Abstract
Aims
Protection of cells from oxidative insult may be possible through direct scavenging of reactive oxygen species, or through stimulation of intracellular antioxidant defense mechanisms by induction of antioxidant gene expression. In this study we investigated the cytoprotective effect of chamomile and elucidated the underlying mechanisms.
Main Methods
The cytoprotective effect of chamomile was examined on H2O2-induced cellular stress in RAW 264.7 murine macrophages.
Key Findings
RAW 264.7 murine macrophages treated with chamomile were protected from cell death caused by H2O2. Treatment with 50 μM H2O2 for 6 h caused significant increase in cellular stress accompanied by cell death in RAW 264.7 macrophages. Pretreatment with chamomile at 10-20 μg/mL for 16 h followed by H2O2 treatment protected the macrophages against cell death. Chamomile exposure significantly increased the expression of antioxidant enzymes viz. heme oxygenase-1 (HO-1), peroxiredoxin-1 (Prx-1), and thioredoxin-1 (Trx-1) in a dose-dependent manner, compared with their respective controls. Chamomile increased nuclear translocation of Nrf2 with increased phosphorylated Nrf2 levels, and binding to the antioxidant response element in the nucleus.
Significance
These molecular findings for the first time provide insights into the mechanisms underlying the induction of phase 2 enzymes through the Keap1-Nrf2 signaling pathway by chamomile, and provide evidence that chamomile possesses antioxidant and cytoprotective properties.
Keywords: chamomile, macrophages, phase II enzymes, oxidative stress, antioxidant defense, inflammation
INTRODUCTION
Chamomile is a member of the daisy family, many of whose species have been used world-wide in folk medicine from ancient times (McKay and Blumberg, 2006; Srivastava et al., 2010) The plants of this family exhibit various biological and pharmacological activities, including anti-inflammatory, antimicrobial, anticancer, antispasmodic and sedative properties (McKay and Blumberg, 2006; Srivastava and Gupta, 2007; Srivastava et al., 2010). Aqueous extract of chamomile in the form of tea has been consumed at a frequency of more than a million cups per day for its anti-inflammatory, analgesic and cytoprotective effects (Srivastava et al., 2010; Kato et al., 2008). The beneficial effects of chamomile are related to the presence of several flavonoid constituents and the core structure consisting of either flavone (apigenin, luteolin) or flavonol-derivatives (quercetin, patuletin) occurring in various forms such as aglyco- mono- and di- glycosides and/or acyl-derivatives. Chamomile also contains high levels of coumarins, hydroxycoumarins, terpenoids, and is one of the richest sources of dietary antioxidants (McKay and Blumberg, 2006; Srivastava et al., 2010; Kato et al., 2008; Carnat et al., 2004). In cell culture, chamomile has been demonstrated to modulate the production of many pro-inflammatory mediators such as superoxide anion, tumor necrosis factor-alpha, interleukins and prostaglandins by selectively targeting cyclooxygenase-2 (Yoo et al., 2009; Smolinski and Pestka, 2003; Lee et al., 2010; Srivastava et al., 2009). We have demonstrated that chamomile inhibits the production of nitric oxide and inducible nitric oxide synthase in a dose-dependent manner without causing toxicity to murine macrophages (Bhaskaran et al., 2010). Moreover, a study has shown that the flavonoids from chamomile has the ability to protect cells from excessive production of reactive oxygen species such as superoxide radical and hydrogen peroxide (H2O2) (Yoo, et al., 2009).
H2O2 is a bi-product of aerobic metabolism and is generated in sites of tissue inflammation. An increased level of H2O2 in cells results in oxidative stress and causes cellular damage (Veal et al., 2007). H2O2 diffuses readily across cellular membranes and is converted into a highly reactive and toxic hydroxyl radical via the heme-catalyzed Fenton reaction. Cells respond to H2O2-induced oxidative stress, in part, by increasing the expression of cytoprotective antioxidants enzymes. Among antioxidant enzymes, heme oxygenase (HO)-1 possesses potent anti-inflammatory and anti-proliferative properties, and catalyzes the degradation of heme to biliverdin, iron and carbon monoxide (Zweier et al., 2011; Otterbein and Choi, 2000). Upregulation of HO-1 expression is recognized as a key event in cellular maintenance of adaptive survival response and cytoprotective defense system. Upregulation of HO-1 is mediated by activation of relevant redox-sensitive cytoplasmic transcriptional regulators, such as nuclear factor E2-related factor 2 (Nrf2), activator protein (AP)-1, nuclear factor kappa B (NF-κB), and cAMP-responsive element-binding protein (CREB) (Otterbein and Choi, 2000). Nrf2 is a member of the CNC-bZIP (cap‘n’collar subfamily of basic leucine-zipper) family of basic leucine zipper proteins and remains inactive in association with Keap1 (Kelch-like ECH associating protein 1) under normal condition in the cytoplasm. Upon oxidative stimulation, Nrf2 dissociates from Keap1, translocates into the nucleus and binds to the antioxidant response element (ARE) in the promoter region of phase II anti-oxidant enzymes such as NAD(P)H:quinone oxidoreductase (NQO1), glutathione S-transferase (GST), glutathione peroxidase (GPx), heme oxygenase (HO)-1, peroxiredoxin (Prx)-1 and thioredoxin (Trx)-1 (Lee et al., 2005; Kaspar et al., 2009). Although the anti-inflammatory effects of chamomile have been well documented, the molecular mechanisms underlying antioxidant activity and cellular protection are not well understood.
