Abstract
Mammalian target of rapamycin complex (MTORC) 2 phosphorylates AGC protein kinases including PKC and regulates cellular functions including cell migration. However, its regulation remains poorly understood. Here we show that LPA induces two phases of PKCδ hydrophobic motif (HM) phosphorylation. The late phase is mediated by Gα12, which specifically activates ARAF, leading to upregulation of the expression of an E3 ubiquitin ligase RFFL and subsequent ubiquitination and degradation of PRR5L. Destabilization of PRR5L, a suppressor of mTORC2-mediated HM phosphorylation of PKCδ, but not AKT, results in PKCδ HM phosphorylation and activation. This Gα12-mediated pathway is critically important for fibroblast migration and pulmonary fibrosis development. Thus, our study unravels a signaling pathway for mTORC2 regulation and fibroblast migration.
INTRODUCTION
MTOR is an evolutionarily conserved Ser or Thr protein kinase. It plays an important role in regulating a wide range of cell activities in response to extracellular stimuli 1–5. This kinase is present in two structurally and functionally distinct protein complexes, mTORC1 and mTORC2. These two complexes share two common components, MLST8 (GβL) and DEPTOR, but other components are distinct. While mTORC1 has RAPTOR and PRAS40 6–10, mTORC2 contains RICTOR, MAPKAP1 (SIN1), PRR5, and PRR5L (Protor-1 or 2) 11–15. The molecular functions of these mTORC components remain poorly defined. Nevertheless, studies suggest that MLST8, RICTOR, RAPTOR, and MAPKAP1 are critical for the complex assembly and/or linking the MTOR kinase to its substrates 3, 4. The function of PPR5L is not known.
The two mTOR complexes are regulated differently. While mTORC1 regulates diverse cellular processes including protein synthesis, ribosome biogenesis, transcription, and autophagy 16–19, mTORC2 regulates AGC kinases, including AKT and PKC, by phosphorylating their HM sites 20–24. MTORC2 also phosphorylates Turn Motifs ofAKT and PKC, but this phosphorylation is independent of growth factor regulation 20, 22. The cellular activities that mTORC2 regulates include actin cytoskeleton reorganization and cell migration 13, 23, 25–27. Many extracellular stimuli including growth factors, G protein-coupled receptor ligands, and cytokines stimulate AKT and PKC HM phosphorylation. The PI3K-mediated regulation of AKT HM phosphorylation was initially attributed to PtdIns(3,4,5)P3-mediated membrane translocation and conformational changes of AKT. However, recent evidence suggests that the mTORC2 kinase may also be regulated by PtdIns(3,4,5)P3 directly 28–30 and indirectly through stimulation of its ribosome association 31. Ribosome-associated mTORC2 promotes cotranslational phosphorylation of AKT Turn Motif and stability of nascent AKT polypeptides 32. It is not known whether there are other mechanisms for extracellular stimuli to regulate mTORC2 or whether mTORC2’s activity toward different substrates can be selectively regulated.
In our recent study of mTORC2 kinase activity regulation by PtdIns(3,4,5)P3 30, we discovered that Gα12, but not Gα13, specifically regulated PKCδ HM phosphorylation in HEK293T and mouse embryonic fibroblasts (MEFs). This led us to uncovering a signaling mechanism by which LPA, via Gα12 and mTORC2, stimulates long term PKCδ HM phosphorylation and activation, which is important for fibroblast migration and pulmonary fibrosis development.
RESULTS
Persistent PKC activation by LPA and Gα12
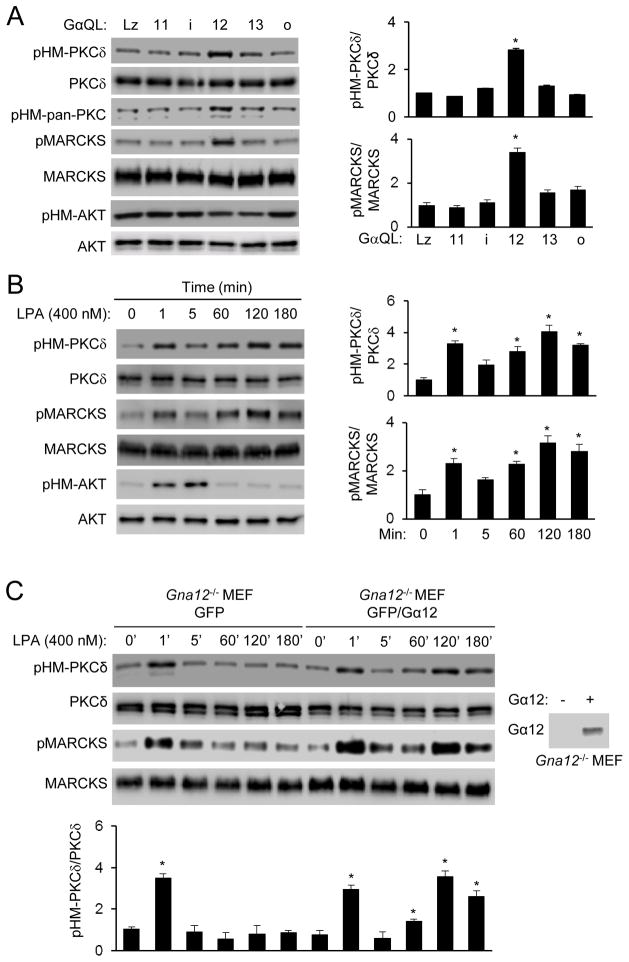
In our recent study of mTORC2 regulation, we found that expression of Gα12QL, but not the activated QL forms of Gαi2, Gα13, GαoA and Gα11, in HEK293T cells stimulated HM phosphorylation of PKCδ (pHM-PKCδ), but not AKT (Fig. 1A & S1A). Expression of Gα12QL could also increase PKC phosphorylation detected by an antibody recognizing the HM phosphorylation of all of PKC isoforms (pHM-pan-PKC) and MARCKS phosphorylation (Fig. 1A). Because MARCKS phosphorylation could be blocked by a PKC inhibitor (Fig. S1B), it is a surrogate marker for PKC activity.
Figure 1. Gα12 is required for LPA-induced late phase PKC HM phosphorylation and activation.
A) Gα12QL, but not other GαQL subunits, stimulates PKC HM and MARCKS phosphorylation. HEK293T cells were transfected with one of the constitutively activated forms of Gα in the absence of serum. Cells were collected 24 hours after transfection for Western analysis. Normalized quantification of immunoblots was performed from independent experiments. Data are presented as means ± S.D. (*, p<0.01, Student’s t-Test, n=3).
B) LPA stimulates PKCδ HM and MARCKS phosphorylation in a time-dependent manner in MEFs. Normalized quantification of immunoblots was performed from independent experiments. Data are presented as means ± S.D. (*, p<0.01, Student’s t-Test, n=3).
C) Late phase PKCδ HM phosphorylation and activation by LPA is dependent on Gα12. Gna12−/− MEFs were infected with retroviruses expressing GFP or GFP-IRES-Gα12. Expression of Gα12 is shown in the side panel. Normalized quantification of immunoblots was performed from independent experiments. Data are presented as means ± S.D. (*, p<0.01, Student’s t-Test, n=3).
LPA, which can signal through the Gα12 or 13 family of G proteins 33, 34, also stimulated PKCδ HM and MARCKS phosphorylation in HEK293T cells (Fig. S1C) or MEFs (Fig. 1B) with two phases; an early phase response peaked before 5 min, and a late one after 1 hour of stimulation. LPA, on the other hand, induced only one phase of AKT HM phosphorylation that peaked before 5 min of the stimulation (Fig. 1B & S1C). The late, but not the early, phase PKC HM phosphorylation depended on Gα12 as determined using the Gα12-null (Gna12−/−) MEFs (Fig. 1C). Importantly, expression of Gα12 in Gna12−/− MEFs restored the late phase stimulation by LPA (Fig. 1C). Gα13-deficiency, however, had no significantly effects on either phase of PKC HM or MARCKS phosphorylation (Fig. S1D; data not shown), suggesting that LPA acts primarily through Gα12 to induce late phase PKC HM and MARCKS phosphorylation. Additionally, LPA appeared to act largely through the type 1 LPA receptor (LPAR1) to stimulate the late phase responses (Fig. S1E).
