Abstract
Periodontal disease and diabetes, two diseases that have achieved epidemic status, share a bi-directional relationship driven by micro-inflammatory processes. The present review frames the current understanding of the pathological processes that appear to link these diseases and advances the hypothesis that reversal of the epidemic is possible through application of interdisciplinary intervention and advancement of oral-systemic personalized medicine. An overview of how Marshfield Clinic’s unique clinical, informatics and bio-repository resources and infrastructures are being aligned to advance oral-systemic personalized medicine is presented as an interventional model with the potential to reverse the epidemic trends seen for these two chronic diseases over the past several decades. The overall vision is to engineer a transformational shift in paradigm from ‘personalized medicine’ to ‘personalized health’.
Keywords: personalized medicine, diabetes, periodontal disease
Introduction
In 2000, Surgeon General David Satcher published a report entitled “Oral Health in America” drawing attention to the persistent ‘silent epidemic’ represented by the largely under-recognized national oral health crisis that has persisted for decades (U.S. Department of Health and Human Services, 2000). Due to lack of a systematic definition and under-diagnosis of conditions affecting the gingiva, estimation of prevalence has been somewhat difficult to characterize (Borrell and Papapanou, 2005; Page and Eke, 2007; Savage et al, 2009). However, existing attempts at epidemiological prevalence projections support Satcher’s characterization, with estimates of 75% of the US population affected by gingivitis, 35% of adults affected by periodontal disease (PD), and approximately 13% afflicted by severe periodontitis, with persons of low socioeconomic status disproportionately affected (Albandar et al, 1999). Emerging evidence that PD impacts exacerbation of other pathological conditions systemically has heightened concern over the high prevalence of oral disease. Of highest concern was mounting evidence demonstrating that diabetes mellitus (DM), another escalating epidemic, and PD represented reciprocal risk and exacerbation factors for each other, presumably mediated by chronic inflammatory pathophysiological mechanisms (Mealey and Oates, 2006). However, a critically important finding of some studies was that, with appropriate oral prophylaxis and intervention, exacerbation of both conditions could be dramatically curtailed. (Khader et al, 2006; Darré et al, 2008; Janket et al, 2005; Simpson et al, 2010; Teeuw et al, 2010; Lalla and Papapanou, 2011).
Shortly after the Surgeon General’s report was released (U.S. Department of Health and Human Services, 2000), Harvey Schenkein (2002) prepared a prescient review predicting the advent a new era of personalized medicine relative to oral health and disease as a result of expanded understanding emanating from applied genomics studies. He proposed a scenario wherein personalized medicine approaches could be harnessed to: 1) facilitate identification and analysis of etiological factors contributing to oral disease; 2) permit assessment of environmental impact on oral health and disease; 3) evaluate host risk from a clinical and genetic perspective; 5) predict response to treatment; and 4) support development of diagnostic, prognostic and therapeutic approaches for application on a personalized basis.
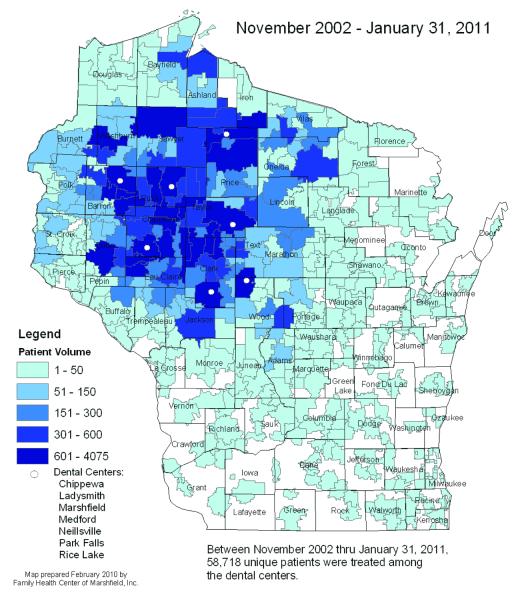
The realization of a growing dental crisis that has disproportionately affected low income and under- or non-insured populations, especially those populations residing in dental professional shortage areas, has been a strong conduit for advancement of personalized medicine at Marshfield Clinic. To put this in perspective, in 2002, the Marshfield Clinic’s Family Health Center (FHC) in Wisconsin found itself in the midst of a dental care crisis. Oral health care access had become a highly significant problem for many Wisconsin residents, with only 22.6% of Medicaid/BadgerCare recipients able to receive dental services due to a continuing decline in participation by most dentists in the Medicaid/BadgerCare insurance program. Of further concern was the prediction that continued attrition was expected over the next 10 years due to an aging dental workforce entering retirement. These statistics, coupled with an anticipated steady increase in demand attributable to expanding numbers of eligible individuals over time and the potential impact on their quality of life in a climate of escalating of healthcare cost, projected that future access to dental care would become increasingly problematic, necessitating urgent, immediate, proactive measures. Recognizing the impact of oral disease on both quality of life and systemic health, author (GN), Executive Director of the Family Health Center (FHC) of Marshfield Clinic in Wisconsin, a designated federally qualified health centre (FQHC), undertook an unprecedented grassroots effort, in collaboration with Wisconsin communities, to set up the first 8 of 15 projected dental clinics across their combined, largely rural, service area. Currently, these 8 dental centres are serving residents from all 72 counties in Wisconsin, with one additional site scheduled to open in 2012. Between November 2002 and January 2011, 58,718 unique patients were treated at these dental centres (Figure 1), 85% of which were under 200% of the poverty level. Long-term plans call for establishment of dental facilities to support approximately 100 to 120 full-time equivalent (FTE) dentists and a slightly higher number of hygienists. To maintain a steady workforce to support the new dental clinics, a new dental school is being created as a pipeline to support provision of dental care in designated regional dental professional shortage areas.
Figure1.
Unique Dental Patients by Zip Codes treated by Marshfield Clinic Family Health Center Dental Centers.
In addition to making huge inroads into providing urgently needed access to state-of-the-art dental care for its population, establishing these dental clinics has coincidentally created a natural laboratory in which to study the impact of improved dental care on a variety of health outcomes in low income populations with high prevalence rates of PD and systemic conditions such as DM. By aligning many unique resources at Marshfield Clinic, including its advanced clinical informatics and health information technology (HIT) as demonstrated by its interrogatable, highly sophisticated, dental-medical electronic health record, an established Personalized Medicine Research Project (PMRP) featuring a population-based biobank of >20,000 DNA samples, and metagenomics research capability, Marshfield Clinic finds itself positioned to accelerate realization of the personalized approach to oral health care envisioned by Schenkein (2002).
Evidence supporting the inter-relationship of PD and DM, two diseases that have achieved epidemic proportions, has been rapidly mounting over the past decade and the strength of the evidence has recently undergone extensive, systematic reviews and meta-analyses that are cited throughout this review. The present review will briefly frame the current understanding relative to the process driving these diseases as it relates to advancing oral-systemic personalized medicine. Further, an overview of Marshfield Clinic’s unique resources will be presented in the context of how these are advancing oral-systemic personalized medicine based on the current conceptual understanding of oral-systemic health and disease. Special emphasis is given to stemming the epidemic tide of PD in the context of DM through advancement of an interdisciplinary approach to disease management and how recognition of this double threat to population health has fuelled efforts to address the expanding dental crisis in Wisconsin.
The periodontitis-diabetes connection: brief summary of the evidence
DM has achieved epidemic proportions with 8.3% of the US population (25.5 million people) affected in 2011, among whom 18.8 million carry a confirmed DM diagnosis, while an estimated 6.7 million (27%) remain officially undiagnosed. Further, an additional 79 million people exhibit pre-diabetes based on impaired glucose tolerance as determined by haemoglobin A1C (HbA1C) and fasting glucose levels (American Diabetes Association, 2011). The US is ranked third among countries globally expected to show the largest increase in diabetic prevalence (Smyth and Heron, 2006). Epidemiological studies suggest that among individuals with a family history of diabetes who present with PD, 27% to 53% are likely impacted by undiagnosed DM (Borrell and Papanou, 2005). Further, the odds ratio of PD among patients with DM is 2 to 5 fold higher than in normoglycemic individuals as supported by meta-analysis (Khader et al, 2006). Incidence of DM has continued to show an upward trend over the past 10 years, and this trend is expected to continue, especially in conjunction with a trend towards a rise in obesity with which diabetes shares a close association. The World Health Organization (WHO) has projected prevalence to triple in the next 10 years (World Health Organization, 2009). Notably, DM prevalence increases with age, with estimates of 26.9% prevalence among the US population over 65 years-of-age (Centers for Disease Control, 2011). Because approximately 90% of DM in adults is Type 2DM (T2DM), the overview of the pathological mechanisms underlying DM presented in this review focuses on T2DM.
Estimates of the pervasiveness of PD have been challenged by lack of a standardized definition (Borrell and Papanou, 2005; Page and Eke 2007; Savage et al, 2009). Designated as a pandemic by the WHO, PD is one of the most pervasive diseases seen in industrialized nations, with greater than 30% of the population impacted and between 5%–15% of the population with PD presenting with more severe destructive forms of the disease (Burt et al, 2005). Prevalence rates of PD similarly rise with age of the population (Burt et al, 2005) and disproportionately affect segments of the population with limited or no access to dental care (Satcher 2002).
Studies postulating an impact of DM on PD were first published in the mid-1960’s (Belting et al, 1964), with PD being designated as the sixth most common complication of DM (Löe, 1993). In the ensuing years, increasing evidence suggests a bidirectional exacerbative impact of these two conditions on each other (Mealey et al, 2006; Taylor 2001; Nagasawa et al, 2010). Furthermore, the presence of PD increased the risk of onset of diabetes-associated complications in patients with DM and/or hyperglycaemia (Jepsen et al, 2011; Saremi et al, 2005; Shultis et al, 2007). A study by Saremi et al (2005) reported a 2.3 fold increase in risk for mortality stemming from ischemic heart disease and an 8.5-fold increase in risk of diabetic nephropathy in patients with DM and severe PD compared to patients with no PD following adjustment for other known risk factors; with a 3.5 fold overall risk of death from cardio-renal pathology in subjects with DM and PD compared to subjects who with no PD (Saremi et al, 2005).
Understanding of the complex pathophysiology linking DM and PD has evolved over the past few decades and has been the focus of a number of excellent recent reviews (Kim and Amar, 2006; Kuo et al, 2008; Chávarry et al, 2009; Taylor and Borgnakke, 2008; Amir et al, 2011; Marigo et al, 2011; Deschner et al, 2011; Lalla and Papapanou, 2011; Santos Tunes et al, 2010; Mealey and Rose, 2008; Jepsen et al, 2011); therefore, it will only be briefly summarized here. Notably, research data have underscored the pivotal contribution of chronic systemic micro-inflammatory and macro-inflammatory processes as the common denominator driving establishment of chronicity, progression, and mutual exacerbation of these two conditions in the absence of intervention (Rial et al, 2011; Genco et al, 2005; King 2008). Detailed reviews of pathological processes supporting emergence of T2DM have recently been published (Rial et al 2011; Donath and Shoelson, 2011; King 2008), and will therefore only be summarized here to provide a context for efforts underway at Marshfield Clinic to advance oral-systemic personalized medicine.
