Abstract
The purpose of this study was to investigate interactions between exposure to supportive family environments and genetic characteristics, which were hypothesized to forecast variations in allostatic load (AL) in a representative sample of 315 rural African American youths. Data on family environments were gathered when youths were 11–13, and genetic data were collected when they were 16, years of age. Data on AL were obtained at the beginning of emerging adulthood, age 19 years. The data analyses revealed that, as predicted, emerging adults exposed to less supportive family environments across preadolescence manifested higher levels of AL when they carried the short (s) allele at the 5-HTTLPR and an allele of DRD4 with 7 or more repeats. This is an E(family environment) × G(5-HTTLPR status) × G(DRD4 status) interaction. These data suggest that African American youths carrying genes that confer sensitivity who are exposed to less supportive family environments may be at greater risk for adverse physical health consequences that AL presages.
The well-documented health disparities between African Americans and members of other ethnic groups do not originate in adulthood, but result from changes in biological processes that occur at earlier stages of development (Geronimus, Hicken, Keene, & Bound, 2006). This may be particularly true for African American youths living in the rural South. These youths have a distinctive profile of characteristics. Risk factors include the chronic poverty and limitations in occupational and educational opportunities endemic to these localities, frequent housing adjustments in response to economic pressures, changing employment status, interpersonal and institutional racism, difficulty in accessing medical care, and marginalization by health care professionals (Dressler, Oths, & Gravlee, 2005). Living in such challenging contexts can have deleterious effects on the functioning of biological stress regulatory systems across the lifespan and, ultimately, on health (Shonkoff, Boyce, & McEwen, 2009).
Recent research suggests that coping with cumulative SES-related stressors elicits a cascade of biological responses that may be functional in the short term but over time may “weather” or damage the systems that regulate the body’s stress response. The concept of allostatic load (AL), a marker of chronic physiological stress and cumulative wear and tear on the body, illustrates the disease-promoting potential of continuous adjustment to stress (McEwen, 2002; Sterling & Eyer, 1988). AL is indexed by elevated physiological activity across multiple systems, including the sympathetic adrenomedullary system, the hypothalamic-pituitary-adrenal (HPA) axis, lipid metabolism, fat deposition, indices of inflammation, and immune functioning (McEwen, 2000; Seeman, McEwen, Rowe, & Singer, 2001). If coping demands are elevated or prolonged, the body mobilizes resources more actively than during less demanding periods. The bodies of individuals with high AL, however, become less efficient at turning off the multiple physiological resources marshaled to deal with chronic demands, even during periods of relative calm. This inability to turn off the demand response sets in motion a cascade of physiological changes that contribute to the development of chronic illnesses including hypertension, cardiac disease, diabetes, and stroke (Seeman et al., 2001).
Despite the pivotal role that AL may play in health disparities, no studies to date have focused on African American youths growing up in either urban or rural Southern contexts. To fill the need for such research and to increase understanding of the factors that forecast AL and the vulnerability it confers, we tested potential interactions between exposure to variations in family supportive environment during preadolescence (ages 11 to 13 years) and well-characterized polymorphisms in the dopamine receptor gene, DRD4, and at the 5-HTT linked polymorphic region (5-HTTLPR) in the serotonin transporter gene, SLC6A4, in forecasting AL at age 19. We hypothesized an E×G×G interaction in which youths who are exposed to less supportive family environments and carry both a copy of a short (s) allele at the 5-HTTLPR and an allele of DRD4 with 7 or more repeats (7+R allele) would evince greater AL. In the following sections, we discuss the hypothesized contributions of supportive family environments, 5-HTTLPR and DRD4 genotypes, and their hypothesized interaction to the development of AL.
Supportive Family Environments and AL
A growing body of recent research has tested the hypothesis that family emotional climate during childhood may contribute to vulnerability to chronic diseases later in life (Repetti, Taylor, & Seeman, 2002; Shonkoff et al., 2009). Because these research efforts can be best described as early in their development, disparate literatures (e.g., family violence and retrospective reports of family emotional climates) have been marshaled to support the hypothesis that exposure to supportive or harsh family environments affects biological processes that carry forward to influence health and well-being. For example, researchers who conducted the Adverse Childhood Experiences Study assessed the medical histories of more than 17,000 adults and found that the rates of cardiovascular disease, autoimmune disorders, and premature death were 1.5 to 2.0 times higher among respondents who were exposed to family violence than among those who were not exposed (Dube et al., 2009). Other studies using retrospective designs in which adults reported conflict and warmth in their families of orientation also suggest that childhood psychosocial environments may have long-term effects on biological responses to stress. Adults who reported conflicted and unresponsive family environments during childhood showed elevated physiological responses to stress in the forms of autonomic and cortisol reactivity and compromised metabolic functioning (Lehman, Taylor, Kiefe, & Seeman, 2005). They also evinced elevated inflammatory profiles (Miller & Chen, 2010) and higher resting systolic blood pressure (Chan, Chen, Hibbert, Wong, & Miller, 2011). Taken together, these findings suggest that exposure to supportive family environments in childhood may forecast AL among rural African Americans at the beginning of emerging adulthood.
