Abstract
Objective
This study examines clinical predictors of symptom deterioration (relapse/recurrence) at the completion of a clinical intervention trial of depressed, low-income, predominantly Hispanic diabetes patients who were randomized to socio-culturally adapted collaborative depression treatment or usual care and no longer met clinically significant depression criteria at 12 months post-trial baseline.
Methods
A sub-cohort of 193 diabetes patients with major depression symptoms at baseline, that were randomized to a 12-month collaborative care intervention (INT) (Problem Solving Therapy and/or pharmacotherapy, telephone symptom monitoring/relapse prevention, behavioral activation and patient navigation support) or enhanced usual care (EUC), and who did not meet major depression criteria at 12 months were subsequently observed over 18 to 24 months.
Results
Post-trial depression symptom deterioration was similar between INT (35.2%) and EUC (35.3%) groups. Among the combined groups, significant predictors of symptom deterioration were baseline history of previous depression and/or dysthymia (odds ratio [OR] =2.66), 12-month PHQ-9 score (OR=1.22), antidepressant treatment receipt during the initial 12-months (OR=2.38), 12-month diabetes symptoms (OR=2.27) and new ICD-9 medical diagnoses in the initial 12 months (OR=1.11) (R2=27%; Max-rescaled R2=37%; Likelihood ratio test, chi-sq=59.79, df=5, p<.0001).
Conclusions
Among predominantly Hispanic diabetes patients in community safety net primary care clinics whose depression had improved over 1 year, more than one third experienced symptom deterioration over the following year. A primary care management depression care protocol that includes ongoing depression symptom monitoring, antidepressant adherence, and diabetes and co-morbid illness monitoring plus depression medication adjustment and behavioral activation may reduce and/or effectively treat depression symptom deterioration.
Keywords: Depression Recurrence and Relapse, Depression Care Disparities, Depression Symptom Monitoring, Depression and Diabetes, Depression in Hispanics
INTRODUCTION
Patients with depression often experience symptom deterioration (i.e., symptom relapse or recurrence back to clinically significant depression), particularly in the presence of medical or psychiatric comorbid illness.1–2 Patients with diabetes are at notably high risk for comorbid depression compared to the general population3 and up to 80% of patients with diabetes and depression will experience a relapse of depressive symptoms over a 5-year period.4–6 Major depression is associated with an increased risk of clinically significant microvascular and macrovascular complications among people with type 2 diabetes which may worsen the course of depression.7 While there is a recently growing body of research on post acute depression treatment relapse and recurrence8–9, much remains to be learned about causal mechanisms and potential effective treatments.. The risk of depression symptom deterioration over time underscores a need for ongoing depression symptom and treatment adherence monitoring, and when indicated, provision of self-management motivation and behavioral activation support, and/or adjustments in antidepressant treatment over time.10–12
Disparities in depression treatment and outcomes among low-income and racially/ethnically diverse populations13–14 present additional challenges to safety net organizations and primary care providers. For example, Hispanics not only have a higher prevalence of diabetes compared to non-Hispanic whites,15 but also have high comorbid depression rates,3 and greater risk of cardiovascular illness, functional disability, and mortality.16 While Hispanics predominantly seek depression treatment from primary care, only 36% of Hispanics with depression receive treatment compared to 60% among non-Hispanic whites.17 A recent study examining the course of depression over 30 months among Latino primary care patients meeting Patient Health Questionnaire-2 depression criteria found that 37% of patients, whose depression had improved at 25 months post study enrollment, experienced a relapse or recurrence of symptoms at 30 months.18
In the Multifaceted Diabetes and Depression Program (MDDP) clinical trial, 387 low-income, predominantly Hispanic diabetes patients with clinically significant depression were randomly assigned to a 12-month primary care depression intervention versus enhanced usual care.19–20 Compared to usual primary care, the MDDP culturally adapted21–22 intervention significantly improved receipt of depression care (84% vs. 32%, p<.001), depression symptoms (≥50% reduction in Patient Health Questionnaire-9 (p<.001) and SCL Symptom Checklist depression scores from baseline (p=.001)) and quality of life over a two-year study period and was found to be cost effective.23 However, group differences narrowed at 18 and 24 months following the 12 month trial.19 In this report, a subset cohort of 193 MDDP patients who no longer met PHQ-9 criteria for major depression at 12 months was observed at 18 and 24 months post-enrollment to examine three key questions: a) What is the incidence of clinically significant symptom deterioration? b) Will symptom deterioration vary by clinical trial group? and c) What demographic and clinical factors predict symptom deterioration? In addition, potential implications of the study findings applicable to developing post-acute depression care management protocols for primary care are discussed.
