Background: Modifications of the RNA polymerase II CTD are necessary for transcriptional regulation.
Results: Perturbation of O-GlcNAc addition and removal showed transcription defects in vitro and in vivo.
Conclusion: O-GlcNAc modification of the CTD functions in transcription initiation.
Significance: These data provide an additional modification of the CTD that acts before the initiation of transcription.
Keywords: O-GlcNAc, Promoters, RNA Polymerase II, Transcription, Transcription Regulation
Abstract
The RNA polymerase II C-terminal domain (CTD), which serves as a scaffold to recruit machinery involved in transcription, is modified post-translationally. Although the O-GlcNAc modification of RNA polymerase II CTD was documented in 1993, its functional significance remained obscure. We show that O-GlcNAc transferase (OGT) modified CTD serine residues 5 and 7. Drug inhibition of OGT and OGA (N-acetylglucosaminidase) blocked transcription during preinitiation complex assembly. Polymerase II and OGT co-immunoprecipitated, and OGT is a component of the preinitiation complex. OGT shRNA experiments showed that reduction of OGT causes a reduction in transcription and RNA polymerase II occupancy at several B-cell promoters. These data suggest that the cycling of O-GlcNAc on and off of polymerase II occurs during assembly of the preinitiation complex. Our results define unexpected roles for both the CTD and O-GlcNAc in the regulation of transcription initiation in higher eukaryotes.
Introduction
Eukaryotic RNA polymerase II has a unique structure at its C terminus, the C-terminal domain (CTD).3 The CTD in humans consists of 52 imperfect repeats of the consensus sequence YSPTSPS (1, 2), with serines 2, 5, and 7 phosphorylated subsequent to transcription initiation (3, 4). Current models state that two species of RNA polymerase II (pol II) exist: unphosphorylated (pol IIA) and phosphorylated (pol IIO) (5). In vitro transcription assays showed that pol IIA was incorporated into preinitiation complexes (PICs) that form on promoter DNA. pol IIO forms upon the initiation of transcription and defines the elongation-specific form of pol II (6–10). However, neither state of the CTD affects the intrinsic catalytic activity of pol II, and in vitro, in purified systems, a CTD-less pol II is capable of directing transcription (11–14). Rather, CTD phosphorylation serves to recruit various post-transcriptional mRNA processing factors to the elongating polymerase (2). The CTD likely also has a role during the initiation of transcription, because of the defects seen in initiation using truncated CTDs (15, 16) and the functional and physical interactions of the Mediator coactivator complex with the CTD (17–22).
However, other work suggests that evidence that pol IIA is the form of pol II entering the PIC should be reconsidered. Kelly et al. (23) show that “unmodified” pol II (pol IIA), (purified from calf thymus as in Ref. 7) contains a population of pol II that is O-GlcNAcylated on the CTD. O-GlcNAc (N-acetylglucosamine) is a post-translational modification added to serine and threonine residues via an O-linkage by O-GlcNAc transferase (OGT) and UDP-GlcNAc (24). Removal of O-GlcNAc is catalyzed by the N-acetyl-β-d-glucosaminidase (OGA) (25). Edman degradation indicates that the threonine at position 4 and the serine at position 5 of the CTD are O-GlcNAcylated (23). There is no O-GlcNAcylation of the elongation-specific phosphorylated CTD (RNA polymerase IIO). Secondly, O-GlcNAc-modified CTD peptides are refractory to further phosphorylation by the Ser-5-specific CTD kinase CDK7, a component of the general transcription factor TFIIH, consistent with the proposal that O-GlcNAc and phosphorylation are often mutually exclusive (26). However, the functional consequences of O-GlcNAc modification of the CTD and whether the O-GlcNAc modification of the CTD has a role in transcription have not been determined.
These data suggest that it is not clear which form of pol II is entering the PIC, as the experiments in Chesnut et al. (7) do not distinguish between a true unmodified pol II and O-GlcNAc-modified pol II. We therefore have attempted to discern the functional significance of GlcNAc-modified RNA pol II. We provide several lines of evidence that pol II is O-GlcNAc-modified and that pol II O-GlcNAcylation occurs in vitro and in vivo. These data suggest that pol II undergoes a cycle of O-GlcNAcylation at the promoter during assembly of the preinitiation complex, which is required for promoter activity and/or regulation. Furthermore, these data point to O-GlcNAc-pol II as being an initiation-specific pol II species.
EXPERIMENTAL PROCEDURES
Reagents
The reagents used were STO45849 (Tim Tec), alloxan (Sigma), 8WG16 (Covance), anti-OGT AL28 or AL24 (Hart laboratory), anti-TBP 3G3 mAb (Chemicon), Dynabeads (Invitrogen), UDP-GlcNAc (Sigma), 110.6 (Hart laboratory), E3 promoter DNA (27), and anti-pol II N20, A20, and F12 (Santa Cruz Biotechnology). Anti-TFIIA (rabbit), anti-TFIIF (rabbit), and anti-TFIIH mAb were provided courtesy of D. Reinberg. O-(2-Acetamido-2-deoxy-d-glucopyranosylidene)amino-N-phenylcarbamate (PUGNAc) and 1,2-dideoxy-2′-methyl-d-glucopyranoso[2,1-d]-2′-thiazoline (GlcNAc-thiazoline (NAGT)) were synthesized by the Medicinal Chemistry Core Facility at Johns Hopkins University. Wheat germ agglutinin (WGA) resin (Vector Laboratories), protein gel/MOPS buffers (Invitrogen) H5/H14 (Covance), TFIIH (ProteinOne), rP-TEFb (ProteinOne), anti-GST antibody (Santa Cruz), and Complete protease inhibitors (Roche Applied Science) were also used.
In Vitro Transcriptions
Assays were as described (28). Briefly, HeLa nuclear extracts (25–50 μg) were incubated with 0.5 mm NTPs and 0.1 μg of supercoiled promoter DNA for 30 min. RNA was extracted and used in primer extension assays with 32P-end-labeled primers. 10% acrylamide/TBE/urea gels were used to separate RNA products, which were visualized by autoradiography. Human RNA polymerase IIA was supplied by the laboratory of D. Reinberg (NYU School of Medicine). Inhibitor concentrations were: STO45849, 0.05–0.4 mm; PUGNAc, 0.2–4 mm (pol IIA add-back experiment (Fig. 5, D and E, 4 mm)). In Figs. 2 and 3, C and D, inhibitors were added concomitantly with NTPs. In Fig. 3, C and D, an equivalent amount of either pol II A or pol IIγ was added to PUGNAc-treated nuclear extract.
FIGURE 5.
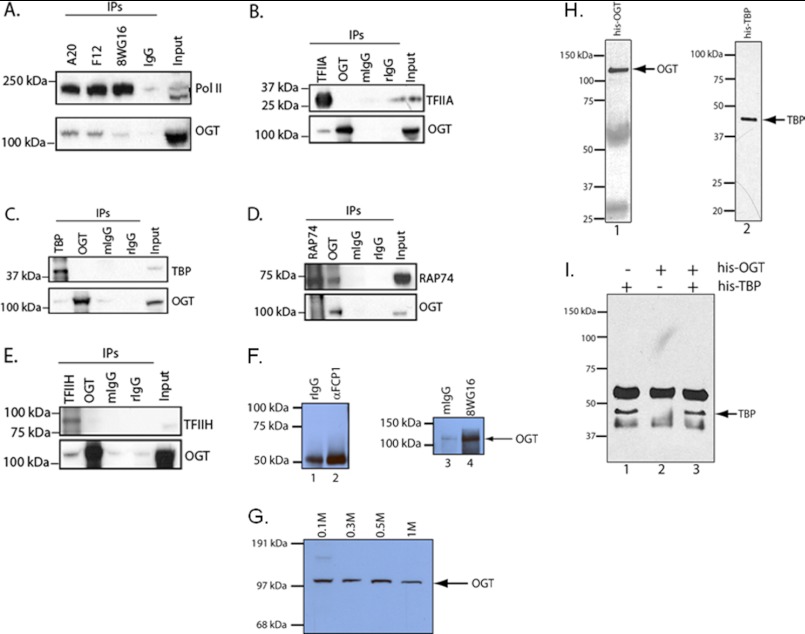
RNA polymerase II and several GTFs bind OGT in nuclear extracts. A, co-immunoprecipitations (IPs) were done using a panel of mAb against RNA polymerase II (A20, F12, and 8WG16) and compared with a control immunoprecipitation using normal mouse IgG serum. The immunoblots were then assayed for either pol II or OGT. B, co-immunoprecipitations of TFIIA (mAb) and OGT (rabbit polyclonal) were compared with normal mouse or rabbit IgG (mIgG and rIgG, respectively). Immunoblots were probed for TFIIA and OGT as indicated. C–E, co-immunoprecipitations for OGT using anti-TBP, anti-TFIIF/RAP74, and anti-TFIIH/ERCC3 mAb, along with an anti-OGT immunoprecipitation (rabbit IgG) as indicated. F, co-immunoprecipitations of FCP1 (Santa Cruz Biotechnology) and RNA pol II (8WG16 mAb) and control rabbit and mouse IgG. The Western blot was probed with the anti-OGT antibody AL24. G, nuclear extracts were fractionated over P11 resin and step-eluted in 0.1, 0.3, 0.5, and 1 m KCl (55) followed by SDS-PAGE and Western blotting for OGT. H, aliquots of recombinant His-OGT and His-TBP (1) were assayed by Western blot with an anti-His tag antibody. I, either His-TBP or His-OGT or a mixture of His-TBP and His-OGT was immunoprecipitated with a TBP mAb. Eluates were run on SDS-PAGE followed by a Western blot using an anti-His tag antibody.
FIGURE 2.
