Summary
The mTOR signaling pathway regulates cell growth, differentiation, proliferation and metabolism. Loss of function mutations in upstream regulators of mTOR have been highly associated with dysplasias, epilepsy and neurodevelopmental disorders. These include tuberous sclerosis, which is due to mutations in TSC1 or TSC2 genes, mutations in phosphatase and tensin homolog (PTEN) as in Cowden syndrome, polyhydramnios, megalencephaly, symptomatic epilepsy syndrome (PMSE) due to mutations in the STE20-related kinase adaptor alpha (STRADalpha), and neurofibromatosis type 1 attributed to neurofibromin 1 mutations. Inhibition of the mTOR pathway with rapamycin may prevent epilepsy and improve the underlying pathology in mouse models with disrupted mTOR signaling, due to PTEN or TSC mutations. However the timing and duration of its administration appear critical in defining the seizure and pathology-related outcomes. Rapamycin application in human cortical slices from patients with cortical dysplasias reduces the 4-aminopyridine induced oscillations. In the multiple-hit model of infantile spasms, pulse high dose rapamycin administration can reduce the cortical overactivation of the mTOR pathway, suppresses spasms and has disease-modifying effects by partially improving cognitive deficits. In post-status epilepticus models of temporal lobe epilepsy, rapamycin may ameliorate the development of epilepsy-related pathology and reduce the expression of spontaneous seizures, but its effects depend on the timing and duration of administration, and possibly the model used. The observed recurrence of seizures and epilepsy-related pathology after rapamycin discontinuation suggests the need for continuous administration to maintain the benefit. However, the use of pulse administration protocols may be useful in certain age-specific epilepsy syndromes, like infantile spasms, whereas repetitive pulse rapamycin protocols may suffice to sustain a long-term benefit in genetic disorders of the mTOR pathway. In summary, mTOR dysregulation has been implicated in several genetic and acquired forms of epileptogenesis. The use of mTOR inhibitors can reverse some of these epileptogenic processes although their effects depend upon the timing and dose of administration as well as the model used.
Keywords: Cortical dysplasia, infantile spasms, tuberous sclerosis, kainic acid, pilocarpine, status epilepticus
Introduction
The mTOR pathway plays an essential role in cell growth, differentiation, proliferation and metabolism via phosphorylation of a number of translational regulators such as ribosomal S6 kinase and initiation factor 4E binding protein 1 (4EBP1) (Inoki et al. 2005; Crino 2011). In turn, the mTOR pathway receives key information from nutrients, growth factors, cytokines, and hormones through tyrosine kinase receptors (Kwiatkowski 2003; Inoki et al. 2005). In brain cells, mTOR is also modulated by glutamate and dopamine receptors (Hoeffer et al. 2010). Not surprisingly, the mTOR pathway has been shown to play a pivotal role during development of the cerebral cortex (Crino 2011). The mTOR pathway is negatively regulated by tumor suppressor genes TSC1 and TSC2, as well as by their upstream regulators including phosphatase and tensin homolog (PTEN), the STE20-related kinase adaptor alpha (STRADalpha and neurofibromin 1 (NF1)(Sulis et al. 2003; Puffenberger et al. 2007; Crino 2011; Ehninger et al. 2011) (Figure 1). Mutations in these genes lead to hyperactivity of the mTOR pathway associated with cellular alterations including abnormal differentiation, proliferation and growth. Clinically, there is a high co-morbidity of these genetic disorders with epilepsy (Table 1). Increasing evidence also implicates mTOR dysregulation in the pathogenesis of acquired forms of epilepsy, such as infantile spasms (IS) and temporal lobe epilepsy (TLE) (Zeng et al. 2009; Raffo et al. 2011).
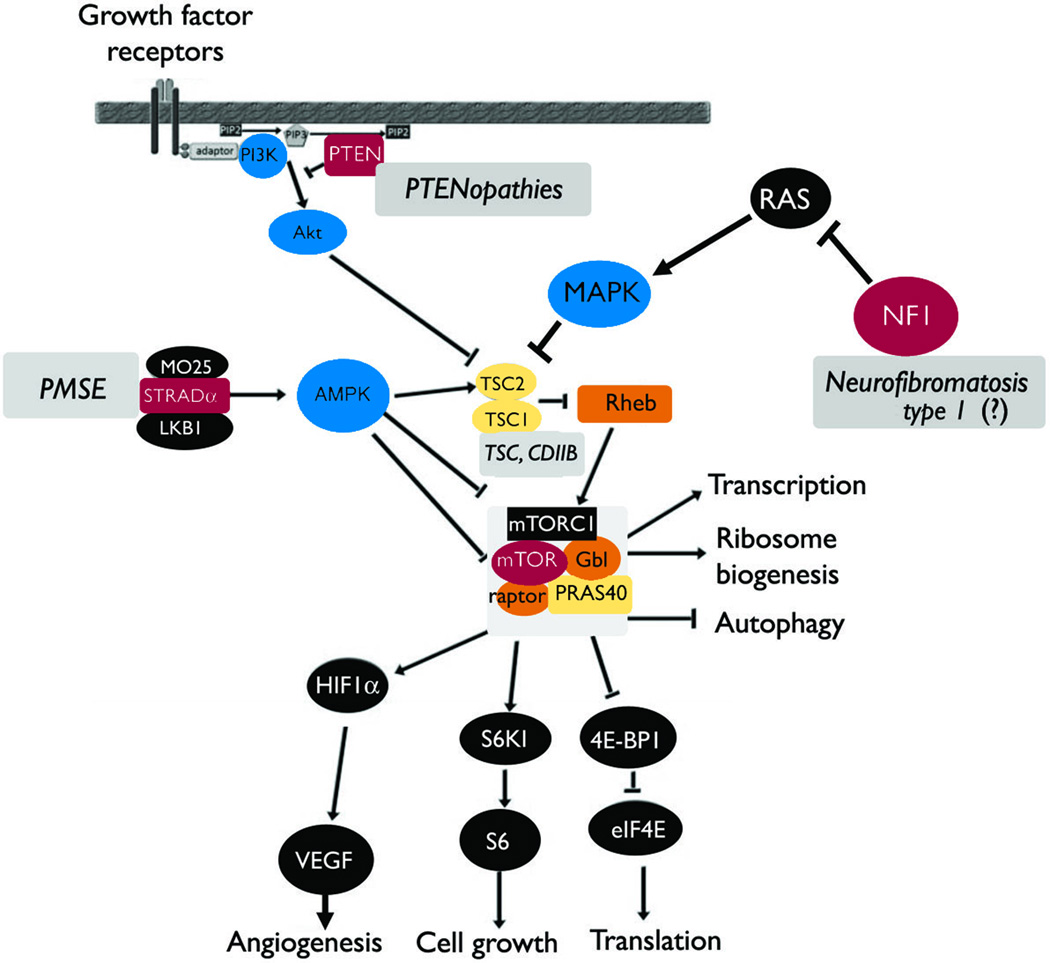
Figure 1. Simplified diagram of the mTORC1 pathway and the main regulators implicated in human epilepsies.
The mTORC1 complex regulates the activity of downstream targets that eventually lead to enhanced translation. Cell growth, angiogenesis, transcription, ribosome biogenesis and inhibit autophagy. Its regulation is complex and only selected pathways known to be implicated in human epilepsies are seen here. TSC1 and TSC2 dimers inhibit mTORC1 activity but when mutations impair their function, diseases such as TSC or CDIIB ensue (Curatolo et al. 2001; Becker et al. 2002; Schonberger et al. 2009; Crino 2011). Other disorders with overactivated mTORC1 include PMSE (mutations of STRADalpha) (Puffenberger et al. 2007; Orlova et al. 2010b), PTENopathies (Cowden syndrome, Bannayan-Riley-Ruvalcaba syndrome and Proteus syndrome) where loss of function mutation of PTEN are seen (Cross 2005; Bastos et al. 2008; Pilarski et al. 2011). Neurofibromatosis type 1, which is due to neurofibromin 1 (NF1) mutations, may also lead to overactivation of the mTOR pathway (Motte et al. 1993; Ruggieri et al. 2009). The incidence of epilepsy, including IS, in neurofibromatosis type 1 is lower than in other neurocutaneous disorders. Although it has been speculated that the association of epilepsy and neurofibromatosis 1 may be more than coincidental, there is no definite proof of a causative role of NF1 in human epilepsies [hence the (?) in the figure] (Motte et al. 1993; Ruggieri et al. 2009).
Table 1.
Genetic dysregulation of the mTOR pathway associated with epilepsy
| Disease | Gene | CNS manifestations | Reference |
|---|---|---|---|
| Tuberous sclerosis | TSC1 TSC2 | Epilepsy, tubers, autism, cognitive impairment, tubers, subependymal nodules, tumors | (Curatolo et al. 2001; Crino 2011) |
| Cowden syndrome | PTEN | Macrocephaly (38%), Lhermitte-Duclos, intellectual disability, epilepsy, ataxia | (Pilarski et al. 2011) |
| Bannayan-Riley-Ruvalcaba syndrome | PTEN | Macrocephaly, epilepsy, developmental delay | (Pilarski et al. 2011) |
| Proteus syndrome | PTEN | 20% of hemimegalencephaly patients, case reports with epilepsy | (Cross 2005; Bastos et al. 2008) |
| Neurofibromatosis type 1 | Neurofibromin 1 | Case reports with epilepsy | (Motte et al. 1993; Ruggieri et al. 2009) |
| Polyhydramnios, megalencephaly, symptomatic epilepsy syndrome (PMSE) | STRADalpha | Macrocephaly, cognitive disability, epilepsy, subependymal dysplasias, ventriculomegaly | (Puffenberger et al. 2007; Orlova et al. 2010b) |
| Focal cortical dysplasia type IIB | TSC1, TSC2 polymorphisms | Focal cortical dysplasias, epilepsy | (Becker et al. 2002; Schonberger et al. 2009) |
Here, we present a composite review on the role of the mTOR pathway in epileptogenesis, based on our presentations at the XI Workshop on the Neurobiology of Epilepsy (WONOEP XI), organized by the Neurobiology Commission of the International League Against Epilepsy in Grottaferrata, Italy (23–27 August 2011). We discuss evidence on the role of mTOR overactivation in the pathogenesis of disorders associated with cortical dysplasias (CDs), IS, and TLE and the potential use of mTOR inhibitors, like rapamycin, in their treatment.
