Abstract
Aging is often associated with increased systolic blood pressure and decreased diastolic blood pressure. Isolated systolic hypertension or an elevated systolic blood pressure without an elevated diastolic blood pressure is a known risk factor for incident heart failure in older adults. In the current study, we examined whether isolated diastolic hypotension, defined as a diastolic blood pressure <60 mm Hg and a systolic blood pressure ≥100 mm Hg, is associated with incident heart failure. Of the 5795 Medicare-eligible community-dwelling adults age ≥65 years in the Cardiovascular Health Study, 5521 were free of prevalent heart failure at baseline. After excluding 145 individuals with baseline systolic blood pressure <100 mm Hg, the final sample included 5376 participants, of whom 751 (14%) had isolated diastolic hypotension. Propensity scores for isolated diastolic hypotension were calculated for each of the 5376 participants and used to match 545 and 2348 participants with and without isolated diastolic hypotension, respectively who were balanced on 58 baseline characteristics. During over 12 years of median follow-up, centrally-adjudicated incident heart failure developed in 25% and 20% of matched participants with and without isolated diastolic hypotension respectively (hazard ratio associated with isolated diastolic hypotension, 1.33; 95% confidence interval, 1.10–1.61; p=0.004). Among the 5376 pre-match individuals, multivariable-adjusted hazard ratio for incident heart failure associated with isolated diastolic hypotension was 1.29 (95% confidence interval, 1.09–1.53; p=0.003). As in isolated systolic hypertension, among community-dwelling older adults without prevalent heart failure, isolated diastolic hypotension is also a significant independent risk factor for incident heart failure.
Keywords: aging, blood pressure, diastolic, heart failure, pulse pressure
Arterial stiffening is common in older adults and may contribute to the elevation of systolic blood pressure (SBP) and the depression of diastolic blood pressure (DBP), and a resultant widening of the pulse pressure (PP), all commonly observed in this population.1 Although isolated systolic hypertension (ISH), defined as isolated elevation of SBP without a concomitant elevation of DBP, is a known risk factor for incident heart failure (HF) in older adults,2, 3 little is known about the effect of an isolated diastolic hypotension (IDH), where DBP is decreased without a concomitant decrease in SBP. The purpose of this study was to examine the association between IDH and incident HF in community-dwelling older adults.
Methods
Study Design and Participants
The Cardiovascular Health Study (CHS) is a National Heart, Lung, and Blood Institute (NHLBI)-funded population-based prospective study of cardiovascular disease in older adults, the details of which have been reported previously.4–6 Briefly, 5888 Medicare-eligible community-dwelling older adults (≥65 years of age) were recruited in two phases: an original cohort of 5201 participants recruited during 1989–1990 was later supplemented with a second cohort of 687 African-American participants recruited during 1992–1993. For the purpose of the current study, we used a de-identified public-use copy of the CHS data obtained from the NHLBI. These data, prepared by the CHS Central Coordinating Center at the University of Washington, Seattle, are identical to the original CHS data except that it contains data on 5795 participants (93 did not consent to be included in the public-use copy of the data).
Of the 5795 participants, 274 had centrally-adjudicated prevalent HF at baseline and hence were excluded from the current analysis. Of the remaining 5521 participants, data on baseline SBP and DBP were available on 5504 participants. Because our definition of IDH excludes patients with SBP <100 mm Hg, we excluded 128 participants with SBP <100 mm Hg. Thus, the final sample size comprised of 5376 participants without baseline HF and with SBP ≥100 mm Hg. We included participants with and without treated hypertension to determine if the effect of IDH on incident HF varied by antihypertensive therapy.
Baseline IDH and Other Measurements
Seated SBP and DBP were measured at baseline in the right arm after a five minute rest with a Hawksley random-zero sphygmomanometer and the average of two measurements corrected for zero values was used.2 IDH was defined as DBP <60 mm Hg (and SBP ≥100 mm Hg), and of the 5376 participants, 751 (14%) had IDH. Because blood pressure is a continuous variable and our definition of IDH is categorical, to understand the clinical characteristics of those with IDH better, we also categorized those with IDH into the following SBP categories: 100–109, 110–119, 120–129, and 130–139 and ≥140 mm Hg. Data on demographics, past medical history, clinical findings, and laboratory variables were collected at baseline and have been described in detail previously.2, 4, 5 Missing values for continuous variables were imputed based on values predicted by age, sex and race.