Briefly, the current characterization of T2DM is that it represents an acquired pathology that phenotypically presents as insulin resistance at the level of the cellular target, is unresponsive to a compensatory increase in production of insulin by pancreatic islet cells and is accompanied by stimulation of both macro- and micro-inflammatory processes. Micro-inflammation is characterized by targeted leukocytic modulation, activation and recruitment via molecular signalling mediated by altered levels of specific pro-inflammatory chemokines and acute phase reactants and concomitant induction of changes in gene expression. Because of its systemic impact, micro-inflammation collaterally exacerbates additional pathologies responsive to hyperinflammatory states, such as PD, retinopathy, neuropathy and nephropathy (reviewed by Rial et al, 2011). A role for macro-inflammatory contribution to PD cannot be ruled out however, especially in the presence of obesity, where PD is exacerbated even in the absence of DM (Genco et al, 2005). In addition, elevation of inflammatory markers in the presence of periodontitis has been reported (Loos 2005). Micro- and macro-inflammatory processes are prone to further escalation by stress emanating from the advancing hyperglycaemic state including oxidative stress resulting in free radical generation (Evans et al,2003), stress to the endoplasmic reticulum from heightened protein production (Araki et al, 2003), and stress associated with dysregulation of lipid metabolism and amyloid deposition (Donath and Shoelson, 2011). In their recent review, Donath and Shoelson (2011) note that, especially in overweight or obese persons, fat accumulation is postulated to result in adipocyte hypoxia from excessive adipocyte expansion, and these cells contribute to the inflammatory milieu by releasing additional inflammatory mediators and adipokines, hormone-like substances with immunomodulatory effects produced exclusively by these cells, and ultimately mediate activation of kinases including nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and Jun N-terminal Kinase (JNK). Kinase signalling in turn, up-regulate important cytokines including tumour necrosis factors (TNF), interleukin-6 (IL-6) and interleukin-1 beta (IL-1β) among others, which further promote inflammation and insulin resistance through ensuing promotion of immune mechanisms. Some of these immune mechanisms become autostimulatory once set into motion due to induction of interleukin-1 receptor 1 (IL1-R1) signalling. Heightened immune response in turn escalates inflammation through further up-regulation of inflammatory mediator production. In the absence of intervention, inflammatory chronicity and eventual tissue destruction ensues at the level of the islet cells while insulin resistance at the cellular level is reinforced. Currently clinical studies targeting intervention at the level of IL1 receptor blockade, NF-κB inhibition or IL1-β appear to be achieving outcomes consistent with reversal of disease processes in early clinical trials (Donath and Shoelson, 2011).
PD is exacerbated by receptor-mediated binding of advanced glycated end-products (AGE) and bacterial lipopolysaccharides (LPS) to Toll-like receptor 4 (TLR4), expressed by cells of the periodontal infrastructural tissue supporting the teeth. AGE and TLR4 receptor binding is associated with release of pro-inflammatory cytokines and other inflammatory mediators. TLR4 receptor activation is particularly sensitive to LPS stimulation emanating from the subgingival microbial biofilm (also called gingival plaque) and perturbations in the microbial composition or ecological homeostasis of the periodontal microbiome. In addition, release of chemotactic and adhesion factors promote recruitment of inflammatory cells from circulation into the inflamed periodontal tissues. If no intervention occurs, inflammatory processes mediated by host immune response to cytokine/chemokine and acute phase responses eventually inflict damage to host tissue by stimulating collagenases, metalloproteases, and neutrophil activity. Damage to the periodontal infrastructure manifests through deepening of subgingival pockets, attachment loss of the teeth from the periodontal tissue, and loss of bone supporting the periodontal tissue. Loss of mucosal barrier integrity at the level of periodontal epithelial basement membrane permits systemic access of microbes and inflammatory mediators (Marigo et al, 2011; Deschner et al, 2011; King 2010; Williams et al, 2008) Hyperglycaemia associated with T2DM also directly activates monocytes and stimulates release of inflammatory mediators from mononuclear cells systemically (Shoelson et al, 2006) and in gingival tissue. Prospective and cross sectional studies have reported elevated systemic levels of pro-inflammatory cytokines and acute phase proteins in connection with both conditions. It is postulated that the chronic low grade inflammatory processes stimulated by infectious processes in the periodontal biofilm contribute to the insulin resistance and impact glycemic control. In support of this theory, meta-analysis of 10 interventional trials demonstrated improvement in HbA1C level, a biomarker of T2DM, following periodontal treatment and reduction in periodontal inflammatory processes. (Santos Tunes et al, 2010).
Importantly, it should be recognized that micro-inflammatory processes that contribute to disease chronicity are modifiable risk factors and responsive to intervention. Poor glycemic control has been identified as an important variable contributing to progression and severity of PD (Weidlich et al, 2008). Further, good glycemic control improves periodontal health and, conversely, improvement in periodontal health impacts positively on glycemic control. In support of this, at least four systematic reviews and meta-analyses in the recent literature have examined clinical trials, and other evidence-based literature report the positive impact of periodontal treatment on glycemic control (Darré et al, 2008; Janket et al, 2005; Simpson et al, 2010; Teeuw et al, 2010). In Janket’s meta-analysis of 10 clinical studies that collectively measured HbA1C outcomes following intervention for PD, a clinically significant absolute reduction in HbA1C of 0.66% was detected, where a 0.2% reduction in HbA1C is associated with a 10% reduction in mortality (Teeuw et al, 2010; Khaw et al, 2004; Mealey and Rose, 2008). Further, clinical trials focussed on interventions that target microinflammatory processes by treating subjects with T2DM with salicylates and interleukin-1 antagonists or molecular inhibition of anti-inflammatory factors suggest that application of immunomodulatory strategies can lower blood glucose levels and concurrently mitigate inflammatory processes and prevalence of complications associated with DM (Donath and Shoelson, 2011). Thus, it theoretically appears possible to simultaneously stem the epidemic increases in prevalence of both PD and DM through interventions that emphasize the importance of glycemic control in conjunction with good oral care. This involves conceptualization of these conditions as treatable, manageable, and potentially preventable through application of proactive, preventive, relatively low cost, interdisciplinary care and education. This holistic, interdisciplinary method of care is an integral component of the personalized medicine approach to PD and DM as it is being advanced at Marshfield Clinic.
Understanding human genetic contribution to dental personalized medicine
At its most fundamental level, personalized medicine can be envisioned as making clinical and treatment decisions for an individual based on probabilities of events given the interactions between genetic, clinical and environmental factors affecting that individual (Kinane and Heart, 2003). By contrast, traditional medicine bases treatment and clinical decisions for individuals on probabilities of population-based observations of outcomes and responses, which may or may not be appropriate at the level of the individual. Assuming the validity of genetic contribution, the following is a brief review of the current understanding regarding the contribution of genetics to PD.
To date, genetic studies of disease association have estimated that there are approximately 307 human ‘periodontitis-associated’ genes. However, these studies are largely underpowered to find all but the largest of genetic effects, with >65% of reported associations based on examination of ≤ 100 patients, and approximately 40% based on ≤ 60 patients that require further validation. Approximately 14% of the 307 genes represent candidates based on biological significance or detection across multiple studies (Diehl et al, 2005; Diehl et al, 2011; Nikolopoulos et al, 2008). Thus, more robust studies are required to test these candidates and explore further genetic associations in the light of advances in understanding of mechanisms underlying PD.
Genetic analysis applying familial and twin studies support a heritable component for aggressive PD, whereas twin studies support a genetic basis for chronic PD. To date, no gene(s) have been clearly associated with causation, although autosomal dominant transmission, albeit with heterogeneity of loci for familial aggressive PD, appears to be operative following correction for ascertainment bias (Kinane et al, 2008). An interesting study of 50 families with affected members presenting with aggressive PD by Diehl et al (2003) found that 38% of variance of immunoglobulin G allotype 2 (IgG2) levels could be explained by genetic contribution (p=0.0006). No correlation was noted following bi-trait variance component analysis of IgG2 levels and aggressive PD susceptibility, suggesting that different genes determine IgG2 levels and disease susceptibility.
One meta-analysis of association studies reports association of single nucleotide polymorphisms (SNPs) occurring in chronic periodontitis in the IL1, IL6, VDR, and CD14 genes (Laine et al, 2010), although further studies are required to validate these in larger, more stringently phenotyped populations. Other studies suggest that moderate and severe PD both share and have distinct genetic components (Brett et al, 2005). The possibility that functional mutations, such as single-nucleotide polymorphisms (SNPs) occurring in pro-inflammatory pathways, may simultaneously impact on association with several conditions, is also of interest to explore. One such example can be found by SNPs identified in the pro-inflammatory cytokine genes for IL1 (including one each in IL1A and 1L1B), that promote periodontal progression (Huynh-Ba et al, 2007; Nikolopoulos et al, 2008). A further example includes SNPs occurring within the IL1 gene cluster that have recently been implicated both in PD and in hyperlipidemia. The impact IL1 SNPs on two distinct pathological conditions is simultaneously amplified by both increasing risk for myocardial infarction (Laine et al, 2010; Keavney, 2010) and promoting diabetes pathogenesis (Santos Tunes et al, 2010). Similarly, expression of IL6, a pro-inflammatory cytokine, increases with age and may contribute to promoting pathogenesis of conditions that are exacerbated by pro-inflammatory mechanisms. Further, IL6 impacts on bone resorption and may thus contribute to bone loss observed in PD.
A recent study by Offenbacher et al (2009) investigated the role of the immune response and proinflammatory processes in gingival health from a host susceptibility perspective. These investigators explored changes in the patterns of whole-transcriptome that occur in humans during the induction and resolution of experimentally-induced gingivitis, the mildest form of PD, on serial biopsy specimens collected pre-induction, during active gingivitis, and after resolution. Whole-transcriptome gene-expression arrays revealed that 131 immune response genes were up- or down-regulated. Transient expression of inflammatory and oxidative stress mediators were predominantly expressed, including interleukin IL1A, IL1B, IL8 (CXCL8), Regulated on Activation, Normal T Expressed and Secreted, (RANTES), colony stimulating factor 3 (CSF3), and superoxide dismutase 2 (SOD2); while there was decreased expression of interferon gamma-induced protein 10 (IP10), interferon inducible T-cell alpha chemoattractant (I-TAC), matrix metalloproteinase 10 (MMP10), and beta 4 defensin (DEFB4). Reversal of these expression patterns was noted when inflammatory processes associated with gingivitis resolved. This study revealed that a subset representing approximately 10% of the immune response genes demonstrated specificity relative to the transcriptome-expression response and maintenance of tissue homeostasis at the biofilm-gingival junction (Offenbacher et al, 2009).
