Abstract
Potential roles for lactate in the energetics of brain activation have changed radically during the past three decades, shifting from waste product to supplemental fuel and signaling molecule. Current models for lactate transport and metabolism involving cellular responses to excitatory neurotransmission are highly debated, owing, in part, to discordant results obtained in different experimental systems and conditions. Major conclusions drawn from tabular data summarizing results obtained in many laboratories are as follows: Glutamate-stimulated glycolysis is not an inherent property of all astrocyte cultures. Synaptosomes from the adult brain and many preparations of cultured neurons have high capacities to increase glucose transport, glycolysis, and glucose-supported respiration, and pathway rates are stimulated by glutamate and compounds that enhance metabolic demand. Lactate accumulation in activated tissue is a minor fraction of glucose metabolized and does not reflect pathway fluxes. Brain activation in subjects with low plasma lactate causes outward, brain-to-blood lactate gradients, and lactate is quickly released in substantial amounts. Lactate utilization by the adult brain increases during lactate infusions and strenuous exercise that markedly increase blood lactate levels. Lactate can be an ‘opportunistic', glucose-sparing substrate when present in high amounts, but most evidence supports glucose as the major fuel for normal, activated brain.
Keywords: astrocyte, brain activation, glucose, lactate shuttling, metabolism, neuron
Glucose is the major fuel for the brain, and its metabolism by different pathways has important functions related to energetics, neurotransmission, oxidation–reduction (redox) reactions, and biosynthesis of essential brain components (Figure 1). For many decades, lactate production in the brain was viewed as a consequence of inadequate oxygen delivery, disruption of oxidative metabolism, or mismatch between glycolytic and oxidative rates (Siesjö, 1978), but more recently, the conceptual role of lactate metabolism and function in the normal brain have undergone major changes, shifting from developmental fuel and glycolytic waste product to include its use as a supplemental fuel and signaling molecule. Starting in the 1970s to 1980s studies carried out in different laboratories with diverse experimental interests related to brain function brought attention to upregulation of glycolysis, lactate production, lactate release into the blood, the possibility of lactate shuttling among cell types within the brain, lactate fueling adult brain during exercise, and roles of lactate in the regulation of blood flow; some of these topics are controversial and highly debated. The experimental paradigm and physiologic status of subjects are critical for interpretation of data, and this review first presents a brief historical overview of studies related to brain lactate transport and metabolism, then compares sets of data to provide a perspective and context within which the consistency of similar experiments and their in vivo relevance can be compared and assessed. Space and reference limitations prevent citation of many important studies, and selected initial reports and reviews for specific topics are cited.
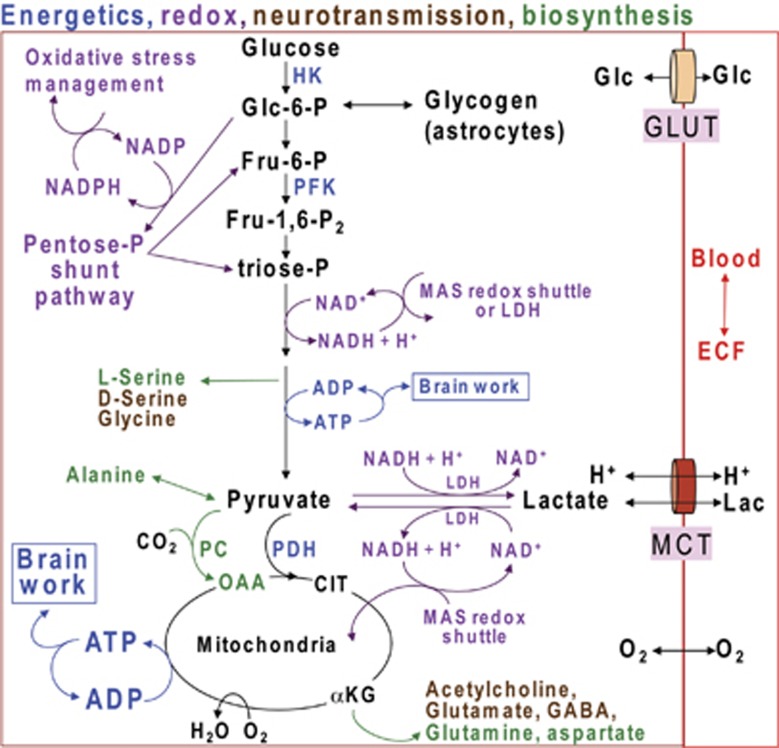
Figure 1.
Multifunctional roles of glucose metabolism. Color coding denotes different functional roles of pathways of glucose metabolism. Glucose (Glc) and lactate (Lac) plus H+ are transported into brain cells from blood or extracellular fluid (ECF) by equilibrative transporters, GLUTs and MCTs (monocarboxylic acid transporters), respectively, whereas oxygen diffuses into brain cells. Energetics (blue) involves ATP production by the glycolytic (glucose to pyruvate; Fru=fructose) and oxidative (pyruvate to CO2 + H2O) pathways. Glycolytic rate is modulated by regulation of hexokinase (HK), phosphofructokinase (PFK), and other enzymes. Glucose is stored as glycogen, mainly in astrocytes. Pyruvate enters the oxidative pathway of the mitochondrial tricarboxylic acid cycle, with formation of 3 CO2 and regeneration of oxaloacetate (OAA). Neurotransmitters and neuromodulators (brown) are synthesized through the glycolytic and oxidative pathways. Other biosynthetic pathways produce amino acids (green) and sugars (not shown) used to synthesize complex carbohydrates for glycoproteins and glycolipids. Net synthesis of a ‘new' four- or five-carbon compound (aspartate, glutamate, glutamine, GABA) requires pyruvate carboxylase (PC), which is only located in astrocytes. CO2 fixation by PC converts pyruvate to OAA. OAA is transaminated to form aspartate or it condenses with acetyl CoA derived from a second pyruvate molecule by the action of pyruvate dehydrogenase (PDH) to form citrate (CIT). Decarboxylation of this ‘new' six-carbon compound forms α-ketoglutarate (αKG) that can be converted to a new molecule of glutamate, glutamine, and GABA. These compounds can also incorporate label from labeled glucose in neurons and astrocytes by means of reversible exchange reactions, but their net synthesis requires the astrocytic PC reaction. Acetylcholine is also derived from glucose through citrate in neurons. Entry of glucose-6-P into the pentose phosphate shunt pathway (purple) results in oxidative decarboxylation of carbon one of glucose and generates NADPH, which is used to detoxify reactive species that can cause oxidative stress. The nonoxidative branch of pentose shunt produces fructose-6-phosphate (Fru-6-P) and triose-P that reenter the glycolytic pathway; nucleotide precursors are also generated by the pentose shunt pathway. NAD+ is required for glycolysis, and it is regenerated from NADH by the malate–aspartate shuttle (MAS) or lactate dehydrogenase (LDH) (purple). The MAS redox shuttle transfers reducing equivalents from the cytosol to the mitochondria and is required to generate pyruvate for oxidation by the tricarboxylic acid cycle; it is also required for oxidative metabolism of lactate. Regeneration of NAD+ by LDH removes pyruvate from the oxidative pathway.
Brief Thematic and Historical Perspective of Brain Lactate Metabolism and Trafficking
Compartmentation of Lactate Metabolism in the Brain
During the 1960 to 1970s, in vivo studies of precursors of brain amino acids revealed compartmentation of metabolism in the brain, with identification of different precursors that preferentially labeled the large (neuronal) glutamate pool and the small (astrocytic) glutamate pool that is the precursor for glutamine; pool labeling assignments were based on the ratio of the specific activity of purified glutamate to that of glutamine (reviewed in the volume edited by Balázs and Cremer (1972)). Early studies have shown that glucose (Cremer, 1964) and lactate labeled the large glutamate pool, whereas butyrate and acetate labeled the small pool (O'Neal and Koeppe, 1966). However, lactate is oxidized by cultured cortical and cerebellar neurons and astrocytes (Dienel and Hertz (2001) and references cited therein) and by both neurons and astrocytes in vivo (Zielke et al, 2007; Zielke et al, 2009).
Rapid Lactate Release from the Brain into the Blood
Hawkins et al (1973) showed that an ammonia injection increases the rate of cerebral glucose utilization (CMRglc) and oxygen consumption (CMRO2) in the rat brain and increases lactate release to blood from 3.5% (as glucose equivalents) of glucose uptake at rest to 15% after ammonia. The brain lactate level was less than that in blood, suggesting sites with locally high lactate levels from which lactate diffused into blood. In humans, positron emission tomographic imaging studies using [11C]glucose detected release of 11C-acidic metabolites into blood within 4 minutes (Blomqvist et al, 1990). During spreading cortical depression, release of 14C-lactate was detectable within 2 minutes after pulse labeling of the rat brain with [6-14C]glucose; maximal lactate efflux equaled 20% of glucose uptake, and [14C]lactate accounted for nearly all of the 14C discharged into the blood (Cruz et al, 1999). In humans given stressful mental testing, lactate release corresponded to 7% of glucose uptake (Madsen et al, 1995). The above studies show that the resting brain also releases small amounts of lactate (∼3% to 7% of glucose uptake), and that lactate efflux quickly increases by 3- to 4-fold during activation. A recent positron emission tomographic study in a resting young adult human brain revealed regional heterogeneity in the mismatch between local rates of glucose and oxygen utilization (Vaishnavi et al, 2010), suggesting that lactate release from various brain structures probably differs under basal conditions.
Lactate Release from Brain Cells
Lactate is released in larger quantities from ‘resting' cultured astrocytes than neurons, but both cell types produce lactate under various conditions (Walz and Mukerji, 1988). Dringen et al (1993) discovered that lactate, not glucose, is released from cultured astrocytes during glycogenolysis, and suggested that lactate may function as fuel for neighboring cells. These and related in vitro studies underlie the widely held notion that astrocytes may be the major source of brain lactate, but the cellular origin and cellular metabolic fate of lactate in vivo remain to be experimentally established.
Underestimation of Metabolic Activation with Labeled Glucose and Lactate Release from the Brain
Functional metabolic brain imaging studies in conscious rats (Collins et al, 1987; Ackermann and Lear, 1989; Adachi et al, 1995; Cruz et al, 2007) and humans (Blomqvist et al, 1990) found that the magnitude of increased CMRglc evoked by sensory stimulation, seizures, spreading depression, and voluntary finger tapping was greatly underestimated (by approximately ⩾50%) with labeled glucose compared with labeled deoxyglucose, suggesting upregulation of glycolysis and rapid lactate release (Collins et al, 1987; Lear and Ackermann, 1989; Lear, 1990). Studies that our laboratory designed to understand the neurobiology underlying the above discrepant results obtained with glucose and deoxyglucose showed that brain lactate is quickly labeled by blood glucose, lactate is readily diffusible, and rapid lactate efflux to the blood causes loss of labeled products from the brain (Adachi et al, 1995; Cruz et al, 1999; Dienel and Cruz, 2009). Focal label retention in activated structures is enhanced by blockade of lactate transporters and astrocytic gap junctions (Cruz et al, 2007), and astrocytes have a much higher rate and capacity for lactate uptake from extracellular fluid and for dispersion within the astrocytic syncytium compared with lactate shuttling from astrocytes to neurons (Gandhi et al, 2009). Most lactate derived from glucose microinfused into interstitial fluid is not locally oxidized, and extracellular metabolites are released through perivascular flow into the lymphatic drainage systems (Ball et al, 2010). Taken together, these findings indicate that increased glycolysis during activation is associated with substantial loss of lactate from the brain through vascular and perivascular drainage systems within 5 minutes in normal subjects with low blood lactate levels (∼0.5 to 1 mmol/L) and modest (∼2-fold) or large (>3- to 8-fold) increases in brain lactate level.
‘Uncoupling' of Cerebral Blood Flow, Oxygen Consumption (CMRO2), and CMRglc During Sensory and Mental Stimulation
In the resting brain, nearly all of the glucose metabolized is oxidized, and many, but not all, studies report that the resting CMRO2/CMRglc ratio is close to the theoretical maximum of 6.0 (i.e., 6 O2 are required to oxidize 1 glucose). However, during activation, disproportionately larger increases in cerebral blood flow (CBF) and CMRglc compared with CMRO2 were reported by Fox and Raichle (1986) and Fox et al (1988), and confirmed in humans (Madsen et al, 1995) and rats (Madsen et al, 1999). The CMRO2/CMRglc ratio falls in most, but not all, activation studies by a variable magnitude, showing that nonoxidative metabolism usually increases much more than oxidative metabolism, which can be either unchanged or increased somewhat (∼10% to 25%), depending on the paradigm and brain structures involved (Dienel and Cruz (2008) and cited references). The basis for this phenomenon (sometimes called aerobic glycolysis) remains to be elucidated, and it contrasts the brain's capacity to increase CMRO2 by 2- to 3-fold during seizures and maintain the increase for 2 hours (Meldrum and Nilsson, 1976; Borgström et al, 1976). The activation-induced CMRO2–CMRglc mismatch is consistent with increased glycolysis without local oxidation of the lactate equivalents generated.
Lactate and Neuronal Function in Brain Slices
Levels of lactate transporters at the blood–brain barrier and enzymes that metabolize ketone bodies decrease drastically after weaning (Cremer, 1982; Vannucci and Simpson, 2003), and blood lactate and ketones are not major fuels for the adult brain unless their concentrations increase markedly. However, during hypoxia/ischemia, glucose/glycogen-derived lactate accumulates in brain tissue. The notion that lactate may ‘jump start' neuronal recovery after restoration of blood flow and oxygen delivery was proposed after the discovery that lactate supported electrically evoked action potentials in brain slices (Schurr et al, 1988; Schurr, 2006). However, other investigators previously found that lactate and other alternative substrates cannot substitute for glucose, and evoked action potentials fail even though ATP levels are maintained (see Figure 4 and related text in Dienel and Hertz (2005)). The ability of lactate to support evoked action potentials depends on the speed of slice preparation and other technical issues that are not fully understood (Okada and Lipton, 2007). Moreover, lactate cannot prevent anoxic depolarization in slices from P12 and P28 rats when glycolysis is completely inhibited (Allen et al (2005) and discussion therein). These findings indicate that lactate oxidation can support cellular functions or contribute to brain energetics under specific experimental conditions. However, glycolytic metabolism of glucose satisfies critical functions (Figure 1) that cannot be fulfilled by lactate or mitochondrially generated ATP, and maintenance of specific brain function requires glucose, not lactate, under many experimental conditions.
Extracellular Lactate Levels Increase During Activating and Pathophysiologic Conditions
Microdialysis (Korf and de Boer, 1990) and microelectrode (Hu and Wilson, 1997a, ) technology enabled monitoring of extracellular glucose and lactate levels. Many investigators have reported ∼2-fold increases in extracellular lactate levels during various behaviors or stresses, and these findings are often used to support the idea that glycolytic flux increases. However, lactate concentration changes must be interpreted with caution (Veech, 1991) because metabolite concentration is the net result of input to and output from a pool, and it does not report flux through the pool.
Lactate and Excitatory Neurotransmission
In 1994, Pellerin and Magistretti (1994) reported that glutamate stimulated CMRglc and lactate release in cultured astrocytes, and proposed that glutamate uptake stimulates astrocytic glycolysis and the lactate serves as fuel for nearby neurons. This concept, the astrocyte–neuron lactate shuttle hypothesis, posits that (1) the two ATP required by astrocytes to dispose of the Na+ taken up with glutamate and to convert glutamate to glutamine are satisfied by glycolysis and (2) there is a predominant cellular compartmentation of glycolytic and oxidative metabolism in astrocytes and neurons, respectively, during excitatory neurotransmission, with lactate shuttling to neurons and neuronal oxidation of lactate as major fuel (Hyder et al, 2006; Pellerin et al, 2007; Pellerin, 2008; Magistretti, 2009; Jolivet et al, 2010).
Cerdán et al (2006) proposed a different mechanism and role for astrocyte–neuron lactate trafficking, i.e., redox shuttling in which reducing equivalents are hypothesized to be transferred from astrocytes to neurons. In this model, lactate release from astrocytes and its uptake and oxidation to pyruvate in neurons transfers NADH to neurons. However, the pyruvate is not retained and oxidized in the neurons. Instead, pyruvate is released, taken up by astrocytes, and reduced to lactate to regenerate NAD+ in the astrocyte. This mechanism could thereby support glycolytic metabolism in astrocytes by means of a transcellular redox shuttle cycle instead of the intracellular, malate–aspartate shuttle (MAS) that transfers reducing equivalents from cytoplasmic NADH to the mitochondria for oxidation and ATP generation (Figure 1).
Discordant metabolic effects of glutamate on cultured astrocytes, complex biochemical and cellular responses to activation, oxidation of lactate by both neurons and astrocytes in vitro and in vivo, and rapid, substantial lactate release from the brain during in vivo activation have been cited as evidence against the brain's use of lactate as a major fuel during normal adult brain activation under physiologic conditions (Hertz et al, 1998, 2004, 2007; Chih et al, 2001; Chih and Roberts, 2003; Dienel and Cruz, 2003, 2004, 2006, 2008; Dienel and Hertz, 2001, 2005; Mangia et al, 2009a; Zielke et al, 2009). In addition, major metabolic responses to activation of the cerebellum in vivo are linked to postsynaptic events, with no detectable effect of blockade of astrocytic glutamate uptake on evoked metabolic activity. The AMPA (2-amino-3-(5-methyl-3-oxo-1,2- oxazol-4-yl)propanoic acid) receptor blockade, not astrocytic glutamate transport inhibition, eliminates stimulus-induced increases in extracellular lactate level, CMRglc, CMRO2, and CBF in the cerebellum in vivo (Caesar et al, 2008), separating metabolic activation from glutamate transport.