The mTOR pathway in cortical dysplasias
Hyperactivity of the mTOR pathway has been evidenced in a number of hypertrophic disorders of the brain including tuberous sclerosis complex (TSC) and Cowden disease (Inoki et al. 2005). Indeed, dysregulation of the mTOR pathway could be a common theme in focal cortical dysplasia (FCD), hemimegalencephaly and TSC, which could be grouped under the term “TORopathies” (Crino 2007; Crino 2009). TSC, a genetic disorder caused by mutations in TSC1 or TSC2 genes that code for hamartin and tuberin respectively, is a prime example of dysregulation of the mTOR pathway. The mutation produces a nosological constellation that includes benign tumors (hamartomas) affecting multiple organs. In the brain, subependymal giant cell astrocytomas and cortical and subcortical tubers are pathognomonic of TSC. Tubers can lead to epileptic activity, seizures, IS, and cognitive disturbances often associated with autism (Orlova et al. 2010a). PTEN is another tumor suppressor gene that counteracts signals for growth and proliferation through the phosphoinositide 3 kinase (PI3K) pathway (Cantley 2002). Inherited mutations of PTEN lead to hamartoma syndromes including Cowden syndrome and Lhermitte-Duclos diseases (Marsh et al. 1999). Cowden syndrome is a multisystem disorder with predisposition to malignant or benign tumors and hamartomas (e.g., breast, thyroid, uterine, skin, gastrointestinal) (Pilarski et al. 2011) (Table 1). Lhermitte-Duclos disease is a dysplastic gangliocytoma of the cerebellum and, according to the more recent diagnostic criteria, it is a pathognomonic feature of Cowden syndrome (Pilarski et al. 2011).
FCD, a malformation of brain development, is the most common cause of epilepsy in pediatric surgical cases (Lerner et al. 2009). FCD, as originally described by Taylor et al., is characterized by cortical dyslamination and the presence of abnormal cell types including cytomegalic neurons and balloon cells (Taylor et al. 1971). According to the most recent classification of CD, three distinct types can be differentiated: CDI, CDII and CDIII (Blumcke et al. 2011). CD type II is of particular importance in the context of the mTOR pathway as it displays neuronal cytomegaly (CDIIA) and balloon cells (CDIIB). It has been suggested that hyperactivity in the mTOR pathway is the cause of abnormal cell size and differentiation in CD (Baybis et al. 2004; Miyata et al. 2004; Ljungberg et al. 2006).
Similarities and differences between TSC and CDIIB
CDIIB shares histopathological similarities with TSC, e.g., presence of cytomegalic pyramidal neurons and balloon cells, which are morphologically indistinguishable from giant cells found in TSC cortical tubers (Blumcke et al. 2011; Cepeda et al. 2012). Histopathological similarities led to the conjecture that CD could represent a forme fruste of TSC (Farrell et al. 1992). In agreement, it has been reported that tuberin and hamartin expression is reduced in CDIIB cases, supporting that FCD could represent a focal form of TSC (Grajkowska et al. 2008). While their morphology is similar, several studies found differences between giant and balloon cells. For example, it has been shown that giant cells in TSC and balloon cells in CDIIB recruit different PI3K molecular cascades allowing distinction of these cells (Schick et al. 2007). In addition, giant and balloon cells display different gene expression profiles. Thus, phospho-S6 (pS6) expression alone in balloon cells does not support mTOR cascade activation in FCD, suggesting these cells derive by distinct pathogenic mechanisms (Baybis et al. 2004). In spite of differences in molecular mechanisms, morphological and functional similarities can also provide clues about their origin.
While in most cerebral pathologies leading to epileptic activity one would be hard pressed to pinpoint a specific cell type responsible for the generation of paroxysmal activity, in the case of TSC and CD, the histopathology is so telling that the answer appears obvious. In both human TSC and CD type IIB, the presence of abnormally enlarged cells (giant/balloon cells and cytomegalic neurons), against a backdrop of dyslaminated cortical mantle within and around the lesion area, led investigators to the assumption that these cells were the source of epileptogenic activity. In contrast, using brain slices from cortical tissue resected for the treatment of pharmacoresistant pediatric epilepsy, we reported essential differences in the electrophysiological profiles of these enlarged cells (Cepeda et al. 2003). Thus, while cytomegalic pyramidal neurons are hyperexcitable and may play an important role in epileptogenesis, giant/balloon cells are unable to generate action potentials and do not integrate synaptic inputs, thus they are unlikely candidates as triggers of epileptic activity (Cepeda et al. 2003). This observation led us to suggest that balloon and giant cells are undifferentiated cells that did not commit to a glial or neuronal phenotype, in line with anatomical reports (Huttenlocher et al. 1984). If neuronal hypertrophy, caused by abnormal activation of the mTOR pathway, leads to cortical hyperexcitability, can mTOR inhibitors restore normal excitability and cell size? Use of animal models and human tissue from TSC and CD patients can help provide an answer.
Effects of mTOR inhibitors in animal models of TSC and CD
As we gain more insights into the mechanisms of TSC and CD, animal models are becoming more accurate at reproducing the histopathology and phenotype of these disorders. A growing number of rodent models are now available. In the case of TSC, deletions of TSC1 or TSC2 genes in specific cell types has been effective at replicating some histopathological features of TSC and, in some cases, the occurrence of spontaneous epileptic activity (Uhlmann et al. 2002; Zeng et al. 2011). While the replication of giant/balloon cells had been particularly difficult, recently created models have now been able to reproduce these abnormal cells (Goto et al. 2011; Magri et al. 2011). In the case of CD, cortical dyslamination can be induced by a wide range of methods such as freezing small cortical areas just after birth (Jacobs et al. 1999), methylazoxymethanol (MAM) exposure in rats (Paredes et al. 2006), or in utero irradiation (Roper 1998). However, an accurate model of CD type IIB has not yet been produced.
The availability of animal models of TSC and CD has allowed examination of pharmacological agents for therapy. While the use of rapamycin and other mTOR inhibitors has been widespread in a number a clinical applications (antifungal, antibiotic, immunosuppressor, anti-tumor), its use in epilepsy has only recently been recognized. In TSC and CD, mTOR inhibitors have been proposed to represent a rational therapy not only for treatment but also for preventing their multiple manifestations (Wong 2011a).
In Eker rats, a spontaneous mutation in TSC2 gene leads to tumor formation, including subependymal giant cell astrocytomas (Yeung et al. 1997). Although no spontaneous seizures are observed, when combined with irradiation, the Eker rats develop a cortical histopathology similar to human TSC and have reduced seizure threshold (Wenzel et al. 2004). Tumor progression can be reduced with rapamycin but a complete eradication is not achieved, probably as a consequence of development of drug resistance or activation of a rapamycin-insensitive pathway during tumor formation (Kenerson et al. 2005). Mice with conditional inactivation of the TSC1 gene in glial cells (Tsc1GFAP CKO mice) develop glial proliferation, progressive epilepsy, and premature death (Zeng et al. 2008). In this TSC model, treatment with rapamycin prevented the development of epilepsy and premature death. Also, in recently described models of TSC induced by removal of TSC1 from embryonic neural progenitor cells leading to development of giant cells, severe epilepsy and premature death, rapamycin extends survival and reduces seizure activity (Goto et al. 2011; Magri et al. 2011). As tubers can be manifested at birth, the value of prenatal administration of rapamycin was examined in another TSC model (TSC1cc Nescre+). A single administration of rapamycin to pregnant dams increased survival of pups but did not prevent brain enlargement and eventual lethality of the mutation (Anderl et al. 2011), indicating the value and limitations of prenatal rapamycin treatment.
Rapamycin is also effective to treat hypertrophy induced by disruption of PTEN. For example, in a model of CD produced by disruption of the PTEN gene in a subset of neurons leading to abnormal EEG activity and seizures, rapamycin administration reduced the severity of epileptic manifestations (Ljungberg et al. 2009). Inactivation of PTEN in cerebellar and dentate gyrus granular cells in mice causes macrocephaly, seizures and premature death (Kwon et al. 2003). Cellular hypertrophy in PTEN conditional inactivation is dependent on mTOR as inhibition with CCI-779 decreases seizure frequency and death rate, suggesting that mTOR inhibitors can be used to treat diseases resulting from PTEN deficiency (Kwon et al. 2003). Similarly, in NS-Pten conditional KO mice, inhibition of mTOR blocks epilepsy progression (Sunnen et al. 2011).
While therapeutic effects of mTOR inhibitors in models of TSC and CD could have been expected, the observation that it also affects the development and manifestations of acquired epilepsies came as a surprise and has sparked intense research and controversy (Wong 2010; Kumar et al. 2011). Rapamycin was shown to suppress mossy fiber sprouting in the pilocarpine model (Huang et al. 2010), leading to the suggestion that rapamycin could prevent epileptogenesis (Zeng et al. 2009). This effect is discussed in more detail in the section on TLE epileptogenesis.
Effects of mTOR inhibitors in Human Cortex
Preliminary studies in Cepeda’s lab tested the effects of rapamycin in tissue samples from pediatric TSC (n=1), CD (n=5) and non-CD (n=2) cases (Cepeda et al. 2010). Epileptiform activity was induced by applying a proconvulsant agent 4-aminopyridine (4-AP), a drug that blocks the A-type K+ current thereby enhancing neurotransmitter release. 4-AP induces paroxysmal activity and CD tissue shows particular sensitivity to this agent (Mattia et al. 1995). When applied to pyramidal neurons from pediatric cortical tissue, 4-AP induces rhythmic, large-amplitude membrane oscillations reminiscent of interictal discharges. In most slices (9 out of 12) from TSC and CD cases, bath application of rapamycin produced decreases in frequency (Figure 2) and/or amplitude of 4-AP oscillations. The latency for these effects was about 20–30 min. Similar effects of rapamycin were not observed in non-CD cases.
