Abstract
Compound 2 (KU-32) is a first-generation novologue (a novobiocin-based, C-terminal, heat shock protein 90 (Hsp90) inhibitor), that decreases glucose-induced death of primary sensory neurons and reverses numerous clinical indices of diabetic peripheral neuropathy in mice. The current study sought to exploit the C-terminal binding site of Hsp90 to determine whether the optimization of hydrogen bonding and hydrophobic interactions of second generation novologues could enhance neuroprotective activity. Using a series of substituted phenylboronic acids to replace the coumarin lactone of 2, we identified electronegative atoms placed at the meta-position of the B-ring exhibit improved cytoprotective activity, which is believed to result from favorable interactions with Lys539 in the Hsp90 C-terminal binding pocket. Consistent with these results, a meta-3-fluorophenyl substituted novologue (13b) exhibited a 14-fold lower ED50 compared to 2 for protection against glucose-induced toxicity of primary sensory neurons.
Introduction
Approximately 26 million Americans are afflicted with either Type 1 or Type 2 diabetes. Despite the use of insulin and oral anti-diabetic medications to help maintain euglycemia, about 60–70% of these individuals develop diabetic peripheral neuropathy (DPN).1 To date, approaches toward the treatment of DPN have centered on pathways/targets directly limited to hyperglycemia (i.e., polyol & hexosamine pathways, advanced glycation end products (AGEs), enhanced oxidative stress, PKC activation)2. Unfortunately, the contribution of these targets/pathways to the progression of DPN differs between individuals and does not concur with biochemical uniformity, and consequently, these approaches have resulted in little success for the management of DPN. As an alternative approach, we have explored the pharmacologic modulation of molecular chaperones to promote a broad cytoprotective response that may enhance a patient's ability to tolerate hyperglycemic insults and improve the symptoms of DPN.
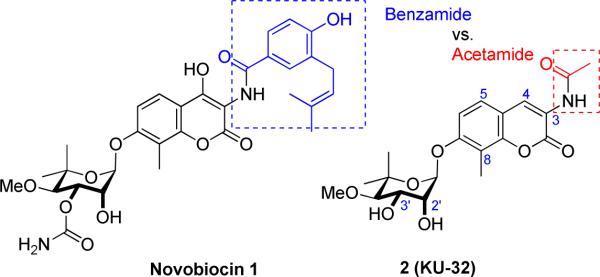
Molecular chaperones, such as heat shock proteins 90 and 70 (Hsp90, Hsp70), are essential for folding nascent polypeptides into their biologically active structures and for the refolding of aggregated and denatured proteins that occur upon cellular stress.3,4 Numerous conditions that cause cell stress can also induce the “heat shock response” (HSR); the transcriptional upregulation of antioxidant genes and chaperones such as Hsp70. Importantly, small molecule inhibition of Hsp90 is sufficient to induce the HSR. Compound 2 (KU-32)5,6 is a small molecule Hsp90 C-terminal inhibitor that is based on novobiocin 1, a naturally occurring antimicrobial agent that inhibits DNA gyrase (Figure1). Although the etiology of DPN is unrelated to the accumulation of one specific mis-folded or aggregated protein, hyperglycemia can increase oxidative stress and the oxidative modification of amino acids7 that impair protein folding,8 decrease mitochondrial protein import9 and promote mitochondrial dysfunction.2, 7a Even in the absence of a single, disease-specific protein aggregate, we have shown that pharmacologic induction of cytoprotective molecular chaperones can improve myelinated and unmyelinated fiber function in cellular models of glucotoxic stress and animal models of DPN.10 Mechanistically, compound 2 was ineffective at preventing neuregulin-induced demyelination of myelinated cultures of sensory neurons prepared from Hsp70.1 and 70.3 double knockout mice, indicating that Hsp70 is necessary for the neuroprotective activity manifested by compound 2. Similarly, weekly treatment with 2 restored normal sensory and motor nerve function in diabetic wild type mice, but was unable to reverse multiple clinical indices of DPN in the diabetic Hsp70 knockout mice.10 Collectively, these studies provide the biological and clinical rationale to support the modulation of molecular chaperones as a viable approach toward the treatment of DPN.
Figure 1.
Chemical structures of novobiocin 1 and 2 (KU-32).
An enviable aspect of 2 is that it induces Hsp70 at concentrations well below those needed to inhibit Hsp90's protein folding ability, thus, it possesses a rather broad therapeutic window that dissociates cytoprotective properties from potentially cytotoxic effects resulting from the degradation of Hsp90-dependent client proteins.4 We have previously shown that molecules containing a benzamide, as found in novobiocin, exhibit anti-proliferative activities, whereas molecules such as 2 containing an acetamide manifest neuroprotective properties. However, these prior studies sought to evaluate structure–activity relationships for novobiocin analogues as anti-cancer agents,11,12 rather than exploring chemical attributes that enhance the neuroprotective properties of novobiocin-based analogs. Therefore, the goal of this work was to determine whether diversification of the scaffold of compound 2 could identify structure–activity relationships (SAR) for novobiocin analogues (novologues) that enhance the neuroprotective properties manifested by Hsp90 C-terminal inhibitors.
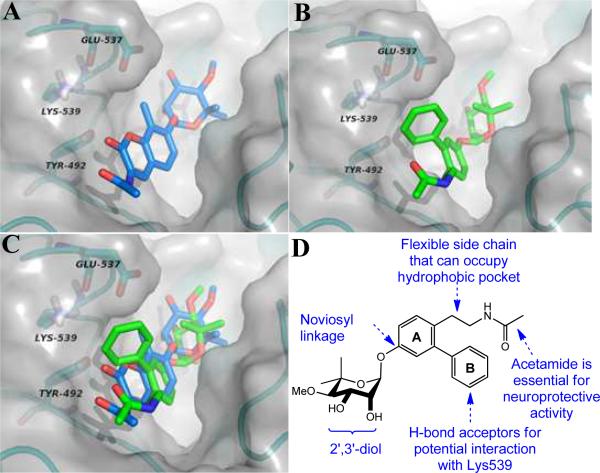
Recently, we used molecular modeling and azide-containing novobiocin derivatives as photoaffinity probes to elucidate, for the first time, the Hsp90 C-terminal binding site.13 As shown in Figure 2 (A–C), compound 2 docks to this region and appears to exhibit binding interactions with both the protein backbone and the amino acid side chains similar to those manifested by novobiocin. Interestingly, the coumarin lactone of 2 appears too distant from Lys539 to provide complementary interactions with this residue. In addition, the 3-amido side chain appears to project into a large hydrophobic pocket that could accommodate more flexible linkers. As a consequence of these observations, the novologue scaffold (Figure 2D) was designed to project the B-ring into the pocket wherein Lys539 resides and to serve as a lead compound for further diversification. In addition, the flexible ethyl amide projecting from the A-ring could accommodate a number of orientations that could better occupy the large hydrophobic pocket that remains vacant in the presence of 2.
Figure 2.
A) Compound 2 docked to Hsp90 C-terminal binding site. B) Novologue docked to Hsp90 C-terminal binding site. C) Overlay of 2 and novologue docked to Hsp90 C-terminal binding site. All structures docked into Hsp90α open homology model. D) Structure of novologue and its attributes.
Based on the novologue design, we envisioned construction of a parallel library to validate this scaffold for use as a neuroprotective agent. The library was designed so that the 3'-carbamate on noviose was omitted; based upon prior studies that showed this group to be detrimental to Hsp90 inhibitory activity.5 In contrast, additional hydrophobic and hydrogen bonding interactions could be provided by the incorporation of functionalities onto the 3-aryl substituent (B-ring), which was designed to provide complementary interactions with Lys539. The 4-ethyl acetamide was included to occupy the binding pocket about the coumarin ring system. Consistent with data obtained from prior studies, the 7-noviosyl linkage was maintained as well the requisite 2',3'-diol. In this article, we report the parallel synthesis of rationally designed novologues as Hsp90 C-terminal inhibitors and assessment of their neuroprotective activities.
Results and Discussion
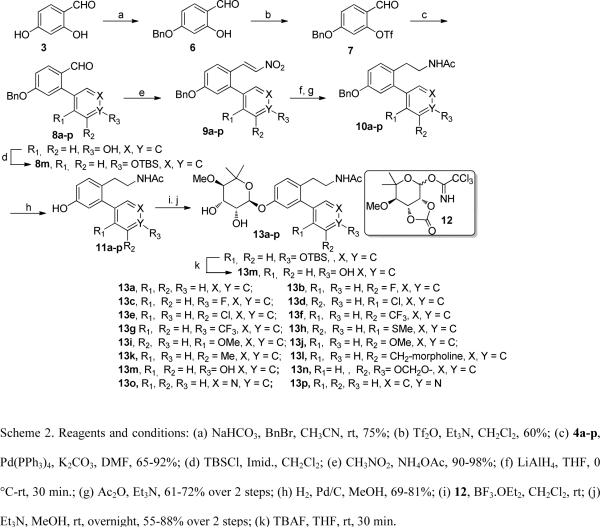
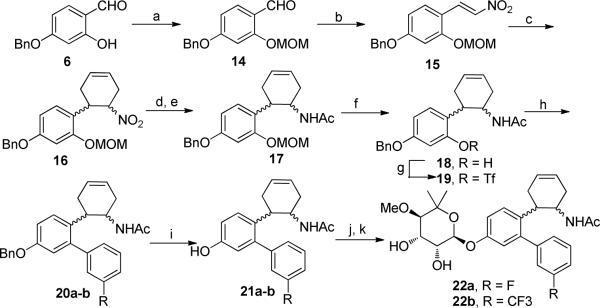
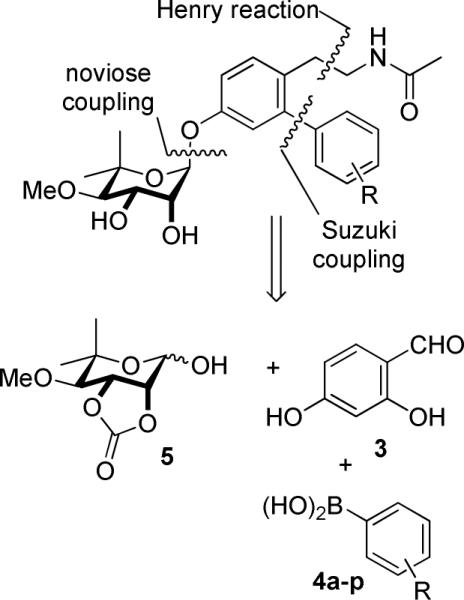
Retrosynthetically, a library of novologues was envisioned for construction via four components (Scheme 1); a resorcinolic benzaldehyde (3), a variety of commercially available boronic acids (4a–p), noviose (5), and the acetamide side chain (Scheme 1). Prior work from our laboratory demonstrated that the trichloroacetimidate of noviose carbonate undergoes rapid coupling with phenols to give the desired α-anomer in high yield.
Scheme 1.
Retrosynthetic analysis for the construction of novologue.
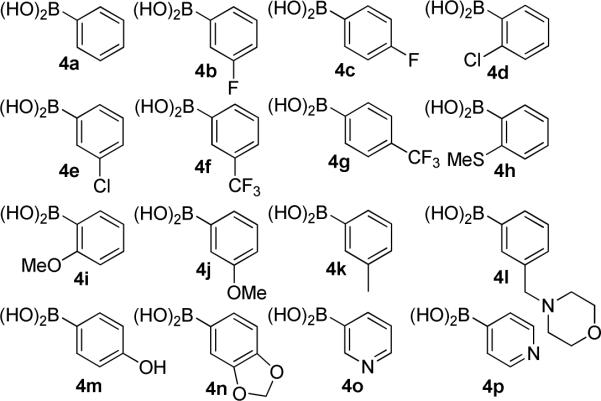
The boronic acids chosen for this study contain both electronic and steric moieties that could aid in elucidation of structure–activity relationships and provide crucial interactions with Lys539 and the surrounding pocket. Towards this goal, phenylboronic acids (Figure 3) containing electronegative atoms at the meta- and para-positions were explored. In addition, hydrogen bond acceptors were included at these locations to provide potential hydrogen bonding interactions with the protonated form of Lys539. To serve as controls, hydrophobic groups (4j, 4k) and a tertiary amine (4l) were included in this series.
Figure 3.
Boronic acids selected for incorporation into novologue X scaffold.
The synthesis of ethyl acetamide side chain containing novologues 13a–p, began with commercially available 2,4-dihydroxybenzaldehyde, 3. The 4-phenol of resorcinolic benzaldehyde 3 was protected as the corresponding benzyl ether 6,14 and the 2-phenol converted to triflate 7 using trifluoromethanesulfonic anhydride and triethylamine (Scheme 2). Compound 7 was subsequently coupled with commercially available aryl boronic acids (4a–p) under standard Suzuki conditions15,16 to give biaryl ring systems 8a–p in good yields. Benzaldehydes 8a–p were converted to the corresponding nitrostyrenes (9a–p), following a Henry reaction with nitromethane and ammonium acetate.17,18 Reduction of the nitro and olefin functionalities with lithium aluminum hydride was followed by acylation of the resulting amines to afford acetamides 10a–p in good yields. The benzyl ether of compounds 10a–p was cleaved under hydrogenolysis conditions to afford phenols 11a–p, which were coupled with the tricloroacetimidate of noviose carbonate 125,19 in the presence of a catalytic amount of boron trifluoride etherate. The resulting noviosylated biaryl systems were exposed to methanolic ammonia to solvolyze the cyclic carbonate and give the desired novologues (13a–p) in good to moderate yields.
Scheme 2.
Synthesis of ethyl acetamide side chain containing novologues.
In addition, two cyclohexene analogues 22a–b were pursued to test our hypothesis regarding the region surrounding the flexible side chain (Scheme 3). Although these molecules contain the same linker length, these analogues contain a bulky cyclohexane tether between the biaryl ring system and the acetamide.
Scheme 3.
Synthesis of cyclohexene containing novologues.
Synthesis of cyclohexene analogues 22a–b began with the previously described phenol 6, which was protected as the methoxymethyl (MOM) ether 1420 before the aldehyde of which was converted to nitrostyrene 15 under Henry conditions.16 The electron deficient nitrostyrene (15) was subjected to a Diels–Alder cycloaddition with excess butadiene to give an enantiomeric mixture of cyclohexene derivative 16 in excellent yield.21 The nitro group of 16 was selectively reduced to the amine via zinc dust and acidic isopropanol,22 followed by acetylation to afford acetamide 17 in 71% yield over two steps. In order to construct the biaryl ring system, the MOM-ether was cleaved to give the phenol, which was then converted to the corresponding triflate, 19. A Suzuki reaction between 19 and 3-fluorophenylboronic acid (4b) or 3- (trifluoromethyl) phenylboronic acid (4f), yielded biaryl compounds 20a or 20b, respectively. Finally, boron trifluoride etherate promoted removal of the benzyl ether23 on compounds 20a–b and gave phenols 21a–b. Lewis acid-catalyzed noviosylation of 21a–b, with activated noviose carbonate (10), followed by methanolysis, afforded an inseperable mixture of diastereomeric products, 22a–b.
Evaluation of Neuroprotective Efficacy
Upon synthesis of ethyl acetamide side chain novologues 13a–p that contain various substitutions on the B-ring (hydrogen bond acceptors, hydrogen bond donors, hydrophobic groups, and a tertiary amine), their neuroprotective efficacy against glucose-induced toxicity of embryonic dorsal root ganglion (DRG) sensory neuron cultures was evaluated. As shown in Table 1, meta-substituted acetamide novologues (13b, 13e and 13f) showed significant protection against glucotoxicity and were comparable to that observed with compound 2. Although the corresponding ortho- and para- substituted (13c, 13d and 13g) derivatives showed significant protection against glucose-induced cell death, they were modestly less effective than novologues 13b, 13e and 13f. However in the case of analogues 13i (ortho-OMe) and 13j (meta-OMe) the opposite trend was observed. Electronegative atoms at the meta-position (F, Cl, CF3) exhibited greater cytoprotective activity, which is believed to result from favorable interactions with Lys539 in the Hsp90 C-terminal binding pocket. Consistent with this hypothesis, increasing the size of the electronegative atom at the meta-position (F to Cl to CF3) resulted in a decrease in neuroprotective activity. Analogue 13b (meta-F) was amongst the most cytoprotective (95%±14) compounds evaluated.
Table 1.
Cell viability data of ethyl acetamide side chain novologues.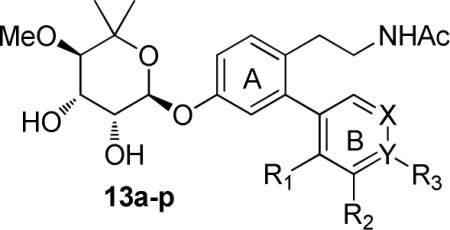
| Entry | R1 | R2 | R3 | X | Y | % of cell viabilitya |
|---|---|---|---|---|---|---|
| 2 | - | - | - | - | - | 86% ± 2 |
| 13a | H | H | H | C | C | 76%±11# |
| 13b | H | F | H | C | C | 95%±14# |
| 13c | H | H | F | C | C | 75%±27# |
| 13d | Cl | H | H | C | C | 71%±21#,* |
| 13e | H | Cl | H | C | C | 90%±23# |
| 13f | H | CF3 | H | C | C | 83%±16# |
| 13g | H | H | CF3 | C | C | 74%±19#,* |
| 13h | SMe | H | H | C | C | 83%±4O# |
| 13i | OMe | H | H | C | C | 92%±10# |
| 13j | H | OMe | H | C | C | 78%±34# |
| 13k | H | Me | H | C | C | 82%±30# |
| 13l | H | CH2-N-morpholine | H | C | C | 83%±26# |
| 13m | H | H | OH | C | C | 67%±10* |
| 13n | H | -OCH2O- | C | C | 83%±18# | |
| 13o | H | H | H | N | C | 61%±7* |
| 13p | H | H | H | C | N | 81%±12# |
In the presence of 1 μM of each novologue + 20 mM excess glucose. Viability in the presence of 20mM excess glucose + DMSO was 54% ± 2.
p<0.05 versus glucose + DMSO;
p<0.05 versus glucose + compound 2 (n=6–24) per novologue.
Electronegative atoms at the ortho- or para-position on ring B (13c, 13d and 13g) manifested activities comparable to the unsubstituted analogue (13a) and were less active than the corresponding meta-substituted analogues (13b, 13e and 13f). Although novologues 13d and 13g manifested protection against neuronal glucotoxicity, they were less effective than our previous compound 2 and 13b. Hydrogen bond donors at the para-position (13m) appeared to be undesired as 13m (para-OH) was unable to provide significant protection against glucotoxicity. It was also somewhat, but not significantly less protective than the unsubstituted analogue (13a).
On the other hand, hydrogen bond acceptors at the para-position (13c and 13g) protected against glucose-induced neuronal death but did not display significantly increased protection compared to the novologue containing a para-position hydrogen bond donor (13m).
Pyridine-containing analogues (13o–p) were also synthesized and evaluated for neuroprotective activity. The 3-pyridine analogue (13o) was unable to protect against glucoseinduce toxicity and was also significantly less protective than the corresponding 4-pyridine analogue, 13p, 2, and the unsubstituted phenyl analogue, 13a. Although the 4-pyridinecontaining analogue (13p) demonstrated a modestly improved neuroprotective activity when compared to the simple phenyl analogue 13a, this difference in efficacy was not significant.