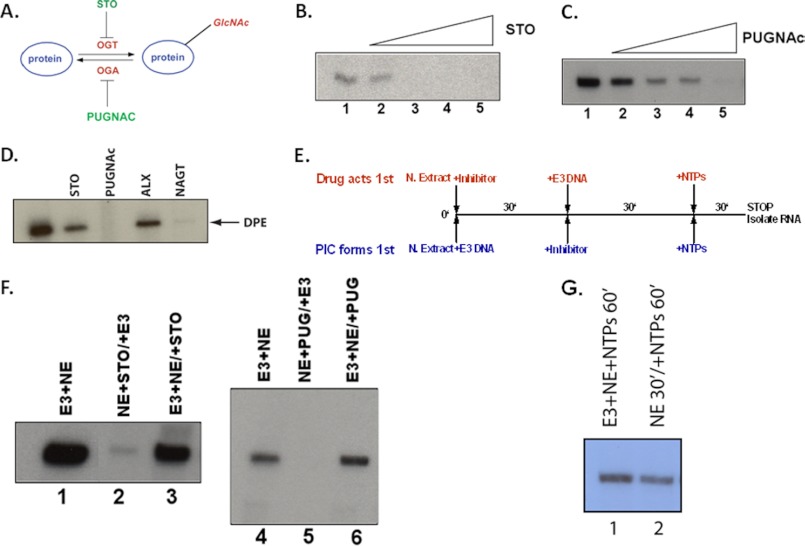
Inhibitors of OGT and OGA block transcription in a cell-free system. A, schematic illustration of the addition and removal of the O-GlcNAc post-translational modification and the targets of the two inhibitors, STO45849 (STO) and PUGNAc. B, titration of the OGT inhibitor STO45849 (0.05–0.4 mm) into an in vitro transcription assay using HeLa nuclear extracts and the adenovirus E3 promoter. C, titration of the OGA inhibitor PUGNAc (0.2–4 mm) in the identical assay system as described in B. RNA products were detected by primer extension assays. D, in vitro transcription assay in crude HeLa nuclear extracts using a DPE-containing promoter (28). STO45849 is an OGT inhibitor (0.4 mm), PUGNAc is an OGA inhibitor (4 mm), alloxan (ALX) (0.5 mm) is an inhibitor of both OGT and OGA, and NAGT is an OGA inhibitor (see “Experimental Procedures”). All transcription products were detected by primer extension assays. E, the schematic shows the experimental design of the transcription assays, where either of the drug inhibitors is allowed to act, either before or after PIC formation. The NE/inhibitor and NE/DNA reactions incubate for 30 min, and then the missing component (either DNA or inhibitor, respectively) is added for an additional 30-min incubation. This is followed by the addition of NTPs for 30 min. The reaction is stopped and RNA, synthesis is detected by primer extension. F, shows the results of these experiments using either STO45849 (OGT inhibitor) or the PUGNAc (OGA inhibitor). The information above the lanes indicates the order of addition of the inhibitors and promoter DNA. For example, NE+STO/E3 indicates that the nuclear extract and the STO45849 inhibitor were incubated together for 30 min, at which time the E3 promoter DNA was then added for 30 min. Finally, the NTPs are added to initiate transcription. G, nuclear extracts are not inactivated by a 30-min preincubation. Lane 1 shows a positive control for transcription where E3 promoter DNA, nuclear extract, and NTPs were added at the same time for 60 min. Lane 2 shows incubation of only nuclear extract and buffer for 30 min prior to the addition of DNA and NTPs.
FIGURE 3.
Recruitment of OGT and pol IIγ to E3 promoter PICs. A, OGT is present in the PIC. Left panel, biotinylated E3 promoter or pSP72 MCS DNA was bound to strepavidin-coated Dynabeads, incubated with HeLa nuclear extracts, and washed three times. Bound proteins were eluted with sample buffer and run on 10% SDS-PAGE, transferred to nitrocellulose, and analyzed by Western blot. Right panel, the amounts of E3 and MCS DNA used in the left panel were run on a 1.5% agarose gel and visualized by EtBr stain. B, the schematic shows the hypothesized step where the PUGNAc inhibitor acts, by blocking the removal of O-GlcNAc from pol IIγ; this results in the accumulation of pol IIγ. Therefore, the titration of unmodified pol IIA should bypass the PUGNAc block in transcription. C, purified pol IIA was titrated into HeLa nuclear extracts treated with 4 mm PUGNAc and containing the E3 promoter; transcription was assayed by primer extension. D, this experiment shows the result of titrating either pol IIA (lanes 3–5) or pol IIγ (lanes 6–8) into nuclear extracts where transcription was blocked by the addition of 4 mm PUGNAc. Identical amounts of RNA polymerase II were used in both titrations, as shown by Western blots in the bottom panel using an N-terminal Rpb1 antibody.
Immobilized Templates
Assays were carried out by incubating 0.5 μg of biotinylated E3 promoter DNA (created by PCR using a biotinylated T7 primer for the upstream primer and SP6 primer) with 10 μl of M280 Dynabeads as per the manufacturer's instructions. The beads were then incubated for 1 h with 50 mg/ml BSA. The beads were briefly washed in H.1 buffer (20 mm Hepes, pH 7.9, 100 mm KCl, 0.2 mm EDTA, and 10% glycerol) and incubated with 50 μl of HeLa nuclear extract, 75 μl of HM.1 buffer (H.1 buffer plus 12.5 mm MgCl2), and 125 μl of H20 plus 2 μg of Escherichia coli DNA (determined by titration)) at room temperature for 30 min. Afterward, the beads were washed three times for 20 min each with H.2, 0.05% Nonidet P-40. Bead pellets were then heated in sample buffer, run on a 4–12% gradient SDS-PAGE, and transferred overnight to nitrocellulose. PUGNAc and alloxan inhibitors were added concomitantly with the DNA and HeLa nuclear extract (PUGNAc at 4 mm final; alloxan at 0.5 mm final).
Recombinant Protein Purification
rOGT, rOGA, and rGST-CTD bacterial expression vectors were transformed into BL21(DE3). Cells were grown to and OD of 0.4 to 0.6 and induced with 1 mm IPTG for 3 h at 37 °C. Cells were resuspended in PBS containing 1% Nonidet P-40, 1 mm EDTA, and Complete protease inhibitors (Roche Applied Science) and lysed by sonication. rOGT, rOGA, and GST-CTD (and CTD mutants) were purified using standard nondenaturing protocols. rOGT and rOGA were purified after sonication and clarification over a nickel-nitrilotriacetic acid-Sepharose HiTrap column using an AKTA purification system (GE Healthcare). Bound proteins were eluted with a 50–250 mm imidazole gradient. Detection was first done using A280 elution profiles and confirmed by SDS-PAGE. GST-CTD proteins were purified over GT-Sepharose HiTrap columns and eluted with a glutathione gradient. All proteins were aliquoted and frozen at −80 °C.
Enzymatic Reactions
For OGT, 3 μg of GST-CTD (and mutants) or 1 μl of RNA pol II, 1 μl (1.5 μg) of rOGT, 5 mm UDP-GlcNAc, 12.5 mm MgCl2, and 50 mm Tris, pH 7.4, were used; the reaction was at 37 °C for 30 min (29). The OGA assay was performed essentially as described (30). P-TEFb labeling of GST-CTD was as described (31). TFIIH kinase assays contained 3 μg of GST-CTD or GST-CTDγ plus partially purified TFIIH fraction and 1 mm ATP under the buffer conditions used for the OGT reactions above.
Western Blots
Western blot assays were performed using nitrocellulose filters (Whatman, 0.45 μm) and Western transfer buffer (Invitrogen). Polyacrylamide gels were either 10% or 4–12% gradient gels (MOPS buffer system, Invitrogen). Western blots were developed with the appropriate primary and secondary antibodies (anti-mouse IgM-HRP, Santa Cruz Biotechnology) and detected by ECL (Pierce). Western blots with the 110.6 mAb were done as described (32). All other Western blots were treated with standard protocols, blocked with either 5% milk/Tris-Tween or 3% BSA/Tris-Tween and washed with Tris-Tween buffer.
Sugar Nucleotide Determination
Nuclear extracts were lyophilized and extracted with 0.75 ml of cold 0.5 n perchloric acid. The suspension was dispersed vigorously for 20 s in an ice bath followed by centrifugation at 15,000 × g for 10 min in a cold room, and the supernatant was collected. The pellet was re-extracted similarly, and both supernatants were pooled. 200 μl of charcoal suspension (30 mg of Mallinckrodt charcoal/ml of 1 n perchloric acid) was added to the cold supernatant and stirred vigorously in an ice bath. After centrifugation as described above, the supernatant was discarded. The charcoal pellet was eluted three times with 750 μl of a solution containing 50% EtOH and 1% NH4OH. After the ethanol was removed, the supernatant was frozen and lyophilized. The bound nucleotide fraction was hydrolyzed and analyzed by Dionex HPLC using a PA10 anion exchange column and pulsed amperometric detection as described previously (33).
Chromatin Immunoprecipitation (ChIP)
A total of 30 million BJAB cells were treated with formaldehyde (37%) to a final concentration of 1% added directly to the culture medium for 15 min at room temperature with gently shaking. Cross-linking was stopped by adding glycine to a final concentration of 0.125 m for 10 min at room temperature with gentle shaking. Cells were harvested by spinning down at 1300 rpm for 5 min. After two washings in PBS, cells were resuspended in lysis buffer (50 mm Hepes, pH 8.0, 150 mm NaCl, 1% Triton-X 100, 0.1% sodium deoxycholate, and 1 mm EDTA with Complete protease inhibitor mixture (Roche Applied Science)). DNA was sheared by sonication in a Bioruptor using the following settings: high energy, 30 s on/30 s off for 30 min. Chromatin immuno-precipitation of DNA fragments was performed using protein G-magnetic beads (Dynal/Invitrogen). First, magnetic beads were washed according to the manufacturer's recommendations. Then, 10 μg of antibody for pol II, O-GlcNAc, OGT, OGA, and the normal mouse and rabbit IgG controls was prebound to 50 μl of washed magnetic beads in 100 μl of binding buffer (0.1 m NaOAc, 0.2% Tween 20, and 0.2% BSA) with rotation for 3 h at 4 °C.