Another noteworthy study by Covani et al (2009) used a genomics approach to define genetic common denominators between DM and PD. The investigators set out to identify genes associated with the highest number of functionally interactive biological pathways associated with each condition. These genes, termed ‘leader genes’ by the authors, were defined independently for DM (n=12), PD (n=5), and sinusitis (n=6). Remarkably, comparison of the resulting ‘leader gene’ analysis for each condition demonstrated bidirectional overlap between four of five PD leader genes with DM leader genes. Overlapping genes included NFκB1, RELA, PIK3R1, and GRB2. Notably, such convergence was not present with any of the six leader genes associated with sinusitis, although infectious/inflammatory mechanisms also are implicated in establishment of pathogenesis and chronicity of this condition.
Metagenomics and microbiomics: what contributes to personalized medicine?
Within the context of PD, another genetic component that needs to be considered is that of the oral and other microbiota in the body, especially those microorganisms associated with periodontal plaque that have been implicated in the pathogenesis of destructive inflammatory responses locally and systemically. Historically, early inroads into metagenomics were made with the analysis by Socransky et al (1998) using a whole genomic approach to characterize the microbial profile associated with PD and health. These investigators contributed recognition of the so-called ‘red complex’, that consists of three periopathogens – P. gingivalis, T. forsythia, and T. denticola – that tend to occur as a group in association with PD. Darveau (2010) postulated that this trio of organisms collectively has the capacity to disrupt innate immune responses.
Evidence is mounting that health depends on the dynamic interplay between the host and the microbial community that harbours in different organs of the body. The microbiome represents the ecological community of commensals, symbiotic, and pathogenic microorganisms that share our body space, and any perturbation in microbiome is likely to have a serious role in health and disease (Lederberg and McCray, 2001; Keijser et al, 2008). Human adults harbour ten times more microbial cells than human cells (Gordon et al, 2005). Consequently, these microbial cells contribute more genomic material (genes, transcripts, proteins, metabolites) than the host; therefore, it is only logical to assume that the microbiota of an organ may play a huge role in the development, modulation, and/or maintenance of some chronic diseases.
Advances made in characterizing oral microbiomes have been recently reviewed (Zarco et al, 2012; Jakubovics and Kolenbrander, 2010; Avila et al, 2009; Xie et al, 2010; Dewhirst et al, 2010; Sakamoto et al, 2004 and 2005) and will be discussed here in the context of how advances in these fields have expanded the understanding of pathological mechanisms contributing to PD and how this impacts on advancing personalized medicine. Like other human organs such as the gut, the human oral cavity harbours a large community of bacteria. Any changes in the dynamics of the oral microflora could initiate the formation of a dental plaque. While the majority of these organisms could be commensals; some are opportunistic pathogens and play a role in oral conditions such as gingivitis and chronic periodontitis. Individuals with chronic periodontitis have an increased risk of developing coronary heart disease (Beck et al, 2005; Kebschull et al, 2010), ischemic stroke (Joshipura et al, 2003), hypertension (Desvarieux et al, 2010), diabetes (Genco et al, 2005), aspiration pneumonia (Scannapieco 1999), bacterial endocarditis (Berbari et al, 1997), renal disease (Kshirsagar et al, 2005; Shultis et al, 2007) and preterm low birth-weight babies (Offenbacher et al, 1998). Similarly, recognition of the threats posed by periodontal diseases to individuals with chronic diseases such as respiratory diseases and osteoporosis is relatively recent (Seymour et al, 2007).
Indeed, some findings from these studies relative to the constituents of the oral microbiome are challenging traditional paradigms that have been advanced to explain disease pathogenesis. As summarized by Zarco et al (2012), the collective microbial genomic material, or metagenome that represents the genetic content of an estimated 700 species of microbes, expands the genomic content of any given human approximately 100 fold and adds systemic attributes that were not genetically endowed by the human genome. Collectively the microbiome systems throughout the body contribute to the global metagenome and establish a unique ecological system within each person that creates a symbiotic relationship with the host. Perturbations in the microbiome or changes in the host interaction with the microbial load may shift the balance within microbiomes and the global ecosystem from one supporting health to one supporting disease. This shift underscores the necessity for a personalized approach to care, since microbiomes will vary with each individual relative to microbial content and environmental characteristics contributed by the microbial milieu and metagenomic content and host responses. These authors emphasize the need to attempt definition of microbiome expression patterns in health and in the context of various diseases.
An important insight afforded by advanced molecular methodological approaches into the composition of the new oral microbiome is its heterogeneity throughout the oral cavity and even within the layers of a biofilm, as dictated by the environment. Further, genotypic characterization of an isolated microbe alone is no longer sufficient to establish relative pathogenic status. As seen with the ‘red complex’ microbial trio, determining relative representation, analysis of other microbes present, and characterization of contextual interaction with the host is critical to establishing the relative contribution to health or disease of any constellation of organisms present in the microbiome. Broad heterogeneity in genomic content, even for any given species, has been noted to endow new virulence factors through horizontal transfer of genetic material and transfer of new functional capacity among species.
The use of culture-independent techniques, such as 16S rDNA sequencing to identify uncultivable microorganisms has proven to be a powerful technique for identifying and characterizing novel pathogens found in clinical samples and the environment. With the advances in sequencing capability from Roche’s 454 DNA sequencer to Illumina’s sequencing by synthesis (SBS) technology, it is now possible to sequence millions of bases at a fraction of the cost compared to 10 years ago. This ability to identify previously uncultivable, therefore unsuspected, pathogens from complex infectious diseases has redefined the etiological agent(s) of a number of complex illnesses and metabolic diseases (Eng et al, 2011). These approaches revealed that approximately 50% of organisms represent uncultivable bacteria and even include newly defined microbes (Wade, 2011). The relative contribution of these organisms to health or disease is totally uncharted territory. Even the existent body of evidence limiting attribution of PD to the periodontal pathogens recognized to date has been challenged by data reporting low relative representation of the these organisms associated with the presence of other previously unidentified, undefined species in samples exhibiting disease (Wade, 2011). Importantly, application of new technologies such as the Human Microbe Identification Microarray (HOMIM), including probes for 300 microbial species, is making it possible to discern a unique genetic profile in patients with refractory PD that distinguishes them from healthy subjects (Colombo et al, 2009). These discoveries suggest a new paradigm wherein definable patterns of microbial profiles within microbiomes and shifts in these patterns in the context of disease may support more accurate characterization of pathogenic processes.
Notably, a new hypothesis recently advanced by Amir et al (2011) proposes that hyperglycaemia associated with T2DM may be responsible for mediating translocation and relocation of gastrointestinal flora systemically. Translocation is postulated to impact the composition of the periodontal microbiome causing perturbation of the periodontal homeostasis and further up-regulation of acute phase response, cytokine signalling, and immune mediated response (Amir et al, 2011). The impact of individual microbiomes on other microbiomes requires further examination. Increasingly, the importance of microbiome-based studies is underlined in identifying the potential role of etiological agents in complex chronic diseases such as DM and PD, where infectious/inflammatory processes are suspected contributors to pathogenesis (Rajilic-Stojanovic et al, 2007).
The central role of informatics contribution to realization of dental-systemic personalized medicine
While the more comprehensive understanding of infectious/inflammatory processes contributing to PD and DM has been facilitated in part by advances in biotechnology, rapid advances in informatics capabilities are also supporting acceleration of translational personalized medicine from bench to bedside. Many of these advances are being driven by an urgent need to manage the massive amounts of data being generated as a consequence of advances in genomic and metagenomic analysis that potentiate high throughput accumulation of both human and microbial data that must then be stored, organized, sorted, analysed, and interpreted in the context of other available data in order to decipher its significance. For example, the oral metagenome, currently under study as a component of the Human Microbiome Project (NIH HMP Working Group et al, 2009) will generate vast amounts of genetic data whose interpretation will be aided by availability of the Human Oral Microbiome Database and, as a reference tool, its auxiliary website (http://www.homd.org) that has catalogued all microbial species presently found in the mouth (Chen et al, 2010). Availability of these resources and analytical toolboxes to analyse their content in order to create accurate microbial phenotypes relative to pathogenic capacity in the context of microbiomes is critical to advancing applicability to personalized medicine. Similar advances in genomic sequencing capabilities are making sequencing technology less cost prohibitive and more accessible. Such efforts will undoubtedly provide increased understanding of the host genomic contribution to health and disease, especially in the context of shifts in profiles of constituent microbiomes of the systemic metagenome in the context of disease. However, these data will also need to be systematically catalogued in searchable, accessible databases for future analyses in the context of health and a myriad of disease entities.
Central to the systematic evaluation of parallel data and distillation of its contribution to the global understanding of health maintenance and aberrations contributing to disease pathogenesis is the development and implementation of validated, standardized phenotypic definitions of the various host and microbial characteristics that contribute to oral (and systemic) health and disease. A phenotype is an observable trait that may define, for example, an organism’s appearance, behaviour, or signs and symptoms of disease among other features. Phenotypes also extend to referencing observable traits or attributes at a molecular level. Lack of uniformity in the clinical phenotypic definition of PD has created significant challenges in comparing and interpreting epidemiological data across studies (Borrell and Papapanou, 2005). Advancing accurate clinical phenotyping capacity also requires advanced biomedical informatics capabilities to sift and analyse clinical and demographic data that will support accurate definition of attributes that allow affected individuals to be systematically distinguished and compared to unaffected individuals at molecular, clinical, and demographic levels in the context of environmental exposures that influence each individual. Ideally, to advance biomedical informatics capacity in this regard requires the availability of integrated dental and medical electronic health records that can be systematically interrogated to create phenotypes.
Characterization of the host dental phenotype is facilitated by availability of an electronic dental record (EDR). There has been a slow increase in the use of computers to store not only administrative data but also clinical information at dental practices across the nation. An American Dental Association (ADA) survey in 2007 (American Dental Association Survey, 2007) showed that approximately 55% of all practitioners maintain their clinical information using an electronic (computer) format. A more recent survey of California dentists showed that about 23% of its dentists report having fully implemented an EDR in their practices (California Healthcare Foundation 2010). The survey also reported that another one-third is either in the process of or planning to do so in the coming years.
More recently there has been a strong push from the federal government for the adoption of the electronic health record (EHR) within all branches of healthcare. This will most likely support the adoption of EDR amongst dental practitioners who treat Medicaid patients. There has been a wide-spread adoption of EDRs in majority of the US dental schools. Additionally, US dental schools, as well as dental schools in Canada and Europe, are either using or adopting some aspects of a common EDR framework (White et al, 2011). The adoption of EDR in dental schools and private practices throughout the nation is resulting in a wide array of clinical data being stored in the individual repositories (databases). This creates a potential opportunity to utilize these clinical data not only to support clinical practice but also to conduct cutting-edge research.
How is Marshfield Clinic ushering in dental personalized medicine?