Neuroprotective activity was also determined for the cyclohexene-containing novologues (22a–b) that contain the fluoro or trifluoromethane substituent at the meta-position of ring B. In general, cyclohexene-containing analogues 22a–b were less efficacious than the corresponding derivatives that contain a flexible side chain (13b versus 22a, and 13f versus 22b). Although not statistically different, novologue 22a (meta-F) exhibited slightly better cytoprotective activity than analogue 22b (meta-CF3), which follows the same trend observed for flexible acetamidecontaining compounds (13b versus 13f). Although these data are inconsistent with our hypothesis that accommodation of the hydrophobic pocket would improve efficacy, the cyclohexene ring may exceed the space allowed in this binding cleft.
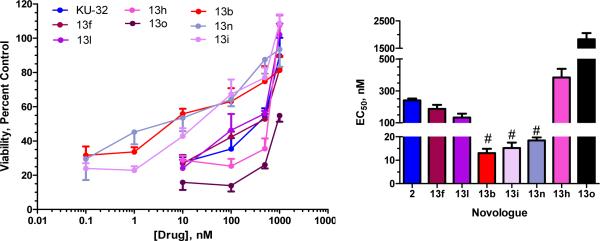
The data in Table 1 clearly support that the majority of novologues synthesized decrease neuronal toxicity induced by hyperglycemic stress. Although some of these compounds appear more effective than KU-32 at 1 μM, the differences were relatively minor. Therefore, to further scrutinize their efficacy, compounds exhibiting high neuroprotective activity were further evaluated for determination of EC50 values. Since the difference in efficacy for novologues with meta-F and meta-CF3 substitutions on 13b and 13f were not significantly different from 2 or each other at 1 μM, the EC50 values for these compounds were determined alongside 13h, 13l, 13n, and 13o. As shown in Figures 4A and 4B, EC50 values were significantly improved upon closer inspection and clear distinctions were obtained. Novologue 13b yielded an EC50 value (13.0 ± 3.6 nM) that was approximately 14-fold lower than compound 2 (240.2 ± 42.5 nM) or 13f (187.7 ± 43.5 nM). Similar results were also observed for novologue 13n, which exhibited an EC50 value of 18.4 ± 3.2 nM. In contrast, novologue 13h which manifested similar efficacy to compound 2 at 1 μM, exhibited an EC50 of 384 ± 108 nM, approximately 1.6-fold greater than 2.
Figure. 4. Determination of EC50 of select novologues.
A) DRG sensory neurons were incubated in the absence or presence of 0.1–1000 nM of the indicated novologue overnight and then subjected to 4 hrs of hyperglycemia. Cell viability was measured as described in Experimental Methods and the data expressed as percent of normoglycemic controls. Under hyperglycemic conditions and in the absence of any novologues, cell viability was 20% ± 7. B) The EC50 was determined using the ECanything function of GraphPad Prism 5.0 and the mean ± SEM (n=3–8) is shown. #, p< 0.05 versus compound 2.
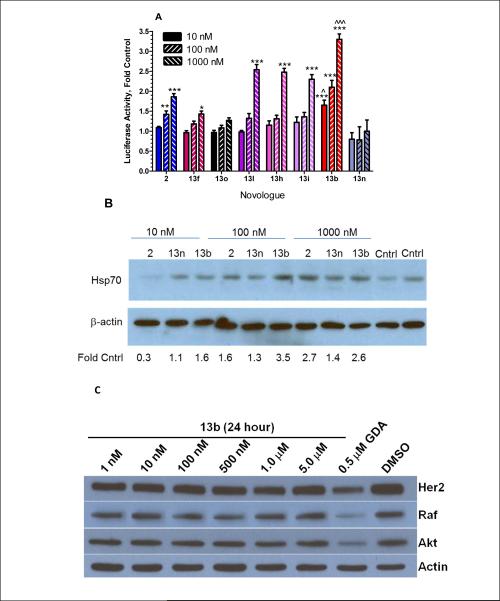
The data in Figure 4 demonstrate that novologues 13b, 13i and 13n are more cytoprotective than the initial lead compound 2. Since we have previously shown that the cytoprotective activity manifested by compound 2 requires Hsp70,10 we determined the ability of the novologues to induce Hsp70, relative to 2. To this end, a luciferase reporter assay was developed in which the expression of luciferase is driven by the human Hsp70 promoter containing two heat shock binding elements.24 Since primary sensory neurons transfect poorly, an immortalized sensory neuron cell line (50B11 cells) was used for transfection.25 Importantly, 50B11 cells have a very low basal level of Hsp70 expression, similar to primary sensory neurons.26 Twenty-four hours after transfection with the reporter, cells were re-seeded into 12 well plates and incubated for an additional 24 h. The cells were then treated for 16 h with 10–1000 nM of the indicated novologues, cell lysates were prepared, luciferase activity assessed and luminescence normalized to total protein per well. Consistent with its increased efficacy in protecting against glucotoxicity, 13b was more effective than 2 at activation of the Hsp70 promoter (Fig. 5A) and also increased expression of Hsp70 protein at lower concentrations relative to KU-32 (Fig. 5B). Although 13i had a similar EC50 as 13b in preventing glucotoxicity, it only activated the Hsp70 promoter at 1 μM and the magnitude of this effect was no better than either 2 or 13b. However, it was surprising that despite the low EC50 of 13n in protecting against glucotoxicity, 13n did not increase luciferase activity at any concentration tested nor did it increase Hsp70 protein expression as effectively as 2 or 13b. These results suggest that 13n likely affects Hsp70 levels indirectly and that the mechanism for neuroprotection may be distinct from that of related novologues.
Figure. 5. Induction of Hsp70 by select novologues in the absence of client protein degradation.
A) 50B11 cells were transfected with a luciferase reporter whose expression was drive by the human Hsp70 promoter. The cells were treated with the indicated concentration of select novologues for 16 hr and luciferase activity assessed. **, p < 0.01 and ***, p < 0.001 versus control; ^, p < 0.05 and ^^^, p< 0.001 versus compound 2 at same concentration. B) DRG sensory neurons were incubated in the presence of DMSO (Cntrl) or 10–1000 nM of the indicated novologue overnight and then subjected to 4 hrs of hyperglycemia. The neurons were harvested and Hsp70 and β-actin levels were determined by immunoblot analysis. Band intensity was quantified using Image J, Hsp70 expression was normalized to the level of β-actin and expressed as a fold control. C) MCF7 cells were treated with the indicated concentrations of 13b for 24 hr, cell lysates were prepared and the levels of the Hsp90 client proteins, Her2, Raf, and Akt determined by immunoblot analysis. As a positive control, some cells were treated with 500 nM geldanamycin (GDA) to induce client protein degradation. The level of β-actin verified equivalent protein loading.
Lastly, we mentioned that an attractive property of the modified novobiocin scaffold of 2 is that it induces Hsp70 at concentrations well below those needed to inhibit Hsp90's protein folding ability10. Therefore, to confirm that this new scaffold manifests similar activity, 13b was evaluated against MCF-7 breast cancer cells that are highly reliant upon the Hsp90 protein folding machinery. As can be observed, no client protein degradation occurred at concentrations up to 5 μM, indicating the potential for a large therapeutic window for this scaffold as well.
Conclusion
Using the recently reported model for the Hsp90 C-terminal binding site, a novologue scaffold was designed to afford putative interactions with previously unoccupied regions of the binding pocket, including Lys539. Through systematic replacement of substituents on the novologue B-ring (see Table 2), compound 13b was identified as a neuroprotective agent that exhibited ~14-fold greater efficacy against glucose-induced toxicity than the lead compound 2. The concentration of 13b needed to manifest neuroprotective activity correlated well with its ability to induce Hsp70 levels, and therefore linking cytoprotection to Hsp70 induction. When combined, these data demonstrate the rationally-designed novologue scaffold provides a promising platform on which diversification of the B-ring can lead to compounds that exhibit better neuroprotective activities.
Table 2.
Cell viability data of cyclohexene analogues.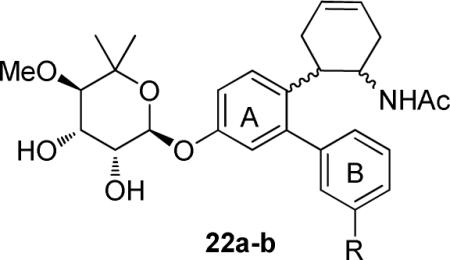
In the presence of 1 μM novologue + 20 mM excess glucose. Viability in the presence of 20mM excess glucose + DMSO was 54% ± 2.
p<0.05 versus glucose + DMSO;
p<0.05 Versus glucose + 2 (n=8) per novologue.
General Experimental Methods
Preparation of Embryonic Dorsal Root Ganglion (DRG) Neuron Cultures
DRG from embryonic day 15-–18 Sprague Dawley rat pups were harvested into Leibovitz's L15 medium (L15) and dissociated with 0.25% trypsin for 30 min at 37°C. The ganglia were sedimented at 1,000 × g for 5 min, resuspended in growth media [phenol red free Neurobasal medium (Gibco, Grand Island, NY) containing 25 mM glucose, 1X B-27 additive, 50 ng/ml nerve growth factor (NGF) (Harlan Bioscience, Indianapolis, IN), 4 mM glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin] and triturated with a fire-polished glass pipette. The cells were cultured on collagen-coated (0.1 mg/mL collagen followed by overnight air drying in a laminar flow hood) black-walled 96-well plates (Corning Incorporated Corning, NY) at a seeding density of 2–3 × 104 cells per well. DRG neurons were re-fed the next day with fresh growth media containing 40 μM fluorodeoxyuridine and 10 μM cytosine β-D-arabinoside (both from Sigma Aldrich, St. Louis, MO) for 2 days to remove proliferating cells. Experiments were performed on DRG neurons on the third day in culture after placing the cells in fresh growth medium.
Glucotoxicity Assay
As immature DRG are susceptible to hyperglycemia-induced death27, an additional 20 mM glucose was added to the growth medium (yielding a total of 45mM glucose) for 4 hours. Preliminary experiments found that 20 mM excess glucose for 4 h was sufficient to induce a reproducible 40–50% loss in neuronal viability. As a result, the toxicity induced by the acute change in glucose concentration makes it a useful model for drug screening.10, 28 Given the short time frame that the neurons are grown in vitro, they are not pure neuronal cultures but instead, highly enriched. Importantly, the contaminating SCs that remain in the culture are resistant to glucose-induced death as we and others have reported previously.6 Unfortunately, the use of highly purified cultures is problematic since the cells extend neurites and establish connections with each other, thus becoming resistant to hyperglycemia-induced death.6a
DRG neurons were incubated overnight with the novologues in the presence of Neurobasal medium, 50 ng/ml NGF and antibiotics only. In order to monitor the efficiency of our the novologues in protecting DRG neurons against glucotoxicity, we made use of Calcein AM (Invitrogen, Carlsbad, CA) to measure cell viability. Hydrolysis of calcein AM to a fluorescent product can only occur in live cells. Excess glucose was added to the cultures for 4 h and cell viability was measured by incubating the cells with 2 μM calcein AM for 30 min in the dark at 37°C. Fluorescence was then measured using a plate reader with excitation and emission wavelengths set to 485nm and 520nm, respectively. The arbitrary fluorescence readings were normalized to the total amount of protein from each respective well of the neuronal cultures. The protein concentrations in each well were determined using the DC protein assay (Bio-Rad). Significant differences in the efficacy of the novologues for increasing cell viability were determined using a Kruskal-Wallis non-parametric ANOVA and Dunn's post-test.
Luciferase Reporter Assay and Client Protein Degradation
A 1.5 kb region upstream of the start codon of the human HSPA1A gene was synthesized by GeneArt (Life Technologies, Grand Island, NY) and a 5' Kpn I and 3' Sac I sites added to direct cloning into the pGL3 basic luciferase reporter plasmid. DNA sequencing verified the integrity of the promoter sequence and the presence of two heat shock elements. 50B11 cells25 were grown in 10 cm dishes in DMEM containing 25 mM glucose, 10% FCS and 5 μg/ml blasticidin. The cells were transfected using lipofectamine and after 24 h, were re-seeded into 24 well plates at a density of 2 × 105 cells per well. The cells were permitted to attach to the plate for 6 h in growth medium and treated with the indicated concentrations of the various novologues for 16 h. Luciferase activity was assessed and normalized to the total protein concentration of each well. Results shown are from triplicate wells obtained in at least three separate experiments. Preliminary experiments validated that the reporter was strongly activated as expected by either heat shock (~ 10 fold) or 250 nM geldanamycin (~4–5 fold). Client protein degradation in MCF7 cells was performed as we have previously described.
Molecular Modeling
Surflex-Dock in Sybyl v8.0 was used for molecular modeling and docking studies. A homology model of Hsp90α based on the open HtpG SAXS structure was used as the receptor, while the protomol was generated using docked Novobiocin as described in reference.13 The energy minimized molecules were then docked with 10 different starting conformations while rotation of rotatable bonds was unrestricted. Visual interpretation and figure preparation were then carried out in Pymol.
Chemistry General
1H NMR were recorded at 400 or 500 MHz (Bruker DRX-400 Bruker with a H/C/P/F QNP gradient probe) spectrometer and 13C NMR spectra were recorded at 125 MHz (Bruker DRX 500 with broadband, inverse triple resonance, and high resolution magic angle spinning HR-MA probe spectrometer); chemical shifts are reported in δ (ppm) relative to the internal reference chloroform-d (CDCl3, 7.27 ppm). FAB (HRMS) spectra were recorded with a LCT Premier (Waters Corp., Milford, MA). The purity of all compounds was determined to be >95%as determined by 1H NMR and 13C NMR spectra, unless otherwise noted. The most active 5 compounds were verified for >95% purity by HPLC analyses. TLC was performed on glass backed silica gel plates (Uniplate) with spots visualized by UV light. All solvents were reagent grade and, when necessary, were purified and dried by standard methods. Concentration of solutions after reactions and extractions involved the use of a rotary evaporator operating at reduced pressure.
5-(Benzyloxy)-2-formylphenyl trifluoromethanesulfonate (7)
Triethylamine (1.02 mL, 7.35 mmol) followed by triflic anhydride (1.38 mL, 6.35 mmol) were added simultaneously to a phenol 6 (1.12 g, 4.91 mmol) in anhydrous CH2Cl2 (10 mL) at 0 °C. Upon completion of the reaction, quenched by the addition of water (50 mL), extracted with CH2Cl2 (3 × 15 mL), washed with saturated aqueous sodium chloride solution, dried over anhydrous Na2SO4, filtered and concentrated. The residue was purified by column chromatography (SiO2, 4:1, Hex:EtOAc) to afford triflate 7 as a yellow oil (1.06 g, 60%).
General procedure for Suzuki coupling reaction of triflate 3 and boronic acids 4a–p:
5-(Benzyloxy)-[1,1'-biphenyl]-2-carbaldehyde (8a)
Tetrakis(triphenylphosphine)palladium(0) (70.4 mg, 0.068 mmol) was added to a mixture of triflate 7 (0.246 g, 0.68 mmol), phenylboronic acid 4a (92 mg, 0.75 mmol), and K2CO3 (0.169 g, 1.2 mmol) in DMF (6.8 mL) under argon atmosphere in a sealed tube. The resulting reaction mixture was sealed and heated to reflux for 16 h. The reaction was cooled to room temperature, quenched with saturated sodium bicarbonate, extracted with EtOAc (3 × 5 mL), washed with saturated aqueous sodium chloride, dried over anhydrous Na2SO4, filtered and concentrated. The crude product was purified by column chromatography (SiO2, 3:1, Hex:EtOAc) to afford 8a (0.16 g, 0.56 mmol, 82%) as an amorphous solid. 1H NMR (400 MHz, CDCl3) δ 9.90 (s, 1H), 8.08 (d, J = 8.7 Hz, 1H), 7.55 – 7.34 (m, 10H), 7.11 (d, J = 8.7 Hz, 1H), 7.0 3 (d, J = 2.4 Hz, 1H), 5.19 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 191.2, 162.8, 148.6, 137.8, 136.0, 130.0, 128.8, 128.4, 127.6, 116.3, 114.7, 70.4; HRMS (FAB) m/z: [M + Na+] for C20H16O2Na, calcd, 311.1042; found, 311.1046.
5-(Benzyloxy)-3'-fluoro-[1,1'-biphenyl]-2-carbaldehyde (8b)
Using 3-flourophenylboronic acid. 1H NMR (500 MHz, CDCl3) δ 9.85 (d, J = 0.7 Hz, 1H), 8.03 (d, J = 8.7 Hz, 1H), 7.49 – 7.33 (m, 6H), 7.20 – 7.13 (m, 2H), 7.13 – 7.08 (m, 2H), 7.03 (d, J = 2.5 Hz, 1H), 5.15 (s, 2H); 13C NMR (125 MHz, CDCl3) δ 190.7, 162.9, 161.7, 147.2, 140.1, 136.0, 130.5, 129.0, 128.6, 127.8, 126.0, 117.1, 116.9, 116.4, 115.5, 115.1, 70.6; HRMS m/z: [M + Na+] for C20H15FO2Na, calcd, 329.0948; found, 329.0952.
5-(Benzyloxy)-4'-fluoro-[1,1'-biphenyl]-2-carbaldehyde (8c)
Using 4-Flourophenylboronic acid. 1H NMR (400 MHz, CDCl3) δ 9.84 (s, 1H), 8.06 (dd, J = 8.7, 1.0 Hz, 1H), 7.49 – 7.40 (m, 4H), 7.40 – 7.32 (m, 3H), 7.21 – 7.13 (m, 2H), 7.12 – 7.06 (dd, J = 8.0, 2.5 Hz, 1H), 7.03 (d, J = 2.2 Hz, 1H), 5.17 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 190.9, 162.8, 147.4, 136.0, 131.7, 131.6, 130.5, 128.8, 128.5, 127.7, 127.6, 116.5, 115.6, 115.4, 114.7, 70.4; HRMS m/z: [M + Na+] for C20H15FO2Na, calcd, 329.0948; found, 329.0944.
5-(Benzyloxy)-2'-chloro-[1,1'-biphenyl]-2-carbaldehyde (8d)
Using 2-Chlorophenylboronic acid. 1H NMR (500 MHz, CDCl3) δ 9.70 (s, 1H), 8.08 (d, J = 8.7 Hz, 1H), 7.55 – 7.49 (m, 1H), 7.49 – 7.32 (m, 8H), 7.17 – 7.12 (dd, J = 8.6, 2.5 Hz, 1H), 6.99 (d, J = 2.6 Hz, 1H), 5.16 (s, 2H); 13C NMR (125 MHz, CDCl3) δ 190.3, 162.9, 145.1, 136.8, 135.9, 133.5, 131.6, 130.0, 129.8, 129.6, 128.8, 128.4, 127.6, 127.6, 126.9, 116.7, 115.1, 70.4; HRMS m/z: [M + Na+] for C20H15ClO2Na, calcd, 345.0658; found, 345.0653.
5-(Benzyloxy)-3'-chloro-[1,1'-biphenyl]-2-carbaldehyde (8e)
Using 3-Chlorophenylboronic acid. 1H NMR (400 MHz, CDCl3) δ 9.85 (s, 1H), 8.04 (d, J = 8.7 Hz, 1H), 7.49 – 7.33 (m, 8H), 7.26 (m, 1H), 7.13 – 7.07 (dd, J = 8.3, 2.8 Hz, 1H), 6.96 (d, J = 2.5 Hz, 1H), 5.17 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 190.4, 162.8, 146.8, 139.7, 135.9, 134.5, 130.5, 129.8, 129.7, 128.8, 128.5, 128.4, 128.3, 127.6, 127.5, 116.3, 115.0, 70.4; HRMS m/z: [M + Cl−] for C20H15Cl2O2, calcd, 341.0505; found, 341.0508.