Antibody-bound magnetic bead complexes were washed twice in 0.1 m NaOAc. Immunoprecipitation of the precleared chromatin was performed by incubating the cross-linked lysate (5 × 106 cells/immunoprecipitation) with the antibody-bound magnetic beads rotating overnight at 4 °C. Immunocomplexes were captured and washed once with each of the following: 1) ChIP lysis buffer (50 mm Hepes, 150 mm NaCl, 1% Triton X-100, 0.1% Na-deoxycholate, 1 mm EDTA); 2) high salt buffer (50 mm Hepes, pH 8.0, 500 mm NaCl, 1% Triton-X 100, and 0.1% sodium deoxycholate); 3) LiCl (Upstate 20-156); and 4) TE in conjunction with a Dynal MPC-S magnet. Finally, washed magnetic beads are resuspended in 100 μl of water.
Unprocessed lysate (equivalent to 10% of the immunoprecipitated material) was processed as “input” material from now on along with the washed beads. DNA was recovered using 100 μl of Chelex-100 (Bio-Rad 142-1253 as 10% slurry in water) per sample at boiling temperature for 10 min. Samples were incubated with 2 μl of proteinase K (10 μg/μl) at 55 °C for 30 min followed by 10 min at 100 °C. Samples were spun down, and the supernatant, which contained the eluted DNA, was collected and transferred to a clean tube. Samples were cleaned using QIAquick spin columns (Qiagen) according to the manufacturer's recommendations and eluted in 30 μl of elution buffer (10 mm Tris HCl, pH 8.5). DNA fragments were tested by quantitative real-time PCR using primers that amplified the pol II/O-GlcNAc overlapping peaks at the promoter of some of the B-cell-specific genes highly expressed in the lymphoma cell line used for this experiment (BCL6, IRF8, MTA3, POU2F1, and POU2F2) along with the amplification of the VIM promoter (vimentin, a non-expressed gene in this lymphoma cell line used as a negative control). The quantitative real-time PCR assay was performed using Syber Green (Applied Biosystems). Standard curves for each pair of primers were generated by doing PCR of serial dilutions of the input material. The “-fold enrichment” per each specific immunoprecipitation sample was determined relative to the nonspecific corresponding immunoprecipitation (mouse or rabbit IgG). The primer sequences used to amplify the pol II/O-GlcNAc overlapping peaks are available upon request.
In calculating the DNA binding enrichment for pol II, O-GlcNAc (RL2), OGT, and mouse and rabbit IgG controls on the gene promoters assayed, serial dilutions of 10% input were run for each primer on the same quantitative real-time PCR plate to get the corresponding standard curve. Second, after applying the straight line formula to all the samples, the specific immunoprecipitation sample results were divided by the corresponding IgG control result. Finally, the data were expressed as fold enrichment relative to 10% input. The calculation described was applied to gene promoters where DNA binding enrichment was expected (highly expressed genes in this lymphoma cell line) as well as to housekeeping gene promoters where the opposite was expected. All samples were run in triplicate for each experiment. At least three biological replicates of each experiment were performed.
Retroviral Production
293-T cells were transfected with the pRSMX_PG -eGFP-Puro vector delivering the shRNA for OGT along with the mutant ecotropic envelope-expressing plasmid, pHIT/EA6 × 3*, as reported previously using Lipofectamine 2000 (34). Virus supernatant was collected at 48 and 72 h post-transfection. BJAB lymphoma cells were spin-infected twice on consecutive days in the presence of 8 μg/ml Polybrene at 2500 rpm for 90 min at room temperature. The cells used for these experiments were engineered to express the bacterial tetracycline repressor; the vector does not express the shRNA delivered until doxycycline is added and can inducibly knock down the expression of the endogenous target gene. Two days after the last round of infection, BJAB cells were treated with puromycin (1 μg/ml) for 1 week to achieve a high percentage of GFP+ cells (98%) and consequently to select stable integrants. The GFP+ cells were shOGT+ as well. After puromycin selection, the expression of the OGT shRNA was induced by adding 20 ng/ml doxycycline to the medium. Samples from uninduced and induced shOGT cultures were collected at different time points according to the experiment.
The following shRNA OGT sequence was used (the hairpin is in bold letters): top, 5′-GATCCCGGCACAAACTTCCGAGTGATTCAAGAGATCACTCGGAAGTTTGTGCCTTTTT-3′; bottom, 5′-AGCTAAAAAGGCACAAACTTCCGAGTGATCTCTTGAATCACTCGGAAGTTTGTGCCGG-3′.
Real-time PCR
RNA from shOGT-infected BJAB cells (uninduced and doxycycline-induced) was prepared with TRIzol (Invitrogen) according to the manufacturer's instructions. A Superscript II first-strand cDNA synthesis kit (Invitrogen) was used to prepare cDNA following the manufacturer's protocol. OGT, BCL6, Oct-1, Oct-2, MTA3, and IRF8 gene mRNA expression levels were achieved by using pretested assay-on-demand probe/primer sets from Applied Biosystems with an ABI 7500 PCR machine for 45 cycles with an annealing temperature of 60 °C. Gene expression was normalized to that of B2MG (encoding β2-microglobulin), and data are presented as the “-fold change” relative to the corresponding shRNA for OGT uninduced infected cells according to the 2-ΔΔCT (change in cycling threshold) method.
Immunoblot Analysis
BJAB shOGT-infected cells were induced and 2 days after doxycycline induction treatment (10 × 106) were lysed in buffer consisting of 50 mm Tris-HCL, pH 7.5, 200 mm NaCl, 50 mm β-glycerophosphate, 1% Tween 20, 0.2% Nonidet P-40, and a mixture of protease inhibitors (Roche Applied Science). Proteins were resolved by 10% SDS-PAGE (Bio-Rad) following immunoblot. Membranes were incubated with the primary antibodies for 1 h. at room temperature. Peroxidase-conjugated goat anti-rabbit (111-035-003, 1:7000 dilution, Jackson Immunoresearch) or peroxidase-conjugated goat anti-mouse (115-035-003, 1:7000 dilution) was used as the secondary antibody with incubation for 30 min at room temperature.
Co-Immunoprecipitations
25 μg of protein G-agarose beads (Roche Applied Science) was mixed with 10 μg of antibody or the corresponding normal mouse and/or normal rabbit IgG control and incubated for 3 h at 4 °C with rotation. HeLa nuclear extract (25 μg) was added to the antibody-beads mixture and incubated overnight with rotation at 4 °C. Beads were washed in HM.1 buffer supplemented with 0.1% Tween 20 twice for 15 min each by rotation at 4 °C. Beads were resuspended in loading buffer and incubated at 75 °C for 5 min. Proteins were separated on 4–12% gradient SDS-polyacrylamide gels and transferred overnight at room temperature for pol II and at 45 v for 3 h at room temperature for the other proteins.
Co-immunoprecipitations of His-OGT and His-TBP were done in BC100 (20 mm Tris, pH 7.9, 100 mm KCl, 0.2 and mm EDTA) for 30 min prior to incubation with 3G3 anti-TBP mAb and protein G-sepharose beads. Beads were washed in BC100, heated in sample buffer, and run on 10% SDS-PAGE. After transferring to nitrocellulose (Whatman), blots were probed with anti-His antibody (Santa Cruz Biotechnology) and developed by ECL (Pierce).
For wheat germ agglutinin affinity purification, 20 μl of WGA-agarose slurry was incubated with 100 μl of HeLa nuclear extract (8 mg/ml) plus 4 mm PUGNAc for 2 h at 4 °C. Beads were washed in H.1 buffer and analyzed by SDS-PAGE and Western blot.
RESULTS
Human RNA Polymerase II Is a Substrate of Both OGT and OGA
Previous work established that CTD peptides were substrates for OGT (26). We first wished to establish that native human RNA polymerase II is in fact a substrate for the O-GlcNAc-modification enzymes. We purified rOGT from bacterial extracts and used that to modify highly purified human RNA polymerase II (Fig. 1A). We assayed for O-GlcNAc by Western blot using 110.6, an anti-O-GlcNAc antibody raised against synthetic O-GlcNAcylated CTD peptides (35). This resulted in a significant O-GlcNAc signal in two separate OGT reactions, indicating the O-GlcNAc modification of the CTD (Fig. 1B).
FIGURE 1.