METHODS
The MDDP randomized trial, approved by the University of Southern California Institutional Review Board, was conducted in Los Angeles County Department of Health Services community clinics. Patients with diabetes, identified from medical charts, provided verbal consent to depression screening by bilingual study recruiters between August 2005 and August 2007; 387 study participants provided written informed study consent and completed a structured baseline interview prior to computer-driven randomization to MDDP collaborative care (INT) or modestly enhanced usual care (EUC). Eligible patients were ≥18 years, English or Spanish speaking, and endorsed one of the 2 cardinal depression symptoms for more than half the days to nearly every day over the last two weeks and scored ≥10 on the PHQ-9 indicating a high likelihood of clinically significant depression. Patients with acute suicidal ideation, excessive current alcohol use, or recent lithium/antipsychotic medication use were ineligible for the trial.
MDDP is based on evidence-based practice guidelines for primary care and is designed to reduce known patient and provider barriers to depression care in the study community public safety net clinics.19–21 The 12-month, team delivered, intervention (INT) included: 1) a choice of starting treatment with either antidepressant medications (AMs) prescribed by the treating primary care physicians (PCPs) (who provided care to both study groups and attended a psychiatrist didactic on the algorithm and depression treatment) or Problem-Solving Therapy (PST) (if initially preferred by patients over AM) provided by bilingual graduate social work diabetes depression clinical specialists (DDCS); 2) application of a stepped care treatment algorithm; 3) DDCS monthly telephone follow-up on symptom monitoring, treatment maintenance, relapse prevention, behavioral activation, and additional booster in-person or telephone PST sessions if indicated; 4) care and service system navigation by the DDCS and/or an assistant patient navigator. A study psychiatrist and the study principal investigator provided weekly telephone DDCS supervision and, if requested, the psychiatrist provided PCP antidepressant medication telephone consultation. During the intervention year, 84% of INT patients received depression treatment (49 PST, 9 AM, 104 both) and 35 (23%) PST-graduated patients also attended ≥1 optional open-ended PST monthly support group sessions. Over 12 months, a dosage increase or change in type of medication was provided to 63 (56%) patients prescribed AM. During the intervention year, 63% of INT patients with baseline moderately severe to severe depression symptoms (i.e., PHQ-9 score ≥15) and 44% of INT with moderate depression symptoms (i.e., PHQ-9 score 10–14) received both PST and AM (chi-sq=6.94, df=1, p=0.01).
EUC patients were informed of their depression and were given patient and family focused depression educational pamphlets (Spanish or English) and a community mental health, financial, social services, transportation, and child care resource list. EUC PCPs were informed of patient PHQ-9 scores and study participation and could prescribe antidepressant medications, provide counseling or refer patients to community mental health care. During the initial year, 32.5% of EUC patients received depression treatment (37 AM, 11 self-reported counseling, 15 both), while 10 (19%) patients prescribed AM had a dosage increment or change in antidepressant.
Of 387 patients enrolled at baseline, 24 month attrition totaled 123 (31.8%). Of these, 48 (12.4%) declined continuing study participation, 29 (7.5%) were no longer receiving care at the clinic, 9 (2.3%) no longer living in the US, 3 (<1%) were deceased, and 34 (8.8%) could not be located. Although average HbA1C values did not differ between attrition and retention groups (9.38% vs. 8.73%, P=0.20), relatively more attrition patients had an HbA1C greater than 7% compared to retention patients (56.9% vs. 43.1%, P=0.02). All other baseline depression, clinical and demographic characteristics were similar between attrition and retention groups. In this report, major depressive disorder is defined as: 1) at least one of the 2 cardinal depression symptoms more than half the days to nearly every day over the past 2 weeks, and 2) having PHQ-9 scores ≥10. Symptom deterioration over a one year post-intervention period was defined as meeting major depression criteria at 18 or 24 months among those who did not meet PHQ-9 criteria for major depression at 12-month follow-up. Standard definitions have defined relapse as occurring prior to 6 months after reaching symptom remission and recurrence as occurring ≥6 months after symptom remission. However in most depression treatment trials only a minority of patients meet remission criteria at study completion, with the majority having residual symptoms. Thus we use the term “symptom deterioration” in this article.
Of 193 patients in the study, 158 (82%) had both 18- and 24-month follow-up data, 24 (12%) had only 18-month, and 11 (6%) had only 24-month follow-up. If participants completed only one follow-up interview post 12-month, the available follow-up data were applied.