5-(Benzyloxy)-3'-(trifluoromethyl)-[1,1'-biphenyl]-2-carbaldehyde (8f)
Using 3-(Trifluoromethyl)phenylboronic acid. 1H NMR (400 MHz, CDCl3) δ 9.82 (s, 1H), 8.05 (m, 1H), 7.72 (m, 1H), 7.67 – 7.64 (td, J = 1.6, 0.8 Hz, 1H), 7.64 – 7.53 (m, 2H), 7.50 – 7.35 (m, 5H), 7.15 – 7.11 (dd, J = 8.7, 2.2 Hz, 1H), 6.96 (d, J = 2.5 Hz, 1H), 5.19 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 190.4, 163.0, 146.8, 138.8, 135.9, 133.4, 131.0, 130.9, 129.0, 129.0, 128.6, 127.8, 127.6, 126.6, 126.5, 125.2, 116.7, 115.2, 70.6; HRMS m/z: [M + Na+] for C21H15F3O2Na, calcd, 379.0922; found, 379.0926.
5-(Benzyloxy)-4'-(trifluoromethyl)-[1,1'-biphenyl]-2-carbaldehyde (8g)
Using 4-(Trifluoromethyl)phenylboronic acid. 1H NMR (400 MHz, CDCl3) δ 9.84 (s, 1H), 8.06 (d, J = 8.7 Hz, 1H), 7.75 (d, J = 8.0 Hz, 2H), 7.55 – 7.49 (m, 2H), 7.49 – 7.34 (m, 6H), 7.17 – 7.12 (dd, J = 9.1, 2.2 Hz, 1H), 6.98 (d, J = 2.5 Hz, 1H), 5.19 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 190.2, 162.9, 146.7, 141.7, 135.9, 130.8, 130.3, 128.9, 128.6, 127.7, 127.5, 125.5, 125.4, 122.8, 116.6, 115.1, 70.5; HRMS m/z: [M + H+] for C21H16F3O2, calcd, 357.1097; found, 357.1096.
5-(Benzyloxy)-2'-(methylthio)-[1,1'-biphenyl]-2-carbaldehyde (8h)
Using 2-(Methylthio)phenylboronic acid. 1H NMR (400 MHz, CDCl3) δ 9.62 (s, 1H), 8.05 (d, J = 8.7 Hz, 1H), 7.47 – 7.32 (m, 6H), 7.30 – 7.23 (m, 2H), 7.24 – 7.20 (m, 1H), 7.13 – 7.09 (m, 1H), 6.93 – 6.90 (m, 1H), 5.17 (s, 2H), 2.36 (d, J = 1.1 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 190.8, 163., 146.3, 138.4, 136.2, 136.1, 130.4, 129.5, 129.1, 128.8, 128.4, 127.8, 127.7, 124.7, 124.6, 116.4, 115.3, 70.4, 15.6; HRMS m/z: [M + H+] for C21H18O2SNa, calcd, 357.0920; found, 357.0923.
5-(Benzyloxy)-2'-methoxy-[1,1'-biphenyl]-2-carbaldehyde (8i)
Using 2- Methoxyphenylboronic acid. 1H NMR (500 MHz, CDCl3) δ 9.73 (s, 1H), 8.07 (d, J = 8.7 Hz, 1H), 7.48 – 7.39 (m, 5H), 7.37 (d, J = 6.5 Hz, 1H), 7.32 – 7.27 (m, 1H), 7.13 – 7.07 (m, 2H), 7.02 (d, J = 8.3 Hz, 1H), 6.98 – 6.95 (dd, J = 2.4, 1.1 Hz, 1H), 5.15 (s, 2H), 3.75 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 191.5, 163.1, 156.6, 144.5, 136.2, 131.4, 130.1, 129.2, 128.8, 128.4, 127.9, 127.7, 126.8, 121.0, 116.9, 114.5, 110.8, 70.3, 55.5; HRMS m/z: [M + H+] for C21H19O3, calcd, 319.1329; found, 319.1333.
5-(Benzyloxy)-3'-methoxy-[1,1'-biphenyl]-2-carbaldehyde (8j)
Using 3- Methoxyphenylboronic acid. 1H NMR (400 MHz, CDCl3) δ 9.93 (s, 1H), 8.06 (d, J = 9.0 Hz, 1H), 7.52 – 7.35 (m, 6H), 7.10 (d, J = 8.6 Hz, 1H), 7.05 – 6.93 (m, 4H), 5.20 (s, 2H), 3.89 (s, 3H); HRMS m/z: [M + Na+] for C21H18O3Na, calcd, 341.1154; found, 341.1150.
5-(Benzyloxy)-3'-methyl-[1,1'-biphenyl]-2-carbaldehyde (8k)
Using 3-Methylphenylboronic acid. 1H NMR (500 MHz, CDCl3) δ 9.85 (d, J = 0.9 Hz, 1H), 8.03 (d, J = 8.6 Hz, 1H), 7.49 – 7.39 (m, 3H), 7.39 – 7.32 (m, 2H), 7.27 (d, J = 8.1 Hz, 1H), 7.22 – 7.16 (m, 2H), 7.09 – 7.05 (ddd, J = 8.8, 2.6, 0.9 Hz, 1H), 6.98 (d, J = 2.5 Hz, 1H), 5.15 (s, 2H), 2.43 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 191.4, 162.8, 148.9, 138.3, 137.9, 136.2, 130.9, 130.1, 129.2, 128.9, 128.5, 128.5, 127.8, 127.3, 116.3, 114.8, 70.5, 21.7; HRMS m/z: [M + H+] for C21H18O2Na, calcd, 325.1205; found, 325.1217.
5-(Benzyloxy)-3'-(morpholinomethyl)-[1,1'-biphenyl]-2-carbaldehyde (8l)
Using 3-(4- Morpholinomethyl)phenylboronic acid pinacol ester.1H NMR (400 MHz, CDCl3) δ 9.87 (s, 1H), 8.83 (d, J = 8.7 Hz, 1H), 7.47 – 7.31 (m, 7H), 7.32 – 7.24 (m, 1H), 7.12 – 7.04 (dd, J = 8.7, 2.5 Hz, 1H), 7.05 (d, J = 2.5 Hz, 1H), 5.17 (s, 2H), 3.79 – 3.68 (t, J = 4.6 Hz, 4H), 3.56 (s, 3H), 2.49 (d, J = 6.5 Hz, 4H); 13C NMR (100 MHz, CDCl3) δ 191.0, 162.7, 148.5, 138.3, 137.8, 136.0, 130.7, 130.2, 129.1, 128.8, 128.4, 127.6, 127.6, 116.4, 114.5, 70.4, 67.1, 63.2, 53.7; HRMS m/z: [M + Na+] for C25H25NO3Na, calcd, 410.1726; found, 410.1730.
5-(Benzyloxy)-4'-hydroxy-[1,1'-biphenyl]-2-carbaldehyde (8m)
Used 4- Hydroxyphenylboronic acid. Partially purified biaryl phenol was treated with TBSCl (1.2 eq.) and imidazole (3 eq.) in CH2Cl2 and stirred for 2 h at room temperature. After reaction was completed by TLC, the resulting reaction mixture was concentrated. The crude product was purified by column chromatography (SiO2, 4:1, Hex:EtOAc) to afford 8m (94%) as an amorphous solid. 1H NMR (500 MHz, CDCl3) δ 9.89 (s, 1H), 8.03 (d, J = 8.7 Hz, 1H), 7.52 – 7.33 (m, 5H), 7.26 (dd, J = 6.6, 1.8 Hz, 2H), 7.05 (dd, J = 8.7, 2.3 Hz, 1H), 7.02 – 6.93 (m, 3H), 5.17 (s, 2H), 1.05 (s, 9H), 0.29 (s, 6H); 13C NMR (125 MHz, CDCl3) δ 191.2, 162.7, 156.0, 148.4, 136.1, 131.2, 130.6, 130.0, 128.7, 128.3, 127.6, 127.5, 120.0, 116.1, 114.3, 70.3, 25.7, 18.3, 4.3; ESI-HRMS m/z : [M + Na]+ for C26H30NaO3Si, calcd, 441.5899, found 441.5896.
2-(Benzo[d][1,3]dioxol-5-yl)-4-(benzyloxy)benzaldehyde (8n)
Using 3,4- (Methylenedioxy)phenylboronic acid. 1H NMR (500 MHz, CDCl3) δ 9.90 (s, 1H), 8.08 (d, J = 8.7 Hz, 1H), 7.48 – 7.39 (m, 4H), 7.39 – 7.35 (m, 1H), 7.06 (d, J = 8.6 Hz, 1H), 6.97 (d, J = 2.5 Hz, 1H), 6.91 – 6.86 (m, 2H), 6.83 – 6.79 (m, 1H), 6.03 (s, 2H), 5.15 (s, 2H); 13C NMR (125 MHz, CDCl3) δ 191.2, 162.8, 148.2, 147.9, 147.9, 136.1, 131.6, 130.2, 128.8, 128.4, 127.7, 127.6, 124.0, 116.2, 114.5, 110.3, 108.3, 101.5, 70.4; HRMS (FAB) m/z: [M + Na+] for C21H16O4Na, calcd, 355.0941; found, 355.0935.
4-(Benzyloxy)-2-(pyridin-3-yl)benzaldehyde (8o)
1H NMR (400 MHz, CDCl3) δ 9.79 (s, 1H), 8.65 (dd, 2H, J = 5.1, 8.3 Hz), 8.01 (d, 1H, J = 8.8 Hz), 7.67 (m, 1H), 7.48–7.26 (m, 6H), 7.09 (dd, 1H, J = 2.4, 8.7 Hz), 6.93 (d, 1H, J = 2.4 Hz), 5.14 (s, 2H); 13C NMR (125 MHz, CDCl3) δ 187.8, 165.3, 160.5, 135.8, 131.2, 129.0, 128.7, 127.8, 120.0, 109.5, 102.1, 91.0, 70.8; HRMS (FAB) m/z: [M + H+] for C19H16NO2, calcd, 290.1181; found, 290.1177.
4-(Benzyloxy)-2-(pyridin-4-yl)benzaldehyde (8p)
1H NMR (500 MHz, CDCl3) δ 9.82 (s, 1H), 8.67 (d, J = 5.9 Hz, 2H), 8.02 (d, J = 8.7 Hz, 1H), 7.49–7.33 (m, 6H), 7.30 (d, J = 6.0 Hz, 1H), 7.15–7.10 (dd, J = 8.6, 2.6 Hz, 1H), 6.95 (d, J = 2.6 Hz, 1H), 5.15 (s, 2H); 13C NMR (125 MHz, CDCl3) δ 189.7, 162.9, 149.8, 145.8, 145.2, 135.7, 131.0, 128.8, 128.5, 127.6, 127.1, 124.6, 116.3, 115.4, 70.5; HRMS (FAB) m/z: [M + H+] for C19H16NO2, calcd, 290.1181; found, 290.1183.
General procedure for Henry Reaction of compounds 8a–p
(E)-5-(Benzyloxy)-2-(2-nitrovinyl)-1,1'-biphenyl (9a)
Nitromethane (1.4 mL) was added to a mixture of aldehyde 8a (0.16g, 0.56mmol) and ammonium acetate (77mg, 1.0mmol) and heated to 50 °C. Upon completion (~15–30 min), the reaction mixture was cooled to RT and purified without work-up by column chromatography (SiO2, 3:1, Hex:EtOAc) to afford nitrostyrene 9a as a yellow oil (182 mg, 0.55 mmol, 98%). 1H NMR (400 MHz, CDCl3) δ 8.02 (d, J = 13.6 Hz, 1H), 7.64 (d, J = 9.5 Hz, 1H), 7.50 – 7.35 (m, 10H), 7.31(d, J = 2.1 Hz, 2H), 7.04 (d, J = 2.5 Hz, 1H), 5.15 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 161.8, 146.1, 138.1, 136.4, 136.3, 135.5, 131.8 131.7, 129.9, 129.2, 128.8, 128.0, 121.3, 117.3, 116.3, 116.0, 115.6, 70.7; HRMS (FAB) m/z: [M+Na+] for C21H18NO3, calcd, 332.1281; found, 332.1290.
(E)-5-(Benzyloxy)-3'-fluoro-2-(2-nitrovinyl)-1,1'-biphenyl (9b)
1H NMR (400 MHz, CDCl3) δ 8.07 (d, J = 13.5 Hz, 1H), 7.65 (d, J = 8.7 Hz, 1H), 7.49 – 7.35 (m, 7H), 7.20 – 7.13 (ddd, J = 9.3, 7.9, 2.6 Hz, 1H), 7.09 – 7.03 (m, 2H), 7.02 (d, J = 2.8 Hz, 2H), 5.16 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 164.0, 161.5, 145.4, 141.4, 137.6, 136.1, 136.0, 130.5, 130.4, 129.6, 128.6, 127.7, 125.7, 121.0, 116.9, 116.6, 115.6, 115.4, 70.5; HRMS m/z: [M + H+] for C21H17FNO3, calcd, 350.1187; found, 350.1185.
(E)-5-(Benzyloxy)-4'-fluoro-2-(2-nitrovinyl)-1,1'-biphenyl (9c)
1H NMR (400 MHz, CDCl3) δ 8.08 (d, J = 13.6 Hz, 1H), 7.64 (d, J = 8.7 Hz, 1H), 7.50 – 7.34 (m, 6H), 7.32 – 7.24 (m, 2H), 7.23 – 7.14 (t, J = 8.3 Hz, 2H), 7.10 – 7.00 (m, 2H), 5.17 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 161.5, 145.7, 137.8, 136.1, 136.0, 131.5, 131.4, 129.6, 128.9, 128.5, 127.7, 121.0, 117.0, 115.9, 115.7, 115.3, 70.4; HRMS m/z: [M + Na+] for C21H16FNO3Na, calcd, 372.1006; found, 372.1011.
(E)-5-(Benzyloxy)-2'-chloro-2-(2-nitrovinyl)-1,1'-biphenyl (9d)
1H NMR (500 MHz, CDCl3) δ 7.85 – 7.75 (m, 1H), 7.74 – 7.66 (m, 1H), 7.55 (m, 1H), 7.53 – 7.34 (m, 8H), 7.31 (d, J = 5.3 Hz, 1H), 7.17 (d, J = 8.3 Hz, 1H), 7.01 (t, J = 2.0 Hz, 1H), 5.20 – 5.11 (m, 2H); 13C NMR (125 MHz, CDCl3) δ 161.4, 143.8, 137.7, 137.0, 135.9, 133.2, 131.4, 130.0, 130.0, 129.3, 128.7, 128.3, 127.6, 127.1, 123.4, 121.5, 117.1, 115.6, 70.3; HRMS m/z: [M + H+] for C21H17ClNO3, calcd, 366.0892; found, 366.0895.
5-(Benzyloxy)-3'-chloro-2-(2-nitrovinyl)-1,1'-biphenyl (9e)
1H NMR (400 MHz, CDCl3) δ 7.95 (d, J = 13.5 Hz, 1H), 7.64 (d, J = 8.8 Hz, 1H), 7.50 – 7.36 (m, 8H), 7.33 (s, 1H), 7.18 (d, J = 7.0 Hz, 1H), 7.09 – 7.04 (m, 1H), 7.00 (d, J = 2.6 Hz, 1H), 5.17 (s, 2H); 13C NMR (125 MHz, CDCl3) δ 145.1, 141.1, 140.9, 137.4, 136.1, 134.7, 129.9, 129.6, 129.6, 129.5, 129.0, 128.8, 128.5, 128.4, 128,0, 127.6, 120.9, 116.9, 115.5, 109.9, 70.4; HRMS m/z: [M + Cl−] for C21H16Cl2NO3, calcd, 400.0513; found, 400.0505.
(E)-5-(Benzyloxy)-2-(2-nitrovinyl)-3'-(trifluoromethyl)-1,1'-biphenyl (9f)
1H NMR (400 MHz, CDCl3) δ 7.90 (d, J = 13.5 Hz, 1H), 7.78 – 7.70 (m, 1H), 7.69 – 7.55 (m, 3H), 7.51 – 7.34 (m, 7H), 7.13 – 7.05 (dd, J = 8.8, 2.6 Hz, 1H), 7.02 (d, J = 2.6 Hz, 1H), 5.17 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 161.6, 155.7, 152.1, 145.1, 140.6, 140.0, 137.2, 136.4, 136.0, 133.2, 129.7, 129.3, 129.0, 128.6, 127.7, 121.0, 117.1, 115.8, 70.6; HRMS m/z: [M + H+] for C22H17F3NO3, calcd, 400.1161; found, 400.1157.
(E)-5-(Benzyloxy)-2-(2-nitrovinyl)-4'-(trifluoromethyl)-1,1'-biphenyl (9g)
Pushed through plug of SiO2. TS1-189: 1H NMR (400 MHz, CDCl3) δ 7.98 – 7.90 (m, 1H), 7.80 (d, J = 8.0 Hz, 2H), 7.68 (d, J = 8.8 Hz, 1H), 7.52 – 7.37 (m, 8H), 7.11 (d, J = 8.8 Hz, 1H), 7.04 (s, 1H), 5.19 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 161.4, 147.8, 144.9, 144.3, 139.8, 138.6, 137.1, 136.4, 135.8, 133.5, 131.2, 129.5, 129.1, 128.8, 128.5, 127.6, 124.2, 120.8, 120.4, 117.0, 115.6, 70.4; HRMS m/z: [M + H+] for C22H17F3NO3, calcd, 400.1155; found, 400.1151.
(E)-(5'-(Benzyloxy)-2'-(2-nitrovinyl)-[1,1'-biphenyl]-2-yl)(methyl)sulfane (9h)
1H NMR (400 MHz, CDCl3) δ 7.71 (d, J = 13.6 Hz, 1H), 7.62 (d, J = 8.6 Hz, 1H), 7.45 – 7.31 (m, 7H), 7.31 – 7.29 (m, 1H), 7.25 – 7.19 (t, J = 7.2 Hz, 1H), 7.13 – 6.99 (m, 2H), 6.95 (d, J = 2.8 Hz, 1H), 5.09 (s, 2H), 2.35 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 161.5, 144.9, 138.0, 137.5, 137.2, 136.1, 135.7, 130.0, 129.4, 129.3, 128.8, 128.4, 127.7, 125.0, 124.9, 121.6, 117.0, 115.8, 70.3, 15.6; HRMS m/z: [M + K+] for C22H19NO3SK, calcd, 416.0718; found, 416.0756.
(E)-5-(Benzyloxy)-2'-methoxy-2-(2-nitrovinyl)-1,1'-biphenyl (9i)
1H NMR (500 MHz, CDCl3) δ 7.86 (d, J = 13.8 Hz, 1H), 7.65 (d, J = 8.7 Hz, 1H), 7.57 – 7.34 (m, 7H), 7.24 – 7.17 (m, 1H), 7.16 – 6.99 (m, 4H), 5.15 (s, 2H), 3.74 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 161.6, 156.4, 143.7, 138.8, 136.3, 135.3, 131.4, 130.4, 128.9, 128.4, 127.7, 122.0, 121.1, 117.5, 115.1, 111.4, 70.4, 55.6; HRMS m/z: [M + H+] for C22H19NO4, calcd, 362.1387; found, 362.1389.