According to family systems theory, researchers must progress beyond an understanding of adolescent development solely in the context of the parent-child relationship by incorporating collective experiences in the broader family unit into family process models. Homes characterized by high levels of cocaregiver communication and support also evince nurturant-responsive parenting, including emotional availability (Sturge-Apple, Davies, & Cummings, 2006). In addition, children and adolescents exposed to high levels of cocaregiver communication and support experience fewer problems in meeting developmental milestones (e.g., Sturge-Apple, Davies, Winter, Cummings, & Schermerhorn, 2008). Consistent with this family systems perspective, supportive family environments among rural African Americans were operationalized as a set of systemic processes that included high levels of caregiving support, cocaregiver communication, and provision of nurturant-involved parenting. Rural African Americans are more likely than are members of other ethnic groups to view child care and parenting as communal tasks, with mothers often relying on husbands, intimate partners, extended family, and community networks to share in child rearing (see Jones, Zalot, Foster, Sterrett, & Chester, 2007, for a review). Cocaregiving communication and support buffer the effects of negative life events on rural African American mothers’ mental health (Brody & Flor, 1996) and provide children with models of cooperation and problem solving; they are also strongly linked to indicators of nurturant-involved parenting—warmth, support, and vigilance in the mother-child relationship (Dorsey, Forehand, & Brody, 2007; Jones et al., 2007).
Serotonin- and Dopamine-Related Genes and Sensitivity to the Environment
An individual’s reactivity to the family environment clearly has multiple determinants, including genetic inheritance. Genes and their interactions with the environments that children encounter have been conjectured to play an important role in the development of AL (Ganzel, Morris, & Wethington, 2010); knowledge to date, however, is more theoretical than empirical. To begin to understand the operation of such interactions among rural African American youths, we proposed that youths carrying genes that enhance sensitivity to the family environment, the 5-HTTLPR s allele and a DRD4 7+R allele, and who are exposed to low levels of supportive family environments would evince elevated AL. In the present study, we do not posit main effects for family environments or genetic sensitivity. Rather, we propose that some youths will be more reactive to less supportive family environments due to their genetic makeups, and these youths will evince higher AL across time. This perspective is consistent with resilience and differential susceptibility theories, in which genetic variations are hypothesized to render individuals more or less susceptible to environmental risks (Caspi, Hariri, Holmes, Uher, & Moffitt, 2010; Cicchetti & Blender, 2006). In the next section, we describe the 5-HTTLPR and DRD4 genotypes and present evidence that they increase sensitivity to the environment.
5-HTTLPR
The serotonin transporter SLC6A4 is a key regulator of serotonergic neurotransmission, localized to 17p13 and consisting of 14 exons and a single promoter. Variation in the promoter region of the gene, the 5-HTTLPR, results in two main variants, a short (s) and a long (l) allele; the presence of the s allele results in lower serotonin transporter availability. The s variant has 12 copies, and the l variant 14 copies, of a 22-bp repeat element. In the current report, we focus on contrasts between individuals with at least one s allele and those with no s alleles. Several bodies of research support its designation as a gene that confers sensitivity. Research on the neuroscience of information processing indicates that carriers of the s allele direct preferential attention toward, and have more difficulty disengaging from, threat-related stimuli (Beevers, Wells, Ellis, & McGeary, 2009) and are more sensitive to financial stressors (Crişan et al., 2009; Roiser et al., 2009). Others have hypothesized that carriers of the s allele will be sensitive to nurturing environments (Belsky, Bakermans-Kranenburg, & Van IJzendoorn, 2007; Ellis & Boyce, 2011); however, this conjecture has not yet been demonstrated empirically. Of particular relevance to the present study, several studies have described increases in cortisol levels among carriers of the s allele, but not among carriers of ll alleles, when presented with aversive or threatening stimuli (Alexander et al., 2009; Gotlib, Joormann, Minor, & Hallmayer, 2008; Way & Taylor, 2010). This literature suggests that the effects of the 5-HTTLPR s allele on the brain’s neural circuitry may shape risk for elevated AL. Consistent with this conjecture are studies indicating that unfavorable rearing environments forecast emotion dysregulation among carriers of the s allele but not among those homozygous for the l allele (Barr et al., 2004; Pauli-Pott, Friedl, Hinney, & Hebebrand, 2009).
DRD4
The 48bp Variable Number Tandem Repeats (VNTRs) in DRD4 that we examined form a polymorphism in exon 3 that codes for a 16 amino acid insert in the dopamine D4 receptor. The VNTR contains 2 to 11 repeats, with the 4-repeat and 7-repeat alleles being most common. Alleles with 7 or more repeats function in a way that yields a protein structure that produces less reactive D4 receptors in both in vitro and in vivo tests of responsiveness, resulting in weaker transmission of intracellular signals for those with a 7+R allele versus an allele with 6 or fewer repeats (6-R; for example, see Levitan et al., 2006). In this report, a 7+R allele was classified as a sensitivity factor.
Two literatures suggest that those who carry a 7+R allele are more sensitive to environmental influences than are those who do not carry it: observational G×E studies, and studies of differential responsiveness to intervention and prevention programming. Observational G×E studies found that insensitive parenting during infancy predicted aggressive, undercontrolled behavior at age 3 years only among children carrying the DRD4 7-repeat allele; parenting did not predict behavior problems in the absence of that allele (see Bakermans-Kranenburg & Van IJzendoorn, 2011, for a review of this literature). The intervention/prevention literature indicates that children and adolescents who carry the DRD4 7-repeat allele are more likely to show behavioral changes (e.g., less oppositional behavior or drug use) than are carriers of the DRD4 4-repeat allele when they participate in parenting interventions (Bakermans-Kranenburg, Van IJzendoorn, Pijlman, Mesman, & Juffer, 2008) or prevention programs (Beach, Brody, Lei, & Philibert, 2010). Thus, this evidence supports the study hypothesis that both the 5-HTTLPR s allele and a DRD4 7+R allele enhance sensitivity to environmental experiences.