Assembly of the Balanced Cohort
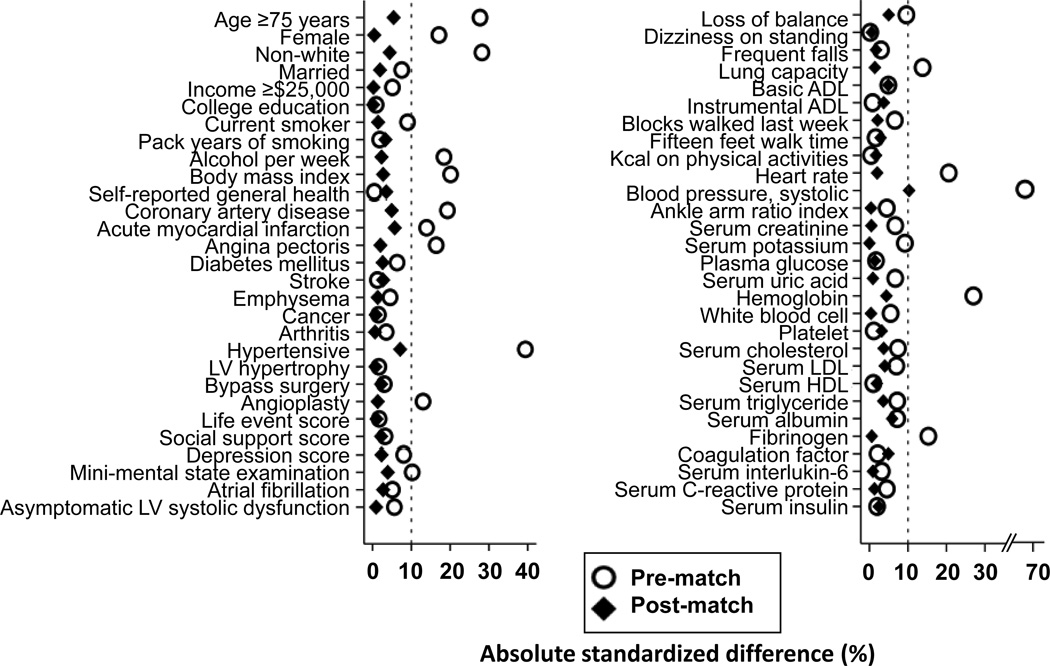
Considering significant imbalances in baseline characteristics between participants with and without IDH (Table 1 and Figure 1), we used propensity score matching to assemble a population in which those with and without IDH would be well-balanced in all measured baseline characteristics.7, 8 Propensity scores for IDH were estimated for each of the 5376 participants using a non-parsimonious multivariable logistic regression model, in which IDH was the dependent variable and 58 baseline characteristics (Figure 1) were used as covariates.3, 9–13 Using a greedy matching protocol we matched each IDH participant with up to five no-IDH participants who had similar propensity scores, thus matching 545 IDH participants to 2348 no-IDH participants.3, 9–13 Absolute standardized differences for all 58 covariates were estimated and presented as Love plots.
Table 1.
Baseline characteristics of older adults without prevalent heart failure by isolated diastolic hypotension (IDH)* before and after propensity score matching
| n (%) or mean (±SD) | Before matching | P value | After matching | P value | ||
|---|---|---|---|---|---|---|
| No IDH (n=4625) |
IDH (n=751) |
No IDH n=2348) |
IDH (n=545) |
|||
| Demographics | ||||||
| Age ≥75 years | 1458 (32%) | 337 (45%) | <0.001 | 890 (38%) | 221 (41%) | 0.253 |
| Female | 2609 (56%) | 486 (65%) | <0.001 | 1452 (62%) | 338 (62%) | 0.938 |
| Non-white | 798 (17%) | 60 (8%) | <0.001 | 237 (10%) | 48 (9%) | 0.364 |
| Education college or higher | 1992 (43%) | 320 (43%) | 0.813 | 1026 (44%) | 238 (434%) | 0.991 |
| Past Medical History | ||||||
| Coronary artery disease | 757 (16%) | 181 (24%) | <0.001 | 441 (19%) | 113 (21%) | 0.297 |
| Hypertension | 2870 (62%) | 321 (43%) | <0.001 | 1169 (50%) | 252 (46%) | 0.135 |
| Diabetes mellitus | 717 (16%) | 134 (18%) | 0.103 | 374 (16%) | 92 (17%) | 0.586 |
| Stroke | 180 (4%) | 31 (4%) | 0.757 | 91 (4%) | 24 (4%) | 0.570 |
| Peripheral artery disease | 568 (12%) | 116 (15%) | 0.016 | 459 (18%) | 86 (24%) | 0.007 |
| Loss of balance | 1041 (23%) | 200 (27%) | 0.013 | 574 (24%) | 145 (27%) | 0.293 |
| Dizzy or light headed on standing up | 939 (20%) | 153 (20%) | 0.965 | 480 (20%) | 110 (20%) | 0.892 |
| Frequent falls | 147 (3%) | 28 (4%) | 0.431 | 79 (3%) | 20 (4%) | 0.724 |
| Self-reported fair to poor general health | 1089 (24%) | 178 (24%) | 0.926 | 509 (22%) | 126 (23%) | 0.464 |
| Current smoker | 537 (12%) | 110 (15%) | 0.018 | 313 (13%) | 70 (13%) | 0.763 |
| Alcohol, units per week | 2.7 (±6.7) | 1.6 (±4.2) | <0.001 | 1.8 (±4.5) | 1.9 (±4.6) | 0.633 |
| Physical Examination | ||||||
| Body mass index, kg/m2 | 26.7 (±4.1) | 25.9 (±3.9) | <0.001 | 26.3 (±4.0) | 26.2 (±3.8) | 0.582 |
| Heart rate, bpm | 68 (±11) | 66 (±11) | <0.001 | 67 (±10) | 67 (±11) | 0.672 |
| Systolic blood pressure, mm Hg | 139 (±21) | 126 (±18) | <0.001 | 131 (±17) | 130 (±18) | 0.028 |
| Diastolic blood pressure, mm Hg† | 74 (±9) | 53 (±5) | <0.001 | 71 (±8) | 54 (±5) | <0.001 |
| Pulse pressure, mm Hg† | 66 (±11) | 73 (±11) | <0.001 | 60 (±16) | 76 (±20) | <0.001 |
| Mini-mental state examination | 27.6 (±2.7) | 27.3 (±2.9) | 0.008 | 27.6 (±2.8) | 27.4 (±2.6) | 0.423 |
| Depression score | 4.6 (±4.5) | 4.9 (±4.8) | 0.036 | 4.6 (±4.6) | 4.7 (±4.7) | 0.624 |
| Cardiopulmonary Testing | ||||||
| Atrial fibrillation by electrocardiogram | 99 (2%) | 11 (2%) | 0.225 | 39 (2%) | 11 (32%) | 0.564 |
| Left ventricular hypertrophy by electrocardiogram | 200 (4%) | 35 (5%) | 0.676 | 100 (4%) | 24 (54%) | 0.881 |
| Borderline or abnormal left ventricular systolic function | 336 (7%) | 66 (9%) | 0.141 | 184 (8%) | 44 (8%) | 0.853 |