Here we show, for the first time, that chamomile induces the expression of HO-1, Prx-1 and Trx-1 and elevates HO activity in murine RAW 264.7 macrophages by stimulating the nuclear translocation of Nrf2 and enhancing its binding to ARE. Our results suggest that upregulation of HO-1 expression and elevation of HO activity induced by chamomile is essential for adaptive cytoprotection and can protect cells from oxidative injury caused by inflammatory products like H2O2.
MATERIALS AND METHODS
Materials
Dry chamomile flower of Egyptian origin was purchased from Bec's Tea Nirvana, Cleveland, Ohio. Cell culture medium, DMEM, fetal bovine serum, penicillin–streptomycin cocktail and phosphate buffered saline were purchased from Cellgro Mediatech, Inc. (Herndon, VA). Anti-HO-1 (sc-136960), anti-Prx-1 (sc-7381), anti-Trx-1 (sc-166393), anti-Nrf2 (sc-722) and anti-β-actin (sc-47778) were obtained from Santa Cruz (Santa Cruz, CA). Anti-p-Nrf2 (ab76026) was obtained from Abcam (Abcam, MA). Anti-histone 3 (05-928) was purchased from Upstate cell signaling solutions (Billerica, MA). Oligonucleotide primers for EMSA were purchased from Invitrogen (Grand Island, NY). Other chemicals, not specifically indicated used in the experiments were of analytical reagent grade or HPLC grade where applicable.
Preparation and analysis of aqueous chamomile extract
Dry chamomile flowers were weighed and crushed to powder with a marble pestle and mortar and a 5% w/v suspension was prepared in a flask by adding hot boiled water. The flask was then placed on a shaker (200 rpm) for 4-5 h and the temperature was maintained at 37°C. After shaking, the flask was brought to room temperature and the suspension was filtered through a series of Whatman filters and finally passed through 0.22 micron filter (Millipore, Billerica, MA). The filtered aqueous extract was freeze-dried, constituents identified by mass spectroscopy and stored at −20° until use. Briefly, analysis of aqueous chamomile extract through HPLC scan at wavelengths ranging from 200 to 590 nm provided a total of 10 peaks most of which correspond to water soluble flavonoids (Srivastava and Gupta, 2009). The identified two major peaks with retention times of 1.15 min (27.7%) and 1.52 min (63.3%) and other minor peaks which together constitute 9% of the total flavonoids (Supplemental Figure 1A). MS-MS confirmation of these two peaks in the aqueous chamomile extract preparation corresponded to apigenin 7-O-glucoside (63.3%) and apigenin 7-O-neohespridoside (27.7%), respectively (Supplemental Figure 1B). For cell culture studies, the dried material from aqueous extract was weighed and dissolved in culture medium to achieve desired concentration.
Cell viability assay
Murine RAW 264.7 macrophages were obtained from the American Type Culture Collection (ATCC) and cultured in Dulbecco's modified essential medium (DMEM) in appropriate culture conditions. Briefly, in the first experiment the cells were treated with 100 μM H2O2 and 20 μg/mL chamomile together, whereas in the second experiment the cells were pretreated with 10- and 20- μg/mL of chamomile for 16 h before their exposure to 50 μM H2O2. Cell respiration, an indicator of cell viability, was used to determine the mitochondrial-dependent reduction of 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) to formazan. After removal of culture media, cells were incubated at 37°C with MTT (0.5 mg/mL) for 1 h. The medium was aspirated and cells were solubilized in dimethyl sulfoxide (100 μL) for at least 15 min in the dark. The extent of reduction of MTT was quantified by optical density measurement at 540 nm.
Flow cytometry-Annexin V Assay
To distinguish the proportion of cells undergoing necrotic and apoptotic deaths, propidium iodide (PI) and annexin V staining assays were employed. Macrophages were treated with 20 μg/mL of aqueous chamomile for 16 h alone or further incubated for 6 h with 50 μM H2O2. Later, the cells were harvested, washed twice with PBS, stained with PI and annexin V and analyzed using FACS cytometer.
Reverse transcriptase-polymerase chain reaction
RAW 264.7 macrophages were treated with 50 μM H2O2 or with 10-40 μg/mL chamomile and total RNA was extracted using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. Reverse transcriptase-PCR was used for amplification of mouse mRNA for HO-1, Prx-1 and Trx-1 using the following primers, HO-1: Forward: 5’-CTGTGTAACCTCTGCTGTTCC-3’ and Reverse: 5’-CCACACTACCTGAGTCTACC- 3’; Prx-1: Forward: 5′-GTGGATTCTCACTTCTGTCATCT -3′ Reverse: 5′-GGCTTATCTGGAATCACACCACG -3′; Trx-1: Forward: 5’-CGTGGTGGACTTCTCTGCTACGTGGTG-3’ Reverse: 5’-GGTCGGCATGCATTTGACTTCACAGTC-3’. The mRNA of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as the loading control. Samples were heated to 95°C for 2 min and subjected to 40 cycles of amplification (1 min at 94°C, 1 min at 58°C, and 1 min at 72°C), followed by 10 min at 72°C for final extension. PCR products were electrophoresed in 2% agarose gel and viewed by ethidium bromide staining.