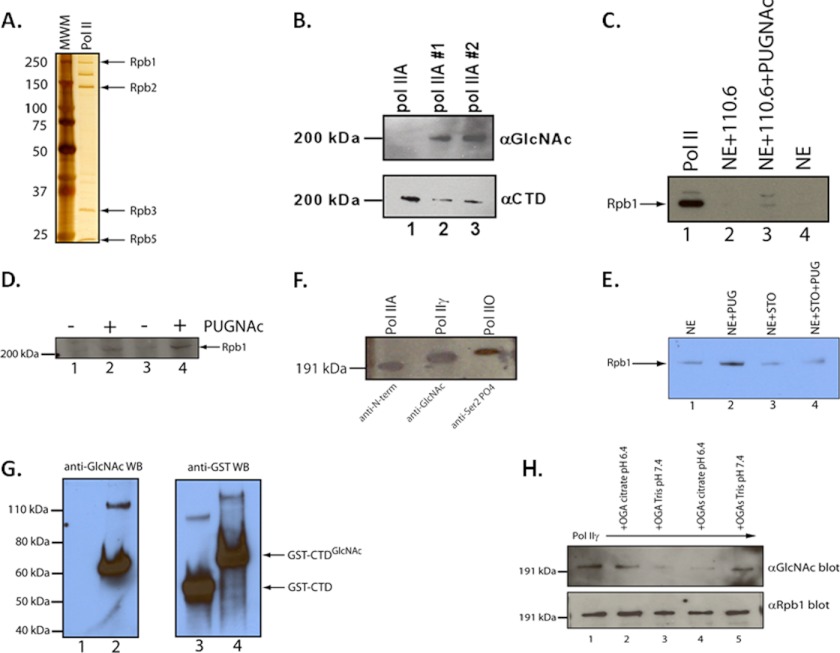
In vitro analysis of the O-GlcNAc modification of human RNA polymerase II. A, depiction of silver-stained SDS-PAGE of purified human RNA polymerase II used in the experiments herein and purified as per Maldonado et al. (55). B, shown is a Western blot analysis of the modification of RNA polymerase II with O-GlcNAc using the anti-O-GlcNAc antibody 110.6 (26). The O-GlcNAc is added by incubating purified RNA polymerase II with rOGT and UDP-GlcNAc. Two independent reactions of pol II with OGT are shown. The lower panel shows pol II detected with the CTD-specific 8WG16 mAb. C, O-GlcNAc-modified Rpb1 subunit of RNA pol II can be detected in nuclear extracts. Rpb1 was detected by Western blot using an antibody recognizing the N terminus of Rpb1. Lane 1, purified human RNA pol II Western blot control; lane 2, immunoprecipitations of HeLa NE using the 110.6 anti-O-GlcNAc IgM mAb plus anti-mouse IgM-agarose beads; lane 3, same immunoprecipitation as in lane 2 with the addition of the OGA inhibitor PUGNAc (4 mm); lane 4, control immunoprecipitation using only anti-mouse IgM-agarose beads incubated with HeLa NE. D, isolation of O-GlcNAc-modified RNA pol II from HeLa NE using WGA-agarose beads. Lanes 1 and 2 and 3 and 4 are duplicate experiments of affinity purification of RNA pol II from HeLa NE with or without the addition of the OGA inhibitor PUGNAc (4 mm). RNA pol II was detected using an N terminus-specific antibody to Rpb1 (N20). E, STO45849 OGT inhibitor blocks O-GlcNAc modification of RNA pol II in nuclear extracts. HeLa NE were incubated as described in D with either PUGNAc (4 mm (lane 2)), STO45849 (0.4 mm STO (lane 3)), or both inhibitors (lane 4). O-GlcNAc-modified proteins were purified with WGA-agarose, eluted in sample buffer, and run on 10% SDS-PAGE. Western blots were done with an anti-N-terminal Rpb1 antibody (F12, Santa Cruz Biotechnology). F, comparison of the migration of unmodified native pol II (pol IIA; N-terminal antibody), pol IIγ (assayed with 110.6 anti-O-GlcNAc antibody), or pol IIO (assayed with the H5 antibody specific for CTD serine 2 phosphorylation) on SDS-PAGE. The resulting Western blots were developed separately and overlaid relative to the molecular weight standards to more easily visualize the mobility differences. pol IIA is the same material as used in A. pol IIγ was made as described in B. pol IIO was made with rP-TEFb and ATP. G, comparison of the relative migrations of GST-CTD and O-GlcNAc-modified GST-CTD in SDS-PAGE. Lanes 1 and 2 show a Western blot of GST-CTD (lane 1) and GST-CTD incubated with rOGT and UDP-GlcNAc (lane 2). The blot was developed with the O-GlcNAc-specific 110.6 antibody. Lanes 3 and 4 are GST-CTD or O-GlcNAc-modified GST-CTD as shown in lane 2. The Western blot (WB) seen in lanes 3 and 4 was developed with an anti-GST antibody. H, analysis of pol IIγ as a substrate for OGA. Two isoforms of OGA (OGA and OGAs) were assayed using pol IIγ (made as described in B.) at two different pH levels, 6.4 and 7.4. Loss of O-GlcNAc was assayed by SDS-PAGE and Western blot following incubation with OGA. Blots were assayed with the 110.6 αO-GlcNAc antibody (upper panel) or with the CTD-specific 8WG16 mAb as a loading control (αRpb1 blot (lower panel)).
We next asked whether endogenous RNA polymerase II (pol II) in nuclear extracts was O-GlcNAcylated. We assayed this in two ways. In the first, we treated HeLa nuclear extracts with the OGA inhibitor PUGNAc (to stabilize any O-GlcNAc on pol II) and immunoprecipitated with 110.6. The immunoprecipitate was analyzed by Western blot using the Rpb1 N-terminal-specific antibody N20. We were able to detect O-GlcNAc-pol II in this manner (Fig. 1C). Our second approach used WGA-agarose beads to affinity purify O-GlcNAc-modified proteins from the nuclear extract (32). Again, these products were assayed in duplicate with the N-terminal pol II antibody N20 by Western blot (Fig. 1D, compare lane 1 with 2 and 3 with 4). Here we detected a slower migrating form of pol II that immunoprecipitated only with the addition of PUGNAc.
Furthermore, the nuclear extracts had the ability to modify RNA pol II with O-GlcNAc. We measured the levels of UDP-GlcNAc in the nuclear extracts and determined that the concentration was 220 ± 14 (three determinations ± 2 S.D.) μmol/mg protein (data not shown; see “Experimental Procedures”). This value is consistent with a concentration of ∼0.5 μm endogenous UDP-GlcNAc and is very similar to the OGT Km for UDP-GlcNAc, meaning that the nuclear extract [UDP-GlcNAc] is within the operational window of OGT (36, 37). Lastly, we incubated nuclear extracts with STO45849, an inhibitor of OGT (Gross et al. (43)), and purified O-GlcNAc-modified proteins with WGA-agarose. Nuclear extracts incubated with STO45849, PUGNAc, or both were compared by Western blotting for pol II. We again observed the increase in O-GlcNAcylated pol II in the presence of PUGNAc (Fig. 1E, compare lanes 1 and 2). We did not see this increase with STO45849, and most importantly, STO45849 blocked the increase in O-GlcNAcylated pol II that we obtained using PUGNAc alone (Fig. 1E, compare lanes 2 and 4). This indicates that the STO45849 blocked OGT activity in the nuclear extracts.
We next compared the mobility of the three forms of pol II using phospho- and O-GlcNAc-specific antibodies in Western blots (Fig. 1F). To best show these small differences, we overlaid the results of the Western blots with the three different antibodies relative to the molecular weight markers. We consistently observed that O-GlcNAc-pol II migrated as an intermediate species, in between unmodified pol IIA and P-TEFb-phosphorylated pol IIO. We refer to the O-GlcNAc-modified form of RNA pol II as pol IIγ. To further support this mobility difference, we performed the same OGT assay using GST-CTD (containing 26 heptad repeats) as a substrate. As seen in Fig. 1G (compare lanes 1 and 2), the GST-CTD was modified by OGT. Furthermore, when comparing the mobility differences between GST-CTD and O-GlcNAc-modified GST-CTD using an anti-GST antibody, we found the O-GlcNAc-modified GST-CTD had a reduced mobility in SDS-PAGE (Fig. 1G, compare lanes 3 and 4). These data are consistent with the determination of sites that can be modified by OGT; the CTD heptad repeat is clearly contained within the derived consensus sequence of sites within proteins that are O-GlcNAcylated (38).
We also asked whether native human pol IIγ was a substrate for OGA. We assayed two isoforms, the full-length OGA and the 75-kDa short isoform, OGAs (39, 40). We assayed for N-acetylglucosaminidase activity using two different pH levels and buffers to optimize OGA activity: pH 6.5 to reproduce conditions of its detection and pH 7.4 to assess a more physiological pH. Following incubation of pol IIγ with either of the OGA isoforms, we assayed for the presence of O-GlcNAc by Western blot (Fig. 1H, upper panel). With both isoforms we obtained almost complete removal of O-GlcNAc from native RNA pol IIγ, although the full-length OGA was more active at pH 7.4 than at 6.4 (and vice versa for OGAs). In any case, pol IIγ, derived from native human pol II, is a legitimate substrate for OGA in vitro. In all cases we did not see any loss of Rpb1 itself, indicating that our loss of O-GlcNAc was not due to a contaminating protease (Fig. 1H, lower panel).
OGT- and OGA-specific Inhibitors Abrogate Transcription in Vitro
To address the functional role of pol IIγ, we began to study O-GlcNAc functions in transcription by utilizing a cell-free transcription system (27, 28, 41, 42). We took advantage of the existence of several inhibitors of both OGT and OGA and asked whether such inhibitors would have transcription defects in our cell-free system. Indeed, STO45849 and PUGNAc, inhibitors of OGT and OGA, respectively (43) (25, 44), significantly blocked transcription from the adenovirus E3 promoter, so chosen because it contains a representative and typical core promoter architecture and is robustly transcribed in vitro (Fig. 2, B and C) (27). We found similar results when assaying a second promoter containing the DPE-class core promoter element (Fig. 2D) (28, 45, 46). In addition to STO and PUGNAc, alloxan, a dual OGT and OGA inhibitor, and 1,2-dideoxy-2′-methyl-α-d-glucopyranoso-[2,1-d]-Δ 2′-thiazoline (NAGT), a second, more selective OGA inhibitor, also abrogated transcription in vitro (Fig. 2D) (47–49). Because we used different core promoter types, we inferred that the O-GlcNAc requirement in transcription is not restricted to one core promoter class and is potentially a widespread phenomenon.