Summary
We investigated exposure to supportive family environments × gene interactions that were hypothesized to lead to variations in AL in a representative sample of 315 rural African Americans. Data on family environments were gathered when youths were 11 to 13 years of age, and genetic data were collected when youths were 16 years of age. AL data were obtained at the beginning of emerging adulthood, at age 19 years. This design is consistent with allostasis theory (McEwen, 2002), which proposes that exposure to contextual challenges requires physiological accommodations that become evident in markers of physiological stress over time. This design allowed us to test an E(supportive family environment) × G(5-HTTLPR) × G(DRD4) hypothesis.
Method
Participants
African American primary caregivers and a target youth selected from each family participated in annual data collections; target youths’ mean age was 11.2 years at the first assessment and 19.2 years at the last assessment. Of the youth in the sample, 53% were female. At baseline, 78% of the caregivers had completed high school or earned a GED. The families resided in nine rural counties in Georgia, in small towns and communities in which poverty rates are among the highest in the nation and unemployment rates are above the national average (Proctor & Dalaker, 2003). Although the primary caregivers in the sample worked an average of 39.4 hours per week, at the first assessment 46.3% lived below federal poverty standards with a median family income per month of $1655; at the last assessment, the proportion was 49.1% with a median income of $1169. The increase in the proportion of families living in poverty and the decrease in family income over time were due to the economic recession that was occurring during 2010, when the last wave of data was collected. Overall, the families can be characterized as working poor.
At the first assessment, 667 families were selected randomly from lists that schools provided of 5th-grade students (see Brody et al., 2004, for a full description). From a sample of 561 at age 19 (a retention rate of 84%), 500 emerging adults were selected randomly to take part in the assessments of AL; of this subsample, 489 agreed to participate. Of the 489 participants for whom AL data were collected, 387 had been genotyped at age 16 for both DRD4 and the 5-HTTLPR. Of these participants’ primary caregivers, 315 indicated that a cocaregiver helped to rear the target youth. These 315 families constituted the sample in the present study. Independent sample t-tests on all study and demographic variables compared families with and without cocaregivers when youths were ages 11 to 13. These analyses did not reveal any significant group mean differences on any variables. An additional attrition analysis revealed no differences on any variable between families who did or did not provide data when youths were 13 and 19 years of age.
Procedure
All data were collected in participants’ homes using a standardized protocol. Primary caregivers consented to their own and the youths’ participation in the study, and youths assented to their own participation. At each wave of data collection, two African American field researchers conducted one visit that lasted approximately 2 hours. The field researchers interviewed the primary caregiver and the target youth separately and privately, with no other family members present or able to overhear the conversation.
Measures
Controlled parental variables
Parents’ ages and the averages of their responses to the Center for Epidemiologic Studies Depression scale (CES–D; Radloff, 1977), which is widely used with community samples, were recorded. Responses to the CES–D were averaged across waves of data collection; the average alpha coefficient was .87.
Preadolescent supportive family environment
Three waves of preadolescent data were collected when the target youths were 11, 12, and 13 years of age.
Coparenting
Coparent communication and support were assessed using the Parenting Convergence Scale (PCS; Ahrons, 1981). The primary caregiver was first asked to identify a person who assisted her or him in caring for the target youth; those who could identify such a person subsequently completed this scale. Of the identified cocaregivers, 45.3% were the youths’ biological fathers, 12.6% were stepfathers, 12.3% were mothers’ romantic partners, 6.5% were grandmothers, 1.6% were aunts, 11.3% were other individuals, and 10.4% were not specified.
Each item on the PCS was rated on a Likert-type scale ranging from 1 (never) to 5 (always). Coparenting communication was measured in reference to the person who helped rear the target youth using the six-item Communication subscale. The items were, “How often do you and this person: make big decisions together about your child’s life; talk about the school or health problems of your child together; plan special events in your child’s life together; make day-to-day decisions together about your child’s life; talk about the good things your child does together; and talk about the way your child acts?” Alphas across the three preadolescent waves ranged from .91 to .93.
Coparenting support was measured using the two-item Support subscale: “When you need help with your child, how often do you go to this person for help?” and “How often would you say that this person is a help to you in raising your child?” Alphas across the three preadolescent waves ranged from .70 to .77.
Nurturant-involved parenting
Primary caregivers also reported nurturant-involved parenting using an instrument that we developed in our previous research with rural African American families (Brody et al., 2001). The nine questions that comprise this measure were rated on a Likert-type scale ranging from 1 (never) to 4 (always). Example items include, “How often do you and your child talk about things that bother him/her?” and “How often do you ask your child what he/she thinks before deciding on family matters that involve your child?” Alphas across the three preadolescent waves ranged from .76 to .82.
The measures of supportive family environment were standardized and averaged across the three data collection waves and then summed to form the supportive family environment construct. Prior to summing, we determined that the individual measures were highly intercorrelated (all ps < .01).