Western blot analysis
Macrophages, grown in 6-well plates to confluence, were incubated with or without H2O2 in the absence or presence of 10-40 μg/mL aqueous chamomile extract. Cells were washed with ice-cold PBS and stored at −70° until further analysis. Frozen plates were put on ice and cells were lysed in 1% Triton X-100, 0.15 M NaCl, and 10 mM Tris-HCl pH 7.4 for 30 min. Lysates were homogenized through a 22 G needle and centrifuged at 10,000 g for 10 min at 4°. The supernatants were collected and protein was measured by the method according to Bradford assay. Cell lysates, containing equal amounts of protein, were boiled in SDS sample buffer for 5 min before running on a 10% SDS–polyacrylamide gel. Proteins were transferred to polyvinylidene fluoride membranes (Invitrogen, Carlsbad, CA). Membranes were blocked with 5% fat-free dry milk in TBS-T pH 8.0 (Tris-buffered saline [50 mM Tris, pH 8.0, 150 mM NaCl] with 0.1% Tween 20) and then incubated with the respective primary antibodies for HO-1, Trx-1 and Prx-1 at appropriate dilutions overnight at 4°. After washing, the membrane was incubated with secondary antibody IgG:horseradish peroxidase conjugate and the enhanced chemiluminescence system (ECL™, Amersham Pharmacia Biotech). Signal intensities were evaluated by densitometric analysis (Kodak Digital Science™ Image Station 2000R Life Science Products).
Nuclear levels of phosphorylated Nrf2 and Electrophoretic mobility gel shift assay (EMSA)
Nuclear lysates were obtained from the macrophages after the respective treatment and evaluated for the nuclear Nrf2 and phosphorylated Nrf2 expressions. EMSA for Nrf2 was performed in the nuclear fraction of RAW 264.7 macrophages incubated for 16 h with or without chamomile (20 μg/mL) and H2O2 (50 μM) using Lightshift™ Chemiluminiscent EMSA kit (Pierce Biotechnology, Rockford, IL) following manufacturer's protocol as previously described (Bhaskaran et al., 2010). The DNA probes used were Nrf2 oligonucleotides as forward (5'-CACGAGCTGCCGGCGCTGTCCACATC -3') and reverse (5’-GATGTGGACAGCGCCGGCAGCTCG TG-3’).
Heme oxygenase activity
Heme oxygenase activity was determined by employing the conventional bilirubin production assay as previously described (Srisook and Cha, 2004). Briefly, microsomal suspension obtained from 50 μM H2O2 or with 10-40 μg/mL chamomile of RAW 264.7 macrophages was added to the reaction mixture containing 0.8 mM NADPH, 2 mM glucose-6-phosphate, 0.2 U glucose-6-phosphate dehydrogenase, 20 μM hemin and 2 mg of rat-liver cytosol in 100 mM potassium phosphate buffer, pH 7.4. The reaction mixture (1.0 ml) was incubated at 37°C for 1 h in the dark and then placed on ice for 2 min to terminate the reaction. The production of bilirubin was quantified by calculating from the difference in absorbance between 464 and 530 nm (extinction coefficient, 40 mM−1 cm−1 for bilirubin). HO activity was expressed as nmoles of bilirubin formed from per mg of microsomal protein per hour.
Statistical analysis
The experiments on cell culture were repeated at least three times. Results are expressed as mean values ± SD. Statistical comparisons were made by ANOVA followed by a Dunnett's multiple comparison test. P values < 0.05 were considered significant.
RESULTS
H2O2 induces cell death of RAW 264.7 cells
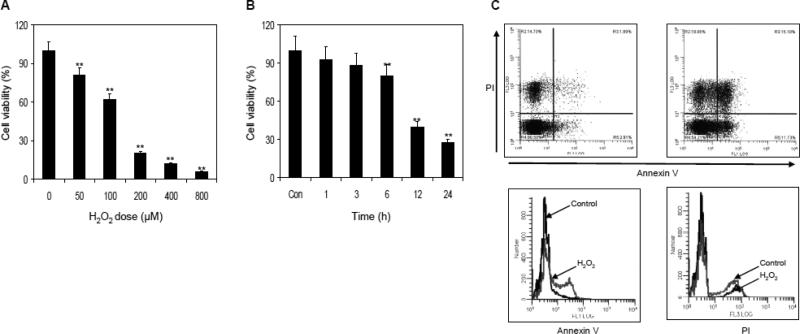
To determine the effect of H2O2 on RAW 264.7 macrophages viability, the cells were treated with various concentrations of H2O2 in dose and time dependent manner. As shown in figure 1A, treatment of macrophages with 50 to 800 μM concentration of H2O2 resulted in 19% to 94% decrease in cell viability observed after 6 h of exposure. Furthermore, exposure of macrophages with 50 μM H2O2 caused 7.5% decrease in cell viability at 1 h which increased to 72.8% in 24 h (Figure 1B). Therefore, for further studies we selected the dose of 50 μM of H2O2 for 6 h of treatment which caused ~20% decrease in cell viability. Upon 6 h exposure to 50 μM H2O2, 15.1% of cells died of apoptosis as shown by the results of PI-annexin V staining (Figure 1C).
Figure 1.
Effect of H2O2 on cell viability and death in RAW 264.7 macrophages. (A) Dose-dependent effect of H2O2 on cell viability. The macrophages were exposed to various concentration of H2O2 for 6 h and cell viability was determined by MTT assay. (B) Exposure of cells to 50 μM H2O2 for various times interval resulted in progressive loss of cell viability. (C) Exposure of cells to 50 μM H2O2 for 6 h caused ~20% of cell death. Data shown are representative of three independent experiments. Mean ± SD; **p<0.001 represent significant differences as compared with the control group. The details are described in ‘materials and methods’ section.