Unique resources have aligned Marshfield Clinic to advance dental personalized medicine and set the stage for ushering in its integration at the clinical level. The key elements already in place that have positioned Marshfield Clinic to advance realization of dental personalized medicine include 1) the Personalized Medicine Research Project (PMRP) and its bio-repository; 2) Marshfield Clinic’s combined dental and medical clinic infrastructure; 3) an integrated medical-dental electronic health record (iEHR) supported by substantial bioinformatics expertise; and 4) a research infrastructure with a dual focus on genetics and metagenomics. How this infrastructure has been integrated to advance dental personalized medicine at Marshfield Clinic is the focus of the second part of this review. It is essential to provide an overview of one of Marshfield Clinic’s oldest and most valuable resources, its EHR, as well as characterising the central role of the EHR in realizing personalized medicine.
Foremost, EHRs provide multiple benefits relative to tracking clinical care, patient outcomes, and maximizing efficiency. Further, the EHR is an invaluable resource for research aimed at assessing and improving patient care. In its landmark report, The Computer-Based Patient Record (CPR): An Essential Technology for Health Care (Dick et al, 1997), the Institute of Medicine (IOM) eloquently formulated a vision for EHRs. The benefits of computer-based records (CPRs) articulated by the IOM include longitudinal and comprehensive patient documentation, improved data quality and validation, built-in decision support functions, universal and secure access, and availability. Since 1997, several other IOM reports have addressed how HIT can reduce medical error (Kohn et al, 2000; Committee on Quality of Health Care in America, 2001) and increase quality in healthcare (Thompson and Brailer, 2004). These benefits of CPRs provide the central rationale supporting the development of the National Health Information Infrastructure (Osheroff et al, 2007).
Integrated medical-dental EHR and Clinical Decision Support System
Marshfield Clinic, the largest, physician-owned, private group medical practice in Wisconsin and one of the largest in the US with 750 physicians representing 86 different medical specialties, is unique among organizations that are successfully harnessing the CPR to improve patient care. Marshfield Clinic physicians and surgeons provide care for approximately 400,000 unique patients residing in the mostly rural counties of central, northern, and western Wisconsin as well as Michigan’s Upper Peninsula through its network of 45 regional centres and in collaboration with local and regional hospitals, with roughly 1.8 million patient visits annually. Coordinating seamless care to this largely rural population across regional centres was a challenge that became greatly facilitated through the establishment of one of the oldest and most comprehensive EHRs in the US. Internally developed by the visionary efforts of its long-standing clinical informatics team, its proprietary EHR known as CattailsMD™, contains coded diagnostic data dating back to 1960, with other coded data and digital documents dating back to the mid-1980s. This EHR provides comprehensive electronic access to diagnoses, procedures, clinical notes, laboratory results, imaging results, pharmacy data, hospital progress notes and discharge summaries. This fully integrated and comprehensive EHR allows real-time electronic access to a patient’s medical record from any of its 45 regional centres and collaborating hospitals using its system. Additionally, it was the first physician practice-developed EHR to receive accreditation from the Certification Commission for Health Information Technology. Early development of the Medical Event Coding by Clinicians Application (MECCA) system has provided Marshfield Clinic with several decades of experience in the use of coded terminology in an EHR. In addition, the EHR contains roughly 70 million text documents. As such, Marshfield Clinic provides a unique environment for informatics research and the perfect laboratory to develop new tools for clinicians and staff. Marshfield Clinic physicians have been routinely using computers as an integral tool to capture and record relevant details of every patient encounter for over a decade. Marshfield Clinic continues to develop much of its own clinical software, thus allowing for rapid integration of new ideas and applications into clinical practice. With the introduction of hand-held wireless tablet computers, Marshfield Clinic is among the first health care provider in the country to put the electronic medical record truly at the source of health care decisions - in physicians’ hands.
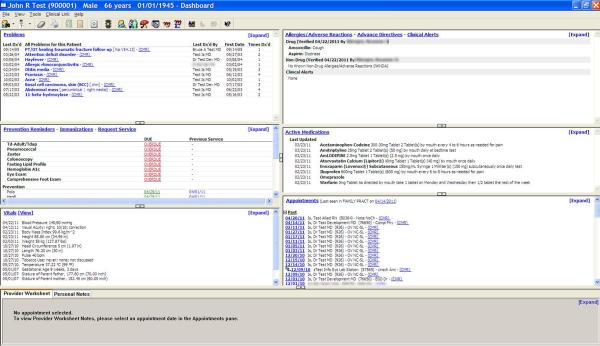
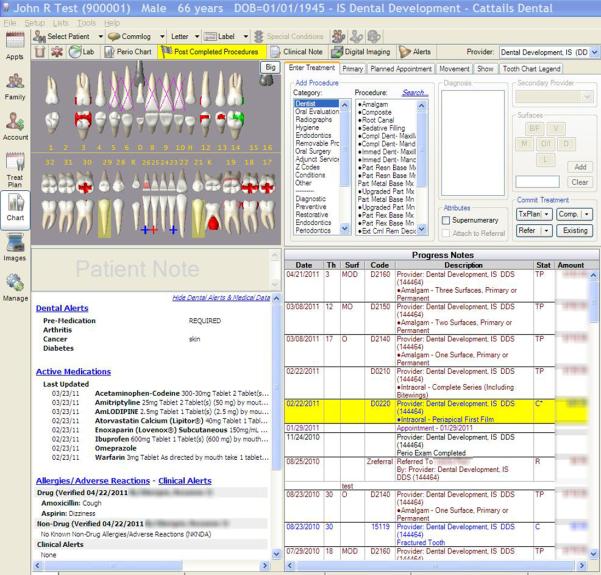
Marshfield Clinic was among the first institutions to respond to the challenge of the IOM, National Academy of Sciences report, “Dental Education at the Crossroads: Challenges and Change” (Committee on the Future of Dental Education, 1995), which called for a greater collaboration and integration between medicine and dentistry at all levels of the health care: patient care, education and research. These developments also addressed the Surgeon General’s call to action to deal with the silent epidemic of oral disease disproportionately affecting the poor of all ages (U.S. Department of Health and Human Services, 2000). As previously described, the partnership between Marshfield Clinic and its Family Health Center led to provision of dental services to rural communities throughout central and northwestern Wisconsin through a series of dental clinics that have become an integral component of Marshfield Clinic. With support from Delta Dental of Wisconsin, Marshfield Clinic was positioned to emerge as an early adopter of an integrated dental medical record, incorporating clinical and practice management functions into its comprehensive EHR. With medical/dental diagnoses, hospital data, imaging studies such as radiographs, and prescription information (including potential drug interactions) all available at the touch of a button, Marshfield Clinic clinicians have in their hands the information needed to make the best clinical decisions. The dental module, CattailsDental™, has been successfully implemented in all seven Marshfield Clinic dental centres. CattailsDental™ is based on an open-source dental software platform, Open Dental of Salem, Oregon. The integrated medical-dental electronic health record (iEHR) system is designed specifically for Marshfield Clinic physicians and dentists to access and share patient health information (Figures 2 & 3). CattailsDental™ includes a dental dashboard; workflow management tools including tooth charting, periodontal charting, and treatment planning; access to centralized medications, allergies, special conditions, problems list, demographics, and Health Insurance Portability and Accountability Act (HIPAA)1 forms; access to medical appointments to support better coordination of care; and highly secure remote access supporting devices such as laptops and computer tablets. Medication Manager is a recently introduced point-of-care integrated electronic prescribing and medication inventory system currently used by all physicians and dentists at Marshfield Clinic. Any changes in a patient’s medication history is available to physicians and dentists in real-time, irrespective of who made the change.
Figure 2.
Physician’s View of the Patient Dashboard.
Figure 3.
Dentist’s View of the Patient Dashboard.
Emerging approaches such as Clinical Decision Support Systems (CDDS) (Sackett et al, 1996) and evidence-based practices (American Recovery and Reinvestment Act, 2009) require availability of all data elements to support clinical decision making, with each element contributing a specific input needed by the system. In response to the American Recovery and Reinvestment Act of 2009 (Committee on Comparative Effectiveness Research, 2009), the IOM recommended comparative effectiveness research priorities as a focus of new national investment. Priority topics included healthcare delivery, health care disparities, oral health, and HIT (Schmittdiel et al, 2004). Effectiveness of EHRs, eHealth, and other technology-based solutions to improve clinical care and self-management are urgently vital. EHRs increasingly have embedded evidence-based physician reminders to address clinical inertia (O’Connor et al, 2009; Peterson et al, 2008; Chi et al, 2010).
Historically, Marshfield Clinic has focused efforts on maximizing the capability of its EHR. With the iEHR almost complete, attention is now being focused on improving decision support at point-of-care. One of the key strategies to reverse the oral health access disparity is to enlist medical providers with appropriate decision support tools delivered through the iEHR system. This is an area targeted for improvement by the IOM, who recognises that true progress in this arena is possible through advances in health informatics and informatics technology. Currently, useful and readily available information to physicians and dentists to manage their patients in a comprehensive way is lacking. Apart from the systemic diseases that are interconnected with PD, there are several oral manifestations of systemic conditions (Chi et al, 2010. See Table 1). Careful examination of the oral cavity may reveal findings indicative of an underlying systemic condition and allow for early diagnosis and treatment. With the iEHR, this information can be used to develop the cross-disciplinary CDDS to improve quality of care, provider workflow, and decision making for physicians and dentists. This infrastructure further provides support for conducting translational oral-systemic disease research.
Table 1.
Oral manifestations of systemic conditions (Chi et al, 2010)
| Clinical Presentation | Associated Condition | Oral Manifestation |
|---|---|---|
| Mucosal pallor and atrophy | Anemia | Mucosal pallor; atrophic glossitis; candidiasis (including angular cheilitis); mucosal burning, pain, or tenderness |
| Oral lesions (including ulcerative, erosive, or white lesions; swelling; erythema) |
Lichen planus | Erosive: diffuse erythema and painful ulceration with peripheral radiating striae |
| Reticular: white lacy striae, especially on bilateral buccal mucosa |
||
| Lupus erythematosus | Oral discoid lesions; honeycomb plaques; raised keratotic plaques; erythema; purpura; petechiae; irregularly shaped ulcers; cheilitis |
|
| Benign mucus membrane pemphigoid |
Diffuse and painful oral ulceration; scarring | |
| Pemphigus vulgaris | Diffuse and painful oral ulceration; positive Nikolsky’s sign |
|
| Crohn disease | Diffuse mucosal swelling; cobblestone mucosa; localized mucogingivitis; deep linear ulceration; fibrous tissue tags, polyps, or nodules; pyostomatitis vegetans (“snail track” ulcers on an erythematous base); possible aphthous-like ulcers |
|
| Behçet syndrome | Recurrent, painful aphthous-like ulcers, usually numerous and especially involving the soft palate and oropharynx |
|
| Change in mucosal pigmentation | Addison disease | Diffuse melanin pigmentation; candidiasis (in patients with autoimmune polyendocrinopathy- candidiasis-ectodermal dystrophy syndrome) |
| Periodontal bleeding and inflammation |
Diabetes mellitus | Gingivitis; periodontitis; candidiasis; generalized atrophy of the tongue papillae; taste dysfunction; salivary dysfunction; burning mouth syndrome; delayed wound healing |
| HIV-associated periodontal disease |
Linear gingival erythema: linear band of erythema along the free gingival margin |
|
| Necrotizing ulcerative gingivitis: ulceration and necrosis of gingival interdental papillae, gingival bleeding and pain, halitosis. |
||
| Necrotizing ulcerative periodontitis: gingival ulceration, necrosis, rapid loss of periodontal attachment, edema, pain, spontaneous hemorrhage |
||
| Thrombocytopenia | Petechiae; purpura; ecchymosis; hemorrhagic bullae; hematomas |
|
| Leukemia | Mucosal bleeding; ulceration; petechiae; diffuse or localized gingival enlargement; secondary infections (e.g., candidiasis, herpes simplex virus infection, periodontal bone loss) |
|
| Dental erosion | Gastroesophageal reflux disease |
Water brash; xerostomia; burning sensation; halitosis; palatal erythema; dental erosion |
| Bulimia and anorexia | Dental erosion; xerostomia; increased caries rate; sialadenosis (especially bilateral parotid enlargement) |
The iEHR is supported by an integrated Enterprise Data Warehouse (iEDW) that houses patients’ medical and dental information. The intent of this sophisticated data warehouse is to be the sole reporting source for high quality integrated information related to patient care, research, and clinical operations. The iEDW is a high quality data repository that has been optimized for data analysis and reporting to support research activities, quality assurance, and decision support at Marshfield Clinic. The iEDW utilizes existing Marshfield databases and other data sources to store key information central to defining patient phenotypes. iEHR data are transferred to the iEDW, where they are linked accurately and completely as longitudinal continuums of care, ensuring that virtually no records are lost. As such, the iEDW serves as an essential resource for the development of research databases. As data is moved from clinical applications into the iEDW, data models and coding standards are normalized, algorithms are applied that ensure data quality, and data is transformed into a data model that supports population-based research.