Data Collection
We examined at three predictors of interest, i.e., baseline demographics and measures of clinical severity, 12-month characteristics, and worsening clinical status between baseline and 12 month follow-up. Demographic data on age, gender, marital status, ethnic group, birthplace, time living in the US, language, education, and employment were collected at baseline interview. Follow-up telephone outcome interviews at 12, 18, 24 months were conducted by bilingual interviewers blinded to treatment assignment. The 20-item Symptom Checklist (SCL) depression scale24 was used as a reliable and valid measure of depression in medical populations that has been shown to be sensitive to change in primary care studies (Cronbach α =0.91). The Patient Health Questionnaire-9 (PHQ-9) was used because it provides both a dichotomous diagnosis of major depression,25 a continuous severity score and has been found to have 73% sensitivity and 98% specificity to a diagnosis of probable major depressive disorder (MDD) based on structured psychiatric interview.26 The Brief Symptom Inventory (BSI)27 assessed anxiety; the Sheehan Disability Scale (SDS) assessed functional impairment.28 Health-related quality of life (QOL) was assessed using the MOS Short-Form Health Survey (SF-12).29 Chronic pain was defined as pain present most of the time for 6 months or more during the past year. The Summary of Diabetes Self-Care Activities Questionnaire assessed self-reported adherence;30 the Whitty 9-item questionnaire assessed diabetes symptoms.31 Glycated hemoglobin (HbA1c) was obtained from medical records (the last test done within 3 months of each follow up interview). Patient self-report of socioeconomic stressors (financial, work, family, legal problems, and community violence worry) were also obtained during each outcome assessment. The dichotomized clinical status changes, worsening versus not worsening, were identified by comparing the clinical outcomes at 12 month follow-up to their baseline values, such as decreasing functional status or increasing diabetes symptoms indicating a worsening status. International Classification of Diseases (ICD-9) codes between 6 months prior to baseline to 12 months post baseline were obtained from electronic medical records in order to identify new medical diagnoses over initial 12 months.
Analyses
Because the intervention ended at 12 months, this Year 2 sample is no longer an intent-to-treat population. We conducted bivariate analyses to compare baseline demographic characteristics, 12-month clinical characteristics, and changes in health status in the initial 12 months such as increasing diabetes symptoms or new ICD-9 diagnoses (from baseline to 12 month follow-up) between patients with and without depressive symptom deterioration with t-test for continuous data and chi-square test for dichotomous data. Variables that were significantly associated with symptom deterioration were jointly entered into a logistic regression model with stepwise selection to identify predictors. To assess whether predictors were affected by randomization status, we also examined randomization group and the interactions between randomization group and each predictor. Randomization group was not a significant covariate, and there were no significant interactions between randomization group and these predictors. Therefore we combined data of subjects from both intervention and control groups for the analysis. All analyses were conducted at 0.05 significance level (2-tailed) using SAS version 9.1 (SAS Institute Inc, Cary, NC).
RESULTS
Sample
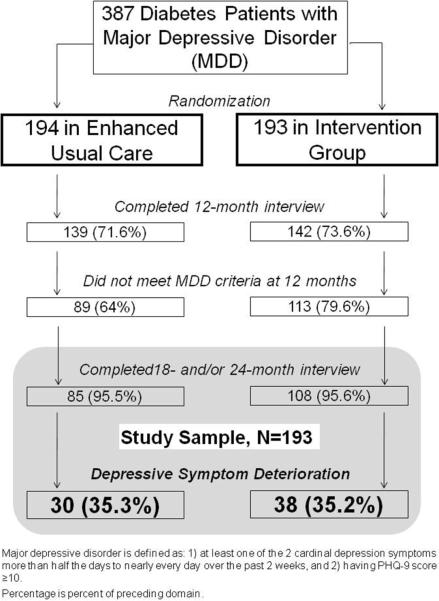
The depressive symptom deterioration study cohort includes 193 MDDP patients (108 INT, 85 EUC) who did not meet criteria for major depressive disorder based on the PHQ-9 at 12 months post-baseline and had completed a follow-up outcome interview at 18 and/or 24 months. (Figure 1) These included 158 (82%) patients who had both 18- and 24-month follow-up data, and 24 (12%) only 18-month, and 11 (6%) only 24-month follow-up. During the initial year, 94% of intervention patients received depression treatment versus 35% EUC. Due to the effectiveness of the MDDP collaborative care intervention previously reported,20 more INT patients met the inclusion criteria of the current study. To assess possible differences between INT and EUC patients, we compared baseline characteristics, 12-month clinical, functional and socioeconomic variables, and worsening clinical status from baseline to 12 months. At baseline, significantly more INT patients reported a history of previous major depression or dysthymia compared to EUC patients (62% v 47%, chi-sq=4.32, df=1, p=0.04); no other significant group differences were found in baseline demographic, 12-month clinical characteristics, or clinical status change over baseline to 12 months.
