Abstract
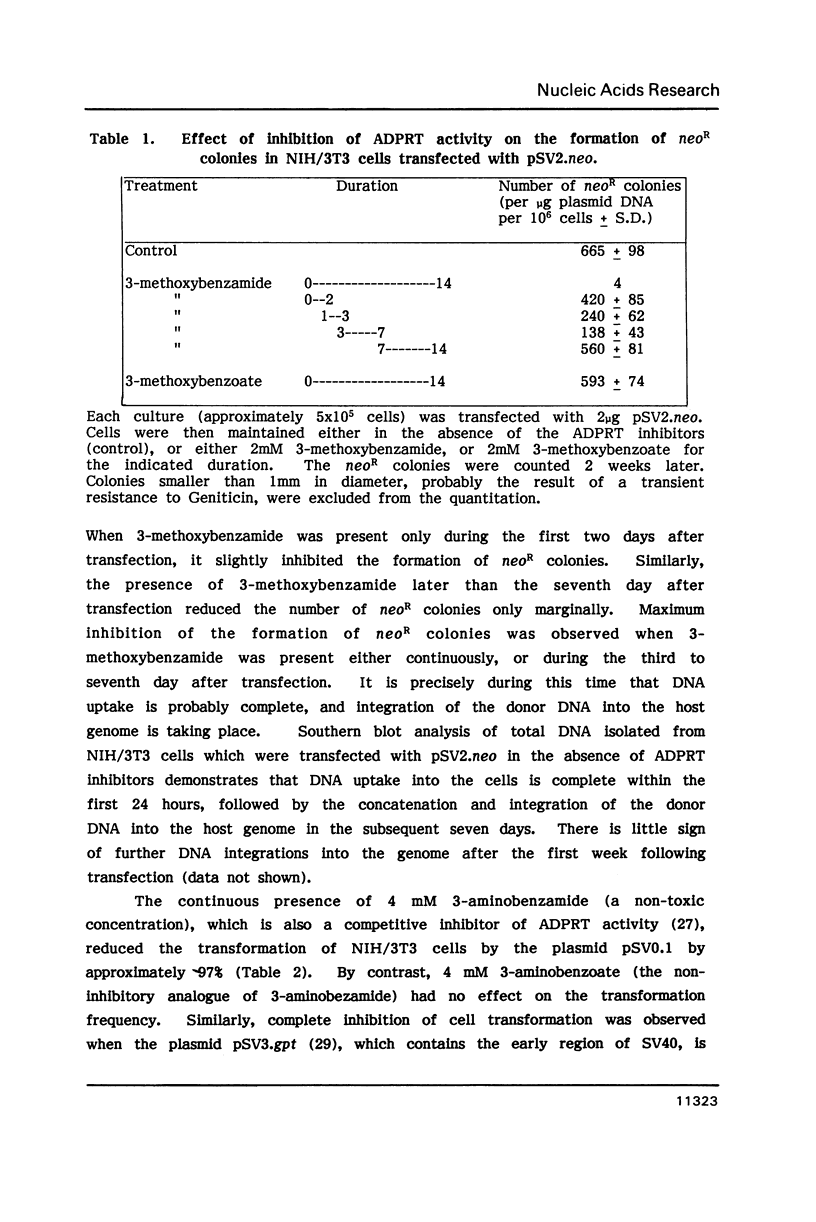
The most commonly used DNA transfection method, which employs the calcium phosphate co-precipitation of the donor DNA, involves several discrete steps (1,2). These include the uptake of the donor DNA by the recipient cells, the transport of the DNA to the nucleus, transient expression prior to integration into the host cell genome, concatenation and integration of the transfected DNA into the host cell genome and finally the stable expression of the integrated genes (2,3). Both the concatenation and the integration of the donor DNA into the host genome involve the formation and ligation of DNA strand-breaks. In the present study we demonstrate that the nuclear enzyme, adenosine diphosphoribosyl transferase (ADPRT, E.C. 2.4.2.30), which is dependent on the presence of DNA strand breaks for its activity (4,5) and necessary for the efficient ligation of DNA strand-breaks in eukaryotic cells (4,6), is required for the integration of donor DNA into the host genome. However, ADPRT activity does not influence the uptake of DNA into the cell, its episomal maintenance or replication, nor its expression either before or after integration into the host genome. These observations strongly suggest the involvement of ADPRT activity in eukaryotic DNA recombination events.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Althaus F. R., Lawrence S. D., He Y. Z., Sattler G. L., Tsukada Y., Pitot H. C. Effects of altered [ADP-ribose]n metabolism on expression of fetal functions by adult hepatocytes. Nature. 1982 Nov 25;300(5890):366–368. doi: 10.1038/300366a0. [DOI] [PubMed] [Google Scholar]
- Benjamin R. C., Gill D. M. ADP-ribosylation in mammalian cell ghosts. Dependence of poly(ADP-ribose) synthesis on strand breakage in DNA. J Biol Chem. 1980 Nov 10;255(21):10493–10501. [PubMed] [Google Scholar]
- Cornelissen A. W., Michels P. A., Borst P., Spanjer W., Versluijs-Broers J. A., Van der Meer C., Farzaneh F., Shall S. Effect of 3-aminobenzamide on antigenic variation of Trypanosoma brucei. Biochem Pharmacol. 1985 Dec 1;34(23):4151–4156. doi: 10.1016/0006-2952(85)90208-4. [DOI] [PubMed] [Google Scholar]
- Creissen D., Shall S. Regulation of DNA ligase activity by poly(ADP-ribose). Nature. 1982 Mar 18;296(5854):271–272. doi: 10.1038/296271a0. [DOI] [PubMed] [Google Scholar]
- Durkacz B. W., Omidiji O., Gray D. A., Shall S. (ADP-ribose)n participates in DNA excision repair. Nature. 1980 Feb 7;283(5747):593–596. doi: 10.1038/283593a0. [DOI] [PubMed] [Google Scholar]
- Farzaneh F., Feon S., Lebby R. A., Brill D., David J. C., Shall S. DNA repair in human promyelocytic cell line, HL-60. Nucleic Acids Res. 1987 Apr 24;15(8):3503–3513. doi: 10.1093/nar/15.8.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzaneh F., Zalin R., Brill D., Shall S. DNA strand breaks and ADP-ribosyl transferase activation during cell differentiation. Nature. 1982 Nov 25;300(5890):362–366. doi: 10.1038/300362a0. [DOI] [PubMed] [Google Scholar]