(E)-5-(Benzyloxy)-3'-methoxy-2-(2-nitrovinyl)-1,1'-biphenyl (9j)
1H NMR (500 MHz, CDCl3) δ 8.04 (d, J = 13.6 Hz, 1H), 7.62 (d, J = 9.5 Hz, 1H), 7.46 – 7.37 (m, 6H), 7.07 – 7.02 (m, 3H), 7.02 – 6.97 (ddd, J = 8.2, 2.6, 0.9 Hz, 1H), 6.88 – 6.84 (m, 1H), 6.84 – 6.80 (dd, J = 2.6, 1.6 Hz, 1H), 5.15 (s, 2H), 3.85 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 161.5, 159.8, 146.8, 140.6, 138.2, 136.2, 135.9, 129.9, 129.5, 129.0, 128.6, 127.7, 122.3, 121.1, 116.8, 115.4, 115.4, 114.1, 70.5, 55.6; HRMS m/z: [M + Na+] for C22H19NO4Na, 384.1212; found, 384.1218.
(E)-5-(Benzyloxy)-3'-methyl-2-(2-nitrovinyl)-1,1'-biphenyl (9k)
1H NMR (500 MHz, CDCl3) δ 8.01 (d, J = 13.6 Hz, 1H), 7.62 (m, 1H), 7.48 – 7.39 (m, 7H), 7.39 – 7.33 (t, J = 7.7 Hz, 1H), 7.14 – 7.07 (m, 2H), 7.05 – 6.99 (m, 2H), 5.15 (s, 2H), 2.43 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 138.4, 135.8, 130.4, 129.5, 129.3, 128.9, 128.7, 128.5, 127.8, 127.8, 126.9, 121.1, 116.8, 115.3, 77.5, 77.4, 77.2, 77.0, 70.5 21.7; HRMS m/z: [M + Na+] for C22H19NO3Nalcd, 368.1263; found, 368.1257.
(E)-4-((5'-(Benzyloxy)-2'-(2-nitrovinyl)-[1,1'-biphenyl]-3-yl)methyl)morpholine (9l)
1H NMR (400 MHz, CDCl3) δ 7.98 (d, J = 13.6 Hz, 1H), 7.63 (d, J = 9.5 Hz, 1H), 7.48 – 7.33 (m, 8H), 7.33 (d, J = 1.7 Hz, 1H), 7.23 – 7.20 (dd, J = 6.7, 1.8 Hz, 1H), 7.08 – 6.99 (m, 2H), 5.15 (d, J = 1.6 Hz, 2H), 3.79 – 3.67 (t, J = 4.1 Hz, 4H), 3.56 (s, 2H), 2.55 – 2.40 (dd, J = 5.7, 3.4 Hz, 4H); 13C NMR (100 MHz, CDCl3) δ 161.5, 146.9, 139.2, 138.5, 138.1, 136.1, 135.8, 130.6, 129.5, 129.3, 128.9, 128.8, 128.5, 128.4, 127.7, 121.0, 116.9, 115.1, 70.4, 67.1, 63.3, 53.8; HRMS m/z: [M + H+] for C26H27N2O4, calcd, 431.1971; found, 431.1974.
(E)-((5'-(Benzyloxy)-2'-(2-nitrovinyl)-[1,1'-biphenyl]-4-yl)oxy)(tert-butyl)dimethylsilane (9m)
1H NMR (400 MHz, CDCl3) δ 8.03 (d, J = 13.7 Hz, 1H), 7.61 (d, J = 8.3 Hz, 1H), 7.49 – 7.33 (m, 6H), 7.17 (d, J = 8.4 Hz, 2H), 7.02 (s, 2H), 6.95 (d, J = 8.5 Hz, 2H), 5.15 (s, 2H), 1.04 (s, 9H), 0.30 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 161.5, 156.2, 146.8, 138.5, 136.2, 135.8, 132.2, 131.0, 129.6, 128.9, 128.5, 127.7, 121.1, 120.4, 116.8, 115.0, 70.4, 25.9, 18.4, −4.1; HRMS (FAB) m/z: [M + Na+] for C27H31NO4SiNa, calcd, 484.1914; found, 484.1936.
(E)-5-(5-(Benzyloxy)-2-(2-nitrovinyl)phenyl)benzo[d][1,3]dioxole (9n)
1H NMR (400 MHz, CDCl3) δ 8.03 (d, J = 13.6 Hz, 1H), 7.59 (d, J = 8.0 Hz, 1H), 7.50 – 7.33 (m, 6H), 7.05 – 6.98 (m, 2H), 6.92 – 6.85 (m, 1H), 6.79 (s, 1H), 6.71(d, J = 7.9 Hz, 1H), 6.03 (s, 2H), 5.17 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 161.4, 148.0, 147.9, 146.5, 138.1, 136.1, 135.7, 132.9, 129.5, 128.8, 128.4, 127.6, 123.6, 121.0, 116.7, 115.0, 109.9, 108.5, 101.5, 70.3; HRMS (FAB) m/z: [M + H+] for C22H18NO5, calcd, 376.1185; found, 376.1160.
(E)-3-(5-(Benzyloxy)-2-(2-nitrovinyl)phenyl)pyridine (9o)
1H NMR (400 MHz, CDCl3) δ 8.70 (dd, J = 4.8, 1.6 Hz, 1H), 8.59 (d, J = 1.6 Hz, 1H), 7.89 (d, J = 13.5 Hz, 1H), 7.68 – 7.60 (m, 2H), 7.47 – 7.32 (m, 8H), 7.12 – 7.06 (dd, J = 8.7, 2.5 Hz, 1H), 7.00 (d, J = 2.6 Hz, 1H), 5.15 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 161.5, 149.9, 149.6, 142.8, 136.9, 136.8, 136.3, 135.8, 134.8, 129.7, 128.8, 128.5, 127.6, 123.4, 121.1, 117.1, 115.8, 70.4; HRMS (FAB) m/z: [M + Na+] for C20H17N2O3, 333.1239; found, 333.1234.
(E)-4-(5-(Benzyloxy)-2-(2-nitrovinyl)phenyl)pyridine (9p)
1H NMR (500 MHz, CDCl3) δ 8.74 (dd, 2H, J = 1.6, 4.4 Hz), 7.91 (d, 1H, J = 13.6 Hz), 7.67 (d, 1H, J = 8.8 Hz), 7.48 (d, 1H, J = 13.4 Hz), 7.41 (m, 5H), 7.25 (dd, 2H, J = 1.6, 4.4 Hz), 7.11 (dd, 1H, J = 2.6, 8.7 Hz), 7.01 (d, 1H, J = 2.5 Hz), 5.17 (s, 2H); 13C NMR (125 MHz, CDCl3) δ 161.2, 150.2, 147.0, 143.7, 136.7, 136.6, 135.8, 128.9, 127.6, 124.5, 120.7, 116.8, 116.1, 70.6; ESI-HRMS m/z calculated for C20H17N2O3 [M + H]+ 333.1239, found 333.1249.
General procedure for preparation of 10a–p from 9a–p
N-(2-(5-(Benzyloxy)-[1,1'-biphenyl]-2-yl)ethyl)acetamide (10a)
Nitrostyrene 9a (182 mg, 0.55 mmol) in THF (0.7 mL) was added dropwise to a solution of Lithiumaluminium hydride (42 mg, 1.12 mmol) in THF (2 mL) under organ atmosphere at RT. Upon completion (nearly immediately) the reaction was quenched by the addition of water (42 μL), 3M NaOH (42 μL), and water (84 μL). The resulted mixture was filtered through a plug of celite, washed with CH2Cl2, and dried over K2CO3. Upon filtration the mixture was concentrated to oil and used without further purification. Acetic anhydride (58 μL, 0.62 mmol) and triethylamine (93 μL, 0.67 mmol) were added to a solution of the crude amine in CH2Cl2 (5.6 mL) under an organ atmosphere at RT. After 3 h the reaction was quenched with saturated aqueous ammonium chloride and extracted with CH2Cl2 (3 × 10 mL); combined organic fractions were washed with saturated aqueous sodiumchloride, dried over Na2SO4, filtered and concentrated. The residue was purified by column chromatography (SiO2; 3:1, Hex:EtOAc) to afford acetamide 10a (0.12 g, 0.35 mmol, 64%). 1H NMR (400 MHz, CDCl3) δ 7.50 – 7.38 (m, 8H), 7.38 – 7.30 (m, 2H), 7.23 (d, J = 8.4 Hz, 1H), 7.01 – 6.95 (dd, J = 8.4, 2.7 Hz, 1H), 6.93 (d, J = 2.7 Hz, 1H), 5.71 (br s, NH), 5.08 (s, 2H), 3.42 – 3.16 (q, J = 7.0 Hz, 2H), 2.89 – 2.64 (t, J = 7.2 Hz, 2H), 1.85 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 170.2, 157.2, 143.4, 141.4, 137.0, 130.8, 129.1, 128.7, 128.6, 128.4, 128.0, 127.6, 127.2, 116.6, 114.2, 70.1, 40.7, 31.9, 23.2; HRMS m/z: [M + K+] for C23H23NO2K calcd, 384.1361; found, 384.1359.
N-(2-(5-(Benzyloxy)-3'-fluoro-[1,1'-biphenyl]-2-yl)ethyl)acetamide (10b)
1H NMR (400 MHz, CDCl3) δ 7.48 – 7.30 (m, 6H), 7.24 – 7.18 (d, J = 8.4 Hz, 1H), 7.12 – 7.04 (m, 2H), 7.04 – 6.92 (ddd, J = 18.6, 8.2, 2.5 Hz, 2H), 6.85 (d, J = 2.7 Hz, 1H), 5.34 (br s, NH), 5.05 (s, 2H), 3.32 – 3.21 (q, J = 6.4, 5.9 Hz, 2H), 2.79 – 2.68 (t, J = 7.1 Hz, 2H), 1.86 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 170.3, 157.3, 143.7, 142.2, 136.9, 131.0, 130.1, 123.0, 128.8, 128.6, 128.2, 127.7, 125.0, 116.5, 116.4, 114.6, 114.4, 70.2, 40.8, 32.0, 23.3; HRMS m/z: [M + H+] for C23H23FNO2, calcd, 364.1713; found, 364.1705.
N-(2-(5-(Benzyloxy)-4'-fluoro-[1,1'-biphenyl]-2-yl)ethyl)acetamide (10c)
1H NMR (400 MHz, CDCl3) δ 7.44 – 7.31 (m, 6H), 7.27 – 7.22 (dd, J = 8.4, 5.5 Hz, 1H), 7.21 – 7.17 (d, J = 8.4 Hz, 1H), 7.12 – 7.05 (m, 3H), 6.96 – 6.91 (dd, J = 8.3, 3.0 Hz, 1H), 5.83 (br s, NH), 5.05 (s, 2H), 3.33 – 3.15 (q, J = 6.7 Hz, 2H), 2.78 – 2.66 (t, J = 7.2 Hz, 2H), 1.87 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 170.5, 157.3, 142.4, 137.0, 130.9, 130.8, 130.7, 128.7, 128.7, 128.2, 127.7, 116.8, 115.5, 115.3, 114.3, 70.2, 40.8, 32.0, 23.1; HRMS m/z: [M + Na+] for C23H22FNO2Na, calcd, 386.1527; found, 386.1529.
N-(2-(5-(Benzyloxy)-2'-chloro-[1,1'-biphenyl]-2-yl)ethyl)acetamide (10d)
1H NMR (500 MHz, CDCl3) δ 7.52 – 7.45 (m, 1H), 7.45 – 7.40 (m, 2H), 7.40 – 7.35 (m, 3H), 7.35 – 7.29 (m, 3H), 7.25 – 7.21 (m, 1H), 7.05 – 6.95 (dd, J = 8.5, 2.8 Hz, 1H), 6.82 (d, J = 2.7 Hz, 1H), 5.93 (d, J = 5.4 Hz, 1H), 5.05 (s, 2H), 3.36 – 3.19 (ddq, J = 19.3, 13.0, 6.1 Hz, 2H), 2.67 – 2.49 (m, 2H), 1.93 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 175.7, 171.0, 157.1, 140.4, 139.8, 136.9, 133.1, 131.3, 130.4, 129.6, 129.0, 128.6, 128.0, 127.6, 126.8, 116.4, 114.9, 70.1, 40.3, 31.8, 22.9; HRMS m/z: [M + H+] for C23H23ClNO2, calcd, 380.1417; found, 380.1415.
N-(2-(5-(Benzyloxy)-3'-chloro-[1,1'-biphenyl]-2-yl)ethyl)acetamide (10e)
1H NMR (500 MHz, CDCl3) δ 7.47 – 7.28 (m, 8H), 7.25 – 7.17 (m, 2H), 6.99 – 6.92 (dd, J = 8.5, 2.7 Hz, 1H), 6.84 (d, J = 2.8 Hz, 1H), 5.46 (br s, NH), 5.06 (s, 2H), 3.34 – 3.25 (m, 2H), 2.83 – 2.68 (t, J = 7.3 Hz, 2H), 2.03 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 171.6, 157.5, 143.2, 142.1, 136.9, 134.3, 131.1, 129.9 129.3, 128.8, 128.3, 127.7, 127.6, 127.5, 116.7, 114.8, 70.3, 46.1, 41.3, 31.7, 22.5, 8.8; HRMS m/z: [M + H+] for C23H23ClNO2, calcd, 380.1412; found, 380.1414.
N-(2-(5-(Benzyloxy)-3'-(trifluoromethyl)-[1,1'-biphenyl]-2-yl)ethyl)acetamide (10f)
1H NMR (400 MHz, CDCl3) δ 7.64 (d, J = 7.7 Hz, 1H), 7.59 – 7.54 (m, 2H), 7.55 – 7.49 (t, J = 7.3 Hz, 1H), 7.47 – 7.32 (m, 5H), 7.24 (d, J = 8.5 Hz, 1H), 7.01 – 6.96 (dd, J = 8.5, 2.7 Hz, 1H), 6.87 (d, J = 2.7 Hz, 1H), 5.90 (br s, NH), 5.06 (s, 2H), 3.34 – 3.23 (q, J = 6.9 Hz, 2H), 2.79 – 2.68 (t, J = 7.3 Hz, 2H), 1.99 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 170.7, 157.4, 142.2, 141.9, 136.9, 132.6, 131.1, 129.0, 128.8, 128.5, 128.2, 127.7, 124.2, 116.7, 114.8, 70.3, 40.8 31.9, 23.0; HRMS m/z: [M + H+] for C24H23F3NO2, calcd, 414.1676; found, 414.1681.
N-(2-(5-(Benzyloxy)-4'-(trifluoromethyl)-[1,1'-biphenyl]-2-yl)ethyl)acetamide (10g)
1H NMR (400 MHz, CDCl3) δ 7.66 (d, J = 8.1 Hz, 2H), 7.46 – 7.23 (m, 8H), 6.99 – 6.94 (dd, J = 8.5, 2.7 Hz, 1H), 6.84 (d, J = 2.7 Hz, 1H), 6.03 (t, J = 5.5 Hz, 1H), 5.06 (s, 2H), 3.33 – 3.19 (dd, J = 14.3, 6.4 Hz, 2H), 2.76 – 2.68 (dd, J = 8.3, 6.6 Hz, 2H), 1.85 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 170.3, 157.1, 145.1, 141.8, 136.8, 130.9, 129.5, 129.1, 128.6, 128.6, 127.5, 125.6, 125.2, 125.2, 122.9, 116.4, 114.6, 70.1, 40.6, 31.9; HRMS m/z: [M + Na+] for C24H22F3NO2Na, calcd, 436.1495; found, 436.1489.
N-(2-(5-(Benzyloxy)-2'-(methylthio)-[1,1'-biphenyl]-2-yl)ethyl)acetamide (10h)
1H NMR (400 MHz, CDCl3) δ 7.48 – 7.30 (m, 7H), 7.28 – 7.18 (m, 2H), 7.14 (s, 1H), 7.03 – 6.98 (ddd, J = 8.5, 2.8, 1.0 Hz, 1H), 6.87 – 6.83 (m, 1H), 5.63 (br s, NH), 5.05 (s, 2H), 3.43 – 3.16 (ddt, J = 42.5, 13.3, 6.6 Hz, 2H), 2.66 – 2.52 (t, J = 6.7 Hz, 2H), 2.39 (d, J = 1.0 Hz, 3H), 1.84 (d, J = 1.0 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 170.3, 157.3, 141.1, 139.1, 137.6, 137.0, 130.6, 129.8, 129.4, 128.7, 128.4, 128.1, 127.7, 124.5, 124.0, 116.5, 115.2, 70.2, 40.1, 31.7, 23.3, 15.2; HRMS m/z: [M + Na+] for C24H25NO2SNa, calcd, 414.1504; found, 414.1509.
N-(2-(5-(Benzyloxy)-2'-methoxy-[1,1'-biphenyl]-2-yl)ethyl)acetamide (10i)
1H NMR (400 MHz, CDCl3) δ 7.47 – 7.30 (m, 5H), 7.22 (d, J = 8.5 Hz, 1H), 7.17 – 7.13 (dd, J = 7.4, 1.9 Hz, 1H), 7.07 – 6.95 (m, 4H), 6.85 (d, J = 2.7 Hz, 1H), 5.51 (br s, NH), 5.07 (s, 2H), 3.77 (s, 3H), 3.44 – 3.18 (m, 2H), 2.68 – 2.56 (td, J = 6.8, 3.7 Hz, 2H), 1.86 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 170.0, 157.2, 156.4, 139.9, 137.1, 131.2, 130.1, 129.2, 128.7, 128.1, 127.8, 120.9, 116.8, 114.4, 111.2, 70.1, 55.8, 40.4, 31.9, 23.5; HRMS m/z: [M + H+] for C24H26NO3, calcd, 376.1913; found, 376.1902.
N-(2-(5-(Benzyloxy)-3'-methoxy-[1,1'-biphenyl]-2-yl)ethyl)acetamide (10j)
1H NMR (400 MHz, CDCl3) δ 7.48 – 7.36 (m, 4H), 7.36 – 7.30 (m, 3H), 7.21 (d, J = 8.4 Hz, 1H), 6.98 – 6.92 (m, 1H), 6.92 – 6.82 (m, 3H), 5.49 (br s, NH), 5.06 (s, 2H), 3.85 (s, 3H), 3.34 – 3.22 (q, J = 6.6, 6.2 Hz, 2H), 2.85 – 2.68 (t, J = 7.2 Hz, 2H), 1.85 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 170.1, 159.5, 157.2, 143.3, 142.9, 137.0, 130.8, 129.5, 128.7, 128.1, 128.1, 127.7, 121.6, 116.5, 114.9, 114.3, 112.7, 70.17, 55.4, 40.8, 32.0, 23.3; HRMS m/z: [M + H+] for C24H25NO3Na, calcd, 398.1732; found, 398.1725.
N-(2-(5-(Benzyloxy)-3'-methyl-[1,1'-biphenyl]-2-yl)ethyl)acetamide (10k)
1H NMR (400 MHz, CDCl3) δ 7.45 (m, 3H), 7.40 (m, 3H), 7.37 – 7.30 (q, J = 7.7, 7.1 Hz, 1H), 7.21 (d, J = 1.4 Hz, 1H), 7.15 – 7.10 (m, 2H), 6.96 (d, J = 8.1 Hz, 1H), 6.90 (s, 1H), 5.51 (br s, NH), 5.08 (s, 2H), 3.34 – 3.24 (q, J = 6.5 Hz, 2H), 2.83 – 2.71 (t, J = 7.0 Hz, 2H), 2.41 (s, 3H), 1.84 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 170.0, 157.2, 143.5, 141.4, 138.0, 137.0, 130.7, 129.9, 128.7, 128.7, 128.3, 128.1, 128.0, 127.6, 126.2, 116.5, 114.2, 70.1, 40.8, 31.9, 23.3, 21.6; ESI-HRMS m/z calculated for C24H25NO2Na [M + Na] 382.1777, found 382.1770.