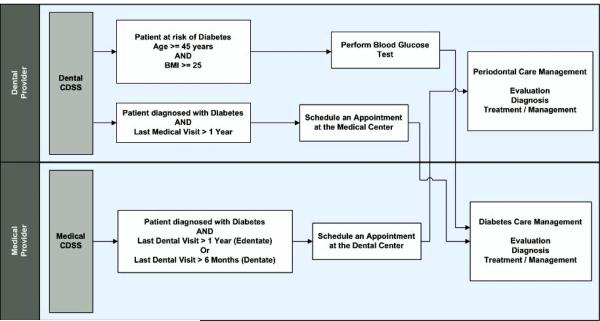
To give an example, the primary care departments at Marshfield Clinic currently use a CDSS (called iList) to create department/physician-based listings of patients whose quality care measures are not at goal for DM, hypertension, heart failure, and coronary artery disease. Patients who are diagnosed with one or more of these disease states and meet the quality care measures will not be displayed in a list. The patient listings allow staff to identify and intervene with patients not meeting the measures. The intent is to provide actionable information to improve patient outcomes and is used as a tracking tool to improve patient care. Any actions taken by physicians or staff members will help patients achieve a quality goal. Per protocol, support staff may call a patient to schedule an overdue fasting lipid panel or add an HgA1c test to a patient’s next scheduled appointment; or a provider may direct support staff to advise a patient to return to the clinic in one month for a blood pressure check. Currently, iList assists in ensuring that diabetic patients are scheduled for needed services such as lab orders and appointments with primary care physicians and non-physician team members by executing the rule-based algorithm. However, as part of this diabetes management protocol, there is no routine oral examination done for the diabetic patient. Additional rules are being developed for application to this diabetes management protocol along with the necessary triggers to filter out the diabetic patients who have not had an oral examination in the last 6 or 12 months for dentate or edentate patients, respectively, within the iList. The developed CDSS combines analytics with oral care referrals for diabetics in a real time clinical care application that provides actionable patient lists for diabetes disease management and prevention.
On the other side, the dental CDSS at the dental site focuses on generating appropriate referral reminders based on previous diagnosis of diabetes and system alerts based on potential risk for diabetes/pre-diabetes. The dental CDSS then utilizes data already available within the integrated medical-dental EHR system or collected as part of patient history taking and examination to trigger the reminders and alerts. The dental CDSS algorithm also evaluates the potential risk for diabetes/pre-diabetes in the undiagnosed population. The dental CDSS utilizes patient’s age, height, and weight already available within the iEHR system or collected as part of history when logging in new patients. Based on specific criteria (e.g. patient’s age ≥45 years and body mass index ≥25) the dental CDSS will trigger an alert to perform a blood glucose test at the dental centre. Appropriate referrals to the medical centres will be made based on the test results.
Clinical phenotyping in dentistry and the Marshfield Clinic experience in clinical phenotyping utilizing PMRP
The term ‘clinical phenotype’ is broadly defined as a clinical manifestation or characteristics of a patient’s condition, where ‘condition’ may refer to such entities as disease (or lack of disease), physical characteristics such as systolic or diastolic blood pressure, gingival inflammation, tooth color, or definition of other factors that impact phenotypic expression. Phenotypic characterization involving infectious processes occur at two levels: 1) host phenotypic expression and response driven by infectious/inflammatory exposure; and 2) microbial phenotypic characterization of the microbial/inflammatory forces advancing the pathological manifestations. Phenotypic expression may vary among organisms with the same genotype due to the environmental influence. As an integral component of the human body, the microbiome imposes environmental signals on an individual.
Dental phenotypes can be identified and defined from multiple sources. Dental radiographs (bite wings and panorex), dental casts, and dental photographs (intra-oral and extra-oral photographs) are some important sources for identifying dental phenotypes. Transforming EDR data into research-appropriate clinical phenotypes could be very beneficial to focusing on the personalized oral health and also translating the results of the research into practice. There has been little exploration focussed on the clinical phenotypes, genotype, and the oral microbial environment of the patients to understand the personal health concept in dental and oral health. Although EDR is a highly valuable source for this purpose, it is not a straight-forward process. EDR data is rarely collected for the purpose of research, and deriving valid phenotypic information from the secondary use of EDR data is complicated. Data collected in the course of routine patient care is not validated to the extent needed to guarantee the presence or absence of a phenotype, making clinical phenotyping an arduous process.
Historically, the lack of precise and appropriate phenotyping has contributed to the often varying and non-reproducible results of some genetic and clinical studies due to variability in phenotypic definition (Borrell and Papapanou, 2005). Care must be taken at multiple levels, with rigorous attention paid to data quality, completeness, comparability and recognition and reduction of clinical heterogeneity. These issues are particularly challenging to address when using data gathered with the EHR to identify phenotypic populations. The Marshfield Clinic’s approach involves development of EDR-based algorithms using a combination of structured data extraction, natural language processing, or other methods to identify patients based on clinical features. The development of a phenotype definition requires in-depth clinician review of records identified by the preliminary EDR-based algorithms.
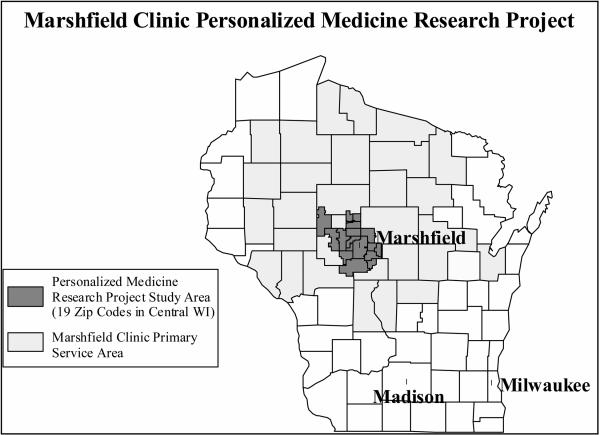
Although clinical phenotyping using the EDR is a time-consuming process involving a multi-disciplinary team to define the clinical attributes of a disease or condition, it is essential. As in all research that attempts to identify and quantify relationships between exposures and outcomes, rigorous characterization of study subjects, or phenotyping, is extremely important. Careful phenotyping is both critical to the eventual results discovered by the study and a source of great challenge due to the variety of phenotyping options and approaches that can be employed using the same data. Marshfield Clinic has had extensive experience in extracting phenotypes utilizing the combined resources of the EHR and the PMRP. Details about the PMRP can be found on the project website at http://www.marshfieldclinic.org/pmrp. The target cohort for PMRP is adults aged 18 years and older who reside in the Marshfield Epidemiologic Study Area (MESA). Established in 1991, MESA comprises a 19-Zip code region centered geographically around Marshfield, Wisconsin and a 9-Zip code area in northern Wisconsin (see Figure 1) and has a total population of approximately 90,000 residents. The majority of residents within MESA use the Marshfield Clinic for their health care, thus allowing for population-based epidemiologic research within the system. Except for the city of Marshfield (which has a population of 19,000), MESA residents reside rurally or in small towns or villages. The annual in- and out-migration is very low, making it ideal for prospective studies. By sharing a combined EHR with neighbouring hospitals, inpatient diagnoses, outpatient diagnoses and procedures are captured for MESA residents. Many MESA residents are also members of the Marshfield Clinic-sponsored health maintenance organization, Security Health Plan, allowing capture of health events that may occur outside the Marshfield Clinic system of care.
Currently >20,000 MESA residents have participated in PMRP (approximately 45% of eligible residents that could be contacted). The demographics and health characteristics of PMRP subjects are similar to adult residents of Wood County in central Wisconsin (98.2% white Caucasian). Thus, the PMRP cohort is ideal for genetic studies, since population stratification is not likely to introduce bias to study results. PMRP was supported with Federal, State and Marshfield Clinic funds, and has a database containing genetic, phenotypic, and environmental information that can be used for large-scale association studies. With over 20,000 participants, the PMRP continues to be one of the largest population-based biobanks in the US.
The PMRP cohort has been used in studies that aim to better understand the role of genetics in various diseases including cataracts (Waudby et al, 2011), endometriosis (McCarty, et al, 2012), Alzheimer disease (Ghebranious et al, 2011), fibromyalgia (Reeser et al, 2011), DM (Wilke et al, 2007), congestive heart failure (Wilke et al, 2009), and breast cancer (Onitilo et al, 2009). Other studies within PMRP relative to personalized medicine include the pharmacogenetics of warfarin clearance (Yazigi et al, 2010) and atorvastin efficacy (Wilke et al, 2008). PMRP is one of several biobank assets that are part of the eMERGE consortium. (McCarty et al, 2011).
Analysis of genetic contribution requires phenotypic characterization of cases and controls to support investigation of genetic association. Marshfield Clinic has developed extensive expertise in developing and validating electronic algorithms for phenotypic definition of disease. Some approaches to defining phenotypes remain in progress while several have been published previously (Wauby et al, 2011; Conway et al, 2011; Wilke et al, 2010).
To investigate the relationship between oral health and systemic health, Marshfield Clinic is currently recruiting subjects for an oral-systemic health research sub-cohort to PMRP. This cohort will also be used to better understand the genetic contributions to PD and caries. A cohort of 2000–3000 individuals is envisioned with iEHR data, DNA, plasma, serum, oral samples (saliva, subgingival plaque), and cross sectional longitudinal clinical tests (HbA1C, C-reactive protein, fasting glucose, urine microalbumin) collected at defined intervals. We aim to build on our past success in recruiting subjects for personalized medicine research, and indeed, these new subjects will become part of PMRP.
