:47–55. doi: 10.1002/jcp.21160. [DOI] [PubMed] [Google Scholar]
- 23.Lin MT, Kuo IH, Chang CC, Chu CY, Chen HY, Lin BR, Sureshbabu M, Shih HJ, Kuo ML. Involvement of hypoxia-inducing factor-1alpha-dependent plasminogen activator inhibitor-1 up-regulation in Cyr61/CCN1-induced gastric cancer cell invasion. J Biol Chem. 2008;283:15807–15815. doi: 10.1074/jbc.M708933200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Buchler P, Reber HA, Tomlinson JS, Hankinson O, Kallifatidis G, Friess H, Herr I, Hines OJ. Transcriptional regulation of urokinase-type plasminogen activator receptor by hypoxia-inducible factor 1 is crucial for invasion of pancreatic and liver cancer. Neoplasia. 2009;11:196–206. doi: 10.1593/neo.08734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pennacchietti S, Michieli P, Galluzzo M, Mazzone M, Giordano S, Comoglio PM. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 2003;3:347–361. doi: 10.1016/s1535-6108(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 26.Ishikawa T, Nakashiro K, Klosek SK, Goda H, Hara S, Uchida D, Hamakawa H. Hypoxia enhances CXCR4 expression by activating HIF-1 in oral squamous cell carcinoma. Oncol Rep. 2009;21:707–712. [PubMed] [Google Scholar]
- 27.Li Y, Qiu X, Zhang S, Zhang Q, Wang E. Hypoxia induced CCR7 expression via HIF-1alpha and HIF-2alpha correlates with migration and invasion in lung cancer cells. Cancer Biol Ther. 2009;8:322–330. doi: 10.4161/cbt.8.4.7332. [DOI] [PubMed] [Google Scholar]
- 28.Kotch LE, Iyer NV, Laughner E, Semenza GL. Defective vascularization of HIF-1alpha-null embryos is not associated with VEGF deficiency but with mesenchymal cell death. Dev Biol. 1999;209:254–267. doi: 10.1006/dbio.1999.9253. [DOI] [PubMed] [Google Scholar]
- 29.Tsuzuki Y, Fukumura D, Oosthuyse B, Koike C, Carmeliet P, Jain RK. Vascular endothelial growth factor (VEGF) modulation by targeting hypoxia-inducible factor-1alpha--> hypoxia response element--> VEGF cascade differentially regulates vascular response and growth rate in tumors. Cancer Res. 2000;60:6248–6252. [PubMed] [Google Scholar]
- 30.Nikitenko LL, Smith DM, Bicknell R, Rees MC. Transcriptional regulation of the CRLR gene in human microvascular endothelial cells by hypoxia. Faseb J. 2003;17:1499–1501. doi: 10.1096/fj.02-0993fje. [DOI] [PubMed] [Google Scholar]
- 31.Sun Q, Zhou H, Binmadi NO, Basile JR. Hypoxia-inducible factor-1-mediated regulation of semaphorin 4D affects tumor growth and vascularity. J Biol Chem. 2009;284:32066–32074. doi: 10.1074/jbc.M109.057166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han ZB, Ren H, Zhao H, Chi Y, Chen K, Zhou B, Liu YJ, Zhang L, Xu B, Liu B, Yang R, Han ZC. Hypoxia-inducible factor (HIF)-1 alpha directly enhances the transcriptional activity of stem cell factor (SCF) in response to hypoxia and epidermal growth factor (EGF) Carcinogenesis. 2008;29:1853–1861. doi: 10.1093/carcin/bgn066. [DOI] [PubMed] [Google Scholar]
- 33.Kelly BD, Hackett SF, Hirota K, Oshima Y, Cai Z, Berg-Dixon S, Rowan A, Yan Z, Campochiaro PA, Semenza GL. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res. 2003;93:1074–1081. doi: 10.1161/01.RES.0000102937.50486.1B. [DOI] [PubMed] [Google Scholar]
- 34.Cheng J, Kang X, Zhang S, Yeh ET. SUMO-specific protease 1 is essential for stabilization of HIF1alpha during hypoxia. Cell. 2007;131:584–595. doi: 10.1016/j.cell.2007.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu Y, Zuo Y, Zhang H, Kang X, Yue F, Yi Z, Liu M, Yeh ET, Chen G, Cheng J. Induction of SENP1 in endothelial cells contributes to hypoxia-driven VEGF expression and angiogenesis. J Biol Chem. 2010;285:36682–36688. doi: 10.1074/jbc.M110.164236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 37.Hayashi M, Sakata M, Takeda T, Yamamoto T, Okamoto Y, Sawada K, Kimura A, Minekawa R, Tahara M, Tasaka K, Murata Y. Induction of glucose transporter 1 expression through hypoxia-inducible factor 1alpha under hypoxic conditions in trophoblast-derived cells. J Endocrinol. 2004;183:145–154. doi: 10.1677/joe.1.05599. [DOI] [PubMed] [Google Scholar]