A contrasting transport-metabolism model that emphasizes concentrations and kinetic properties of cellular glucose and lactate transporters predicts that neurons take up most glucose during activation and release lactate to astrocytes, i.e., a neuron-to-astrocyte lactate shuttle (Simpson et al, 2007; Mangia et al, 2009b, 2011; DiNuzzo et al, 2010a, ). A mechanism that may explain, in part, increased neuronal lactate production during activation comes from in vitro studies of regulation of mitochondrial metabolism by calcium (Bak et al, 2009; Contreras and Satrústegui, 2009). In brief, extramitochondrial Ca2+ binds to the aspartate–glutamate carrier (aralar) that is predominant in neurons and a component of the MAS. The MAS transfers reducing equivalents from cytoplasmic NADH to the mitochondria and regenerates NAD+ to maintain glycolytic flux and produce pyruvate for oxidative metabolism (Figure 1). Small [Ca2+] signals stimulate MAS activity, whereas large [Ca2+] signals arising from Ca2+ entry into the mitochondria via the Ca2+ uniporter activate pyruvate, α-ketoglutarate, and isocitrate dehydrogenases and increase tricarboxylic acid (TCA) cycle flux (Pardo et al (2006) and cited references). However, Ca2+ activation of the MAS and TCA cycle are competitive, with preferential retention of α-ketoglutarate in the TCA cycle, thereby limiting its role in the MAS; low MAS activity would cause lactate production to increase in activated neurons (Bak et al, 2009; Contreras and Satrústegui, 2009) and it would impair neuronal lactate oxidation (Figure 1).
To sum up, the role of lactate during activation has been a difficult, controversial topic owing, in part, to technical difficulties associated with comprehensive, quantitative in vivo assays of metabolism and metabolite trafficking and to temporal-spatial limitations of current methodology. Brain lactate metabolism is complex and in vivo studies are required to establish its role during brain activation.
Lactate is Fuel for the Human Brain when Exercise Increases Blood Lactate Levels
During strenuous physical work, human plasma lactate increases from ∼0.5 to 1 mmol/L to 20 to 30 mmol/L, and whole-brain studies of metabolic activity during exercise reveal progressive increases in brain lactate uptake and metabolism as work load and plasma lactate levels increase (Ide et al, 1999, 2000). Blood lactate is oxidized in the brain and more glucose is also consumed during exhaustive exercise, but there is also a decline in the oxygen/(glucose+½ lactate) utilization ratio from ∼6 to as low as 1.7, and there is a large, unexplained excess carbohydrate taken up into brain that is not accounted for by oxidative metabolism or tissue metabolite accumulation or release (Dalsgaard, 2006; Quistorff et al, 2008; van Hall et al, 2009).
Lactate can Stimulate Vasodilation
Gap junction-coupled astrocytes can avidly take up lactate from extracellular fluid and are poised to discharge it from their endfeet into perivascular fluid where pulsatile pressure can drive the lactate along the vasculature (Gandhi et al (2009); Ball et al (2007, 2010) and cited references). Several studies have reported that lactate increases vasodilation by different mechanisms (Hein et al, 2006; Yamanishi et al, 2006; Gordon et al, 2008), and continuous lactate release from the activated brain may serve a signaling function to increase blood flow and fuel delivery to the brain. As glucose delivery to the brain exceeds demand for glucose over a wide range of CMRglc (Cremer et al, 1983; Hargreaves et al, 1986), lactate release and its use as a blood flow regulator need not be a ‘waste' of fuel, because lactate can be used by peripheral tissues as fuel or as a gluconeogenic substrate.
Summary
Evidence for increased glycolysis and lactate release from the brain to the blood during brain activation in normal subjects with low plasma glucose levels during normal and pathophysiological conditions has accumulated since the 1970s. Strenuous exercise increases blood lactate levels and floods the brain with an alternative substrate that is oxidized in increased amounts. Flooding experiments in cultured cells and brain slices also show lactate oxidation and reduced glucose utilization, and these assays mimic strenuous exercise, not sedentary subjects. Lactate is generated and oxidized by neurons and astrocytes, but the magnitude and direction of cell-to-cell lactate shuttling coupled to its oxidation or release from the brain remains to be established in vivo. Continuous lactate release may serve an important CBF-regulatory function.
Aspects of Experimental Systems Relevant to Interpretation of Lactate as Brain Fuel
The lactate literature is very extensive and involves many different experimental systems. Experiments often focus on specific aspects of a more complex system, and comparative data interpretation requires a broad perspective, context, and attention to experimental details.
Properties and Physiology of the Experimental System
Assessment of all studies must take into account age, nutritional status, anesthesia, and physiologic state. Brain growth and metabolic and functional development have enormous spurts between 10 and 21 days, with slower increases thereafter (Baquer et al, 1975). Particular care must be taken when translating findings obtained in cells or tissue from prenatal, early postnatal, and weanling subjects to the adult brain owing to downregulation of specific transport and metabolic activities after weaning and to continued brain grown for weeks after weaning. Brain slices obtained from immature or adult brains have cell–cell interactions acquired through normal development, but they are damaged by preparative procedures and postmortem ischemia and have lower metabolic rates than in vivo owing to deafferentation. Slices have no blood flow and are dependent on diffusion of fuel and oxygen from the incubation medium. Cultured cells derived from embryonic and newborn animals have very low levels of metabolic enzymes when the tissue is harvested, and transport and metabolic capability may be geared to the early prenatal or postnatal and suckling stages of development, i.e., for use of lactate and ketone bodies more than glucose. Different cell types and brain regions mature at different ages, and neurons that survive tissue dissociation and multiply in culture are considered to be recently postmitotic neurons that have not developed a lot of processes. Cerebral cortical neuronal cultures obtained from ∼15-day-old embryos are used as a model system for GABAergic neurons (culture conditions apparently select against glutamatergic neurons; Yu et al (1984)), and cerebellar granule neurons obtained from ∼7-day-old postnatal rodents are used as a model system for glutamatergic neurons (Schousboe et al (1985); Hertz et al (1988) and cited references). Harvest age, culture duration, conditions, medium composition, and cellular development during culturing influence characteristics of cultures (Hertz et al (1998), Hertz (2004) and cited references), as well as any acquired pathophysiology during culturing (e.g., 15 to 30 mmol/L glucose causes diabetic complications; Gandhi et al (2010)). The capacity to use glucose or lactate by cultured astrocytes and neurons grown for <2 weeks in vitro need not be equivalent to the adult brain.
Lactate Concentration and Utilization Rates
Brain lactate concentration in normal, carefully handled resting subjects is ∼0.2 to 1 μmol/g, and it approximately doubles during activation. The quantity of lactate that accumulates in the brain during an activation episode is <5% of the pyruvate formed from glucose (Dienel et al, 2007a). The lactate level in a normal resting brain is linearly related to that of pyruvate (Dienel and Cruz, 2008), and its increase during activation probably reflects an increase in pyruvate concentration. The arterial plasma lactate level in resting subjects is often lower than that in the brain, and it increases with physical activity. However, even during exhaustive exercise, human brain lactate does not accumulate above ∼1 mmol/L (Quistorff et al, 2008). Large increases in brain lactate level are abnormal (Siesjö, 1978), and metabolic assays using high, flooding doses of lactate (greater than ∼3 mmol/L) mimic brain pathology or physically active subjects.
Fractional Contribution of Lactate to Overall Metabolism
Lactate is sometimes called a ‘preferred substrate' compared with glucose. Within this context, the notion of ‘equi-caloric' concentrations of glucose and lactate (1 glucose=2 lactate) is sometimes used as a framework for testing relative concentrations of each substrate. However, this is a specious concept because glycolysis is highly regulated (by activation and inhibition) at many steps, whereas lactate dehydrogenase (LDH)-mediated formation of pyruvate from lactate is an equilibrative reaction (lactate + NAD+ ↔ pyruvate + NADH + H+) that is not governed by metabolic demand nor fine-tuned by intricate regulation. Lactate concentration is influenced by pyruvate level, pH, NADH/NAD ratio, and other reactions coupled to the NADH–NAD redox system (Veech, 1991). Lactate cannot fulfill many functions of glucose metabolism (Figure 1) and elevated concentrations of lactate reduce glucose utilization in a concentration-dependent manner in cultured astrocytes (Swanson and Benington, 1996; Rodrigues et al, 2009), cultured neurons (Bouzier-Sore et al, 2006), and brain in vivo (Wyss et al, 2011). This feature of lactate utilization is consistent with its use as an opportunistic, glucose-sparing substrate when available in blood in high amounts, as during intense exercise.
Transport of glucose and of lactate plus H+ is equilibrative, and unidirectional uptake rates will increase with substrate concentration until the transporters are saturated. Brain glucose levels are typically ∼20% to 25% that of arterial plasma, and once hexokinase is saturated (its Km for glucose is ∼0.05 mmol/L), further increases in glucose concentration do not increase glucose utilization rate in the rat brain in vivo (Orzi et al, 1988) or in isolated synaptosomes (Bradford et al, 1978) owing to feedback-regulatory mechanisms that coordinate CMRglc with ATP demand and ADP availability as cosubstrate for reactions that produce ATP. In contrast, lactate-pyruvate interconversion is driven by concentration gradients. The higher the lactate level, the more pyruvate plus NADH + H+ will be generated until inhibitory levels of pyruvate are reached or MAS activity to regenerate cytoplasmic NAD+ becomes limiting (Figure 1). It must be noted that the actual metabolic situation in brain tissue is probably much more complex because of compartmentation of intracellular pyruvate/lactate pools and differential fates of pyruvate in different pools that are not considered in this simplified discussion (Cruz et al, 2001; Rodrigues et al, 2009). Utilization of pyruvate derived from either substrate by the oxidative TCA cycle pathways would then be governed by the same regulatory steps that modulate the rates of the pyruvate dehydrogenase reaction, TCA cycle, and oxidative phosphorylation.
Different monocarboxylic acid transporter (MCT) and LDH isoforms are present in neurons and astrocytes. These isoforms can influence the concentration dependence of the proportion of lactate taken up and metabolized by either cell type because of differences in their Kms, Vmaxs, and LDH inhibition by pyruvate, but do not govern the direction of lactate flow (see discussions by Veech (1991), Chih et al (2001), Chih and Roberts (2003), Gandhi et al (2009), and Quistorff and Grunnet (2011a, 2011b). Lactate transport and its oxidation to pyruvate generate intracellular H+, and, depending on buffering capacity, reduced intracellular pH may inhibit phosphofructokinase and glycolytic rate. Depletion of NAD+ by the LDH reaction will reduce its availability for glycolysis (Figure 1). Metabolites generated by lactate oxidation (citrate, ATP, and other TCA cycle compounds) can inhibit brain phosphofructokinase in a very complex manner that depends on the levels of many modulators of this enzyme and pH (Passonneau and Lowry, 1963; Lowry and Passonneau, 1966). Of interest is the lack of effect of glutamate (Passonneau and Lowry, 1963), 10 to 100 mmol/L glucose, 2 mmol/L creatine, 0.2 mmol/L pyruvate, 3 mmol/L lactate, 0.06 mmol/L acetyl CoA, and 0.3 mmol/L α-ketoglutarate on the activity of brain phosphofructokinase (Krzanowski and Matschinsky, 1969). In contrast, 5 to 10 mmol/L lactate inhibits skeletal muscle phosphofructokinase (Costa Leite et al, 2007), suggesting that there may be different regulatory mechanisms involving lactate in the muscle compared with the brain, as observed for TCA cycle intermediates that can modulate phosphofructokinase from the rat brain but not the rat heart (Passonneau and Lowry, 1963).
Many investigators have used high-lactate flooding experiments, and a critical issue that is not always addressed in competitive substrate assays is dilution of labeled pyruvate when labeled glucose or lactate is the tracer. This is important because pyruvate concentration is 10- to 13-fold lower than lactate owing to the LDH equilibrium constant. To interpret inhibition of metabolism of pyruvate derived from glucose compared with that derived from lactate, the specific activity or fractional enrichment of pyruvate (i.e., the ratio of the labeled to unlabeled pyruvate) must be determined and used to calculate the effects of different concentrations of lactate or glucose added to the assay. For example, if labeled glucose generates pyruvate with a specific activity of 1, and addition of unlabeled lactate reduces pyruvate specific activity to 0.5 and the amount of glucose oxidized by 50%, then lactate had no effect on glucose oxidation. In other words, lactate only depressed pyruvate specific activity and, therefore, reduced the fraction of labeled pyruvate that entered the oxidative pathway. Increasing the level of unlabeled lactate will overwhelm labeling of pyruvate by glucose, whereas increasing glucose concentration will not have much effect on pyruvate labeled by lactate because of regulated metabolism of glucose.
Summary
High levels of extracellular lactate can ‘flood the system' and provide a nonregulated source of pyruvate, thereby influencing glucose utilization. However, a ‘preference' for lactate that arises from fine-tuned regulation glycolytic enzyme activities by many metabolites is not the same as preferring one of different candies of identical composition and caloric content. If brain-derived lactate was highly ‘preferred' over blood-borne glucose as fuel, why would any lactate be released from the brain? Other factors must be involved in substrate utilization. The apparent simplicity of brain lactate metabolism and trafficking during brain activation in vivo is deceptive, and knowledge of pyruvate specific activity or fractional enrichment is necessary to interpret effects of lactate on glucose utilization. Unresolved issues include the cellular origin of lactate released into the extracellular fluid, flux through lactate pools, routes for dispersal and release of lactate, and the contribution of lactate oxidation to energetics of brain activation in neurons and astrocytes.
Unexplained Discordant Results Underlie Lactate-Related Controversies
When viewed in isolation, various studies may seem to support or oppose a model for brain lactate metabolism, but when evaluated within a broad context of different data sets related to the same issue, each set can ‘speak for itself' and trends or anomalies are easily recognized.
Glutamate Transport-Evoked Glycolysis is not a Robust, Intrinsic Property of all Cultured Astrocytes
Increased CMRglc and lactate production by cultured astrocytes exposed to glutamate in the culture medium is reproducibly observed in some laboratories but not in many others (Table 1). Responsive pure astrocyte cultures have different temporal responses to glutamate compared with astrocytes in mixed astrocyte–neuron cultures (Table 1). The basis for the presence or absence of a glycolytic response to glutamate is unknown (Hertz et al, 1998), but may be related to oxidative metabolism of glutamate, which stimulates astrocytic respiration and is oxidized in greater amounts with increasing extracellular level (Table 1). Use of ATP generated from glutamate oxidation to extrude sodium is consistent with the increase in CMRglc evoked by nonmetabolizable D-aspartate (Table 1). In the cerebellum in vivo, glutamate transport blockade has no effect on metabolic activation and lactate increase, whereas these changes are eliminated by AMPA receptor inhibition (Caesar et al, 2008), ruling out astrocytic glutamate transport-induced glycolysis as a major factor governing blood flow-metabolism upregulation.
Table 1. Discordant metabolic responses of cultured astrocytes to glutamate exposure.
| Preparation | Glutamate concentration (μmol/L) | Glucose utilizationa | Glucose uptakea | Lactate productiona | O2 utilizationa | Metabolite oxidation (substrate)a | Reference |
|---|---|---|---|---|---|---|---|
| Forebrain, cerebral cortical, or striatal astrocytes | 10–500 | +15 to +180 | +40 to +55 | Pellerin and Magistretti, 1994; Takahashi et al, 1995; Debernardi et al, 1999; Chatton et al, 2003 | |||
| 10–1,000 | +7 to −60 | 0 to −27 | 0 to −60 | −25 to −75 (glucose) | Hertz et al, 1998; Swanson et al, 1990; Peng et al, 2001; Qu et al, 2001; Gramsbergen et al, 2003; Liao and Chen, 2003; Dienel and Cruz, 2004 | ||
| 100 | +55 | Eriksson et al, 1995 | |||||
| 100 → 500 | 15% → 40% (glutamate) | McKenna et al, 1996 | |||||
| 200 | 0b | Prebil et al, 2011 | |||||
| 20,000 | +18b | ||||||
| (D-Aspartate, 500–1,000) | +20 | Peng et al, 2001 | |||||
| Hippocampal astrocytes in mixed astrocyte–neuron cultures | 500 | 2-NBDG, +110c 6-NBDG, +180c | Loaiza et al, 2003 | ||||
| 50, Acute | −20b | +130 | Bittner et al, 2011 | ||||
| 50, 20 minutes | +275b | ||||||
| 5, 20 minutes | +300b | ||||||
| (50, D-Aspartate) | 0b |
NBDG, N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)2-deoxyglucose.
Magnitude of response is expressed as approximate percentage change owing to treatment, 100[(treated−control)/control]. For details, see the original references and Tables 5 and 6 of Dienel and Cruz (2004). Except where noted, glucose utilization was assayed with deoxyglucose. Effects of D-aspartate, a nonmetabolizable substrate for the glutamate transporter, were also tested in some studies.
Fluorescence resonance energy transfer (FRET) assays based on nanosensors that bind to intracellular glucose and report glucose concentration; glucose utilization is based on the change in glucose concentration, which does not reveal the pathway(s) that consume the glucose (glycolysis, pentose phosphate shunt pathway, glycogen synthesis, or sorbitol production if glucose levels are high).
Uptake assays were 10 minutes and measured change in fluorescence with time. 6-NBDG reflects only transport, whereas 2-NBDG uptake can reflect both transport and phosphorylation because there was no washout of unmetabolized precursor.
Brain Activation In Vivo Activates Glycogenolysis and Oxidative Metabolism in Astrocytes
Glycogen turnover is very slow under resting conditions, but astrocytes have significant resting oxidative activity, calculated to be ∼15% to 38% of total oxidative metabolism of glucose (Hyder et al, 2006; Duarte et al, 2011; Hertz, 2011). The astrocytic filopodial processes that surround and interact with synaptic structures contain mitochondria (Lovatt et al, 2007; Pardo et al, 2011; Lavialle et al, 2011) and have the potential to oxidize glucose, glycogen, and glutamate during activation. If brain activation stimulated only glycolysis in astrocytes, it would be reasonable to assign the ATP derived from this pathway toward the energetics of glutamate uptake. However, this is not the case. In vivo studies have shown that glycogenolysis, TCA cycle flux, and pyruvate carboxylation (a biosynthetic pathway involving the TCA cycle that also generates NADH and ATP; see Figure 1 and Hertz et al (2007)) are all increased in astrocytes in vivo under activating conditions (Table 2).