Figure 1.
Sample Selection
Depressive Symptom Deterioration
The rate of depressive symptom deterioration over the 1-year post study period was 35% in both study groups. Randomization group was not a significant covariate, and there were no significant interactions between randomization group and potential predictors, thus intervention and control patients were combined to examine predictors of symptom deterioration. Table 1 reports bivariate baseline demographics, 12-month clinical outcomes, and health status changes over initial12 months between patients with and without symptom deterioration. Patients with symptom deterioration were significantly more educated, more likely to have reported baseline history of previous major depression or dysthymia, and chronic pain, had significantly higher 12-month depression symptom (SCL-20, PHQ-9) and anxiety scores, and a greater number of self-reported socioeconomic stressors. Over baseline to 12 months, symptom deterioration patients were also more likely to have greater functional impairment, new ICD-9 medical conditions, more diabetes symptoms and complications. Intervention patients with symptom deterioration were also more likely to have received antidepressants plus PST over the 12 months after baseline. The length of treatment received during the one-year trial did not vary between deterioration and non-deterioration patients (length of time on AM: 8.16±4.03 vs. 7.69±4.69 months, p=0.6; PST: 8.71±6.29 vs. 8.21±5.33 sessions, p=0.67).
Table 1.
Baseline, 12 Month Characteristics, and Health Status Changes Over Initial 12 Months of Patients With and Without Depressive Symptom Deterioration
| Deterioration, n=68 | Non-deterioration, n=125 | Test value | df | P | |
|---|---|---|---|---|---|
| Baseline Characteristics | |||||
| Female | 59 (86.8) | 106 (84.8) | 0.14 | 1 | 0.71 |
| Hispanic | 65 (95.6) | 124 (99.2) | 2.83 | 1 | 0.09 |
| Age | 53.34±9.70 | 54.51±8.69 | −0.86 | 191 | 0.39 |
| Foreign born | 62 (91.2) | 119 (95.2) | 1.22 | 1 | 0.27 |
| Living in the US ≥10 years | 63 (92.6) | 110 (88.0) | 1.02 | 1 | 0.31 |
| Primary language - Spanish | 57 (83.8) | 113 (90.4) | 1.81 | 1 | 0.18 |
| Less than high school education | 52 (76.5) | 113 (90.4) | 6.89 | 1 | 0.01 |
| Unemployed | 49 (72.1) | 101 (80.8) | 1.94 | 1 | 0.16 |
| Married | 35 (51.5) | 69 (55.2) | 0.25 | 1 | 0.62 |
| History of previous major depression or dysthymia | 52 (76.5) | 55 (44.0) | 18.80 | 1 | <.0001 |
| Chronic Pain | 26 (38.2) | 29 (23.2) | 4.89 | 1 | 0.03 |
| 12-month Clinical Characteristics | |||||
| Depression SCL-20 score | 0.83±0.52 | 0.46±0.38 | 5.27 | 106.3 | <.0001 |
| Depression PHQ-9 score | 6.76±3.54 | 3.82±3.02 | 6.08 | 191 | <.0001 |
| Received antidepressant during previous 12 months | 44 (64.7) | 44 (35.2) | 15.46 | 1 | 0.0001 |
| Received PST/self-reported counseling during previous 12 months | 42 (61.8) | 72 (57.6) | 0.32 | 1 | 0.57 |
| Received both antidepressant and PST/counseling during previous 12 months | 34 (50.0) | 36 (28.8) | 8.56 | 1 | 0.003 |
| SF-12 physical function | 39.75±12.02 | 43.06±10.78 | −1.96 | 191 | 0.05 |
| SDS functional impairment | 3.42±3.08 | 1.63±2.35 | 4.17 | 110.2 | 0.0001 |
| Anxiety BSI score | 3.16±2.85 | 1.54±2.48 | 4.13 | 191 | 0.0001 |
| Number of socioeconomic stressorsa | 2.25±1.59 | 1.59±1.83 | 2.50 | 191 | 0.01 |
| Diabetes symptoms | 1.73±0.49 | 1.44±0.45 | 4.18 | 191 | <.0001 |
| Diabetes complications | 1.38±1.09 | 0.96±0.87 | 2.75 | 113.3 | 0.01 |
| Diabetes selfcare management | 3.21±1.12 | 3.53±1.39 | −1.61 | 191 | 0.11 |
| Worsened Status Over Initial 12 | |||||
| Months | |||||