- 38.Calvert JW, Cahill J, Yamaguchi-Okada M, Zhang JH. Oxygen treatment after experimental hypoxia-ischemia in neonatal rats alters the expression of HIF-1alpha and its downstream target genes. J Appl Physiol. 2006;101:853–865. doi: 10.1152/japplphysiol.00268.2006. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y, Li YM, Tian RF, Liu WP, Fei Z, Long QF, Wang XA, Zhang X. The expression and significance of HIF-1alpha and GLUT-3 in glioma. Brain Res. 2009;1304:149–154. doi: 10.1016/j.brainres.2009.09.083. [DOI] [PubMed] [Google Scholar]
- 40.Obach M, Navarro-Sabate A, Caro J, Kong X, Duran J, Gomez M, Perales JC, Ventura F, Rosa JL, Bartrons R. 6-Phosphofructo-2-kinase (pfkfb3) gene promoter contains hypoxia-inducible factor-1 binding sites necessary for transactivation in response to hypoxia. J Biol Chem. 2004;279:53562–53570. doi: 10.1074/jbc.M406096200. [DOI] [PubMed] [Google Scholar]
- 41.Lum JJ, Bui T, Gruber M, Gordan JD, DeBerardinis RJ, Covello KL, Simon MC, Thompson CB. The transcription factor HIF-1alpha plays a critical role in the growth factor-dependent regulation of both aerobic and anaerobic glycolysis. Genes Dev. 2007;21:1037–1049. doi: 10.1101/gad.1529107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodriguez H, Drouin R, Holmquist GP, Akman SA. A hot spot for hydrogen peroxide-induced damage in the human hypoxia-inducible factor 1 binding site of the PGK 1 gene. Arch Biochem Biophys. 1997;338:207–212. doi: 10.1006/abbi.1996.9820. [DOI] [PubMed] [Google Scholar]
- 43.Kress S, Stein A, Maurer P, Weber B, Reichert J, Buchmann A, Huppert P, Schwarz M. Expression of hypoxia-inducible genes in tumor cells. J Cancer Res Clin Oncol. 1998;124:315–320. doi: 10.1007/s004320050175. [DOI] [PubMed] [Google Scholar]
- 44.Ullah MS, Davies AJ, Halestrap AP. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1alpha-dependent mechanism. J Biol Chem. 2006;281:9030–9037. doi: 10.1074/jbc.M511397200. [DOI] [PubMed] [Google Scholar]
- 45.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 46.Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 47.Corn PG, Ricci MS, Scata KA, Arsham AM, Simon MC, Dicker DT, El-Deiry WS. Mxi1 is induced by hypoxia in a HIF-1-dependent manner and protects cells from c-Myc-induced apoptosis. Cancer Biol Ther. 2005;4:1285–1294. doi: 10.4161/cbt.4.11.2299. [DOI] [PubMed] [Google Scholar]
- 48.Zhang H, Gao P, Fukuda R, Kumar G, Krishnamachary B, Zeller KI, Dang CV, Semenza GL. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell. 2007;11:407–420. doi: 10.1016/j.ccr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 49.Takubo K, Goda N, Yamada W, Iriuchishima H, Ikeda E, Kubota Y, Shima H, Johnson RS, Hirao A, Suematsu M, Suda T. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell. 2010;7:391–402. doi: 10.1016/j.stem.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 50.Ezashi T, Das P, Roberts RM. Low O2 tensions and the prevention of differentiation of hES cells. Proc Natl Acad Sci USA. 2005;102:4783–4788. doi: 10.1073/pnas.0501283102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Covello KL, Kehler J, Yu H, Gordan JD, Arsham AM, Hu CJ, Labosky PA, Simon MC, Keith B. HIF-2alpha regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 2006;20:557–570. doi: 10.1101/gad.1399906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Helczynska K, Kronblad A, Jogi A, Nilsson E, Beckman S, Landberg G, Pahlman S. Hypoxia promotes a dedifferentiated phenotype in ductal breast carcinoma in situ. Cancer Res. 2003;63:1441–1444. [PubMed] [Google Scholar]
- 53.Jogi A, Ora I, Nilsson H, Poellinger L, Axelson H, Pahlman S. Hypoxia-induced dedifferentiation in neuroblastoma cells. Cancer Lett. 2003;197:145–150. doi: 10.1016/s0304-3835(03)00095-8. [DOI] [PubMed] [Google Scholar]