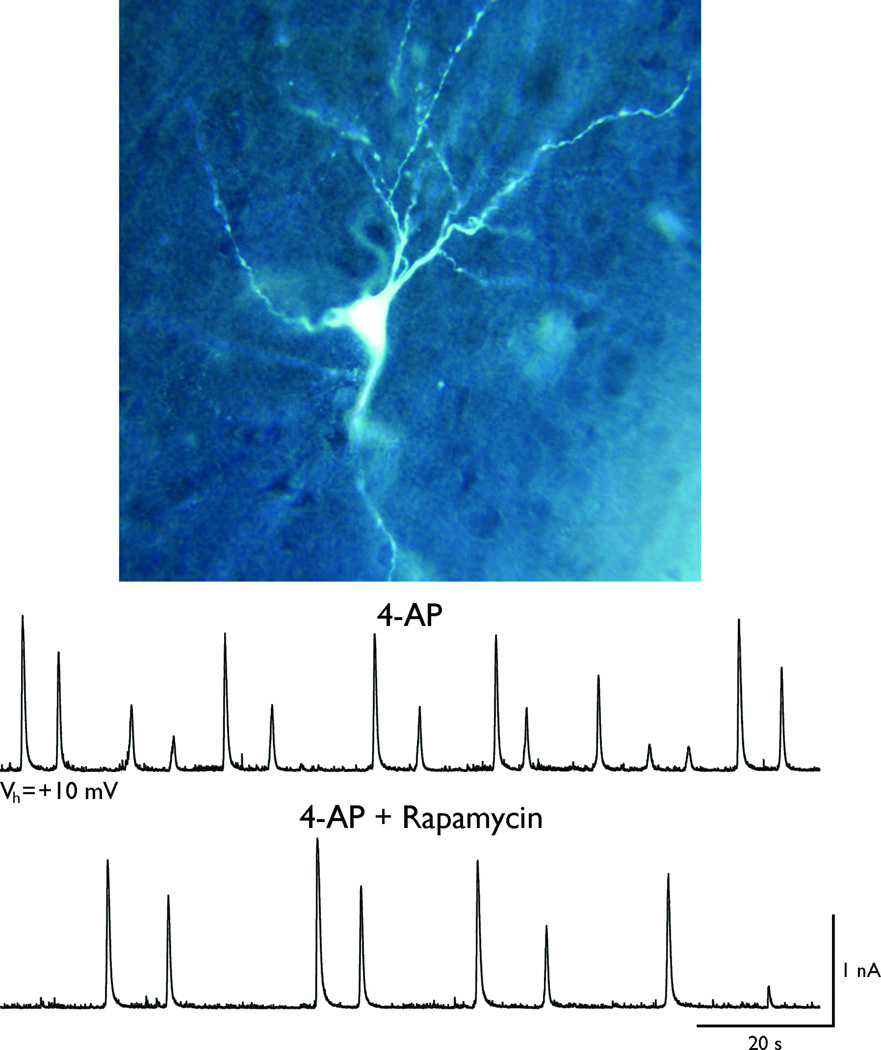
Figure 2. Acute effects of rapamycin in human cortical dysplasia.
A cortical pyramidal neuron was recorded in a slice from a patient with CD (0.87 yr, parietal cortex). The membrane potential was held at +10 mV and interictal-like activity was induced with 4-AP (100 µM). This proconvulsant agent induced membrane oscillations reminiscent of paroxysmal discharges. Bath application of rapamycin (2 µM) reduced both the amplitude and frequency of 4-AP oscillations, suggesting that acute application of rapamycin can reduce cortical excitability in cases of CD. The data are from Carlos Cepeda’s group.
The mTOR pathway in infantile spasms epileptogenesis
IS are epileptic seizures which manifest as clusters of flexion or extension tonic spasms in infants and represent the key feature of West syndrome, an infantile epileptic encephalopathy consisting of the triad IS, hypsarrhythmia (chaotic, epileptiform and high amplitude interictal EEG) and psychomotor delay or arrest (Pellock et al. 2010). However, the classical hypsarrhythmic EEG may not always be present (Curatolo et al. 2001). IS are often associated with neurodevelopmental deficits and appearance of other, difficult to treat, epileptic seizures (Hrachovy et al. 2003; Pellock et al. 2010). The currently preferred treatments typically include hormonal therapies such as adrenocorticotropic hormone (ACTH), high dose glucocorticoids, and vigabatrin (Mackay et al. 2004; Pellock et al. 2010). Patients with IS of structural/metabolic etiology have the poorest prognosis in regards to both epilepsy and neurodevelopmental outcomes after such treatments (Lombroso 1983; Mackay et al. 2004; Karvelas et al. 2009; Pellock et al. 2010). Among the responders, even in the absence of identifiable etiology, neurodevelopmental outcomes are not always normal (Lux et al. 2005) emphasizing the need to find better therapies to stop spasms and improve disease processes.
Around 200 human disorders have been linked with IS (Frost et al. 2005). The relatively high co-morbidity between disorders of the mTOR pathway and IS has drawn attention upon the potential role of mTOR signaling in the pathogenesis of IS and their treatment (Chudomelova et al. 2010; Raffo et al. 2011). IS are diagnosed in 38% of patients with TSC, whereas 5–10% of patients with IS have TSC (Sidenvall et al. 1995; Curatolo et al. 2001; Riikonen 2001; Karvelas et al. 2009; Bombardieri et al. 2010; Chu-Shore et al. 2010; Osborne et al. 2010). Other genetic disorders associated with IS include those with genetic defects of PTEN, which are present in ~20% of patients with hemimegalencephaly (Cross 2005; Bastos et al. 2008) (Table 1). CDIIB is also a common cause of IS which, as shown in the previous section, associates with dysregulated mTOR pathway (Becker et al. 2002; Baybis et al. 2004; Crino 2007; Crino 2009; Schonberger et al. 2009). Furthermore, IS have been described in case reports of patients with Neurofibromatosis type 1 (NF1). Although this association between IS and NF1 has been proposed to be more than purely coincidental (Motte et al. 1993; Ruggieri et al. 2009), a causative link has not been demonstrated and the incidence of IS in NF1 patients is certainly lower than in other genetic diseases of the mTOR pathway, like TSC. Neurofibromin 1, the gene implicated in NF1 is known to overactivate the mTOR pathway, by inactivating TSC2 (Johannessen et al. 2005). Patients with mutations of STRADalpha have also been reported to have IS (Puffenberger et al. 2007). Although it is certainly possible that the high association of these genetic “TORopathies” with IS may be due to shifting the onset of associated epilepsy to the early ages of life when IS are a highly prevalent seizure type, recent evidence from animal models supports a pathogenic role of mTOR pathway in IS per se.
Work from Galanopoulou’s lab has recently reported that mTOR dysregulation may be involved in the pathogenesis of IS of non-genetic etiology as well, using the multiple-hit rat model of symptomatic IS (Scantlebury et al. 2010; Raffo et al. 2011). According to the multiple-hit protocol, rats receive right intracerebral injections of doxorubicin and lipopolysaccharide at postnatal day 3 (PN3), which result in a right cortical/subcortical lesion, intentionally induced to simulate some of the pathologies of symptomatic IS (Scantlebury et al. 2010). Although the protocol maintains the intraperitoneal injection of p-chlorophenylalanine (a serotonin depleter) at postnatal day 5 (PN5), to increase the frequency of spasms, p-chlorophenylalanine is not necessary for the induction of spasms, which are observed in clusters between PN4–13. Similar to the human condition, the doxorubicin/lipopolysaccharide/p-chlorophenylalanine treated pups (DLP pups) also manifest other types of seizures, learning and memory problems, as well as abnormal social interactions and stereotypical behaviors, which are reminiscent of the higher risk for neurodevelopmental decline reported in infants with symptomatic IS. Furthermore, similar to the symptomatic IS which are less responsive to available treatments, DLP spasms do not respond to ACTH (Scantlebury et al. 2010).
Rapamycin, given after the onset of spasms, suppresses spasms and normalizes pS6-immunoreactivity (pS6-ir) in the cortical layers in a dose-dependent manner in the DLP model (Raffo et al. 2011). Very high doses (6 mg/kg i.p.) suppress spasms and normalize pS6-ir acutely, whereas low and moderate doses (1–3 mg/kg i.p.) decrease spasms and pS6-ir but with a few days delay, depending on the dose. These suggested a relative state of rapamycin-resistance at the early stages of IS, that can be bypassed by early exposure to very high doses of rapamycin. A possible hypothesis is that in the early period of spasms there is excessive activation of the mTORC1 pathway, due to increased activity of upstream regulators that can only be overriden by increasing rapamycin dose. Indeed, in prior studies, acute suppression of spasms was observed in the DLP model by combined mTORC1/mTORC2 inhibition, which reduces the positive feedback of mTORC2 pathway upon mTORC1 activity (Galanopoulou 2010).
Interestingly, early suppression of spasms with a high dose, 3-day pulse protocol (DLP-RAP633 group: 6 mg/kg i.p. at PN4, and 3 mg/kg/day i.p. at PN5 and PN6) sufficed to stop spasms without rebounds after its discontinuation. This can be attributed to the fact that pathological overactivation of downstream targets of the mTORC1 pathway, i.e. pS6-ir, is time-limited to the period when spasms manifest but not after their resolution (Raffo et al. 2011). Consequently, these findings raise the possibility that in IS of acquired etiology, rapamycin use may not need to be continuous but could be given only during the acute phase of spasms, limiting therefore the associated potential side effects of chronic unnecessary exposure to the drug. It is unclear which specific mechanisms lead to the pathological activation of the mTOR pathway in the DLP rat model of IS. It is possible that the activation of the mTOR pathway can be directly related to the activation of cell injury and survival pathways, as well as the local inflammation induced by doxorubicin and lipopolysaccharide (Weinstein et al. 2000; Wallin et al. 2010; Xu et al. 2011). However, these pathways can also be involved in several other pathologies associated with IS due to structural-metabolic etiologies, including hypoxia-ischemia, dysplastic lesions (Carloni et al. 2010; Aronica et al. 2011). Another possibility is that the overactivation of mTOR pathway may be an epiphenomenon of DLP spasms. However, the observation that mTOR inhibition with rapamycin suppresses spasms indicates that mTOR signaling is involved in their generation in the DLP model.