PRR5L ubiquitination and destabilization
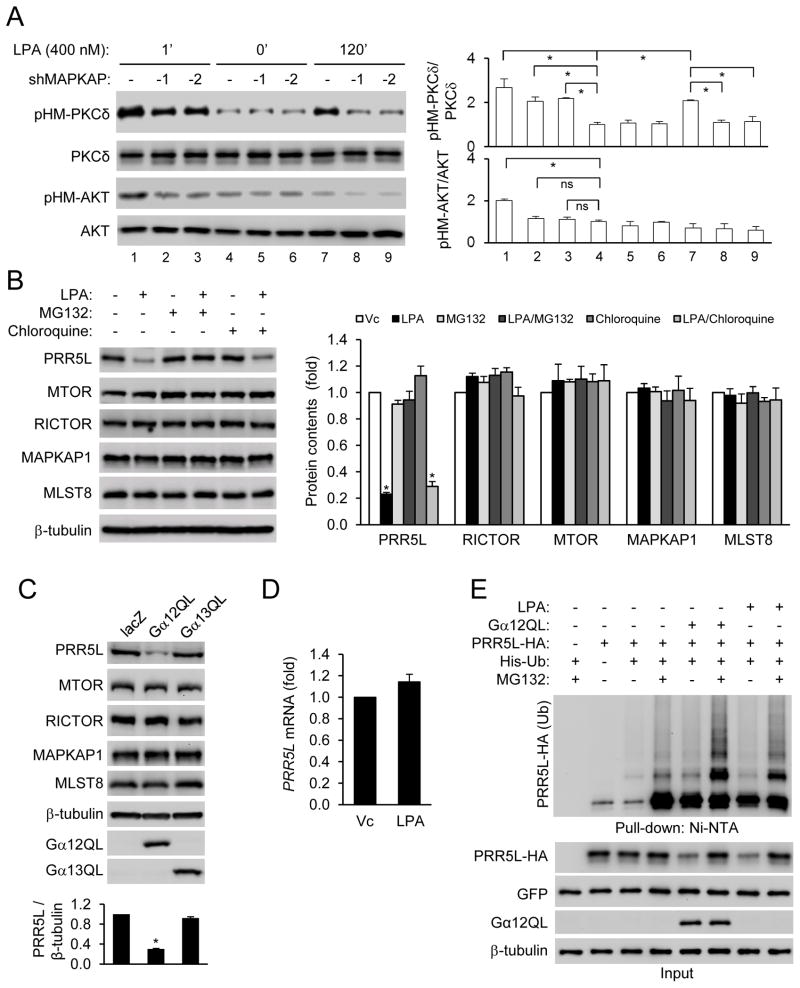
PKC HM phosphorylation may be mediated by mTORC2 3, 4. We thus knocked down an mTORC2 core component MAPKAP1, which caused a strong inhibition of the late, but not the early, phase PKCδ HM and MARCKS phosphorylation in response to LPA, while abrogating AKT HM phosphorylation (Fig. 2A). These results suggest that the two phases of PKCδ HM phosphorylation may be regulated differently. Consistent with this conclusion, mTORC2 pulled down by a RICTOR antibody from WT MEFs treated with LPA for 120 min, but not from those treated for 1 min, showed increased ability to phosphorylate PKC in an in vitro kinase assay (Fig. S2A). Of note, mTORC2 from the long term LPA treatment did not stimulate AKT HM phosphorylation (Fig. S2A). Because PKC could autophosphorylate its HM site (Fig. S2B), the early phase PKC HM phosphorylation may be the result of autophosphorylation probably as the result of PKC activation by LPA through phospholipase C35.
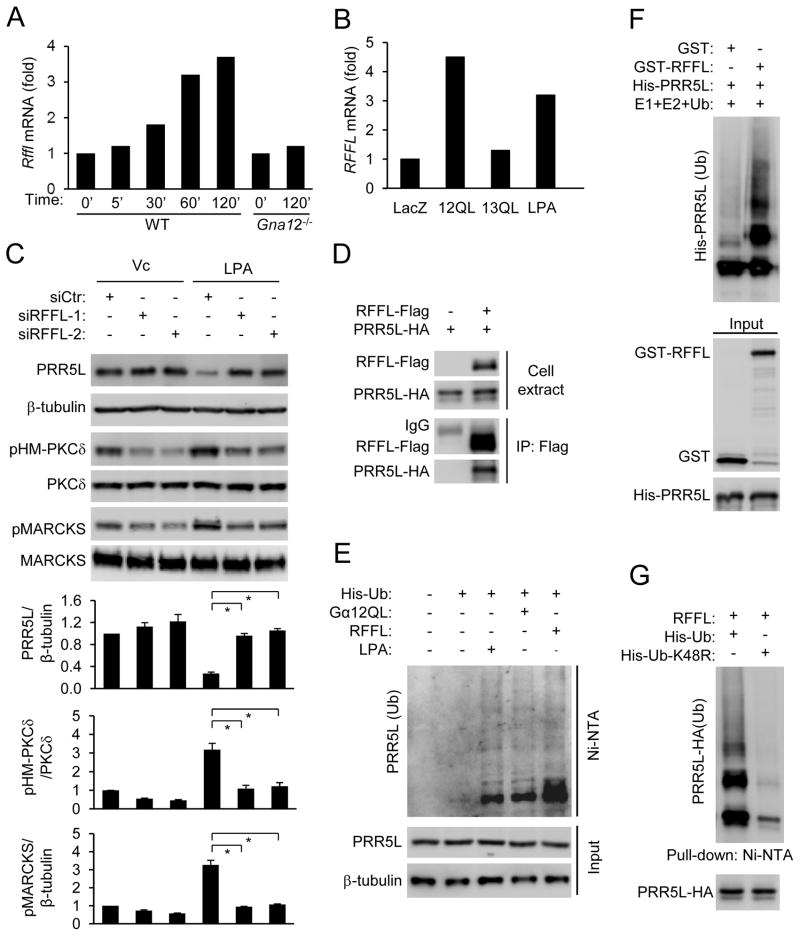
Figure 2. LPA and Gα12 destabilize PRR5L by inducing PRR5L ubiquitination.
A) Late phase PKCδ HM phosphorylation is dependent on the mTORC2 complex. WT MEFs were infected with retrovirus expressing a MAPKAP1 shRNA and treated with LPA (400 nM). Normalized quantification of immunoblots was performed from independent experiments. Data are presented as means ± S.D. (*, p<0.01, Student’s t-Test, n=3).
B) LPA treatment reduces the contents of PRR5L. HEK293T cells were pre-treated with MG132 (10 μM) or Chloroquine (100 μM) for 6 hrs, followed by LPA (5 μM) for 2 hrs. Normalized quantification of immunoblots was performed from independent experiments. Data are presented as means ± S.D. (*, p<0.01, Student’s t-Test, n=3).
C) Gα 12QL expression reduces the content of PRR5L. HEK293T cells were transfected with LacZ, Gα 12QL, or Gα 13QL for 24 hrs before Western analysis. Normalized quantification of immunoblots was performed from independent experiments. Data are presented as means ± S.D. (*, p<0.01, Student’s t-Test, n=3).
D) LPA does not alter the PRR5L mRNA concentration. HEK293T cells were treated with LPA (5 μM) for 2 hrs prior to RNA isolation and qRT-PCR analysis. Data are presented as means ± S.D. (n=3).
E) LPA treatment or Gα12QL expression stimulates ubiquitination of PRR5L. HEK293T cells were transfected and treated with or without LPA (5 μM, 2 hrs) in the presence or absence of MG132 (10 μM, 6 hrs before LPA treatment) pre-treatment. Whole-cell lysates (Input) and Ni-NTA precipitates were analyzed by Western blotting 24 hrs after transfection.
To understand how LPA may induce the late phase PKCδ HM phosphorylation through mTORC2, we examined the possibility that LPA treatment might alter the protein contents of the mTORC2 components. While LPA did not alter the contents of the core mTORC2 components including MTOR, RICTOR, MAPKAP1, and MLST1, it reduced the content of PRR5L, an mTORC2-binding protein with no clear function, in HEK293T cells (Fig. 2B) in a time dependent manner (Fig. S1C). LPA also destabilized PRR5L in WT MEFs (Fig. S2C,D). Expression of Gα12QL, but not Gα13QL, also led to a reduction in the protein content of PRR5L, but not other mTORC2 components (Fig. 2C).