- 54.Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, Ruas JL, Poellinger L, Lendahl U, Bondesson M. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–628. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 55.Semenza GL. Involvement of oxygen-sensing pathways in physiologic and pathologic erythropoiesis. Blood. 2009;114:2015–2019. doi: 10.1182/blood-2009-05-189985. [DOI] [PubMed] [Google Scholar]
- 56.Jiang Y, Xue ZH, Shen WZ, Du KM, Yan H, Yu Y, Peng ZG, Song MG, Tong JH, Chen Z, Huang Y, Lubbert M, Chen GQ. Desferrioxamine induces leukemic cell differentiation potentially by hypoxia-inducible factor-1 alpha that augments transcriptional activity of CCAAT/enhancer-binding protein-alpha. Leukemia. 2005;19:1239–1247. doi: 10.1038/sj.leu.2403734. [DOI] [PubMed] [Google Scholar]
- 57.Kim JS, Cho EW, Chung HW, Kim IG. Effects of Tiron, 4,5-dihydroxy-1,3-benzene disulfonic acid, on human promyelotic HL-60 leukemia cell differentiation and death. Toxicology. 2006;223:36–45. doi: 10.1016/j.tox.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 58.Zhang J, Song LP, Huang Y, Zhao Q, Zhao KW, Chen GQ. Accumulation of hypoxia-inducible factor-1 alpha protein and its role in the differentiation of myeloid leukemic cells induced by all-trans retinoic acid. Haematologica. 2008;93:1480–1487. doi: 10.3324/haematol.13096. [DOI] [PubMed] [Google Scholar]
- 59.Liu W, Guo M, Xu YB, Li D, Zhou ZN, Wu YL, Chen Z, Kogan SC, Chen GQ. Induction of tumor arrest and differentiation with prolonged survival by intermittent hypoxia in a mouse model of acute myeloid leukemia. Blood. 2006;107:698–707. doi: 10.1182/blood-2005-03-1278. [DOI] [PubMed] [Google Scholar]
- 60.Zhang J, Chen GQ. Hypoxia-HIF-1alpha-C/EBPalpha/Runx1 signaling in leukemic cell differentiation. Pathophysiology. 2009;16:297–303. doi: 10.1016/j.pathophys.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 61.Zhao XY, Zhao KW, Jiang Y, Zhao M, Chen GQ. Synergistic induction of galectin-1 by CCAAT/enhancer binding protein alpha and hypoxia-inducible factor 1alpha and its role in differentiation of acute myeloid leukemic cells. J Biol Chem. 2011;286:36808–36819. doi: 10.1074/jbc.M111.247262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao XY, Chen TT, Xia L, Guo M, Xu Y, Yue F, Jiang Y, Chen GQ, Zhao KW. Hypoxia inducible factor-1 mediates expression of galectin-1: the potential role in migration/invasion of colorectal cancer cells. Carcinogenesis. 2010;31:1367–1375. doi: 10.1093/carcin/bgq116. [DOI] [PubMed] [Google Scholar]
- 63.Amarilio R, Viukov SV, Sharir A, Eshkar-Oren I, Johnson RS, Zelzer E. HIF1alpha regulation of Sox9 is necessary to maintain differentiation of hypoxic prechondrogenic cells during early skeletogenesis. Development. 2007;134:3917–3928. doi: 10.1242/dev.008441. [DOI] [PubMed] [Google Scholar]
- 64.Hammond EM, Dorie MJ, Giaccia AJ. ATR/ATM targets are phosphorylated by ATR in response to hypoxia and ATM in response to reoxygenation. J Biol Chem. 2003;278:12207–12213. doi: 10.1074/jbc.M212360200. [DOI] [PubMed] [Google Scholar]
- 65.Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, Giaccia AJ. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379:88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- 66.Yu J, Wang Z, Kinzler KW, Vogelstein B, Zhang L. PUMA mediates the apoptotic response to p53 in colorectal cancer cells. Proc Natl Acad Sci USA. 2003;100:1931–1936. doi: 10.1073/pnas.2627984100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hsieh JK, Kletsas D, Clunn G, Hughes AD, Schachter M, Demoliou-Mason C. p53, p21 (WAF1/CIP1), and MDM2 involvement in the proliferation and apoptosis in an in vitro model of conditionally immortalized human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2000;20:973–981. doi: 10.1161/01.atv.20.4.973. [DOI] [PubMed] [Google Scholar]