Following the publication of rapamycin effects in the DLP model, a study using the prenatal betamethasone / postnatal N-methyl-D-aspartate (NMDA) model, reported that rapamycin (3 mg/kg/day i.p.) given prior to the induction of NMDA-induced emprosthotonic seizures at PN15 had no anticonvulsant effect (Chachua et al. 2011). These results corroborate the findings in the DLP model, according to which low doses of rapamycin have no anticonvulsant effect on the acute stage of spasms. However, further comparisons between the 2 studies are hindered by the different administration protocols (pre- vs post-treatment), different dose sensitivity of PN4 vs PN15 rats (higher doses are generally needed in PN15 than in PN4 pups), and the lack of evidence for mTORC1 overactivation in the betamethasone/NMDA model.
As there is no prior report of acute anticonvulsant effects of rapamycin in models of status epilepticus (SE) (Zeng et al. 2009), the acute anti-IS effects that were observed in the DLP model, could therefore reflect either the existence of distinct dynamics in the networks generating spasms not applicable to other seizure types, or may be dose-related. It would be interesting to test whether similar dose-related acute effects can be seen in vitro as well. Interestingly, there was no antiseizure or antiepileptogenic effect of rapamycin on other emerging seizures in the DLP model, suggesting that the networks generating and controlling spasms are distinct from those implicated in other types of seizures (Raffo et al. 2011). Despite this, significant improvement of learning, memory and exploratory ability was noted in the rapamycin treated DLP pups when tested at PN16–19, including in the DLP-RAP633-treated group, which indicates disease modification (Raffo et al. 2011). Indeed, the importance of mTOR signaling in cognitive processing is well known not only from the animal studies but also from the known association of TSC with neurodevelopmental deficits (Curatolo et al. 2004; Jansen et al. 2008; Ehninger et al. 2009; Chu-Shore et al. 2010). It is worth noting that, whereas DLP pups had a clear cognitive benefit from rapamycin, naïve pups treated with rapamycin had worse performance in the early learning trials of the Barnes maze testing, even though eventually they did learn the task. These observations indicate that the effects of rapamycin on IS are disease-modifying rather than simple pharmacologic effects of the drug and underline the important differences in the effects of pre- vs post-treatment protocols in preclinical drug testing.
At present, there are no clinical case reports or trials of mTOR inhibitors in patients with IS. Case reports of successful anticonvulsant effects of rapamycin in patients with focal-onset seizures in TSC patients exist (Muncy, et al. 2009), while larger clinical trials of similar mTOR inhibitors are currently underway (Krueger et al. 2010). Of particular interest would be to do clinical trials of mTOR inhibitors in patients with IS due to TSC or other genetic “TORopathies” (Crino 2007; Crino 2009). The observations in the DLP model raise the possibility that other subgroups of patients with IS, not necessarily due to genetic “TORopathies”, may also be a candidate target population for clinical trials of pulse mTOR inhibitor protocols. It would however be of the outmost importance to identify clinically appropriate biomarkers of mTOR activity for selection of target patient population and treatment implementation, so as to enhance the therapeutic benefit and minimize the potentially adverse effects due to unnecessary prolonged exposure to mTOR inhibitors.
The mTOR pathway in temporal lobe epileptogenesis
TLE is the most frequent form of epilepsy in the adult population. Recently, several research groups have shown that rapamycin might have seizure suppressing and possibly antiepileptogenic effects in post-SE rat models in which TLE develops after a latent period of several weeks (Zeng et al. 2009; Huang et al. 2010). The mechanisms by which this is accomplished are not precisely known. A common finding in these studies is that rapamycin treatment reduces mossy fiber sprouting and neuronal cell loss although the latter appears to depend on the timing of the treatment (Table 2). Since brain inflammation may play a causal role in the development of epilepsy (Vezzani et al. 2011) and since rapamycin has been shown to decrease microglia activation after traumatic brain injury (Erlich et al. 2007), the hypothesis was put forward that the drug may exert its antiepileptogenic action after SE via a decrease of inflammation. To test this, Gorter et al. used a post SE rat model in which SE was induced via electrical stimulation of the angular bundle. This leads to SE that lasts an average of 8 hours, with characteristic neuropathology consisting of neuronal loss, mossy fiber sprouting and strong astro- and microgliosis mainly in limbic brain regions (Gorter et al. 2001). Inflammation and activation of the immune response are other characteristic responses during the latent period, which lasts about 8 days on average (Gorter et al. 2006). EEG activity was measured in rapamycin- and vehicle-treated rats during 6 weeks after electrically induced SE. One hour before sacrifice, rats were intravenously injected with fluorescein in order to assess blood brain barrier (BBB) leakage. Brains were investigated using immunocytochemical and histochemical methods. The same rapamycin treatment protocol was used as previously reported in the kainate rat model, except that the treatment started 4 hours after the onset of SE instead of 24 hours after SE. Rapamycin injection did not alter SE duration compared to vehicle post SE rats. Epilepsy did not develop in 25% of the rapamycin-treated rats, while all vehicle-treated animals showed a progressive increase of seizures starting 1 week after SE. In the other 75% of the rapamycin-treated rats, significantly less seizures were observed compared to controls. Rapamycin treatment significantly reduced mossy fiber sprouting and was also neuroprotective, although considerable mossy fiber sprouting and cell loss were still observed. Nevertheless, astrogliosis and expression of various inflammation markers were not reduced by the treatment. Interestingly, BBB leakage could be hardly detected in the rapamycin-treated group, while this was prominent in the vehicle-treated group. Whether the effects on BBB leakage in rapamycin-treated rats are a consequence of seizure suppressing properties of the drug, or contribute to a real antiepileptogenic effect will be studied in the near future.
Table 2.
Studies on the effects of rapamycin treatment on the development of epilepsy in TLE animal models.
| Reference | TLE animal model |
Start and duration Rapamycin Treatment |
Suppression of seizures |
Suppression of epilepsy development |
Suppression of mossy fiber sprouting |
Neuroprotective effects |
|---|---|---|---|---|---|---|
| (Zeng et al. 2009) | Kainic-acid induced SE in rats | 3 days before SE or 24 hrs after SE; Up to 7 weeks |
No acute effects on behavioral seizures | Yes | Yes | Yes, only when started before SE induction |
| (Buckmaster et al. 2009) | Pilocarpine induced SE in rats | Rapamycin infusions into the dentate gyrus started 3–10 hrs after SE or 2 months after SE; 1–2 months | Not done | Not done | Yes, but regrowth after discontinuati on of treatment. No effect if given 2 months after SE | No |
| (Huang et al. 2010) | Pilocarpine induced SE in rats | 10 weeks after SE; 3 week treatment in chronic phase |
Less spontaneous seizures during treatment | Seizures recur after rapamycin discontinuati on | Yes | Not done |
| (Buckmaster et al. 2011) | Pilocarpine induced SE in mice | Starting 24 hrs after SE; entire period |
No | No | Yes | No |
| (van Vliet et al. 2011) | Electrically induced SE in rats | Starting 4 hrs after SE; 6 weeks | Not done | Yes | Yes | Yes |
| (Sliwa et al. 2012) | Amygdala stimulation model of TLE in rats | Starting 24 hrs after stimulation; 2 weeks | Not done | No | No | Not done |
The mTOR pathway in other etiologies of epilepsy
Overactivation of the mTOR pathway and neuroprotective effects of rapamycin or related mTOR inhibitors have also been found in other etiologies of epilepsy, such as in traumatic brain injury (Erlich et al. 2007; Park et al. 2011), and in neonatal hypoxiaischemia (Carloni et al. 2010). However, the antiepileptogenic potential of mTOR inhibitors in these settings is still under investigation.
Antiseizure or antiepileptogenic effects of mTOR inhibition?
In certain experimental epilepsy models, mTOR inhibition has been proposed to have antiseizure or antiepileptogenic effects or both [also reviewed in (Wong 2011b)]. Often, distinction between these effects is not definitive with the available reported experimental data and given the complex mechanisms of rapamycin mechanisms of action. Furthermore, such effects are not universal and seem to depend upon the experimental conditions and administration protocols, animal model or species used (Buckmaster et al. 2009; Sliwa et al. 2012), and type of seizures studied (Raffo et al. 2011).
According to a recently proposed terminology (Galanopoulou et al. 2012), antiseizure treatments “reduce or stop seizures irrespective of any effects on the underlying epileptic state and disease progression”. Among the in vivo studies, evidence for acute antiseizure effects (within 2 hours) has only been reported in the multiple-hit model of IS (Raffo et al. 2011). Of note, this was dose-dependent and perhaps seizure-specific, as only the high dose of rapamycin suppressed DLP spasms, whereas rapamycin did not appear to modify the frequency of other types of seizures (Raffo et al. 2011). In the in vivo TLE models, rapamycin was not found to have similar acute antiseizure effects (Zeng et al. 2009), although in many of these TLE studies the acute effects were not elaborated. In contrast, many, but not all, studies in CD, IS, or TLE models demonstrate seizure reduction after repetitive or chronic administration, if rapamycin is started after seizure onset [(Table 2) and (Ljungberg et al. 2009; Zhou et al. 2009; Huang et al. 2010; Raffo et al. 2011)]. It is not easy to determine whether these reflect delayed antiseizure effects or whether they are linked to the concomitantly observed disease modifying or antiepileptogenic actions.
A recently proposed definition of antiepileptogenic treatment advises that such treatments should “either prevent or delay the onset of epilepsy, if given prior to epilepsy onset”, or that they “could alleviate seizure severity, prevent or reduce epilepsy progression” or pharmacoresponsiveness if given after epilepsy onset (Galanopoulou et al. 2012). Accepting this terminology, there is strong evidence that rapamycin may prevent epilepsy and ameliorate its progression in the TSC/PTEN genetic models of epilepsy (Zeng et al. 2008; Sunnen et al. 2011). The study in the multiple-hit model of IS has supported that pulse rapamycin may improve the natural course of IS, for a period extending beyond the time of rapamycin discontinuation, although no benefit was seen for other emerging seizures within the timespan of this study (Raffo et al. 2011). In the TLE models, contrasting reports exist as to whether rapamycin has antiepileptogenic effects (Table 2). It is possible that model or species-related factors, the timing and protocols of rapamycin administration or other methodological differences may contribute to these diverse effects. However, to this date, there is no experimental evidence that rapamycin has resulted in the “cure” of any of these epilepsy models, i.e., to the “permanent reversal of the epilepsy, such that no seizures occur after treatment withdrawal” (Galanopoulou et al. 2012).