Emerging adult AL
The protocol for measuring AL in emerging adulthood when youths were 19 years of age was based on procedures that Evans (2003) developed for field studies involving children and adolescents. Resting blood pressure was monitored with a Critikon Dinamap Pro 100 (Critikon; Tampa, FL) while the youth sat reading quietly. Seven readings were taken every 2 minutes, and the average of the second through the seventh readings was used as the resting index. This procedure yields highly reliable indices of chronic resting blood pressure (Kamarck et al., 1992). Assays of overnight (8 p.m. to 8 a.m.) urinary catecholamines and cortisol were assayed beginning on the evening of data collection. All urine that the emerging adult voided during this time was stored on ice in a container with metabisulfite as a preservative. Total volume was recorded, and four 10-ml samples were randomly extracted and deep frozen at −80° C until subsequent assays were completed. The pH of two of these samples was adjusted to 3 to inhibit oxidation of catecholamines. The frozen urine was delivered to the Emory University Hospital medical laboratory in Atlanta, Georgia, for assaying. Total unbound cortisol was assayed with a radioimmune assay (Contreras, Hane, & Tyrrell, 1986). Epinephrine and norepinephrine were assayed with high-pressure liquid chromatography with electrochemical detection (Riggin & Kissinger, 1977). Creatinine was assayed to control for differences in body size and incomplete urine voiding (Tietz, 1976). Technicians blind to the participants’ cumulative risk status assayed the samples.
AL was calculated by summing the standardized scores of six indicators: overnight cortisol, epinephrine, and norepinephrine; resting diastolic and systolic blood pressure; and fat deposits measured via body mass index (weight in kilograms divided by the square of height in meters). Prior studies of AL in adults (Seeman, Singer, Ryff, Dienberg Love, & Levy-Storms, 2002) and in children (Evans, 2003) used similar metrics, combining multiple physiological indicators of risk into a total AL index.
Genotyping
Participants’ DNA was obtained using Oragene DNA kits (Genetek; Calgary, Alberta, Canada). Participants rinsed their mouths with tap water, then deposited 4 ml of saliva in the Oragene sample vial. The vial was sealed, inverted, and shipped via courier to a central laboratory in Iowa City, where samples were prepared according to the manufacturer’s specifications. Genotype at DRD4 was determined for each participant as described by Lichter et al. (1993). For tests of the G×E hypotheses, DRD4 status was dummy coded; participants carrying a 7+R allele were assigned a code of 1 (n = 143, 45.4% of the sample), and participants who carried a 6-R allele were assigned a code of 0 (n = 172, 54.6% of the sample). Genotype at the 5-HTTLPR was determined for each sample as described by Bradley, Dodelzon, Sandhu, and Philibert (2005) using the primers F-GGCGTTGCCGCTCTGAATGC and R-GAGGGACTGA GCTGGACAAC CAC, standard Taq polymerase and buffer, standard dNTPs with the addition of 100μmM7-deaza GTP, and 10% DMSO. The resulting polymerase chain reaction products were electrophoresed on a 6% nondenaturing polyacrylamide gel, and products were visualized using silver staining. Genotype was then called by two individuals blind to the study hypotheses and other information about the participants. Of the sample, 5.4% were homozygous for the s allele (ss), 36.5% were heterozygous (sl), and 58.1% were homozygous for the l allele (ll). Consistent with prior research (Hariri et al., 2005), genotyping results were used to form two groups of participants: those homozygous for the l allele (coded as 0, n = 183, 58.1%) and those with either one or two copies of the s allele (coded as 1, n = 132, 41.9%). None of the DRD4 or 5-HTTLPR alleles deviated from Hardy–Weinberg equilibrium.
Results
Plan of Analysis for the Study Hypothesis
Linear regression analysis was used to test the study hypothesis. Three regression models were executed on AL. Family per capita income, youth gender, parental age, and parental depression were controlled in all models. The first model was designed to examine associations among AL, supportive family environment, 5-HTTLPR status, and the interaction of supportive family environment with 5-HTTLPR status. The second model was executed to estimate the main effects of supportive family environment, DRD4 status, and the interaction of supportive family environment with DRD4 status. The third model estimated the contributions of supportive family environment, 5-HTTLPR status, DRD4 status, their respective two-way interactions, and the three-way supportive family environment × 5-HTTLPR status × DRD4 status interaction. The results of these analyses are presented in Table 1.
Table 1.
Supportive Family Environment and Genetic Diversity as Predictors of Allostatic Load (N = 315)
| Allostatic Load
|
||||||
|---|---|---|---|---|---|---|
| Model 1
|
Model 2
|
Model 3
|
||||
| Predictors | Estimate | SE | Estimate | SE | Estimate | SE |
| 1. Per capita income | −.001 | .001 | −.001 | .001 | −.001 | .001 |
| 2. Gender (male) | .519 | .341 | .504 | .343 | .593 | .338 |
| 3. Parents’ age | −.073** | .026 | −.066* | .025 | −.072** | .025 |
| 4. Parental depression | −.000 | .022 | .002 | .022 | .003 | .021 |
| 5. Supportive family environment | .121 | .097 | .009 | .098 | .030 | .125 |
| 6. 5-HTTLPR (short allele) | .421 | .347 | - | - | −.187 | .453 |
| 7. 5-HTTLPR × supportive family environment | −.326* | .143 | - | - | −.040 | .189 |
| 8. DRD4 (7+R allele) | - | - | .547 | .343 | −.054 | .437 |
| 9. DRD4 × supportive family environment | - | - | −.080 | .144 | .217 | .188 |
| 10. 5-HTTLPR × DRD4 | - | - | - | - | 1.663* | .687 |
| 11. 5-HTTLPR × DRD4 × supportive family environment | - | - | - | - | −.682* | .287 |
SE = standard error. 7+R = allele of DRD4 with 7 or more repeats.
p < .05.
p < .01.