Microbiome research at Marshfield Clinic
The availability of saliva and subgingival plaque collected in the context of the oral-systemic sub-cohort of PMRP will provide an important resource for microbiome characterization in patients with and without PD or DM. Dental samples are being obtained from individuals with no PD as well as mild to severe PD at the time of their visit to a Marshfield Clinic’s dental centre. Accurate phenotyping of these patients is accomplished through conduct of a complete oral examination by trained, calibrated dental examiners. Periodontal status is assessed at six sites per tooth (mesiobuccal, midbuccal, distobuccal, mesiolingual, midlingual, and distolingual) for all teeth present. Probing depth (mm) and location of the gingival margin in relation to the cementoenamel junction is measured using a UNC-15 manual probe (HuFriedy, Chicago, Illinois, USA) (Desvarieux et al, 2010). Dentists perform a full-mouth clinical examination including inspection of the teeth, oral mucosa, and periodontal tissues to characterize oral health status of all individuals. Inclusion criteria for subjects with untreated PD include no antibiotic usage in the past 3 months, no routine dental care in the past 12 months, at least eight sites with a pocket depth >4mm, and six sites with attachment loss levels >4mm. Inclusion criteria for subjects defined as having no PD include no periodontal pockets deeper than 3 mm, no antibiotic use in the past 3 months, and no clinical evidence of PD or other concurrent oral disease. In addition, patients are assessed for diabetic status by determining HbA1C levels. Collection of these specimens will allow characterization of microbiome in the presence of PD and following resolution of infectious processes. Further, comparisons of microbiomes in patients with and without DM are planned to gain insights into how the environment impacts the metagenomic footprint in the presence and absence of glycemic control.
Summary
Global epidemic prevalence of PD and DM and growing evidence supporting bidirectional exacerbation of these conditions on each other present an escalating health crisis requiring immediate intervention. In addition, evidence suggests that PD may be a risk factor for a broad spectrum of other chronic systemic conditions. Notably, cost effective interventions for PD are available, rendering it a modifiable risk factor for a large percentage of the affected population. At Marshfield Clinic, we are pursuing oral-systemic personalized medicine as an interventional approach to the growing problem. Advancement of personalized medicine is possible because we are positioned to align essential resources to advance the definition of phenotype, genotype, and environmental factors contributing to disease and health. These resources include the iEHR, iEDW, PMRP, a state of the art dental clinic infrastructure spanning much of the state and targeting service to high risk populations, and a research focus on oral microbiomes, metagenomics, and genetic and physiological factors regulating host response and interaction with infectious processes. This line of research will advance the understanding of pathogenic mechanisms contributing to PD and other systemic conditions such as DM and contribute further to developing personalized approaches to intervention and prevention. Harnessing these interdisciplinary resources into a multifaceted approach intended to move oral systemic personalized medicine further into the clinical arena will have more collateral benefits. In addition to advancing metagenomic and human genetic research relative to the oral cavity, advancing microbiomics and integrated dental HIT, we are also positioned to engage in clinical, translational, and comparative effectiveness research. Finally, because the dental infrastructural model in which we are advancing personalized medicine was put into place to address regional healthcare disparities, our efforts are ultimately establishing transformational care models that will inform and shape health policy for the future. In conclusion, we are envisioning a shift in paradigm from ‘personalized medicine’ to ‘personalized health’.
Figure 4.
Clinical Decision Support System (CDSS) to Support Cross-disciplinary Plan of Care Process Model
Figure 5.
Personalized Medicine Research Project (PMRP) recruitment area.
Acknowledgements
Research presented in this paper is supported by grant 1UL1RR025011 from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources, and NHGRI grant # 1U01HG006389-01 “IRIS: Incorporating Research into Saving Sight,” National Institutes of Health, and a grant from Delta Dental of Wisconsin. The authors thank Steven J. Schrodi, PhD for critically reviewing the manuscript and providing helpful comments. The authors also thank Marie Fleisner of the Marshfield Clinic Research Foundation for editorial assistance in the preparation and submission of this article.
Key to Abbreviations
- ADA
American Dental Association
- AGE
advanced glycated end-products
- CDDS
Clinical Decision Support Systems
- CPRs
computer-based records
- CSF3
colony stimulating factor 3
- DEFB4
beta 4 defensin
- DM
diabetes mellitus
- EDR
electronic dental record
- FHC
Clinic’s Family Health Center
- FQHC
federally qualified health centre
- FTE
full-time equivalent
- HbA1C
haemoglobin A1C
- HER
electronic health record
- HIT
health information technology
- HOMIM
Human Microbe Identification Microarray
- iEDW
integrated Enterprise Data Warehouse
- iEHR
integrated medical-dental electronic health record
- IL1-R1
interleukin-1 receptor 1
- IL-1β
interleukin-1 beta
- IL-6
interleukin-6
- IOM
Institute of Medicine
- IP10
interferon gamma-induced protein 10
- I-TAC
interferon inducible T-cell alpha chemoattractant
- JNK
Jun N-terminal Kinase
- LPS
lipopolysaccharides
- MESA
Marshfield Epidemiologic Study Area
- MMP10
matrix metalloproteinase 10
- PD
periodontal disease
- PMRP
Personalized Medicine Research Project
- RANTES
Regulated on Activation, Normal T Expressed and Secreted
- SBS
sequencing by synthesis
- SNP
single-nucleotide polymorphism
- SOD2
superoxide dismutase 2
- T2DM
Type 2DM
- TLR4
Toll-like receptor 4
- TNF
tumour necrosis factors
- WHO
World Health Organization
Footnotes
HIPPA is a US law designed to provide privacy standards to protect patients’ medical records and other health information provided to health plans, doctors, hospitals and other health care providers. Developed by the U.S. Department of Health and Human Services, these standards provide patients with access to their medical records and more control over how their personal health information is used and disclosed. HIPAA took effect on April 14, 2003.
Author Contributions: Glurich researched and authored the topic background, compiled the initial draft, critically revised late drafts, and edited the final draft. Acharya authored the IT component of the topic, critically revised the late drafts, and edited the final draft. Shukla contributed to drafting of some sections of the topic background, critical revision of the late drafts, and editing of the final draft. Nycz contributed text to topic background on dental infrastructure and review of the final draft. Brilliant initiated the idea and manuscript structure, provided guidance through the writing process, contributed text on the PMRP infrastructure and studies, participated in critically revising/editing the final draft, had full access to all study data and takes responsibility for the integrity and the accuracy of the materials presented in this article. All authors approved the final manuscript.
References
- Albandar JM, Brunelle JA, Kingman A. Destructive periodontal disease in adults age 30 years and older in the United States. J Periodontol. 1999;70:13–29. doi: 10.1902/jop.1999.70.1.13. [DOI] [PubMed] [Google Scholar]
- American DentalAssociation SurveyCenter . 2006 Technology Survey. ADA; Chicago, IL: 2007. Available at: http://www.ada.org/1440.aspx. [Google Scholar]
- American Diabetes Association Diabetes Statistics:Data from the 2011 National Diabetes Fact Sheet. 2011 Available at: http://www.diabetes.org/diabetes-basics/diabetes-statistics/
- American Recoveryand Reinvestment Act of2009. Available at: http://www.recovery.gov/About/Pages/The_Act.aspx.
- Amir J, Waite M, Tobler J, et al. The role of hyperglycemia in mechanisms of exacerbated inflammatory responses within the oral cavity. Cell Immunol. 2011;272:45–52. doi: 10.1016/j.cellimm.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki E, Oyadomari S, Mori M. Impact of endoplasmic reticulum stress pathway on pancreatic beta-cells and diabetes mellitus. Exp Biol Med (Maywood) 2003;228:1213–1217. doi: 10.1177/153537020322801018. [DOI] [PubMed] [Google Scholar]
- Avila M, Ojcius DM, Yilmaz O. The oral microbiota: living with a permanent guest. DNA Cell Biol. 2009;28:405–411. doi: 10.1089/dna.2009.0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck JD, Eke P, Heiss G, et al. Periodontal disease and coronary heart disease: a reappraisal of the exposure. Circulation. 2005;112:19–24. doi: 10.1161/CIRCULATIONAHA.104.511998. [DOI] [PubMed] [Google Scholar]
- Belting SM, Hiniker JJ, Dummett CO. Influence of diabetes mellitus on severity of periodontal disease. J Periodontol. 1964;35:476–480. [Google Scholar]
- Berbari EF, Cockerill FR, 3rd, Steckelberg JM. Infective endocarditis due to unusual or fastidious microorganisms. Mayo Clin Proc. 1997;72:532–542. doi: 10.4065/72.6.532. [DOI] [PubMed] [Google Scholar]
- Borrell LN, Papapanou PN. Analytical epidemiology of periodontitis. J Clin Periodontol. 2005;32:132–158. doi: 10.1111/j.1600-051X.2005.00799.x. [DOI] [PubMed] [Google Scholar]
- Brett PM, Zygogianni P, Griffiths GS, et al. Functional Gene Polymorphisms in Aggressive and Chronic Periodontitis. J Dent Res. 2005;84:1149–1153. doi: 10.1177/154405910508401211. [DOI] [PubMed] [Google Scholar]
- Burt B, Research, Science and Therapy Committee of the American Academy of Periodontology Position Paper: Epidemiology of Periodontal Diseases. J Periodontol. 2005;76:1406–1419. doi: 10.1902/jop.2005.76.8.1406. [DOI] [PubMed] [Google Scholar]
- California Healthcare Foundation Health Information Technology in California Dental Practices: Survey Findings. 2010 Available at: http://www.chcf.org/publications/2010/08/health-information-technology-in-california-dental-practices-survey-findings.