- 68.Chipuk JE, Green DR. Cytoplasmic p53: bax and forward. Cell Cycle. 2004;3:429–431. [PubMed] [Google Scholar]
- 69.Hansson LO, Friedler A, Freund S, Rudiger S, Fersht AR. Two sequence motifs from HIF-1alpha bind to the DNA-binding site of p53. Proc Natl Acad Sci USA. 2002;99:10305–10309. doi: 10.1073/pnas.122347199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen D, Li M, Luo J, Gu W. Direct interactions between HIF-1 alpha and Mdm2 modulate p53 function. J Biol Chem. 2003;278:13595–13598. doi: 10.1074/jbc.C200694200. [DOI] [PubMed] [Google Scholar]
- 71.An WG, Kanekal M, Simon MC, Maltepe E, Blagosklonny MV, Neckers LM. Stabilization of wild-type p53 by hypoxia-inducible factor 1alpha. Nature. 1998;392:405–408. doi: 10.1038/32925. [DOI] [PubMed] [Google Scholar]
- 72.Li J, Zhang X, Sejas DP, Bagby GC, Pang Q. Hypoxia-induced nucleophosmin protects cell death through inhibition of p53. J Biol Chem. 2004;279:41275–41279. doi: 10.1074/jbc.C400297200. [DOI] [PubMed] [Google Scholar]
- 73.Buhler K, Plaisance I, Dieterle T, Brink M. The human urocortin 2 gene is regulated by hypoxia: identification of a hypoxia-responsive element in the 3'-flanking region. Biochem J. 2009;424:119–127. doi: 10.1042/BJ20090311. [DOI] [PubMed] [Google Scholar]
- 74.Bruick RK. Expression of the gene encoding the proapoptotic Nip3 protein is induced by hypoxia. Proc Natl Acad Sci USA. 2000;97:9082–9087. doi: 10.1073/pnas.97.16.9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vande Velde C, Cizeau J, Dubik D, Alimonti J, Brown T, Israels S, Hakem R, Greenberg AH. BNIP3 and genetic control of necrosis-like cell death through the mitochondrial permeability transition pore. Mol Cell Biol. 2000;20:5454–5468. doi: 10.1128/mcb.20.15.5454-5468.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim JY, Ahn HJ, Ryu JH, Suk K, Park JH. BH3-only protein Noxa is a mediator of hypoxic cell death induced by hypoxia-inducible factor 1alpha. J Exp Med. 2004;199:113–124. doi: 10.1084/jem.20030613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kamura T, Koepp DM, Conrad MN, Skowyra D, Moreland RJ, Iliopoulos O, Lane WS, Kaelin WG, Jr., Elledge SJ, Conaway RC, Harper JW, Conaway JW. Rbx1, a component of the VHL tumor suppressor complex and SCF ubiquitin ligase. Science. 1999;284:657–661. doi: 10.1126/science.284.5414.657. [DOI] [PubMed] [Google Scholar]
- 78.Lisztwan J, Imbert G, Wirbelauer C, Gstaiger M, Krek W. The von Hippel-Lindau tumor suppressor protein is a component of an E3 ubiquitin-protein ligase activity. Genes Dev. 1999;13:1822–1833. doi: 10.1101/gad.13.14.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stebbins CE, Kaelin WG, Jr., Pavletich NP. Structure of the VHL-ElonginC-ElonginB complex: implications for VHL tumor suppressor function. Science. 1999;284:455–461. doi: 10.1126/science.284.5413.455. [DOI] [PubMed] [Google Scholar]
- 80.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Kaelin WG, Jr., Lane WS. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 81.Jeong JW, Bae MK, Ahn MY, Kim SH, Sohn TK, Bae MH, Yoo MA, Song EJ, Lee KJ, Kim KW. Regulation and destabilization of HIF-1alpha by ARD1-mediated acetylation. Cell. 2002;111:709–720. doi: 10.1016/s0092-8674(02)01085-1. [DOI] [PubMed] [Google Scholar]
- 82.Chowdhury R, Hardy A, Schofield CJ. The human oxygen sensing machinery and its manipulation. Chem Soc Rev. 2008;37:1308–1319. doi: 10.1039/b701676j. [DOI] [PubMed] [Google Scholar]