Test of the Study Hypothesis
In all models, 5-HTTLPR and DRD4 were dummy coded. Participants with ss or sl genotypes at the 5-HTTLPR were assigned a code of 1, those with ll genotypes were assigned a code of 0, and the genetic status predictor was regressed on AL. For DRD4, participants carrying a 7+R allele were assigned a code of 1 and those with a 6-R allele were assigned a code of 0. The first model, presented in Table 1, Model 1, revealed a significant interaction between supportive family environments and 5-HTTLPR status. Interpretation of this interaction awaited the results of the test of the E×G×G interaction. We also examined the possibility that a gene-environment correlation could exist between 5-HTTLPR status or DRD4 status and one or more of the subscales that comprised the supportive family environment construct. None of the correlations approached statistical significance.
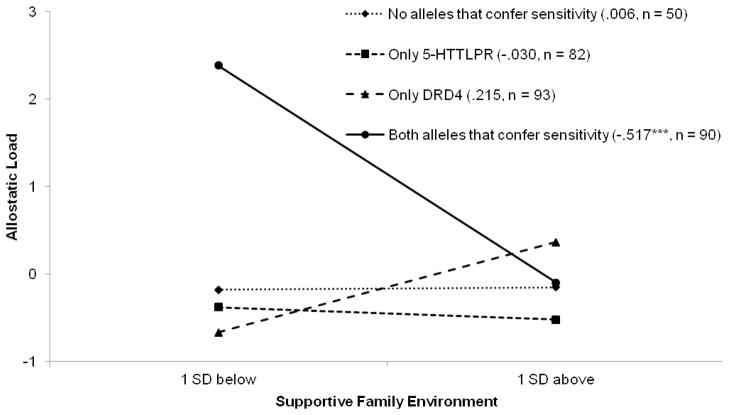
The second model, presented in Table 1, Model 2, was designed to determine whether supportive family environments interacted with DRD4 status; this interaction was not significant. The last model tested the primary E×G×G study hypothesis; the results are presented in Table 1, Model 3. The hypothesized supportive family environment × 5-HTTLPR status × DRD4 status interaction significantly predicted AL beyond the previous variables in the model, β = −.682, p < .02. To interpret this finding, we plotted AL for supportive family environments at 1 standard deviation below and 1 standard deviation above the sample mean for youths with no sensitivity alleles, only 5-HTTLPR sensitivity, only DRD4 sensitivity, or both 5-HTTLPR and DRD4 sensitivity. These data are illustrated in Figure 1. Consistent with the study hypothesis, compared with youths who carried one or no genes conferring sensitivity, youths carrying two genes that confer sensitivity evinced the highest AL when they were exposed to unsupportive family environments. Of particular importance was the finding that youths exposed to highly supportive family environments evinced low AL whether they carried two, one, or no genes that confer sensitivity.
Figure 1.
The effect of supportive family environment on allostatic load by 5-HTTLPR and DRD4 genotypes. Numbers in parentheses refer to slopes for each genotype group.
***p < .001.
Discussion
In this study, we found that exposure to family environments characterized by low levels of support across preadolescence interacts with the s allele at the 5-HTTLPR and a 7+R allele of DRD4 to predict AL during emerging adulthood at age 19. Individuals carrying these alleles exhibit higher AL when they are exposed to less supportive family environments. To the best of our knowledge, this is the first article to present data on family environment × sensitivity gene interactions that forecast variations in AL in an African American, or any other, population. Consistent with the study hypothesis, we did not find main effects for supportive family environments or sensitivity genes on AL. Instead, we predicted and found that exposure to less supportive family environments can exert a long-term effect on AL for youths who carry genes that render them more sensitive to the environment. These findings are consistent with propositions that poor health and health disparities during adulthood are tied to experiences earlier in life, particularly for persons growing up with the stressors associated with low SES (Shonkoff et al., 2009). The findings are also consistent with theoretical propositions that some individuals are more susceptible to environmental systems than others. Thus, not all children and adolescents who lack supportive family environments will develop physiological dysregulation arising from exposure to SES-related contextual stress. The present results also reinforce research conducted with animals and humans indicating that warm and supportive family environments have beneficial effects on the functioning of biological stress regulatory mechanisms across the life span and, ultimately, on health (Repetti, Taylor, & Saxbe, 2007). The observed buffering effects of exposure to supportive family environments among rural African Americans extends this literature.