OGT and OGA Function during PIC Assembly
These data suggest that the inhibition of O-GlcNAc cycling by OGT and OGA inhibitors causes a defect in transcription. We further tested this hypothesis to ask when during the transcription process the inhibitors act. Excluding ribonucleotides from the assay separates the in vitro transcription system into two steps. The first step, the assembly of the PIC onto the promoter DNA, is done simply by mixing nuclear extract and promoter DNA. After this assembly step, the addition of NTPs initiates transcription. We assayed for a defect in PIC assembly by incubating the OGT inhibitor, STO45849, or the OGA inhibitor, PUGNAc, with nuclear extracts either before or after a 30-min incubation of nuclear extract with E3 promoter DNA, i.e. before or after PIC assembly (Fig. 2E). We found that the addition of either STO45849 or PUGNAc before PIC assembly resulted in the complete abrogation of transcription. However, if PIC assembly occurred first, the subsequent addition of either inhibitor did not significantly decrease transcription from the E3 promoter (Fig. 2F) nor did a 30-min mock incubation have any effect on transcription (Fig. 2G). These results show that both the addition and removal of O-GlcNAc occur before or during PIC assembly and before the initiation of transcription. If this was an early elongation defect, then we would expect to see transcription decrease regardless of when the inhibitors were added, because the NTPs were always added afterward.
OGT Is Stably Recruited to the PIC and Associates with RNA Polymerase II
Given the functional associations between OGT and pol II, we asked whether we could detect OGT as part of the PIC of the adenovirus E3 promoter. We assayed this via immobilized DNA templates of either the E3 promoter or non-promoter DNA, represented by the multiple cloning site (MCS) of the parent vector of the E3 promoter template, pSP72. Biotinylated E3 promoter DNA was bound to strepavidin-magnetic beads and incubated with HeLa nuclear extracts. After a 30-min incubation period, the bound proteins were eluted in sample buffer and analyzed by SDS-PAGE and Western blot (Fig. 3A). We consistently observed higher than background levels of pol II and TBP, both of which would be expected to be recruited to the E3 promoter. Likewise, we found that OGT was recruited to the promoter above the background levels seen when using MCS DNA. Although we used similar amounts of each DNA, the MCS DNA was ∼3–4-fold more concentrated on a molar basis, because of its smaller size (Fig. 3A, right panel). Therefore these background protein levels are probably an overestimate.
Because the PUGNAc inhibitor blocks the removal of the O-GlcNAc from the CTD by the OGA, we reasoned that the addition of the unmodified form of pol II (pol IIA, purified from HeLa cells) would bypass this block (Fig. 3B). This experiment also served as a very important control; if the removal of O-GlcNAc from another protein was necessary for transcription, and if this removal was blocked in this assay by PUGNAc, then one would not expect the pol IIA to bypass the PUGNAc block in transcription. We found that titration of purified pol IIA resulted in the alleviation of the PUGNAc-mediated block in transcription (Fig. 3C).
A second prediction was that O-GlcNAc-modified pol II would not bypass the PUGNAc block. To test this, we first modified purified RNA polymerase II with rOGT and UDP-GlcNAc (Fig. 1B). We then titrated an equivalent amount of either pol IIA or pol IIγ into the in vitro transcription system containing PUGNAc. In contrast to the pol IIA titration, the pol IIγ did not rescue the PUGNAc block to transcription (Fig. 3D). Titration of rOGT and UDP-GlcNAc had no effect (data not shown). These results suggest that RNA polymerase II is functionally the target of the PUGNAc block and that removal of O-GlcNAc from pol II is necessary for transcription to occur.
Serines 5 and 7 of the CTD of RNA Polymerase II Are Necessary for OGT Activity
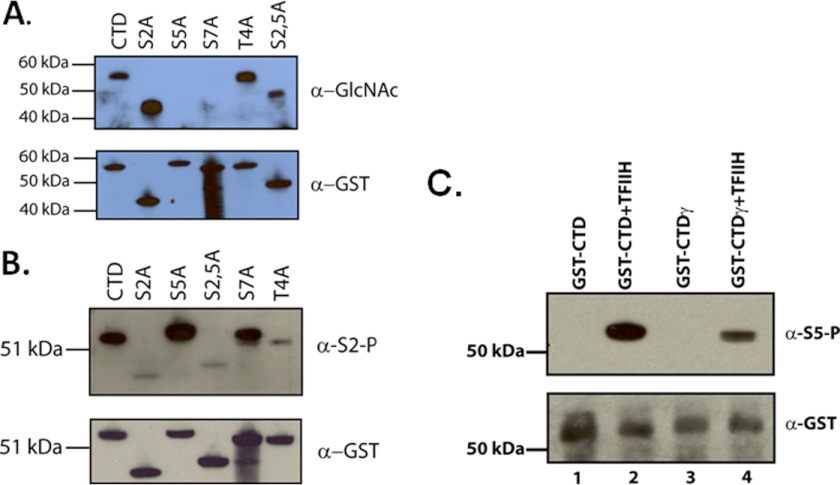
The human CTD consists of 52 repeats of the heptad consensus sequence YSPTSPS. Kelly et al. (23) determined by Edman degradation of calf thymus RNA pol II that both Thr-4 and Ser-5 of the CTD are O-GlcNAc-modified. We wished to corroborate this result by assaying a panel of CTD alanine substitution mutants (kindly provided by Shona Murphy). The appropriate GST-CTD (containing ∼26 heptad repeats) proteins were incubated with rOGT and UDP-GlcNAc and separated by SDS-PAGE. Western blots of the reaction products show that serine residues 5 and 7 specifically are necessary for OGT activity (Fig. 4A). Because alanine substitutions formally do not distinguish between a residue being modified and a requirement for that residue for substrate modification, we could not rule out the latter. However, given the Edman degradation results that serine 5 is O-GlcNacylated (23), these results are likely because serine 5 is modified directly by OGT. Secondly, it is well known now that serine residues are particularly labile in Edman reactions (50). Thus, it is likely that the amount of serine O-GlcNAcylation in the CTD in the findings of Kelly et al. (23) is an underestimate, whereas the O-GlcNAc on threonine might be an overestimate. It is apparent that either both of the serine residues are O-GlcNAcylated or that one residue is required for the accurate modification of the other, because both alanine substitutions completely abrogated O-GlcNAcylation. It is also possible that O-GlcNAcylation at serine 5 or 7 may be required for subsequent O-GlcNAcylation of threonine 4. Lastly, as a control for the integrity of the S5A and S7A CTD mutants, we assessed their efficacy as a substrate for the serine 2-specific CTD kinase, P-TEFb. In the experiment presented in Fig. 4B, we clearly observe that both mutants are efficiently phosphorylated, whereas the S2A mutant is not phosphorylated. Thus, the results obtained with OGT and the Ser-5 and Ser-7 mutants are likely because of the alanine substitutions themselves and not the nonfunctional substrates.
FIGURE 4.
OGT modifies the CTD at serine positions 5 and 7. A, GST-CTD or GST-CTD containing single alanine substitutions at serine positions 2 or 5, a threonine to alanine substitution at position 4, or a double substitution at positions 2 and 5 of the CTD (each containing ∼26 heptad repeats and each confirmed by sequencing). The lower panel is an example of the loading of the GST-CTD proteins in each lane as assayed by anti-GST antibody. The upper panel shows the results of incubations of the CTD substrates with rOGT and UDP-GlcNAc. The O-GlcNAc modification was detected using the 110.6 anti-O-GlcNAc antibody. B, control phosphorylation reactions with P-TEFb kinase and the GST-CTD or GST-mutant CTDs as described in A. Phosphorylation was detected by Western blot with the anti-phosphoserine 2 antibody H5. The lower panel indicates the loading of each protein as indicated in an anti-GST Western blot. C, O-GlcNAc-modified CTD was assayed for its efficiency as a substrate for TFIIH phosphorylation. GST-CTD or O-GlcNAc-modified GST-CTD (GST-CTDγ) was incubated with native TFIIH and ATP, and phosphorylation was assayed by Western blot with the serine 5-specific H14 antibody. The bottom panel illustrates the loading of equivalent amounts of GST-CTD as assayed by an anti-GST antibody. GST-CTDγ was produced with rOGT and UDP-GlcNAc.
Serine residues 5 and 7 are sites of CTD phosphorylation by the general transcription factor TFIIH, in which activity is concomitant with the initiation of transcription (4). Because there is an overlap between CTD residues modified by OGT and TFIIH, we asked whether O-GlcNAc-modified CTD would interfere with TFIIH phosphorylation of the CTD. We used GST-CTDγ as a substrate for TFIIH and assayed for phosphorylation by Western blot using the serine 5-specific antibody H14 (Fig. 4C). As with Comer and Hart's (26) results using rCAK and CTD peptides, we found that native TFIIH did not phosphorylate GST-CTDγ as well as unmodified GST-CTD. These data indicate that O-GlcNAcylation and serine 5 phosphorylation are mutually exclusive events.
Co-immunoprecipitations Reveal Interactions between OGT and the General Transcription Machinery
Given the data showing that OGT was present in a PIC, we asked whether RNA polymerase II and other general transcription factors (GTFs) interacted with OGT. Such information would shed light on how OGT is recruited to the PIC. To do these experiments, we immunoprecipitated pol II, TFIIA, TBP, the RAP74 subunit of TFIIF, and the ERRC3 subunit of TFIIH. We then probed these samples on immunoblots using an OGT antibody (Fig. 5). We found three antibodies to pol II that co-immunoprecipitated OGT, whereas the normal mouse IgG did not (Fig. 5A). We also found that anti-TFIIA, anti-TBP, and anti-TFIIH (ERCC3 subunit) antibodies co-immunoprecipitated OGT (Fig. 5, B, C, and E). The only GTF co-immunoprecipitated with the OGT antibody was the RAP74 subunit of TFIIF and perhaps ERCC3 (Fig. 5, D and E). We also did not observe any interactions of OGT with the FCP1 CTD phosphatase (Fig. 5F). Because these are crude nuclear extracts, it is likely that multiple interactions between the GTFs and OGT are stably maintained. Consistent with these data is the fact that P11 fractionation of nuclear extract revealed OGT in all four elution steps, each containing different subsets of the GTFs (Fig. 5G). We also assayed for potential interactions with recombinant OGT and TBP. We mixed together His-OGT and His-TBP (Fig. 5H) and immunoprecipitated with a TBP mAb. Using an anti-His antibody we did not detect any His-OGT, despite recovery of His-TBP (Fig. 5I). From these data we concluded that OGT interacts with several members of the general transcriptional machinery that are known components of the preinitiation complexes that form on promoters.