Table 2. Stimulus-induced increased metabolism in astrocytes in the rat or human brain in vivo.
| Experimental condition and substrate fuel |
Percentage increase in pathway activity |
References | |
|---|---|---|---|
| Glycogenolysis (glycolysis) | TCA cycle | ||
| Sensory stimulation-cerebral cortex, conscious rat | |||
| Glycogen consumption (stimulation and recovery) | 14–32 | Cruz and Dienel, 2002 | |
| Release of label from glycogen | 22 | Dienel et al, 2007a | |
| Anesthetized rat | |||
| Acetate oxidation | 14 | Wyss et al, 2009 | |
| Metabolic washout of acetate-derived label | 93 | ||
| Conscious human—washout of acetate label | 62 | Wyss et al, 2009 | |
| Acoustic stimulation-inferior colliculus, conscious rat | |||
| Release of label from glycogen | 12 | Dienel et al, 2007a | |
| Acetate oxidation | 15–18 | Cruz et al, 2005 | |
| Visual stimulation-conscious rat | |||
| Release of label from glycogen, superior colliculus | 13 | Dienel et al, 2007a | |
| Acetate oxidation superior colliculus | 25–30 | Dienel et al, 2007b | |
| Acetate oxidation lateral geniculate | 14–20 | ||
| Operant training, conscious rat | Dienel et al, 2003 | ||
| Acetate oxidation in five brain regions | 15–24 | ||
| Spreading cortical depression-cerebral cortex | |||
| Conscious rat, acetate and butyrate oxidation | 15–40 | Dienel et al, 2001 | |
| Anesthetized rat, glycogen consumption | 10–28 | Krivanek, 1958 | |
| Pyruvate carboxylation, whole brain | |||
| Pentobarbital-anesthetized to awake rat | ∼400 | Öz et al, 2004 | |
TCA, tricarboxylic acid.
Glycogen and pyruvate carboxylase are located predominantly in astrocytes, and acetate is preferentially oxidized by astrocytes. These markers can be used to reveal changes in pathway fluxes in astrocytes in the brain in vivo. The fate of glycogen-derived pyruvate in vivo is not known; hence, glycogenolysis is considered to reflect glycolysis. As (1) calculated TCA cycle rates determined with [13C]acetate and [13C]glucose are similar (Hyder et al, 2006) and (2) calculated [14C]acetate oxidation is within the range of that estimated for astrocytic glucose oxidation (Cruz et al, 2005), it is likely that acetate oxidation rate reflects glucose oxidation rate in astrocytes. Pyruvate carboxylation is part of the anaplerotic pathway located in astrocytes (not neurons) for de novo synthesis of aspartate, glutamate, glutamine, and GABA from glucose (Figure 1). Increased anaplerotic flux generates ATP through NADH formed by pyruvate and isocitrate dehydrogenase reactions.
In our studies of acoustic stimulation of conscious rats that assayed both glucose utilization by all cells and acetate oxidation by astrocytes in the inferior colliculus in vivo, CMRglc increased by 0.49 μmol/g per min (from 0.71 to 1.20 μmol/g per min, or 69%) and acetate oxidation increased a minimal mean value of 0.02 μmol/g per min (from 0.126 to 0.146 μmol/g per min, or 16%) (see Table 5 in Cruz et al (2005)). Assuming 2 ATP produced by glycolysis and 32 ATP from the oxidative pathways (<38 ATP owing to proton leak), the increase in glycolysis in all cells would produce 2 × 0.49=0.98 μmol ATP/g per min, and the increase in astrocytic oxidative metabolism would generate 32 × 0.02=0.64 μmol ATP/g per min. Thus, a minimal estimate of the contribution of increased astrocytic oxidative metabolism (assuming that changes in acetate oxidation reflect those of glucose) is 65% that of total glycolysis in all cells. If half of the glucose is metabolized in astrocytes, then increased oxidative metabolism produces a similar amount of ATP as the increase in glycolysis. If all glycolytic ATP were used to power Na+-K+-ATPase to extrude sodium taken up with glutamate into astrocytes, then other unidentified, upregulated energy-requiring processes consume at least half of the additional ATP generated by the astrocytes. Contributions of glycogenolysis and oxidative flux related to pyruvate carboxylase activity (Table 2) are not included and would increase the total ATP produced by astrocytes further. Although speculative and approximate, this calculation suggests that working astrocytes are not well understood, and that further experiments are required to evaluate the energetics of astrocytic activation in vivo.
Cultured Neurons and Presynaptic Endings from the Adult Brain Increase Glucose Utilization
Arguments used by Jolivet et al (2010) in support of the need of neurons for lactate as fuel during activation include inability of neurons to increase glucose transport (citing Porras et al (2004) and a few other studies) and glycolysis (citing Herrero-Mendez et al (2009)). The Bolaños–Almeida–Moncada group has carried out an elegant series of studies (reviewed by Bolaños and Almeida (2010)) designed to elucidate the basis for high sensitivity of cultured cerebral cortical neurons to respiratory inhibition by nitric oxide (NO) and neuronal inability to increase glycolysis when treated with NO (Table 3). In brief, they showed that the enzyme 6-phosphofructo-2-kinase/fructose 2,6-bisphosphatase isoform 3 (Pfkfb3) that makes a potent allosteric activator of 6-phosphofructo-1-kinase (i.e., PFK, see Figure 1) is constantly degraded in cultured cortical neurons but not in cultured cortical astrocytes. Their cortical neurons have a lower glycolytic rate than do astrocytes, and neurons divert glucose-6-phosphate into the pentose phosphate shunt pathway to produce NADPH for management of oxidative stress (Figure 1). The study by Herrero-Mendez et al (2009) extended these findings by showing upregulation of neuronal Pfkfb3 confers to neurons the ability to increase glycolysis and lactate production at the expense of glucose-6-P flux into the pentose shunt pathway (Table 3). Bolaños and Almeida (2010) stated that different regulatory mechanisms may operate in other preparations and brain regions.
Table 3. Metabolic responses of cultured neurons derived from different brain regions to activating conditions.
| Brain region and harvest agea | Treatment | Response magnitudeb | Reference |
|---|---|---|---|
| Cerebral cortex | (Cortical neurons, a model for GABAergic neuronsa) | ||
| E14 | 5 → 50 mmol/L K+ | 10% ↑ DG phosphorylation | Peng et al, 1994 |
| 20% ↑ [U-14C]glucose to 14CO2 (low rate) | |||
| 20% ↓ [U-14C]lactate to 14CO2 | |||
| 115% ↑ [2-14C]pyruvate to 14CO2 (very low rate) | |||
| E16–17 | 1.4 μmol/L nitric oxide | 85% ↓ O2 uptake, no change in lactate production | Almeida et al, 2001 |
| 100 μmol/L Glutamate | No change in lactate production | Almeida et al, 2004 | |
| Overexpression of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3 | 550% ↑glycolysis, 190% ↑lactate level, 50% ↓pentose phosphate shunt pathway (PPP) flux (control neurons: PPP flux=200% glycolytic flux) | Herrero-Mendez et al, 2009; Bolaños and Almeida, 2010 | |
| E17 | Hypoxia, 3 days | 200% ↑ glucose utilization | Malthankar-Phatak et al, 2008 |
| 330% ↑ lactate concentration in medium | |||
| E16–17 | Hypoxia for 24 hours | 200% ↑ lactate concentration in medium | Sher, 1990 |
| E15 | 55 mmol/L K+ | 50% ↑ cycling ratio for glutamate=increased TCA cycle activity | Waagepetersen et al, 2000 |
| E18 | 33 μmol/L Glutamate | 200–250% ↑ oxygen consumption with glucose substrate | Gleichmann et al, 2009 |
| 7–32 μmol/L FCCP | 200–250% ↑ oxygen consumption with glucose substrate | ||
| E15 | 25 nmol Dinitrophenol | 20% ↑ oxygen consumption with glucose substrate | Jameson et al, 1984 |
| E17 | 2 μmol/L amyloid-β1–42 , 4 days | 200% ↑ [1-14C]glucose to 14CO2; 155% ↑ [6-14C]glucose to 14CO2; 205% ↑ pentose phosphate shunt pathway | Soucek et al, 2003 |
| Cerebellum | (Cerebellar granule neurons, a model for glutamatergic neuronsa) | ||
| PN7 | 5 → 50 mmol/L K+ | 75% ↑ DG phosphorylation | Peng et al, 1994 |
| 120% ↑ [U-14C]glucose to 14CO2 | |||
| 20% ↑ [U-14C]lactate to 14CO2 | |||
| 110% ↑ [2-14C]pyruvate to 14CO2 | |||
| PN7 | 50 μmol/L Glutamate | 30% ↑ DG phosphorylation | Peng and Hertz, 2002 |
| 500 μmol/L Glutamate | 40% ↑ DG phosphorylation | ||
| 5.4 → 55 mmol/L K+ | 75% ↑ lactate production rate; 75% ↑ [U-14C]glucose to 14CO2 | ||
| PN8 | 25 nmol Dinitrophenol | 43% ↑ oxygen consumption with glucose substrate | Jameson et al, 1984 |
| PN6–7 | Hypoxia, 7h | 100% ↑ lactate production | Sonnewald et al, 1994 |
| PN8 | 100 μmol/L Glutamate | 115% ↑ DG uptake plus phosphorylation (10 minute assay) | Minervini et al, 1997 |
| 100 μmol/L NMDA | 180% ↑ DG uptake plus phosphorylation (10 minute assay) | ||
| 60 μmol/L Kainate | 220% ↑ DG uptake plus phosphorylation (10 minute assay) | ||
| 100 μmol/L Quisqualate | 55% ↑ DG uptake plus phosphorylation (10 minute assay) | ||
| PN5–7 | Respiration assays in 25 mmol/L K+ 2 μmol/L FCCP | 175% ↑ oxygen consumption with glucose substrate | Jekabsons and Nicholls, 2004 |
| 250 μmol/L Glutamate + 25 μmol/L glycine | 32–60% ↑ oxygen consumption with glucose substrate | ||
| 300 μmol/L NMDA | 33–36% ↑ oxygen consumption with glucose substrate | ||
| PN5–7 | Respiration assays in 3.9 mmol/L K+ 3 μmol/L FCCP | 250–325% ↑ oxygen consumption with glucose substrate | Yadava and Nicholls, 2007 |
| 100 μmol/L Glutamate + 10 μmol/L glycine | 250–325% ↑ oxygen consumption with glucose substrate | ||
| PN7–8 | DG assays in 3.9 mmol/L K+ | Ward et al, 2007 | |
| 100 μmol/L Glutamate + 10 μmol/L glycine for 10 minutes, then new medium with no glutamate + [3H]DG for 20 minutes | 50% ↑ DG phosphorylation (reflecting ↑ glucose utilization) | ||
| Hippocampus | |||
| Neurons in mixed | 500 μmol/L Glutamate or 20 μmol/L AMPA | 75–80% ↓ 2- or 6-NBDG uptake—fast, reversible | Porras et al, 2004 |
| Neuron–astrocyte | 75 μmol/L veratridine | No change in 6-NBDG uptake for ∼10 minutes, then 70% ↓ | |
| Cultures (PN1–3) | 40 mmol/L KCl | No effect on 6-NBDG uptake | |
| Neurons | 100 μmol/L Glutamate, 10 minutes | No effect on DG uptake | Patel and Brewer, 2003 |
| (E18) | 1 μmol/L FCCP + 10 mg/mL | 200% ↑ DG uptake at 5 minutes | |
| oligomycin 100 μmol/L Glutamate + 1 μmol/L FCCP + 10 mg/mL oligomycin | 135% ↑ DG uptake at 5 minutes | ||
| Neurons | Acute anoxia | 40% ↑ DG uptake | Yu et al, 2008 |
| (PN0) | Acute anoxia after hypoxic preconditioning, 20 minutes/day for 6 days | 90% ↑ DG uptake | |
AMPA, 2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl)propanoic acid; DG, deoxyglucose; NBDG, N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)2-deoxyglucose; TCA, tricarboxylic acid.
The age at which tissue was obtained for cultured cells is denoted by E=embryonic or PN=postnatal, followed by the age in days. Cerebral cortical neuronal cultures obtained from ∼15-day-old embryos are used as a model system for GABAergic neurons, and cerebellar granule neurons obtained from ∼7 day-old postnatal rodents are used as a model system for glutamatergic neurons (Yu et al (1984); Schousboe et al (1985); Hertz et al (1988) cited references). It must be noted that culture conditions and duration, composition of the culture medium, and cellular development during time in culture influence characteristics of the cultures (e.g., Hertz et al (1998); Hertz (2004) cited references).
Magnitude of response is expressed as approximate percentage change owing to treatment, 100[(treated−control)/control)]. DG assays are typically used to measure hexokinase-dependent phosphorylation as a measure of glucose utilization. 2-NBDG is a fluorescent glucose analog that is transported and phosphorylated, whereas 6-NBDG is transported but not phosphorylated. Brief assays (<5 minutes) with DG or 2-NBDG measure mainly transport (uptake), intermediate duration assays (5–10 minutes) can represent transport plus phosphorylation depending on washout of unmetabolized precursor from cells at the end of the assay, and longer assays with DG or 2-NBDG reflect mainly phosphorylation that can be overestimated somewhat if unmetabolized precursor is not completely washed out. 6-NBDG accumulation reflects transport until intracellular and extracellular levels equilibrate. See legend to Table 4 for more details related to sites of action of metabolic inhibitors.
In fact, many laboratories have shown that different types of cultured neurons can substantially upregulate glucose metabolism, whereas a few preparations have no or small responses. Many cerebral cortical neuron preparations (a model for GABAergic neurons) do respond to many treatments (e.g., depolarization, hypoxia, exposure to glutamate, treatment with uncouplers or amyloid-β) with quite large increases in glycolysis and glucose-supported respiration, indicating that glucose transport must increase in parallel (Table 3). Cultured cerebellar granule cell neurons (a model for glutamatergic neurons) also exhibit large metabolic responses to depolarization, uncouplers, hypoxia, glutamate, NMDA, and other conditions (Table 3). Cerebral glucose utilization in cultured hippocampal neurons increases during exposure to uncouplers and anoxia, and is not affected by glutamate (Table 3). Conversely, hippocampal neurons in mixed astrocyte–neuron cultures exhibit reduced NBDG uptake upon glutamate exposure, delayed inhibition of NBDG uptake after veratridine exposure, and no response to depolarization (Table 3). The inability of neuronal cultures to respond to activating conditions by increasing CMRglc, glycolysis, pentose shunt flux, or respiration is an exception, not the rule (Table 3).
Synaptosomes embody the metabolic capabilities of nerve endings from the mature brain, although their capacity may be reduced by losses of soluble enzymes, ATP, Pi, and phosphocreatine during preparative procedures. Synaptosomes isolated from adult brain regions, including the hippocampus and cerebral cortex from different species, have high metabolic capacity and respond with large increases in glucose-supported respiration to depolarization, uncouplers, anoxia, enhanced ion fluxes, and NO donors (Table 4). Inhibition of MAS with aminooxyacetate reduces uncoupler-evoked respiration (Table 4). Glycolysis and glucose-supported respiration in hippocampal and cortical synaptosomes are enhanced by K+ and veratridine (Table 4), sharply contrasting the responses of hippocampal neurons in mixed cultures (Table 3). The magnitude of response to Na+-stimulated glucose oxidation increases with developmental age, and is much higher in synaptosomes isolated from adult compared with the immature brain (Table 4).