Chamomile protects RAW 264.7 cells from H2O2-mediated cellular injury
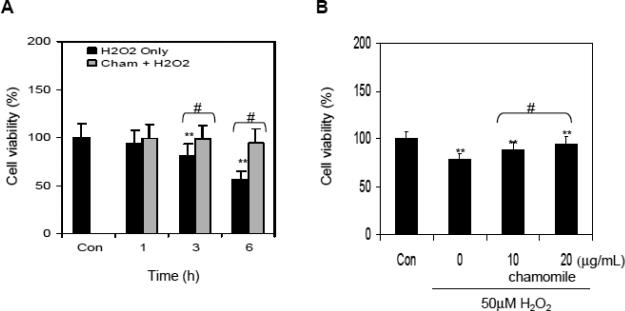
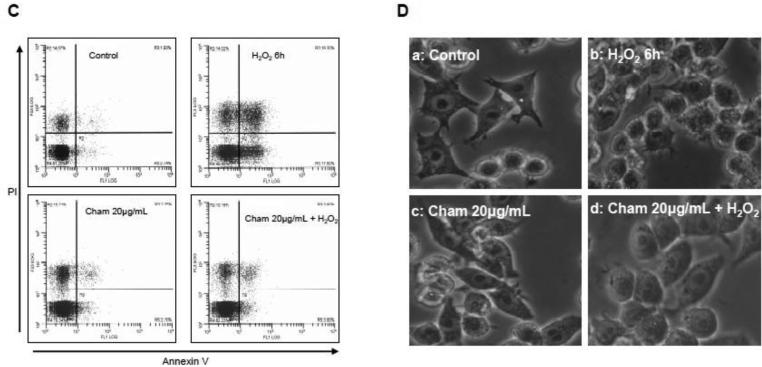
Next we examined whether chamomile could ameliorate toxicity of H2O2 in RAW 264.7 macrophages. The cells were initially treated with 100 μM H2O2 and 20 μg/mL chamomile together. Exposure of cells with 20 μg/mL chamomile and 100 μM H2O2 resulted in a marked increase in cell viability in time-dependent manner (Figure 2A). In subsequent experiments, cells were pretreated with various concentrations of chamomile before exposing them to 50 μM H2O2. Cells exposed to 10 and 20 μg/mL chamomile for 16 h significantly reduced the cytotoxicity of H2O2 by enhancing the cell viability by 20.4% at 10 μg/mL with further increase to 41.2% at 20 μg/mL of chamomile treatment (Figure 2B). To further confirm the protective activity of chamomile, RAW 264.7 macrophages were stained with annexin V and PI and subjected to flow cytometric analysis. Treatment of RAW 264.7 macrophages with 50 μM H2O2 for 6 h caused significant increase in apoptosis (1.93% in control versus 18.30% after H2O2 treatment), whereas pretreatment with 20 μg/mL chamomile resulted in marked decrease in H2O2-mediated apoptosis (3.80%) in these cells. Treatment with chamomile alone did not induce apoptosis in these cells (Figure 2C). The results were further confirmed by analyzing the cells under light microscopy after various treatments (Figure 2D). Overall, these results suggested that pre-treatment of cells with chamomile might result in the enhancement of antioxidant capacity and protected the cells from cell death caused by H2O2.
Figure 2.
Effect of chamomile treatment on H2O2 -mediated cell death in RAW 264.7 macrophages. (A) Viability of cells treated with 20 μg/mL chamomile was assessed together with exposure to 100 μM H2O2 using the MTT assay. Data shown are representative of three independent experiments. Mean ± SD **p<0.001 compared to control and #p<0.001 compared to H2O2 treated cells. (B) Viability of cells pretreated with 10 and 20 μg/mL concentrations of chamomile for 16 h was determined at 6 h after exposure to 50 μM H2O2. Data shown are representative of three independent experiments. Mean ± SD **p<0.001 compared to control and #p<0.001 compared to H2O2. (C) Cells were treated with 20 μg/mL aqueous chamomile for 16 h alone or further incubated for 6 h with 50 μM H2O2, stained with PI and annexin V for 15 min and analyzed by using Fluorescence Activated Cell Sorter (FACS) two times in duplicate. Data shown are representative FACS graphs. (D) Photographs of macrophages (sub panel a-d) showing cells after treatment with H2O2, chamomile alone and chamomile with H2O2. The details are described in ‘materials and methods’ section.
Chamomile induces expression of several antioxidant proteins
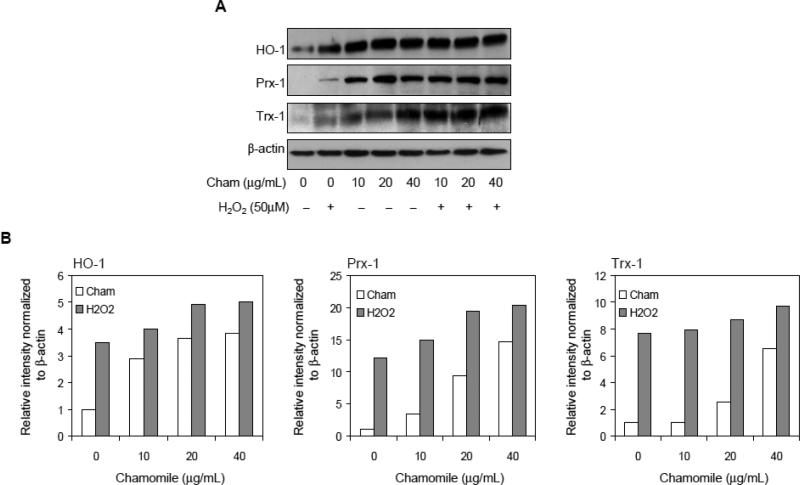
Antioxidant proteins like heme oxygenase-1 (HO-1), peroxiredoxin (Prx) and thioredoxin (Trx) protect the cells from oxidative injury caused by reactive oxygen species (ROS) (Zweier et al, 2011; Otterbein and Choi, 2000). Induction of these antioxidant proteins is essential for the protection of cells from oxidative stress caused by ROS like H2O2. Exposure of cells with 10-40 μg/mL chamomile alone for 16 h resulted in significant increase in the protein expression of HO-1, Trx-1 and Prx-1. Treatment with chamomile at 10-40μg/mL doses resulted in 129 to 285% increase in HO-1, 246 to 1363% increase in Prx-1 and 3 to 552% in Trx-1 protein expression after 16 h post-treatment. Furthermore, treatment of cells with 16 h chamomile followed by 50 μM H2O2 for 6 h, caused further increase in the expression of antioxidant proteins viz. 14.5% to 43.4% in HO-1, 18.1 to 67.2% in Prx-1, and 3.2 to 26.3% in Trx-1 which allows the protection against oxidative injury caused by excess H2O2 (Figure 3A&B).