OGT shRNA Reduces Transcription and RNA Polymerase II Promoter Occupancy
The in vitro data points to pol IIγ having a specific function in PIC formation, and the in vivo ChIP and ChIP-seq data clearly position pol IIγ at the transcription start site of genes. Therefore, we wished to test these ideas and the emerging model (see Fig. 7) by reducing OGT expression in vivo and asking what effects that would have on transcription and ChIP-based detection of factors at promoters. The OGT shRNA was assembled into a lentivirus delivery system that would infect B-cells with high efficiency. Infected cells were selected and individual stable cells lines established. OGT shRNA was induced with the addition of doxycycline, and cells were processed for protein and RNA at 2 days after shRNA induction.
FIGURE 7.
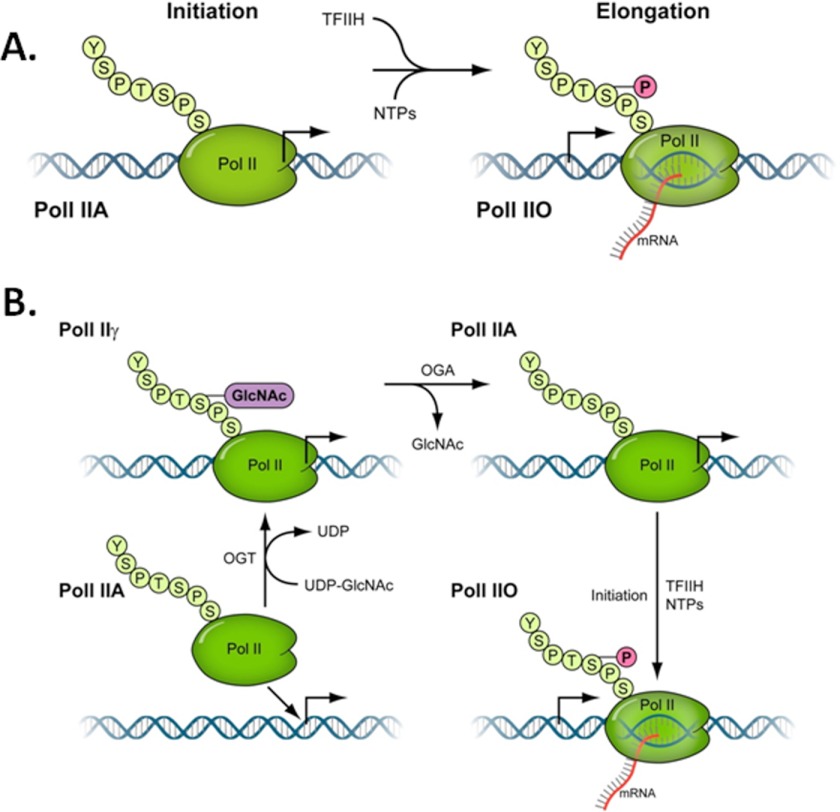
A cycle of O-GlcNAcylation on promoter bound RNA polymerase II CTD. A, the prevailing model of CTD modification of RNA pol II, derived from the work of Dahmus and Reinberg (7–10) and others, is that the unmodified form of pol II (pol IIA) is the initiation-specific form and is the only form of pol II that can form a preinitiation complex. After the initiation of transcription, TFIIH phosphorylates the CTD at serine 5 residues and this becomes the O-form of pol II (pol IIO) (5). B, shown is a schematic illustration of a model that depicts the steps involved in O-GlcNAc-dependent modification of RNA polymerase II. The cycling of O-GlcNAc begins with the arrival of the unmodified pol IIA to the promoter, recruited by other members of the transcriptional machinery (not shown). The pol IIA is converted to pol IIγ by the action of the OGT enzyme and UDP-GlcNAc while both proteins are on the promoter. The O-GlcNAc is subsequently removed by the action of the OGA, converting pol IIγ back to pol IIA. This removal of O-GlcNAc is necessary for the subsequent initiation/elongation-dependent phosphorylation events by TFIIH and P-TEFb (pol IIO).
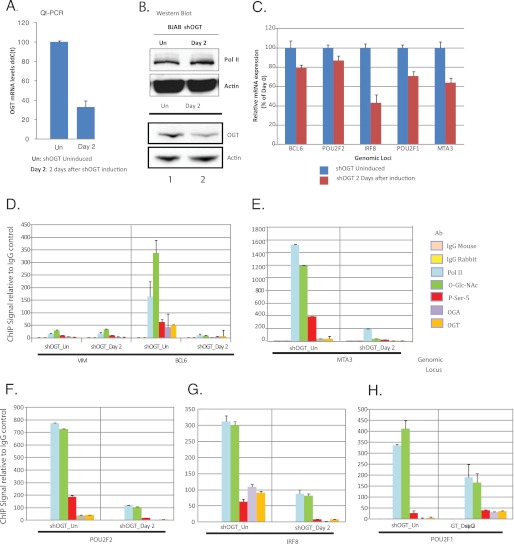
In Fig. 6A we found that at day 2 post-doxycycline, OGT mRNA levels were reduced 70% relative to the uninduced cells. We also saw ∼50% OGT reduction at the protein level, as assayed by Western blots on whole cell extracts. In contrast, we did not observe any reduction in actin protein levels. We also assayed for decreases in pol II levels by Western blot, comparing uninduced cells to cells at day 2 after shOGT induction. The pol II levels were identical before and after OGT shRNA induction (Fig. 6B). We then assayed several highly expressed B-cell genes and found that OGT shRNA reduced RNA levels 20–60% (Fig. 6C). Lastly, we assayed for the presence of RNA polymerase II, O-GlcNAc, phospho-CTD (serine 5), OGT, and OGA by single locus ChIP (Fig. 6, D–H). We noted significant reductions in O-GlcNAc and OGT, as expected. We also found that both RNA polymerase II and serine 5-phosphorylated RNA polymerase CTD were significantly reduced on all five promoters examined. These data strikingly illustrate the essential requirement of OGT and O-GlcNAc for RNA polymerase II promoter occupancy and transcription.
FIGURE 6.
ShRNA targeting of OGT reduces transcription and RNA polymerase II promoter occupancy. A, shown in the left panel are OGT mRNA levels before and 2 days after OGT shRNA induction, represented as relative ddC(t) levels. The right panel depicts Western blots of whole cell extracts from cells before (shOGT_Un) and 2 days after OGT shRNA induction (shOGT_Day 2), probing for either OGT or actin. Qt-PCR, quantitative real-time PCR. B, control experiment assaying for levels of pol II before and after induction of the OGT shRNA. Day 2 post-induction is the day used for the ChIP-seq and single locus ChIP assays. Whole cell lysates from the indicated periods were run on SDS-PAGE and immunoblotted with either a pol II antibody (N20) or and actin antibody (loading control). C, shown are the relative mRNA levels for five highly expressed B-cell genes before and after OGT shRNA induction: BCL6, POU2F2 (Oct2), IRF8, POU2F1 (Oct1), and MTA3. d–h, shown are normalized single locus ChIPs for vimentin (VIM (D)), BCL6 (D), POU2F (E), IRF8 (F), POU2F1 (G), and MTA3 (H) using either control rabbit or mouse IgG antibodies (yellow and tan bar, respectively), 8WG16 anti-pol II mAb (light blue bar), RL2 anti-O-GlcNAc (green bar), phospho-serine 5 pol II CTD mAb (red bar), anti-OGA (purple bar), or anti-OGT antibodies (orange bar). Error bars are derived from triplicate experiments.
DISCUSSION
The RNA Polymerase IIA/PIC Model
The current model of PIC formation contains pol IIA as the species of pol II capable of forming a PIC. These are based largely on the pioneering experiments of Dahmus and colleagues (7) in the late 1980s and early 1990s, culminating in experiments showing that only pol IIA forms transcriptionally competent PICs. The exclusion of pol IIO is also documented by them and others as well (7, 51). As we mentioned in the introduction, other data imply that pol IIA may not be the only form of pol II associated with the PIC. Kelly et al. (23) show that “unmodified” pol II (pol IIA, purified from calf thymus as described in Ref. 7) contains a population of pol II that is O-GlcNAcylated on the CTD. So the pol II used in experiments described in Ref. 23 to establish the form of pol II that associates with the PIC is likely a mixture of both pol IIA and pol IIγ. There are several additional caveats to those experiments, including their use of cytoplasmic S100 extracts and not nuclear extracts and the addition of 0.08% Sarkosyl to the transcription reactions (7). We are unaware of any literature in which similar experiments have been done in crude human systems, and data on yeast transcription systems are not relevant because Saccharomyces cerevisiae do not have O-GlcNAc.