Mechanisms of action
Antiseizure actions could be due to diverse effects on voltage- and ligand-gated channels, neurotransmitter receptors and signaling pathways, which could diminish epileptic activity. Early electrophysiological studies demonstrated that rapamycin can regulate neuronal excitability. Thus, application of rapamycin prolongs mean open channel time of Ca2+ -dependent K+ channels (Terashima et al. 1998). Further evidence that rapamycin can reduce neuronal excitability was the demonstration that it increases Kv1.1 expression in cortical and hippocampal neurons (Raab-Graham et al. 2006). In addition, rapamycin reduces AMPA receptor surface expression normally seen after blockade of synaptic activity, suggesting that the mTOR pathway plays an important role in regulating glutamate receptor surface expression (Wang et al. 2006). Some of the protective effects of rapamycin and its analogs may not be solely due to effects on the mTOR pathway. For example, modification of rapamycin at the mTOR binding region yields novel immunophilin ligands that could inhibit L-type Ca2+ channels, thus explaining neuroprotective effects (Ruan et al. 2008).
In hippocampal or cortical neurons rapamycin did not affect cell size, nor did it have overt effects on neuronal firing or Na+ and K+ channels (Victor et al. 1995; Daoud et al. 2007; Ruegg et al. 2007). However, in a fraction of cells acute application of rapamycin reduced synaptic inputs and the duration of bicuculline-induced bursts (Ruegg et al. 2007), an effect reminiscent of the observations reported by Cepeda and colleagues in human TSC. Overall this indicates that rapamycin does not increase cell excitability and in some cases it may reduce it. In agreement, a recent study demonstrated that chronic administration of rapamycin reduces cortical excitability (Hauptman et al. 2010). While acute administration to cortical slices in physiological conditions had negligible effects, chronic treatment led to changes in membrane and synaptic properties consistent with reduced overall excitability including hyperpolarized resting membrane potentials, increased rheobase, and increased GABAA receptor-mediated synaptic activity (Hauptman et al. 2010).
Rapamycin could also exert antiepileptogenic effects through direct or indirect actions on cell growth and morphology, axonal sprouting, anti-inflammatory actions, or modification of epileptogenic circuit dynamics (Wong 2011a; Wong 2011b). If one accepts that cytomegaly can lead to neuronal hyperexcitability and that rapamycin can prevent or reverse abnormal cell growth, it can be suggested that, at least in some conditions (e.g., in the setting of TSC1/TSC2 or PTEN loss of function) mTOR inhibition may affect disease progression. Further, a multitude of epilepsies induce plastic changes including mossy fiber sprouting, cell proliferation, and other processes that require mTOR activation. If brain derived neurotrophic factor (BDNF) increases after an epileptogenic insult, and if BDNF activates the mTOR pathway to induce mossy fiber sprouting and neurogenesis, then inhibition of this pathway could prevent epilepsy development. In fact, as mentioned earlier, intra-hippocampal or systemic administration of rapamycin prevents mossy fiber sprouting (Buckmaster et al. 2009; Huang et al. 2010).
Conclusions
Rapamycin is not an innocuous drug and has undesirable side effects. The search for selective agonists targeting specific effectors of the mTOR pathway can lead to the discovery of better drugs to treat epilepsy. A number of mTOR pathway inhibitors are now being used in clinical trials to treat some TSC manifestations (Krueger et al. 2010). If the work in animal models is corroborated, the therapeutic potential of these agents could be extended to other pathological substrates of epileptic activity, not just limited to genetic etiologies. Preclinical studies have suggested that rapamycin may have “epileptostatic” effects based on studies in models of genetic “TORopathies” and TLE, preventing the development of epilepsy-related pathology and, in certain cases, the development of epilepsy itself, although this benefit may be lost after treatment discontinuation. Yet, these effects depend upon the dosing and timing of administration, as well as the model of epilepsy used. Furthermore, pulse rapamycin treatment may have anti-IS and disease modifying effects for early life epilepsies with acquired dysregulation of the mTOR pathway. It will be essential, however, to generate clinically relevant biomarkers to guide in the selection of target patient populations in order to optimize the therapeutic potential of mTOR inhibitors while limiting untoward side effects.
Acknowledgements
ASG would like to acknowledge funding support from the following sources: NINDS/NICHD NS62947 (ASG), Autism Speaks, NINDS NS20253 (PI: Solomon L. Moshé) and the Heffer Family Foundation. ASG would like to thank Drs Antonietta Coppola, Solomon Moshé, Tomonori Ono, and Emmanuel Raffo, as well as Mr Stephen Briggs, Mrs Qianyun Li, Mrs Wei Liu, and Mrs Hong Wang for their valuable contributions. ASG has received speaker’s honorarium by Novartis and royalties by Morgan & Claypool Publishers.
JG acknowledges funding support from the Nationaal Epilepsie Fonds (11-03) and NWO (Veni grant 863.08.017 to EvV) and thanks his collaborators, Drs. Erwin van Vliet, Grazia Forte, Linda Holtman, Wytse Wadman and Eleonora Aronica.
CC acknowledges funding support from NIH grant NS 38992 and thanks his collaborators Drs. Gary W. Mathern, Michael S. Levine, Harry V. Vinters, Jason S. Hauptman, and Véronique M. André.
We confirm that we have read the Journal’s position on issues relating to ethical publication. This review is consistent with these guidelines.
Appendix
Extracts of the panel discussion
Albert Becker (University of Bonn Medical Center, Bonn, Germany): My question is about the side effects of chronic rapamycin treatment. Could you comment about using mTOR inhibition transiently after a transient insult versus chronically.
Jan Gorter: Well we have not tried transient application, we only used chronic application so far and I cannot say whether transient use will have a positive effect. But from the literature in mice, when you stop the treatment too early, the seizures will come back, therefore we have to give continuous, daily rapamycin treatment in order to have an effect. What I heard in a previous meeting, and I am not sure whether this has been published yet, is that when you stop the treatment in status epilepticus models, that seizures come back, but I don’t know the time and the length of the treatment. But I think that Aristea used a pulse treatment for 3 days in her model and has seen an effect.
Aristea Galanopoulou: It depends on the model that you are studying. In our model of IS which is not a genetic model but an acquired model, we were able with a 3-day treatment of very high doses of rapamycin, not only to abort spasms but also to observe disease modification down the road. If we look at the literature, Jan is correct, in the kainic acid and pilocarpine SE models, if you discontinue rapamycin it may lead to reappearance of some of the pathology that is associated with epileptogenesis. One interesting paper by Ljungberg et al (Ljungberg et al. 2009) in a genetic model (NS-PTEN model) showed a reversal of the pathology and epileptic activity following chronic treatment with very high doses of rapamycin for weeks, and the benefits of rapamycin lasted for at least 3 weeks off treatment (Ljungberg et al. 2009). In a follow up study, epilepsy recurred 4–7 weeks off rapamycin, but both the underlying pathology (mossy fiver sprouting in the dentate) and the epileptic activity were inhibited with repetitive pulses of rapamycin (Sunnen et al. 2011). In summary, both our study in the multiple hit model of IS as well as the subsequent study in the NS-PTEN model raise the possibility that, in certain conditions, rapamycin need not be given continuously, but pulse high dose treatments may suffice to produce therapeutic effect, and minimize the risk and severity of associated adverse effects. The availability of clinically relevant surrogate endpoints to guide treatment implementation will be most useful in such protocols.
Asla Pitkanen (University of Eastern Finland, Kuopio, Finland): Paul Buckmaster showed that there was no response to rapamycin-treated mice. What is your opinion on that? And Carlos Cepeda showed today that rapamycin may be anticonvulsant. It is still unclear to me whether rapamycin may affect convulsion or it may be through the mTOR pathway. This is very important to try and interpret the antiepileptogenesis data because seizure recordings are typically done while the animals are on rapamycin treatment. So whether rapamycin is directly anticonvulsant or whether its effect is via circuitry re-organization. So I was wondering how do you interpret these antiepileptogenesis data.
Jan Gorter: When we started our study, there were a few studies that showed that rapamycin did not have any anticonvulsant action. Studies by Peter Crino and others did not find any effect on sodium or potassium currents (Ruegg et al. 2007). Of course, as we heard during this meeting, Carlos Cepeda and colleagues have shown a clear excitability-reducing effect by rapamycin in tissue where mTOR is activated to begin with. That is why we cannot exclude that some of the effects we see may be anticonvulsant effects. Buckmaster showed that in mice at least it does not have antiepileptogenic or anticonvulsant effect (Buckmaster et al. 2011). In all published studies so far, the reduction of mossy fiber sprouting is a common finding in mice as well as in rats (see table 2).
Carlos Cepeda: Let me first make clear that we did not propose that rapamycin is necessarily anticonvulsant, we just said that it reduces cortical excitability. There are many possible mechanisms by which this may happen. We have seen for example that chronic injections of rapamycin produce a hyperpolarization of many cortical neurons. Another effect of rapamycin is the increase in Ca2+ -activated K+ currents, which reduces excitability. We were very surprised that in the acute situation rapamycin could produce such profound effects. However, this is really not unprecedented. Ruegg and colleagues showed that within one hour you can see effects of rapamycin in some neurons (Ruegg et al. 2007). In the human tissue we can see already within half an hour a decrease in cortical excitability.