LPA did not alter the PRR5L mRNA concentrations in HEK293T cells (Fig. 2D) or MEFs (data not shown), but the proteasome inhibitor MG132 inhibited LPA-induced PRR5L destabilization (Fig. 2B). By contrast, the lysosome inhibitor chloroquine had no effect (Fig. 2B). These results suggest that LPA may induce destabilization of PRR5L via the proteasome, which often requires polyubiquitination of its target proteins. Indeed, LPA treatment or Gα12QL expression stimulated PRR5L ubiquitination (Fig. 2E).
PRR5L inhibits PKC HM phosphorylation
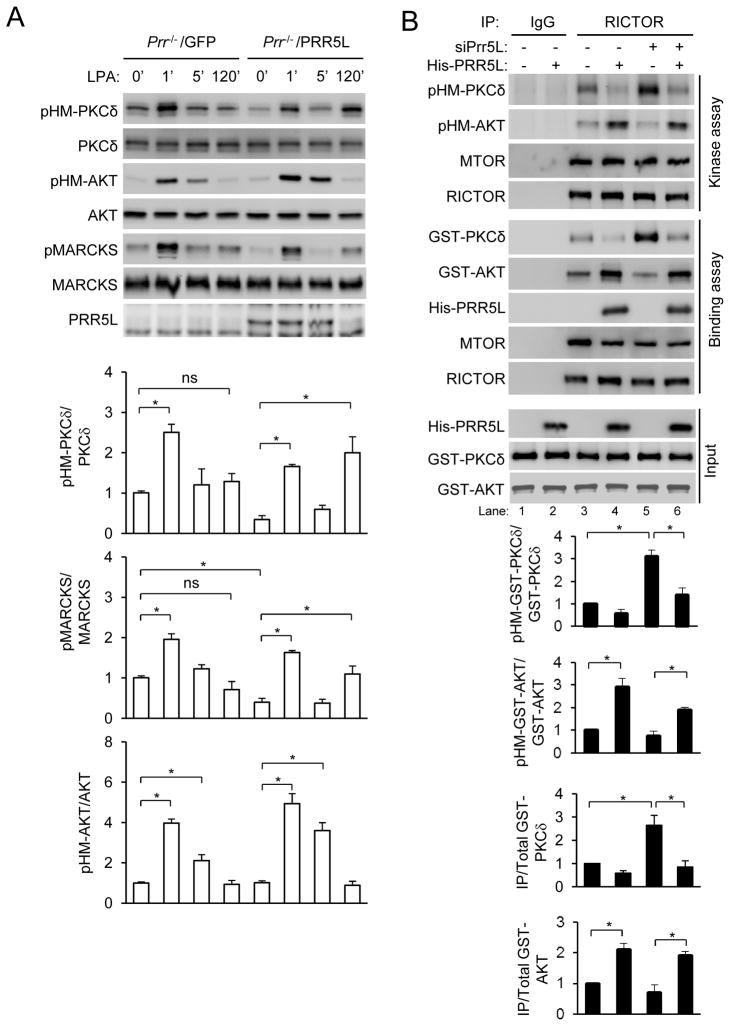
LPA failed to elicit the late phase PKCδ HM phosphorylation without noticeable effects on the early phase PKCδ and AKT HM phosphorylation in MEFs derived from Prr5−/−;Prr5l−/− mice (Fig. 3A). In addition, expression of PRR5L in these Prr5−/−;Prr5l−/− MEFs restored the late phase responses apparently by reducing the basal PKCδ HM phosphorylation (Fig. 3A). These results, together with the observation that LPA destabilizes PRR5L protein (Fig. 2), suggest that PRR5L may be a suppressor of PKC HM phosphorylation. LPA may hence induce the late phase PKC HM phosphorylation by destabilizing PRR5L protein. Consistent with this hypothesis, siRNA-mediated knockdown PRR5L increased PKCδ HM and MARCKS phosphorylation (Fig. S3A).
Figure 3. PRR5L inhibits mTORC2-mediated PKCδ HM phosphorylation.
A) Responses to LPA stimulation in Prr5−/−;Prr5l−/− MEFs. Prr5−/−;Prr5l−/− (Prr−/−) MEFs and those infected with PRR5L expressing retrovirus were stimulated with LPA (400 nM). Normalized quantification of immunoblots was performed from independent experiments. Data are presented as means ± S.D. (*, p<0.01, Student’s t-Test, n=3).
B) PRR5L regulates mTORC2’s substrate preference. WT MEFs were transfected with a PRR5L siRNA for 72 hrs. Immunoprecipitation was carried out using a control antibody (IgG) or RICTOR antibody. The immunocomplexes were used to phosphorylate recombinant GST-PKCδ or GST-AKT protein in an in vitro kinase assay or to interact with the GST-PKCδ or GST-AKT protein in a cell-free binding assay as described in the “Methods.” Normalized quantification of immunoblots was performed from independent experiments. Data are presented as means ± S.D. (* p<0.01 vs. LacZ-transfected or vehicle treatment (Vc) controls, Student’s t-Test, n=3).
We next examined the effect of PRR5L in a cell-free system and found that mTORC2 immunoprecipitated by the RICTOR antibody from cells transfected with the PRR5L siRNA showed increased ability to phosphorylate PKCδ HM site compared to mTROC2 from cells transfected with a control siRNA (Fig. 3B). Addition of PPR5L protein inhibited PKC HM phosphorylation by mTORC2 (Fig. 3B). Furthermore, mTORC2 from PRR5L knockdown cells showed stronger interactions with PKCδ protein than mTORC2 from the control cells (Fig. 3B). Addition of recombinant PRR5L protein attenuated the interactions (Fig. 3B). Addition of a PRR5L mutant protein that lacks its N-terminal 95 amino acid and does not bind to mTORC2 14 did not suppress the phosphorylation or binding (Fig. S3B). These effects of PRR5L siRNA transfection and protein addition are consistent with the observation that each MAPKAP1 molecule in mTORC2 immunoprecipitated from wildtype cells appeared to be occupied by at least one PRR5L molecule, whereas LPA treatment reduced the stoichiometry of MAPKAP1 to PRR5L to below 1 (Fig. S3C).
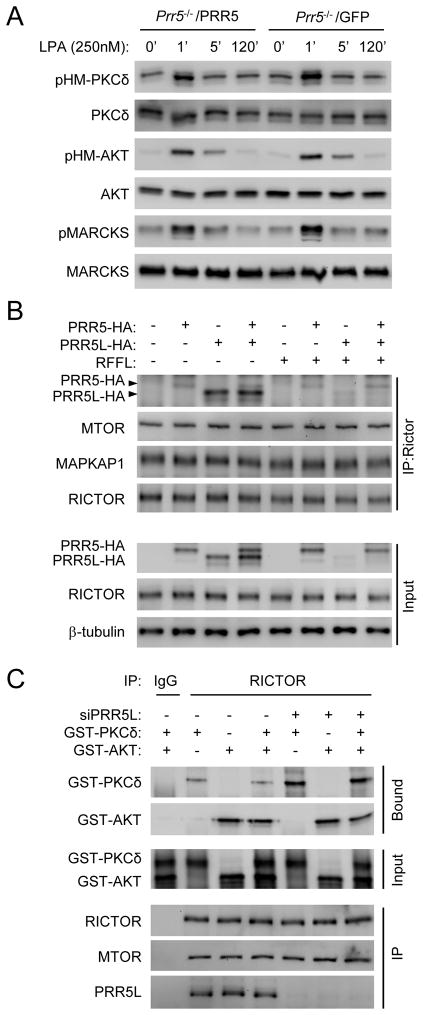
Expression of the PRR5L homolog PRR5, unlike that of PRR5L, in Prr5−/−;Prr5l−/− MEFs did not restore the late phase PKCδ HM phosphorylation, nor did it affect the early phase responses (Fig. 4A). Moreover, PRR5 and PRR5L did not compete for binding to mTORC2 (Fig. 4B). These results together suggest that PRR5 may not share the function of PRR5L in regulating PKC phosphorylation. In contrast to the effects on PKCδ, addition of the PRR5L protein enhanced AKT HM phosphorylation by and its interaction with mTORC2 (Fig. 3B). However, PRR5L knockdown only slightly attenuated AKT HM phosphorylation or interaction with mTORC2 (Fig. 3B), suggesting that endogenous PRR5L may have a stronger impact on PKC than AKT regulation. Given that AKT and PKC did not seem to compete for binding to mTORC2 (Fig. 4C), PRR5L may bind to a site on mTORC2 that affects more the regulation of PKC HM phosphorylation than that of AKT.
Figure 4. PRR5 does not regulate PKCδ HM phosphorylation.