- 83.Kaelin WG Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 84.Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- 85.Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 86.Berra E, Benizri E, Ginouves A, Volmat V, Roux D, Pouyssegur J. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. EMBO J. 2003;22:4082–4090. doi: 10.1093/emboj/cdg392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baek JH, Mahon PC, Oh J, Kelly B, Krishnamachary B, Pearson M, Chan DA, Giaccia AJ, Semenza GL. OS-9 interacts with hypoxia-inducible factor 1alpha and prolyl hydroxylases to promote oxygen-dependent degradation of HIF-1alpha. Mol Cell. 2005;17:503–512. doi: 10.1016/j.molcel.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 88.Yoo YG, Kong G, Lee MO. Metastasis-associated protein 1 enhances stability of hypoxia-inducible factor-1alpha protein by recruiting histone deacetylase 1. EMBO J. 2006;25:1231–1241. doi: 10.1038/sj.emboj.7601025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baek JH, Liu YV, McDonald KR, Wesley JB, Hubbi ME, Byun H, Semenza GL. Spermidine/spermine-N1-acetyltransferase 2 is an essential component of the ubiquitin ligase complex that regulates hypoxia-inducible factor 1alpha. J Biol Chem. 2007;282:23572–23580. doi: 10.1074/jbc.M703504200. [DOI] [PubMed] [Google Scholar]
- 90.Li Z, Wang D, Messing EM, Wu G. VHL protein-interacting deubiquitinating enzyme 2 deubiquitinates and stabilizes HIF-1alpha. EMBO Rep. 2005;6:373–378. doi: 10.1038/sj.embor.7400377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li Z, Wang D, Na X, Schoen SR, Messing EM, Wu G. Identification of a deubiquitinating enzyme subfamily as substrates of the von Hippel-Lindau tumor suppressor. Biochem Biophys Res Commun. 2002;294:700–709. doi: 10.1016/S0006-291X(02)00534-X. [DOI] [PubMed] [Google Scholar]
- 92.Lim JH, Park JW, Kim SJ, Kim MS, Park SK, Johnson RS, Chun YS. ATP6V0C competes with von Hippel-Lindau protein in hypoxia-inducible factor 1alpha (HIF-1alpha) binding and mediates HIF-1alpha expression by bafilomycin A1. Mol Pharmacol. 2007;71:942–948. doi: 10.1124/mol.106.030296. [DOI] [PubMed] [Google Scholar]
- 93.Moon EJ, Jeong CH, Jeong JW, Kim KR, Yu DY, Murakami S, Kim CW, Kim KW. Hepatitis B virus X protein induces angiogenesis by stabilizing hypoxia-inducible factor-1alpha. FASEB J. 2004;18:382–384. doi: 10.1096/fj.03-0153fje. [DOI] [PubMed] [Google Scholar]
- 94.Gradin K, McGuire J, Wenger RH, Kvietikova I, fhitelaw ML, Toftgard R, Tora L, Gassmann M, Poellinger L. Functional interference between hypoxia and dioxin signal transduction pathways: competition for recruitment of the Arnt transcription factor. Mol Cell Biol. 1996;16:5221–5231. doi: 10.1128/mcb.16.10.5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Isaacs JS, Jung YJ, Mimnaugh EG, Martinez A, Cuttitta F, Neckers LM. Hsp90 regulates a von Hippel Lindau-independent hypoxia-inducible factor-1 alpha-degradative pathway. J Biol Chem. 2002;277:29936–29944. doi: 10.1074/jbc.M204733200. [DOI] [PubMed] [Google Scholar]
- 96.Minet E, Mottet D, Michel G, Roland I, Raes M, Remacle J, Michiels C. Hypoxia-induced activation of HIF-1: role of HIF-1alpha-Hsp90 interaction. FEBS Lett. 1999;460:251–256. doi: 10.1016/s0014-5793(99)01359-9. [DOI] [PubMed] [Google Scholar]
- 97.Liu YV, Baek JH, Zhang H, Diez R, Cole RN, Semenza GL. RACK1 competes with HSP90 for binding to HIF-1alpha and is required for O(2)-independent and HSP90 inhibitor-induced degradation of HIF-1alpha. Mol Cell. 2007;25:207–217. doi: 10.1016/j.molcel.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu YV, Semenza GL. RACK1 vs. HSP90: competition for HIF-1 alpha degradation vs. stabilization. Cell Cycle. 2007;6:656–659. doi: 10.4161/cc.6.6.3981. [DOI] [PubMed] [Google Scholar]
- 99.Baek JH, Liu YV, McDonald KR, Wesley JB, Zhang H, Semenza GL. Spermidine/spermine N(1)-acetyltransferase-1 binds to hypoxia-inducible factor-1alpha (HIF-1alpha) and RACK1 and promotes ubiquitination and degradation of HIF-1alpha. J Biol Chem. 2007;282:33358–33366. doi: 10.1074/jbc.M705627200. [DOI] [PubMed] [Google Scholar]