- Centers for Disease Control and Prevention . National Diabetes Fact Sheet: Fast Facts on Diabetes. US Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA: 2011. Available at: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. [Google Scholar]
- Chávarry NG, Vettore MV, Sansone C, Sheiham A. The relationship between diabetes mellitus and destructive periodontal disease: a meta-analysis. Oral Health Prev Dent. 2009;7:107–127. [PubMed] [Google Scholar]
- Chen T, Yu WH, Izard J, Baranova OV, Lakshmanan A, Dewhirst FE. The Human Oral Microbiome Database: a web accessible resource for investigating oral microbe taxonomic and genomic information. Database (Oxford) 2010;2010:baq013. doi: 10.1093/database/baq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi AC, Neville BW, Krayer JW, Gonsalves WC. Oral manifestations of systemic disease. Am Fam Physician. 2010;82:1381–1388. [PubMed] [Google Scholar]
- Colombo AP, Boches SK, Cotton SL, et al. Comparisons of subgingival microbial profiles of refractory periodontitis, severe periodontitis, and periodontal health using the human oral microbe identification microarray. J Periodontol. 2009;80:1421–1432. doi: 10.1902/jop.2009.090185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee on Comparative Effectiveness Research Prioritization. Institute of Medicine . Initial National Priorities for Comparative Effectiveness Research. National Academies Press; Washington, DC: 2009. [Google Scholar]
- Committee on Quality of Health Care in America. Institute of Medicine . Crossing the Quality Chasm: A New Health System for the 21st Century. National Academies Press; Washington, DC: 2001. [Google Scholar]
- Committee on the Future of Dental Education. Institute of Medicine . In: Dental Education at the Crossroads: Challenges and Change. Field MJ, editor. National Academies Press; Washington, DC: 1995. [PubMed] [Google Scholar]
- Conway M, Berg RL, Carrell D, et al. Analyzing the heterogeneity and complexity of electronic health record oriented phenotyping algorithms. AMIA Annu Symp Proc. 2011;2011:274–283. [PMC free article] [PubMed] [Google Scholar]
- Covani U, Marconcini S, Derchi G, Barone A, Giacomelli L. Relationship between human periodontitis and type 2 diabetes at a genomic level: a data-mining study. J Periodontol. 2009;80:1265–1273. doi: 10.1902/jop.2009.080671. [DOI] [PubMed] [Google Scholar]
- Darré L, Vergnes JN, Gourdy P, Sixou M. Efficacy of periodontal treatment on glycaemic control in diabetic patients: A meta-analysis of interventional studies. Diabetes Metab. 2008;34:497–506. doi: 10.1016/j.diabet.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol. 2010;8:481–490. doi: 10.1038/nrmicro2337. [DOI] [PubMed] [Google Scholar]
- Deschner J, Haak T, Jepsen S, et al. [Diabetes mellitus and periodontitis. Bidirectional relationship and clinical implications. A consensus document] Internist (Berl) 2011;52:466–477. doi: 10.1007/s00108-011-2835-2. [DOI] [PubMed] [Google Scholar]
- Desvarieux M, Demmer RT, Jacobs DR, Jr, et al. Periodontal bacteria and hypertension: the oral infections and vascular disease epidemiology study (INVEST) J Hypertens. 2010;28:1413–1421. doi: 10.1097/HJH.0b013e328338cd36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhirst FE, Chen T, Izard J, et al. The human oral microbiome. J Bacteriol. 2010;192:5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee on Improving the Patient Record. Institute of Medicine . In: The Computer-Based Patient Record: An Essential Technology for Health Care. Dick RS, Steen EB, Detmer DE, editors. National Academies Press; Washington, DC: 1997. [PubMed] [Google Scholar]
- Diehl SR, Wu T, Burmeister JA, et al. Evidence of a substantial genetic basis for IgG2 levels in families with aggressive periodontitis. J Dent Res. 2003;82:708–712. doi: 10.1177/154405910308200910. [DOI] [PubMed] [Google Scholar]
- Diehl SR, Wu T, Michalowicz BS, et al. Quantitative measures of aggressive periodontitis show substantial heritability and consistency with traditional diagnoses. J Periodontol. 2005;76:279–288. doi: 10.1902/jop.2005.76.2.279. [DOI] [PubMed] [Google Scholar]
- Diehl SR, Chou CH, Kuo F, Huang Cy. Genetic factors and Periodontal Disease. In: Newman MG, Takei H, Carranza FA, Klokkevold PR, editors. Carranza’s Clinical Periodontology. 11th ed Elsevier-Saunders; St. Louis, MO: 2011. pp. 274–284. [Google Scholar]
- Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- Eng G, Chen A, Vess T, Ginsburg G. Genome technologies and personalized dental medicine. Oral Dis. 2011 doi: 10.1111/j.1601-0825.2011.01876.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes. 2003;52:1–8. doi: 10.2337/diabetes.52.1.1. [DOI] [PubMed] [Google Scholar]
- Genco RJ, Grossi SG, Ho A, Nishimura F, Murayama Y. A proposed model linking inflammation to obesity, diabetes, and periodontal infections. J Periodontol. 2005;76:2075–2084. doi: 10.1902/jop.2005.76.11-S.2075. [DOI] [PubMed] [Google Scholar]
- Ghebranious N, Mukesh B, Giampietro PF, et al. A pilot study of gene/gene and gene/environment interactions in Alzheimer disease. Clin Med Res. 2011;9:17–25. doi: 10.3121/cmr.2010.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JI, Ley RE, Wilson R, et al. Extending our view of self: the human gut microbiome initiative (HGMI) 2005 Available at: http://www.genome.gov/Pages/Research/Sequencing/SeqProposals/HGMISeq.pdf.
- Huynh-Ba G, Lang NP, Tonetti MS, Salvi GE. The association of the composite IL-1 genotype with periodontitis progression and/or treatment outcomes: a systematic review. J Clin Periodontol. 2007;34:305–317. doi: 10.1111/j.1600-051X.2007.01055.x. [DOI] [PubMed] [Google Scholar]
- Jakubovics NS, Kolenbrander PE. The road to ruin: the formation of disease-associated oral biofilms. Oral Dis. 2010;16:729–739. doi: 10.1111/j.1601-0825.2010.01701.x. [DOI] [PubMed] [Google Scholar]
- Janket SJ, Wightman A, Baird AE, Van Dyke TE, Jones JA. Does periodontal treatment improve glycemic control in diabetic patients? A meta-analysis of intervention studies. J Dent Res. 2005;84:1154–1159. doi: 10.1177/154405910508401212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepsen S, Kebschull M, Deschner J. [Relationship between periodontitis and systemic diseases] Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2011;54:1089–1096. doi: 10.1007/s00103-011-1348-4. [DOI] [PubMed] [Google Scholar]
- Joshipura KJ, Hung HC, Rimm EB, Willett WC, Ascherio A. Periodontal disease, tooth loss, and incidence of ischemic stroke. Stroke. 2003;34:47–52. doi: 10.1161/01.str.0000052974.79428.0c. [DOI] [PubMed] [Google Scholar]
- Kaur G, Holtfreter B, Rathmann W, et al. Association between type 1 and type 2 diabetes with periodontal disease and tooth loss. J Clin Periodontol. 2009;36:765–774. doi: 10.1111/j.1600-051X.2009.01445.x. [DOI] [PubMed] [Google Scholar]
- Keavney B. The interleukin 1 cluster, dyslipidemia and risk of myocardial infarction. BMC Medicine. 2010;8:6. doi: 10.1186/1741-7015-8-6. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee on Quality of Health Care in America. Institute of Medicine . In: To Err Is Human: Building a Safer Health System. Kohn LT, Corrigan JM, Donaldson MS, editors. National Academies Press; Washington, DC: 2000. [PubMed] [Google Scholar]
- Kebschull M, Demmer RT, Papapanou PN. “Gum bug, leave my heart alone”--epidemiologic and mechanistic evidence linking periodontal infections and atherosclerosis. J Dent Re. 2010;89:879–902. doi: 10.1177/0022034510375281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keijser BJ, Zaura E, Huse SM, et al. Pyrosequencing analysis of the oral microflora of healthy adults. J Dent Res. 2008;87:1016–1020. doi: 10.1177/154405910808701104. [DOI] [PubMed] [Google Scholar]
- Khader YS, Dauod AS, El-Qaderi SS, Alkafajei A, Batayha WQ. Periodontal status of diabetics compared with nondiabetics: a meta-analysis. J Diabetes Complications. 2006;20:59–68. doi: 10.1016/j.jdiacomp.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med. 2004;141:413–420. doi: 10.7326/0003-4819-141-6-200409210-00006. [DOI] [PubMed] [Google Scholar]
- Kim J, Amar S. Periodontal disease and systemic conditions: a bidirectional relationship. Odontology. 2006;94:10–21. doi: 10.1007/s10266-006-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinane D, Bouchard P, Group E of European Workshop on Periodontology Periodontal diseases and health: Consensus report of the Sixth European Workshop on Periodontology. J Clin Periodontol. 2008;35:333–337. doi: 10.1111/j.1600-051X.2008.01278.x. [DOI] [PubMed] [Google Scholar]
- Kinane DF, Hart TC. Genes and gene polymorphisms associated with periodontal disease. Crit Rev Oral Biol Med. 2003;14:430–449. doi: 10.1177/154411130301400605. [DOI] [PubMed] [Google Scholar]
- King GL. The role of inflammatory cytokines in diabetes and its complications. J Periodontol. 2008;79:1527–1534. doi: 10.1902/jop.2008.080246. [DOI] [PubMed] [Google Scholar]
- Kshirsagar AV, Moss KL, Elter JR, Beck JD, Offenbacher S, Falk RJ. Periodontal disease is associated with renal insufficiency in the Atherosclerosis Risk In Communities (ARIC) study. Am J Kidney Dis. 2005;45:650–657. doi: 10.1053/j.ajkd.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Kuo LC, Polson AM, Kang T. Associations between periodontal diseases and systemic diseases: a review of the inter-relationships and interactions with diabetes, respiratory diseases, cardiovascular diseases and osteoporosis. Public Health. 2008;122:417–433. doi: 10.1016/j.puhe.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Laine ML, Loos BG, Crielaard W. Gene polymorphisms in chronic periodontitis. Int J Dent. 2010;2010:324719. doi: 10.1155/2010/324719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalla E, Papapanou PN. Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat Rev Endocrinol. 2011 doi: 10.1038/nrendo.2011.106. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Lederberg J, Mccray AT. Ome sweet omics – a genealogical treasury of words. The Scientist. 2001;15:8–10. [Google Scholar]
- Löe H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care. 1993;16:329–334. [PubMed] [Google Scholar]
- Loos BG. Systemic markers of inflammation in periodontitis. J Periodontol. 2005;76:2106–2115. doi: 10.1902/jop.2005.76.11-S.2106. [DOI] [PubMed] [Google Scholar]
- Marigo L, Cerreto R, Giuliani M, Somma F, Lajolo C, Cordaro M. Diabetes mellitus: biochemical, histological and microbiological aspects in periodontal disease. Eur Rev Med Pharmacol Sci. 2011;15:751–758. [PubMed] [Google Scholar]