A) Inability of PRR5 to restore LPA responses in Prr5−/−;Prr5l−/− (Prr−/−) MEFs. Prr5−/−;Prr5l−/− (Prr−/−) MEFs and those infected with PRR5 expressing retrovirus were stimulated with LPA (400 nM).
B) The binding of PRR5 to mTORC2 is not affected by the presence or absence of PRR5L. HEK293 cells were transfected with PRR5-HA or PRR5L-HA in the presence or absence of RFFL. Expression of RFFL resulted in reduction in PRR5L levels. Cells were subject to anti-RICTOR immunoprecipitation.
C) PKCδ and AKT do not compete for mTORC2 binding. Anti-RICTOR immunoprecipitates from MEFs were incubated with GST-AKT1, GST-PKCδ, or both, and bound AKT1 or PKCδ were detected by Western analysis.
RFFL is an E3 ligase for PRR5L
Because LPA or Gα12-mediated destabilization of PRR5L depended on protein synthesis (Fig. S4A), LPA and Gα12 may upregulate the expression of an E3 ubiquitin ligase that catalyzes the polyubiquitination of PRR5L. Microarray gene expression analysis revealed that LPA upregulated the expression of 846 genes by more than 2 folds in HEK293T cells. Among these genes, there were 4 verified or putative E3 ligases (RFFL, TRAF6, WWP2, and RNF157). Quantitative RT-PCR analysis indicated that the expression of Rffl was upregulated by LPA in a Gα12-dependent manner (Fig. 5A). RFFL expression was also upregulated by Gα12QL, but not Gα13QL (Fig. 5B). Importantly, the RFFL siRNAs reversed LPA-mediated destabilization of PRR5L and increases in PKC-HM and MARCKS phosphorylation (Fig. 5C). The siRNAs also inhibited LPA or Gα12QL-induced ubiquitination of PRR5L (Fig. S4B). Thus, RFFL may be an E3 ligase for PRR5L.
Figure 5. RFFL is the E3 ligase for PRR5L.
A) LPA induces Rffl expression dependently of Gα12. WT or Gna12−/− MEFs were stimulated with LPA, and the relative expression levels of Rffl were determined by qRT-PCR.
B) Gα12, but not Gα13, upregulates RFFL expression. HEK293T cells were transfected with Gα12QL or Gα13QL for 24 hrs or treated with LPA (5 μM) for 2 hrs. Relative expression levels of RFFL were determined by qRT-PCR.
C) Knockdown of RFFL abolishes the effects of LPA on PRR5L stability regulation and PKC HM and MARCKS phosphorylation. HEF293T cells were transfected with the RFFL siRNAs for 72 hrs and stimulated with LPA (5 μM, 2hrs) before Western analysis. Normalized quantification of immunoblots was performed from independent experiments. Data are presented as means ± S.D. (*, p<0.01, Student’s t-Test, n=3).
D) RFFL interacts with PRR5L. HEK293T cells were transfected with RFFL-Flag and PRR5L-HA as indicated, and immunoprecipitation was carried out using an anti-Flag antibody 24 hrs after transfection.
E) LPA treatment, expression of RFFL, or Gα12 stimulates endogenous PRR5L polyubiquitination. HEK293T cells were transfected with His-ubiquitin, Gα12QL, and/or RFFL-FLAG for 24 hrs and pretreated with MG132. Some of the samples were stimulated with LPA (5 μM, 2 hrs). Cell lysates were subjected to Ni-NTA bead pull-down, followed by Western analysis with a PRR5L antibody.
F) RFFL catalyzes PRR5L ubiquitination in vitro.
G) Expression of K48R ubiquitin blocks PRR5L polyubiquitination by RFFL.
The E3 ubiquitin ligases often bind to their substrates. Indeed, RFFL and PRR5L coimmunoprecipitated in HEK293T cells (Fig. 5D & S4C), and their recombinant protein interacted (Fig. S4D). Additionally, expression of RFFL, like Gα12QL expression or LPA treatment, was able to stimulate ubiquitination of endogenous PRR5L (Fig. 5E), and recombinant RFFL catalyzed ubiquitination of PRR5L in an in vitro assay (Fig. 5F). RFFL-mediated PRR5L poly-ubiquitination is ubiquitin Lys48-linked (Fig. 5G) and RFFL Ring domain-dependent (Fig. S4E). All of these results together support the conclusion that RFFL is the E3 ligase that catalyzes PRR5L polyubiquitination in response to Gα12QL expression or LPA treatment.
RAF regulates Rffl expression via ERK
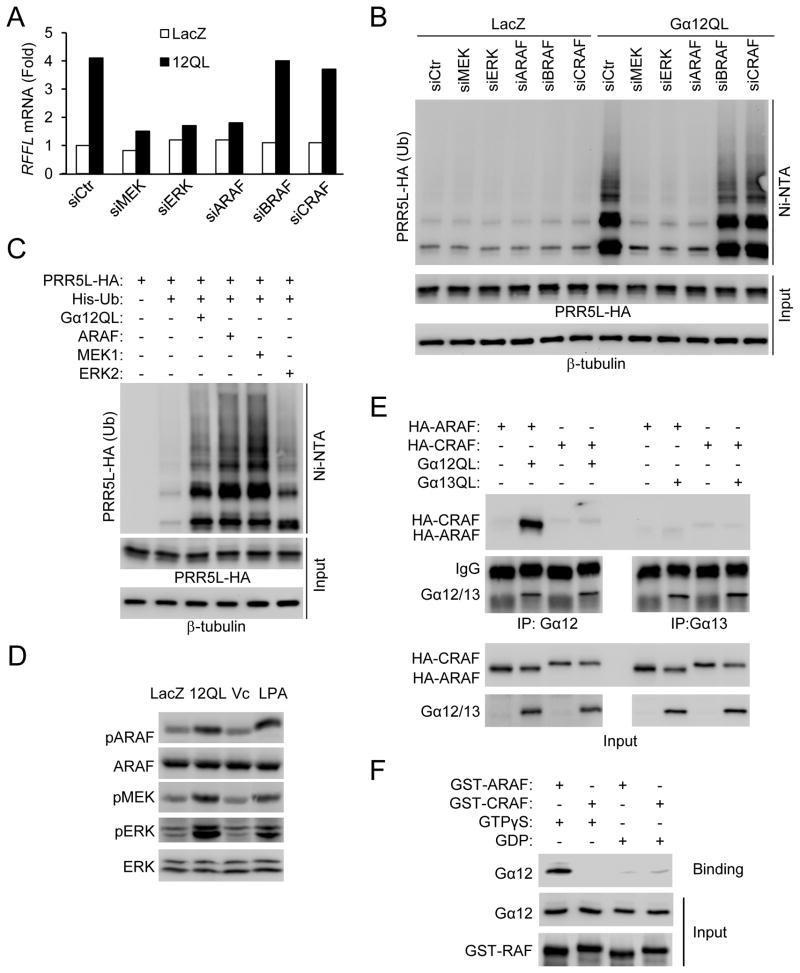
The Gα12 family of G proteins has been implicated in regulation of the MAP kinases 33. While expression of Gα12 or Gα13 stimulated p38 kinase phosphorylation, only Gα12QL, but not Gα13QL, potently stimulated MEK and ERK phosphorylation (Fig. S5A). LPA has been previously shown to stimulate ERK phosphorylation 36, which we confirmed (Fig. S5A). Consistent with these results, MEK inhibitors PD98059 and U0126 inhibited LPA-induced upregulation of RFFL expression (Fig. S5B), and knockdown of MEK or ERK by siRNAs blocked Gα12QL-induced upregulation of RFFL expression (Fig. 6A) and polyubiquitination of PRR5L (Fig. 6B). Together with the result that overexpression of MEK1 or ERK2 stimulated RFFL expression (Fig. S5C) and polyubiquitination of PRR5L (Fig. 6C), we concluded that LPA and Gα12 upregulated RFFL expression via a MEK-ERK pathway.
Figure 6. RAF upregulates RFFL expression via MEK and ERK.
A) Knockdown of ARAF, MEK1 and 2, or ERK1 and 2 by siRNAs blocks Gα 12QL-induced upregulation of RFFL expression. HEK293T cells were cotransfected with the indicated siRNA and the plasmid encoding LacZ or Gα 12QL. Seventy-two hours later, cells were harvested for q-RT-PCR analysis.