| Worsened SF-12 physical function | 39 (57.4) | 61 (48.8) | 1.29 | 1 | 0.26 |
| Worsened SF-pain interference | 23 (33.8) | 48 (38.4) | 0.40 | 1 | 0.53 |
| Worsened SDS functional impairment | 36 (52.9) | 42 (33.6) | 6.84 | 1 | 0.01 |
| Number of new ICD-9 diagnoses | 7.22±4.41 | 5.57±3.53 | 2.66 | 114.4 | 0.01 |
| Increased number of socioeconomic stressors | 38 (55.9) | 38 (30.4) | 11.98 | 1 | 0.001 |
| Increased diabetes symptoms | 42 (61.8) | 50 (40.0) | 8.36 | 1 | 0.004 |
| Increased diabetes complications | 35 (51.5) | 41 (32.8) | 6.43 | 1 | 0.01 |
| Less diabetes selfcare management | 34 (50.0) | 54 (43.2) | 0.82 | 1 | 0.36 |
| Increased HbA1c levelb | 35 (58.3) | 64 (61.5) | 0.16 | 1 | 0.69 |
| Increased BMI | 40 (58.8) | 57 (45.6) | 3.08 | 1 | 0.08 |
Data are mean±SD or frequency (%); T-test for continuous data; χ2 test for dichotomous data. SCL-20: 20-item Symptom Checklist; PHQ-9: Patient Health Questionnaire-9; PST: Problem-Solving Therapy; SF-12: Short-Form Health Survey; SDS: Sheehan Disability Scale; BSI: Brief Symptom Inventory; ICD-9: International Classification of Diseases, Ninth Revision; HbA1c: Glycated Hemoglobin; BMI: Body Mass Index.
Work, unemployment, financial problems, marital/family conflicts, child/caregiving problems, cultural conflicts, legal or immigration problems, serious illness or death of family member, community violence or crime.
29 patients were excluded due to no HbA1c test done (8 patients with symptom deterioration, 21 nondeterioration).
Variables that were significantly associated with symptom deterioration in the univariate models in Table 1 were jointly entered into a logistic regression model with stepwise selection to identify predictors of odds of having symptom deterioration. History of previous depression and/or dysthymia reported at the trial baseline, receipt of antidepressant treatment during the 12 months post-baseline, the 12-month PHQ-9 score, frequency of diabetes symptoms at 12 months, and increasing ICD-9 diagnoses over the initial 12 months were significant predictors of greater odds of having depressive symptom deterioration jointly in the final model (R2=27%; Max-rescaled R2=37%; Likelihood ratio test, chi-sq=59.79, df=5, p<.0001). (Table 2)
Table 2.
Predictors of Depressive Symptom Deterioration at 18 and/or 24 Months
| Variable | Beta Estimate | OR (95% CI) | Wald Chi-Square [df=1] | P |
|---|---|---|---|---|
| Baseline history of previous major depression or dysthymia | 0.98 | 2.66 (1.25 – 5.65) | 6.48 | 0.01 |
| PHQ-9 score at 12 months | 0.20 | 1.22 (1.08 – 1.37) | 10.92 | 0.001 |
| Received antidepressant during initial 12 months | 0.87 | 2.38 (1.17 – 4.86) | 5.70 | 0.02 |
| Frequency of diabetes symptoms at 12 months | 2.27 (1.08 – 4.77) | 4.72 | 0.03 | |
| Number of new ICD-9 diagnoses over initial 12 months | 0.11 | 1.11 (1.01 – 1.22) | 5.18 | 0.02 |
PHQ-9: Patient Health Questionnaire-9; ICD-9: International Classification of Diseases, Ninth Revision Logistic Regression Model R2=27%; Max-rescaled R2=37%; Likelihood ratio test, chi-square=59.79, df=5, p<.0001
DISCUSSION
Depression symptom deterioration in this population of low-income, predominantly Hispanic patients with diabetes did not vary by randomized study group. Depression symptom deterioration was significantly associated with trial baseline reported history of previous depression and dysthymia as well as 12-month PHQ-9 scores. In addition, increasing diabetes symptoms and ICD-9 diagnoses in the initial 12-month period as well as having received antidepressant therapy in the post-baseline 12-month period was significantly associated with symptom deterioration over 18–24 months post trial.