Figure 3.
Effect of chamomile and H2O2 on the expression of antioxidant protein expression in RAW 264.7 macrophages. The cells were pretreated with 10-40 μg/mL chamomile for 16 h alone or prior to 50 μM H2O2 exposure. (A) Levels of HO-1, Prx-1 and Trx-1 expression were determined with and without H2O2 and chamomile exposure. The levels of antioxidant proteins were increased after chamomile treatment. β-actin levels were measured to ensure equal protein loading. (B) Bar graphs show relative levels of proteins estimated by densitometry of immunoblots normalized to β-actin. The details are described in ‘materials and methods’ section.
Chamomile activates nuclear translocation of Nrf2 and increased binding to DNA in response to oxidative stress
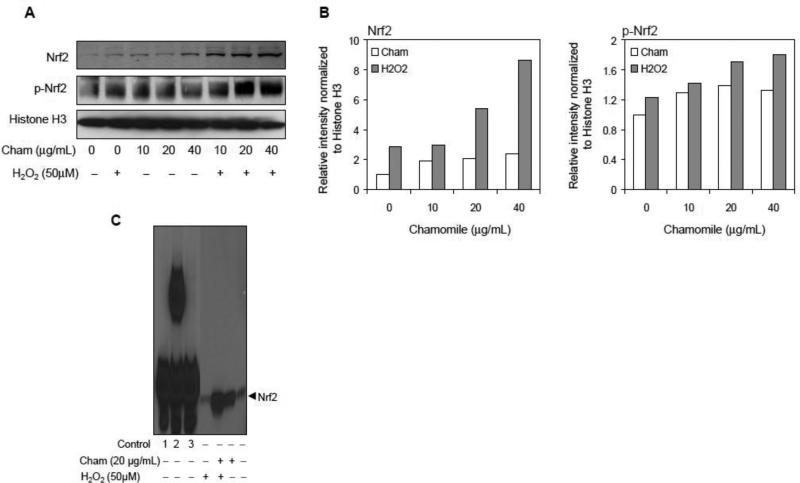
Since Nrf2 is a key redox sensitive transcription factor whose translocation from the cytoplasm to the nucleus and binding to the antioxidant response element (ARE) sequences localized in the promoter regions of genes that encode several antioxidant proteins increases as a result of oxidative stress (Lee et al., 2005; Kaspar et al., 2009), therefore, next we determined the effect of chamomile and H2O2 on nuclear translocation of Nrf2. As shown in figure 4A, treatment of cells with chamomile for 10-40 μg/mL for 16 h resulted in 92 to 106% increase in Nrf2 and 30 to 33% increase in p-Nrf2 levels in the nuclear fraction of macrophages. Furthermore, treatment of cells with chamomile for 16 h followed by 50 μM H2O2 for 6 h caused further increase in the nuclear expression of 1.4 to 198.2% in Nrf2 and 15.4 to 46.3% in p-Nrf2, respectively (Figure 4B). In support of these observations, the levels of Nrf2 translocated into the nucleus and bound to ARE were higher with chamomile than the corresponding controls (Figure 4C).
Figure 4.
Effect of chamomile and H2O2 on protein expression of Nrf2, p-Nrf2 and nuclear Nrf2/ARE binding in RAW 264.7 cells. The cells were pretreated with 10-40 μg/mL chamomile for 16 h alone or prior to 50 μM H2O2 exposure. (A) Chamomile increased nuclear translocation of Nrf2 and p-Nrf2. Histone H3 levels were measured to ensure equal protein loading. (B) Bar graphs show protein levels of Nrf2 and p-Nrf2 after various treatments estimated by densitometry of immunoblots normalized to histone H3. (C) EMSA assay. EMSA was performed to determine the effect of chamomile on the nuclear translocation of Nrf2 dimers and their binding to the ARE. Controls: #1 Biotin-EBNA control DNA, #2 Biotin-EBNA control DNA+EBNA extract, #3 Biotin-EBNA control DNA + EBNA extract + 20-fold molar excess of unlabeled EBNA DNA. The details are described in ‘materials and methods’ section.