| Medications | ||||||
| Angiotensin-converting enzyme inhibitors | 319 (7%) | 28 (4%) | 0.001 | 131 (6%) | 21 (4%) | 0.104 |
| Beta-blockers | 593 (13%) | 96 (13%) | 0.977 | 324 (14%) | 63 (12%) | 0.166 |
| Calcium channel blockers | 568 (12%) | 106 (14%) | 0.159 | 291 (12%) | 62 (11%) | 0.513 |
| Diuretics | 1263 (27%) | 208 (28%) | 0.825 | 637 (27%) | 150 (28%) | 0.852 |
| Any antihypertensive drug | 2133 (46%) | 331 (44%) | 0.297 | 1055 (45%) | 235 (43%) | 0.443 |
| Laboratory Values | ||||||
| Serum creatinine, mg/dL | 0.96 (±0.38) | 0.94 (±0.33) | 0.106 | 0.94 (±0.36) | 0.94 (±0.34) | 0.922 |
| Serum uric acid, mg/dL | 5.67 (±1.51) | 5.57 (±1.49) | 0.090 | 5.61 (±1.50) | 5.60 (±1.50) | 0.850 |
| Serum glucose, mg/dL | 111 (±36) | 112 (±38) | 0.658 | 110 (±37) | 111 (±35) | 0.766 |
| Serum insulin, mcU/mL | 17 (±24) | 16 (±24) | 0.606 | 16 (±23) | 17 (±27) | 0.593 |
| Serum potassium, mEq/L | 4.16 (±0.38) | 4.19 (±0.38) | 0.020 | 4.17 (±0.37) | 4.17 (±0.38) | 0.994 |
| Total cholesterol, mg/dL | 212 (±39) | 209 (±40) | 0.057 | 212 (±38) | 211 (±40) | 0.424 |
| Serum albumin, g/dL | 4.00 (±0.28) | 3.98 (±0.31) | 0.059 | 3.99 (±0.28) | 3.98 (±0.32) | 0.200 |
| Serum C-reactive protein, mg/dL | 4.6 (±7.7) | 5.0 (±9.7) | 0.217 | 4.6 (±7.4) | 4.5 (±8.6) | 0.767 |
| Hemoglobin, g/dL | 14.1 (±1.3) | 13.7 (±1.3) | <0.001 | 13.9 (±1.3) | 13.8 (±1.3) | 0.357 |
Defined as an average diastolic blood pressure <60 mm Hg and systolic blood pressure ≥100 mm Hg
Diastolic blood pressure and pulse pressure are related to the exposure variable, IDH, and are expected to be imbalanced and are presented as baseline descriptive purposes only
Figure 1.
Love plot displaying absolute standardized differences in baseline characteristics between older adults with and without isolated diastolic hypotension (IDH), before and after propensity score matching
Incident Heart Failure and Other Outcomes
The primary outcome for this study was centrally-adjudicated incident HF during a median follow-up of over 12 years, which has been described previously.5, 14–19 Briefly, self-reports of physician-diagnosed HF ascertained during semi-annual visits were adjudicated by CHS Events Committee through the examination of participant’s medical records for clinical symptoms, physical signs, and other supporting evidence including medications commonly used for HF and follow-up surveillances. Secondary outcomes were all-cause mortality and other incident cardiovascular events.
Statistical Analysis
For descriptive analyses the Pearson’s chi-square test, student t-test, analysis of variance, Wilcoxon rank-sum test, and paired sample t-test were used as appropriate for between group comparisons. We used Kaplan Meier and Cox proportional hazard analyses to estimate the associations between IDH and outcomes. Subgroup analyses were performed to determine the homogeneity of this association. Sensitivity analyses were performed (1) to quantify the degree of a hidden bias that would need to be present to invalidate our conclusions based on the propensity-matched cohort;20 (2) to estimate the multivariable-adjusted association of IDH with incident HF in the 5376 pre-match participants using the 58 covariates used in the propensity model; and (3) to estimate the effect of IDH in 3141 pre-match participants without hypertension (after excluding 157 and 2078 participants with and without IDH who had SBP ≥140 mm Hg). All statistical tests were two-tailed with 95% confidence levels and a p-value <0.05 was considered significant. SPSS for Windows Version 15 (Chicago, IL) was used for all data analysis.