N-(2-(5-(Benzyloxy)-3'-(morpholinomethyl)-[1,1'-biphenyl]-2-yl)ethyl)acetamide (10l)
1H NMR (400 MHz, CDCl3) δ 7.47 – 7.30 (m, 7H), 7.28 (s, 1H), 7.24 – 7.18 (m, 2H), 6.98 – 6.93 (dd, J = 8.4, 2.8 Hz, 1H), 6.89 (d, J = 2.7 Hz, 1H), 5.40 (s, 1H), 5.05 (s, 2H), 3.75 – 3.69 (t, J = 4.7 Hz, 4H), 3.55 (s, 2H), 3.36 – 3.22 (q, J = 6.9 Hz, 2H), 2.80 – 2.68 (t, J = 7.1 Hz, 2H), 2.47 (m, 4H), 1.85 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 170.0, 157.3, 143.4, 141.5, 138.0, 137.1, 130.9, 123.0, 128.7, 128.7, 128.4, 128.2, 128.2, 128.0, 127.7, 116.8, 114.1, 70.2, 67.1, 63.5, 53.8, 40.6, 32.1, 23.4; HRMS m/z: [M + H+] for C28H33N2O3, calcd, 445.2491; found, 445.2494.
N-(2-(5-(Benzyloxy)-4'-((tert-butyldimethylsilyl)oxy)-[1,1'-biphenyl]-2-yl)ethyl)acetamide (10m)
1H NMR (500 MHz, CDCl3) δ 7.44 (d, J = 7.5 Hz, 3H), 7.42 – 7.36 (dt, J = 10.5, 5.7 Hz, 3H), 7.36 – 7.31 (m, 1H), 7.21 – 7.14 (m, 3H), 6.94 – 6.86 (m, 2H), 5.08 (s, 2H), 3.34 – 3.23 (q, J = 6.7 Hz, 2H), 2.75 (t, J = 7.1 Hz, 2H), 1.74 (s, 3H), 1.97 (s, 9H), 0.25 (s, 6H); 13C NMR (125 MHz, CDCl3) δ 169.9, 157.3, 155.0, 143.3, 137.2, 134.5, 130.8, 130.2, 128.7, 128.1, 127.7, 120.0, 116.8, 114.0, 70.2, 53.6, 40.7, 32.1, 25.8, 23.4, 18.4, −4.2; HRMS (FAB) m/z: [M + Na+] for C29H37NO3SiNa, calcd, 498.2440; found, 498.2447.
N-(2-(Benzo[d][1,3]dioxol-5-yl)-4-(benzyloxy)phenethyl)acetamide (10n)
1H NMR (400 MHz, CDCl3) δ 7.49 – 7.36 (m, 5H), 7.34 (d, J = 4.4 Hz, 1H), 7.20 (d, J = 8.3 Hz, 1H), 6.96 – 6.89 (dd, J = 8.4, 2.8 Hz, 1H), 6.90 – 6.84 (m, 2H), 6.81 – 6.73 (m, 1H), 6.00 (s, 2H), 5.69 – 5.60 (t, J = 5.8 Hz, 1H), 5.06 (s, 2H), 3.42 – 3.16 (m, 2H), 2.93 – 2.68 (t, J = 7.3 Hz, 2H), 1.87 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 170.4, 157.2, 147.5, 146.8, 143.0, 137.0, 135.2, 130.8, 129.3, 128.8, 128.1, 127.6, 123.2, 122.4, 116.7, 114.1, 109.7, 108.3, 101.2, 70.1, 40.7, 31.9, 23.2; HRMS (FAB) m/z: [M + Na+] for C24H23NO4Na, 412.1519; found, 412.1524.
N-(4-(Benzyloxy)-2-(pyridin-3-yl)phenethyl)acetamide (10o)
1H NMR (400 MHz, CDCl3) δ 8.69 – 8.52 (dd, J = 18.2, 4.0 Hz, 2H), 7.71 – 7.63 (dt, J = 7.8, 2.0 Hz, 1H), 7.49 – 7.31 (m, 7H), 7.06 – 6.97 (dd, J = 8.5, 2.8 Hz, 1H), 6.84 (d, J = 2.8 Hz, 1H), 5.06 (s, 2H), 3.36 – 3.20 (q, J = 6.5 Hz, 2H), 2.78 – 2.67 (dd, J = 8.1, 6.6 Hz, 2H), 1.90 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 170.1, 157.5, 149.6, 148.5, 139.5, 136.9, 131.2, 129.0, 128.8, 128.3, 127.7, 123.5, 116.9, 115.0, 70.3, 40.7, 32.2, 23.5; HRMS (FAB) m/z: [M + H+] for C22H23N2O2, calcd, 347.1759; found, 347.1754.
N-(4-(Benzyloxy)-2-(pyridin-4-yl)phenethyl)acetamide (10p)
1H NMR (400 MHz, CDCl3) δ 8.66 (d, J = 5.1 Hz, 2H), 7.46 – 7.39 (m, 5H), 7.36 (s, 1H), 7.30 (s, 2H), 7.06 – 7.01 (m, 1H), 6.84 (d, J = 2.7 Hz, 1H), 5.94 (d, J = 4.8 Hz, 1H), 5.09 (s, 2H), 3.35 – 3.23 (dd, J = 14.5, 6.4 Hz, 2H), 2.74 (t, J = 7.5 Hz, 2H), 1.90 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 171.4, 158.1, 156.3, 137.2, 132.3, 132.2, 130.8, 128.7, 128.5, 129.7, 127.5, 117.9, 106.2, 103.0, 69.9, 41.1, 29.7, 29.6, 23.1; HRMS (FAB) m/z: [M + Na+] for C22H22N2O2Na, calcd, 369.1579; found, 369.1573.
General hydrogenolysis procedure for compounds 10a–p
N-(2-(5-Hydroxy-[1,1'-biphenyl]-2-yl)ethyl)acetamide (11a)
Palladium on carbon (10%, 5 mg) was added to 10a (120 mg, 0.35 mmol) in degassed MeOH (3.5 mL) and the solution was placed under an atmosphere of H2. After 12 h, the solution was diluted with CH2Cl2 and filtered through Celite. The eluent was concentrated to afford a yellow solid, which was purified by column chromatography (SiO2, 100:5, CH2Cl2:MeOH) to afford phenol 11a (64 mg, 0.25mmol, 79%) as a pale yellow amorphous solid. 1H NMR (400 MHz, CDCl3) δ 7.25 – 7.14 (m, 5H), 7.11 – 7.05 (m, 1H), 6.90 (d, J = 8.3 Hz, 1H), 6.64 (d, J = 8.3 Hz, 1H), 6.59 (d, J = 2.5 Hz, 1H), 5.61 (t, J = 5.5 Hz, 1H), 3.12 – 3.02 (m, 2H), 2.55 (t, J = 7.1 Hz, 2H), 1.66 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 171.2, 155.2, 143.4, 141.6, 130.8, 129.1, 128.4, 127.2, 127.2, 117.4, 115.0, 41.1, 31.8, 23.2; HRMS m/z: [M + Na+] for C16H17NO2Na, calcd, 278.1151; found, 278.1155.
N-(2-(3'-Fluoro-5-hydroxy-[1,1'-biphenyl]-2-yl)ethyl)acetamide (11b)
1H NMR (500 MHz, MeOD) δ 7.88 (s, 1H), 7.39 (d, J = 7.5 Hz, 1H), 7.16 – 6.99 (m, 4H), 6.77 (d, J = 8.1 Hz, 1H), 6.62 (d, J = 2.6 Hz, 1H), 3.15 (t, J = 6.6 Hz, 2H), 2.66 (t, J = 7.4 Hz, 2H), 1.80 (s, 3H); 13C NMR (125 MHz, MeOD) δ 173.1, 164.8, 162.9, 156.7, 145.5, 143.3, 132.0, 131.0, 128.3, 126.1, 117.6, 115.9, 114.7, 41.8, 32.8, 22.5; HRMS m/z: [M + Na+] for C16H16FNO2Na, calcd, 296.1063; found, 296.1059.
N-(2-(4'-Fluoro-5-hydroxy-[1,1'-biphenyl]-2-yl)ethyl)acetamide (11c)
1H NMR (400 MHz, MeOD) δ 7.26 – 7.20 (m, 2H), 7.11 – 7.03 (m, 3H), 6.71 (dd, J = 8.3, 2.5 Hz, 1H), 6.56 (d, J = 2.5 Hz, 1H), 3.07 (t, J = 7.6 Hz, 2H), 2.60 (t, J = 7.6 Hz, 2H), 1.78 (s, 3H); 13C NMR (100 MHz, MeOD) δ 173.0, 156.7, 143.5, 139.2, 131.9, 131.9, 131.8, 128.5, 117.8, 116.0, 115.8, 115.7, 41.8, 32.9, 22.5; HRMS m/z: [M + Na+] for C16H16FNO2Na, calcd, 296.1063; found, 296.1065.
N-(2-(2'-Chloro-5-hydroxy-[1,1'-biphenyl]-2-yl)ethyl)acetamide (11d)
1H NMR (400 MHz, CDCl3) δ 8.37 (br s, OH), 7.45 – 7.39 (m, 1H), 7.32 – 7.24 (m, 2H), 7.21 – 7.15 (m, 1H), 7.09 (d, J = 8.3 Hz, 1H), 6.85 (dd, J = 8.3, 2.5 Hz, 1H), 6.68 (d, J = 2.6 Hz, 1H), 5.62 (s, 1H), 3.40 – 3.14 (m, 2H), 2.63 – 2.44 (dd, J = 7.1, 5.1 Hz, 2H), 1.86 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 171.1, 155.1, 140.5, 140.0, 133.2, 131.4, 130.5, 129.7, 129.0, 127.7, 126.9, 117.3, 115.7, 40.5, 31.8, 23.3; HRMS m/z: [M + H+] for C16H17ClNO2, 290.0948; found, 290.0941.
N-(2-(3'-Chloro-5-hydroxy-[1,1'-biphenyl]-2-yl)ethyl)acetamide (11e)
1H NMR (500 MHz, CDCl3) δ 7.40–7.09 (m, 5H), 6.83 – 6.76 (dq, J = 8.1, 4.9, 3.8 Hz, 1H), 6.76 – 6.67 (dd, J = 18.3, 2.7 Hz, 1H), 3.34 – 3.23 (p, J = 6.6 Hz, 2H), 2.77 – 2.64 (dt, J = 14.3, 7.2 Hz, 2H), 1.76 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 170.8, 154.9, 143.6, 141.6, 131.0, 130.9, 129.7, 129.2, 128.5, 127.5, 117.4, 115.5, 115.0, 41.0, 32.0, 23.4; HRMS m/z: [M + Na+] for C16H16ClNO2Na, calcd, 312.0762; found, 312.0788.
N-(2-(5-Hydroxy-3'-(trifluoromethyl)-[1,1'-biphenyl]-2-yl)ethyl)acetamide (11f)
1H NMR (400 MHz, CDCl3) δ 7.64 – 7.39 (m, 4H), 7.07 (s, 1H), 6.82 (s, 1H), 6.73 (s, 1H), 6.00 (s, 1H), 3.34 – 3.18 (q, J = 6.8 Hz, 2H), 2.66 (t, J = 7.0 Hz, 2H), 1.87 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 171.3, 155.4, 142.4, 141.8, 132.6, 131.0, 130.8, 128.9, 126.9, 125.8, 125.8, 124.0, 117.3, 115.6, 60.7, 41.0, 21.2; HRMS m/z: [M + Na+] for C17H16F3NO2Na, calcd, 346.1031; found, 346.1040.
N-(2-(5-Hydroxy-4'-(trifluoromethyl)-[1,1'-biphenyl]-2-yl)ethyl)acetamide (11g)
1H NMR (400 MHz, CDCl3) δ 7.54 (d, 2H, J = 8.0 Hz), 7.31 (d, 2H, J = 8.0 Hz), 7.03 (d, 1H, J = 8.3 Hz), 6.72 (dd, 1H, J = 2.5, 8.3 Hz), 6.59 (d, 1H, J = 2.5 Hz), 4.09 (br s, 2H), 3.10 (t, J = 7.5 Hz, 2H), 2.56 (t, 2H, J = 7.5 Hz), 1.76 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 170.5, 155.1, 146.3, 141.7, 130.8, 129.5, 127.1, 125.1 (q, J = 4.2 Hz), 116.9, 116.5, 115.3, 45.6, 40.6, 23.0; HRMS m/z: [M + H+] for C17H16F3NO2Na, calcd, 346.1031; found, 346.1025.
N-(2-(5-Hydroxy-2'-(methylthio)-[1,1'-biphenyl]-2-yl)ethyl)acetamide (11h)
1H NMR (500 MHz, CDCl3) δ 7.40 – 7.34 (m, 1H), 7.25 – 7.14 (m, 3H), 7.12 – 7.07 (m, 1H), 6.86 – 6.82 (dd, J = 8.4, 2.7 Hz, 1H), 6.68 (d, J = 2.7 Hz, 1H), 5.51 (br s, NH), 3.42 – 3.16 (m, 2H), 2.55 (t, J = 6.8 Hz, 2H), 2.37 (s, 3H), 1.85 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 170.5, 154.5, 141.2, 139.1, 137.6, 130.7, 123.0, 128.8, 128.5, 124.6, 124.0, 117.3, 115.6, 40.2, 31.6, 23.4, 15.2; HRMS m/z: [M + Na+] for C17H19NO2SNa, calcd, 324.1034; found, 324.1035.
N-(2-(5-Hydroxy-2'-methoxy-[1,1'-biphenyl]-2-yl)ethyl)acetamide (11i)
1H NMR (400 MHz, CDCl3) δ 7.52 (br s, OH), 7.41 – 7.31 (m, 1H), 7.14 – 7.07 (dd, J = 8.4, 6.4 Hz, 1H), 7.05 – 6.94 (m, 3H), 6.83 – 6.76 (dd, J = 8.3, 2.7 Hz, 1H), 6.70 (d, J = 2.7 Hz, 1H), 5.55 (s, 1H), 3.76 (s, 3H), 3.41 – 3.17 (ddt, J = 34.4, 13.1, 6.5 Hz, 2H), 2.57 (t, J = 6.9 Hz, 2H), 1.85 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 171.0, 156.4, 155.1, 139.9, 131.3, 130.5, 130.1, 129.1, 128.5, 121.0, 117.7, 115.2, 111.4, 55.9, 40.7, 31.7, 23.3; HRMS m/z: [M + Na+] for C17H19NO3Na, calcd, 308.1263; found, 308.1264.
N-(2-(5-Hydroxy-3'-methoxy-[1,1'-biphenyl]-2-yl)ethyl)acetamide (11j)
1H NMR (400 MHz, CDCl3) δ 7.83 (br s, OH), 7.30 – 7.24 (m, 1H), 7.06 (d, J = 8.2 Hz, 1H), 6.90 – 6.70 (m, 5H), 5.59 (t, J = 5.7 Hz, 1H), 3.79 (s, 3H), 3.33 – 3.19 (q, J = 6.9 Hz, 2H), 2.69 (t, J = 7.1 Hz, 2H), 1.85 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 171.1, 159.4, 155.1, 143.3, 143.0, 130.9, 129.5, 127.3, 121.7, 117.2, 115.1, 115.0, 112.6, 55.4, 41.1, 31.8, 23.3; HRMS m/z: [M + H+] for C17H20NO3, calcd, 286.1443; found, 286.1436.
N-(2-(5-Hydroxy-3'-methyl-[1,1'-biphenyl]-2-yl)ethyl)acetamide (11k)
1H NMR (400 MHz, CDCl3) δ 7.50 (br s, OH), 7.30 – 7.24 (m, 1H), 7.15 (d, J = 7.6 Hz, 1H), 7.09 – 7.03 (m, 3H), 6.80 (d, J = 7.6 Hz, 1H), 6.73 (s, 1H), 5.53 (br s, NH), 3.31 – 3.21 (q, J = 6.7 Hz, 2H), 2.71 (t, J = 7.0 Hz, 2H), 2.37 (s, 3H), 1.85 (s, 3H); 13C NMR (1001 MHz, CDCl3) δ 170.9, 155.0, 143.6, 141.6, 138.1, 130.8, 1230.0, 128.3, 128.0, 127.4, 126.3, 117.4, 114.9, 41.1, 31.8, 23.3, 21.7; HRMS m/z: [M + Na+] for C17H19NO2Na, calcd, 292.1308; found, 292.1314.
N-(2-(5-Hydroxy-3'-(morpholinomethyl)-[1,1'-biphenyl]-2-yl)ethyl)acetamide (11l)
1H NMR (500 MHz, CDCl3) δ 7.36 – 7.23 (m, 4H), 7.16 (d, J = 7.2 Hz, 1H), 7.07 (d, J = 8.2 Hz, 1H), 6.74 – 6.69 (dd, J = 8.2, 2.7 Hz, 1H), 6.62 (d, J = 2.6 Hz, 1H), 5.50 (br s, NH), 3.74 (m, 4H), 3.53 (s, 3H), 3.29 – 3.20 (q, J = 6.7 Hz, 2H), 2.69 (t, J = 7.0 Hz, 2H), 2.49 (t, J = 4.8 Hz, 4H), 1.87 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 170.5, 155.0, 143.4, 141.7, 130.9, 130.2, 128.4, 128.2, 117.5, 115.0, 66.9, 63.4, 53.8, 40.8, 32.0, 23.4; HRMS m/z: [M + H+] for C21H27N2O3, calcd, 355.2022; found, 355.2024.
N-(2-(4'-((Tert-butyldimethylsilyl)oxy)-5-hydroxy-[1,1'-biphenyl]-2-yl)ethyl)acetamide (11m)
1H NMR (500 MHz, CDCl3) δ 7.16 – 7.10 (d, J = 6.7 Hz, 2H), 7.10 – 7.06 (d, J = 8.2 Hz, 1H), 7.00 (br s, OH), 6.91 – 6.84 (d, J = 8.4 Hz, 2H), 6.79 – 6.72 (m, 2H), 5.38 (s, 1H), 3.34 – 3.21 (q, J = 6.6 Hz, 2H), 2.78 – 2.64 (t, J = 6.9 Hz, 2H), 1.93 – 1.81 (s, 3H), 1.00 (s, 9H), 0.24 (s, 6H); 13C NMR (125 MHz, CDCl3) δ 170.7, 155.0, 154.9, 143.3, 134.6, 130.9, 130.3, 127.8, 120.0, 117.5, 114.7, 41.0, 32.0, 26.0, 23.4, 18.4, −4.1; HRMS (FAB) m/z: [M + Na+] for C22H31NO3SiNa, calcd, 408.1965; found, 408.1960.
N-(2-(Benzo[d][1,3]dioxol-5-yl)-4-hydroxyphenethyl)acetamide (11n)
1H NMR (500 MHz, CDCl3) δ 8.00 (br s, OH), 7.08 – 6.98 (d, J = 8.3 Hz, 1H), 6.81 – 6.73 (m, 2H), 6.73 – 6.68 (m, 2H), 6.68 – 6.64 (dd, J = 7.9, 1.7 Hz, 1H), 5.97 – 5.92 (s, 2H), 5.70 – 5.63 (t, J = 5.7 Hz, 1H), 3.29 – 3.21 (td, J = 7.1, 5.6 Hz, 2H), 2.75 – 2.63 (t, J = 7.2 Hz, 2H), 1.89 – 1.81 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 171.1, 155.1, 147.5, 146.8, 143.0, 135.4, 130.8, 127.4, 122.4, 117.5, 114.9, 109.8, 108.3, 101.2, 41.1, 31.9, 23.3; HRMS (FAB) m/z: [M + Na+] for C17H17NO4Na, calcd, 322.1050; found, 322.1022.