Most G×E research has been guided by the diathesis-stress framework in which individuals are viewed as carrying risk alleles, genetic variants that render them more vulnerable than others to adverse conditions. Other researchers have proposed an alternative differential susceptibility framework (Belsky & Pluess, 2009; Ellis, Boyce, Belsky, Bakermans-Kranenburg, & Van IJzendoorn, 2011). They maintain that individuals thought to be at genetic risk in the presence of adversity are really genetically primed to be more sensitive to environmental influences; they are not only more vulnerable to environmental adversity, but also more likely to benefit from environmental support. To date, the differential sensitivity framework has been supported mainly by data from studies that investigate parenting effects on youth outcomes such as depression and conduct problems (Belsky & Pluess, 2009), prosocial behavior (Knafo, Israel, & Ebstein, 2011), and attachment style (Bakermans-Kranenburg & Van IJzendoorn, 2007). The outcome of the present study supports the diathesis-stress perspective, with youths who carry susceptibility genes evincing higher AL at age 19 when they experienced less supportive family environments. Clearly, future research will address the possibility of boundary conditions on differential susceptibility effects. The present results suggest that more research is needed to determine whether genetic susceptibility effects extend to biological as well as psychosocial outcomes.
It is unclear how or through what mechanisms family environments buffer development of AL. Emotionally supportive family environments may decrease the need for youth to be hypervigilant in their social milieus because the family provides an arena of comfort and sense of safety that is generalized to other contexts. Fewer mobilizations of stress responses and less wear and tear on bodily systems would provide evidence for such a reduction in vigilance. Emotionally supportive family environments may also facilitate the development of emotion regulation that attenuates or downregulates reactivity to negative contextual events (cf. Repetti et al., 2002). Clearly, research is needed to identify intermediate processes that link the interaction of family environments and genes that confer sensitivity to AL.
Limitations of the present study should be noted. Future replications of this prospective study with younger rural African American children are indicated. Understanding the complex relationships among early family environments, genes that confer sensitivity, and indicators of AL, particularly among African American children living in impoverished rural Southern contexts, is highly relevant to public health. It is not known whether the results of this study generalize to European American or Latino families living in either rural or urban communities. In attempting future replications, researchers should note whether their study populations differ in the prevalence of the polymorphisms included in this study. Again, such studies are needed because of their importance for understanding the development of AL and the vulnerability it confers. Finally, implications for prevention should be considered. The present results suggest that youths who carry sensitivity genes and are exposed to family environments that offer little support are at high risk for hypertension, obesity, and dysregulation across biological regulatory systems. Research on the design of preventive interventions for these youths may yield approaches that are effective in deterring their subsequent development of chronic disease.
Acknowledgments
This research was supported by Award Number R01HD030588 from the National Institute of Child Health and Human Development and Award Number P30DA027827 from the National Institute on Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Child Health and Human Development, the National Institute on Drug Abuse, or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/fam
Contributor Information
Gene H. Brody, Center for Family Research, University of Georgia
Tianyi Yu, Center for Family Research, University of Georgia.
Yi-fu Chen, Center for Family Research, University of Georgia.
Steven M. Kogan, Department of Child and Family Development and Center for Family Research, University of Georgia
Gary W. Evans, Department of Design and Environmental Analysis and Department of Human Development, Cornell University
Michael Windle, Department of Behavioral Sciences and Health Education, Emory University.
Meg Gerrard, Department of Psychiatry, Dartmouth College.
Frederick X. Gibbons, Department of Psychological and Brain Sciences, Dartmouth College
Ronald L. Simons, Department of Sociology, University of Georgia
Robert A. Philibert, Department of Psychiatry, University of Iowa
References
- Ahrons CR. The continuing coparental relationship between divorced spouses. American Journal of Orthopsychiatry. 1981;51:415–428. doi: 10.1111/j.1939-0025.1981.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Alexander N, Kuepper Y, Schmitz A, Osinsky R, Kozyra E, Hennig J. Gene–environment interactions predict cortisol responses after acute stress: Implications for the etiology of depression. Psychoneuroendocrinology. 2009;34:1294–1303. doi: 10.1016/j.psyneuen.2009.03.017. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, Van IJzendoorn MH. Research review: Genetic vulnerability or differential susceptibility in child development: The case of attachment. Journal of Child Psychology and Psychiatry. 2007;48:1160–1173. doi: 10.1111/j.1469-7610.2007.01801.x. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, Van IJzendoorn MH. Differential susceptibility to rearing environment depending on dopamine-related genes: New evidence and a meta-analysis. Development and Psychopathology. 2011;23:39–52. doi: 10.1017/S0954579410000635. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, Van IJzendoorn MH, Pijlman FTA, Mesman J, Juffer F. Experimental evidence for differential susceptibility: Dopamine D4 receptor polymorphism (DRD4 VNTR) moderates intervention effects on toddlers’ externalizing behavior in a randomized controlled trial. Developmental Psychology. 