Table 4. Metabolic responses of synaptosomes to activating conditions.
| Substratea and treatmentb | Response magnitudec | Tissue source and Reference |
|---|---|---|
| Electrical stimulation | 20–65% ↑respiration rate, 10–70% ↑ glycolysis | Seven brain regions from adult rat, sheep, or rabbit |
| Glucose + 50 mmol/L K+ | 15% ↑ respiration rate, 30% ↑ glycolysis | Bradford, 1975 |
| 10 mmol/L glucose + 24 mmol/L KCl | 137% ↑ respiration rate | Cerebral cortex, adult rat |
| 85% ↑ [1-14C]pyruvate decarboxylation | Schaffer and Olson, 1980 | |
| 10 mmol/L glucose + 40 mmol/L KCl | 69% ↑ respiration rate | Adult rat forebrain |
| Erecińska et al, 1991 | ||
| 5 mmol/L pyruvate + 40 mmol/L KCl | 63% ↑ respiration rate | |
| 9.5 mmol/L [U-14C]glucose + 72 mmol/L Na+ | 180% ↑ glucose oxidation to 14CO2 | Whole brain from adult or immature rats |
| + 100 μmol/L 2,4-dinitrophenol | 370% ↑ glucose oxidation to 14CO2 | Diamond and Fishman, 1973 |
| Developmental age from 1 to 90 days | 500%↑ Na+-stimulated glucose oxidation to 14CO2 | |
| Developmental age from 10 to 20 days | 200%↑ Na+-stimulated glucose oxidation to 14CO2 | |
| 1 mmol/L [U-14C]glucose + 100 μmol/L veratridine | 290% ↑ glucose oxidation to 14CO2 | Adult rat forebrain |
| Harvey et al, 1982 | ||
| 10 mmol/L glucose + 100 μmol/L veratridine | 300% ↑ respiration rate | Adult rat hippocampal mossy fiber synaptosomes |
| Terrian et al, 1988 | ||
| 10 mmol/L glucose + 0.5 μmol/L FCCP | Immediate 500% ↑ respiration rate | Cerebral cortex, 4–8-week-old guinea pig |
| 10 mmol/L glucose + 100 μmol/L veratridine, followed by 0.5 μmol/L FCCP | Immediate veratridine-induced 175% ↑ respiration, followed by an immediate additional 65% FCCP-evoked ↑ respiration | Scott and Nicholls, 1980 |
| 1.5 mmol/L glucose + [3,4-14C]glucose + 100 μmol/L 2,4-dinitrophenol | 120% ↑ glucose oxidation to 14CO2 | Adult rat forebrain Ksiezak and Gibson, 1981a,1981b |
| 1.5 mmol/L glucose, severe hypoxia (O2 tension 19 torr → <1 torr) | 250% ↑ lactate production rate | |
| 5 mmol/L glucose + 1 mmol/L cyanide or 6 μmol/L rotenone | Block respiration, 9-fold increase glycolysis | Cerebral cortex, adult guinea pig Kauppinen and Nicholls, 1986a |
| 5 mmol/L glucose + Nitrogen atmosphere (anoxia ) | Ten-fold increase in glycolysis | |
| 5 mmol/L glucose + A23187 (divalent cation ionophore) | Three-fold stimulation respiration, 5-fold stimulation glycolysis | |
| 10 mmol/L glucose + anoxia | 335% ↑ lactate amount produced | Adult rat forebrain |
| White et al, 1989 | ||
| 10 mmol/L glucose + 1 μmol/L FCCP | 900% ↑ glycolysis maintained for at least 30 minutes 500% ↑ respiration | Cerebral cortex, adult guinea pig Kauppinen and Nicholls, 1986b |
| 10 mmol/L glucose + 1 mmol/L arsenite (inhibit pyruvate oxidation) | 35% inhibition respiration, 3-fold increase in glycolysis | |
| 5 mmol/L glucose + 45 mmol/L KCl | 55% ↑ glycolysis, 47% ↑pyruvate decarboxylation | Cerebral cortex, 6–10-week-old guinea pig Kauppinen et al, 1989 |
| 5 mmol/L glucose + 75 μmol/L veratridine | 250% ↑ glycolysis, 290% ↑pyruvate decarboxylation | |
| 5 mmol/L glucose + 1 μmol/L Cl-CCP | 650% ↑pyruvate decarboxylation | |
| 10 mmol/L glucose + anoxia | 2,100% ↑ lactate synthesis rate | Adult rat cerebral cortex |
| 10 mmol/L glucose + 10 μmol/L veratridine | 160% ↑ respiration rate, 75% ↓ lactate synthesis rate | Gleitz et al, 1993 |
| 10 mmol/L glucose + 1 mmol/L nitroprusside or 100 μmol/L S-nitrocysteine | Aerobic conditions: 20–30% ↑ lactate synthesis rate; 70% ↑ lactate amount at 15 minutes | Adult rat forebrain Erecińska et al, 1995 |
| 10 mmol/L glucose + 10 μmol/L rotenone | 900% ↑ lactate synthesis rate | |
| 10 mmol/L glucose + 40 μmol/L veratridine, 5 minutes | 210% ↑ respiration, 515% ↑ lactate production | Adult rat forebrain Erecińska et al, 1996 |
| 15 minutes | 135% ↑ respiration, 620% ↑ lactate production | |
| 10 mmol/L glucose + 10 μmol/L monensin, 5 minutes | 73% ↑ respiration, 1,100% ↑ lactate production | |
| 15 minutes | 70% ↑ respiration, 580% ↑ lactate production | |
| 10 mmol/L glucose + 5 μmol/L nigericin, 5 minutes | 58% ↑ respiration, 465% ↑ lactate production | |
| 15 minutes | 8% ↑ respiration, 200% ↑ lactate production | |
| 10 mmol/L glucose + 100 (young rats) or 50 (old rats) μmol/L veratridine | 120 or 110% ↑ respiration in synaptosomes from young or old rats, respectively | Whole brain from 3-month (young) or 24 (old)-month rats Joyce et al, 2003 |
| 10 mmol/L glucose + 240 nmol/L FCCP | 150 or 88% ↑ respiration in synaptosomes from young or old rats, respectively | |
| 15 mmol/L glucose + 4 μmol/L FCCP | 300% ↑ respiration rate | Cerebral cortex, 17–20 day-old mouse |
| 10 mmol/L pyruvate + 4 μmol/L FCCP | 230% ↑ respiration | Choi et al, 2009 |
| 15 mmol/L glucose + 10 mmol/L pyruvate + 4 μmol/L FCCP | 225% ↑ respiration rate | |
| 15 mmol/L glucose + 2–5 μmol/L veratridine | 100–135% ↑respiration, 200–300%, ↑extracellular acidification (i.e., ↑glycolysis with lactate production and release) | |
| 15 mmol/L glucose + 10 mmol/L pyruvate + 2–5 μmol/L veratridine | 210–165% ↑respiration, 180–250% ↑extracellular acidification | |
| 15 mmol/L glucose + 10–100 μmol/L AOAA | No effect on basal respiration rate | |
| 15 mmol/L glucose + 4 μmol/L FCCP + 10 μmol/L AOAA | 15% inhibition of maximal FCCP-evoked rate | |
| + 30 μmol/L AOAA | 30% inhibition of maximal rate | |
| + 100 μmol/L AOAA | 45% inhibition of maximal rate | |
| 15 mmol/L glucose + 10 mmol/L pyruvate + 0.05–1 mmol/L 4-aminopyridine | 35% ↑respiration rate compared with without 4-aminopyridine | |
| 15 mmol/L glucose + 10 mmol/L pyruvate + 4 μmol/L FCCP | Values normalized by number of bioenergetically competent synaptosomes: | Cerebral cortex or striatum from 3 to 4 month-old mice |
| 447 or 452 ↑ respiration rate in dopamine transporter-enriched synaptosomes from the striatum or cortex, respectively | Choi et al, 2011 | |
| 538 or 542 ↑ respiration rate in residual nondopaminergic synaptosomes from the striatum or cortex, respectively |
Synaptosomes are heterogeneous populations of presynaptic nerve endings that contain mitochondria and are capable of glycolytic and oxidative metabolism of glucose and other substrates. Glycolysis generates ATP plus pyruvate when reducing equivalents are transferred from cytoplasmic NADH to the mitochondria by the malate–aspartate shuttle (MAS). NADH can also be oxidized to regenerate NAD+ by production of lactate, which is sometimes used as a surrogate marker for glycolytic pathway flux. Oxidation of pyruvate through the tricarboxylic acid cycle generates NADH and FADH2. The electron transport chain transfers electrons from NADH and FADH2 to oxygen, along with extrusion of protons from the mitochondrial matrix. Proton reentry into the matrix through ATP synthetase drives ATP synthesis. In well-coupled mitochondria, respiration rate (oxygen consumption or uptake) is coupled to ATP synthesis, and ‘respiratory control' is exerted by energy demand, i.e., ADP availability. Respiration in the absence of ATP synthesis is low and arises from proton leakage into the matrix.
Synaptosomal energetics can be modulated by treatment with compounds that inhibit specific metabolic or transport reactions, abolish respiratory control, or increase energy demand. The MAS can be blocked by aminooxyacetate (AOAA), which inhibits pyridoxal-dependent enzymes (e.g., aminotransferases), thereby reducing activity of the malate–aspartate shuttle, preventing reoxidation of cytosolic NADH, and blocking the ability of mitochondria to use glycolytic pyruvate. 4-CIN (α-cyanocinnamate) inhibits monocarboxylic acid transporters and pyruvate transport into the mitochondria. Electron transport can be inhibited at different sites, complex I (NADH dehydrogenase complex) by rotenone; complex II (succinate dehydrogenase flavoprotein complex) by 2-nitropropionate, malonate, or methylmalonate; complex III (cytochrome bc1 complex) by antimycin A; and complex IV (cytochrome a1a3 or cytochrome oxidase) by cyanide, azide, nitric oxide, carbon monoxide, or anoxia. Oligomycin inhibits proton reentry into the mitochondrial matrix through the ATP synthase and blocks ATP synthesis; the residual respiration reflects the proton leak into the matrix. FCCP (carbonylcyanide-p-trifluoromethoxy-phenylhydrazone), Cl-CCP (carbonylcyanide-m-chlorophenylhydrazone), and dinitrophenol are uncouplers or protonophores that allow proton reentry into the mitochondrial matrix and relieve respiratory control by diverting the proton current away from the ATP synthase and reducing the capacity to generate ATP. Uncouplers stimulate respiration, and FCCP treatment can reveal maximal respiratory capacity, i.e., the available capacity of nerve endings or cells for substrate delivery and electron transport to increase ATP synthesis in response to increased ATP demand. Ion movements can also be altered to increase ATP demand and stimulate metabolism. Increased extracellular K+ levels are a consequence of neuronal activity, and depolarization of cells or nerve endings with high concentrations of KCl stimulates energy production. Veratridine prevents inactivation of voltage-activated sodium channels and causes intracellular Na+ to increase, thereby increasing ATP use by Na+-K+-ATPase; veratridine also causes intracellular Ca2+ to increase, followed by glutamate release. 4-Aminopyridine (4-AP) is an inhibitor of A-type K+ channels that causes synaptosomes to fire repetitive action potentials. NMDA, N-methyl-D-aspartate, is an agonist for a class of ionotropic glutamate receptors. Nigericin and monensin are ionophores that exchange H+ for K+ or Na+, respectively, whereas A23187 exchanges a divalent cation (Ca2+ or Mg2+) for 2H+. For more details regarding synaptosomal bioenergetics and responses to various treatments, see Nicholls (2003, 2009, 2010) and Erecińska et al (1996).
Magnitude of response is expressed as approximate percentage change owing to treatment, 100[(treated−control)/control], or, when indicated (i.e., as ‘–fold' change), treated relative to control ratio.
To summarize, many preparations of cortical, cerebellar, and hippocampal neurons and synaptosomes upregulate various pathways of glucose metabolism under many different conditions. However, cultured neurons derived from different brain regions may not have the same metabolic capacities or responses to the same treatment. Synaptosomes are one structure of adult brain neurons that is readily isolated, and these nerve terminals can increase glycolysis and respiration by 5- to 10-fold in vitro. Glucose transport and glycolytic flux must increase in parallel with glucose-supported respiration. Citation of selected metabolic studies that support a point of view (Jolivet et al, 2010) does not provide an appropriate perspective of the field.
Neurons can Quickly Upregulate Glucose Transport Capacity During Activation
Glutamate inhibits NBDG transport into cultured neurons (Porras et al, 2004; Table 3) and stimulates glucose transport into cultured astrocytes (Loaiza et al, 2003; Table 1). These findings have been interpreted by Pierre et al (2009) as rerouting of glucose from neurons to astrocytes during glutamatergic neurotransmission, so neurons would depend on astrocyte-derived lactate as a fuel, in accordance with the astrocyte-neuron lactate shuttle hypothesis. However, these results sharply contrast those from other neuronal cultures that exhibit glutamate-induced increases in CMRglc and 2- to 3-fold stimulation of glucose-supported respiration by glutamate in cultured cerebral cortical neurons and cerebellar granule neurons (Table 3). Moreover, nerve endings isolated from both immature and adult brains are capable of large increases in glycolysis and glucose-supported respiration (Table 4). Therefore, neuronal glucose transport must increase simultaneously with stimulation of its utilization.
Neuronal glucose transport capacity is enhanced within minutes by treatment of cultured neurons with glutamate, bicuculline, and a NO donor by increasing cell-surface expression of the neuronal glucose transporter (GLUT)3 throughout the neuronal processes and soma (Table 5). Upregulation of the GLUT3 protein level is slower than cell-surface translocation, and is stimulated in vivo by conditions that affect CMRglc in the brain (Table 5). Glucose transport into neurons is critical for brain function, and in the adult rat brain, GLUT3 is localized in synaptic terminals, small neuronal processes, and postsynaptic structures, with significant intracellular localization (Leino et al, 1997). Glucose transporter-3 deficiency causes serious developmental abnormalities in mice (Table 5). Taken together, the rapid increases in glucose transport capacity by cultured hippocampal, cortical, and cerebellar neurons and synaptosomes plus increased glucose utilization show that neurons require and consume more glucose when activated.
Table 5. Neuronal glucose transporter GLUT3: treatment-evoked changes in cell surface expression and total protein level, and functional abnormalities in mice with GLUT3 deficiency.
| Preparationa | Treatmenta | Duration | Response Magnitude b | Reference |
|---|---|---|---|---|
| Hippocampal neurons (E18) | 100 μmol/L Glutamate + 1 μmol/L FCCP + | 5–20 minutes | 60% ↑GLUT3 surface expression 130% ↑DG transport | Patel and Brewer, 2003 |
| 10 mg/mL oligomycin | ||||
| Cerebellar granule neurons (PN7) | 100 μmol/L Glutamate + 10 μmol/L glycine | 30–60 minutes | 30–55% ↑GLUT3 surface expression | Weisová et al, 2009 |
| Cerebral cortical neurons (E18) | 50 μmol/L Bicuculline + 100 μmol/L 4- | 15 minutes | 400% ↑GLUT3 surface expression | Ferreira et al, 2011 |
| Aminopyridine (AP) | 30 minutes | 1,900% ↑GLUT3 surface expression | ||
| 50 μmol/L NOR3 (nitric oxide donor) | 15 minutes | 1,400% ↑GLUT3 surface expression | ||
| Hippocampal neurons (E18) | 50 μmol/L Bicuculline + 100 μmol/L 4-AP | 15 minutes | 300% ↑GLUT3 surface expression | |
| 50 μmol/L Bicuculline + 100 μmol/L 4-AP | 15 minutes | 80% ↑GLUT3 surface expression in dendrites | ||
| 50 μmol/L NOR3 (nitric oxide donor) | 5 minutes | 700% ↑GLUT3 surface expression | ||
| 50 μmol/L Bicuculline + 100 μmol/L 4-AP | 15 minutes | 60% ↑2-NBDG mainly phosphorylation | ||
| Cerebellar granule | 5 → 15 or 25 mmol/L KCl | 8 days | 150–200% ↑GLUT3 protein level | Maher and Simpson, |
| neurons (PN8) | 250–550% ↑DG transport | 1994 | ||
| 15 mmol/L KCl + 150 μmol/L NMDA | 80% ↑GLUT3 protein level versus 15 mmol/L KCl | |||
| 60% ↑DG transport versus 15 mmol/L KCl | ||||
| Adult rat | Repeated hypoglycemia | 4 days | 50% ↑GLUT3 protein level in forebrain | Lee et al, 2000 |
| Water deprivation | 2–3 days | 40–55% ↑GLUT3 protein level in neurohypophysis | Vannucci et al, 1994 | |
| Streptozotocin diabetes | 2 weeks | 50% ↑GLUT3 protein level | ||
| Streptozotocin diabetes + 6 hours/day restraint stress | 7 days | 10–20% ↑GLUT3 protein level in regions of the hippocampus | Reagan et al, 1999 | |
| Heterozygous GLUT3-deficient mice | Tests were carried out with 7-day-old or 2–12-month-old mice | Developmental abnormalities leading to autism spectrum disorders including abnormal spatial learning, working memory, and cognitive flexibility. Animals also have EEG seizures and perturbed social behavior | Zhao et al, 2010 |
The age at which tissue was obtained for cultured cells is denoted by E=embryonic or PN=postnatal, followed by the age in days. Bicuculline is a GABA receptor antagonist; see legends to Tables 3 and 4 for more details about actions of inhibitors.
Magnitude of response is expressed as approximate percentage change owing to treatment, 100 [(treated−control)/control]. Deoxyglucose (DG) assays are typically used to measure glucose phosphorylation, but brief assays are also used to measure transport. 2-NBDG is a fluorescent glucose analog that is transported and phosphorylated; the 15 minutes assay in this study probably reflects mainly phosphorylation.
Dendritic Spine Energetics: Is Monocarboxylic Acid Transporter-2 used for Lactate Release?
Neuronal MCT2 and AMPA receptor GluR2/3 are colocalized in postsynaptic densities of glutamatergic synapses between parallel fibers and Purkinje cells in the cerebellum (Bergersen et al, 2001, 2005), and these two proteins are translocated to the cell surface from intracellular stores in parallel under activating conditions (Pierre et al, 2009). Monocarboxylic acid transporter-2 localization and trafficking are claimed to facilitate uptake of astrocyte-derived lactate as oxidative fuel for these glutamatergic spines (Bergersen et al, 2005, 2007; Pierre et al, 2009). However, spines do not contain the mitochondria (Bergersen et al, 2001, 2002); hence, lactate, ADP, and phosphate must diffuse through the spine neck to the mitochondria in the dendritic shaft, followed by lactate oxidation and synthesis of ATP, then diffusion of ATP back to postsynaptic density for its utilization. This scenario does not include glucose transport and metabolism in spines, and to understand the energetics of dendritic structures more fully, it is important to know the relative levels of GLUT3 compared with MCT2 in presynaptic and postsynaptic structures and to evaluate glucose and lactate metabolism in these structures.
Most dendritic spines have very few mitochondria, in contrast to the shafts (Li et al, 2004; Bourne and Harris, 2008). Postsynaptic densities contain glycolytic enzymes that synthesize ATP (Wu et al, 1997), and GLUT3 is localized in synaptic endings and postsynaptic structures (Leino et al, 1997). Calcium clearance in activated cultured cerebellar granule neurons and in Purkinje cells in brain slices relies on glycolysis to power the plasma membrane Ca2+-ATPase in the soma, dendrites, and spines, and inhibition of mitochondrial ATP generation does not affect operation of this pump (Ivannikov et al, 2010). These findings underscore the importance of glycolysis in neuronal dendritic spines and show that diffusion of ATP from the dendritic shaft into the spine cannot support calcium pumping at the plasma membrane of spines. Therefore, trafficking of MCT2 might be required to release lactate generated by glycolysis in the spine into extracellular fluid, so that high glycolytic flux can be maintained within the spine at the site of the postsynaptic density. Avid lactate uptake by nearby astrocytes could then oxidize or disperse and discharge the lactate to more remote locations (Gandhi et al, 2009).