REFERENCES
- Anderl S, Freeland M, Kwiatkowski DJ, Goto J. Therapeutic value of prenatal rapamycin treatment in a mouse brain model of tuberous sclerosis complex. Hum Mol Genet. 2011;20:4597–4604. doi: 10.1093/hmg/ddr393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronica E, Crino PB. Inflammation in epilepsy: clinical observations. Epilepsia. 2011;52(Suppl 3):26–32. doi: 10.1111/j.1528-1167.2011.03033.x. [DOI] [PubMed] [Google Scholar]
- Bastos H, da Silva PF, de Albuquerque MA, Mattos A, Riesgo RS, Ohlweiler L, Winckler MI, Bragatti JA, Duarte RD, Zandona DI. Proteus syndrome associated with hemimegalencephaly and Ohtahara syndrome: report of two cases. Seizure. 2008;17:378–382. doi: 10.1016/j.seizure.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Baybis M, Yu J, Lee A, Golden JA, Weiner H, McKhann G, 2nd, Aronica E, Crino PB. mTOR cascade activation distinguishes tubers from focal cortical dysplasia. Ann Neurol. 2004;56:478–487. doi: 10.1002/ana.20211. [DOI] [PubMed] [Google Scholar]
- Becker AJ, Urbach H, Scheffler B, Baden T, Normann S, Lahl R, Pannek HW, Tuxhorn I, Elger CE, Schramm J, Wiestler OD, Blumcke I. Focal cortical dysplasia of Taylor's balloon cell type: mutational analysis of the TSC1 gene indicates a pathogenic relationship to tuberous sclerosis. Ann Neurol. 2002;52:29–37. doi: 10.1002/ana.10251. [DOI] [PubMed] [Google Scholar]
- Blumcke I, Thom M, Aronica E, Armstrong DD, Vinters HV, Palmini A, Jacques TS, Avanzini G, Barkovich AJ, Battaglia G, Becker A, Cepeda C, Cendes F, Colombo N, Crino P, Cross JH, Delalande O, Dubeau F, Duncan J, Guerrini R, Kahane P, Mathern G, Najm I, Ozkara C, Raybaud C, Represa A, Roper SN, Salamon N, Schulze-Bonhage A, Tassi L, Vezzani A, Spreafico R. The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia. 2011;52:158–174. doi: 10.1111/j.1528-1167.2010.02777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombardieri R, Pinci M, Moavero R, Cerminara C, Curatolo P. Early control of seizures improves long-term outcome in children with tuberous sclerosis complex. Eur J Paediatr Neurol. 2010;14:146–149. doi: 10.1016/j.ejpn.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Ingram EA, Wen X. Inhibition of the mammalian target of rapamycin signaling pathway suppresses dentate granule cell axon sprouting in a rodent model of temporal lobe epilepsy. J Neurosci. 2009;29:8259–8269. doi: 10.1523/JNEUROSCI.4179-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster PS, Lew FH. Rapamycin suppresses mossy fiber sprouting but not seizure frequency in a mouse model of temporal lobe epilepsy. J Neurosci. 2011;31:2337–2347. doi: 10.1523/JNEUROSCI.4852-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- Carloni S, Girelli S, Scopa C, Buonocore G, Longini M, Balduini W. Activation of autophagy and Akt/CREB signaling play an equivalent role in the neuroprotective effect of rapamycin in neonatal hypoxia-ischemia. Autophagy. 2010;6:366–377. doi: 10.4161/auto.6.3.11261. [DOI] [PubMed] [Google Scholar]
- Cepeda C, André VM, Hauptman JS, Rao SP, Joshi PR, Chen JY, Mathern GW, Levine MS. Differential sensitivity of cortical neurons to 4-aminopyridine and rapamycin in diverse forms of pediatric epilepsy. Society for Neuroscience Annual Meeting; San Diego, CA. Society for Neuroscience; 2010. [Google Scholar]
- Cepeda C, Andre VM, Hauptman JS, Yamazaki I, Huynh MN, Chang JW, Chen JY, Fisher RS, Vinters HV, Levine MS, Mathern GW. Enhanced GABAergic network and receptor function in pediatric cortical dysplasia Type IIB compared with Tuberous Sclerosis Complex. Neurobiol Dis. 2012;45:310–321. doi: 10.1016/j.nbd.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Hurst RS, Flores-Hernandez J, Hernandez-Echeagaray E, Klapstein GJ, Boylan MK, Calvert CR, Jocoy EL, Nguyen OK, Andre VM, Vinters HV, Ariano MA, Levine MS, Mathern GW. Morphological and electrophysiological characterization of abnormal cell types in pediatric cortical dysplasia. J Neurosci Res. 2003;72:472–486. doi: 10.1002/jnr.10604. [DOI] [PubMed] [Google Scholar]
- Chachua T, Yum MS, Veliskova J, Velisek L. Validation of the rat model of cryptogenic infantile spasms. Epilepsia. 2011;52:1666–1677. doi: 10.1111/j.1528-1167.2011.03220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu-Shore CJ, Major P, Camposano S, Muzykewicz D, Thiele EA. The natural history of epilepsy in tuberous sclerosis complex. Epilepsia. 2010;51:1236–1241. doi: 10.1111/j.1528-1167.2009.02474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudomelova L, Scantlebury MH, Raffo E, Coppola A, Betancourth D, Galanopoulou AS. Modeling new therapies for infantile spasms. Epilepsia. 2010;51(Suppl 3):27–33. doi: 10.1111/j.1528-1167.2010.02605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crino PB. Focal brain malformations: a spectrum of disorders along the mTOR cascade. Novartis Found Symp. 2007;288:260–272. doi: 10.1002/9780470994030.ch18. discussion 272–281. [DOI] [PubMed] [Google Scholar]
- Crino PB. Focal brain malformations: seizures, signaling, sequencing. Epilepsia. 2009;50(Suppl 9):3–8. doi: 10.1111/j.1528-1167.2009.02289.x. [DOI] [PubMed] [Google Scholar]
- Crino PB. mTOR: A pathogenic signaling pathway in developmental brain malformations. Trends Mol Med. 2011 doi: 10.1016/j.molmed.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Cross JH. Neurocutaneous syndromes and epilepsy-issues in diagnosis and management. Epilepsia. 2005;46(Suppl 10):17–23. doi: 10.1111/j.1528-1167.2005.00353.x. [DOI] [PubMed] [Google Scholar]
- Curatolo P, Porfirio MC, Manzi B, Seri S. Autism in tuberous sclerosis. Eur J Paediatr Neurol. 2004;8:327–332. doi: 10.1016/j.ejpn.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Curatolo P, Seri S, Verdecchia M, Bombardieri R. Infantile spasms in tuberous sclerosis complex. Brain Dev. 2001;23:502–507. doi: 10.1016/s0387-7604(01)00300-x. [DOI] [PubMed] [Google Scholar]
- Daoud D, Scheld HH, Speckmann EJ, Gorji A. Rapamycin: brain excitability studied in vitro. Epilepsia. 2007;48:834–836. doi: 10.1111/j.1528-1167.2006.00976.x. [DOI] [PubMed] [Google Scholar]
- Ehninger D, de Vries PJ, Silva AJ. From mTOR to cognition: molecular and cellular mechanisms of cognitive impairments in tuberous sclerosis. J Intellect Disabil Res. 2009;53:838–851. doi: 10.1111/j.1365-2788.2009.01208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehninger D, Silva AJ. Rapamycin for treating Tuberous sclerosis and Autism spectrum disorders. Trends Mol Med. 2011;17:78–87. doi: 10.1016/j.molmed.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlich S, Alexandrovich A, Shohami E, Pinkas-Kramarski R. Rapamycin is a neuroprotective treatment for traumatic brain injury. Neurobiol Dis. 2007;26:86–93. doi: 10.1016/j.nbd.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Farrell MA, DeRosa MJ, Curran JG, Secor DL, Cornford ME, Comair YG, Peacock WJ, Shields WD, Vinters HV. Neuropathologic findings in cortical resections (including hemispherectomies) performed for the treatment of intractable childhood epilepsy. Acta Neuropathol. 1992;83:246–259. doi: 10.1007/BF00296786. [DOI] [PubMed] [Google Scholar]
- Frost JD, Jr, Hrachovy RA. Pathogenesis of infantile spasms: a model based on developmental desynchronization. J Clin Neurophysiol. 2005;22:25–36. doi: 10.1097/01.wnp.0000149893.12678.44. [DOI] [PubMed] [Google Scholar]
- Galanopoulou AS. Palomid 529, a combined TORC1/TORC2 inhibitor suppresses spasms in a rat model of infantile spasms. Ann Neurol. 2010;68(S14):S1. [Google Scholar]
- Galanopoulou AS, Buckmaster PS, Staley KJ, Moshe SL, Perucca E, Engel J, Jr, Loscher W, Noebels JL, Pitkanen A, Stables J, White HS, O'Brien TJ, Simonato M. Identification of new epilepsy treatments: Issues in preclinical methodology. Epilepsia. 2012 doi: 10.1111/j.1528-1167.2011.03391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorter JA, van Vliet EA, Aronica E, Breit T, Rauwerda H, Lopes da Silva FH, Wadman WJ. Potential new antiepileptogenic targets indicated by microarray analysis in a rat model for temporal lobe epilepsy. J Neurosci. 2006;26:11083–11110. doi: 10.1523/JNEUROSCI.2766-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorter JA, van Vliet EA, Aronica E, Lopes da Silva FH. Progression of spontaneous seizures after status epilepticus is associated with mossy fibre sprouting and extensive bilateral loss of hilar parvalbumin and somatostatin-immunoreactive neurons. Eur J Neurosci. 2001;13:657–669. doi: 10.1046/j.1460-9568.2001.01428.x. [DOI] [PubMed] [Google Scholar]
- Goto J, Talos DM, Klein P, Qin W, Chekaluk YI, Anderl S, Malinowska IA, Di Nardo A, Bronson RT, Chan JA, Vinters HV, Kernie SG, Jensen FE, Sahin M, Kwiatkowski DJ. Regulable neural progenitor-specific Tsc1 loss yields giant cells with organellar dysfunction in a model of tuberous sclerosis complex. Proc Natl Acad Sci U S A. 2011;108:E1070–E1079. doi: 10.1073/pnas.1106454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grajkowska W, Kotulska K, Matyja E, Larysz-Brysz M, Mandera M, Roszkowski M, Domanska-Pakiela D, Lewik-Kowalik J, Jozwiak S. Expression of tuberin and hamartin in tuberous sclerosis complex-associated and sporadic cortical dysplasia of Taylor's balloon cell type. Folia Neuropathol. 2008;46:43–48. [PubMed] [Google Scholar]