- Francis G. E., Gray D. A., Berney J. J., Wing M. A., Guimaraes J. E., Hoffbrand A. V. Role of ADP-ribosyl transferase in differentiation of human granulocyte-macrophage progenitors to the macrophage lineage. Blood. 1983 Nov;62(5):1055–1062. [PubMed] [Google Scholar]
- Francis G. E., Ho A. D., Gray D. A., Berney J. J., Wing M. A., Yaxley J. J., Ma D. D., Hoffbrand A. V. DNA strand breakage and ADP-ribosyl transferase mediated DNA ligation during stimulation of human bone marrow cells by granulocyte-macrophage colony stimulating activity. Leuk Res. 1984;8(3):407–415. doi: 10.1016/0145-2126(84)90080-8. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Greer W. L., Kaplan J. G. DNA strand breaks in murine lymphocytes: induction by purine and pyrimidine analogues. Biochem Biophys Res Commun. 1983 Sep 30;115(3):834–840. doi: 10.1016/s0006-291x(83)80010-2. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Johnstone A. P. Rejoining of DNA strand breaks is an early nuclear event during the stimulation of quiescent lymphocytes. Eur J Biochem. 1984 Apr 16;140(2):401–406. doi: 10.1111/j.1432-1033.1984.tb08116.x. [DOI] [PubMed] [Google Scholar]
- Johnstone A. P., Williams G. T. Role of DNA breaks and ADP-ribosyl transferase activity in eukaryotic differentiation demonstrated in human lymphocytes. Nature. 1982 Nov 25;300(5890):368–370. doi: 10.1038/300368a0. [DOI] [PubMed] [Google Scholar]
- Klobutcher L. A., Miller C. L., Ruddle F. H. Chromosome-mediated gene transfer results in two classes of unstable transformants. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3610–3614. doi: 10.1073/pnas.77.6.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Ohashi Y., Ueda K., Kawaichi M., Hayaishi O. Activation of DNA ligase by poly(ADP-ribose) in chromatin. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3604–3607. doi: 10.1073/pnas.80.12.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purnell M. R., Whish W. J. Novel inhibitors of poly(ADP-ribose) synthetase. Biochem J. 1980 Mar 1;185(3):775–777. doi: 10.1042/bj1850775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scangos G. A., Huttner K. M., Juricek D. K., Ruddle F. H. Deoxyribonucleic acid-mediated gene transfer in mammalian cells: molecular analysis of unstable transformants and their progression to stability. Mol Cell Biol. 1981 Feb;1(2):111–120. doi: 10.1128/mcb.1.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scangos G., Ruddle F. H. Mechanisms and applications of DNA-mediated gene transfer in mammalian cells - a review. Gene. 1981 Jun-Jul;14(1-2):1–10. doi: 10.1016/0378-1119(81)90143-8. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Williams G. T. Specific inhibition of the differentiation of Trypanosoma cruzi. J Cell Biol. 1984 Jul;99(1 Pt 1):79–82. doi: 10.1083/jcb.99.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G. T. Trypanosoma cruzi: inhibition of intracellular and extracellular differentiation by ADP-ribosyl transferase antagonists. Exp Parasitol. 1983 Dec;56(3):409–415. doi: 10.1016/0014-4894(83)90086-3. [DOI] [PubMed] [Google Scholar]