B) Knockdown of ARAF, MEK1 and 2 or ERK1 and 2 by siRNA blocks Gα 12QL-induced ubiquitination of PRR5L. HEK293T cells were transfected with the indicated siRNA for 48 hrs, followed by transfection with the indicated plasmids. Twenty-hours later, cells were treated with MG132 for 8 hrs before lysis and precipitation with Ni-NTA beads.
C) Overexpression of ARAF, MEK1, or ERK2 stimulates PRR5L ubiquitination. HEK293T cells were transfected with the plasmids as indicated for 24 hrs. The cells were treated with MG132 for 8 hrs before lysis and pull-down with Ni-NTA beads.
D) LPA and Gα12 stimulate ARAF phosphorylation at Ser-299. HEK293T cells were transfected with Gα12QL or treated with LPA (5 μM, 2hrs), followed by immunoblotting.
E) Gα12 interacts with ARAF. HEK293T cells transfected with the plasmids as indicated. Immunoprecipitation was carried out using a Gα12 or Gα13 antibody in the presence of AMF as described in the “Methods.”
F) Recombinant Gα12 interacts with ARAF, but not CRAF, in a GTP-dependent manner.
The RAF protein kinases act upstream of MEK. Knockdown of ARAF, but not BRAF or CRAF, by siRNAs inhibited Gα12QL-mediated upregulation of RFFL expression and polyubiquitination of PRR5L (Fig. 6A–B). Knockdown of ARAF also blocked LPA- or Gα12QL-induced increases in MEK, ERK and PKCδ HM phosphorylation (Fig. S5D), while knockdown of MEK blocked Gα12QL-induced increases in ERK and PKCδ HM phosphorylation (Fig. S5E). Furthermore, LPA treatment or Gα12QL expression increased ARAF phosphorylation (Fig. 6D), and ARAF expression increased RFFL expression (Fig. S5C), ERK phosphorylation (Fig. S5E), and PRR5L polyubiquitination (Fig. 6C). Because expression of a dominant negative mutant of H-Ras, one of the upstream regulators of the Raf proteins, did not significantly inhibit LPA-mediated RFFL expression or ERK phosphorylation (Fig. S5B &S5F), we examined whether Gα12 could interact with ARAF. Gα12QL and ARAF co-immunoprecipitated in HEK293T cells (Fig. 6E). By contrast, coimmunoprecipitation of Gα12QL with CRAF or of Gα13QL with ARAF or CRAF was not strongly detected (Fig. 6E), suggesting that Gα12 may preferentially activate ARAF. Moreover, recombinant Gα12 protein directly interacted with ARAF, but not CRAF, in a GTP-dependent manner (Fig. 6F). All of these results indicate that LPA acts preferentially through Gα12 to regulate RFFL expression, PRR5L polyubiquitination, and PKCδ HM phosphorylation through an ARAF-MEK-ERK pathway.
Role of Gα12-PKCδ in fibroblast cell migration
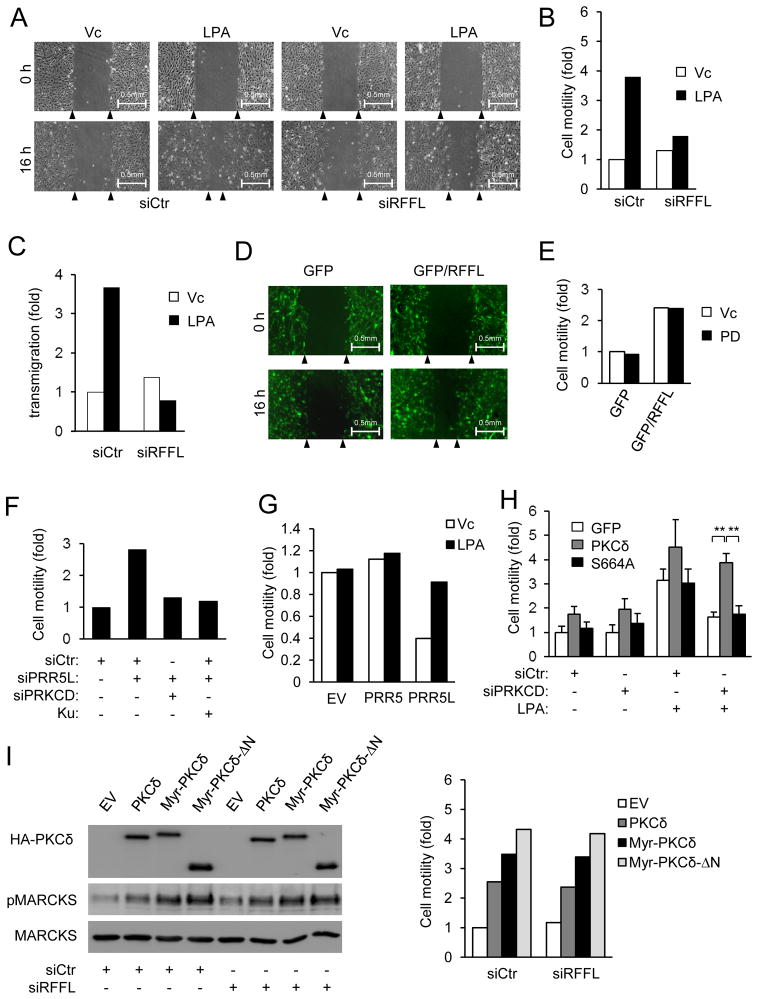
LPA is a potent stimulator of fibroblast migration 35, and its effect on MEF migration depended on Gα12, because it failed to stimulate Gna12−/− MEF migration (Fig. S6A). Expression of Gα12 in Gna12−/− MEFs restored their ability to migrate in response to LPA (Figure S6B) as well as the late phase PKCδ-HM and MARCKs phosphorylation (Figure 1C). Moreover, knockdown of RFFL inhibited LPA-induced MEF migration (Fig. 7A–C), whereas RFFL expression (Fig. 7D–E) or PRR5L knockdown (Fig. 7F) stimulated MEF migration. These results together indicate that Gα12-mediated upregulation of RFFL and destabilization of PRR5L are important for cell migration in response to LPA. Although PRR5 and PRR5L-deficiency abrogated LPA-induced migration, expression of PRR5L, but not PRR5, restored the LPA response by suppressing the basal migration of Prr5−/−;Prr5l−/−MEFs (Fig. 7G). This is consistent with our conclusion that LPA destabilizes PRR5L to induce cell migration.
Figure 7. The RFFL-PRR5L pathway has an important role in regulation of cell migration.
A–C) RFFL is required for LPA-induced fibroblast migration. MEFs were transfected with the control or RFFL siRNA for 72 hrs and analyzed in a scratch migration assay (A–B) or a transwell assay (C). Representative phase contrast images of the migration assays at 0 and 16 hrs after gap creation are shown (A). Experiments were repeated three times, and a representative experiment is shown.
D–E) Expression of RFFL increases cell migration. MEFs were infected with retroviruses expressing GFP or GFP-IRES-RFFL and treated with or without PD98059. Experiments were repeated three times, and a representative experiment is shown.
F) Knockdown of PRR5L increases the migration of MEFs. MEFs were transfected with the indicated siRNAs in the presence or absence of Ku-0063794 (Ku, 50 nM), followed by the scratch migration assay. Experiments were repeated three times, and a representative experiment is shown.
G) Expression of PRR5L in Prr5−/−;Prr5l−/− MEFs restores LPA-induced migration. Prr5−/−;Prr5l−/−MEFs and those infected with PRR5L expressing retrovirus were tested in a scratch migration assay (LPA, 400 nM). Experiments were repeated three times, and a representative experiment is shown.
H) PKCδ knockdown inhibits LPA-induced MEF migration. MEFs were infected with retrovirus expressing GFP, PKCδ, and its mutant S664A and transfected with the indicated siRNAs, followed by the scratch migration assay (LPA, 400 nM). The data are presented as means ± S.D. (**, p<0.05, Student’s t-Test, n=3).
I) Expression of WT and myristylated PKCδ stimulates MEF migration. MEFs were infected with retrovirus expressing GFP, WT PKCδ, myristylated PKCδ (myri-PKCδ) or myristylated PKCδ lacking N-terminal 280 amino acids (myri-PKCδ-ΔN) and transfected with the control siRNA or siRFFL. Western analysis and cell migration assay were performed 72 hours later. Experiments were repeated three times, and a representative experiment is shown.