- 100.Amir S, Wang R, Simons JW, Mabjeesh NJ. SEPT9_v1 up-regulates hypoxia-inducible factor 1 by preventing its RACK1-mediated degradation. J Biol Chem. 2009;284:11142–11151. doi: 10.1074/jbc.M808348200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kong X, Lin Z, Liang D, Fath D, Sang N, Caro J. Histone deacetylase inhibitors induce VHL and ubiquitin-independent proteasomal degradation of hypoxia-inducible factor 1alpha. Mol Cell Biol. 2006;26:2019–2028. doi: 10.1128/MCB.26.6.2019-2028.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kong X, Alvarez-Castelao B, Lin Z, Castano JG, Caro J. Constitutive/hypoxic degradation of HIF-alpha proteins by the proteasome is independent of von Hippel Lindau protein ubiquitylation and the transactivation activity of the protein. J Biol Chem. 2007;282:15498–15505. doi: 10.1074/jbc.M700704200. [DOI] [PubMed] [Google Scholar]
- 103.Zhou J, Schmid T, Frank R, Brune B. PI3K/Akt is required for heat shock proteins to protect hypoxia-inducible factor 1alpha from pVHL-independent degradation. J Biol Chem. 2004;279:13506–13513. doi: 10.1074/jbc.M310164200. [DOI] [PubMed] [Google Scholar]
- 104.van de Sluis B, Groot AJ, Vermeulen J, van der Wall E, van Diest PJ, Wijmenga C, Klomp LW, Vooijs M. COMMD1 Promotes pVHL and O2-Independent Proteolysis of HIF-1alpha via HSP90/70. PLoS One. 2009;4:e7332. doi: 10.1371/journal.pone.0007332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Koh MY, Darnay BG, Powis G. Hypoxia-associated factor, a novel E3-ubiquitin ligase, binds and ubiquitinates hypoxia-inducible factor 1alpha, leading to its oxygen-independent degradation. Mol Cell Biol. 2008;28:7081–7095. doi: 10.1128/MCB.00773-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Koh MY, Powis G. HAF: the new player in oxygen-independent HIF-1alpha degradation. Cell Cycle. 2009;8:1359–1366. doi: 10.4161/cc.8.9.8303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ravi R, Mookerjee B, Bhujwalla ZM, Sutter CH, Artemov D, Zeng Q, Dillehay LE, Madan A, Semenza GL, Bedi A. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1alpha. Genes Dev. 2000;14:34–44. [PMC free article] [PubMed] [Google Scholar]
- 108.Nieminen AL, Qanungo S, Schneider EA, Jiang BH, Agani FH. Mdm2 and HIF-1alpha interaction in tumor cells during hypoxia. J Cell Physiol. 2005;204:364–369. doi: 10.1002/jcp.20406. [DOI] [PubMed] [Google Scholar]
- 109.Bae MK, Ahn MY, Jeong JW, Bae MH, Lee YM, Bae SK, Park JW, Kim KR, Kim KW. Jab1 interacts directly with HIF-1alpha and regulates its stability. J Biol Chem. 2002;277:9–12. doi: 10.1074/jbc.C100442200. [DOI] [PubMed] [Google Scholar]
- 110.Kang X, Li J, Zou Y, Yi J, Zhang H, Cao M, Yeh ET, Cheng J. PIASy stimulates HIF1alpha SUMOylation and negatively regulates HIF1alpha activity in response to hypoxia. Oncogene. 2010;29:5568–5578. doi: 10.1038/onc.2010.297. [DOI] [PubMed] [Google Scholar]
- 111.Carbia-Nagashima A, Gerez J, Perez-Castro C, Paez-Pereda M, Silberstein S, Stalla GK, Holsboer F, Arzt E. RSUME, a small RWD-containing protein, enhances SUMO conjugation and stabilizes HIF-1alpha during hypoxia. Cell. 2007;131:309–323. doi: 10.1016/j.cell.2007.07.044. [DOI] [PubMed] [Google Scholar]
- 112.Amir S, Wang R, Matzkin H, Simons JW, Mabjeesh NJ. MSF-A interacts with hypoxia-inducible factor-1alpha and augments hypoxia-inducible factor transcriptional activation to affect tumorigenicity and angiogenesis. Cancer Res. 2006;66:856–866. doi: 10.1158/0008-5472.CAN-05-2738. [DOI] [PubMed] [Google Scholar]
- 113.Cai Q, Lan K, Verma SC, Si H, Lin D, Robertson ES. Kaposi's sarcoma-associated herpesvirus latent protein LANA interacts with HIF-1 alpha to upregulate RTA expression during hypoxia: Latency control under low oxygen conditions. J Virol. 2006;80:7965–7975. doi: 10.1128/JVI.00689-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tandle AT, Calvani M, Uranchimeg B, Zahavi D, Melillo G, Libutti SK. Endothelial monocyte activating polypeptide-II modulates endothelial cell responses by degrading hypoxia-inducible factor-1alpha through interaction with PSMA7, a component of the proteasome. Exp Cell Res. 2009;315:1850–1859. doi: 10.1016/j.yexcr.