Results
Baseline Characteristics
Compared with participants without IDH, those with IDH were more likely to be older, female, Caucasian, current smokers, have coronary artery disease, but less likely to have hypertension, and have similar orthostatic symptoms, all of which were balanced after matching (Table 1 and Figure 1). Overall, 267 (5%) had DBP ≥90 mm Hg, and among the remaining 5109 participants, 1995 (39%) had ISH and 3114 (61%) had normal SBP. Among the 751 older adults with IDH, 41% had normal SBP, 38% had pre-hypertension, and 21% had ISH of whom respectively 37%, 48% and 53% received anti-hypertensive therapy (Table 2). Mean DBP was similar across SBP categories regardless of the use of anti-hypertensive drugs (Table 2).
Table 2.
Baseline characteristics of the 751 pre-match participants with isolated diastolic hypotension (IDH), by baseline systolic blood pressure (SBP)
| Variable | IDH (Total) |
SBP (mm Hg) | P value | ||||
|---|---|---|---|---|---|---|---|
| 100–109 | 110–119 | 120–129 | 130–139 | >=140 | |||
| Number (%), mean | 751 (100) | 126 (17) | 184 (25) | 158 (21) | 126 (17) | 157 (21) | |
| Mean age | 75 | 73 | 74 | 74 | 75 | 77 | <0.001 |
| Female | 486 (65%) | 81 (64%) | 105 (57%) | 105 (67%) | 82 (65%) | 113 (72%) | 0.073 |
| Hypertension | 321 (43%) | 24 (19%) | 44 (24%) | 52 (33%) | 44 (35%) | 157 (100%) | <0.001 |
| Coronary artery disease | 181 (24%) | 33 (26%) | 42 (23%) | 43 (27%) | 26 (21%) | 37 (24%) | 0.707 |
| Diabetes | 134 (18%) | 18 (14%) | 27 (15%) | 28 (18%) | 21 (17%) | 40 (26%) | 0.069 |