N-(4-Hydroxy-2-(pyridin-3-yl)phenethyl)acetamide (11o)
1H NMR (400 MHz, CDCl3) δ 8.54 (s, 2H), 7.72 (d, J = 7.9 Hz, 1H), 7.42 – 7.34 (dd, J = 8.0, 4.8 Hz, 1H), 7.14 (d, J = 8.4 Hz, 1H), 6.90 – 6.84 (dd, J = 8.3, 2.7 Hz, 1H), 6.73 (d, J = 2.7 Hz, 1H), 5.82 (t, J = 5.9 Hz, 2H), 3.33 – 3.19 (q, J = 6.8 Hz, 2H), 2.69 (t, J = 7.2 Hz, 2H), 1.85 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 170.7, 156.1, 149.1, 147.7, 138.8, 138.0, 131.4, 127.3, 123.7, 117.5, 116.4, 100.2, 40.9, 32.0, 23.4; HRMS (FAB) m/z: [M + H+] for C15H17N2O2, calcd, 257.1290; found, 257.1297.
N-(4-Hydroxy-2-(pyridin-4-yl)phenethyl)acetamide (11p)
1H NMR (400 MHz, CDCl3) δ 8.69 – 8.60 (m, 2H), 7.25 (d, J = 1.5 Hz, 2H), 7.17 (d, J = 8.4 Hz, 1H), 6.90 – 6.83 (dd, J = 8.4, 2.7 Hz, 1H), 6.70 (d, J = 2.7 Hz, 1H), 6.02 (br s, OH), 5.47 (s, 1H), 3.33 – 3.24 (q, J = 7.0 Hz, 2H), 2.71 (t, J = 7.4 Hz, 2H), 1.90 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 173.0, 157.1, 152.8, 149.7, 149.6, 141.3, 132.4, 128.0, 126.2, 117.2, 117.1, 116.9, 41.8, 32.8, 22.5; HRMS (FAB) m/z: [M + Na+] for C15H16N2O2Na, calcd, 279.1104; found, 279.1109.
General procedure for activated Noviose carbamate coupling and followed by methanolysis of compounds 11a–p
Borontrifluoride etherate (6.2 μL, 0.05 mmol) was added to 11a (0.25 mmol) and activated noviose (0.2 mmol) in 2.5 mL anhydrous CH2Cl2. After stirring at room temperature for 2 h, triethylamine (150 μL) was added and concentrated. The residue was partially purified via column chromatography (SiO2, 100:8 CH2Cl2:acetone) to give noviose coupled product as a colorless foam, which was used directly for next step.
Triethylamine (0.22 mL, 10%) was added to the cyclic carbonate (100 mg, 0.22 mmol) in MeOH (2.2 mL). After 12 h, the solvent was concentrated and the residue was purified via column chromatography (SiO2, 10:1, CH2Cl2:Acetone) to afford inseparable diastereomers 13a (see following experimental section for diastereoselectivities) as a colorless amorphous solids. Compounds 13b-m were synthesized using this procedure.
N-(2-(5-(((3R,4S,5R)-3,4-Dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yl)oxy)-[1,1'-biphenyl]-2-yl)ethyl)acetamide (13a)
Colorless amorphous solid (63% yield over 2 steps); 1H NMR (500 MHz, CDCl3) δ 7.41 – 7.28 (m, 3H), 7.28 – 7.18 (dt, J = 5.9, 3.2 Hz, 2H), 7.13 (m, 1H), 6.97 (m, 1H), 6.92 – 6.78 (dd, J = 7.6, 2.7 Hz, 1H), 5.55 – 5.47 (dd, J = 7.7, 2.7 Hz, 1H), 5.39 (m, 1H), 4.14 (m, 2H), 3.58 – 3.46 (m, 3H), 3.34 – 3.15 (m, 4H), 3.03 (d, J = 5.5 Hz, 1H), 2.77 – 2.65 (m, 2H), 1.84 – 1.76 (m, 3H), 1.31 (d, J = 4.9 Hz, 3H), 1.21 – 1.10 (m, 3H); 13C NMR (125 MHz, CDCl3) δ 170.3, 155.4, 143.5, 141.4, 130.8, 129.5, 129.2, 128.5, 127.4, 118.2, 115.2, 98.1, 84.5, 78.4, 71.5, 68.8, 62.0, 40.8, 32.1, 29.2, 23.4, 23.1; HRMS m/z: [M + H+] for C24H32NO6, calcd, 430.2224; found, 430.2227.
N-(2-(5-(((3R,4S,5R)-3,4-Dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yl)oxy)-3'-fluoro-[1,1'-biphenyl]-2-yl)ethyl)acetamide (13b)
Colorless amorphous solid (51% yield over 2 steps); 1H NMR (500 MHz, CDCl3) δ 7.39 (dd, 1H, J = 7.9, 13.9 Hz), 7.22 (d, 1H, J = 8.5 Hz), 7.07 (dd, 2H, J = 7.5, 10.5 Hz), 7.02 (dd, 1H, J = 2.8, 8.4 Hz), 6.99 (m, 1H), 6.91 (d, 1H, J = 2.7 Hz), 5.34 (d, 1H, J = 1.3 Hz), 5.28 (s, 1H), 4.20 (d, 1H, J = 2.2 Hz), 3.80 (m, 1H), 3.63 (s, 3H), 3.30 (d, 1H), 3.28 (m, 2H), 2.75 (t, 2H, J = 7.2 Hz), 2.63 (m, 2H, J = 15.9 Hz), 1.87 (s, 3H), 1.41 (s, 3H), 1.28 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 169.9, 163.5–161.6 (d, J = 251 Hz) 155.0, 143.2 (d, J = 7.8 Hz), 142.1 (d, J = 1.8 Hz), 130.9, 130.1, 130.0 (d, J = 8.8 Hz), 124.8 (d, J = 2.8 Hz), 118.0, 116.0 (d, J = 8.8 Hz), 115.4, 114.3 (d, J = 21.6 Hz), 93.8, 84.2, 76.0, 71.3, 71.1, 62.0, 40.4, 32.0, 28.6, 23.3, 18.5; HRMS m/z: [M + H+] for C24H31FNO6, calcd, 448.2180; found, 448.2174. This material was determined to be 95.6% pure (retention time = 6.401) by HPLC (Phenomenex Luna C-18, 5 μm, 10 × 250 mm column eluting with 30% CH3CN, 70% H2O, flow rate 5.0 mL/min).
N-(2-(5-(((3R,4S,5R)-3,4-Dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yl)oxy)-4'-fluoro-[1,1'-biphenyl]-2-yl)ethyl)acetamide (13c)
Colorless amorphous solid (57% yield over 2 steps); 1H NMR (500 MHz, CDCl3) δ 7.25 (dd, 2H, J = 5.4, 8.6 Hz), 7.18 (d, 1H, J = 8.5 Hz), 7.10 (t, 2H, J = 8.7 Hz), 7.01 (dd, 1H, J = 2.7, 8.5 Hz), 6.87 (d, 1H, J = 2.7 Hz), 5.54 (d, 1H, J = 2.2 Hz), 5.37 (t, 1H, J = 5.2 Hz), 4.20 (dd, 1H, J = 3.3, 9.1 Hz), 4.15 (m, 1H), 3.59 (s, 3H), 3.33 (d, 1H, J = 9.1 Hz), 3.26 (q, 2H, J = 6.9 Hz), 2.97 (s, 1H), 2.81 (s, 1H), 2.72 (t, 2H, J = 7.3), 1.87 (s, 3H), 1.36 (s, 3H), 1.22 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 170.2, 163.2–161.3 (d, J = 250 Hz), 155.3, 142.3, 137.2 (d, J = 3.2 Hz), 130.8, 130.8, 130.7, 129.5, 118.1, 115.4, 115.3, 115.3, 97.9, 84.4, 78.3, 71.4, 68.7, 62.0, 40.6, 32.1, 29.1, 23.4, 23.1; HRMS m/z: [M + Na+] for C24H30FNO6, calcd, 470.1955; found, 470.1958.
N-(2-(2'-Chloro-5-(((3R,4S,5R)-3,4-dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yl)oxy)-[1,1'-biphenyl]-2-yl)ethyl)acetamide (13d)
Colorless amorphous solid (62% yield over 2 steps); 1H NMR (500 MHz, CDCl3) δ 7.46 (m, 1H), 7.31 (m, 2H), 7.21 (m, 2H), 7.03 (m, 1H), 6.86 (dd, 1H, J = 2.7, 13.2 Hz), 5.55 (m, 1H), 5.42 (s, 1H), 4.20 (dt, 1H, J = 3.0, 9.1 Hz), 4.14 (m, 1H), 3.59 (s, 3H), 3.33 (dd, 1H, J = 2.5, 9.1 Hz), 3.26 (ddt, 2H, J = 4.8, 6.8, 9.3 Hz), 3.11 (s, 1H), 2.93 (s, 1H), 2.58 (tq, 2H, J = 7.1, 14.2 Hz), 1.86 (s, 3H), 1.35 (d, 3H, J = 2.4 Hz), 1.20 (t, 3H, J = 5.8 Hz); 13C NMR (125 MHz, CDCl3) δ 170.2, 155.2, 140.6, 140.5, 139.8, 133.4, 131.4, 130.5, 129.8, 126.9, 118.1, 117.9, 116.05, 97.9, 84.5, 78.4, 71.5, 71.4, 68.7, 62.1, 62.0, 40.2, 40.2, 32.1, 32.1, 29.3, 29.2, 23.5, 23.1, 23.0; HRMS m/z: [M + Na+] for C24H30ClNO6Na, 486.1659; found, 486.1652.
N-(2-(3'-Chloro-5-(((3R,4S,5R)-3,4-dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yl)oxy)-[1,1'-biphenyl]-2-yl)ethyl)acetamide (13e)
Colorless amorphous solid (55% yield over 2 steps); 1H NMR (500 MHz, CDCl3) δ 7.35 (m, 2H), 7.28 (m, 1H), 7.18 (m, 2H), 7.03 (dd, 1H, J = 2.7, 8.5 Hz), 6.87 (d, 1H, J = 2.7 Hz), 5.55 (t, 1H, J = 2.5 Hz), 5.34 (m, 1H), 4.21 (dd, 1H, J = 3.1, 9.1 Hz), 4.16 (m, 1H), 3.60 (s, 3H), 3.34 (dd, 1H, J = 1.9, 9.1 Hz), 3.28 (m, 2H), 2.75 (dt, 4H, J = 7.3, 14.5 Hz), 1.88 (s, 3H), 1.37 (s, 3H), 1.22 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 170.1, 155.4, 143.5, 142.0, 134.3, 131.0, 130.9, 129.8, 129.4, 128.5, 127.6, 127.4, 118.2, 115.7, 97.9, 84.6, 78.4, 71.5, 68.7, 62.1, 40.8, 32.1, 29.2, 23.6, 23.1; HRMS m/z: [M + Na+] for C24H30ClNO6Na, calcd, 486.1659; found, 486.1642.
N-(2-(5-(((3R,4S,5R)-3,4-Dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yl)oxy)-3'-(trifluoromethyl)-[1,1'-biphenyl]-2-yl)ethyl)acetamide (13f)
Colorless amorphous solid (52% yield over 2 steps); 1H NMR (500 MHz, CDCl3) δ 7.64 (d, 1H, J = 7.7 Hz), 7.55 (t, 2H, J = 7.6 Hz), 7.49 (m, 1H), 7.23 (d, 1H, J = 8.5 Hz), 7.06 (dd, 1H, J = 2.7, 8.4 Hz), 6.89 (d, 1H, J = 2.7 Hz), 5.56 (d, 1H, J = 2.2 Hz), 5.31 (s, 1H), 4.19 (m, 2H), 3.60 (s, 3H), 3.34 (d, 1H, J = 9.1 Hz), 3.29 (dd, 2H, J = 7.0, 13.3 Hz), 2.72 (t, 2H, J = 7.3 Hz), 2.69 (s, 1H), 2.64 (s, 1H), 1.87 (s, 3H), 1.37 (s, 3H), 1.22 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 170.1, 155.4, 142.1, 141.9, 132.6, 131.0, 130.7 (q, J = 31.5 Hz), 129.4, 129.0, 125.9 (q, J = 3.6, 7.2 Hz), 125.3, 124.2 (q, J = 3.6, 7.2 Hz), 123.1, 118.0, 115.8, 97.9, 84.4, 77.4, 71.4, 68.7, 62.0, 40.6, 32.1, 29.8, 29.2, 23.4, 23.0; HRMS m/z: [M + Na+] for C25H30F3NO6Na, 520.1923; found, 520.1932. This material was determined to be 97.2% pure (retention time = 7.631) by HPLC (Phenomenex Luna C-18, 5 μm, 10 × 250 mm column eluting with 30% CH3CN, 70% H2O, flow rate 5.0 mL/min).
N-(2-(5-(((3R,4S,5R)-3,4-Dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yl)oxy)-4'-(trifluoromethyl)-[1,1'-biphenyl]-2-yl)ethyl)acetamide (13g)
Colorless amorphous solid (49% yield over 2 steps); 1H NMR (400 MHz, CDCl3) δ 7.70 (d, J = 7.6 Hz, 2H), 7.43 (d, J = 7.9 Hz, 2H), 7.24 (d, J = 8.4 Hz, 1H), 7.09 – 7.03 (dd, J = 8.6, 2.7 Hz, 1H), 6.90 (d, J = 2.7 Hz, 1H), 5.55 (d, J = 2.3 Hz, 1H), 5.33 (m, 1H), 4.26 – 4.11 (m, 2H), 3.60 (s, 3H), 3.36 – 3.25 (m, 3H), 2.74 (t, J = 7.4 Hz, 2H), 2.56 (br s, 2OH), 1.88 (s, 3H), 1.37 (s, 3H), 1.22 (s, 3H); 13C NMR (125 MHz, MeOD) δ 173.1, 156.8, 146.9, 143.2, 132.1, 130.9, 130.7, 130.5, 130.2, 126.3, 126.2, 124.7, 118.5, 116.8, 100.1, 85.3, 79.5, 72.8, 69.5, 62.1, 41.7, 32.9, 29.2, 23.6, 22.5; HRMS m/z: [M + Na+] for C25H30F3NO6Na, 520.1923; found, 520.1934.
N-(2-(5-(((3R,4S,5R)-3,4-Dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yl)oxy)-2'-(methylthio)-[1,1'-biphenyl]-2-yl)ethyl)acetamide (13h)
Colorless amorphous solid (63% yield over 2 steps); 1H NMR (400 MHz, CDCl3) δ 7.36 (t, 1H, J = 7.0 Hz), 7.27 (m, 3H), 7.09 (m, 1H), 7.01 (m, 1H), 6.87 (s, 1H), 5.64 (s, 1H), 5.54 (m, 1H), 4.16 (m, 2H), 3.32 (d, 2H, J = 8.8 Hz), 3.27 (m, 2H), 3.06 (s, 1H), 2.56 (t, 2H, J = 6.2 Hz), 2.36 (d, 3H, J = 7.6 Hz), 1.83 (s, 3H), 1.33 (s, 3H), 1.20 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 170.3, 155.1, 155.0, 141.0, 138.9, 130.5, 130.1, 129.8, 128.4, 124.6, 124.2, 118.3, 116.2, 115.9, 97.9, 84.5, 78.3, 71.5, 68.7, 62.0, 53.6, 40.1, 31.7, 29.3, 23.3, 15.3, 15.2; HRMS m/z: [M + Na+] for C25H33NO6SNa, calcd, 498.1926; found, 498.1925. This material was determined to be 95% pure (retention time = 7.465) by HPLC (Phenomenex Luna C-18, 5 μm, 10 × 250 mm column eluting with 30% CH3CN, 70% H2O, flow rate 5.0 mL/min).
N-(2-(5-(((3R,4S,5R)-3,4-Dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yl)oxy)-2'-methoxy-[1,1'-biphenyl]-2-yl)ethyl)acetamide (13i)
Colorless amorphous solid (41% yield over 2 steps); 1H NMR (500 MHz, CDCl3) δ 7.36 (ddd, 1H, J = 1.8, 7.6, 8.2 Hz), 7.18 (d, 1H, J = 8.3 Hz), 7.12 (t, 1H, J = 5.8 Hz), 7.02 (m, 3H), 6.87 (dd, 1H, J = 2.3, 11.3 Hz), 5.54 (s, 1H), 5.39 (s, 1H), 4.21 (dt, 1H, J = 3.3, 9.0 Hz), 4.15 (m, 1H), 3.77 (d, 3H, J = 6.9 Hz), 3.60 (s, 3H), 3.33 (d, 1H, J = 8.7 Hz), 3.29 (m, 2H), 2.73 (s, 1H), 2.66 (s, 1H), 2.60 (dd, 2H, J = 6.5, 12.8 Hz), 1.84 (s, 3H), 1.37 (s, 3H), 1.24 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 170.0, 156.4, 155.2, 139.9, 131.2, 130.8, 130.2, 130.0, 129.2, 120.9, 118.6, 118.3, 115.7, 115.2, 111.4, 111.2, 98.0, 97.9, 84.5, 78.2, 71.4, 68.7, 62.0, 55.9, 55.9, 40.3, 31.9, 30.2, 29.3, 29.2, 23.4, 23.1; HRMS m/z: [M + H+] for C25H34NO7, calcd, 460.2335; found, 460.2336. This material was determined to be 96.1% pure (retention time = 5.057) by HPLC (Phenomenex Luna C-18, 5 μm, 10 × 250 mm column eluting with 30% CH3CN, 70% H2O, flow rate 5.0 mL/min).
N-(2-(5-(((3R,4S,5R)-3,4-Dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yl)oxy)-3'-methoxy-[1,1'-biphenyl]-2-yl)ethyl)acetamide (13j)
Colorless amorphous solid (53% yield over 2 steps); 1H NMR (400 MHz, CDCl3) δ 7.31 (t, J = 7.9 Hz, 1H), 7.17 (d, J = 8.5 Hz, 1H), 7.02 – 6.96 (dd, J = 8.5, 2.7 Hz, 1H), 6.92 – 6.83 (m, 4H), 6.81 (d, J = 1.5 Hz, 2H), 5.54 (d, J = 2.2 Hz, 1H), 5.45 (s, 1H), 4.25 – 4.16 (dd, J = 9.1, 3.2 Hz, 1H), 4.17 – 4.10 (dd, J = 3.3, 2.2 Hz, 1H), 3.82 (s, 3H), 3.58 (s, 3H), 3.39 – 3.20 (m, 3H), 3.24 (br s, OH), 2.97 (br s, OH), 2.75 (t, J = 7.1 Hz, 2H), 1.85 (s, 3H), 1.35 (s, 3H), 1.20 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 170.4, 159.5, 155.3, 143.3, 142.8, 130.8, 129.5, 129.5, 121.7, 118.0, 115.3, 115.1, 112.7, 98.1, 84.5, 78.4, 71.5, 68.7, 62.0, 55.4, 40.9, 32.0, 29.1, 23.4, 23.1; HRMS m/z: [M + H+] for C25H34NO7, calcd, 460.2335; found, 460.2322.