Net Transport of Lactate Across the Blood–Brain Barrier In Vivo
Arteriovenous differences are used to evaluate brain uptake and release of compounds, but limited access to venous drainage systems restricts these assays to the whole brain, cerebral cortex, and eye. Small amounts of lactate (∼5% of glucose uptake) are released from resting brain, and during activation, lactate release increases to 15% to 22% of glucose influx (Tables 6A and 6B). Importantly, lactate release can occur even when global brain lactate levels are lower than blood (Table 6A), presumably owing to locally high brain lactate levels. Krebs (1972) noted that the eye is highly glycolytic, and lactate release from the eye exceeds that from the brain, ranging from ∼20% to 100% of glucose uptake (Table 6C). Lactate is also released from the human brain during stressful cognitive testing (Table 6E). When blood lactate levels increase during sensory stimulation (Table 6D) or graded exercise (Table 6E), lactate enters the brain in progressively increasing amounts. Activation is associated with lactate release, but strenuous physical activity increases blood lactate level and brain uptake.
Table 6. Lactate release from the brain and eye during activation and lactate uptake during exercise.
| Tissue and experimental condition | Glucose | Lactate | Lactate flux (%glucose influx)a | Reference |
|---|---|---|---|---|
| A. Conscious rat brain, acute ammonia challengeb | Hawkins et al, 1973 | |||
| Before ammonia injection | ||||
| Arterial (A) blood level (mmol/L) | 5.10 | 2.64 | ||
| Cerebral venous (V) blood (mmol/L) | 4.53 | 2.68 | ||
| A–V difference (mmol/L) | 0.57 | −0.04 | −4% | |
| Brain level (μmol/g) | 0.75 | 1.25 | ||
| 4–5 minutes after ammonia injection | ||||
| Arterial blood level (mmol/L) | 5.74 | 3.20 | ||
| Cerebral venous blood (mmol/L) | 4.97 | 3.43b | ||
| A–V difference (mmol/L) | 0.77* | −0.23**b | −15% | |
| Brain level (μmol/g) | 1.05** | 2.39**b | ||
| B. Conscious rat brain, spreading cortical depressionc | Adachi et al, 1995; Cruz et al, 1999 | |||
| Arterial blood level (mmol/L) | 7.32 | 0.87 | ||
| A–V difference (mmol/L) | 0.62 | −0.27* | −22% | |
| Brain level (μmol/g) | 1.2 | 8.5** | ||
| C. Eye, anesthetized animalsd | ||||
| Rabbit retina | ||||
| A–V difference (mmol/L)e | 0.39 | −0.37 | −47% | Krebs, 1972 |
| Rate of consumption or production (μmol/min) | Wang and Bill, 1997 | |||
| Dark | 0.204 | −0.160 | −39% | |
| Light 10 lux | 0.197 | −0.153 | −39% | |
| Light 150 lux | 0.206 | −0.146 | −35% | |
| Light | 0.221 | −0.212 | −48% | |
| 4 Hz flicker | 0.258*** | −0.242** | −47% | |
| Pig outer retina, A–V difference (mmol/L) | Wang et al, 1997a | |||
| Dark | 0.304** | −0.372** | −61% | |
| Light | 0.182 | −0.160 | −44% | |
| Pig inner retina, A–V difference (mmol/L) | Wang et al, 1997b | |||
| Dark | 0.731* | −0.296 | −20% | |
| Light | 0.625 | −0.324 | −26% | |
| Cat outer retina, use or release rate (μmol/min) | Wang et al, 1997c | |||
| Dark | 0.236** | −0.409** | −87% | |
| Light | 0.123 | −0.253 | −103% | |
| D. Conscious rat brain, generalized sensory stimulatione | Madsen et al, 1999 | |||
| Before stimulation | ||||
| Arterial blood (mmol/L) | 6.81 | 0.50 | ||
| A–V difference (mmol/L) | 0.68 | −0.08 | −6% | |
| Brain (μmol/g) | 2.8 | 1.0 | ||
| After 5 minutes of stimulation | ||||
| Arterial blood level (mol/L) | 7.81* | 1.9* | ||
| A–V difference (mmol/L) | 0.60 * | +0.02* | +2% * | |
| Brain level (μmol/g) | 3.1 | 1.9* | ||
| E. Conscious human braine | LactateArterial (mmol/L) | (A−V)Lactate (mmol/L) | ||
| Rest | 0.46 | −0.04 | −5% | Madsen et al, 1995 |
| Cognitive activation + stress | 0.47 | −0.06*** | −7%** | |
| Rest | 0.94 | −0.04 | −4% | Dalsgaard, 2006 |
| Light exercise | 0.99 | −0.05 | −5% | |
| Moderate exercise | 3.16 | +0.12* | +11%* | |
| Maximal exercise | 6.95 | +0.50** | +41%** | |
| Early recovery | 14.9 | +0.71** | +44%** | |
Positive arteriovenous differences (A−V) across the brain indicate uptake into brain, whereas negative values denote efflux from the brain. Tabulated data are mean values from the cited references.
*P<0.05, **P<0.01; ***P<0.001 versus control.
Lactate flux from or to the brain (in glucose equivalents) is expressed as percentage of glucose uptake, i.e., 100[(A−V)lactate/2]/(A−V)glucose.
Note that lactate was released from the brain in ammonia-loaded rats even though the brain lactate level is lower than that in blood. As lactate transport is passive and concentration gradient-driven, these results suggest locally high brain lactate levels that exceed the average value in tissue (see text).
Efflux of 14C-labeled lactate from the brain to blood during spreading cortical depression in the conscious rat was detectable within 2 minutes after an intravenous pulse of [6-14C]glucose, and between 2 and 8 minutes after the pulse of [14C]glucose the efflux of [14C]lactate from brain was equivalent to that of unlabeled lactate indicating rapid equilibration with glycolytic intermediates and efflux of lactate derived from brain metabolism of blood-borne glucose (Cruz et al, 1999).
The retina of the pig and cat is more metabolically active in the dark compared with light (contrasting the rabbit), presumably because light inhibits rod metabolism by inhibiting the dark current (Wang and Bill (1997) and references cited therein).
Under ‘resting' conditions, there is a slight efflux of lactate from the brain to blood as long as the blood lactate is relatively low (A, D; also see Madsen et al (1998); Linde et al (1999); Schmalbruch et al (2002)), in sharp contrast to the eye that releases large amounts of lactate under resting and activated conditions (C). When blood lactate increases above that in the brain during physical movement (D, rat) with moderate and strenuous exercise (E, human), lactate influx increases markedly and it becomes a significant brain fuel (Dalsgaard, 2006; Quistorff et al, 2008; van Hall et al, 2009).
Extracellular Lactate as Fuel During Activation
Changes in extracellular metabolite levels can be measured with high temporal resolution using enzyme-linked sensors. Decreases in extracellular lactate level evoked by electrical stimulation (Hu and Wilson, 1997a, 1997b) are assumed to be caused by neuronal lactate metabolism and are cited as evidence supporting the astrocyte-neuron lactate shuttle hypothesis (Bergersen, 2007; Pellerin et al, 2007). Metabolite levels reported by Hu and Wilson (1997b) are expressed as percentage of basal level, and percentage data hinder quantitative comparisons between glucose and lactate utilization because percentage changes do not account for differences in substrate concentration and delivery. Interpretation of percentage data in terms of relative consumption rates can be quite misleading, and these values were, therefore, converted to concentrations and used to calculate utilization rates (Table 7). Stimulation for 1, 2, 3, or 4 seconds had no or little effect on extracellular glucose and lactate levels, and only 5-second stimuli evoked changes (Hu and Wilson, 1997b). Minimal CMRglc was calculated based on glucose delivered to the resting brain (hyperemic responses to activation are rapid but not quantified in this study; hence, the additional glucose delivered during a stimulus was not included in calculated CMRglc) plus extracellular glucose consumed during the stimulus. The resulting rate during the first stimulus is ∼5-fold higher than resting CMRglc (Table 7). This value is much higher than those evoked by strong physiologic stimuli (∼50% to 100%), raising the possibility of seizure-like activity. Maximal lactate utilization rate during the first stimulus was only 4% of glucose plus lactate utilization. During subsequent stimuli, the extracellular lactate level increased and percentage decreases were larger, contrasting the lower baseline for extracellular glucose and lower percentage decreases during stimulation. Minimal CMRglc increased 4.5- to 6-fold during subsequent stimuli, and maximal lactate utilization was ∼20% to 30% of the total (Table 7). Maximal lactate utilization contributed a trivial fraction to metabolism during the first episode and <1/3 of the total (ignoring upregulation of glucose delivery and utilization) during ensuing stimulus events.
Table 7. Glucose utilization increases 5–6-fold and greatly exceeds lactate utilization after electrical stimulation of hippocampal dentate gyrus in vivoa.
| Experimental interval | Glucose | Lactate |
|---|---|---|
| Concentration in extracellular fluid (ECF), resting rat brain (μmol/mL)b | 2.6 | 0.75 |
| Resting glucose utilization rate (CMRglc) in dentate gyrus in vivo (μmol/g per min)c | 0.66 | |
| 12 seconds interval following the first 5 seconds electrical stimulation | ||
| Percentage decrease in ECF concentration after stimulationd | 20 | 7 |
| Amount consumed from ECF (μmol/mL)d | 0.52 | 0.05 |
| Glc delivered to the resting brain from blood during 12 seconds (μmol/mL)c | 0.13 | – |
| Total Glc consumed from ECF + minimum Glc delivered; lac lost from ECF (all as Glc equivalents, μmol/mL)e | 0.65 | 0.025 |
| Calculated utilization rate during 12 seconds (all as glucose equivalents, μmol/g per min) | 3.25 | 0.13 |
| Minimum relative increase in CMRglc after first stimulus compared with rest in vivo | 4.9-fold | |
| Maximal lactate utilization as percentage of total lactate + glucose utilization | 4% | |
| 12 seconds interval following subsequent 5 seconds electrical stimuli | ||
| Interinterval extracellular fluid concentration (μmol/mL)f | 2.08 | 1.28 |
| Percentage decrease in ECF concentration after stimulationf | 10–20 | 28 |
| Amount consumed from ECF (μmol/mL)d | 0.21–0.42 | 0.36 |
| Glc delivered to the resting brain from blood during 12 seconds (μmol/mL)c | 0.13 | – |
| Glc equivalent of net interinterval increase in ECF lactate level (μmol/mL) (i.e., glucose consumed that is converted to lactate and released to ECF)f | 0.26 | – |
| Total Glc consumed=(ECF loss + minimum Glc delivered + Lac accumulated in ECF); Lac consumed=loss from ECF (all as Glc equivalents, μmol/mL)f | 0.60–0.81 | 0.18 |
| Calculated utilization rate during 12 seconds (all as glucose equivalents, μmol/g per min)f | 3.0–4.05 | 0.9 |
| Minimum relative increase CMRglc after subsequent stimuli compared with rest in vivof | 4.5–6.1-fold | |
| Maximal lactate utilization as percentage of total lactate + glucose utilizatione | 22–30% | |
Hu and Wilson (1997b) implanted glucose (Glc) and lactate (Lac) sensors into the dentate gyrus of the rat hippocampus, gave single or repeated electrical stimuli of 5-second duration, and measured temporal changes in extracellular glucose and lactate levels that were reported as percentage of the respective control values; actual concentrations were not stated. Shorter stimuli (1, 2, 3 seconds) did not produce detectable changes in lactate level, and those after 4 seconds were minor and variable.
Extracellular fluid (ECF) brain glucose level is from Hu and Wilson (1997a) who used the same glucose sensor and experimental paradigm as did Hu and Wilson (1997b); absolute values for extracellular lactate were estimated as follows. Values for total brain tissue lactate level in various regions of normal resting rat brain range from ∼0.2 to 0.6 μmol/g in our laboratory (Dienel et al, 2002, 2007a; Cruz and Dienel, 2002); similar percentage increases also occur in the human brain (Mangia et al, 2007b). To allow for higher interlaboratory values up to ∼1 μmol/g, an intermediate value for resting brain [lac]=0.75 was used for calculations in this table. For simplicity, extracellular and intracellular Glc and Lac levels are taken to be equivalent, with the caveat that extracellular Glc would be somewhat higher than intracellular Glc, whereas the converse would be true for Lac. All ECF lactate is assumed to be derived from metabolism of brain glucose. Where stated, lactate (Lac) concentration is divided by 2 to obtain glucose (Glc) equivalents of lactate.
Glucose utilization rate is the average of mean values from four subregions of the dentate gyrus of conscious rats from the study of Wree et al, 1993. Glucose delivery to the resting brain is equal to glucose utilization rate at steady state; glucose delivered during a 12-second interval is (0.66 μmol/g per min)(0.2 minutes)=0.13 μmol/g. This value does not include the additional stimulus-induced increases in delivery and metabolism of blood-borne glucose and of any brain glycogen consumed; calculated Glc utilization rates (CMRglc) are, therefore, minimal values.
Percentage changes in ECF concentrations at ∼12 seconds after the 5-seconds electrical stimulus are from Hu and Wilson, 1997b. Derived values were calculated from estimated initial concentrations and % changes.
Minimal glucose utilization rate over a 12-second interval after a 5-second electrical stimulus was estimated as total Glc equivalents consumed (i.e., for the first stimulus: net decrease in extracellular Glc + Glc delivered to the resting brain; for subsequent stimuli: net Glc decrease + Glc delivered + Glc equivalents of Lac accumulated) divided by 0.2 minutes. It must be noted that minimal CMRglc values after the first or subsequent stimuli are about 5–6-fold higher than normal resting rate. As this increase greatly exceeds values generally observed after very strong physiological stimuli (approximately 50–100%), the 5-second electrical stimulus may have induced local seizure activity. Calculated lactate utilization rate was based only on the net fall in lactate level, which may not be due only to metabolism, i.e., some lactate release to blood and lactate diffusion beyond the range of the sensor can contribute to a decrease in concentration; lactate utilization estimates are, therefore, maximal rates. Thus, calculated lactate utilization rates expressed as percentages of the calculated glucose plus lactate utilization rates are overestimates.
The increase in extracellular lactate level during subsequent stimuli ranged from 140 to 200% of the basal level (Hu and Wilson, 1997b), and a mean of 170% was used to calculate the higher level after repeated stimuli; new basal ECF glucose level was set at 20% below the initial resting value, and transient decrements were set at 10–20% of the lower basal glucose level. The increase in basal lactate level is attributed to glucose metabolism to lactate and release to ECF. Calculations were made as described for the first stimulus, except that the glucose equivalent to the increase in ECF lactate is included in the CMRglc total. This calculation also does not include the likelihood that CMRglc increased between the subsequent stimuli, because interinterval ECF glucose level fell by 0.52 μmol/mL from 2.6 to 2.08 μmol/mL even though blood flow and glucose delivery probably also increased.
To sum up, the static extracellular lactate content is unlikely to be a major brain fuel owing to its low level (∼0.5 to 2 μmol lactate/g or ∼0.25 to 1 μmol glucose equivalents/g) and small extracellular fluid volume (20% of brain or ∼0.2 g/g brain). The overall glucose utilization rate for the brain is ∼0.7 μmol/g per min and is supported by a >1.5-fold excess of glucose influx from the blood. Total lactate in the brain could only meet glucose demand for ∼1 minute, and extracellular lactate for a much shorter time. For lactate produced in the brain to serve as a significant fuel, there must be a large transcellular flux through the lactate pool.
Changes in Metabolite Concentrations Need not Predict Flux Magnitude or Direction
Concentration changes arise from input–output differences, and without further information they cannot be used to evaluate shifts in metabolic rate. For example, during studies of sensory stimulation of nonfasted, conscious rats, the animals moved around, causing arterial plasma glucose and lactate levels to increase. These changes were accompanied by increases in brain glucose and lactate concentrations, presumably owing to transport down their concentration gradients (Table 8). Interpretation of increased brain glucose level as reflecting reduced CMRglc would be wrong, because CMRglc increased by 27% to 57% and glycogen turnover increased. Net accumulation of lactate in the brain corresponded to <2% of the pyruvate produced from glucose, and some lactate could be derived from glycogen (Table 8). To summarize, the large percentage changes in lactate concentration reflect small quantities and do not reflect glucose flux through the pyruvate pool.