2008;44:293–300. doi: 10.1037/0012-1649.44.1.293. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Shannon C, Parker C, Dvoskin RL, Becker ML, …Higley JD. Rearing condition and rh5-HTTLPR interact to influence limbic-hypothalamic-pituitary-adrenal axis response to stress in infant macaques. Biological Psychiatry. 2004;55:733–738. doi: 10.1016/j.biopsych.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Beach SRH, Brody GH, Lei MK, Philibert RA. Differential susceptibility to parenting among African American youths: Testing the DRD4 hypothesis. Journal of Family Psychology. 2010;24:513–521. doi: 10.1037/a0020835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beevers CG, Wells TT, Ellis AJ, McGeary JE. Association of the serotonin transporter gene promoter region (5-HTTLPR) polymorphism with biased attention for emotional stimuli. Journal of Abnormal Psychology. 2009;118:670–681. doi: 10.1037/a0016198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Bakermans-Kranenburg MJ, Van IJzendoorn MH. For better and for worse: Differential susceptibility to environmental influences. Current Directions in Psychological Science. 2007;16:300–304. doi: 10.1111/j.1467-8721.2007.00525.x. [DOI] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Bradley SL, Dodelzon K, Sandhu HK, Philibert RA. Relationship of serotonin transporter gene polymorphisms and haplotypes to mRNA transcription. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2005;136:58–61. doi: 10.1002/ajmg.b.30185. [DOI] [PubMed] [Google Scholar]
- Brody GH, Flor DL. Coparenting, family interactions, and competence among African American youths. In: McHale JP, Cowan PA, editors. Understanding how family-level dynamics affect children’s development: Studies of two-parent families. San Francisco, CA: Jossey-Bass; 1996. pp. 77–91. [DOI] [PubMed] [Google Scholar]
- Brody GH, Ge X, Conger RD, Gibbons FX, Murry VM, Gerrard M, Simons RL. The influence of neighborhood disadvantage, collective socialization, and parenting on African American children’s affiliation with deviant peers. Child Development. 2001;72:1231–1246. doi: 10.1111/1467-8624.00344. [DOI] [PubMed] [Google Scholar]
- Brody GH, Murry VM, Gerrard M, Gibbons FX, Molgaard V, McNair LD, Neubaum-Carlan E. The Strong African American Families program: Translating research into prevention programming. Child Development. 2004;75:900–917. doi: 10.1111/j.1467-8624.2004.00713.x. [DOI] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: The case of the serotonin transporter gene and its implications for studying complex diseases and traits. American Journal of Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan M, Chen E, Hibbert AS, Wong JHK, Miller GE. Implicit measures of early-life family conditions: Relationships to psychosocial characteristics and cardiovascular disease risk in adulthood. Health Psychology. 2011;30:570–578. doi: 10.1037/a0024210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Blender JA. A multiple-levels-of-analysis perspective on resilience: Implications for the developing brain, neural plasticity, and preventive interventions. Annals of the New York Academy of Sciences. 2006;1094:248–258. doi: 10.1196/annals.1376.029. [DOI] [PubMed] [Google Scholar]
- Contreras LN, Hane S, Tyrrell JB. Urinary cortisol in the assessment of pituitary-adrenal function: utility of 24-hour and spot determinations. Journal of Clinical Endocrinology and Metabolism. 1986;62:965–969. doi: 10.1210/jcem-62-5-965. [DOI] [PubMed] [Google Scholar]
- Crişan LG, Pană S, Vulturar R, Heilman RM, Szekely R, Drugă B, …Miu AC. Genetic contributions of the serotonin transporter to social learning of fear and economic decision making. Social Cognitive and Affective Neuroscience. 2009;4:399–408. doi: 10.1093/scan/nsp019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsey S, Forehand R, Brody G. Coparenting conflict and parenting behavior in economically disadvantaged single parent African American families: The role of maternal psychological distress. Journal of Family Violence. 2007;22:621–630. doi: 10.1007/s10896-007-9114-y. [DOI] [Google Scholar]
- Dressler WW, Oths KS, Gravlee CC. Race and ethnicity in public health research: Models to explain health disparities. Annual Review of Anthropology. 2005;34:231–252. doi: 10.1146/annurev.anthro.34.081804.120505. [DOI] [Google Scholar]
- Dube SR, Fairweather D, Pearson WS, Felitti VJ, Anda RF, Croft JB. Cumulative childhood stress and autoimmune diseases in adults. Psychosomatic Medicine. 2009;71:243–250. doi: 10.1097/PSY.0b013e3181907888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT. Differential susceptibility to the environment: Toward an understanding of sensitivity to developmental experiences and context. Development and Psychopathology. 2011;23:1–5. doi: 10.1017/S095457941000060X. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, Van IJzendoorn MH. Differential susceptibility to the environment: An evolutionary–neurodevelopmental theory. Development and Psychopathology. 2011;23:7–28. doi: 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- Evans GW. A multimethodological analysis of cumulative risk and allostatic load among rural children. Developmental Psychology. 2003;39:924–933. doi: 10.1037/0012-1649.39.5.924. [DOI] [PubMed] [Google Scholar]
- Ganzel BL, Morris PA, Wethington E. Allostasis and the human brain: Integrating models of stress from the social and life sciences. Psychological Review. 2010;117:134–174. doi: 10.1037/a0017773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among Blacks and Whites in the United States. American Journal of Public Health. 2006;96:826–833. doi: 10.2105/AJPH.2004.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J, Minor KL, Hallmayer J. HPA axis reactivity: A mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biological Psychiatry. 2008;63:847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, Weinberger DR. A susceptibility gene for affective disorders and the response of the human amygdala. Archives of General Psychiatry. 2005;62:146–152. doi: 10.1001/archpsyc.62.2.146. Retrieved from http://archpsyc.ama-assn.org/cgi/content/abstract/62/2/146. [DOI] [PubMed] [Google Scholar]