Construction of a Model of a pol IIγ-containing PIC
The data in Fig. 1 establish that the nuclear extracts are competent, in and of themselves, to convert pol IIA to pol IIγ. This means that OGT and especially UDP-GlcNAc are present in sufficient quantities to accomplish this conversion. The data in Fig. 2 functionally show that disruption of either OGT or OGA activity abrogates transcription and this transcription defect occurs prior to PIC assembly. The in vitro data in Fig. 3A and the in vivo ChIP data in Fig. 6 show that OGT maps to promoters and is likely part of the PIC. Furthermore, OGT interacts with several GTFs (Fig. 5). Supporting the PIC interpretation further are CTD serine-to-alanine substitutions (Fig. 4), which show that serine residues 5 and 7, also targets of the initiation-specific TFIIH kinase, are required for OGT modification of the CTD. Lastly, the in vitro experiment in Fig. 3 suggests that pol II itself is the direct target of the OGA inhibition. This is supported by the lack of rescue when adding pol IIγ. This result is not surprising, as the PUGNAc results in the accumulation of pol IIγ (Fig. 1). There are two other possible interpretations of this experiment, however, which preclude its standing alone. 1) The addition of pol IIA simply sets up a new population of PICs de novo. However, this does not explain why then only pol IIA but not pol IIγ rescues the PUGNAc block. 2) Why does pol IIA work at all if the OGT and UDP-GlcNAc convert pol IIA to pol IIγ, which then should be blocked from returning to the A-form by PUGNAc? Note that the likely presence of phosphatases in the experiments of Dahmus and colleagues (7) creates the same caveat to their interpretation. Nevertheless, Fig. 2, E and F, suggests that there is a window of OGT and OGA requirements that perhaps is somehow passed by in the rescue experiment. The limitations of this one experiment, however, are overcome with the in vivo ChIP experiments with pol II, which support the interpretation of the in vitro data. There pol IIγ is localized to promoters and the recruitment of pol II to the promoter is abrogated by the loss of OGT (Fig. 6). These genomic distributions of pol II and O-GlcNAc are very similar to the results obtained by ChIP-chip analysis of Caenorhabditis elegans (52).
From our in vitro and in vivo functional analysis, we infer that pol IIγ is a promoter-specific species of pol II. We think that the most likely interpretation of our data is that a cycle of O-GlcNAcylation on the RNA polymerase II CTD occurs on the promoter. This cycle, the “γ-cycle,” creates a form of RNA polymerase II that we term pol IIγ. pol IIγ is created on the promoter during PIC assembly and is subsequently converted back to an unmodified form before the initiation of transcription (Fig. 7). The complete γ-cycle is necessary for PIC formation, because a block to either step in the cycle, the addition or removal of O-GlcNAc, results in transcription inhibition.
These data open the possibility that pol II is converted from the γ-form immediately to the O-form upon the initiation of transcription, and therefore pol IIA may exist only transiently in the PIC. Secondly, it is now possible that the unmodified form of pol II, pol IIA, exists only as an artifact of its purification, because of the activity of ubiquitous hexosaminidases and the absence of hexosaminidase inhibitors during purification. Indeed, the first purifications of RNA polymerase II from calf thymus contained the pol IIγ form (23). Thirdly, these data further support the contention that the content of the PIC is much more complex than suggested in PIC models to date; it is quite likely that many other factors besides OGT and OGA, such as CK2, PARP, and GAPDH, are involved in PIC formation and are present on promoters (28, 53, 54).
The data presented herein argues strongly that there are additional, novel steps in the assembly of PICs on promoters, through either the regulation of the activity of the OGT and OGA or through the temporal regulation of their recruitment to the promoter. It is clear that the regulation of transcription initiation is becoming increasingly complex, far beyond our imagination, and that multiple regulatory steps occur in the proximal promoter region. The continuation of these functional biochemistry studies is mandatory if we are to accurately understand the regulation of transcription in disease and development, as well as the diverse array of promoters and activators that exist in the genome.
Acknowledgments
We thank Kaoru Sakabe for advice, discussions, and early experimental support, Jeff Corden and Shona Murphy for GST-CTD expression vectors, Dirk Eick for critical reading of the manuscript, and D. Reinberg for purified human RNA pol II and antibodies. We also thank Jason Piotrowski for expert technical assistance and Dr. Alfonso Fernandez for experimental assistance.
This work was supported, in whole or in part, by National Institutes of Health Intramural Research Program grants from NCI, Center for Cancer Research (to B. A. L.) and NIDDK (to J. A. H.) and by National Institutes of Health grants NIH R01-CA42486 and R01-DK61671 (to G. W. H.).
- CTD
- C-terminal domain
- pol
- polymerase
- PIC
- preinitiation complex
- OGT
- O-GlcNAc transferase
- OGA
- O-GlcNAc aminidase
- OGAs
- 75-kDa short isoform OGA
- PUGNAc
- O-(2-acetamido-2-deoxy-d-glucopyranosylidene)amino-N-phenylcarbamate
- NAGT
- 1,2-dideoxy-2′-methyl-d-glucopyranoso[2,1-d]-2′-thiazoline
- MCS
- multiple cloning site
- GTF
- general transcription factor
- WGA
- wheat germ agglutinin
- DPE
- downstream promoter element
- NAGT
- 1,2-dideoxy-2′-methyl-d-glucopyranoso[2,1-d]-2′-thiazoline
- NE
- nuclear extract
- r
- recombinant (e.g. rOGT)
- P-TEFB
- positive elongation factor b
- TBP
- TATA-binding protein.
REFERENCES
- 1. Dahmus M. E. (1996) Reversible phosphorylation of the C-terminal domain of RNA polymerase II. J. Biol. Chem. 271, 19009–19012 [DOI] [PubMed] [Google Scholar]
- 2. Phatnani H. P., Greenleaf A. L. (2006) Phosphorylation and functions of the RNA polymerase II CTD. Genes Dev. 20, 2922–2936 [DOI] [PubMed] [Google Scholar]
- 3. Saunders A., Core L. J., Lis J. T. (2006) Breaking barriers to transcription elongation. Nat. Rev. Mol. Cell Biol. 7, 557–567 [DOI] [PubMed] [Google Scholar]
- 4. Egloff S., Murphy S. (2008) Cracking the RNA polymerase II CTD code. Trends Genet. 24, 280–288 [DOI] [PubMed] [Google Scholar]
- 5. Sims R. J., 3rd, Belotserkovskaya R., Reinberg D. (2004) Elongation by RNA polymerase II: the short and long of it. Genes Dev. 18, 2437–2468 [DOI] [PubMed] [Google Scholar]
- 6. Kang M. E., Dahmus M. E. (1993) RNA polymerases IIA and IIO have distinct roles during transcription from the TATA-less murine dihydrofolate reductase promoter. J. Biol. Chem. 268, 25033–25040 [PubMed] [Google Scholar]
- 7. Chesnut J. D., Stephens J. H., Dahmus M. E. (1992) The interaction of RNA polymerase II with the adenovirus-2 major late promoter is precluded by phosphorylation of the C-terminal domain of subunit IIa. J. Biol. Chem. 267, 10500–10506 [PubMed] [Google Scholar]
- 8. Laybourn P. J., Dahmus M. E. (1990) Phosphorylation of RNA polymerase IIA occurs subsequent to interaction with the promoter and before the initiation of transcription. J. Biol. Chem. 265, 13165–13173 [PubMed] [Google Scholar]
- 9. Payne J. M., Laybourn P. J., Dahmus M. E. (1989) The transition of RNA polymerase II from initiation to elongation is associated with phosphorylation of the carboxyl-terminal domain of subunit IIa. J. Biol. Chem. 264, 19621–19629 [PubMed] [Google Scholar]
- 10. Lu H., Flores O., Weinmann R., Reinberg D. (1991) The nonphosphorylated form of RNA polymerase II preferentially associates with the preinitiation complex. Proc. Natl. Acad. Sci. U.S.A. 88, 10004–10008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buratowski S., Sharp P. A. (1990) Transcription initiation complexes and upstream activation with RNA polymerase II lacking the C-terminal domain of the largest subunit. Mol. Cell. Biol. 10, 5562–5564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim W. Y., Dahmus M. E. (1989) The major late promoter of adenovirus-2 is accurately transcribed by RNA polymerases IIO, IIA, and IIB. J. Biol. Chem. 264, 3169–3176 [PubMed] [Google Scholar]
- 13. Mäkelä T. P., Tassan J. P., Nigg E. A., Frutiger S., Hughes G. J., Weinberg R. A. (1994) A cyclin associated with the CDK-activating kinase MO15. Nature 371, 254–257 [DOI] [PubMed] [Google Scholar]
- 14. Serizawa H., Conaway J. W., Conaway R. C. (1993) Phosphorylation of C-terminal domain of RNA polymerase II is not required in basal transcription. Nature 363, 371–374 [DOI] [PubMed] [Google Scholar]
- 15. Lux C., Albiez H., Chapman R. D., Heidinger M., Meininghaus M., Brack-Werner R., Lang A., Ziegler M., Cremer T., Eick D. (2005) Transition from initiation to promoter proximal pausing requires the CTD of RNA polymerase II. Nucleic Acids Res. 33, 5139–5144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meininghaus M., Chapman R. D., Horndasch M., Eick D. (2000) Conditional expression of RNA polymerase II in mammalian cells. Deletion of the carboxyl-terminal domain of the large subunit affects early steps in transcription. J. Biol. Chem. 275, 24375–24382 [DOI] [PubMed] [Google Scholar]