- McCarty CA, Berg RL, Welter JD, Kitchner TE, Kemnitz JW. A novel gene-environment interaction involved in endometriosis. Int J Gynaecol Obstet. 2012;116:61–63. doi: 10.1016/j.ijgo.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty CA, Chisholm RL, Chute CG, et al. The eMERGE Network: a consortium of biorepositories linked to electronic medical records data for conducting genomic studies. BMC Med Genomics. 2011;4:13. doi: 10.1186/1755-8794-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mealey BL, Oates TW, American Academy of Periodontology Diabetes mellitus and periodontal diseases. Periodontol. 2006;77:1289–1303. doi: 10.1902/jop.2006.050459. [DOI] [PubMed] [Google Scholar]
- Mealey BL, Rose LF. Diabetes mellitus and inflammatory periodontal diseases. Curr Opin Endocrinol Diabetes Obes. 2008;15:135–141. doi: 10.1097/MED.0b013e3282f824b7. [DOI] [PubMed] [Google Scholar]
- Nagasawa T, Noda M, Katagiri S, et al. Relationship between periodontitis and diabetes - importance of a clinical study to prove the vicious cycle. Intern Med. 2010;49:881–885. doi: 10.2169/internalmedicine.49.3351. [DOI] [PubMed] [Google Scholar]
- NIH HMP Working Group. Peterson J, Garges S, et al. The NIH Human Microbiome Project. Genome Res. 2009;19:2317–2323. doi: 10.1101/gr.096651.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolopoulos GK, Dimou NL, Hamodrakas SJ, Bagos PG. Cytokine gene polymorphisms in periodontal disease: a meta-analysis of 53 studies including 4178 cases and 4590 controls. J Clin Periodontol. 2008;35:754–767. doi: 10.1111/j.1600-051X.2008.01298.x. [DOI] [PubMed] [Google Scholar]
- O’Connor PJ, Sperl-Hillen J, Johnson PE, Rush WA, Crain AL. Customized feedback to patients and providers failed to improve safety or quality of diabetes care: a randomized trial. Diabetes Care. 2009;32:1158–1163. doi: 10.2337/dc08-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offenbacher S, Barros SP, Paquette DW, et al. Gingival transcriptome patterns during induction and resolution of experimental gingivitis in humans. J Periodontol. 2009;80:1963–1982. doi: 10.1902/jop.2009.080645. [DOI] [PubMed] [Google Scholar]
- Offenbacher S, Beck JD, Lieff S, Slade G. Role of periodontitis in systemic health: spontaneous preterm birth. J Dent Educ. 1998;62:852–858. [PubMed] [Google Scholar]
- Onitilo AA, McCarty CA, Wilke RA, et al. Estrogen receptor genotype is associated with risk of venous thromboembolism during tamoxifen therapy. Breast Cancer Res Trea. 2009;115:643–650. doi: 10.1007/s10549-008-0264-2. [DOI] [PubMed] [Google Scholar]
- Osheroff JA, Teich JM, Middleton B, Steen EB, Wright A, Detmer DE. A roadmap for national action on clinical decision support. J Am Med Inform Assoc. 2007;14:141–145. doi: 10.1197/jamia.M2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 2007;78:1387–1399. doi: 10.1902/jop.2007.060264. [DOI] [PubMed] [Google Scholar]
- Peterson KA, Radosevich DM, O’Connor PJ, et al. Improving Diabetes Care in Practice: findings from the TRANSLATE trial. Diabetes Care. 2008;31:2238–2243. doi: 10.2337/dc08-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajilić-Stojanović M, Smidt H, de Vos WM. Diversity of the human gastrointestinal tract microbiota revisited. Environ Microbiol. 2007;9:2125–2136. doi: 10.1111/j.1462-2920.2007.01369.x. [DOI] [PubMed] [Google Scholar]
- Reeser JC, Payne E, Kitchner T, McCarty CA. Apolipoprotein e4 genotype increases the risk of being diagnosed with posttraumatic fibromyalgia. PM R. 2011;3:193–197. doi: 10.1016/j.pmrj.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Rial NS, Choi K, Nguyen T, Snyder B, Slepian MJ. Nuclear factor kappa B (NF-κB): A novel cause for diabetes, coronary artery disease and cancer initiation and promotion? Med Hypotheses. 2011 doi: 10.1016/j.mehy.2011.09.034. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312:71–72. doi: 10.1136/bmj.312.7023.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto M, Huang Y, Ohnishi M, Umeda M, Ishikawa I, Benno Y. Changes in oral microbial profiles after periodontal treatment as determined by molecular analysis of 16S rRNA genes. J Med Microbiol. 2004;53:563–571. doi: 10.1099/jmm.0.45576-0. [DOI] [PubMed] [Google Scholar]
- Sakamoto M, Umeda M, Benno Y. Molecular analysis of human oral microbiota. J Periodontal Res. 2005;40:277–285. doi: 10.1111/j.1600-0765.2005.00793.x. [DOI] [PubMed] [Google Scholar]
- Santos Tunes R, Foss-Freitas MC, Nogueira-Filho Gda R. Impact of Periodontitis on the Diabetes-Related Inflammatory Status. J Can Dent Assoc. 2010;76:a35. [PubMed] [Google Scholar]
- Saremi A, Nelson RG, Tulloch-Reid M, et al. Periodontal disease and mortality in type 2 diabetes. Diabetes Care. 2005;28:27–32. doi: 10.2337/diacare.28.1.27. [DOI] [PubMed] [Google Scholar]
- Savage A, Eaton KA, Moles DR, Needleman I. A systematic review of definitions of periodontitis and methods that have been used to identify this disease. J Clin Periodontol. 2009;36:458–467. doi: 10.1111/j.1600-051X.2009.01408.x. [DOI] [PubMed] [Google Scholar]
- Scannapieco FA. Role of oral bacteria in respiratory infection. J Periodontol. 1999;70:793–802. doi: 10.1902/jop.1999.70.7.793. [DOI] [PubMed] [Google Scholar]
- Schenkein HA. Finding genetic risk factors for periodontal diseases: is the climb worth the view? Periodontol 2000. 2002;30:79–90. doi: 10.1034/j.1600-0757.2002.03008.x. [DOI] [PubMed] [Google Scholar]
- Schmittdiel J, McMenamin SB, Halpin HA, et al. The use of patient and physician reminders for preventive services: results from a National Study of Physician Organizations. Prev Med. 2004;39:1000–1006. doi: 10.1016/j.ypmed.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Seymour GJ, Ford PJ, Cullinan MP, Leishman S, Yamazaki K. Relationship between periodontal infections and systemic disease. Clin Microbiol Infect. 2007;13(Suppl 4):3–10. doi: 10.1111/j.1469-0691.2007.01798.x. [DOI] [PubMed] [Google Scholar]
- Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultis WA, Weil EJ, Looker HC, et al. Effect of periodontitis on overt nephropathy and end-stage renal disease in type 2 diabetes. Diabetes Care. 2007;30:306–311. doi: 10.2337/dc06-1184. [DOI] [PubMed] [Google Scholar]
- Simpson TC, Needleman I, Wild SH, Moles DR, Mills EJ. Treatment of periodontal disease for glycaemic control in people with diabetes. Cochrane Database Syst Rev. 2010;5 doi: 10.1002/14651858.CD004714.pub2. CD004714. [DOI] [PubMed] [Google Scholar]
- Smyth S, Heron A. Diabetes and obesity: the twin epidemics. Nat Med. 2006;12:75–80. doi: 10.1038/nm0106-75. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- Taylor GW. Bidirectional interrelationships between diabetes and periodontal diseases: an epidemiologic perspective. Ann Periodontol. 2001;6:99–112. doi: 10.1902/annals.2001.6.1.99. [DOI] [PubMed] [Google Scholar]
- Taylor GW, Borgnakke WS. Periodontal disease: associations with diabetes, glycemic control and complications. Oral Dis. 2008;14:191–203. doi: 10.1111/j.1601-0825.2008.01442.x. [DOI] [PubMed] [Google Scholar]
- Teeuw WJ, Gerdes VE, Loos BG. Effect of periodontal treatment on glycemic control of diabetic patients: a systematic review and meta-analysis. Diabetes Care. 2010;33:421–427. doi: 10.2337/dc09-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson TG, Brailer DJ. The decade of health information technology: delivering consumer-centric and information-rich health care - framework for strategic action. U.S. Department of Health and Human Services, Office of the National Coordinator for Health Information Technology; Washington, DC: 2004. [Google Scholar]
- U.S. Department of Health and Human Services . Oral health in America: A report of the surgeon general (executive summary) U.S. Department of Health and Human Services, National Institute of Dental and Craniofacial Research, National Institutes of Health; Rockville, MD: 2000. Available at: http://silk.nih.gov/public/hck1ocv.@www.surgeon.fullrpt.pdf. [Google Scholar]
- Wade WG. Has the use of molecular methods for the characterization of the human oral microbiome changed our understanding of the role of bacteria in the pathogenesis of periodontal disease? J Clin Periodontol. 2011;38:7–16. doi: 10.1111/j.1600-051X.2010.01679.x. [DOI] [PubMed] [Google Scholar]
- Waudby CJ, Berg RL, Linneman JG, et al. Cataract research using electronic health records. BMC Ophthalmol. 2011;11:32. doi: 10.1186/1471-2415-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidlich P, Cimões R, Pannuti CM, Oppermann RV. Association between periodontal diseases and systemic diseases. Braz Oral Re. 2008;22(Suppl 1):32–43. doi: 10.1590/s1806-83242008000500006. [DOI] [PubMed] [Google Scholar]
- White JM, Kalenderian E, Stark PC, Ramoni RL, Vaderhobli R, Walji MF. Evaluating a dental diagnostic terminology in an electronic health record. J Dent Educ. 2011;75:605–615. [PMC free article] [PubMed] [Google Scholar]
- Wilke RA, Berg RL, Linneman JG, et al. Quantification of the clinical modifiers impacting high-density lipoprotein cholesterol in the community: Personalized Medicine Research Project. Prev Cardiol. 2010;13:63–68. doi: 10.1111/j.1751-7141.2009.00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke RA, Berg RL, Linneman JG, Zhao C, McCarty CA, Krauss RM. Characterization of low-density lipoprotein cholesterol-lowering efficacy for atorvastatin in a population-based DNA biorepository. Basic Clin Pharmacol Toxicol. 2008;103:354–359. doi: 10.1111/j.1742-7843.2008.00291.x. [DOI] [PubMed] [Google Scholar]
- Wilke RA, Berg RL, Peissig P, et al. Use of an electronic medical record for the identification of research subjects with diabetes mellitus. Clin Med Res. 2007;5:1–7. doi: 10.3121/cmr.2007.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke RA, Simpson RU, Mukesh BN, et al. Genetic variation in CYP27B1 is associated with congestive heart failure in patients with hypertension. Pharmacogenomics. 2009;10:1789–1797. doi: 10.2217/pgs.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RC, Barnett AH, Claffey N, et al. The potential impact of periodontal disease on general health: a consensus view. Curr Med Res Opin. 2008;24:1635–1643. doi: 10.1185/03007990802131215. [DOI] [PubMed] [Google Scholar]
- World Health Organization . Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. World Health Organization; Geneva, Switzerland: 2009. [Google Scholar]
- Xie G, Chain PS, Lo CC, et al. Community and gene composition of a human dental plaque microbiota obtained by metagenomic sequencing. Mol Oral Microbiol. 2010;25:391–405. doi: 10.1111/j.2041-1014.2010.00587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazigi N, Yale S, Caldwell M, et al. Modeling clearance of warfarin. J Gen Intern Med. 2010;25(Suppl 3):S334. (Abstract) [Google Scholar]
- Zarco M, Vess T, Ginsburg G. The oral microbiome in health and disease and the potential impact on personalized dental health. Oral Dis. 2012;18:109–120. doi: 10.1111/j.1601-0825.2011.01851.x. [DOI] [PubMed] [Google Scholar]