- Jones DJ, Zalot AA, Foster SE, Sterrett E, Chester C. A review of childrearing in African American single mother families: The relevance of a coparenting framework. Journal of Child and Family Studies. 2007;16:671–683. doi: 10.1007/s10826-006-9115-0. [DOI] [Google Scholar]
- Kamarck TW, Jennings JR, Debski TT, Glickman-Weiss E, Johnson PS, Eddy MJ, Manuck SB. Reliable measures of behaviorally-evoked cardiovascular reactivity from a PC-based test battery: Results from student and community samples. Psychophysiology. 1992;29:17–28. doi: 10.1111/j.1469-8986.1992.tb02006.x. [DOI] [PubMed] [Google Scholar]
- Knafo A, Israel S, Ebstein RP. Heritability of children’s prosocial behavior and differential susceptibility to parenting by variation in the dopamine receptor D4 gene. Development and Psychopathology. 2011;23:53–67. doi: 10.1017/S0954579410000647. [DOI] [PubMed] [Google Scholar]
- Lehman BJ, Taylor SE, Kiefe CI, Seeman TE. Relation of childhood socioeconomic status and family environment to adult metabolic functioning in the CARDIA study. Psychosomatic Medicine. 2005;67:846–854. doi: 10.1097/01.psy.0000188443.48405.eb. [DOI] [PubMed] [Google Scholar]
- Levitan RD, Masellis M, Lam RW, Kaplan AS, Davis C, Tharmalingam S, Kennedy JL. A birth-season/DRD4 gene interaction predicts weight gain and obesity in women with seasonal affective disorder: A seasonal thrifty phenotype hypothesis. Neuropsychopharmacology. 2006;31:2498–2503. doi: 10.1038/sj.npp.1301121. [DOI] [PubMed] [Google Scholar]
- Lichter JB, Barr CL, Kennedy JL, Van Tol HHM, Kidd KK, Livak KJ. A hypervariable segment in the human dopamine receptor D4 (DRD4) gene. Human Molecular Genetics. 1993;2:767–773. doi: 10.1093/hmg/2.6.767. [DOI] [PubMed] [Google Scholar]
- McEwen BS. The neurobiology of stress: From serendipity to clinical relevance. Brain Research. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Sex, stress and the hippocampus: Allostasis, allostatic load and the aging process. Neurobiology of Aging. 2002;23:921–939. doi: 10.1016/S0197-4580(02)00027-1. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E. Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychological Science. 2010;21:848–856. doi: 10.1177/0956797610370161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli-Pott U, Friedl S, Hinney A, Hebebrand J. Serotonin transporter gene polymorphism (5-HTTLPR), environmental conditions, and developing negative emotionality and fear in early childhood. Journal of Neural Transmission. 2009;116:503–512. doi: 10.1007/s00702-008-0171-z. [DOI] [PubMed] [Google Scholar]
- Proctor BD, Dalaker J. Poverty in the United States: 2002 (Current Population Reports, P60-222) Washington, DC: U.S. Bureau of the Census; 2003. Retrieved from http://www.dlc.org/documents/Census_2002_Poverty.pdf. [Google Scholar]
- Radloff LS. The CES–D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- Repetti RL, Taylor SE, Saxbe D. The influence of early socialization experiences on the development of biological systems. In: Grusec JE, Hastings PD, editors. Handbook of socialization: Theory and research. New York, NY: Guilford Press; 2007. [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin. 2002;128:330–336. doi: 10.1037/0033-2909.128.2.330. [DOI] [PubMed] [Google Scholar]
- Riggin RM, Kissinger PT. Determination of catecholamines in urine by reverse-phase liquid chromatography with electrochemical detection. Analytical Chemistry. 1977;49:2109–2111. doi: 10.1021/ac50021a052. [DOI] [PubMed] [Google Scholar]
- Roiser JP, de Martino B, Tan GCY, Kumaran D, Seymour B, Wood NW, Dolan RJ. A genetically mediated bias in decision making driven by failure of amygdala control. Journal of Neuroscience. 2009 May 6;29:5985–5991. doi: 10.1523/JNEUROSCI.0407-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: McArthur studies of successful aging. Proceedings of the National Academy of Sciences of the USA. 2001;98:4770–4775. doi: 10.1073/pnas.081072698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman TE, Singer BH, Ryff CD, Dienberg Love G, Levy-Storms L. Social relationships, gender, and allostatic load across two age cohorts. Psychosomatic Medicine. 2002;64:395–406. doi: 10.1097/00006842-200205000-00004. Retrieved from http://www.psychosomaticmedicine.org/content/64/3/395.abstract. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: Building a new framework for health promotion and disease prevention. Journal of the American Medical Association. 2009 Jun 3;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Sterling P, Eyer J. Allostasis: A new paradigm to explain arousal pathology. In: Fisher S, Reason J, editors. Handbook of life stress, cognition and health. Oxford, UK: Wiley; 1988. pp. 629–649. [Google Scholar]
- Sturge-Apple ML, Davies PT, Cummings EM. Hostility and withdrawal in marital conflict: Effects on parental emotional unavailability and inconsistent discipline. Journal of Family Psychology. 2006;20:227–238. doi: 10.1037/0893-3200.20.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturge-Apple ML, Davies PT, Winter MA, Cummings EM, Schermerhorn A. Interparental conflict and children’s school adjustment: The explanatory role of children’s internal representations of interparental and parent-child relationships. Developmental Psychobiology. 2008;44:1678–1690. doi: 10.1037/a0013857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietz NW, editor. Fundamentals of clinical chemistry. 2. Philadelphia, PA: Saunders; 1976. [Google Scholar]
- Way BM, Taylor SE. The serotonin transporter promoter polymorphism is associated with cortisol response to psychosocial stress. Biological Psychiatry. 2010;67:487–492. doi: 10.1016/j.biopsych.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]