In this study, the vast majority of intervention patients received both psychotherapy and antidepressants. Most intervention patients received antidepressants as part of stepped care, with the majority of patients choosing Problem Solving Therapy as the first step in the process of care. Patients with persistent symptoms were encouraged by their social worker PST therapist and their primary care physician to augment PST with an antidepressant medication and were encouraged to consider depression as a medical condition that affects a person's mood, thoughts, behavior and body. In epidemiologic terms, this is called a likely “severity confounder”, namely receipt of antidepressants is confounded by depression severity and persistence of symptoms. The receipt of antidepressants might also be viewed as a proxy for severe depression or for psychotherapy-refractory depression.
The finding that an increase in diabetes symptoms and in new ICD-9 diagnoses predicted 24 month depression symptom deterioration suggests that worsening overall physical health status predicts worsening depression status. This is consistent with evidence of bidirectional effects of depression and diabetes32 and recent evidence that among diabetics, incident cardiovascular procedures over a five year follow-up predicted depression at five years.33
Study results in this low-income, minority population further underscore a need to consider factors that may contribute to symptom deterioration, such as new or increasing socioeconomic stress, barriers to care access, patient discomfort with AM treatment, new medical co-morbid illness, and depression stigma19 as well as general deterioration in psychosocial functioning.34 Results from our qualitative study of Hispanic patients underscore these issues as depression was perceived as a serious condition linked to the accumulation of social stressors, while concerns about depression treatments included fears about the addictive and harmful properties of antidepressants, worries about taking too many pills, and the stigma attached to taking psychotropic medications.
The fact that intervention patients did not fare better with respect to the rate of symptom deterioration is likely attributable to post-trial barriers to community care system sustainability. For example, the clinics did not have staff social workers or a consulting psychiatrist to assist the primary care physicians. However, in part based on the MDDP study as well as the growing body of research on primary adoption of a major role in depression management within the Los Angeles County Department of Health Services (the second largest public care system in the U.S.), efforts are underway to enhance the sustainability of depression care management for diabetes patients. In addition, a current clinical sustainability study is testing the effectiveness and cost implications of using technologies such as automated speech recognition depression symptom monitoring calls (in English and Spanish) and the application of an online registry to facilitate collaborative provider communication and has begun hiring social workers to provide therapy when indicated. In addition, the similarity in rates of symptom deterioration between Intervention and EUC groups in the MDDP study underscore a need for effective relapse prevention interventions.35 However, sustaining mental health care within primary safety net care systems,36 may also have to consider ways to reduce cost of these services such as utilizing automated telephone monitoring calls or other technology applications.
Potential study limitations include that a relatively high rate of antidepressant medication prescription in controls may, in part, be attributable to the enhanced usual care design in which all patients were screened by study recruiters and clinic physicians were notified of patients meeting study depression criteria and all primary care physicians participated in the didactic depression sessions and were given copies of the study stepped-care algorithm. Acutely suicidal patients were excluded at baseline and referred for further clinical evaluation and treatment. The same primary care practitioners treated both study group patients raising the potential of a spillover effect on quality of depression treatment. EUC patients may have also benefited from receipt of the linguistically and idiomatically adapted patient and family educational pamphlets.
CONCLUSION
Primary safety net mental health care, with efforts to reduce depression symptom deterioration, will likely require reorganization to sustain the benefits of culturally sensitive collaborative depression care. Such reorganization will likely need to include integrated team care routine symptom and treatment adherence monitoring, patient motivational and behavioral activation, as well as patient-centered navigation assistance to reduce practical barriers to continuation of care. The overarching aim is not only to sustain depression improvement and reduce potentially negative health consequences among diabetes patients, but to reduce disparities in access to care.
Acknowledgments
The study is supported by R01 MH068468 from the National Institute of Mental Health (PI, Dr. Ell) and K-24 MH069741 (Katon) From the National Institute of Mental Health.
Trial Registration: NCT00709150, clinicaltrials.gov/ct/gui
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article. Dr. Kapetanovic contributed to this article in his personal capacity. The views expressed are his own and do not necessarily represent the views of the National Institutes of Health or United States Government.
We appreciate the contributions of Dr. Michael Roybal and Dr. Stanley Leong study clinic medical directors, their staff and participating patients.