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 115.van de Sluis B, Mao X, Zhai Y, Groot AJ, Vermeulen JF, van der Wall E, van Diest PJ, Hofker MH, Wijmenga C, Klomp LW, Cho KR, Fearon ER, Vooijs M, Burstein E. COMMD1 disrupts HIF-1alpha/beta dimerization and inhibits human tumor cell invasion. J Clin Invest. 2010;120:2119–2130. doi: 10.1172/JCI40583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fatyol K, Szalay AA. The p14ARF tumor suppressor protein facilitates nucleolar sequestration of hypoxia-inducible factor-1alpha (HIF-1alpha) and inhibits HIF-1-mediated transcription. J Biol Chem. 2001;276:28421–28429. doi: 10.1074/jbc.M102847200. [DOI] [PubMed] [Google Scholar]
- 117.Hagele S, Behnam B, Borter E, Wolfe J, Paasch U, Lukashev D, Sitkovsky M, Wenger RH, Katschinski DM. TSGA10 prevents nuclear localization of the hypoxia-inducible factor (HIF)-1alpha. FEBS Lett. 2006;580:3731–3738. doi: 10.1016/j.febslet.2006.05.058. [DOI] [PubMed] [Google Scholar]
- 118.Hogenesch JB, Gu YZ, Jain S, Bradfield CA. The basic-helix-loop-helix-PAS orphan MOP3 forms transcriptionally active complexes with circadian and hypoxia factors. Proc Natl Acad Sci USA. 1998;95:5474–5479. doi: 10.1073/pnas.95.10.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hogenesch JB, Gu YZ, Moran SM, Shimomura K, Radcliffe LA, Takahashi JS, Bradfield CA. The basic helix-loop-helix-PAS protein MOP9 is a brain-specific heterodimeric partner of circadian and hypoxia factors. J Neurosci. 2000;20:RC83. doi: 10.1523/JNEUROSCI.20-13-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Takahata S, Sogawa K, Kobayashi A, Ema M, Mimura J, Ozaki N, Fujii-Kuriyama Y. Transcriptionally active heterodimer formation of an Arnt-like PAS protein, Arnt3, with HIF-1a, HLF, and clock. Biochem Biophys Res Commun. 1998;248:789–794. doi: 10.1006/bbrc.1998.9012. [DOI] [PubMed] [Google Scholar]
- 121.Koyanagi S, Kuramoto Y, Nakagawa H, Aramaki H, Ohdo S, Soeda S, Shimeno H. A molecular mechanism regulating circadian expression of vascular endothelial growth factor in tumor cells. Cancer Res. 2003;63:7277–7283. [PubMed] [Google Scholar]
- 122.Chilov D, Hofer T, Bauer C, Wenger RH, Gassmann M. Hypoxia affects expression of circadian genes PER1 and CLOCK in mouse brain. FASEB J. 2001;15:2613–2622. doi: 10.1096/fj.01-0092com. [DOI] [PubMed] [Google Scholar]
- 123.Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002;16:1466–1471. doi: 10.1101/gad.991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mahon PC, Hirota K, Semenza GL. FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001;15:2675–2686. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Carrero P, Okamoto K, Coumailleau P, O'Brien S, Tanaka H, Poellinger L. Redox-regulated recruitment of the transcriptional coactivators CREB-binding protein and SRC-1 to hypoxia-inducible factor 1alpha. Mol Cell Biol. 2000;20:402–415. doi: 10.1128/mcb.20.1.402-415.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ema M, Hirota K, Mimura J, Abe H, Yodoi J, Sogawa K, Poellinger L, Fujii-Kuriyama Y. Molecular mechanisms of transcription activation by HLF and HIF1alpha in response to hypoxia: their stabilization and redox signal-induced interaction with CBP/p300. EMBO J. 1999;18:1905–1914. doi: 10.1093/emboj/18.7.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Semenza GL. Oxygen homeostasis. Wiley Interdiscip Rev Syst Biol Med. 2:336–361. doi: 10.1002/wsbm.69. [DOI] [PubMed] [Google Scholar]
- 128.Arany Z, Huang LE, Eckner R, Bhattacharya S, Jiang C, Goldberg MA, Bunn HF, Livingston DM. An essential role for p300/CBP in the cellular response to hypoxia. Proc Natl Acad Sci USA. 1996;93:12969–12973. doi: 10.1073/pnas.93.23.12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kato H, Tamamizu-Kato S, Shibasaki F. Histone deacetylase 7 associates with hypoxia-inducible factor 1alpha and increases transcriptional activity. J Biol Chem. 2004;279:41966–41974. doi: 10.1074/jbc.M406320200. [DOI] [PubMed] [Google Scholar]