To investigate the importance of PKC in MEF migration, we treated MEFs with the PKC inhibitor Go6850 or a PKC activator PMA. While Go6850 inhibited LPA-mediated MEF migration (Fig. S6D), PMA stimulated the migration of both WT and Gna12−/− MEFs (Fig. S6E). Moreover, knockdown of PKCδ inhibited MEF migration in response to LPA (Fig. 7H & S6F) or PRR5L knockdown (Fig. 7F). Expression of wildtype PKCδ, but not the mutant with its HM site mutated (S664A), was able to restore the migration phenotype of PKCδ knockdown, indicating the importance of this phosphorylation for MEF migration (Fig. 7H). Furthermore, expression of myristylated PKCδ, which exhibited elevated activity (Fig. 7I), could stimulate MEF migration even in the presence of RFFL siRNA (Fig. 7I). These data together indicate persistent PKCδ activation is sufficient for driving cell migration.
Role of Gα12 in pulmonary fibrosis
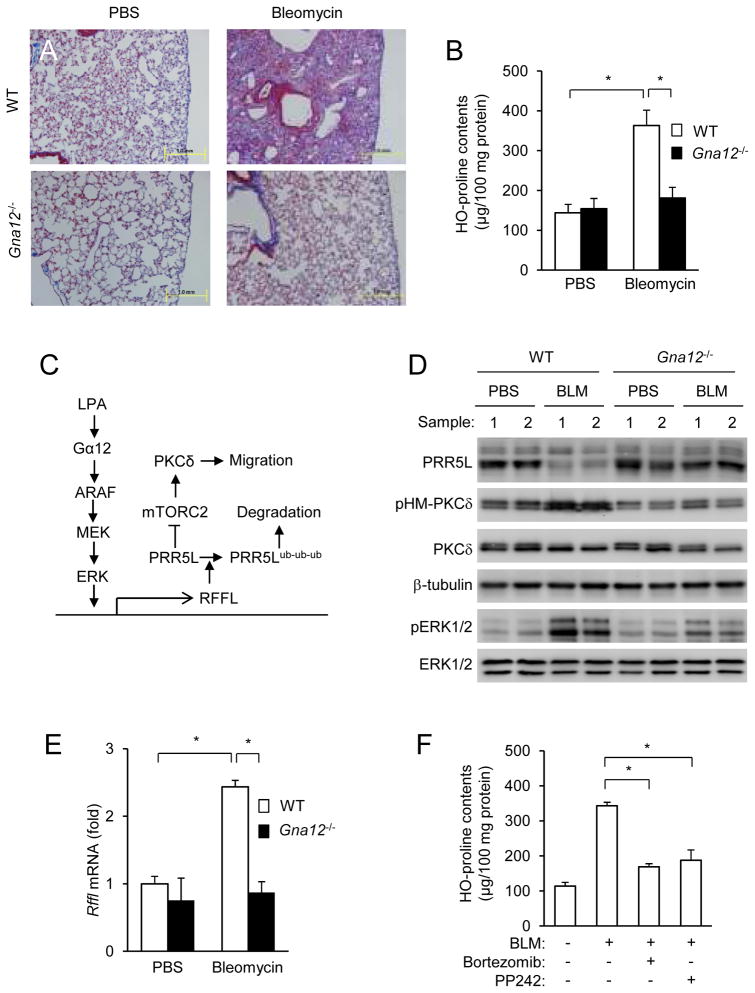
Mouse genetic studies showed that Gα12 and Gα13 proteins functioned largely redundantly in vivo 33, 37, but some distinct in vivo functions of Gα13 have emerged 38–40. Although distinct functions of Gα12 were shown in cultured cells 41–43, there is a lack of such evidence in vivo. The type 1 receptor of LPA, which can couple to Gα12, has an important role in bleomycin-induced pulmonary fibrosis primarily due to the role of LPA in fibroblast migration 44. The involvement of Gα12 in LPA-induced MEF migration suggests that Gα12-deficiency should have a significant role in lung fibrosis. Indeed, Gα12-deficiency showed significant reduction in pulmonary fibrosis (Fig. 8A–B). Consistent to the signaling pathway delineated in cultured cells (Fig. 8C), there were decreases in the PRRL5 protein contents and increases in the levels of Rffl mRNA, accompanied by increases in ERK and PKCδ HM phosphorylation in bleomycin-treated WT lungs compared to PBS-treated (Fig. 8D,E). These bleomycin-induced changes markedly diminished in Gα12-deficient samples (Fig. 8D,E), suggesting that the involvement of Gα12 in pulmonary fibrosis may be attributed to its regulation of PKCδ HM phosphorylation via the signaling pathway depicted in Fig. 8C. Consistent with this conclusion, the proteasome inhibitor Bortezomib 45 or mTOR inhibitor PP242 46 inhibited bleomycin-induced pulmonary fibrosis (Fig. 8F), accompanied by the inhibition of PKCδ HM, but not ERK, phosphorylation (Fig. S7A). Importantly, Bortezomib stabilized PRR5L protein with little effect on its mRNA level, whereas PP242 only blocked PKCδ phosphorylation (Fig. S7A–B).
Figure 8. Gα12 has an important role in bleomycin-induced pulmonary fibrosis.
A–B) Gα12-deficiency impedes pulmonary fibrosis development. Gα12-deficient mice and their WT littermates (12 weeks old) were administered with bleomycin or PBS through intratracheal injection. Fourteen days later, the lungs from these mice were collected for histological examination (Trichrome staining, A) and hydroxyproline content determination (B). The data are presented as means ± S.D. (*, p<0.05, Student’s t-Test; PBS treated, n=5; bleomycin treated, n=6).
C) A model for the regulation of mTORC2-mediated long-term PKC HM phosphorylation. LPA acts through Gα12 to activate ARAF, which leads to polyubiquitination and destabilization of PRR5L. PRR5L inhibits PKCδ HM phosphorylation by mTORC2. Thus, its absence results in persistent PKCδ HM phosphorylation and activation that is important for the migration of fibroblast cells.
D–E) Gα12-deficiency blocks bleomycin-induced changes. Lungs from bleomycin-treated Gα12-deficient and their WT littermate mice were subjected to Western (C; results from two pairs are shown) and qRT-PCR (D) analyses. The data in D are presented as means ± S.D. (*, p<0.05, Student’s t-Test, PBS treated, n=5; bleomycin treated, n=6).
F) Effects of a proteasome and an mTOR inhibitor on bleomycin-induced lung fibrosis. Mice were administered with bleomycin or PBS, followed by treatment with Bortezomib or PP242. The data are presented as means ± S.D. (*, p<0.05, n=5, Student’s t-Test).
DISCUSSION
In this report, we observed that LPA induces two phases of PKC HM phosphorylation and characterized a signaling mechanism by which LPA regulates the late phase phosphorylation through its regulation of mTORC2. As depicted in Figure 7C, LPA acts through Gα12 to specifically activate ARAF, which in turn upregulates RFFL, leading to the polyubiquitination and destabilization of PRR5L, a component of mTORC2 and suppressor of PKC HM phosphorylation. The lack of PRR5L promotes PKCδ HM phosphorylation and activation by mTORC2. Thus, our study revealed a role for RFFL in mTORC2 regulation by identifying PRR5L as its substrate. RFFL is a Ring domain-containing ubiquitin E3 ligase, which is also known as rififylin and CARP2 47, 48 and has only two previously reported putative substrates, p53 and RIP 49, 50. There is a homolog of RFFL, RNF34 (also known as CARP1), which shares less than 50% amino acid identity with RFFL. RNF34 is unlikely to be involved in LPA and Gα12-mediated regulation of mTORC2, because neither Gα12QL nor LPA regulated its expression level in HEK293T or MEF cells (data not shown).