- Hauptman JS, Cepeda CJA, Mathern GW, Levine MS. The effects of mTOR modulation on the excitability of neocortical neurons and networks. Neuroscience Meeting Planner; San Diego, CA. 2010. Program No. 657.13. [Google Scholar]
- Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33:67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrachovy RA, Frost JD., Jr Infantile epileptic encephalopathy with hypsarrhythmia (infantile spasms/West syndrome) J Clin Neurophysiol. 2003;20:408–425. doi: 10.1097/00004691-200311000-00004. [DOI] [PubMed] [Google Scholar]
- Huang X, Zhang H, Yang J, Wu J, McMahon J, Lin Y, Cao Z, Gruenthal M, Huang Y. Pharmacological inhibition of the mammalian target of rapamycin pathway suppresses acquired epilepsy. Neurobiol Dis. 2010;40:193–199. doi: 10.1016/j.nbd.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR, Heydemann PT. Fine structure of cortical tubers in tuberous sclerosis: a Golgi study. Ann Neurol. 1984;16:595–602. doi: 10.1002/ana.410160511. [DOI] [PubMed] [Google Scholar]
- Inoki K, Corradetti MN, Guan KL. Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet. 2005;37:19–24. doi: 10.1038/ng1494. [DOI] [PubMed] [Google Scholar]
- Jacobs KM, Kharazia VN, Prince DA. Mechanisms underlying epileptogenesis in cortical malformations. Epilepsy res. 1999;36:165–188. doi: 10.1016/s0920-1211(99)00050-9. [DOI] [PubMed] [Google Scholar]
- Jansen FE, Vincken KL, Algra A, Anbeek P, Braams O, Nellist M, Zonnenberg BA, Jennekens-Schinkel A, van den Ouweland A, Halley D, van Huffelen AC, van Nieuwenhuizen O. Cognitive impairment in tuberous sclerosis complex is a multifactorial condition. Neurology. 2008;70:916–923. doi: 10.1212/01.wnl.0000280579.04974.c0. [DOI] [PubMed] [Google Scholar]
- Johannessen CM, Reczek EE, James MF, Brems H, Legius E, Cichowski K. The NF1 tumor suppressor critically regulates TSC2 and mTOR. Proc Natl Acad Sci U S A. 2005;102:8573–8578. doi: 10.1073/pnas.0503224102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karvelas G, Lortie A, Scantlebury MH, Duy PT, Cossette P, Carmant L. A retrospective study on aetiology based outcome of infantile spasms. Seizure. 2009;18:197–201. doi: 10.1016/j.seizure.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Kenerson H, Dundon TA, Yeung RS. Effects of rapamycin in the Eker rat model of tuberous sclerosis complex. Pediatr Res. 2005;57:67–75. doi: 10.1203/01.PDR.0000147727.78571.07. [DOI] [PubMed] [Google Scholar]
- Krueger DA, Care MM, Holland K, Agricola K, Tudor C, Mangeshkar P, Wilson KA, Byars A, Sahmoud T, Franz DN. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med. 2010;363:1801–1811. doi: 10.1056/NEJMoa1001671. [DOI] [PubMed] [Google Scholar]
- Kumar G, Mazzuferi M, Otoul C, Jacobs T, Kaminski RM. A randomized, blinded preclinical trial of rapamycin in pilocarpine mouse model of chronic epilepsy. Neuroscience Meeting Planner; Washington, DC. 2011. Program No. 560.08. [Google Scholar]
- Kwiatkowski DJ. Tuberous sclerosis: from tubers to mTOR. Ann Hum Genet. 2003;67:87–96. doi: 10.1046/j.1469-1809.2003.00012.x. [DOI] [PubMed] [Google Scholar]
- Kwon CH, Zhu X, Zhang J, Baker SJ. mTor is required for hypertrophy of Pten-deficient neuronal soma in vivo. Proc Natl Acad Sci U S A. 2003;100:12923–12928. doi: 10.1073/pnas.2132711100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner JT, Salamon N, Hauptman JS, Velasco TR, Hemb M, Wu JY, Sankar R, Donald Shields W, Engel J, Jr, Fried I, Cepeda C, Andre VM, Levine MS, Miyata H, Yong WH, Vinters HV, Mathern GW. Assessment and surgical outcomes for mild type I and severe type II cortical dysplasia: a critical review and the UCLA experience. Epilepsia. 2009;50:1310–1335. doi: 10.1111/j.1528-1167.2008.01998.x. [DOI] [PubMed] [Google Scholar]
- Ljungberg MC, Bhattacharjee MB, Lu Y, Armstrong DL, Yoshor D, Swann JW, Sheldon M, D'Arcangelo G. Activation of mammalian target of rapamycin in cytomegalic neurons of human cortical dysplasia. Ann Neurol. 2006;60:420–429. doi: 10.1002/ana.20949. [DOI] [PubMed] [Google Scholar]
- Ljungberg MC, Sunnen CN, Lugo JN, Anderson AE, D'Arcangelo G. Rapamycin suppresses seizures and neuronal hypertrophy in a mouse model of cortical dysplasia. Dis Model Mech. 2009;2:389–398. doi: 10.1242/dmm.002386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombroso CT. A prospective study of infantile spasms: clinical and therapeutic correlations. Epilepsia. 1983;24:135–158. doi: 10.1111/j.1528-1157.1983.tb04874.x. [DOI] [PubMed] [Google Scholar]
- Lux AL, Edwards SW, Hancock E, Johnson AL, Kennedy CR, Newton RW, O'Callaghan FJ, Verity CM, Osborne JP. The United Kingdom Infantile Spasms Study (UKISS) comparing hormone treatment with vigabatrin on developmental and epilepsy outcomes to age 14 months: a multicentre randomised trial. Lancet Neurol. 2005;4:712–717. doi: 10.1016/S1474-4422(05)70199-X. [DOI] [PubMed] [Google Scholar]
- Mackay MT, Weiss SK, Adams-Webber T, Ashwal S, Stephens D, Ballaban-Gill K, Baram TZ, Duchowny M, Hirtz D, Pellock JM, Shields WD, Shinnar S, Wyllie E, Snead OC., 3rd Practice parameter: medical treatment of infantile spasms: report of the American Academy of Neurology and the Child Neurology Society. Neurology. 2004;62:1668–1681. doi: 10.1212/01.wnl.0000127773.72699.c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magri L, Cambiaghi M, Cominelli M, Alfaro-Cervello C, Cursi M, Pala M, Bulfone A, Garcia-Verdugo JM, Leocani L, Minicucci F, Poliani PL, Galli R. Sustained Activation of mTOR Pathway in Embryonic Neural Stem Cells Leads to Development of Tuberous Sclerosis Complex-Associated Lesions. Cell stem cell. 2011;9:447–462. doi: 10.1016/j.stem.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Marsh DJ, Kum JB, Lunetta KL, Bennett MJ, Gorlin RJ, Ahmed SF, Bodurtha J, Crowe C, Curtis MA, Dasouki M, Dunn T, Feit H, Geraghty MT, Graham JM, Jr, Hodgson SV, Hunter A, Korf BR, Manchester D, Miesfeldt S, Murday VA, Nathanson KL, Parisi M, Pober B, Romano C, Eng C, et al. PTEN mutation spectrum and genotype-phenotype correlations in Bannayan-Riley-Ruvalcaba syndrome suggest a single entity with Cowden syndrome. Hum Mol Genet. 1999;8:1461–1472. doi: 10.1093/hmg/8.8.1461. [DOI] [PubMed] [Google Scholar]
- Mattia D, Olivier A, Avoli M. Seizure-like discharges recorded in human dysplastic neocortex maintained in vitro. Neurology. 1995;45:1391–1395. doi: 10.1212/wnl.45.7.1391. [DOI] [PubMed] [Google Scholar]
- Miyata H, Chiang AC, Vinters HV. Insulin signaling pathways in cortical dysplasia and TSC-tubers: tissue microarray analysis. Ann Neurol. 2004;56:510–519. doi: 10.1002/ana.20234. [DOI] [PubMed] [Google Scholar]
- Motte J, Billard C, Fejerman N, Sfaello Z, Arroyo H, Dulac O. Neurofibromatosis type one and West syndrome: a relatively benign association. Epilepsia. 1993;34:723–726. doi: 10.1111/j.1528-1157.1993.tb00452.x. [DOI] [PubMed] [Google Scholar]
- Orlova KA, Crino PB. The tuberous sclerosis complex. Ann N Y Acad Sci. 2010a;1184:87–105. doi: 10.1111/j.1749-6632.2009.05117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlova KA, Parker WE, Heuer GG, Tsai V, Yoon J, Baybis M, Fenning RS, Strauss K, Crino PB. STRADalpha deficiency results in aberrant mTORC1 signaling during corticogenesis in humans and mice. J Clin Invest. 2010b;120:1591–1602. doi: 10.1172/JCI41592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne JP, Lux AL, Edwards SW, Hancock E, Johnson AL, Kennedy CR, Newton RW, Verity CM, O'Callaghan FJ. The underlying etiology of infantile spasms (West syndrome): information from the United Kingdom Infantile Spasms Study (UKISS) on contemporary causes and their classification. Epilepsia. 2010;51:2168–2174. doi: 10.1111/j.1528-1167.2010.02695.x. [DOI] [PubMed] [Google Scholar]
- Paredes M, Pleasure SJ, Baraban SC. Embryonic and early postnatal abnormalities contributing to the development of hippocampal malformations in a rodent model of dysplasia. J Comp Neurol. 2006;495:133–148. doi: 10.1002/cne.20871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Zhang J, Qiu J, Zhu X, Degterev A, Lo EH, Whalen MJ. Combination therapy targeting Akt and mammalian target of rapamycin improves functional outcome after controlled cortical impact in mice. J Cereb Blood Flow Metab. 2011;32:330–340. doi: 10.1038/jcbfm.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellock JM, Hrachovy R, Shinnar S, Baram TZ, Bettis D, Dlugos DJ, Gaillard WD, Gibson PA, Holmes GL, Nordl DR, O'Dell C, Shields WD, Trevathan E, Wheless JW. Infantile spasms: a U.S. consensus report. Epilepsia. 2010;51:2175–2189. doi: 10.1111/j.1528-1167.2010.02657.x. [DOI] [PubMed] [Google Scholar]