N-(2-(5-(((3R,4S,5R)-3,4-Dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yl)oxy)-3'-methyl-[1,1'-biphenyl]-2-yl)ethyl)acetamide (13k)
Colorless amorphous solid (44% yield over 2 steps); 1H NMR (400 MHz, CDCl3) δ 7.32 – 7.27 (m, 1H), 7.16 (d, J = 6.6 Hz, 2H), 7.10 – 7.04 (m, 2H), 6.99 (d, J = 8.5 Hz, 1H), 6.88 (s, 1H), 5.55 (s, 1H), 5.41 (s, 1H), 4.25 – 4.08 (m, 2H), 3.57 (s, 3H), 3.37 – 3.20 (m, 5H), 2.75 (t, J = 7.0 Hz, 2H), 2.39 (s, 3H), 1.83 (s, 3H), 1.35 (s, 3H), 1.20 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 170.4, 155.3, 143.6, 141.3, 138.1, 130.8, 130.0, 129.5, 128.3, 128.1, 126.3, 118.1, 115.1, 98.1, 84.5, 78.4, 71.5, 68.7, 62.0, 40.9, 32.0, 29.2, 23.4, 23.1, 21.7; HRMS m/z: [M + H+] for C25H33NO6Na, calcd, 466.2206; found, 466.2203.
N-(2-(5-(((3R,4S,5R)-3,4-Dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yl)oxy)-3'-(morpholinomethyl)-[1,1'-biphenyl]-2-yl)ethyl)acetamide (13l)
Colorless amorphous solid (47% yield over 2 steps); 1H NMR (500 MHz, CDCl3) δ 7.41 – 7.29 (m, 2H), 7.27 (m, 1H), 7.19 (d, J = 8.1 Hz, 2H), 7.04 – 6.99 (dd, J = 8.5, 2.7 Hz, 1H), 6.91 (d, J = 2.7 Hz, 1H), 5.55 (d, J = 2.4 Hz, 1H), 5.35 (s, 1H), 4.26 – 4.18 (dd, J = 9.0, 3.3 Hz, 1H), 4.15 (t, J = 2.8 Hz, 1H), 3.72 (t, J = 4.7 Hz, 4H), 3.59 (s, 3H), 3.56 (s, 2H), 3.34 (d, J = 9.0 Hz, 1H), 3.30 – 3.21 (q, J = 6.7 Hz, 2H), 2.75 (t, J = 7.1 Hz, 2H), 2.58– 2.41 (m, 6H), 1.85 (s, 3H), 1.36 (s, 3H), 1.23 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 155.4, 143.4, 141.5, 137.8, 130.9, 130.1, 129.6, 128.5, 128.3, 128.2, 118.2, 115.3, 98.1, 84.6, 78.4, 71.5, 68.8, 67.1, 63.5, 62.0, 53.8, 40.7, 32.2, 29.2, 23.5, 23.2; HRMS (FAB) m/z: [M + Na+] for C29H40N2O7Na, calcd, 551.2728; found, 551.2734.
N-(2-(5-(((3R,4S,5R)-3,4-Dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yl)oxy)-4'-hydroxy-[1,1'-biphenyl]-2-yl)ethyl)acetamide (13m)
After cyclic carbonate hydrolysis following the same procedure as compound 13a, the crude TBS protected compound was dissolved in THF (2 mL) and tertrabutylammonium fluoride (1.5 eq.) was added drop wise at 0 °C under argon atmosphere. After 1 h the reaction was quenched with water and extracted with EtOAc (3 × 10 mL); combined organic fractions were washed with saturated aqueous sodium chloride, dried over anhydrous Na2SO4, filtered and concentrated. The residue was purified by column chromatography (SiO2; 10:1, CH2Cl2:acetone) to afford acetamide 13m as a amorphous solid (40% yield over 3 steps). 1H NMR (500 MHz, MeOD) δ 7.20 (d, J = 8.4 Hz, 1H), 7.15 – 7.08 (d, J = 8.4 Hz, 2H), 6.96 (dd, J = 8.4, 2.6 Hz, 1H), 6.85 – 6.79 (m, 3H), 5.45 (d, J = 2.4 Hz, 1H), 4.12 (dd, J = 9.3, 3.3 Hz, 1H), 3.96 (t, J = 2.8 Hz, 1H), 3.59 (s, 3H), 3.21 (d, J = 9.3, Hz, 1H), 3.16 (dd, J = 8.5, 6.5 Hz, 2H), 2.70 (dd, J = 8.5, 6.5 Hz, 2H), 1.84 (s, 3H), 1.32 (s, 3H), 1.18 (s, 3H); 13C NMR (125 MHz, MeOD) δ 173.1, 157.7, 156.6, 144.7, 134.0, 131.7, 131.2, 131.1, 118.9, 116.0, 115.7, 100.1, 85.4, 79.4, 72.8, 69.5, 62.1, 41.8, 33.0, 29.2, 23.6, 22.5; HRMS (FAB) m/z: [M + Na+] for C24H31NO7Na, calcd, 468.1998; found, 468.1999.
N-(2-(Benzo[d][1,3]dioxol-5-yl)-4-(((3R,4S,5R)-3,4-dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yl)oxy)phenethyl)acetamide (13n)
Colorless amorphous solid (51% yield over 2 steps); 1H NMR (500 MHz, CDCl3) δ 7.15 (d, J = 8.5 Hz, 1H), 7.00 – 6.96 (dd, J = 8.5, 2.7 Hz, 1H), 6.88 (d, J = 2.6 Hz, 1H), 6.84 (d, J = 7.9 Hz, 1H), 6.76 (d, J = 1.6 Hz, 1H), 6.74 – 6.69 (m, 1H), 6.01 (s, 2H), 5.54 (d, J = 2.4 Hz, 1H), 5.40 (s, 1H), 4.21 (dd, J = 9.1, 3.3 Hz, 1H), 4.14 (t, J = 2.7 Hz, 2H), 3.58 (s, 3H), 3.33 (d, J = 9.1 Hz, 1H), 3.30 – 3.23 (q, J = 6.9 Hz, 2H), 3.11 (br s, OH), 2.92 (br s, OH), 2.74 (t, J = 7.2 Hz, 2H), 1.86 (s, 3H), 1.34 (s, 3H), 1.20 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 170.2, 155.4, 147.7, 147.0, 143.1, 135.3, 130.8, 129.7, 122.6, 118.3, 115.2, 109.9, 108.4, 101.3, 98.1, 84.6, 78.4, 71.5, 68.8, 62.0, 40.8, 32.1, 29.2, 23.4, 23.2; HRMS (FAB) m/z: [M + Na+] for C25H31NO8Na, calcd, 496.1947; found, 496.1940. This material was determined to be 98.4% pure (retention time = 4.384) by HPLC (Phenomenex Luna C-18, 5 μm, 10 × 250 mm column eluting with 40% CH3CN, 60% H2O, flow rate 5.0 mL/min).
N-(4-(((3R,4S,5R)-3,4-Dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yl)oxy)-2-(pyridin-3-yl)phenethyl)acetamide (13o)
Colorless amorphous solid (37% yield over 2 steps); 1H NMR (500 MHz, CDCl3) δ 8.55 (d, J = 3.9 Hz, 1H), 8.49 (s, 1H), 7.60 (m, 1H), 7.35 (dd, J = 7.8, 4.5 Hz, 1H), 7.20 (d, J = 8.5 Hz, 1H), 7.05 – 6.99 (dd, J = 8.4, 2.7 Hz, 1H), 6.85 (d, J = 2.6 Hz, 1H), 5.52 (d, J = 2.4 Hz, 1H), 5.36 (s, 1H), 4.14 (dd, J = 3.4, 9.1 Hz, 1H), 4.10 (t, J = 2.7 Hz, 1H), 3.59 (s, 3H), 3.31 (d, J = 9.0 Hz, 1H), 3.27 – 3.20 (m, 2H), 2.68 (t, J = 7.3 Hz, 2H), 1.86 (s, 3H), 1.33 (s, 3H), 1.17 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 170.2, 155.5, 149.8, 148.7, 139.5, 136.8, 131.1, 131.0, 130.6, 129.8, 123.4, 118.3, 118.2, 116.1, 98.0, 84.5, 78.5, 71.4, 68.7, 62.1, 40.7, 32.2, 29.2, 23.5, 23.1; HRMS (FAB) m/z: [M + Na+] for C23H31N2O6, calcd, 431.2182; found, 431.2194.
N-(4-(((3R,4S,5R)-3,4-Dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yl)oxy)-2-(pyridin-4-yl)phenethyl)acetamide (13p)
Colorless amorphous solid (42% yield over 2 steps); 1H NMR (400 MHz, CDCl3) δ 8.73 – 8.63 (dd, J = 5.7, 3.9 Hz, 2H), 7.27 – 7.23 (m, 3H), 7.11 – 7.03 (m, 1H), 6.86 (t, J = 2.8 Hz, 1H), 5.55 (d, J = 2.3 Hz, 1H), 5.41 – 5.31 (m, 2H), 4.26 – 4.13 (m, 2H), 4.05 (d, J = 6.9 Hz, 1H), 3.61 (s, 3H), 3.36 – 3.25 (m, 2H), 2.78 – 2.71 (dd, J = 8.3, 6.8 Hz, 2H), 1.90 (s, 3H), 1.39 (s, 3H), 1.24 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 170.1, 155.5, 149.8, 140.5, 131.4, 129.1, 124.4, 117.9, 116.3, 98.0, 94.1, 84.5, 71.5, 71.4, 68.7, 62.1, 40.7, 32.2, 29.2, 28.8, 23.5, 23.1, 18.7; HRMS (FAB) m/z: [M + Na+] for C23H30N2O6Na, calcd, 453.2001; found, 453.1972.
(Z)-4-(Benzyloxy)-2-(methoxymethoxy)-1-(2-nitrovinyl)benzene (15)
Nitromethane (11.5 mL) was added to a mixture of aldehyde 14 (1.24g, 4.6 mmol) and ammonium acetate (0.63 g, 8.2 mmol) and heated to 50 °C. Upon completion (~20 min), the reaction mixture was cooled to room temperature and purified without work-up by column chromatography (SiO2, 4:1, Hex:EtOAc) to afford nitrostyrene 15 as a colorless oil (1.22 g, 84%). 1H NMR (400 MHz, CDCl3) δ 8.17 (d, J = 13.4 Hz, 1H), 7.80 (d, J = 13.6 Hz, 1H), 7.50 – 7.32 (m, 6H), 6.88 (d, J = 2.5 Hz, 1H), 6.67 (m, 1H), 5.30 (s, 2H), 5.12 (s, 2H), 3.52 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 163.4, 159.0, 136.1, 136.0, 135.6, 133.5, 128.8, 128.5, 127.7, 127.7, 113.0, 108.6, 102.2, 94.7, 70.5, 56.6; HRMS (FAB) m/z: [M + Na+] for C17H17NO5Na, calcd, 338.1004; found, 338.1007.
4'-(Benzyloxy)-2'-(methoxymethoxy)-2-nitro-1,2,3,6-tetrahydro-1,1'-biphenyl (16)
Nitrostyrene 15 (0.65 g, 2.06 mmol) was dissolved in toluene (0.6 mL) in a 2 mL sealed tube and cooled to −78 °. Butadiene was bubbled into the solution to double the volume and then the tube was sealed and heated to reflux for 48 h. To prevent bumping of the butadiene gas, the tube was cooled again to −78 °C and used directly in purification by column chromatography (SiO2; 3:1, Hex:EtOAc) to afford cyclohexene adduct 16 (0.72 g, 95%). 1H NMR (500 MHz, CDCl3) δ 7.40 (m, 4H), 7.36 – 7.28 (m, 1H), 7.06 (d, J = 8.4 Hz, 1H), 6.80 (d, J = 2.4 Hz, 1H), 6.56 (dd, J = 8.4, 2.5 Hz, 1H), 5.86 – 5.77 (m, 1H), 5.71 (ddd, J = 9.8, 5.1, 2.3 Hz, 1H), 5.27 – 5.20 (m, 1H), 5.20 (s, 2H), 5.00 (s, 2H), 3.70 (dt, J = 17.0, 8.7 Hz, 1H), 3.49 (s, J = 12.7 Hz, 3H), 2.84 – 2.74 (m, 1H), 2.71 (ddd, J = 13.2, 8.4, 1.5 Hz, 1H), 2.45 (m, 2H); 13C NMR (125 MHz, CDCl3) δ 159.2, 156.1, 136.9, 129.3, 128.6, 127.0, 122.5, 120.9, 107.8, 120.6, 94.6, 85.6, 70.1, 31.5, 31.3, 29.7.
N-(4'-(Benzyloxy)-2'-(methoxymethoxy)-1,2,3,6-tetrahydro-[1,1'-biphenyl]-2-yl)acetamide (17)
Nitro compound 16 (0.23 g, 0.62 mmol) was dissolved in isopropanol (12.4 mL) and aqeous 1M HCl (6.2 mL). Zinc dust (811 mg, 12.4 mmol) were added sequentially and the mixture was stirred vigorously at 50 °C for 1.5 h. After cooling to room temperature, saturated NaHCO3 (8 mL) was added and the resulting mixture was stirred for an additional 20 min. The solids were removed by filtration and the remaining solution was extracted with CH2Cl2 (3 × 20 mL). The organic layers were combined and washed with aqueous saturated sodium chloride solution, dried over Na2SO4, filtered and concentrated to afford amine as a colorless oil (0.20 g, 0.59 mmol, 95%), which was used for next reaction without further purification.
Acetic anhydride (62 μL, 0.65 mmol) and triethylamine (95 μL, 0.68 mmol) were added to a solution of the amine (0.62 mmol) in CH2Cl2 (6.2 mL) at room temperature. After 3 h the reaction was quenched with saturated aqueous ammonium chloride and extracted with CH2Cl2 (3 × 10 mL); combined organic fractions were washed with saturated aqueous NaCl, dried over anhydrous Na2SO4, filtered and concentrated. The residue was purified by column chromatography (SiO2; 3:1, Hex:EtOAc) to afford acetamide 17 (0.17 g, 74%). 1H NMR (500 MHz, CDCl3) δ 7.42 (d, J = 7.8 Hz, 2H), 7.39 – 7.33 (t, J = 7.2 Hz, 2H), 7.33 – 7.28 (m, 1H), 7.12 (d, J = 8.5 Hz, 1H), 6.77 (s, 1H), 6.63 (d, J = 8.5 Hz, 1H), 5.90 (d, J = 8.4 Hz, 1H), 5.71 (d, J = 36.2 Hz, 2H), 5.17 (s, 2H), 5.02 (s, 2H), 4.36 – 4.23 (dtd, J = 13.8, 10.4, 9.9, 7.2 Hz, 1H), 3.50 (s, 3H), 3.31 – 3.22 (dd, J = 18.6, 7.9 Hz, 1H), 2.59 (d, J = 17.3 Hz, 1H), 2.33 (s, 2H), 2.02 – 1.93 (m, 1H), 1.74 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 169.8, 158.3, 156.1, 136.9, 128.5, 128.3, 127.9, 127.6, 126.7, 125.0, 124.4, 108.0, 102.9, 95.6, 70.0, 56.2, 48.8, 37.4, 33.0, 32.6, 23.1; HRMS (FAB) m/z: [M + Na+] for C23H27NO4Na, calcd, 404.1832; found, 404.1827.
N-(4'-(Benzyloxy)-2'-hydroxy-1,2,3,6-tetrahydro-[1,1'-biphenyl]-2-yl)acetamide (18)
Catalytic amount of conc. HCl (few drops) was added to MOM protected phenol 17 (0.27 g, 0.71 mmol) in methanol (7.1 mL) and stirred vigorously at 50 °C for overnight. Upon completion the reaction mixture was concentrated and residue was purified by column chromatography (SiO2; 5:100, MeOH: CH2Cl2) to afford phenol 18 (0.19 g, 81%). 1H NMR (400 MHz, CDCl3) δ 8.86 (s, 1H), 7.41 – 7.25 (m, 5H), 7.01 (d, J = 8.5 Hz, 1H), 6.73 (d, J = 2.4 Hz, 1H), 6.48 (d, J = 6.0 Hz, 1H), 5.73 (m, 1H), 5.65 (m, 1H), 4.96 (s, 2H), 4.26 (m, 1H), 3.42 (m, 1H), 2.55 – 2.12 (m, 4H), 1.98 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 173.2, 158.3, 155.4, 136.9, 128.5, 128.0, 127.9, 127.6, 127.0, 123.9, 121.1, 107.2, 103.4, 69.9, 51.9, 50.0, 36.6, 31.6, 21.0; HRMS (FAB) m/z: [M + Na+] for C21H23NO3Na, calcd, 360.1576; found, 360.1571.
2'-Acetamido-4-(benzyloxy)-1',2',3',6'-tetrahydro-[1,1'-biphenyl]-2-yl trifluoromethanesulfonate (19)
A solution of phenol (0.19 g, 0.58 mmol) in anhydrous CH2Cl2 (5.8 mL), triethylamine (0.12 mL, 0.87 mmol) followed by N-phenyl-bis(trifluoromethanesulfonimide) (0.31 g, 0.87 mmol) were added at 0 °C. Upon completion the reaction was quenched by addition of water (50 mL), washed with saturated aqueous NaCl solution, dried (Na2SO4), filtered and concentrated. The residue was purified by column chromatography (SiO2, 3:1, Hex:EtOAc) to afford triflate 19 as a pale yellow oil (0.23 g, 0.49 mmol, 85%). 1H NMR (400 MHz, CDCl3) δ 7.45 – 7.31 (m, 6H), 7.00 (d, J = 11.2 Hz, 1H), 6.84 (d, J = 2.4 Hz, 1H), 5.70 (m, 2H), 5.60 (d, J = 9.3 Hz, 1H), 5.04 (s, 2H), 4.53 – 4.38 (dt, J = 15.2, 10.2 Hz, 1H), 3.18 – 3.03 (td, J = 11.2, 5.2 Hz, 1H), 2.63 – 2.50 (dd, J = 16.2, 4.2 Hz, 1H), 2.42 – 2.32 (m, 1H), 2.28 – 2.15 (m, 1H), 2.11 – 1.97 (t, J = 14.5 Hz, 1H), 1.71 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 169.7, 158.2, 147.6, 136.0, 129.9, 128.8, 128.5, 128.1, 127.7, 126.1, 125.4, 115.8, 108.2, 70.7, 48.3, 38.5, 34.8, 33.7, 23.2; HRMS (FAB) m/z: [M + Na+] for C22H22F3NO5SNa, calcd, 492.106318; found, 492.1067.
N-(4'-(Benzyloxy)-3"-fluoro-1,2,3,6-tetrahydro-[1,1':2',1"-terphenyl]-2-yl)acetamide (20a)
Followed same Suzuki coupling procedure as described above for 8a. 1H NMR (400 MHz, CDCl3) δ 7.47 – 7.30 (m, 7H), 7.13 – 7.05 (t, J = 8.9 Hz, 1H), 7.05 – 7.00 (t, J = 7.2 Hz, 2H), 6.97 (d, J = 10.9 Hz, 1H), 6.80 (d, J = 2.7 Hz, 1H), 5.72 – 5.53 (m, 2H), 5.06 (s, 2H), 4.91 (d, J = 8.7 Hz, 1H), 4.36 – 4.24 (m, 1H), 2.90 – 2.75 (dd, J = 19.2, 8.2 Hz, 1H), 2.59 – 2.45 (dt, J = 16.3, 4.4 Hz, 1H), 2.36 (m, 2H), 1.75 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 169.3, 156.8, 143.9, 142.3, 137.0, 132.6, 130.2, 130.2, 128.8, 128.5, 128.2, 127.8, 126.7, 125.2, 125.0, 116.4, 116.2, 116.0, 115.2, 114.5, 114.3, 70.2, 49.4, 40.5, 35.3, 33.4, 23.5; HRMS (FAB) m/z: [M + Na+] for C27H26FNO2Na, calcd, 438.1840; found, 438.1818.