Table 8. Changes in metabolite concentrations and pathway fluxes during brain activation in conscious rats and in brain slices.
| Metabolite | Rest | Activationa | Net change (% of rest) | Reference |
|---|---|---|---|---|
| Glucoseb | Dienel et al, 2007a | |||
| Arterial plasma (mmol/L) | 10.6 | 12.9* | 2.4 (22%) | |
| Cerebral cortex (μmol/g) | 2.38 | 2.82** | 0.44 (19%) | |
| Inferior colliculus (μmol/g) | 2.36 | 3.08** | 0.62 (31%) | |
| Lactateb | ||||
| Arterial plasma (mmol/L) | 0.92 | 1.31 | 0.39 (42%) | |
| Cerebral cortex (μmol/g) | 0.34 | 0.85*** | 0.51 (150%) | |
| Inferior colliculus (μmol/g) | 0.34 | 0.52* | 0.18 (53%) | |
| Glycogen (unlabeled and 14C-labeled)b | ||||
| Cerebral cortex (μmol/g) | 4.60 | 3.96** | −0.64 (−14%) | |
| Inferior colliculus (μmol/g) | 4.11 | 3.99 | −0.12 (−3%) | |
| Cerebral cortex ([nCi/g]/ISA) | 2.31 | 1.75* | −0.56 (−24%) | |
| Inferior colliculus ([nCi/g]/ISA) | 2.35 | 1.96** | −0.39 (−17%) | |
| CMRglc (μmol/min per g)c | ||||
| Overall cerebral cortex | 1.07 | 1.36** | 0.29 (27%) | |
| Sensory cortex | 1.06 | 1.66*** | 0.60 (57%) | |
| Inferior colliculus | 1.54 | 1.98*** | 0.44 (29%) | |
| Pyruvate formed (μmol/g during 10-minute interval)d | ||||
| Overall cerebral cortex | 21.4 | 27.2 | 5.8 (27%) | |
| Sensory cortex | 21.2 | 33.2 | 12.0 (57%) | |
| Inferior colliculus | 30.7 | 39.6 | 8.9 (29%) | |
| Lactate accumulated/pyruvate formed (%) | ||||
| Overall cerebral cortex | 1.9% | |||
| Inferior colliculus | 0.5% | |||
| Lactate accumulated/glycogen consumed (%)e | ||||
| Overall cerebral cortex | 40% | |||
| Inferior colliculus | 75% | |||
| Brain tissue concentration (μmol/g)f | ||||
| NAD+ | 0.2–0.4, 0.9g | Ranges are from values in frozen- or microwave-inactivated adult mouse, rat, guinea pig, or cat brain tissue from Lowryet al (1964), Bonavita et al (1970), Duffy et al (1975), Medina et al (1980), Welsh (1980), Garofalo et al (1988) | ||
| NADH | 0.01–0.03 | |||
| NADP+ | 0.01–0.02 | |||
| NADPH | 0.005–0.01 | |||
| Calculated ratiosf | Data from Veech et al, 1973; Miller et al, 1973; Howse and Duffy, 1975 | |||
| Free cytoplasmic [NAD+]/[NADH] (LDH reaction) | 670–715 | |||
| Free cytoplasmic [NADP+]/[NADPH] (IDH reaction) | 0.01 | |||
| Mitochondrial [NAD+]/[NADH] (GDH reaction) | 0.5–1.5 | |||
| NAD(P)H fluorescence, brain slices (ΔF/Fo, %) | Cyanide, rotenone | Approximate ranges for NAD(P)H transients evoked by electrical stimulation | Representative values determined in brain slices by Kasischke et al (2004), Shuttleworth et al (2003), Brennan et al (2006), Galeffi et al (2007) | |
| Initial dip | Subsequent overshoot | |||
| +37 to +70% | −1% to −5% | +3% to +10% | ||
*P<0.05, **P<0.01, ***P<0.001
Brain activation was achieved by generalized sensory (gentle brushing of whiskers, head, back, tail), acoustic, and visual stimulation of conscious rats for 10 or 30 minutes. Values are group means from vehicle-treated control animals that were assayed during rest or activation.
Metabolite levels were measured in arterial plasma and ethanol extracts of funnel-frozen brain from carefully handled, sequestered rats that were prelabeled for 30 minutes by an intravenous pulse of [1-14C]glucose then given an additional 10 minutes of rest or activation. Unlabeled glycogen is reported as glucosyl units, whereas labeled glycogen is reported as nCi 14C recovered in purified glycogen per gram tissue divided by the integrated specific activity (ISA) in arterial plasma to normalize each value to its exposure to labeled precursor.
Local rates of glucose utilization were assayed during rest and activation with the fully quantitative deoxyglucose method using a 30-minute experimental interval.
Pyruvate formed by the glycolytic pathway during the 10-minutes assay interval was calculated as (CMRglc)(2 pyruvate/glucose)(10 minutes).
The net increase in lactate levels at the end of the 10-minute experimental period was divided by the amount of pyruvate formed from glucose or by the pyruvate equivalents of the glycogen consumed during the 10-minute time interval.
Tissue concentrations are the sum of bound and free pyridine nucleotides. Cultured astrocytes are reported to have 50–100% higher NAD+ levels than cultured neurons, but the mitochondrial NAD+ levels are similar in both cell types, indicating that neurons have a greater proportion of NAD+ in their mitochondria (Pieper et al (2000); Alano et al (2007) and references cited therein).
The higher value for NAD+ was in brain slices from 7-day-old rats from Zeng et al (2007) and is similar to the range of values in cultured brain cells (assuming 100 mg protein/g cultured astrocytes and neurons). Calculated ratios are estimates for the free cytoplasmic pools (based on components of the lactate dehydrogenase (LDH) or isocitrate dehydrogenase (IDH) reactions) and the mitochondrial pool (based on components of the glutamate dehydrogenase (GDH) reaction); the mitochondrial ratio is considered to be more reduced, but values are less reliable owing to complexities of mitochondrial metabolism and assumptions made for the calculation (see discussion in cited references and Siesjö (1978)).
Endogenous fluorescent compounds, NADH, NADPH, and FAD, are commonly used in microscopic studies to localize and evaluate redox changes during activation (Shuttleworth, 2010). Activation-induced changes in fluorescence (ΔF/F) are generally very small (<10%) and are far below the responses to metabolic inhibitors (Table 8). The total concentrations of these redox compounds are quite low and the calculated cytoplasmic NAD+/NADH ratio is very high, indicating that most of this total cofactor pool is not fluorescent (Table 8). Thus, the baseline fluorescence (F) and the induced response (ΔF) correspond to only to a small fraction of the total amount of NAD++NADH. Owing to low cofactor concentration and high glucose metabolic rates, cofactor oxidation-reduction turnover that accompanies pathway fluxes is high. Glycolytic or oxidative rate information cannot be obtained from ΔF/F.
Glucose-Sparing Action of Alternative Substrates that Increase in Blood During Abnormal or Specific Conditions
In the 1960s, studies of human brain metabolism during prolonged starvation revealed that ketone body oxidation could account for ∼60% of the oxygen consumed. Ketone bodies spared glucose oxidation while permitting glycolysis and release of lactate and pyruvate from the brain (Table 9). Glucose-sparing effects of ketone bodies in different organs have been attributed, in part, to increased citrate levels and inhibition by citrate of phosphofructokinase, causing reduced glucose oxidation and release of lactate as gluconeogenic substrate (Robinson and Williamson, 1980). High levels of ketone bodies (2.5 to 17 mmol/L) and lactate (4 to 8 mmol/L) also reduce glucose oxidation in brain slices and in infused, starved, or fat-fed rats (Table 9). Some studies report that exercising humans with elevated lactate levels (4 to 14 mmol/L) have reduced brain CMRglc, whereas other studies find increased glucose and lactate metabolism during strenuous exercise (Table 9). Rats exercising at 85% of maximal respiratory rate had heterogeneous regional increases in CMRglc and no decreases (Table 9). High levels of three oxidative substrates, lactate, glutamine, and pyruvate, in tissue culture media reduce glucose utilization in astrocytes and neurons in a dose-dependent manner (Table 9). When cultured forebrain neurons were incubated with lactate and glucose (1 mmol/L of each substrate), lactate was calculated to contribute 75% to total oxidative metabolism (Bouzier-Sore et al, 2006; Table 9). This conclusion sharply contrasts the quite small, 4% to 8%, contribution of lactate to oxidative metabolism in the brain of humans infused with lactate to achieve plasma and brain lactate levels of ∼0.6 to 4.1 mmol/L and ∼0.4 to 3 μmol/g, respectively (Boumezbeur et al, 2010). The responses of cultured neurons do not correspond to the adult human brain, presumably because of developmental differences affecting transport and metabolism. These findings indicate that translation of results of studies in immature cultured cells to the adult brain in vivo must establish similar metabolic and transport capabilities. Lactate can, but does not necessarily, reduce glucose utilization in vivo.
Table 9. Utilization of alternative fuel and inhibition of glucose utilization by high concentrations of ketone bodies and lactate.
| Preparationa | Treatmenta | Response magnitudeb | Reference |
|---|---|---|---|
| Obese human | 5–6 weeks starvation, arterial blood levels of ketone bodies: BHB=6.7 mmol/L, AcAc=1.2 mmol/L | Ketone body oxidation accounted for 60% of CMRO2, spared glucose oxidation, with continued glycolysis and lactate/pyruvate release from the brain into the blood | Owen et al, 1967 |
| Rat brain slices | 5 mmol/L lactate or pyruvate | Reduced glucose uptake by 30% or 36%, respectively with no change in oxygen consumption | Rolleston and Newsholme, 1967 |
| 5 mmol/L AcAc or 10 mmol/L BHB | No change in glucose uptake, decreased glucose oxidation 10 or 23%, increased lactate formation 21 or 46%, respectively; did not alter oxygen consumption | ||
| 4.2 or 8.3 mmol/L lactate | Reduced 8.3 mmol/L [U-14C]glucose → 14CO2 by 28 or 41% respectively; 8.3 mmol/L increased glycogen 13% | Ide et al, 1969 | |
| 4.2 or 8.3 mmol/L BHB | Reduced 8.3 mmol/L [U-14C]glucose → 14CO2 by 33 or 49% respectively; 8.3 mmol/L increased glycogen 34% | ||
| 4.2 or 16.7 mmol/L BHB | Reduced lactate oxidation 20–65%, depending on label position | ||
| Adult rat | Up to 96 hours starvation or ketone body infusion; blood ketone levels up to 2.5–5 mmol/L | Increased arteriovenous difference across the brain with increasing ketone body concentration in blood | Hawkins et al, 1971 |
| 48 hours starvation or 24 hours starvation + BHB infusion; blood ketone body level 2.5 or 6.5 mmol/L | Reduce glucose utilization 17–21%, increase lactate release to blood 800 or 1,700%, reduce calculated glucose oxidation 33 or 67%, increases O2 consumption owing to ketone bodies from 0.6% to 25% or 60%, respectively, for the starved and infused animals. Brain citrate levels increased and may inhibit glycolysis owing to citrate inhibition of phosphofructokinase | Ruderman et al, 1974 | |
| High fat diet raised blood BHB levels over the range 1–5.5 mmol/L | Decrease glucose utilization in anesthetized rat brain ∼10% per mmol/L increase in plasma ketone body concentration | LaManna et al, 2009 | |
| Lactate infusion increase plasma level from 1.3±0.6 to 5.8±1.6 mmol/L | Reduced glucose utilization in anesthetized rat by a mean of 38%, in dose-dependent manner | Wyss et al, 2011 | |
| Running wheel exercise at 85% of maximal O2 uptake | Heterogeneous increases in CMRglc in most brain regions ranging from about 30–165% no decreases observed in any structure during exercise (plasma glucose approximately 8.5–10 mmol/L; lactate not measured) | Vissing et al, 1996 | |
| Cultured cortical astrocytes (from PN1 rat, used at 25–30 DIV) | 1, 2, 3, 5, 10 mmol/L lactate | Reduced glucose utilization by 15%, 25%, 40%, 50%, or 60%, respectively | Swanson and Benington, 1996 |
| 1, 2, 3, 5, 10 mmol/L glutamine | Reduced glucose utilization by 15%, 20%, 45%, 40%, or 50%, respectively | ||
| (from PN0–2 rat, used at 14 DIV) | 0.5, 1, 2.5, 5 mmol/L lactate 0.25, 0.5, 1, 2.5 mmol/L pyruvate | Reduced glycolytic contribution to lactate labeling by 20%, 40%, 55%, 70% (lactate) or by 25%, 35%, 60%, 70% (pyruvate), respectively | Rodrigues et al, 2009 |
| Cultured forebrain neurons (From PN0 rat; used at 5 DIV) | 5.5 mmol/L [1-13C]glucose + 1.1, 5.5. or 11 mmol/L lactate | 55% and 68% decreases in fractional enrichment of glutamate C4 at 5.5 and 11 mmol/L extracellular lactate. Calculated contribution of 1.1, 5.5, and 11 mmol/L exogenous lactate is 46%, 90%, and 93%, respectively, of neuronal oxidative metabolism | Bouzier-Sore et al, 2003 |
| 1.1 mmol/L glucose + 1.1 mmol/L lactate | Calculated relative contribution of lactate and glucose to neuronal oxidative metabolism is 75% and 25%, respectively | Bouzier-Sore et al, 2006 | |
| Human brain | [3-13C]lactate infusion to produce ∼0.5–3 mmol/L plasma lactate, corresponding to brain lactate levels of ∼0.4 to 3 μmol/g | Plasma glucose levels were approximately 6–7 mmol/L and estimated brain glucose level is ∼1–1.4 μmol/g, assuming a brain/plasma ratio of 0.2. Lactate oxidation contributes a maximum of about 4–8% of total oxidative metabolism in a concentration-dependent manner | Boumezbeur et al, 2010 |
| Lactate infused to raise plasma lactate from 0.6 to 4.1 mmol/L | Plasma glucose was about 5.3 mmol/L. Lactate infusion caused an ∼17% ↓in whole brain CMRglc assayed with [18F]FDG (∼5% per mmol/L increase in plasma lactate) | Smith et al, 2003 | |
| Graded mild to exhaustive exercise and recovery | Assays of blood flow and arteriovenous differences for oxygen, glucose, and lactate across the brain at intervals during mild, moderate, and exhaustive exercise. Calculated cumulative uptake of glucose, lactate, and oxygen progressively increase with time. CMRO2/CMR(glc+0.5 lac) ratio is substantially reduced during and after strenuous exercise | Quistorff et al, 2008 | |
| [1-13C]lactate infusions during rest and cycling exercise (75% of maximal O2 uptake) to achieve plasma lactate levels of 0.9, 3.9, and 6.9 mmol/L | Blood lactate contributed 8%, 19%, and 27%, at the respective plasma lactate levels, to cerebral energy metabolism | van Hall et al, 2009 | |
| Cycling exercise at 30, 55, and 75% of maximal O2 uptake. Plasma lactate rose from ∼0.5 to 14 mmol/L | Brain CMRglc assayed with [18F]FDG. CMRglc decreased with increased exercise intensity and fell in proportion to plasma lactate level (∼3.5% per mmol/L increase in plasma lactate) | Kemppainen et al, 2005 |
Ketone bodies, acetoacetate (AcAc) and β-hydroxybutyrate (BHB) levels in blood increase during prolonged fasting, starvation, and high fat diets. In many tissues including the brain, oxidative metabolism of ketone bodies spares glucose by reducing glucose oxidation, maintaining glycolysis, and releasing lactate, which can be converted back to glucose by gluconeogenesis (Robinson and Williamson, 1980). Although details of the mechanisms of regulation of glucose metabolism when alternative substrates are oxidized are not fully established, elevated ketone body metabolism is associated with an increased concentration of citrate, which, along with other metabolic regulators, is an inhibitor of brain phosphofructokinase within the physiological range (Passonneau and Lowry, 1963). Conceivably, increased lactate concentrations in blood and extracellular fluid could also increase intracellular citrate levels and contribute to glucose sparing by reduction of glycolysis and glucose oxidation. Brains were harvested for tissue culture at indicated postnatal (PN) age in days, and used for experiments after maintaining the cells in tissue culture for the indicated days in vitro (DIV). It must be noted that inhibition of glucose metabolism by lactate in cultured astrocytes and neurons is much higher than observed in normal brain in vivo, suggesting a strong influence of developmental stage, levels of glycolytic and oxidative enzymes and transporters, and relative glycolytic and oxidative fluxes in cultured cells compared with the normal adult brain. Many studies have shown that labeled glucose preferentially labels the large (neuronal) glutamate pool, but in vivo microdialysis studies have shown that fluorocitrate inhibits oxidation of lactate and glucose, indicating that astrocytes oxidize ∼50% of interstitial lactate and ∼35% of interstitial glucose (Zielke et al, 2007).
Magnitude of response is expressed as approximate percentage change owing to treatment, 100[(treated−control)/control].
Evaluation of Roles of Lactate by Monocarboxylic Acid Transporter Inhibition
As lactate shuttling among brain cells is very difficult to evaluate, an MCT inhibitor (e.g., α-cyano-4-hydroxycinnamate or 4-CIN) is often used to assess effects of extracellular lactate on neuronal function, and decrements caused by transport blockade are inferred to reflect insufficient lactate fuel. However, these types of studies are difficult to interpret because low levels of 4-CIN severely inhibit pyruvate transport into the mitochondria from the rat heart and liver (<10 μmol/L) and brain (100 μmol/L), and 100 μmol/L markedly inhibits glucose-supported synaptosomal respiration during activation (Table 10). In addition to blocking plasma membrane lactate transport, 250 μmol/L 4-CIN also reduces oxidation of lactate and glucose owing to impairment of mitochondrial pyruvate transport (Table 10). Thus, the 10% compensatory increase in neuronal NBDG transport and 20% decrease in neuronal intracellular acidification in the presence of 5 mmol/L lactate plus 100 μmol/L 4-CIN (to preferentially inhibit neuronal MCT2 compared with astrocytic MCT1 or MCT4) (Erlichman et al, 2008; Table 10) could have arisen from reduced neuronal pyruvate oxidation, lactate uptake, or both. Even if the 4-CIN-evoked 10% increase in NBDG transport reflects only the magnitude of the astrocyte-neuron lactate shuttle hypothesis, the quantitative effect of blockade of lactate shuttling on neuronal glucose transport (and metabolism) is small.