PRR5L and its homolog PRR5 were identified as mTORC2-associated proteins 51–53. They, unlike many of the other mTORC2 components, are not involved in the assembly of the mTORC2 complexes. Their roles in mTORC2 functions have remained unclear until recently. The finding that PRR5-deficiency appears to affect specifically SGK1 phosphorylation but not AKT or PKC HM phosphorylation 54 suggests that PRR5 may regulate mTORC2 activity in a substrate specific manner. Our result showing that PRR5L specifically suppresses mTORC2-mediated HM phosphorylation of PKC, but not AKT, bolsters the idea. Moreover, we provided a biochemical basis for the selectivity by showing that PRR5L favors the binding of mTORC2 to AKT over PKC. Although addition of recombinant PRR5L protein enhanced the substrate preference of mTORC2 for AKT, the role of PRR5L in AKT HM phosphorylation in vivo does not appear to be as prominent as that in PKC HM phosphorylation, because PRR5L knockdown or knockout had a weak effect on AKT HM phosphorylation. This lack of a strong effect may be because PRR5L’s effect is marginalized by other regulatory factors for AKT, such as PtdIns(3,4,5)P3. However, we do not exclude the possibility that PRR5L may have a significant role in regulating AKT HM phosphorylation under a different context. Our results also suggest that PRR5 and PRR5L are not functional redundant, at least, in the regulation of PKC HM phosphorylation. Expression of PRR5 in Prr5−/−;Prr5l−/− MEFs did not suppress basal PKC HM phosphorylation or migration, nor did it restore the responsiveness to LPA. In addition, RFFL did not coimmunoprecipitate with or stimulate polyubiquitination of PRR5 in overexpressed HEK293T cells (data not shown). Nevertheless, together with the selective effect of PRR5-deficiency on SGK phosphorylation 54, we suggest that the PRR5 and PRR5L proteins may function to modulate the activity of mTORC2 in a substrate-depending manner.
Although mTORC2 was shown to play an important role in cell migration 13, 23, 25, 26, the mechanisms have been remained largely elusive. The stimulation of late phase PKC HM phosphorylation by mTORC2 may be a mechanism by which mTORC2 regulates fibroblast migration. The importance of the late phase PKC HM phosphorylation in cell migration was illustrated by the observation that Gα12-deficiency, on which LPA-induced fibroblast migration depends, specifically diminished the late phase PKC HM phosphorylation without affecting the early phase PKC HM phosphorylation or activation. The fact that knockdown of PRR5L or overexpression of RFFL, which are used by Gα12 and LPA to regulate the late phase PKC HM phosphorylation, stimulates cell migration supports the conclusion. Because fibroblasts migrate at a very slow pace, it is reasonable that sustained PKC activation is required for the process. A transcription and protein stability-based regulatory mechanism would be more suitable for generating sustained responses than those using Ca2+ and lipid-derived second messengers, which are often produced in rather transient manners in cells.
Although persistent PKCδ activation appeared to be sufficient for driving cell migration, we do not know how HM phosphorylation leads to PKC activation. Nor do we know how PKCδ regulates fibroblast migration, even though some previous studies have suggested that PKCδ plays an important role in cell migration 55–58. Cell migration is a complex process, and a large number of signaling mechanisms have been characterized for their involvement in the regulation. These signaling pathways may work in concert with others; some may be redundant of others, and some may function in a context-dependent manner to achieve optimal cell migration. It is hence important to know how PKC HM phosphorylation functions in relation to other signaling pathways in cell migration regulation. Additionally, because all of the three Raf isoforms, when overexpressed, could upregulate RFFL (data not shown), it would be interesting to know whether the RFFL-PKC pathway is also used by growth factors, which activate Raf via Ras, or by activated Ras and RAF mutants, which are frequently found in cancers, to regulate cell, particularly tumor cell, migration. Finally, it is important to know how Gα12, but not Gα13, specifically activated ARAF, but not CRAF. Work is under way to investigate these questions.
Supplementary Material
Acknowledgments
We thank Bing Su for discussion and the anti-MAPKAP1 antibody, Michelle Orsulak for technical help. This work is supported by NIH grants (HL070694, HL080706 and CA139395 to W.D. and GM61454 and GM074001 to T.K.) and a Ellison Medical Foundation grant (AG-SS-2190-08 to M.I.S.).
Footnotes
AUTHOR CONTRIBUTIONS:
X.G. conceived the initial idea, planed and performed some of the experiments, analyzed the data, and participated in manuscript preparation. J.W. and C.W. performed some of the experiments. E.S., T.K. S.S., and D. A. provided critical experimental materials. S.O. and M.I.S. provided critical research materials and comments and helped in manuscript preparation. D.W. conceived the initial idea, planed the experiments, analyzed the data, and prepared the manuscript.
References
- 1.Polak P, Hall MN. mTOR and the control of whole body metabolism. Curr Opin Cell Biol. 2009;21:209–218. doi: 10.1016/j.ceb.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 2.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 3.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageingNature reviews. Molecular cell biology. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foster KG, Fingar DC. Mammalian target of rapamycin (mTOR): conducting the cellular signaling symphony. J Biol Chem. 2010;285:14071–14077. doi: 10.1074/jbc.R109.094003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang J, Manning BD. A complex interplay between Akt, TSC2 and the two mTOR complexes. Biochem Soc Trans. 2009;37:217–222. doi: 10.1042/BST0370217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fonseca BD, Smith EM, Lee VH, MacKintosh C, Proud CG. PRAS40 is a target for mammalian target of rapamycin complex 1 and is required for signaling downstream of this complex. J Biol Chem. 2007;282:24514–24524. doi: 10.1074/jbc.M704406200. [DOI] [PubMed] [Google Scholar]
- 7.Hara K, et al. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–189. doi: 10.1016/s0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- 8.Kim DH, et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 9.Kim DH, et al. GbetaL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol Cell. 2003;11:895–904. doi: 10.1016/s1097-2765(03)00114-x. [DOI] [PubMed] [Google Scholar]
- 10.Kovacina KS, et al. Identification of a proline-rich Akt substrate as a 14–3-3 binding partner. J Biol Chem. 2003;278:10189–10194. doi: 10.1074/jbc.M210837200. [DOI] [PubMed] [Google Scholar]
- 11.Jacinto E, et al. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 12.Pearce LR, et al. Identification of Protor as a novel Rictor-binding component of mTOR complex-2. Biochem J. 2007;405:513–522. doi: 10.1042/BJ20070540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarbassov DD, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 14.Woo SY, et al. PRR5, a novel component of mTOR complex 2, regulates platelet-derived growth factor receptor beta expression and signaling. J Biol Chem. 2007;282:25604–25612. doi: 10.1074/jbc.M704343200. [DOI] [PubMed] [Google Scholar]
- 15.Yang Q, Inoki K, Ikenoue T, Guan KL. Identification of Sin1 as an essential TORC2 component required for complex formation and kinase activity. Genes Dev. 2006;20:2820–2832. doi: 10.1101/gad.1461206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacinto E, Lorberg A. TOR regulation of AGC kinases in yeast and mammals. Biochem J. 2008;410:19–37. doi: 10.1042/BJ20071518. [DOI] [PubMed] [Google Scholar]
- 17.Mamane Y, Petroulakis E, LeBacquer O, Sonenberg N. mTOR, translation initiation and cancer. Oncogene. 2006;25:6416–6422. doi: 10.1038/sj.onc.1209888. [DOI] [PubMed] [Google Scholar]
- 18.Tremblay F, Marette A. Amino acid and insulin signaling via the mTOR/p70 S6 kinase pathway. A negative feedback mechanism leading to insulin resistance in skeletal muscle cells. J Biol Chem. 2001;276:38052–38060. doi: 10.1074/jbc.M106703200. [DOI] [PubMed] [Google Scholar]
- 19.Um SH, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 20.Facchinetti V, et al. The mammalian target of rapamycin complex 2 controls folding and stability of Akt and protein kinase C. EMBO J. 2008;27:1932–1943. doi: 10.1038/emboj.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Martinez JM, Alessi DR. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1) Biochem J. 2008;416:375–385. doi: 10.1042/BJ20081668. [DOI] [PubMed] [Google Scholar]
- 22.Ikenoue T, Inoki K, Yang Q, Zhou X, Guan KL. Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling. EMBO J. 2008;27:1919–1931. doi: 10.1038/emboj.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loewith R, et al. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell. 2002;10:457–468. doi: 10.1016/s1097-2765(02)00636-6. [DOI] [PubMed] [Google Scholar]