N-(4'-(Benzyloxy)-3"-(trifluoromethyl)-1,2,3,6-tetrahydro-[1,1':2',1"-terphenyl]-2-yl)acetamide (20b)
Followed same Suzuki coupling procedure as described above for 8a. 1H NMR (400 MHz, CDCl3) δ 7.73 – 7.30 (m, 10H), 7.05 (d, J = 8.7 Hz, 1H), 6.85 (s, 1H), 5.66 (m, 2H), 5.16 (d, J = 8.5 Hz, 1H), 5.08 (s, 2H), 4.43 – 4.29 (m, 1H), 2.90 – 2.74 (q, J = 10.0, 9.0 Hz, 1H), 2.50 (d, J = 17.7 Hz, 1H), 2.40 – 2.28 (dd, J = 6.9, 3.9 Hz, 2H), 1.75 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 169.7, 156.9, 142.4, 141.9, 136.9, 132.6, 131.1, 130.8, 129.1, 128.7, 128.6, 128.2, 127.7, 126.6, 126.0, 125.9, 125.0, 124.3, 124.2, 116.3, 115.3, 70.2, 49.4, 40.6, 35.2, 33.1, 23.4; HRMS (FAB) m/z: [M + Na+] for C28H26F3NO2Na, calcd, 488.1813; found, 488.1812.
N-(3"-Fluoro-4'-hydroxy-1,2,3,6-tetrahydro-[1,1':2',1"-terphenyl]-2-yl)acetamide (21a)
1,2-Ethanedithiol (0.22 mL, 2.66 mmol) and BF3.OEt2 (0.176 mL, 1.4 mmol) were added to benzyl ether 20a (64 mg, 0.14 mmol) in CH2Cl2 (1.8 mL). After 8h at room temperature, reaction mixture was concentrated and purified by column chromatography (SiO2, 1:10, MeOH: CH2Cl2) to afford phenol 21a as an amorphous solid (45 mg, 0.12 mmol, 86%) 1H NMR (500 MHz, CDCl3) δ 8.98 (s, 1H), 7.40 – 7.34 (q, J = 7.1, 6.2 Hz, 1H), 7.27 (d, J = 7.6 Hz, 1H), 7.10 – 7.01 (m, 2H), 6.96 (d, J = 9.4 Hz, 1H), 6.84 – 6.79 (dd, J = 8.5, 2.6 Hz, 1H), 6.68 (d, J = 2.6 Hz, 1H), 5.73 – 5.52 (m, 2H), 4.51 – 4.38 (dt, J = 9.9, 5.0 Hz, 1H), 2.88 – 2.77 (q, J = 9.5, 7.9 Hz, 1H), 2.43 (d, J = 17.3 Hz, 1H), 2.34 (m, 2H), 2.18 (s, 1H), 1.77 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 170.5, 163.6–161.7 (d, J = 244.0 Hz), 155.2, 144.2 (d, J = 7.6 Hz), 142.3, 130.8, 130.1 (d, J = 8.4 Hz), 128.2, 127.0, 125.0 (d, J = 2.2 Hz), 124.7, 116.5, 116.3 (d, J = 20.3 Hz), 115.9, 114.2 (d, J = 20.3 Hz, 49.6, 40.8, 35.5, 33.5, 23.2; HRMS (FAB) m/z: [M + Na+] for C21H25FNO2Na, 348.1376; found, 348.1379.
N-(4'-Hydroxy-3"-(trifluoromethyl)-1,2,3,6-tetrahydro-[1,1':2',1"-terphenyl]-2-yl)acetamide (21b)
Followed same procedure as for 21a. 1H NMR (400 MHz, CDCl3) δ 9.20 (s, 1H), 7.66 – 7.56 (m, 4H), 7.29 (d, J = 8.5 Hz, 1H), 6.82 – 6.72 (d, J = 10.5 Hz, 1H), 6.65 (s, 1H), 5.65 (m, 1H), 5.54 (m, 1H), 5.21 (d, J = 9.7 Hz, 2H), 4.56 – 4.33 (m, 1H), 2.76 – 2.61 (m, 1H), 2.46 – 2.24 (m, 3H), 1.75 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 170.5, 155.3, 142.7, 142.0, 132.6, 130.8, 130 (q, J = 32.5 Hz), 128.8, 128.4, 126.9, 126.0 (m), 124.7, 124.0 (m), 116.7, 116.0, 49.6, 40.9, 35.6, 33.5, 23.2; HRMS (FAB) m/z: [M + Na+] for C21H20F3NO2Na, calcd, 398.1344; found, 398.1346.
N-(4'-(((3R,4S,5R)-3,4-Dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yl)oxy)-3"-fluoro-1,2,3,6-tetrahydro-[1,1':2',1"-terphenyl]-2-yl)acetamide: (22a)
Followed the same noviose coupling procedure as described above for 13a to afford 22a as a inseparable mixture of diastereomers. 1H NMR (500 MHz, CDCl3) δ 7.32 (ddd, 1H, J = 6.0, 7.9, 13.9 Hz), 7.22 (dd, 1H, J = 2.8, 8.7 Hz), 7.00 (m, 2H), 6.94 (d, 1H, J = 7.6 Hz), 6.87 (m, 1H), 6.77 (dd, 1H, J = 2.7, 8.7 Hz), 5.59 (m, 1H), 5.52 (m, 1H), 5.49 (d, 1/2H, J = 2.4 Hz), 5.45 (d, 1/2H, J = 2.4 Hz), 4.81 (dd, 1H, J = 2.5, 8.8 Hz), 4.21 (m, 1H), 4.12 (m, 1H), 4.07 (m, 1H), 3.52 (s, 3H), 3.25 (dd, 1H, J = 0.9, 9.0 Hz), 2.89 (br s, 1H), 2.76 (m, 1H), 2.67 (s, 1H), 2.26 (m, 2H), 1.69 (m, 1H), 1.65 (s, 3/2H), 1.64 (s, 3/2H), 1.29 (s, 3/2H), 1.28 (s, 3/2H), 1.13 (s, 3/2H), 1.12 (s, 3/2H); 13C NMR (125 MHz, CDCl3) δ 169.4, 169.4, 163.7–161.7 (d, J = 249.0 Hz), 154.9, 154.7, 143.0 (dd, J = 1.7, 8.5 Hz), 142.2 (d, J = 1.7 Hz), 133.4, 133.3, 130.2 (dd, J = 1.7, 8.5 Hz), 128.4 (d, J = 5.0Hz), 126.6 (d, J = 3.2 Hz), 125.1 (d, J = 3.6 Hz), 125.0 (m), 117.2, 116.9, 116.6, 116.3 (dd, J = 13.4, 20.9 Hz), 116.2, 114.3 (dd, J = 1.5, 20.9 Hz), 98.0, 97.7, 84.5, 84.4, 78.3, 78.3, 77.4, 71.5, 71.4, 68.8, 62.0, 61.9, 49.6, 49.6, 40.5, 40.5, 35.2, 35.1, 33.4, 29.2, 23.6, 23.5, 23.2, 23.1; HRMS (FAB) m/z: [M + Na+] for C28H34FNO6Na, 522.2262; found, 522.2267.
N-(4'-(((3R,4S,5R)-3,4-Dihydroxy-5-methoxy-6,6-dimethyltetrahydro-2H-pyran-2-yl)oxy)-3"-(trifluoromethyl)-1,2,3,6-tetrahydro-[1,1':2',1"-terphenyl]-2-yl)acetamide (22b)
Followed the same noviose coupling procedure as described above for 13a to afford 22b as a inseparable mixture of diastereomers.1H NMR (500 MHz, CDCl3) δ 7.65 (d, 1H, J = 8.2 Hz), 7.56 (t, 1H, J = 7.7 Hz), 7.49 (s, 1H), 7.45 (d, 1H, J = 7.6 Hz), 7.32 (dd, 1H, J = 3.0, 8.7 Hz), 7.08 (td, 1H, J = 2.7, 8.6 Hz), 6.85 (dd, 1H, J = 2.7, 8.5 Hz), 5.65 (m, 1H), 5.59 (m, 1H), 5.57 (d, 1/2H, J = 2.4 Hz), 5.53 (d, 1/2H, J = 2.3 Hz), 4.90 (t, 1H, J = 8.2 Hz), 4.30 (m, 1H), 4.19 (dd, 1H, J = 4.3, 8.2 Hz), 4.14 (m, 1H), 3.59 (s, 3/2H), 3.59 (s, 3/2H), 3.33 (d, 1H, J = 9.0 Hz), 3.17 (s, 1H), 2.95 (s, 1H), 2.76 (m, 1H), 2.49 (m, 1H), 2.33 (m, 1H), 1.74 (m, 1H), 1.73 (s, 3/2H), 1.72 (s, 3/2H), 1.36 (s, 3/2H), 1.35 (s, 3/2H), 1.21 (s, 3/2H), 1.20 (s, 3/2H); 13C NMR (100 MHz, CDCl3) δ 169.4, 169.4, 155.0, 154.8, 142.3, 141.8, 133.4, 133.3, 132.8, 132.6, 131.8 (dq, J = 2.2, 32.5 Hz), 129.1, 128.6, 126.5, 125.9 (q, J = 3.2, 7.0 Hz), 125.0, 124.2, 117.6, 117.0, 116.8, 98.1, 97.8, 84.5, 84.4, 78.4, 78.3, 71.3, 71.3, 68.7, 68.7, 61.9, 61.9, 49.4, 49.3, 40.5, 40.5, 35.1, 35.0, 33.1, 29.0, 29.0, 23.4, 23.4, 23.1, 23.0; HRMS (FAB) m/z: [M + Na+] for C29H34F3NO6Na, Calcd, 572.2230; found, 572.2227.
Supplementary Material
Acknowledgements
This work was supported by grants from The National Institutes of Health to BSJB [CA120458, CA109265] and RTD [NS054847, DK073594] and by the Juvenile Diabetes Research Foundation (RTD).
ABBREVIATIONS USED
- DPN
diabetic peripheral neuropathy
- DNA
deoxyribonucleic acid
- SAR
structure activity relationships
- AGEs
advanced glycation end products enhanced oxidative stress
- Hsp90 and Hsp70
heat shock protein 90 and 70
Footnotes
Supporting Information. 1H and 13C NMR spectra for all new compounds and HPLC traces for most active compounds. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.Veves A, Backonja M, Malik RA. Painful diabetic neuropathy:Epidemiology, natural history, early diagnosis, and treatment options. Pain Med. 2008;9:660–674. doi: 10.1111/j.1526-4637.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 2.Tomlinson DR, Gardiner NJ. Glucose neurotoxicity. Nat Rev Neurosci. 2008;9(1):36–45. doi: 10.1038/nrn2294. [DOI] [PubMed] [Google Scholar]
- 3.Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62(6):670–84. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peterson LB, Blagg BS. To fold or not to fold: modulation and consequences of Hsp90 inhibition. Future Med Chem. 2009;1(2):267–283. doi: 10.4155/fmc.09.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burlison JA, Neckers L, Smith AB, Maxwell A, Blagg BSJ. Novobiocin: Redesigning a DNA Gyrase Inhibitor for Selective Inhibition of Hsp90. Journal of the American Chemical Society. 2006;128:15529–15536. doi: 10.1021/ja065793p. [DOI] [PubMed] [Google Scholar]
- 6.(a) Zhang L, Yu C, Vasquez FE, Galeva N, Onyango I, Swerdlow RH, Dobrowsky RT. Hyperglycemia alters the schwann cell mitochondrial proteome and decreases coupled respiration in the absence of superoxide production. J Proteome Res. 2010;9(1):458–471. doi: 10.1021/pr900818g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhang L, Zhao H, Blagg BS, Dobrowsky RT. A C-Terminal Heat Shock Protein 90 Inhibitor Decreases Hyperglycemia-induced Oxidative Stress and Improves Mitochondrial Bioenergetics in Sensory Neurons. J. Proteome Res. 2012;11:129–137. doi: 10.1021/pr300056m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Obrosova IG. Diabetes and the peripheral nerve. Biochim Biophys Acta. 2009;10:931–940. doi: 10.1016/j.bbadis.2008.11.005. [DOI] [PubMed] [Google Scholar]; (b) Akude E, Zherebitskaya E, Roy Chowdhury SK, Girling K, Fernyhough P. 4-Hydroxy-2-Nonenal Induces Mitochondrial Dysfunction and Aberrant Axonal Outgrowth in Adult Sensory Neurons that Mimics Features of Diabetic Neuropathy. Neurotox Res. 2009;1:28–38. doi: 10.1007/s12640-009-9074-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muchowski PJ, Wacker JL. Modulation of neurodegeneration by molecular chaperones. Nat Rev Neurosci. 2005;6(1):11–22. doi: 10.1038/nrn1587. [DOI] [PubMed] [Google Scholar]
- 9.Baseler WA, Dabkowski ER, Williamson CL, Croston TL, Thapa D, Powell MJ, Razunguzwa TT, Hollander JM. Proteomic alterations of distinct mitochondrial subpopulations in the type 1 diabetic heart: contribution of protein import dysfunction. Am J Physiol Regul Integr Comp Physiol. 2011;300(2):R186–200. doi: 10.1152/ajpregu.00423.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Urban MJ, Li C, Yu C, Lu Y, Krise JM, McIntosh MP, Rajewski RA, Blagg BSJ, Dobrowsky RT. Inhibiting Heat Shock Protein 90 Reverses Sensory Hypoalgesia in Diabetic Mice. ASN Neuro. 2010;2:189–199. doi: 10.1042/AN20100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burlison JA, Avila C, Vielhauer G, Lubbers DJ, Holzbeierlein J, Blagg BS. Development of novobiocin analogues that manifest anti-proliferative activity against several cancer cell lines. J Org Chem. 2008;73(6):2130–2137. doi: 10.1021/jo702191a. [DOI] [PubMed] [Google Scholar]
- 12.Donnelly AC, Mays JR, Burlison JA, Nelson JT, Vielhauer G, Holzbeierlein J, Blagg BSJ. The Design, Synthesis, and Evaluation of Coumarin Ring Derivatives of the Novobiocin Scaffold that Exhibit Antiproliferative Activity. J. Org. Chem. 2008;73(22):8901–8920. doi: 10.1021/jo801312r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matts RL, Dixit A, Peterson LB, Sun L, Voruganti S, Kalyanaraman P, Hartson SD, Verkhivker GM, Blagg BSJ. Elucidation of the Hsp90 C-Terminal Inhibitor Binding Site. ACS Chem. Biol. 2011;6:800–807. doi: 10.1021/cb200052x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee M, Gubernator NG, Sulzer D, Sames D. Development of pH-Responsive Fluorescent False Neurotransmitters. Journal of the American Chemical Society. 2010;132(26):8828–8830. doi: 10.1021/ja101740k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grasa GA, Viciu MS, Huang J, Zhang C, Trudell ML, Nolan SP. Suzuki–Miyaura Cross-Coupling Reactions Mediated by Palladium/Imidazolium Salt Systems. Organometallics. 2002;21(14):2866–2873. [Google Scholar]
- 16.Olson JP, Gichinga MG, Butala E, Navarro HA, Gilmour BP, Carroll FI. Synthesis and evaluation of 1,2,4-methyltriazines as mGluR5 antagonists. Organic & Biomolecular Chemistry. 2011;9(11):4276–4286. doi: 10.1039/c0ob01190h. [DOI] [PubMed] [Google Scholar]
- 17.Fuganti C, Sacchetti A. Biocatalytic enantioselective approach to 3-aryl-2- nitropropanols: Synthesis of enantioenriched (R)-5-methoxy-3-aminochroman, a key precursor to the antidepressant drug Robalzotan. Journal of Molecular Catalysis B: Enzymatic. 2010;66(3–4):276–284. [Google Scholar]
- 18.Wood K, Black DS, Kumar N. Ring closing metathesis strategies towards functionalised 1,7-annulated 4,6-dimethoxyindoles. Tetrahedron. 2011;67(22):4093–4102. [Google Scholar]
- 19.Kusuma BR, Peterson LB, Zhao H, Vielhauer G, Holzbeierlein J, Blagg BSJ. Targeting the Heat Shock Protein 90 Dimer with Dimeric Inhibitors. Journal of Medicinal Chemistry. 2011;54(18):6234–6253. doi: 10.1021/jm200553w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oozeki H, Tajima R, Nihei K.-i. Molecular design of potent tyrosinase inhibitors having the bibenzyl skeleton. Bioorg Med Chem Lett. 2008;18:5252–5254. doi: 10.1016/j.bmcl.2008.08.053. [DOI] [PubMed] [Google Scholar]
- 21.Bryce MR, Gardiner JM. Functionalised (+/−)-cephalotaxine analogues. Journal of the Chemical Society, Chemical Communications. 1989;(16):1162–1164. [Google Scholar]
- 22.Pei Z, Li X, von Geldern TW, Madar DJ, Longenecker K, Yong H, Lubben TH, Stewart KD, Zinker BA, Backes BJ, Judd AS, Mulhern M, Ballaron SJ, Stashko MA, Mika AK, Beno DWA, Reinhart GA, Fryer RM, Preusser LC, Kempf-Grote AJ, Sham HL, Trevillyan JM. Discovery of ((4R,5S)-5-Amino-4-(2,4,5- trifluorophenyl)cyclohex-1-enyl)-(3- (trifluoromethyl)-5,6-dihydro- [1,2,4]triazolo[4,3- a]pyrazin-7(8H)-yl)methanone (ABT-341), a Highly Potent, Selective, Orally Efficacious, and Safe Dipeptidyl Peptidase IV Inhibitor for the Treatment of Type 2 Diabetes. Journal of Medicinal Chemistry. 2006;49(22):6439–6442. doi: 10.1021/jm060955d. [DOI] [PubMed] [Google Scholar]
- 23.Andrieux CP, Farriol M, Gallardo I, Marquet J. Thermodynamics and kinetics of homolytic cleavage of carbon-oxygen bonds in radical anions obtained by electrochemical reduction of alkyl aryl ethers. Journal of the Chemical Society, Perkin Transactions 2. 2002;(5):985–990. [Google Scholar]
- 24.Calamini B, Silva MC, Madoux F, Hutt DM, Khanna S, Chalfant MA, Saldanha SA, Hodder P, Tait BD, Garza D, Balch WE, Morimoto RI. Smallmolecule proteostasis regulators for protein conformational diseases. Nat Chem Biol. 2012;8(2):185–196. doi: 10.1038/nchembio.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen W, Mi R, Haughey N, Oz M, Höke A. Immortalization and characterization of a nociceptive dorsal root ganglion sensory neuronal line. J. Periph. Nerv. Sys. 2007;12(2):121–130. doi: 10.1111/j.1529-8027.2007.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang L, Zhao H, Blagg BSJ, Dobrowsky RT. C-Terminal Heat Shock Protein 90 Inhibitor Decreases Hyperglycemia-induced Oxidative Stress and Improves Mitochondrial Bioenergetics in Sensory Neurons. J. Proteome Res. 2012;11:2581–2593. doi: 10.1021/pr300056m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vincent AM, Kato K, McLean LL, Soules ME, Feldman EL. Sensory Neurons and Schwann Cells Respond to Oxidative Stress by Increasing Antioxidant Defense Mechanisms. Antioxid Redox Signal. 2009;11:425–438. doi: 10.1089/ars.2008.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vincent AM, Stevens MJ, Backus C, McLean LL, Feldman EL. Cell culture modeling to test therapies against hyperglycemia-mediated oxidative stress and injury. Antioxid Redox Signal. 2005;7(11–12):1494–1506. doi: 10.1089/ars.2005.7.1494. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.