Table 10. Effects of α-cyano-4-hydroxycinnamate (4-CIN) on mitochondrial pyruvate oxidation and transport and oxidation of glucose and lactate.
| Preparationa | 4-CIN dose | Response magnitude | Reference |
|---|---|---|---|
| Isolated mitochondria | 1–100 μmol/L | 6 μmol/L=Ki for pyruvate transport rat liver mitochondria; | Halestrap, 1975; Halestrap and Denton, 1974; Halestrap and Denton, 1975 |
| 1.5 μmol/L=Ki for pyruvate transport rat heart mitochondria | |||
| 25 μmol/L almost complete inhibition of pyruvate oxidation in rat heart mitochondria | |||
| 100 μmol/L almost complete inhibition of pyruvate oxidation in rat brain, adipose tissue, kidney cortex, or blowfly flight muscle mitochondria | |||
| Cultured astrocytes from PN0 rats used at 12–14 DIV and cortical neurons from E16 rats and used at 7 DIV | 250 μmol/L | Reduced oxidation of lactate and glucose by 43% and 46%, respectively, in astrocytes and by 87 and 58%, respectively, in neurons. 4-CIN did not reduce glucose transport. | McKenna et al, 2001 |
| Cortical synaptosomes | 10–100 μmol/L 4-CIN | No effect on basal respiration rate with 15 mmol/L glucose substrate | Choi et al, 2009 |
| 4 μmol/L FCCP + 10 μmol/L 4-CIN | 35% inhibition of maximal FCCP-evoked respiration rate | ||
| + 30 μmol/L 4-CIN | 50% inhibition of maximal respiration rate | ||
| + 100 μmol/L 4-CIN | 60% inhibition of maximal respiration rate | ||
| Oocytes transfected with MCT1 or MCT2 | 100 μmol/L | 26% or 66% inhibition of lactate transport rate by MCT1 or MCT2, respectively. Ki for 50% lactate transport inhibition for MCT2 and MCT1 are 24 μmol/L and 425 μmol/L, respectively | Bröer et al, 1999 |
| Brain slice | 100 μmol/L | Astrocytes—no effect on 2-NBDG uptake; no effect on intracellular acidification when 5 mmol/L lactate was added to the aCSF perfusate containing 26 mmol/L bicarbonate and 10 mmol/L glucose | Erlichman et al, 2008 |
| Neurons—10% increase in 2-NBDG uptake; 20% reduction in intracellular acidification when 5 mmol/L lactate was added to aCSF |
aCSF, artificial cerebrospinal fluid.
Age at tissue harvest (embryonic, E, or postnatal, PN) and duration of culture (days in vitro, DIV) are indicated for cultured cells. The monocarboxylic acid transporter, MCT1, is predominantly in astrocytes, whereas the higher-affinity MCT2 is neuronal.
Metabolic Modeling and Simulation Studies
Metabolic modeling is necessary to calculate glucose utilization and glucose oxidation rates from labeling studies carried out in vivo. The autoradiographic [14C]deoxyglucose method uses a two-compartment model (blood and brain) that takes into account the kinetic differences in rates of transport and phosphorylation of deoxyglucose and glucose (Sokoloff et al, 1977). The procedure assays the first irreversible step of glucose utilization, the hexokinase step, which corresponds to the overall rate of glucose consumption at steady state. [14C]Glucose autoradiographic and biochemical assays evaluate labeled metabolites retained in the tissue at the end of the experimental period which must be short owing to label loss. 13C-Magnetic resonance spectroscopic studies use programmed infusions to maintain constant arterial plasma [13C]glucose concentrations, and to measure temporal profiles of incorporation of label from [13C]glucose into amino acids derived from the TCA cycle. Compartmental modeling enables calculation of glucose oxidation rates in neurons and astrocytes, glutamate–glutamine cycling, and rates of other pathways, depending on the label position and precursor (Mason and Rothman, 2004). 13C-Magnetic resonance spectroscopic assays focus on the oxidative pathways because the glycolytic pools (glucose to pyruvate/lactate) quickly equilibrate with arterial plasma [13C]glucose, and once this occurs, no kinetic information can be obtained from these compounds to estimate glycolytic rate.
In their revised, more comprehensive model for coupling of glucose metabolism with synaptic activity, Hyder et al, 2006 predict (see their Figure 5) that most of the glucose consumed during activation is used glycolytically by astrocytes, with significant lactate shuttling to neurons and lactate oxidation by neurons. This model also predicts very little (a few percent) lactate release from the brain, contrasting the much greater label release (∼50% see above, ‘Underestimation of metabolic activation with labeled glucose') based on autoradiographic and biochemical studies of brain activation in conscious rats assayed in parallel with [6-14C]glucose and [14C]deoxyglucose (Collins et al, 1987; Ackermann and Lear, 1989; Adachi et al, 1995; Cruz et al, 1999, 2007). The basis for the quantitative differences in the fate of lactate in the 14C- and 13C-magnetic resonance spectroscopic assays remains to be established. Assays of total glucose metabolized and rates of glycolytic, glycogenolytic, oxidative, and anaplerotic (i.e., biosynthetic) pathways are required to have a fuller understanding of brain metabolic activation and roles of lactate.
Metabolic modeling and computer-based simulations are also very useful to predict pathway fluxes in neurons and astrocytes under various test conditions. Calculated rates and predicted outcomes are critically influenced by model assumptions that define the metabolic capabilities and energetic demands of neurons and astrocytes and their subcellular compartments, the magnitude of metabolic activation, cellular concentrations of glucose and lactate transporters, kinetic properties of the endothelial, neuronal, and astrocytic nutrient transporters, and other factors. Model assumptions govern the predicted cellular consumption of glucose, the cellular origin of lactate, and the direction of lactate shuttling (i.e., the astrocyte to neuron or neuron to astrocyte), and the magnitude and duration of lactate concentration changes. Different models, modeling principles, and model assumptions underlie discordant conclusions related to the roles of lactate and glucose in brain activation derived from computer-based simulation studies. Interested readers are referred to studies by Aubert et al (2005, 2007), Aubert and Costalat (2007), Simpson et al (2007), DiNuzzo et al (2010a,2010b), Mangia et al (2009b), Barros and Deitmer (2010), Occhipinti et al (2010), and Calvetti and Somersalo (2011) and commentaries by Jolivet et al (2010) and Mangia et al (2011) for detailed discussions of assumptions and limitations of transport and metabolic models and of simulations derived from them.
Concluding Comments
A wealth of data obtained over several decades in many laboratories shows that cultured neurons and synaptosomes are capable of greatly increasing glucose transport, glycolysis, and glucose-supported respiration under many experimental conditions that increase energy demand. The emphasis of this review is on measured data that directly or indirectly relate to brain lactate metabolism. Modeling and simulation studies are also very useful to predict outcomes, as well as to suggest and design critical experiments. Data in Tables 1 to 10 identify strong trends and some discordant findings, and elucidation of the basis for apparently discrepant results will help understand important characteristics of brain cells. Incorporation of results from in vitro studies into models describing the cellular basis of glucose utilization must accommodate these major data sets, as well as two very different physiologic situations involving brain lactate transport and metabolism in vivo, outward and inward lactate concentration gradients.
Outwardly Directed Lactate Concentration Gradient from the Activated Brain to Blood in Physically Inactive Subjects with Low Plasma Lactate Levels
Brain activation usually causes disproportionately greater increases in CBF and CMRglc compared with CMRO2 (Dienel and Cruz, 2004, 2008). Glycolytic activation increases intracellular lactate concentration, causing lactate to diffuse down its concentration gradient to extracellular fluid. Lactate can then be avidly taken up into astrocytes (Gandhi et al, 2009), channeled through the astrocytic syncytium through gap junctions, and discharged from astrocytic endfeet to perivascular fluid and the vasculature, where it may serve as a signaling molecule for blood flow regulation. Rapid efflux of labeled lactate from the brain during activation contributes to the ∼50% underestimates of CMRglc by labeled glucose in autoradiographic and positron emission tomographic studies. Generation and release of unlabeled lactate contributes to the decrease in CMRO2/CMRglc ratio during activation. It must be noted that the small increases in CMRO2, if any, during activation reflect oxygen consumed by oxidation of all compounds. As lactate utilization must consume oxygen, the maximal contribution of any increase in lactate shuttling to total oxidation during activation cannot exceed the ΔCMRO2. For example, if CMRglc increases by 50% and CMRO2 increases 20%, this increase in CMRO2 corresponds to oxidation of the additional pyruvate derived from glucose, glycogen, and lactate, and oxidation of any other compounds in neurons and astrocytes. If neurons account for half of the additional oxygen consumed by direct metabolism of glucose-derived pyruvate, then lactate shuttling cannot exceed half of the net increase in CMRO2, or 10% in this example.
Inwardly Directed Lactate Concentration Gradient from the Blood to the Entire Brain in Subjects with High Blood Lactate Levels
Lactate flooding during lactate infusions and strenuous exercise (and in vitro assays) eliminates local lactate concentration gradients in tissues arising from focal activation and enables lactate to serve as an opportunistic, supplemental fuel for cells throughout the entire brain. However, lactate oxidation during flooding conditions and partial inhibition of glucose utilization by lactate do not prove directed cell-to-cell lactate shuttling or its use as a major fuel under other situations. Biochemical regulatory mechanisms take place and can modulate glucose utilization by different mechanisms. Lactate uptake with H+ and H+ production by the LDH (Figure 1) can reduce intracellular pH (depending on buffering capacity), lactate conversion to pyruvate reduces NAD+ availability for glycolysis, and lactate oxidation generates ATP and citrate. Acidification, ATP, and citrate can inhibit phosphofructokinase in a very complex, concentration-dependent manner that is influenced by other modulators of this enzyme and can reduce CMRglc. Notably, some studies have shown that glucose uptake and utilization does increase (Table 9) during strenuous exercise in rats (Vissing et al, 1996) and in humans who also have increased lactate uptake and oxidation (Quistorff et al, 2008). High lactate levels that arise during strenuous exercise or hypoxic episodes may be ‘biologically intended' to be glucose-sparing, similar to ketone bodies during starvation.
Cell-Type Specific Production of Lactate, Cell-to-Cell Lactate Shuttling, and Lactate Oxidation or Release
Many studies carried out in different laboratories over several decades show the high glycolytic and respiratory capacity of nerve endings and cultured neurons. These findings are consistent with high neuronal glucose utilization in vivo and they negate assertions that neurons cannot upregulate glucose transport and glycolytic metabolism. During low-level lactate infusions into resting humans, lactate oxidation by the brain contributes <8% to total TCA cycle flux. Moreover, blockade of lactate-pyruvate transporters in brain slices with 4-CIN evokes only a 10% increase in neuronal NBDG uptake. Although modeling predicts significant lactate shuttling, direct, strong in vivo evidence for astrocyte-to-neuron lactate shuttling coupled to local neuronal lactate oxidation as a major fuel is lacking.
Taken together, many independent lines of evidence obtained in vivo and in vitro support the conclusion that glucose, not lactate, is the major brain fuel during activation and that neurons may be a major source of lactate during activation. Small or no increases in CMRO2 during activation compared with CBF and CMRglc indicate preferential upregulation of nonoxidative metabolism of glucose, but most of the ATP generated during activating conditions comes from the oxidative pathway. In pulse-labeling assays, CMRglc is greatly underestimated when assayed with labeled glucose owing to rapid label loss arising from lactate efflux, decarboxylation reactions, and label spreading (Cruz et al, 1999, 2007). Most lactate generated from glucose microinfused into the brain is not locally oxidized (Ball et al, 2010). Lactate dispersal and release can be mediated by astrocytes (Gandhi et al, 2009), and blockade of lactate transporters and gap junctions increase focal label retention in activated structures (Cruz et al, 2007). Strong Ca2+ signals in neuronal mitochondrial reduce MAS activity, which would increase neuronal lactate production and reduce any neuronal lactate utilization (Bak et al, 2009; Contreras and Satrústegui, 2009). Specific neuronal structures and activities depend on glycolysis, including dendritic spines that lack mitochondria (Li et al, 2004; Bourne and Harris, 2008), the plasma membrane calcium pump (Ivannikov et al, 2010), and glutamate loading into synaptic vesicles (Ikemoto et al, 2003). The cost for a neuron to package one glutamate is one ATP, which is half that required by astrocytes for glutamate–glutamine cycling (one ATP for sodium extrusion and one for glutamine synthesis). In cultured glutamatergic neurons, glucose, not lactate, utilization is enhanced by NMDA-induced glutamate release (Bak et al, 2009). These findings support neuronal upregulation of glycolysis during excitatory neurotransmission, and strong compartmentation of glycolysis in astrocytes during brain activation is considered unlikely.
Failure of glutamate transport blockade to reduce stimulus-evoked lactate increases, and metabolic activation in the cerebellum (as does an AMPA receptor blocker; Caesar et al (2008)) is consistent with the low predicted ATP cost for astrocytic participation in glutamate–glutamine cycling compared with postsynaptic and other signaling events (Attwell and Laughlin, 2001). Astrocytes increase glycogenolysis and oxidative metabolism during activation, besides their presumed use of blood glucose. Glycogenolysis generates glucose-6-phosphate that serves as fuel for astrocytes and can also inhibit astrocytic hexokinase activity, providing a mechanism to divert blood-borne glucose for use by neurons (DiNuzzo et al, 2010b). Small increases in astrocytic oxidative metabolism during activation in vivo produce substantial portion of the total increase in ATP generated in astrocytes during activation. The sites and processes consuming the ATP are not known, but fine perisynaptic processes of astrocytes contain mitochondria, endowing these structures with high oxidative capacity that can be used to power many processes linked to neurotransmission, including glutamate uptake and sodium extrusion, regulation of extracellular [K+] (Hertz et al, 2007), and glutamate-evoked calcium waves (Cornell-Bell et al, 1990a). Astrocytic processes are dynamic structures, their formation is stimulated by glutamate exposure, and they advance and retract from active synapses by actin-dependent mechanisms that involve ATP hydrolysis (Cornell-Bell et al, 1990b; Reichenbach et al, 2010). Further work is required to include these processes in the energetics of working astrocytes in vivo.
Release of lactate from the resting and activated brain even though it can serve as an oxidative fuel is an important, unresolved issue that probably involves many factors, including the following: (1) the rapid-onset hyperemic response delivers more fuel to the brain; (2) glucose supply to brain exceeds demand by a factor of at least 1.5 in normoglycemic subjects over a wide range of CMRglc and glucose levels in rats (Cremer et al, 1983; Hargreaves et al, 1986) and humans (Shestov et al (2011) and references cited therein); (3) lactate release to perivascular fluid may enhance the hyperemic response during activation by causing vasodilation; (iv) spatial–temporal interactions of increased energy demand (e.g., plasma membrane ion pumps) during activation may preferentially depend on glycolysis and channeling of lactate, with its discharge from astrocytic endfeet to perivascular space. Excess glucose delivery and high-capacity neuronal glucose transport and metabolism support the conclusion that neurons do not need lactate as supplemental fuel under normal activating conditions. In contrast, lactate flooding of the brain owing to elevated blood levels would normally occur when whole-body glycolytic metabolism may exceed overall oxidative metabolism, such as during strenuous physical work, exercise, and hypoxia. High blood lactate levels abolish brain lactate efflux gradients and can evoke glucose-sparing responses in brain and other organs. Alternative substrates can also substantially contribute to brain energetics when glucose supply is inadequate, e.g., during hypoglycemia or intense brain activity. Most in vivo evidence supports the brain's use of glucose as its major fuel under normal activating conditions in sedentary or modestly physically active subjects.
In conclusion, detailed studies of brain energy metabolism and neurotransmission and their interrelationships during the past 40 years have substantially increased our understanding of the cellular contributions to brain function, imaging, and spectroscopic studies. Development of new approaches to resolve discordant results and extend current technologies is expected to have a high impact on the use of metabolic imaging techniques to assess cellular functions in vivo and to evaluate human brain diseases.
Acknowledgments
The author thanks Dr David Attwell for his critical review of the manuscript and valuable suggestions. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institute Of Diabetes And Digestive And Kidney Diseases, National Institute of Neurological Diseases and Stroke, or the National Institutes of Health.
The author declares no conflict of interest.
Footnotes
This work was supported by National Institutes of Health grants DK081936 and NS038230.