Chamomile increases the mRNA levels of HO-1 and elevates HO activity
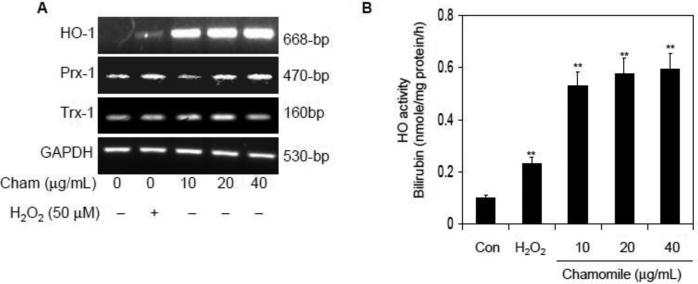
Next we determined the effect of chamomile in the induction of mRNA levels of various antioxidant enzymes. As shown in figure 5A, exposure of cells with chamomile at 10-40 μg/mL doses caused a significant increase in the mRNA levels of HO-1 compared to Prx-1 and Trx-1. Chamomile appeared to stimulate the HO-1 mRNA expression to an even greater extent than that stimulated by 50 μM H2O2 treatment. Chamomile also increased HO activity to a greater level than induced by H2O2. Compared to H2O2, a 143 to 156% increase in HO activity was noted after treatment with 10-40 μg/mL of chamomile (Figure 5B). Thus it appears that increased HO activity and expression is a cytoprotective response by macrophages, and chamomile has ability to significantly upregulate these cellular events, thereby providing cellular protection against cytotoxicity caused by H2O2.
Figure 5.
Effect of chamomile and H2O2 on mRNA levels of HO-1, Prx-1 and Trx-1; and HO enzyme activity in RAW 264.7 macrophages. (A) Levels of HO-1, Prx-1 and Trx-1 mRNA expressed at 16 h after exposure to 10-40 μg/mL doses of chamomile as well as 50 μM H2O2 alone. Chamomile increased HO-1 mRNA expression in a dose-dependent manner. GAPDH mRNA expression level was used to ensure equal loading. (B) Chamomile increases HO activity to a greater level than H2O2 exposure. Macrophages were treated with 10-40 μg/mL chamomile for 16 h and HO activity was determined by employing the bilirubin production assay. Data shown are representative of three independent experiments. Mean ± SD **p<0.001 compared to control. H2O2 (50 μM) was used as a control for HO activity. The details are described in ‘materials and methods’ section.
DISCUSSION
In this study, we demonstrate for the first time that chamomile induces both the nuclear accumulation of Nrf2 protein and the induction of phase II enzymes, including HO-1, Trx-1 and Prx-1 in RAW 264.7 macrophages.
Macrophages are normal cells that are vital for the recognition and elimination of microbial pathogens, and the survival of macrophages is of critical importance to the host defense system (Grom and Mellins, 2010). Several previous studies have demonstrated that the virulence of some bacteria is due to their ability to trigger the death of activated macrophages via stimulating ROS production. This oxidative burst produces large amount of singlet oxygen which is readily converted to H2O2 (Grom and Mellins, 2010; Zhu et al., 2008). Our studies confirm that exposure of macrophages to H2O2 results in a time-dependent loss of viability. However, pretreatment with different concentration of chamomile, with or without H2O2, greatly diminished loss of cell viability compared with respective controls, which was conformed by morphological observations. Further, to discriminate the percentage of cells undergoing death, propidium iodide and annexin V staining was used. We demonstrated that chamomile treatment of macrophages induced antioxidant activity and prevented H2O2-induced apoptosis. However, some necrosis was observed in both treated and untreated macrophages which might be due to loss of membrane integrity during cell harvesting. Our findings indicate that inhibition of ROS formation may be important for cytoprotection against oxidative damage.
It is well recognized that the induction of phase II antioxidant enzymes confers significant biological protection against deleterious effects of endogenous and exogenous ROS and other toxicants on cellular system (D'Autréaux and Toledano, 2007). Antioxidant proteins like Prx and Trx protects cells from oxidative cytotoxicity caused by ROS. Prx reduces hydroperoxides using the electrons provided from Trx, whereas Trx on its own facilitates the reduction of oxidized proteins by catalyzing the cysteine thioldisulfide exchange reaction (Nordberg and Arnér, 2001; Myers and Myers, 2009). The levels of these antioxidant proteins increase markedly in cells exposed to chamomile for 16 h; however, we could not detect significant upregulation of Prx-1 and Trx-1 at the message level in RAW 264.7 cells treated with H2O2. Therefore, it seems that the protective effects of these antioxidant enzymes against oxidative damage by H2O2 may be due to their interlinked anti-oxidative properties; this concept warrants further evaluation.
In an attempt to explore the possible molecular mechanisms underlying the cytoprotective effects of chamomile, we focused on the upregulation of HO-1 expression and increase in HO activity. Exposure of RAW 264.7 cells up to 40 μg/mL chamomile enhanced the expression of HO-1 mRNA, protein and HO activity in a dose-dependent manner without causing significant toxicity. This result is consistent with previous studies showing that several dietary agents, including curcumin, hesperidin, quercetin, resveratrol and green tea polyphenols, induce HO-1 levels in macrophages (Kim et al., 2008; Wu et al., 2006; Chow et al., 2005; Lin et al., 2005; Yu et al., 2010). Chamomile elevates HO activity and induces HO-1 expression to afford protection of macrophages against cytotoxicity and protects cells from H2O2-induced cell death by inducing antioxidant enzymes including HO-1. The most significant finding in our present study is the demonstration of the involvement of the Nrf2 pathway in chamomile-mediated HO-1 gene induction. The electrophoretic mobility shift assay revealed that nuclear ARE binding activity was significantly increased by chamomile treatment in RAW 264.7 macrophages. Additionally, chamomile also increased Nrf2 nuclear translocation, suggesting that increased expression of the Nrf2 protein may play a key role in chamomile-induced HO-1 gene activation.