- 130.Land SC, Tee AR. Hypoxia-inducible factor 1alpha is regulated by the mammalian target of rapamycin (mTOR) via an mTOR signaling motif. J Biol Chem. 2007;282:20534–20543. doi: 10.1074/jbc.M611782200. [DOI] [PubMed] [Google Scholar]
- 131.Ao A, Wang H, Kamarajugadda S, Lu J. Involvement of estrogen-related receptors in transcriptional response to hypoxia and growth of solid tumors. Proc Natl Acad Sci USA. 2008;105:7821–7826. doi: 10.1073/pnas.0711677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lim JH, Lee YM, Chun YS, Chen J, Kim JE, Park JW. Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1alpha. Mol Cell. 2010;38:864–878. doi: 10.1016/j.molcel.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 133.Moon HE, Ahn MY, Park JA, Min KJ, Kwon YW, Kim KW. Negative regulation of hypoxia inducible factor-1alpha by necdin. FEBS Lett. 2005;579:3797–3801. doi: 10.1016/j.febslet.2005.05.072. [DOI] [PubMed] [Google Scholar]
- 134.Budde A, Schneiderhan-Marra N, Petersen G, Brune B. Retinoblastoma susceptibility gene product pRB activates hypoxia-inducible factor-1 (HIF-1) Oncogene. 2005;24:1802–1808. doi: 10.1038/sj.onc.1208369. [DOI] [PubMed] [Google Scholar]
- 135.Kaidi A, Williams AC, Paraskeva C. Interaction between beta-catenin and HIF-1 promotes cellular adaptation to hypoxia. Nat Cell Biol. 2007;9:210–217. doi: 10.1038/ncb1534. [DOI] [PubMed] [Google Scholar]
- 136.Sanchez-Elsner T, Botella LM, Velasco B, Corbi A, Attisano L, Bernabeu C. Synergistic cooperation between hypoxia and transforming growth factor-beta pathways on human vascular endothelial growth factor gene expression. J Biol Chem. 2001;276:38527–38535. doi: 10.1074/jbc.M104536200. [DOI] [PubMed] [Google Scholar]
- 137.Koshiji M, Kageyama Y, Pete EA, Horikawa I, Barrett JC, Huang LE. HIF-1alpha induces cell cycle arrest by functionally counteracting Myc. EMBO J. 2004;23:1949–1956. doi: 10.1038/sj.emboj.7600196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tendler DS, Bao C, Wang T, Huang EL, Ratovitski EA, Pardoll DA, Lowenstein CJ. Intersection of interferon and hypoxia signal transduction pathways in nitric oxide-induced tumor apoptosis. Cancer Res. 2001;61:3682–3688. [PubMed] [Google Scholar]
- 139.Koshiji M, To KK, Hammer S, Kumamoto K, Harris AL, Modrich P, Huang LE. HIF-1alpha induces genetic instability by transcriptionally downregulating MutSalpha expression. Mol Cell. 2005;17:793–803. doi: 10.1016/j.molcel.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 140.Zhang W, Tsuchiya T, Yasukochi Y. Transitional change in interaction between HIF-1 and HNF-4 in response to hypoxia. J Hum Genet. 1999;44:293–299. doi: 10.1007/s100380050163. [DOI] [PubMed] [Google Scholar]
- 141.Peng ZG, Zhou MY, Huang Y, Qiu JH, Wang LS, Liao SH, Dong S, Chen GQ. Physical and functional interaction of Runt-related protein 1 with hypoxia-inducible factor-1alpha. Oncogene. 2008;27:839–847. doi: 10.1038/sj.onc.1210676. [DOI] [PubMed] [Google Scholar]
- 142.Yang L, Jiang Y, Wu SF, Zhou MY, Wu YL, Chen GQ. CCAAT/enhancer-binding protein alpha antagonizes transcriptional activity of hypoxia-inducible factor 1 alpha with direct protein-protein interaction. Carcinogenesis. 2008;29:291–298. doi: 10.1093/carcin/bgm262. [DOI] [PubMed] [Google Scholar]
- 143.van de Sluis B, Mao X, Zhai Y, Groot AJ, Vermeulen JF, van der Wall E, van Diest PJ, Hofker MH, Wijmenga C, Klomp LW, Cho KR, Fearon ER, Vooijs M, Burstein E. COMMD1 disrupts HIF-1alpha/beta dimerization and inhibits human tumor cell invasion. J Clin Invest. 2010;120:2119–2130. doi: 10.1172/JCI40583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lee YM, Lim JH, Chun YS, Moon HE, Lee MK, Huang LE, Park JW. Nutlin-3, an Hdm2 antagonist, inhibits tumor adaptation to hypoxia by stimulating the FIH-mediated inactivation of HIF-1alpha. Carcinogenesis. 2009;30:1768–1775. doi: 10.1093/carcin/bgp196. [DOI] [PubMed] [Google Scholar]