| Mean blood pressure in mm Hg in persons not receiving any antihypertensive drug | |||||||
| SBP | 125 | 105 | 115 | 124 | 134 | 155 | <0.001 |
| DBP | 54 | 55 | 54 | 54 | 54 | 53 | 0.197 |
| PP | 71 | 50 | 61 | 70 | 80 | 102 | <0.001 |
| Any antihypertensive drugs | 331 (44%) | 45 (36%) | 68 (37%) | 74 (47%) | 61 (48%) | 83 (53%) | 0.008 |
| Mean blood pressure in mm Hg in persons receiving any antihypertensive drug | |||||||
| SBP | 129 | 106 | 115 | 124 | 134 | 152 | <0.001 |
| DBP | 54 | 54 | 53 | 54 | 54 | 54 | 0.200 |
| PP | 75 | 52 | 62 | 70 | 80 | 99 | <0.001 |
DBP, diastolic blood pressure; PP, pulse pressure
Association of IDH with Incident HF
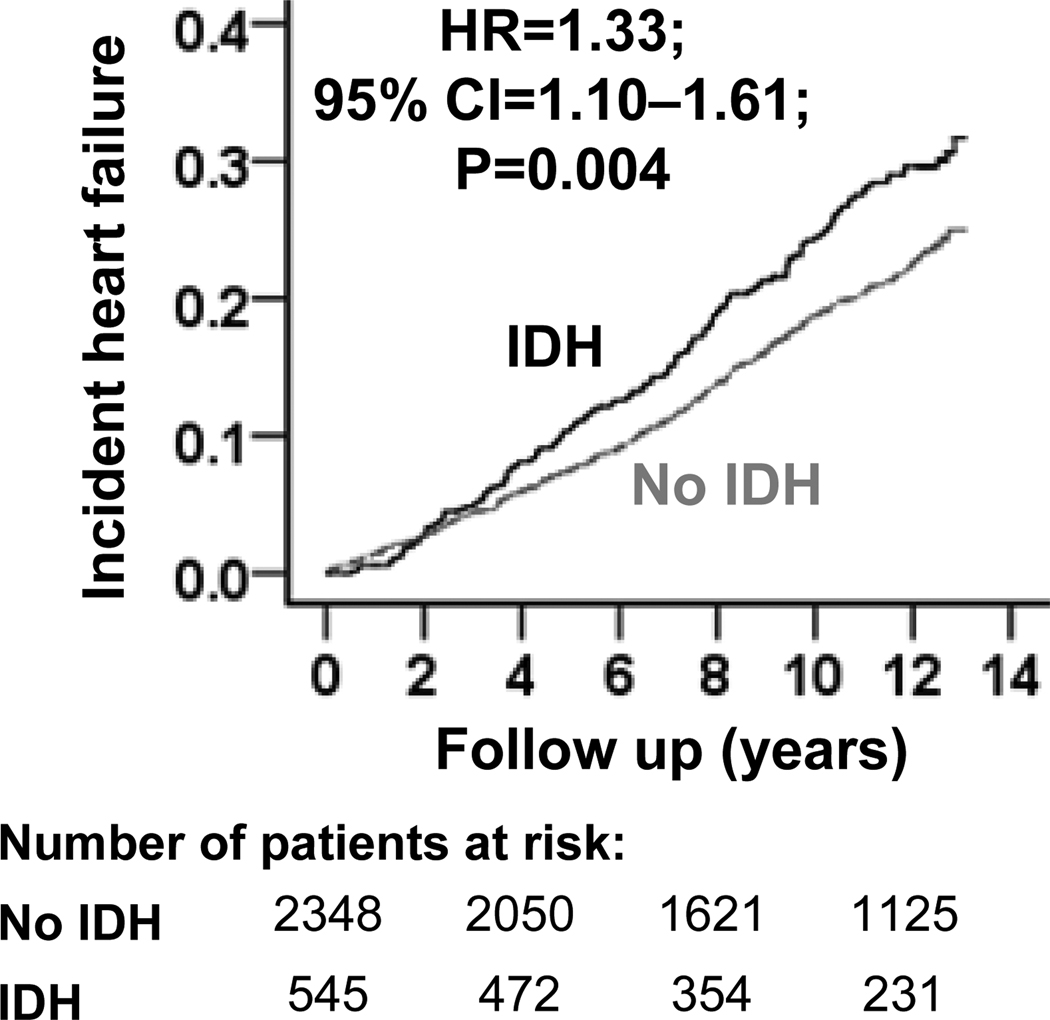
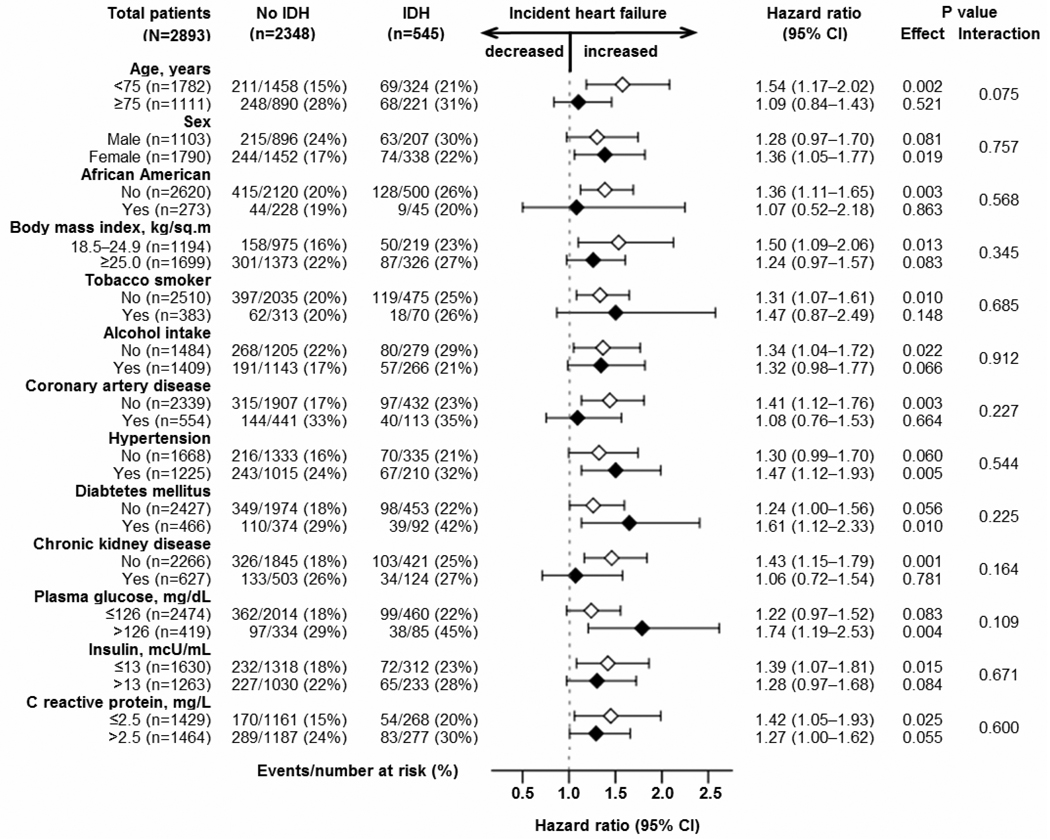
During over 12 years of median follow-up, incident HF developed in 25% and 20% of matched participants with and without IDH, respectively (hazard ratio {HR} when IDH was compared with no-IDH, 1.33; 95% confidence interval {CI}, 1.10–1.61; p=0.004; Table 3 and Figure 2). A hidden binary covariate that is a near-perfect predictor of incident HF would need to increase the odds of IDH by 17% to explain away this association. This association was homogenous across a wide spectrum of matched participants (Figure 3). Pre-match associations of IDH with incident HF are displayed in Table 3. The multivariable-adjusted HR for IDH-associated incident HF in pre-match participants with SBP <140 mm Hg was 1.43 (95% CI, 1.16–1.77; p=0.001).
Table 3.