Studies have demonstrated that phase II gene inducers react much more significantly with Keap1 than with Nrf2 (Surh et al., 2008). Both Nrf2 and Keap1 contain multiple cysteine residues and all cysteine on murine Keap1 were found to react with the phase II inducers (Surh et al., 2008; Giudice et al., 2010). In our study chamomile was shown to stimulate Nrf2 activation and translocation, possibly by dissociating the Nrf2-Keap1 complex. Further studies are needed to evaluate the interaction between chamomile and Keap1 resulting in Nrf2 activation.
Several upstream signaling pathways such as MAPKs, PI3K-Akt, protein kinase C, casein kinase-2 and RAS are known to activate Nrf2 and increase HO-1 expression (Kong et al., 2001; Ferrándiz and Devesa, 2008 and references therein). Our studies indicate that increased p-Nrf2 levels in the nucleus may be the result of phosphorylation by upstream kinase(s). The MAPK signaling cascade is stimulated by many extracellular stimuli and serves as a signal transduction pathway for stress responses. Studies have shown a transient increase in ERK1/2 after treatment with apigenin, a major aglycone constituent of chamomile (Llorens et al., 2002), which might be involved in the activation and nuclear translocation of Nrf2. Further studies are needed to elucidate the mechanistic pathway by which Nrf2 is phosphorylated and translocated into the nucleus by chamomile.
CONCLUSIONS
Taken together, these results suggest that chamomile activation of Nrf2 enhances its nuclear translocation to promote the synthesis of several key antioxidant enzymes in RAW 264.7 macrophages. In conclusion, our findings demonstrate that chamomile is a naturally-occurring antioxidant capable of protecting macrophages from cytotoxicity caused by H2O2. These findings are the first to elucidate the molecular basis for the antioxidant and cytoprotective properties of chamomile.
Supplementary Material
ACKNOWLEDGEMENTS
Financial Support: This work was supported by grants from United States Public Health Services RO1 AT002709 and RO1 CA108512. The authors are thankful to Dr. Gregory T. MacLennan for assistance in reviewing the manuscript.
ABBREVIATIONS
- ARE
antioxidant response element
- EMSA
electrophoretic mobility shift assay
- HPLC
high performance liquid chromatography
- Keap1
Kelch-like ECH-associated protein 1
- PRX
peroxiredoxin
- HO-1
heme oxygenase-1
- H2O2
hydrogen peroxide
- ROS
reactive oxygen species
- Nrf2
nuclear factor (erythroid-derived 2)-like 2
- TRX
thioredoxin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bhaskaran N, Shukla S, Srivastava JK, Gupta S. Chamomile: an anti-inflammatory agent inhibits inducible nitric oxide synthase expression by blocking RelA/p65 activity. International Journal of Molecular Medicine. 2010;26(6):935–940. doi: 10.3892/ijmm_00000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnat A, Carnat AP, Fraisse D, Ricoux L, Lamaison JL. The aromatic and polyphenolic composition of Roman camomile tea. Fitoterapia. 2004;75(1):32–38. doi: 10.1016/j.fitote.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Chow JM, Shen SC, Huan SK, Lin HY, Chen YC. Quercetin, but not rutin and quercitrin, prevention of H2O2-induced apoptosis via anti-oxidant activity and heme oxygenase 1 gene expression in macrophages. Biochemical Pharmacology. 2005;69(12):1839–1851. doi: 10.1016/j.bcp.2005.03.017. [DOI] [PubMed] [Google Scholar]
- D'Autréaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nature Review Molecular Cell Biology. 2007;8(10):813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- Ferrándiz ML, Devesa I. Inducers of heme oxygenase-1. Current Pharmaceutical Design. 2008;14(5):473–486. doi: 10.2174/138161208783597399. [DOI] [PubMed] [Google Scholar]
- Giudice A, Arra C, Turco MC. Review of molecular mechanisms involved in the activation of the Nrf2-ARE signaling pathway by chemopreventive agents. Methods Molecular Biology. 2010;647:37–74. doi: 10.1007/978-1-60761-738-9_3. [DOI] [PubMed] [Google Scholar]
- Grom AA, Mellins ED. Macrophage activation syndrome: advances towards understanding pathogenesis. Current Opinion Rheumatology. 2010;22(5):561–566. doi: 10.1097/01.bor.0000381996.69261.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspar JW, Niture SK, Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radical Biology Medicine. 2009;47:1304–1309. doi: 10.1016/j.freeradbiomed.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A, Minoshima Y, Yamamoto J, Adachi I, Watson AA, Nash RJ. Protective effects of dietary chamomile tea on diabetic complications. Journal Agriculture Food Chemistry. 2008;56(17):8206–8211. doi: 10.1021/jf8014365. [DOI] [PubMed] [Google Scholar]
- Kim KM, Pae HO, Zhung M, Ha HY, Ha YA, Chai KY, Cheong YK, Kim JM, Chung HT. Involvement of anti-inflammatory heme oxygenase-1 in the inhibitory effect of curcumin on the expression of pro-inflammatory inducible nitric oxide synthase in RAW264.7 macrophages. Biomedical Pharmacotherapy. 2008;62(9):630–636. doi: 10.1016/j.biopha.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Kong AN, Owuor E, Yu R, Hebbar V, Chen C, Hu R, Mandlekar S. Induction of xenobiotic enzymes by the MAP kinase pathway and the antioxidant or electrophile response element (ARE/EpRE). Drug Metabolism Review. 2001;33(3-4):255–271. doi: 10.1081/dmr-120000652. [DOI] [PubMed] [Google Scholar]