- 17. Gerber H. P., Hagmann M., Seipel K., Georgiev O., West M. A., Litingtung Y., Schaffner W., Corden J. L. (1995) RNA polymerase II C-terminal domain required for enhancer-driven transcription. Nature 374, 660–662 [DOI] [PubMed] [Google Scholar]
- 18. Kim Y. J., Björklund S., Li Y., Sayre M. H., Kornberg R. D. (1994) A multiprotein mediator of transcriptional activation and its interaction with the C-terminal repeat domain of RNA polymerase II. Cell 77, 599–608 [DOI] [PubMed] [Google Scholar]
- 19. Koh S. S., Ansari A. Z., Ptashne M., Young R. A. (1998) An activator target in the RNA polymerase II holoenzyme. Mol. Cell 1, 895–904 [DOI] [PubMed] [Google Scholar]
- 20. Liao S. M., Taylor I. C., Kingston R. E., Young R. A. (1991) RNA polymerase II carboxy-terminal domain contributes to the response to multiple acidic activators in vitro. Genes Dev. 5, 2431–2440 [DOI] [PubMed] [Google Scholar]
- 21. Myers L. C., Gustafsson C. M., Bushnell D. A., Lui M., Erdjument-Bromage H., Tempst P., Kornberg R. D. (1998) The Med proteins of yeast and their function through the RNA polymerase II carboxy-terminal domain. Genes Dev. 12, 45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thompson C. M., Koleske A. J., Chao D. M., Young R. A. (1993) A multisubunit complex associated with the RNA polymerase II CTD and TATA-binding protein in yeast. Cell 73, 1361–1375 [DOI] [PubMed] [Google Scholar]
- 23. Kelly W. G., Dahmus M. E., Hart G. W. (1993) RNA polymerase II is a glycoprotein. Modification of the COOH-terminal domain by O-GlcNAc. J. Biol. Chem. 268, 10416–10424 [PubMed] [Google Scholar]
- 24. Haltiwanger R. S., Blomberg M. A., Hart G. W. (1992) Glycosylation of nuclear and cytoplasmic proteins. Purification and characterization of a uridine diphospho-N-acetylglucosamine:polypeptide β-N-acetylglucosaminyltransferase. J. Biol. Chem. 267, 9005–9013 [PubMed] [Google Scholar]
- 25. Gao Y., Wells L., Comer F. I., Parker G. J., Hart G. W. (2001) Dynamic O-glycosylation of nuclear and cytosolic proteins: cloning and characterization of a neutral, cytosolic β-N-acetylglucosaminidase from human brain. J. Biol. Chem. 276, 9838–9845 [DOI] [PubMed] [Google Scholar]
- 26. Comer F. I., Hart G. W. (2001) Reciprocity between O-GlcNAc and O-phosphate on the carboxyl-terminal domain of RNA polymerase II. Biochemistry 40, 7845–7852 [DOI] [PubMed] [Google Scholar]
- 27. Lee D. H., Gershenzon N., Gupta M., Ioshikhes I. P., Reinberg D., Lewis B. A. (2005) Functional characterization of core promoter elements: the downstream core element is recognized by TAF1. Mol. Cell. Biol. 25, 9674–9686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lewis B. A., Sims R. J., 3rd, Lane W. S., Reinberg D. (2005) Functional characterization of core promoter elements: DPE-specific transcription requires the protein kinase CK2 and the PC4 coactivator. Mol. Cell 18, 471–481 [DOI] [PubMed] [Google Scholar]
- 29. Lubas W. A., Frank D. W., Krause M., Hanover J. A. (1997) O-Linked GlcNAc transferase is a conserved nucleocytoplasmic protein containing tetratricopeptide repeats. J. Biol. Chem. 272, 9316–9324 [DOI] [PubMed] [Google Scholar]
- 30. Lazarus B. D., Love D. C., Hanover J. A. (2006) Recombinant O-GlcNAc transferase isoforms: identification of O-GlcNAcase, yes tyrosine kinase, and tau as isoform-specific substrates. Glycobiology 16, 415–421 [DOI] [PubMed] [Google Scholar]
- 31. Zhou M., Halanski M. A., Radonovich M. F., Kashanchi F., Peng J., Price D. H., Brady J. N. (2000) Tat modifies the activity of CDK9 to phosphorylate serine 5 of the RNA polymerase II carboxyl-terminal domain during human immunodeficiency virus type 1 transcription. Mol. Cell. Biol. 20, 5077–5086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zachara N. E., Hart G. W., Cole R. N., Gao Y. (2002) Detection and Analysis of Proteins Modified by O-Linked N-Acetylglucosamine, Curr. Protoc. Mol. Biol. Chapter 17, Unit 17.6 [DOI] [PubMed] [Google Scholar]
- 33. Hanover J. A., Forsythe M. E., Hennessey P. T., Brodigan T. M., Love D. C., Ashwell G., Krause M. (2005) A Caenorhabditis elegans model of insulin resistance: altered macronutrient storage and dauer formation in an OGT-1 knock-out. Proc. Natl. Acad. Sci. U.S.A. 102, 11266–11271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ngo V. N., Davis R. E., Lamy L., Yu X., Zhao H., Lenz G., Lam L. T., Dave S., Yang L., Powell J., Staudt L. M. (2006) A loss-of-function RNA interference screen for molecular targets in cancer. Nature 441, 106–110 [DOI] [PubMed] [Google Scholar]
- 35. Comer F. I., Vosseller K., Wells L., Accavitti M. A., Hart G. W. (2001) Characterization of a mouse monoclonal antibody specific for O-linked N-acetylglucosamine. Anal. Biochem. 293, 169–177 [DOI] [PubMed] [Google Scholar]
- 36. Kreppel L. K., Hart G. W. (1999) Regulation of a cytosolic and nuclear O-GlcNAc transferase. Role of the tetratricopeptide repeats. J. Biol. Chem. 274, 32015–32022 [DOI] [PubMed] [Google Scholar]
- 37. Lubas W. A., Hanover J. A. (2000) Functional expression of O-linked GlcNAc transferase. Domain structure and substrate specificity. J. Biol. Chem. 275, 10983–10988 [DOI] [PubMed] [Google Scholar]
- 38. Chalkley R. J., Thalhammer A., Schoepfer R., Burlingame A. L. (2009) Identification of protein O-GlcNAcylation sites using electron transfer dissociation mass spectrometry on native peptides. Proc. Natl. Acad. Sci. U.S.A. 106, 8894–8899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Comtesse N., Maldener E., Meese E. (2001) Identification of a nuclear variant of MGEA5, a cytoplasmic hyaluronidase and a β-N-acetylglucosaminidase. Biochem. Biophys. Res. Commun. 283, 634–640 [DOI] [PubMed] [Google Scholar]
- 40. Kim E. J., Kang D. O., Love D. C., Hanover J. A. (2006) Enzymatic characterization of O-GlcNAcase isoforms using a fluorogenic GlcNAc substrate. Carbohydr. Res. 341, 971–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lewis B. A., Kim T. K., Orkin S. H. (2000) A downstream element in the human β-globin promoter: evidence of extended sequence-specific transcription factor IID contacts. Proc. Natl. Acad. Sci. U.S.A. 97, 7172–7177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lewis B. A., Orkin S. H. (1995) A functional initiator element in the human β-globin promoter. J. Biol. Chem. 270, 28139–28144 [DOI] [PubMed] [Google Scholar]
- 43. Gross B. J., Kraybill B. C., Walker S. (2005) Discovery of O-GlcNAc transferase inhibitors. J. Am. Chem. Soc. 127, 14588–14589 [DOI] [PubMed] [Google Scholar]
- 44. Dong D. L., Hart G. W. (1994) Purification and characterization of an O-GlcNAc selective N-acetyl-β-d-glucosaminidase from rat spleen cytosol. J. Biol. Chem. 269, 19321–19330 [PubMed] [Google Scholar]
- 45. Burke T. W., Kadonaga J. T. (1996) Drosophila TFIID binds to a conserved downstream basal promoter element that is present in many TATA-box-deficient promoters. Genes Dev. 10, 711–724 [DOI] [PubMed] [Google Scholar]
- 46. Burke T. W., Kadonaga J. T. (1997) The downstream core promoter element, DPE, is conserved from Drosophila to humans and is recognized by TAFII60 of Drosophila. Genes Dev. 11, 3020–3031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Konrad R. J., Zhang F., Hale J. E., Knierman M. D., Becker G. W., Kudlow J. E. (2002) Alloxan is an inhibitor of the enzyme O-linked N-acetylglucosamine transferase. Biochem. Biophys. Res. Commun. 293, 207–212 [DOI] [PubMed] [Google Scholar]
- 48. Lee T. N., Alborn W. E., Knierman M. D., Konrad R. J. (2006) Alloxan is an inhibitor of O-GlcNAc-selective N-acetyl-β-d-glucosaminidase. Biochem. Biophys. Res. Commun. 350, 1038–1043 [DOI] [PubMed] [Google Scholar]
- 49. Macauley M. S., Whitworth G. E., Debowski A. W., Chin D., Vocadlo D. J. (2005) O-GlcNAcase uses substrate-assisted catalysis. Kinetic analysis and development of highly selective mechanism-inspired inhibitors. J. Biol. Chem. 280, 25313–25322 [DOI] [PubMed] [Google Scholar]
- 50. Reason A. J., Dell A., Romero P. A., Herscovics A. (1991) Specificity of the mannosyltransferase which initiates outer chain formation in Saccharomyces cerevisiae. Glycobiology 1, 387–391 [DOI] [PubMed] [Google Scholar]
- 51. Gebara M. M., Sayre M. H., Corden J. L. (1997) Phosphorylation of the carboxy-terminal repeat domain in RNA polymerase II by cyclin-dependent kinases is sufficient to inhibit transcription. J. Cell. Biochem. 64, 390–402 [PubMed] [Google Scholar]
- 52. Love D. C., Ghosh S., Mondoux M. A., Fukushige T., Wang P., Wilson M. A., Iser W. B., Wolkow C. A., Krause M. W., Hanover J. A. (2010) Dynamic O-GlcNAc cycling at promoters of Caenorhabditis elegans genes regulating longevity, stress, and immunity. Proc. Natl. Acad. Sci. U.S.A. 107, 7413–7418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pavri R., Lewis B., Kim T. K., Dilworth F. J., Erdjument-Bromage H., Tempst P., de Murcia G., Evans R., Chambon P., Reinberg D. (2005) PARP-1 determines specificity in a retinoid signaling pathway via direct modulation of mediator. Mol. Cell 18, 83–96 [DOI] [PubMed] [Google Scholar]
- 54. Zheng L., Roeder R. G., Luo Y. (2003) S phase activation of the histone H2B promoter by OCA-S, a coactivator complex that contains GAPDH as a key component. Cell 114, 255–266 [DOI] [PubMed] [Google Scholar]
- 55. Maldonado E., Drapkin R., Reinberg D. (1996) Purification of human RNA polymerase II and general transcription factors. Methods Enzymol. 274, 72–100 [DOI] [PubMed] [Google Scholar]