Association of isolated diastolic hypotension (IDH)* with incident heart failure and all-cause mortality.
| Outcomes | Number of events (%) | Absolute risk difference† |
Hazard ratio (95% confidence interval) |
P value | |
|---|---|---|---|---|---|
| No IDH | IDH | ||||
| Incident heart failure | |||||
| Before matching (n=5376) | n=4625 | n=751 | |||
| Unadjusted | 929 (20%) | 189 (25%) | + 5% | 1.28 (1.10–1.50) | 0.002 |
| Multivariable-adjusted | --- | --- | --- | 1.29 (1.09–1.53) | 0.003 |
| After matching (n=2893) | n=2348 | n=545 | |||
| Propensity-matched | 459 (20%) | 137 (25%) | + 5% | 1.33 (1.10–1.61) | 0.004 |
| All-cause mortality | |||||
| Before matching (n=5376) | n=4625 | n=751 | |||
| Unadjusted | 1997 (43%) | 381 (51%) | + 8% | 1.19 (1.07–1.33) | 0.002 |
| Multivariable-adjusted | --- | --- | --- | 1.14 (1.01–1.29) | 0.031 |
| After matching (n=2893) | n=2348 | n=545 | |||
| Propensity-matched | 1039 (44%) | 266 (49%) | + 5% | 1.12 (0.98–1.28) | 0.096 |
Defined as an average diastolic blood pressure <60 mm Hg and systolic blood pressure ≥100 mm Hg
Absolute risk differences were calculated by subtracting the percent of events in the isolated diastolic hypotension group from the percent of events in the no isolated diastolic hypotension group (before values were rounded)
Figure 2.
Kaplan-Meier plot for incident heart failure by isolated diastolic hypotension (IDH). (CI=confidence interval; HR=hazard ratio)
Figure 3.
Association of baseline isolated diastolic hypotension (IDH) with incident heart failure in subgroups of propensity score-matched older adults (IDH=isolated diastolic hypotension; CI=confidence interval)
Association of IDH with Other Outcomes
All-cause mortality occurred in 49% and 44% of matched participants with and without IDH respectively (HR associated with IDH, 1.12; 95% CI, 0.98–1.28; p=0.096; Table 3). IDH had no significant association with acute myocardial infarction (HR, 0.94; 95% CI, 0.69–1.27; p=0.664), angina pectoris (HR, 1.04; 95% CI, 0.83–1.32; p=0.724), stroke (HR, 0.95; 95% CI, 0.74–1.24; p=0.720), transient ischemic attack (HR, 1.21; 95% CI, 0.72–2.04; p=0.475), and peripheral arterial disease (HR, 0.81; 95% CI, 0.48–1.36; p=0.420).
Discussion
The results of the current analysis demonstrate that IDH was an independent risk factor for incident HF among community-dwelling older adults, which was similar in magnitude to the effect of ISH on incident HF in this cohort.3 We also demonstrate that the effect of IDH on incident HF was homogeneous across a wide spectrum of older adults, including those with SBP <140 mm Hg. Although diastolic hypotension has been shown to be associated with adverse cardiovascular outcomes,21 to the best of our knowledge, this is the first report of a propensity-matched study identifying IDH as a significant independent risk factor for centrally-adjudicated incident HF in community-dwelling older adults.
As in ISH and wide PP, IDH is also primarily a manifestation of impaired aortic compliance associated with aging which results in an rise in SBP and a fall in DBP thus widening the PP. 22–26,27 The resultant increased afterload and myocardial oxygen demand may result in myocardial ischemia and subsequent systolic and diastolic dysfunction.28, 29 Poor outcomes associated with a DBP ≤60 mm Hg in frail older adults has been reported to be independent of aortic compliance and LVEF.30 Hypertension or uncontrolled SBP is unlikely to explain the association between IDH and incident HF as the association persisted after excluding those with SBP ≥140 mm Hg. In addition, the use of anti-hypertensive drugs is unlikely to explain this association, as the mean SBP and DBP were similar regardless of the use of antihypertensive drugs. Although both ISH and IDH are associated with wide PP, the role of SBP and DBP in these two conditions may differ. For example, despite a similar PP (70 mm Hg), DBP may not play a role in the development of incident HF in ISH (150/80 mm Hg) while SBP may not play a role in the development of incident HF in IDH (120/50 mm Hg).31 Further studies are needed to elucidate the detailed mechanisms by which IDH is associated with incident HF.
In patients with hypertension and coronary artery disease, a low DBP has been shown to be associated with poor outcomes and it has been suggested that excessive reduction in DBP should be avoided in these patients.30, 32 Despite the fact that in our study the mean SBP and DBP were similar regardless of antihypertensive therapy, data from ISH suggest that antihypertensive therapy is often associated with concomitant lowering of DBP.33 Because intensive lowering of SBP may not reduce major cardiovascular events,34 taken together with the findings of the current study, it may be reasonable to keep DBP at or above 60 mm Hg in older adults with ISH. Future hypertension guidelines need to focus on optimal DBP parameters for older adults receiving antihypertensive therapy.
Several limitations of our study need to be acknowledged. We had no data on dosages of antihypertensive drugs used to determine if those with IDH were receiving higher doses. It is possible that participants without IDH at baseline may have developed IDH during follow-up and this regression dilution may have underestimated the associations observed in our study.35 The association of IDH with outcomes may vary across various subsets of SBP, which needs to be examined in the future. Despite the displayed balance in all measured baseline covariates, bias due to residual imbalance or imbalance in an unmeasured covariate is possible. However, findings from our sensitivity analysis suggest that the association of IDH with incident HF was rather insensitive to an unmeasured covariate.