References
- 1.Burcusa SL, Iacono WG. Risk for recurrence in depression. Clin Psychol Rev. 2007;27(8):959–985. doi: 10.1016/j.cpr.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richards D. Prevalence and clinical course of depression: A review. Clin Psychol Rev. 2011;31(7):1117–1125. doi: 10.1016/j.cpr.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Li C, Ford ES, Strine TW, Mokdad AH. Prevalence of depression among U.S. adults with diabetes: findings from the 2006 behavioral risk factor surveillance system. Diabetes Care. 2008;31(1):105–107. doi: 10.2337/dc07-1154. [DOI] [PubMed] [Google Scholar]
- 4.Lustman P, Griffith LS, Freedland KE, Clouse RE. The course of major depression in diabetes. Gen Hosp Psychiatry. 1997;19(2):138–143. doi: 10.1016/s0163-8343(96)00170-3. [DOI] [PubMed] [Google Scholar]
- 5.Katon W, Rutter C, Ludman EJ, Von Korff M, Lin E, Simon G, et al. A Randomized trial of relapse prevention of depression in primary care. Arch Gen Psychiatry. 2001;58(3):241–247. doi: 10.1001/archpsyc.58.3.241. [DOI] [PubMed] [Google Scholar]
- 6.Katon WJ. The comorbidity of diabetes mellitus and depression. Am J Med. 2008;121(11 Suppl 2):S8–15. doi: 10.1016/j.amjmed.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin EHB, Rutter CR, Katon W, Heckbert SR, Ciechanowski P, Oliver MM, et al. Depression and advanced complications of diabetes: A prospective cohort study. Diabetes Care. 2010;33(2):264–269. doi: 10.2337/dc09-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beshai S, Dobson KS, Bockting CL, Quigley L. Relapse and recurrence prevention in depression: current research and future prospects. Clin Psychol Rev. 2011;31(8):1349–1360. doi: 10.1016/j.cpr.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Monroe SM, Harkness KL. Recurrence in major depression: a conceptual analysis. Psychol Rev. 2011;118(4):655–674. doi: 10.1037/a0025190. [DOI] [PubMed] [Google Scholar]
- 10.Riihimäki KA, Vuorilehto MS, Melartin TK, Isometsä ET. Five-year outcome of major depressive disorder in primary health care. Psychol Med. 2011 doi: 10.1017/S0033291711002303. doi.10.1017/S0033291711002303. [DOI] [PubMed] [Google Scholar]
- 11.Culpepper L. Why do you need to move beyond first-line therapy for major depression? J Clin Psychiatry. 2010;71(Suppl 1):4–9. doi: 10.4088/JCP.9104su1c.01. [DOI] [PubMed] [Google Scholar]
- 12.Segal ZV, Bieling P, Young T, MacQueen G, Cooke R, Martin L, et al. Antidepressant monotherapy vs sequential pharmacotherapy and mindfulness-based cognitive therapy, or placebo, for relapse prophylaxis in recurrent depression. Arch Gen Psychiatry. 2010;67(12):1256–1264. doi: 10.1001/archgenpsychiatry.2010.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bao Y, Alexopoulos GS, Casalino LP, Ten Have TR, Donohue JM, Post EP, et al. Collaborative depression care management and disparities in depression treatment and outcomes. Arch Gen Psychiatry. 2011;68(6):627–636. doi: 10.1001/archgenpsychiatry.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osborn CY, Trott HW, Buchowski MS, Patel KA, Kirby LD, Hargreaves MK, et al. Racial disparities in the treatment of depression in low-income persons with diabetes. Diabetes Care. 2010;33(5):1050–1054. doi: 10.2337/dc09-1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lanting LC, Joung IM, Mackenbach JP, Lamberts SW, Bootsma AH. Ethnic differences in mortality, end-stage complications, and quality of care among diabetic patients. Diabetes Care. 2005;28(9):2280–2288. doi: 10.2337/diacare.28.9.2280. [DOI] [PubMed] [Google Scholar]
- 16.Gross R, Olfson M, Gameroff MJ, Carasquillo O, Shea S, Feder A, et al. Depression and glycemic control in Hispanic primary care patients with diabetes. J Gen Intern Med. 2005;20(5):460–466. doi: 10.1111/j.1525-1497.2005.30003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alegría M, Chatterji P, Wells K, Cao Z, Chen CN, Takeuchi D, et al. Disparity in depression treatment among racial and ethnic minority populations in the United States. Psychiatr Serv. 2008;59(11):1264–1272. doi: 10.1176/appi.ps.59.11.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Interian A, Ang A, Gara MA, Rodriguez MA, Vega WA. The long-term trajectory of depression among Latinos in primary care and its relationship to depression care disparities. Gen Hosp Psych. 2011;33(2):94–101. doi: 10.1016/j.genhosppsych.