- Lee JM, Li J, Johnson DA, Stein TD, Kraft AD, Calkins MJ, Jakel RJ, Johnson JA. Nrf2, a multi-organ protector? FASEB Journal. 2005;19(9):1061–1066. doi: 10.1096/fj.04-2591hyp. [DOI] [PubMed] [Google Scholar]
- Lee SH, Heo Y, Kim YC. Effect of German chamomile oil application on alleviating atopic dermatitis-like immune alterations in mice. Journal Veterinary Science. 2010;11(1):35–41. doi: 10.4142/jvs.2010.11.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HY, Shen SC, Chen YC. Anti-inflammatory effect of heme oxygenase 1: glycosylation and nitric oxide inhibition in macrophages. Journal Cell Physiology. 2005;202(2):579–590. doi: 10.1002/jcp.20160. [DOI] [PubMed] [Google Scholar]
- Llorens F, Garcia L, Itarte E, Gómez N. Apigenin and LY294002 prolong EGF-stimulated ERK1/2 activation in PC12 cells but are unable to induce full differentiation. FEBS Letters. 2002;510(3):149–153. doi: 10.1016/s0014-5793(01)03252-5. [DOI] [PubMed] [Google Scholar]
- McKay DL, Blumberg JB. A review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.). Phytotherapy Research. 2006;20(7):519–530. doi: 10.1002/ptr.1900. [DOI] [PubMed] [Google Scholar]
- Myers CR, Myers JM. The effects of acrolein on peroxiredoxins, thioredoxins, and thioredoxin reductase in human bronchial epithelial cells. Toxicology. 2009;257(1-2):95–104. doi: 10.1016/j.tox.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordberg J, Arnér ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radical Biology Medicine. 2001;31(11):1287–1312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- Otterbein LE, Choi AM. Heme oxygenase: colors of defense against cellular stress. Am J Physiol Lung Cellular Molecular Physiology. 2000;279(6):L1029–1037. doi: 10.1152/ajplung.2000.279.6.L1029. [DOI] [PubMed] [Google Scholar]
- Smolinski AT, Pestka JJ. Modulation of lipopolysaccharide-induced proinflammatory cytokine production in vitro and in vivo by the herbal constituents apigenin (chamomile), ginsenoside Rb(1) (ginseng) and parthenolide (feverfew). Food Chemical Toxicology. 2003;41(10):1381–1390. doi: 10.1016/s0278-6915(03)00146-7. [DOI] [PubMed] [Google Scholar]
- Srisook K, Cha YN. Biphasic induction of heme oxygenase-1 expression in macrophages stimulated with lipopolysaccharide. Biochemical Pharmacology. 2004;68(9):1709–1720. doi: 10.1016/j.bcp.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Srivastava JK, Gupta S. Antiproliferative and apoptotic effects of chamomile extract in various human cancer cells. Journal Agriculture Food Chemistry. 2007;55(23):9470–9478. doi: 10.1021/jf071953k. [DOI] [PubMed] [Google Scholar]
- Srivastava JK, Gupta S. Extraction, Characterization, stability and biological activity of flavonoids isolated from chamomile flowers. Molecular Cellular Pharmacology. 2009;1(3):138–147. doi: 10.4255/mcpharmacol.09.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava JK, Pandey M, Gupta S. Chamomile, a novel and selective COX-2 inhibitor with anti-inflammatory activity. Life Sciences. 2009;85(19-20):663–669. doi: 10.1016/j.lfs.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava JK, Shankar E, Gupta S. Chamomile: A herbal medicine of the past with bright future. Molecular Medicine Report. 2010;3(6):895–901. doi: 10.3892/mmr.2010.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh YJ, Kundu JK, Na HK. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Medicine. 2008;74(13):1526–1539. doi: 10.1055/s-0028-1088302. [DOI] [PubMed] [Google Scholar]
- Veal EA, Day AM, Morgan BA. Hydrogen peroxide sensing and signaling. Molecular Cell. 2007;26(1):1–14. doi: 10.1016/j.molcel.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Wu CC, Hsu MC, Hsieh CW, Lin JB, Lai PH, Wung BS. Upregulation of heme oxygenase-1 by epigallocatechin-3-gallate via the phosphatidylinositol 3-kinase/Akt and ERK pathways. Life Sciences. 2006;78(25):2889–2897. doi: 10.1016/j.lfs.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Yoo KM, Hwang IK, Moon B. Comparative flavonoids contents of selected herbs and associations of their radical scavenging activity with antiproliferative actions in V79-4 cells. Journal Food Science. 2009;74(6):C419–25. doi: 10.1111/j.1750-3841.2009.01191.x. [DOI] [PubMed] [Google Scholar]
- Yu HP, Hwang TL, Hwang TL, Yen CH, Lau YT. Resveratrol prevents endothelial dysfunction and aortic superoxide production after trauma hemorrhage through estrogen receptor-dependent hemeoxygenase-1 pathway. Critical Care Medicine. 2010;38(4):1147–1154. doi: 10.1097/CCM.0b013e3181cd124e. [DOI] [PubMed] [Google Scholar]
- Zhu H, Jia Z, Zhang L, Yamamoto M, Misra HP, Trush MA, Li Y. Antioxidants and phase 2 enzymes in macrophages: regulation by Nrf2 signaling and protection against oxidative and electrophilic stress. Experimental Biology Medicine (Maywood) 2008;233(4):463–474. doi: 10.3181/0711-RM-304. [DOI] [PubMed] [Google Scholar]
- Zweier JL, Chen CA, Druhan LJ. S-glutathionylation reshapes our understanding of endothelial nitric oxide synthase uncoupling and nitric oxide/reactive oxygen species-mediated signaling. Antioxidant Redox Signaling. 2011;14(10):1769–1775. doi: 10.1089/ars.2011.3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.