Perspectives
Isolated diastolic hypotension (IDH), defined as a diastolic blood pressure <60 mm Hg with a systolic blood pressure ≥100 mm Hg, is an independent risk factor for incident heart failure in community-dwelling older adults, the magnitude of which is similar to the effect of isolated systolic hypertension on incident heart failure.
Acknowledgements
The Cardiovascular Health Study (CHS) was conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the CHS investigators. This manuscript was prepared using a limited access dataset obtained from the NHLBI and does not necessarily reflect the opinions or views of the CHS or the NHLBI.
Sources of Funding
Dr. Ahmed is supported by the National Institutes of Health through grants (R01-HL085561, R01-HL085561-S and R01-HL097047) from the National Heart, Lung, and Blood Institute and a generous gift from Ms. Jean B. Morris of Birmingham, Alabama.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Franklin SS, Gustin Wt, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age-related changes in blood pressure. The framingham heart study. Circulation. 1997;96:308–315. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- 2.Psaty BM, Furberg CD, Kuller LH, Borhani NO, Rautaharju PM, O'Leary DH, Bild DE, Robbins J, Fried LP, Reid C. Isolated systolic hypertension and subclinical cardiovascular disease in the elderly. Initial findings from the cardiovascular health study. JAMA. 1992;268:1287–1291. [PubMed] [Google Scholar]
- 3.Ekundayo OJ, Allman RM, Sanders PW, Aban I, Love TE, Arnett D, Ahmed A. Isolated systolic hypertension and incident heart failure in older adults: A propensity-matched study. Hypertension. 2009;53:458–465. doi: 10.1161/HYPERTENSIONAHA.108.119792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, O'Leary DH, Psaty B, Rautaharju P, Tracy RP, Weiler PG. The cardiovascular health study: Design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 5.Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, Gardin JM, Rutledge JE, Boineau RC. Predictors of congestive heart failure in the elderly: The cardiovascular health study. J Am Coll Cardiol. 2000;35:1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 6.Gottdiener JS, McClelland RL, Marshall R, Shemanski L, Furberg CD, Kitzman DW, Cushman M, Polak J, Gardin JM, Gersh BJ, Aurigemma GP, Manolio TA. Outcome of congestive heart failure in elderly persons: Influence of left ventricular systolic function. The cardiovascular health study. Ann Intern Med. 2002;137:631–639. doi: 10.7326/0003-4819-137-8-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 7.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 8.Rubin DB. Using propensity score to help design observational studies: Application to the tobacco litigation. Health Serv Outcomes Res Methodol. 2001;2:169–188. [Google Scholar]
- 9.Ahmed A, Husain A, Love TE, Gambassi G, Dell'Italia LJ, Francis GS, Gheorghiade M, Allman RM, Meleth S, Bourge RC. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: An observational study using propensity score methods. Eur Heart J. 2006;27:1431–1439. doi: 10.1093/eurheartj/ehi890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ekundayo OJ, Dell'italia LJ, Sanders PW, Arnett D, Aban I, Love TE, Filippatos G, Anker SD, Lloyd-Jones DM, Bakris G, Mujib M, Ahmed A. Association between hyperuricemia and incident heart failure among older adults: A propensity-matched study. Int J Cardiol. 2009;142:279–287. doi: 10.1016/j.ijcard.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filippatos GS, Ahmed MI, Gladden JD, Mujib M, Aban IB, Love TE, Sanders PW, Pitt B, Anker SD, Ahmed A. Hyperuricaemia, chronic kidney disease, and outcomes in heart failure: Potential mechanistic insights from epidemiological data. Eur Heart J. 2011;32:712–720. doi: 10.1093/eurheartj/ehq473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pitt B, Ahmed A, Love TE, Krum H, Nicolau J, Cardoso JS, Parkhomenko A, Aschermann M, Corbalan R, Solomon H, Shi H, Zannad F. History of hypertension and eplerenone in patients with acute myocardial infarction complicated by heart failure. Hypertension. 2008;52:271–278. doi: 10.1161/HYPERTENSIONAHA.107.109314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowling CB, Pitt B, Ahmed MI, Aban IB, Sanders PW, Mujib M, Campbell RC, Love TE, Aronow WS, Allman RM, Bakris GL, Ahmed A. Hypokalemia and outcomes in patients with chronic heart failure and chronic kidney disease: Findings from propensity-matched studies. Circ Heart Fail. 2010;3:253–260. doi: 10.1161/CIRCHEARTFAILURE.109.899526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, Cruise RG, Theroux S. Surveillance and ascertainment of cardiovascular events. The cardiovascular health study. Ann Epidemiol. 1995;5:278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 15.Psaty BM, Kuller LH, Bild D, Burke GL, Kittner SJ, Mittelmark M, Price TR, Rautaharju PM, Robbins J. Methods of assessing prevalent cardiovascular disease in the cardiovascular health study. Ann Epidemiol. 1995;5:270–277. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 16.Aurigemma GP, Gottdiener JS, Shemanski L, Gardin J, Kitzman D. Predictive value of systolic and diastolic function for incident congestive heart failure in the elderly: The cardiovascular health study. J Am Coll Cardiol. 2001;37:1042–1048. doi: 10.1016/s0735-1097(01)01110-x. [DOI] [PubMed] [Google Scholar]