- 24.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 25.Jacinto E, et al. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6:1122–1128. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt A, Kunz J, Hall MN. TOR2 is required for organization of the actin cytoskeleton in yeast. Proc Natl Acad Sci U S A. 1996;93:13780–13785. doi: 10.1073/pnas.93.24.13780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu L, Das S, Losert W, Parent CA. mTORC2 regulates neutrophil chemotaxis in a cAMP- and RhoA-dependent fashion. Developmental cell. 2010;19:845–857. doi: 10.1016/j.devcel.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Copp J, Manning G, Hunter T. TORC-specific phosphorylation of mammalian target of rapamycin (mTOR): phospho-Ser2481 is a marker for intact mTOR signaling complex 2. Cancer Res. 2009;69:1821–1827. doi: 10.1158/0008-5472.CAN-08-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soliman GA, et al. mTOR Ser-2481 autophosphorylation monitors mTORC-specific catalytic activity and clarifies rapamycin mechanism of action. J Biol Chem. 2010;285:7866–7879. doi: 10.1074/jbc.M109.096222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gan X, Wang J, Su B, Wu D. Evidence for direct activation of mTORC2 kinase activity by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 2011;286:10998–11002. doi: 10.1074/jbc.M110.195016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zinzalla V, Stracka D, Oppliger W, Hall MN. Activation of mTORC2 by Association with the Ribosome. Cell. 2011;144:757–768. doi: 10.1016/j.cell.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 32.Oh WJ, et al. mTORC2 can associate with ribosomes to promote cotranslational phosphorylation and stability of nascent Akt polypeptide. EMBO J. 2010;29:3939–3951. doi: 10.1038/emboj.2010.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Worzfeld T, Wettschureck N, Offermanns S. G(12)/G(13)-mediated signalling in mammalian physiology and disease. Trends in pharmacological sciences. 2008;29:582–589. doi: 10.1016/j.tips.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Riobo NA, Manning DR. Receptors coupled to heterotrimeric G proteins of the G12 family. Trends in pharmacological sciences. 2005;26:146–154. doi: 10.1016/j.tips.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Choi JW, et al. LPA receptors: subtypes and biological actions. Annu Rev Pharmacol Toxicol. 2010;50:157–186. doi: 10.1146/annurev.pharmtox.010909.105753. [DOI] [PubMed] [Google Scholar]
- 36.Karagiosis SA, Chrisler WB, Bollinger N, Karin NJ. Lysophosphatidic acid-induced ERK activation and chemotaxis in MC3T3-E1 preosteoblasts are independent of EGF receptor transactivation. J Cell Physiol. 2009;219:716–723. doi: 10.1002/jcp.21720. [DOI] [PubMed] [Google Scholar]
- 37.Herroeder S, et al. Guanine nucleotide-binding proteins of the G12 family shape immune functions by controlling CD4+ T cell adhesiveness and motility. Immunity. 2009;30:708–720. doi: 10.1016/j.immuni.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 38.Gong H, et al. G protein subunit Galpha13 binds to integrin alphaIIbbeta3 and mediates integrin “outside-in” signaling. Science. 2010;327:340–343. doi: 10.1126/science.1174779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moers A, et al. G13 is an essential mediator of platelet activation in hemostasis and thrombosis. Nature Med. 2003;9:1418–1422. doi: 10.1038/nm943. [DOI] [PubMed] [Google Scholar]
- 40.Shan D, et al. The G protein G alpha(13) is required for growth factor-induced cell migration. Developmental cell. 2006;10:707–718. doi: 10.1016/j.devcel.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 41.Radhika V, Hee Ha J, Jayaraman M, Tsim ST, Dhanasekaran N. Mitogenic signaling by lysophosphatidic acid (LPA) involves Galpha12. Oncogene. 2005;24:4597–4603. doi: 10.1038/sj.onc.1208665. [DOI] [PubMed] [Google Scholar]
- 42.Ki SH, Choi MJ, Lee CH, Kim SG. Galpha12 specifically regulates COX-2 induction by sphingosine 1-phosphate. Role for JNK-dependent ubiquitination and degradation of IkappaBalpha. J Biol Chem. 2007;282:1938–1947. doi: 10.1074/jbc.M606080200. [DOI] [PubMed] [Google Scholar]
- 43.Won HY, Min HJ, Lee WH, Kim SG, Hwang ES. Galpha12 is critical for TCR-induced IL-2 production and differentiation of T helper 2 and T helper 17 cells. Biochem & Biophys Res Com. 2010;394:811–816. doi: 10.1016/j.bbrc.2010.03.079. [DOI] [PubMed] [Google Scholar]
- 44.Tager AM, et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med. 2008;14:45–54. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- 45.Neubert K, et al. The proteasome inhibitor bortezomib depletes plasma cells and protects mice with lupus-like disease from nephritis. Nat Med. 2008;14:748–755. doi: 10.1038/nm1763. [DOI] [PubMed] [Google Scholar]
- 46.Janes MR, et al. Effective and selective targeting of leukemia cells using a TORC1/2 kinase inhibitor. Nat Med. 2010;16:205–213. doi: 10.1038/nm.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coumailleau F, et al. Over-expression of Rififylin, a new RING finger and FYVE-like domain-containing protein, inhibits recycling from the endocytic recycling compartment. Mol Biol Cell. 2004;15:4444–4456. doi: 10.1091/mbc.E04-04-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McDonald ER, 3rd, El-Deiry WS. Suppression of caspase-8- and -10-associated RING proteins results in sensitization to death ligands and inhibition of tumor cell growth. Proc Natl Acad Sci USA. 2004;101:6170–6175. doi: 10.1073/pnas.0307459101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang W, et al. CARPs are ubiquitin ligases that promote MDM2-independent p53 and phospho-p53ser20 degradation. J Biol Chem. 2007;282:3273–3281. doi: 10.1074/jbc.M610793200. [DOI] [PubMed] [Google Scholar]
- 50.Liao W, et al. CARP-2 is an endosome-associated ubiquitin ligase for RIP and regulates TNF-induced NF-kappaB activation. Current biology : CB. 2008;18:641–649. doi: 10.1016/j.cub.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woo SY, et al. PRR5, a novel component of mTOR complex 2, regulates platelet-derived growth factor receptor beta expression and signaling. J Biol Chem. 2007;282:25604–25612. doi: 10.1074/jbc.M704343200. [DOI] [PubMed] [Google Scholar]
- 52.Thedieck K, et al. PRAS40 and PRR5-like protein are new mTOR interactors that regulate apoptosis. PLoS One. 2007;2:e1217. doi: 10.1371/journal.pone.0001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pearce LR, et al. Identification of Protor as a novel Rictor-binding component of mTOR complex-2. The Biochemical journal. 2007;405:513–522. doi: 10.1042/BJ20070540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pearce LR, Sommer EM, Sakamoto K, Wullschleger S, Alessi DR. Protor-1 is required for efficient mTORC2-mediated activation of SGK1 in the kidney. The Biochemical journal. 2011 doi: 10.1042/BJ20102103. [DOI] [PubMed] [Google Scholar]
- 55.Bai X, et al. Protein kinase C{delta} deficiency accelerates neointimal lesions of mouse injured artery involving delayed reendothelialization and vasohibin-1 accumulation. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:2467–2474. doi: 10.1161/ATVBAHA.110.215723. [DOI] [PubMed] [Google Scholar]
- 56.Zhao CT, et al. PKCdelta regulates cortical radial migration by stabilizing the Cdk5 activator p35. Proc Natl Acad Sci USA. 2009;106:21353–21358. doi: 10.1073/pnas.0812872106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu B, et al. Protein kinase C-delta regulates migration and proliferation of vascular smooth muscle cells through the extracellular signal-regulated kinase 1/2. Journal of vascular surgery : official publication, the Society for Vascular Surgery [and] International Society for Cardiovascular Surgery, North American Chapter. 2007;45:160–168. doi: 10.1016/j.jvs.2006.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chou WH, et al. Neutrophil protein kinase Cdelta as a mediator of stroke-reperfusion injury. The Journal of clinical investigation. 2004;114:49–56. doi: 10.1172/JCI21655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Y, et al. Different roles of G protein subunits beta1 and beta2 in neutrophil function revealed by gene expression silencing in primary mouse neutrophils. J Biol Chem. 2010;285:24805–24814. doi: 10.1074/jbc.M110.142885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hart MJ, et al. Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Galpha13. Science. 1998;280:2112–2114. doi: 10.1126/science.280.5372.2112. [DOI] [PubMed] [Google Scholar]
- 61.Kozasa T, et al. p115 RhoGEF, a GTPase activating protein for Galpha12 and Galpha13. Science. 1998;280:2109–2111. doi: 10.1126/science.280.5372.2109. [DOI] [PubMed] [Google Scholar]
- 62.Gu JL, Muller S, Mancino V, Offermanns S, Simon MI. Interaction of G alpha(12) with G alpha(13) and G alpha(q) signaling pathways. Proc Natl Acad Sci USA. 2002;99:9352–9357. doi: 10.1073/pnas.102291599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tager AM, et al. Inhibition of pulmonary fibrosis by the chemokine IP-10/CXCL10. Am J Respir Cell Mol Biol. 2004;31:395–404. doi: 10.1165/rcmb.2004-0175OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.