- Pilarski R, Stephens JA, Noss R, Fisher JL, Prior TW. Predicting PTEN mutations: an evaluation of Cowden syndrome and Bannayan-Riley-Ruvalcaba syndrome clinical features. J Med Genet. 2011;48:505–512. doi: 10.1136/jmg.2011.088807. [DOI] [PubMed] [Google Scholar]
- Puffenberger EG, Strauss KA, Ramsey KE, Craig DW, Stephan DA, Robinson DL, Hendrickson CL, Gottlieb S, Ramsay DA, Siu VM, Heuer GG, Crino PB, Morton DH. Polyhydramnios, megalencephaly and symptomatic epilepsy caused by a homozygous 7-kilobase deletion in LYK5. Brain. 2007;130:1929–1941. doi: 10.1093/brain/awm100. [DOI] [PubMed] [Google Scholar]
- Raab-Graham KF, Haddick PC, Jan YN, Jan LY. Activity- and mTOR-dependent suppression of Kv1.1 channel mRNA translation in dendrites. Science. 2006;314:144–148. doi: 10.1126/science.1131693. [DOI] [PubMed] [Google Scholar]
- Raffo E, Coppola A, Ono T, Briggs SW, Galanopoulou AS. A pulse rapamycin therapy for infantile spasms and associated cognitive decline. Neurobiol Dis. 2011;43:322–329. doi: 10.1016/j.nbd.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riikonen R. Epidemiological data of West syndrome in Finland. Brain Dev. 2001;23:539–541. doi: 10.1016/s0387-7604(01)00263-7. [DOI] [PubMed] [Google Scholar]
- Roper SN. In utero irradiation of rats as a model of human cerebrocortical dysgenesis: a review. Epilepsy res. 1998;32:63–74. doi: 10.1016/s0920-1211(98)00040-0. [DOI] [PubMed] [Google Scholar]
- Ruan B, Pong K, Jow F, Bowlby M, Crozier RA, Liu D, Liang S, Chen Y, Mercado ML, Feng X, Bennett F, von Schack D, McDonald L, Zaleska MM, Wood A, Reinhart PH, Magolda RL, Skotnicki J, Pangalos MN, Koehn FE, Carter GT, Abou-Gharbia M, Graziani EI. Binding of rapamycin analogs to calcium channels and FKBP52 contributes to their neuroprotective activities. Proc Natl Acad Sci U S A. 2008;105:33–38. doi: 10.1073/pnas.0710424105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegg S, Baybis M, Juul H, Dichter M, Crino PB. Effects of rapamycin on gene expression, morphology, and electrophysiological properties of rat hippocampal neurons. Epilepsy res. 2007;77:85–92. doi: 10.1016/j.eplepsyres.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggieri M, Iannetti P, Clementi M, Polizzi A, Incorpora G, Spalice A, Pavone P, Pratico AD, Elia M, Gabriele AL, Tenconi R, Pavone L. Neurofibromatosis type 1 and infantile spasms. Childs Nerv Syst. 2009;25:211–216. doi: 10.1007/s00381-008-0706-5. [DOI] [PubMed] [Google Scholar]
- Scantlebury MH, Galanopoulou AS, Chudomelova L, Raffo E, Betancourth D, Moshe SL. A model of symptomatic infantile spasms syndrome. Neurobiol Dis. 2010;37:604–612. doi: 10.1016/j.nbd.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schick V, Majores M, Engels G, Hartmann W, Elger CE, Schramm J, Schoch S, Becker AJ. Differential Pi3K-pathway activation in cortical tubers and focal cortical dysplasias with balloon cells. Brain Pathol. 2007;17:165–173. doi: 10.1111/j.1750-3639.2007.00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonberger A, Niehusmann P, Urbach H, Majores M, Grote A, Holthausen H, Blumcke I, Deckert M, Becker AJ. Increased frequency of distinct TSC2 allelic variants in focal cortical dysplasias with balloon cells and mineralization. Neuropathology. 2009;29:559–565. doi: 10.1111/j.1440-1789.2009.01018.x. [DOI] [PubMed] [Google Scholar]
- Sidenvall R, Eeg-Olofsson O. Epidemiology of infantile spasms in Sweden. Epilepsia. 1995;36:572–574. doi: 10.1111/j.1528-1157.1995.tb02569.x. [DOI] [PubMed] [Google Scholar]
- Sliwa A, Plucinska G, Bednarczyk J, Lukasiuk K. Post-treatment with rapamycin does not prevent epileptogenesis in the amygdala stimulation model of temporal lobe epilepsy. Neurosci Lett. 2012;509:105–109. doi: 10.1016/j.neulet.2011.12.051. [DOI] [PubMed] [Google Scholar]
- Sulis ML, Parsons R. PTEN: from pathology to biology. Trends Cell Biol. 2003;13:478–483. doi: 10.1016/s0962-8924(03)00175-2. [DOI] [PubMed] [Google Scholar]
- Sunnen CN, Brewster AL, Lugo JN, Vanegas F, Turcios E, Mukhi S, Parghi D, D'Arcangelo G, Anderson AE. Inhibition of the mammalian target of rapamycin blocks epilepsy progression in NS-Pten conditional knockout mice. Epilepsia. 2011;52:2065–2075. doi: 10.1111/j.1528-1167.2011.03280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DC, Falconer MA, Bruton CJ, Corsellis JA. Focal dysplasia of the cerebral cortex in epilepsy. J Neurol Neurosurg Psychiatry. 1971;34:369–387. doi: 10.1136/jnnp.34.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashima A, Nakai M, Hashimoto T, Kawamata T, Taniguchi T, Yasuda M, Maeda K, Tanaka C. Single-channel activity of the Ca2+-dependent K+ channel is modulated by FK506 and rapamycin. Brain res. 1998;786:255–258. doi: 10.1016/s0006-8993(97)01435-2. [DOI] [PubMed] [Google Scholar]
- Uhlmann EJ, Wong M, Baldwin RL, Bajenaru ML, Onda H, Kwiatkowski DJ, Yamada K, Gutmann DH. Astrocyte-specific TSC1 conditional knockout mice exhibit abnormal neuronal organization and seizures. Ann Neurol. 2002;52:285–296. doi: 10.1002/ana.10283. [DOI] [PubMed] [Google Scholar]
- van Vliet E, Holtman L, Wadman WJ, Aronica E, Gorter JA. mTOR inhibition by rapamycin prevents development of epilepsy but not brain inflammation after electrically induced status epilepticus in the rat. WONOEP Proceedings, Grottaferrata, Italy. 2011 [Google Scholar]
- Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol. 2011;7:31–40. doi: 10.1038/nrneurol.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor RG, Thomas GD, Marban E, O'Rourke B. Presynaptic modulation of cortical synaptic activity by calcineurin. Proc Natl Acad Sci U S A. 1995;92:6269–6273. doi: 10.1073/pnas.92.14.6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin JJ, Guan J, Prior WW, Edgar KA, Kassees R, Sampath D, Belvin M, Friedman LS. Nuclear phospho-Akt increase predicts synergy of PI3K inhibition and doxorubicin in breast and ovarian cancer. Sci Transl Med. 2010;2 doi: 10.1126/scitranslmed.3000630. 48ra66. [DOI] [PubMed] [Google Scholar]
- Wang Y, Barbaro MF, Baraban SC. A role for the mTOR pathway in surface expression of AMPA receptors. Neurosci lett. 2006;401:35–39. doi: 10.1016/j.neulet.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Weinstein SL, Finn AJ, Dave SH, Meng F, Lowell CA, Sanghera JS, DeFranco AL. Phosphatidylinositol 3-kinase and mTOR mediate lipopolysaccharide-stimulated nitric oxide production in macrophages via interferon-beta. J Leukoc Biol. 2000;67:405–414. doi: 10.1002/jlb.67.3.405. [DOI] [PubMed] [Google Scholar]
- Wenzel HJ, Patel LS, Robbins CA, Emmi A, Yeung RS, Schwartzkroin PA. Morphology of cerebral lesions in the Eker rat model of tuberous sclerosis. Acta Neuropathol. 2004;108:97–108. doi: 10.1007/s00401-004-0865-8. [DOI] [PubMed] [Google Scholar]
- Wong M. Mammalian target of rapamycin (mTOR) inhibition as a potential antiepileptogenic therapy: From tuberous sclerosis to common acquired epilepsies. Epilepsia. 2010;51:27–36. doi: 10.1111/j.1528-1167.2009.02341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M. Mammalian target of rapamycin (mTOR) activation in focal cortical dysplasia and related focal cortical malformations. Exp Neurol. 2011a doi: 10.1016/j.expneurol.2011.10.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M. Rapamycin for treatment of epilepsy: antiseizure, antiepileptogenic, both, or neither? Epilepsy curr. 2011b;11:66–68. doi: 10.5698/1535-7511-11.2.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Chen K, Kobayashi S, Timm D, Liang Q. Resveratrol Attenuates Doxorubicin-Induced Cardiomyocyte Death Via Inhibition of S6k1-Mediated Autophagy. J Pharmacol Exp Ther. 2011 doi: 10.1124/jpet.111.189589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung RS, Katsetos CD, Klein-Szanto A. Subependymal astrocytic hamartomas in the Eker rat model of tuberous sclerosis. Am J Pathol. 1997;151:1477–1486. [PMC free article] [PubMed] [Google Scholar]
- Zeng LH, Rensing NR, Wong M. The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J Neurosci. 2009;29:6964–6972. doi: 10.1523/JNEUROSCI.0066-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng LH, Rensing NR, Zhang B, Gutmann DH, Gambello MJ, Wong M. Tsc2 gene inactivation causes a more severe epilepsy phenotype than Tsc1 inactivation in a mouse model of tuberous sclerosis complex. Hum Mol Genet. 2011;20:445–454. doi: 10.1093/hmg/ddq491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng LH, Xu L, Gutmann DH, Wong M. Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann Neurol. 2008;63:444–453. doi: 10.1002/ana.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Blundell J, Ogawa S, Kwon CH, Zhang W, Sinton C, Powell CM, Parada LF. Pharmacological inhibition of mTORC1 suppresses anatomical, cellular, and behavioral abnormalities in neural-specific Pten knock-out mice. J Neurosci. 2009;29:1773–1783. doi: 10.1523/JNEUROSCI.5685-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]