- 17.Gardin JM, McClelland R, Kitzman D, Lima JA, Bommer W, Klopfenstein HS, Wong ND, Smith VE, Gottdiener J. M-mode echocardiographic predictors of six- to seven-year incidence of coronary heart disease, stroke, congestive heart failure, and mortality in an elderly cohort (the cardiovascular health study) Am J Cardiol. 2001;87:1051–1057. doi: 10.1016/s0002-9149(01)01460-6. [DOI] [PubMed] [Google Scholar]
- 18.Schellenbaum GD, Rea TD, Heckbert SR, Smith NL, Lumley T, Roger VL, Kitzman DW, Taylor HA, Levy D, Psaty BM. Survival associated with two sets of diagnostic criteria for congestive heart failure. American journal of epidemiology. 2004;160:628–635. doi: 10.1093/aje/kwh268. [DOI] [PubMed] [Google Scholar]
- 19.Schellenbaum GD, Heckbert SR, Smith NL, Rea TD, Lumley T, Kitzman DW, Roger VL, Taylor HA, Psaty BM. Congestive heart failure incidence and prognosis: Case identification using central adjudication versus hospital discharge diagnoses. Ann Epidemiol. 2006;16:115–122. doi: 10.1016/j.annepidem.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 20.Rosenbaum PR. Sensitivity to hidden bias. In: Rosenbaum PR, editor. Observational studies. New York, NY: Springer-Verlag; 2002. pp. 105–170. [Google Scholar]
- 21.Benetos A, Zureik M, Morcet J, Thomas F, Bean K, Safar M, Ducimetiere P, Guize L. A decrease in diastolic blood pressure combined with an increase in systolic blood pressure is associated with a higher cardiovascular mortality in men. J Am Coll Cardiol. 2000;35:673–680. doi: 10.1016/s0735-1097(99)00586-0. [DOI] [PubMed] [Google Scholar]
- 22.Lakatta EG, Levy D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part i: Aging arteries: A "set up" for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 23.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:3384–3390. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 24.Dart AM, Kingwell BA. Pulse pressure--a review of mechanisms and clinical relevance. J Am Coll Cardiol. 2001;37:975–984. doi: 10.1016/s0735-1097(01)01108-1. [DOI] [PubMed] [Google Scholar]
- 25.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–943. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- 26.Schlatmann TJ, Becker AE. Histologic changes in the normal aging aorta: Implications for dissecting aortic aneurysm. Am J Cardiol. 1977;39:13–20. doi: 10.1016/s0002-9149(77)80004-0. [DOI] [PubMed] [Google Scholar]
- 27.Chae CU, Pfeffer MA, Glynn RJ, Mitchell GF, Taylor JO, Hennekens CH. Increased pulse pressure and risk of heart failure in the elderly. JAMA. 1999;281:634–639. doi: 10.1001/jama.281.7.634. [DOI] [PubMed] [Google Scholar]
- 28.Milnor WR. Arterial impedance as ventricular afterload. Circ Res. 1975;36:565–570. doi: 10.1161/01.res.36.5.565. [DOI] [PubMed] [Google Scholar]
- 29.Urschel CW, Covell JW, Sonnenblick EH, Ross J, Jr, Braunwald E. Effects of decreased aortic compliance on performance of the left ventricle. Am J Physiol. 1968;214:298–304. doi: 10.1152/ajplegacy.1968.214.2.298. [DOI] [PubMed] [Google Scholar]
- 30.Protogerou AD, Safar ME, Iaria P, Safar H, Le Dudal K, Filipovsky J, Henry O, Ducimetiere P, Blacher J. Diastolic blood pressure and mortality in the elderly with cardiovascular disease. Hypertension. 2007;50:172–180. doi: 10.1161/HYPERTENSIONAHA.107.089797. [DOI] [PubMed] [Google Scholar]
- 31.Domanski M, Mitchell G, Pfeffer M, Neaton JD, Norman J, Svendsen K, Grimm R, Cohen J, Stamler J. Pulse pressure and cardiovascular disease-related mortality: Follow-up study of the multiple risk factor intervention trial (mrfit) JAMA. 2002;287:2677–2683. doi: 10.1001/jama.287.20.2677. [DOI] [PubMed] [Google Scholar]
- 32.Messerli FH, Mancia G, Conti CR, Hewkin AC, Kupfer S, Champion A, Kolloch R, Benetos A, Pepine CJ. Dogma disputed: Can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous? Ann Intern Med. 2006;144:884–893. doi: 10.7326/0003-4819-144-12-200606200-00005. [DOI] [PubMed] [Google Scholar]
- 33.Somes GW, Pahor M, Shorr RI, Cushman WC, Applegate WB. The role of diastolic blood pressure when treating isolated systolic hypertension. Arch Intern Med. 1999;159:2004–2009. doi: 10.1001/archinte.159.17.2004. [DOI] [PubMed] [Google Scholar]
- 34.Cushman WC, Evans GW, Byington RP, Goff DC, Jr, Grimm RH, Jr, Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC, Ismail-Beigi F. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clarke R, Shipley M, Lewington S, Youngman L, Collins R, Marmot M, Peto R. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. American journal of epidemiology. 1999;150:341–353. doi: 10.1093/oxfordjournals.aje.a010013. [DOI] [PubMed] [Google Scholar]