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ell K, Katon W, Xie B, Lee PJ, Kapetanovic S, Guterman J, et al. One year post collaborative depression care trial outcomes among predominantly Hispanic diabetes safety net patients. Gen Hosp Psychiatry. 2011;33(5):436–442. doi: 10.1016/j.genhosppsych.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ell K, Katon W, Xie B, Lee PJ, Kapetanovic S, Guterman J, et al. Collaborative care management of major depression among low-income, predominantly Hispanics with diabetes: a randomized controlled trial. Diabetes Care. 2010;33(4):706–713. doi: 10.2337/dc09-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ell K, Katon W, Cabassa LJ, Xie B, Lee PJ, Kapetanovic S, et al. Depression and diabetes among low-income Hispanics: design elements of a socio-culturally adapted collaborative care model randomized controlled trial. International J Psychiatry Med. 2009;39(2):113–132. doi: 10.2190/PM.39.2.a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cabassa LJ, Hansen MC, Palinkas LA, Ell K. Azúcar y Nervios: explanatory models and treatment experiences of Hispanics with diabetes and depression. Soc Sci Med. 2008;66(12):2413–2414. doi: 10.1016/j.socscimed.2008.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hay JW, Katon WJ, Ell K, Lee PJ, Guterman J. Cost effectiveness analyses of collaborative care management of major depression among low-income, predominantly Hispanics with diabetes. Value in Health. doi: 10.1016/j.jval.2011.09.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Derogatis LR, Lipman RS, Rickels K, Uhlenhuth EH, Covi L. The Hopkins Symptom Checklist (HSCL): a measure of primary symptom dimensions. Mod Probl Pharmacopsychiatry. 1974;7(0):79–110. doi: 10.1159/000395070. [DOI] [PubMed] [Google Scholar]
- 25.Wittkampf KA, Naeije L, Schene AH, Huyser J, van Weert HC. Diagnostic accuracy of the mood module of the Patient Health Questionnaire: a systematic review. Gen Hosp Psychiatry. 2007;29(5):388–395. doi: 10.1016/j.genhosppsych.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Huang FY, Chung H, Kroenke K, Delucchi KL, Spitzer RL. Using the Patient Health Questionnaire-9 to measure depression among racially and ethnically diverse primary care patients. J Gen Intern Med. 2006;21(6):547–552. doi: 10.1111/j.1525-1497.2006.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med. 1983;13(3):595–605. [PubMed] [Google Scholar]
- 28.Sullivan M, Katon W, Russo J, Dobie R, Sakai C. A randomized trial of nortriptyline for severe chronic tinnitus: effects on depression, disability, and tinnitus symptoms. Arch Intern Med. 1993;153(19):2251–2259. [PubMed] [Google Scholar]
- 29.Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care. 2004;42(9):851–859. doi: 10.1097/01.mlr.0000135827.18610.0d. [DOI] [PubMed] [Google Scholar]
- 30.Toobert DJ, Hampson SE, Russell E. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care. 2000;23(7):943–950. doi: 10.2337/diacare.23.7.943. [DOI] [PubMed] [Google Scholar]
- 31.Whitty P, Steen N, Eccles M, McColl E, Hewison J, Meadows K, et al. A new self-completion outcome measure for diabetes: Is it responsive to change? Qual Life Res. 1997;6(5):407–413. doi: 10.1023/a:1018443628933. [DOI] [PubMed] [Google Scholar]
- 32.Golden SH, Lazo M, Carnethon M, Bertoni AG, Schreiner PJ, Diez Roux AV, et al. Examining a bidirectional association between depressive symptoms and diabetes. JAMA. 2008;299(23):2751–2750. doi: 10.1001/jama.299.23.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katon W, Russo J, Lin EH, Heckbert SR, Ciechanowski P, Ludman EJ, et al. Depression and diabetes: factors associated with major depression at 5-year follow-up. Psychosomatics. 2009;50(6):570–579. doi: 10.1176/appi.psy.50.6.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vittengl JR, Clark LA, Jarrett RB. Deterioration in psychosocial functioning predicts relapse/recurrence after cognitive therapy for depression. J Affect Disord. 2009;112(1–3):135–143. doi: 10.1016/j.jad.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dobson KS, Hollon SD, Dimidjian S, Schmaling KB, Kohlenberg RJ, Gallop RJ, et al. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the prevention of relapse and recurrence in major depression. J Consult Clin Psychol. 2008;76(3):468–477. doi: 10.1037/0022-006X.76.3.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katon WJ, Lin EH, Von Korff M, Ciechanowski P, Ludman EJ, Young B, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med. 2010;363(27):2611–2620. doi: 10.1056/NEJMoa1003955. [DOI] [PMC free article] [PubMed] [Google Scholar]