References
- Ackermann RF, Lear JL. Glycolysis-induced discordance between glucose metabolic rates measured with radiolabeled fluorodeoxyglucose and glucose. J Cereb Blood Flow Metab. 1989;9:774–785. doi: 10.1038/jcbfm.1989.111. [DOI] [PubMed] [Google Scholar]
- Adachi K, Cruz NF, Sokoloff L, Dienel GA. Labeling of metabolic pools by [6-14C]glucose during K+-induced stimulation of glucose utilization in rat brain. J Cereb Blood Flow Metab. 1995;15:97–110. doi: 10.1038/jcbfm.1995.11. [DOI] [PubMed] [Google Scholar]
- Alano CC, Tran A, Tao R, Ying W, Karliner JS, Swanson RA. Differences among cell types in NAD(+) compartmentalization: a comparison of neurons, astrocytes, and cardiac myocytes. J Neurosci Res. 2007;85:3378–3385. doi: 10.1002/jnr.21479. [DOI] [PubMed] [Google Scholar]
- Allen NJ, Káradóttir R, Attwell D. A preferential role for glycolysis in preventing the anoxic depolarization of rat hippocampal area CA1 pyramidal cells. J Neurosci. 2005;25:848–859. doi: 10.1523/JNEUROSCI.4157-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida A, Almeida J, Bolaños JP, Moncada S. Different responses of astrocytes and neurons to nitric oxide: the role of glycolytically generated ATP in astrocyte protection. Proc Natl Acad Sci USA. 2001;98:15294–15299. doi: 10.1073/pnas.261560998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida A, Moncada S, Bolaños JP. Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat Cell Biol. 2004;6:45–51. doi: 10.1038/ncb1080. [DOI] [PubMed] [Google Scholar]
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Aubert A, Costalat R. Compartmentalization of brain energy metabolism between glia and neurons: insights from mathematical modeling. Glia. 2007;55:1272–1279. doi: 10.1002/glia.20360. [DOI] [PubMed] [Google Scholar]
- Aubert A, Costalat R, Magistretti PJ, Pellerin L. Brain lactate kinetics: modeling evidence for neuronal lactate uptake upon activation. Proc Natl Acad Sci USA. 2005;102:16448–16453. doi: 10.1073/pnas.0505427102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert A, Pellerin L, Magistretti PJ, Costalat R. A coherent neurobiological framework for functional neuroimaging provided by a model integrating compartmentalized energy metabolism. Proc Natl Acad Sci USA. 2007;104:4188–4193. doi: 10.1073/pnas.0605864104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak LK, Walls AB, Schousboe A, Ring A, Sonnewald U, Waagepetersen HS. Neuronal glucose but not lactate utilization is positively correlated with NMDA-induced neurotransmission and fluctuations in cytosolic Ca2+ levels. J Neurochem. 2009;109 (Suppl 1:87–93. doi: 10.1111/j.1471-4159.2009.05943.x. [DOI] [PubMed] [Google Scholar]
- Balázs R, Cremer JE.1972Metabolic Compartmentation in the Brain(eds)New York: John Wiley & Sons [Google Scholar]
- Ball KK, Cruz NF, Mrak RE, Dienel GA. Trafficking of glucose, lactate, and amyloid-beta from the inferior colliculus through perivascular routes. J Cereb Blood Flow Metab. 2010;30:162–176. doi: 10.1038/jcbfm.2009.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball KK, Gandhi GK, Thrash J, Cruz NF, Dienel GA. Astrocytic connexin distributions and rapid, extensive dye transfer via gap junctions in the inferior colliculus: Implications for [14C]glucose metabolite trafficking. J Neurosci Res. 2007;85:3267–3283. doi: 10.1002/jnr.21376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baquer NZ, McLean P, Greenbaum AL.1975Systems relationships and control of metabolic pathways in developing brainIn: Normal and pathological development of energy metabolism. (Hommes FA, van den Berg CJ, eds),London: Academic Press; 109–131. [Google Scholar]
- Barros LF, Deitmer JW. Glucose and lactate supply to the synapse. Brain Res Rev. 2010;63:149–159. doi: 10.1016/j.brainresrev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Bergersen L, Rafiki A, Ottersen OP. Immunogold cytochemistry identifies specialized membrane domains for monocarboxylate transport in the central nervous system. Neurochem Res. 2002;27:89–96. doi: 10.1023/a:1014806723147. [DOI] [PubMed] [Google Scholar]
- Bergersen L, Waerhaug O, Helm J, Thomas M, Laake P, Davies AJ, Wilson MC, Halestrap AP, Ottersen OP. A novel postsynaptic density protein: the monocarboxylate transporter MCT2 is co-localized with delta-glutamate receptors in postsynaptic densities of parallel fiber-Purkinje cell synapses. Exp Brain Res. 2001;136:523–534. doi: 10.1007/s002210000600. [DOI] [PubMed] [Google Scholar]
- Bergersen LH. Is lactate food for neurons? Comparison of monocarboxylate transporter subtypes in brain and muscle. Neuroscience. 2007;145:11–19. doi: 10.1016/j.neuroscience.2006.11.062. [DOI] [PubMed] [Google Scholar]
- Bergersen LH, Magistretti PJ, Pellerin L. Selective postsynaptic co-localization of MCT2 with AMPA receptor GluR2/3 subunits at excitatory synapses exhibiting AMPA receptor trafficking. Cereb Cortex. 2005;15:361–370. doi: 10.1093/cercor/bhh138. [DOI] [PubMed] [Google Scholar]
- Bittner CX, Valdebenito R, Ruminot I, Loaiza A, Larenas V, Sotelo-Hitschfeld T, Moldenhauer H, San Martín A, Gutiérrez R, Zambrano M, Barros LF. Fast and reversible stimulation of astrocytic glycolysis by K+ and a delayed and persistent effect of glutamate. J Neurosci. 2011;31:4709–4713. doi: 10.1523/JNEUROSCI.5311-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomqvist G, Stone-Elander S, Halldin C, Roland PE, Widén L, Lindqvist M, Swahn CG, Långström B, Wiesel FA. Positron emission tomographic measurements of cerebral glucose utilization using [1-11C]D-glucose. J Cereb Blood Flow Metab. 1990;10:467–483. doi: 10.1038/jcbfm.1990.89. [DOI] [PubMed] [Google Scholar]
- Bolaños JP, Almeida A. The pentose-phosphate pathway in neuronal survival against nitrosative stress. IUBMB Life. 2010;62:14–18. doi: 10.1002/iub.280. [DOI] [PubMed] [Google Scholar]
- Bonavita V, Guarneri R, Amore G. Nicotinamide adenine dinucleotides in the convulsant rat brain. J Neurochem. 1970;17:1613–1614. doi: 10.1111/j.1471-4159.1970.tb03732.x. [DOI] [PubMed] [Google Scholar]
- Borgström L, Chapman AG, Siesjö BK. Glucose consumption in the cerebral cortex of rat during bicuculline-induced status epilipticus. J Neurochem. 1976;27:971–973. doi: 10.1111/j.1471-4159.1976.tb05165.x. [DOI] [PubMed] [Google Scholar]
- Boumezbeur F, Petersen KF, Cline GW, Mason GF, Behar KL, Shulman GI, Rothman DL. The contribution of blood lactate to brain energy metabolism in humans measured by dynamic 13C nuclear magnetic resonance spectroscopy. J Neurosci. 2010;30:13983–13991. doi: 10.1523/JNEUROSCI.2040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne JN, Harris KM. Balancing structure and function at hippocampal dendritic spines. Annu Rev Neurosci. 2008;31:47–67. doi: 10.1146/annurev.neuro.31.060407.125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzier-Sore AK, Voisin P, Bouchaud V, Bezancon E, Franconi JM, Pellerin L. Competition between glucose and lactate as oxidative energy substrates in both neurons and astrocytes: a comparative NMR study. Eur J Neurosci. 2006;24:1687–1694. doi: 10.1111/j.1460-9568.2006.05056.x. [DOI] [PubMed] [Google Scholar]
- Bouzier-Sore AK, Voisin P, Canioni P, Magistretti PJ, Pellerin L. Lactate is a preferential oxidative energy substrate over glucose for neurons in culture. J Cereb Blood Flow Metab. 2003;23:1298–1306. doi: 10.1097/01.WCB.0000091761.61714.25. [DOI] [PubMed] [Google Scholar]
- Bradford HF.1975Isolated nerve terminals as an in vitro preparation for the study of dynamic aspects of transmitter metabolism and releaseIn: Handbook of Psychopharmacology. Vol. 1, Biochemical Principles and Techniques in Neuropharmacology (Iversen LL, Iverseh SD, Snyder SH, eds),New York, NY: Plenum Press; 191–252. [Google Scholar]
- Bradford HF, Ward HK, Thomas AJ. Glutamine–a major substrate for nerve endings. J Neurochem. 1978;30:1453–1459. doi: 10.1111/j.1471-4159.1978.tb10477.x. [DOI] [PubMed] [Google Scholar]
- Brennan AM, Connor JA, Shuttleworth CW. NAD(P)H fluorescence transients after synaptic activity in brain slices: predominant role of mitochondrial function. J Cereb Blood Flow Metab. 2006;26:1389–1406. doi: 10.1038/sj.jcbfm.9600292. [DOI] [PubMed] [Google Scholar]
- Bröer S, Bröer A, Schneider HP, Stegen C, Halestrap AP, Deitmer JW. Characterization of the high-affinity monocarboxylate transporter MCT2 in Xenopus laevis oocytes. Biochem J. 1999;341:529–535. doi: 10.1042/0264-6021:3410529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caesar K, Hashemi P, Douhou A, Bonvento G, Boutelle MG, Walls AB, Lauritzen M. Glutamate receptor-dependent increments in lactate, glucose and oxygen metabolism evoked in rat cerebellum in vivo. J Physiol. 2008;586:1337–1349. doi: 10.1113/jphysiol.2007.144154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvetti D, Somersalo E. Dynamic activation model for a glutamatergic neurovascular unit. J Theor Biol. 2011;274:12–29. doi: 10.1016/j.jtbi.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Cerdán S, Rodrigues TB, Sierra A, Benito M, Fonseca LL, Fonseca CP, García-Martín ML. The redox switch/redox coupling hypothesis. Neurochem Int. 2006;48:523–530. doi: 10.1016/j.neuint.2005.12.036. [DOI] [PubMed] [Google Scholar]
- Chatton JY, Pellerin L, Magistretti PJ. GABA uptake into astrocytes is not associated with significant metabolic cost: implications for brain imaging of inhibitory transmission. Proc Natl Acad Sci USA. 2003;100:12456–12461. doi: 10.1073/pnas.2132096100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chih CP, Lipton P, Roberts EL., Jr Do active cerebral neurons really use lactate rather than glucose. Trends Neurosci. 2001;24:573–578. doi: 10.1016/s0166-2236(00)01920-2. [DOI] [PubMed] [Google Scholar]
- Chih CP, Roberts EL., Jr Energy substrates for neurons during neural activity: a critical review of the astrocyte-neuron lactate shuttle hypothesis. J Cereb Blood Flow Metab. 2003;23:1263–1281. doi: 10.1097/01.WCB.0000081369.51727.6F. [DOI] [PubMed] [Google Scholar]
- Choi SW, Gerencser AA, Lee DW, Rajagopalan S, Nicholls DG, Andersen JK, Brand MD. Intrinsic bioenergetic properties and stress sensitivity of dopaminergic synaptosomes. J Neurosci. 2011;31:4524–4534. doi: 10.1523/JNEUROSCI.5817-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SW, Gerencser AA, Nicholls DG. Bioenergetic analysis of isolated cerebrocortical nerve terminals on a microgram scale: spare respiratory capacity and stochastic mitochondrial failure. J Neurochem. 2009;109:1179–1191. doi: 10.1111/j.1471-4159.2009.06055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins RC, McCandless DW, Wagman IL. Cerebral glucose utilization: comparison of [14C]deoxyglucose and [6-14C]glucose quantitative autoradiography. J Neurochem. 1987;49:1564–1570. doi: 10.1111/j.1471-4159.1987.tb01028.x. [DOI] [PubMed] [Google Scholar]
- Contreras L, Satrústegui J. Calcium signaling in brain mitochondria: interplay of malate aspartate NADH shuttle and calcium uniporter/mitochondrial dehydrogenase pathways. J Biol Chem. 2009;284:7091–7099. doi: 10.1074/jbc.M808066200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990a;247:470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- Cornell-Bell AH, Thomas PG, Smith SJ. The excitatory neurotransmitter glutamate causes filopodia formation in cultured hippocampal astrocytes. Glia. 1990b;3:322–334. doi: 10.1002/glia.440030503. [DOI] [PubMed] [Google Scholar]
- Costa Leite T, Da Silva D, Guimarães Coelho R, Zancan P, Sola-Penna M. Lactate favours the dissociation of skeletal muscle 6-phosphofructo-1-kinase tetramers down-regulating the enzyme and muscle glycolysis. Biochem J. 2007;408:123–130. doi: 10.1042/BJ20070687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer JE. Amino acid metabolism in rat brain studied with 14C-labelled glucose. J Neurochem. 1964;11:165–185. doi: 10.1111/j.1471-4159.1964.tb06127.x. [DOI] [PubMed] [Google Scholar]
- Cremer JE. Substrate utilization and brain development. J Cereb Blood Flow Metab. 1982;2:394–407. doi: 10.1038/jcbfm.1982.45. [DOI] [PubMed] [Google Scholar]
- Cremer JE, Cunningham VJ, Seville MP. Relationships between extraction and metabolism of glucose, blood flow, and tissue blood volume in regions of rat brain. J Cereb Blood Flow Metab. 1983;3:291–302. doi: 10.1038/jcbfm.1983.44. [DOI] [PubMed] [Google Scholar]
- Cruz F, Villalba M, García-Espinosa MA, Ballesteros P, Bogónez E, Satrústegui J, Cerdán S. Intracellular compartmentation of pyruvate in primary cultures of cortical neurons as detected by (13)C NMR spectroscopy with multiple (13)C labels. J Neurosci Res. 2001;66:771–781. doi: 10.1002/jnr.10048. [DOI] [PubMed] [Google Scholar]
- Cruz NF, Adachi K, Dienel GA. Metabolite trafficking during K+-induced spreading cortical depression: rapid efflux of lactate from cerebral cortex. J Cereb Blood Flow Metab. 1999;19:380–392. doi: 10.1097/00004647-199904000-00004. [DOI] [PubMed] [Google Scholar]
- Cruz NF, Ball KK, Dienel GA. Imaging focal brain activation in conscious rats: metabolite spreading and release contribute to underestimation of glucose utilization with [14C]glucose. J Neurosci Res. 2007;85:3254–3266. doi: 10.1002/jnr.21193. [DOI] [PubMed] [Google Scholar]
- Cruz NF, Dienel GA. High brain glycogen levels in brains of rats with minimal environmental stimuli: Implications for metabolic contributions of working astrocytes. J Cereb Blood Flow Metab. 2002;22:1476–1489. doi: 10.1097/01.WCB.0000034362.37277.C0. [DOI] [PubMed] [Google Scholar]
- Cruz NF, Lasater A, Zielke HR, Dienel GA. Activation of astrocytes in brain of conscious rats during acoustic stimulation: acetate utilization in working brain. J Neurochem. 2005;92:934–947. doi: 10.1111/j.1471-4159.2004.02935.x. [DOI] [PubMed] [Google Scholar]
- Dalsgaard MK. Fuelling cerebral activity in exercising man. J Cereb Blood Flow Metab. 2006;26:731–750. doi: 10.1038/sj.jcbfm.9600256. [DOI] [PubMed] [Google Scholar]
- Debernardi R, Magistretti PJ, Pellerin L. Trans-inhibition of glutamate transport prevents excitatory amino acid-induced glycolysis in astrocytes. Brain Res. 1999;850:39–46. doi: 10.1016/s0006-8993(99)02022-3. [DOI] [PubMed] [Google Scholar]
- Diamond I, Fishman RA. Development of Na+-stimulated glucose oxidation in synaptosomes. J Neurochem. 1973;21:1043–1050. doi: 10.1111/j.1471-4159.1973.tb07558.x. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Ball KK, Cruz NF. A glycogen phosphorylase inhibitor selectively enhances local rates of glucose utilization in brain during sensory stimulation of conscious rats: implications for glycogen turnover. J Neurochem. 2007a;102:466–478. doi: 10.1111/j.1471-4159.2007.04595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA, Cruz NF. Neighborly interactions of metabolically-activated astrocytes in vivo. Neurochem Int. 2003;43:339–354. doi: 10.1016/s0197-0186(03)00021-4. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Cruz NF. Nutrition during brain activation: does cell-to-cell lactate shuttling contribute significantly to sweet and sour food for thought. Neurochem Int. 2004;45:321–351. doi: 10.1016/j.neuint.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Cruz NF. Astrocyte activation in working brain: energy supplied by minor substrates. Neurochem Int. 2006;48:586–595. doi: 10.1016/j.neuint.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Cruz NF. Imaging brain activation: simple pictures of complex biology. Ann NY Acad Sci. 2008;1147:139–170. doi: 10.1196/annals.1427.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA, Cruz NF. Exchange-mediated dilution of brain lactate specific activity: implications for the origin of glutamate dilution and the contributions of glutamine dilution and other pathways. J Neurochem. 2009;109 (Suppl 1:30–37. doi: 10.1111/j.1471-4159.2009.05859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA, Cruz NF, Ball K, Popp D, Gokden M, Baron S, Wright D, Wenger GR. Behavioral training increases local astrocytic metabolic activity but does not alter outcome of mild transient ischemia. Brain Res. 2003;961:201–212. doi: 10.1016/s0006-8993(02)03945-8. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Hertz L. Glucose and lactate metabolism during brain activation. J Neuroscience Res. 2001;66:824–838. doi: 10.1002/jnr.10079. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Hertz L. Astrocytic contributions to bioenergetics of cerebral ischemia. Glia. 2005;50:362–388. doi: 10.1002/glia.20157. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Liu K, Cruz NF. Local uptake of (14)C-labeled acetate and butyrate in rat brain in vivo during spreading cortical depression. J Neurosci Res. 2001;66:812–820. doi: 10.1002/jnr.10063. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Schmidt KC, Cruz NF. Astrocyte activation in vivo during graded photic stimulation. J Neurochem. 2007b;103:1506–1522. doi: 10.1111/j.1471-4159.2007.04859.x. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Wang RY, Cruz NF. Generalized sensory stimulation of conscious rats increases labeling of oxidative pathways of glucose metabolism when the brain glucose-oxygen uptake ratio rises. J Cereb Blood Flow Metab. 2002;22:1490–1502. doi: 10.1097/01.WCB.0000034363.37277.89. [DOI] [PubMed] [Google Scholar]
- DiNuzzo M, Mangia S, Maraviglia B, Giove F. Changes in glucose uptake rather than lactate shuttle take center stage in subserving neuroenergetics: evidence from mathematical modeling. J Cereb Blood Flow Metab. 2010a;30:586–602. doi: 10.1038/jcbfm.2009.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNuzzo M, Mangia S, Maraviglia B, Giove F. Glycogenolysis in astrocytes supports blood-borne glucose channeling not glycogen-derived lactate shuttling to neurons: evidence from mathematical modeling. J Cereb Blood Flow Metab. 2010b;30:1895–1904. doi: 10.1038/jcbfm.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringen R, Gebhardt R, Hamprecht B. Glycogen in astrocytes: possible function as lactate supply for neighboring cells. Brain Res. 1993;623:208–214. doi: 10.1016/0006-8993(93)91429-v. [DOI] [PubMed] [Google Scholar]
- Duarte JM, Lanz B, Gruetter R. Compartmentalized cerebral metabolism of [1,6-C]glucose determined by in vivo13C NMR spectroscopy at 14.1 T. Front Neuroenergetics. 2011;3:3. doi: 10.3389/fnene.2011.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy TE, Howse DC, Plum F. Cerebral energy metabolism during experimental status epilepticus. J Neurochem. 1975;24:925–934. doi: 10.1111/j.1471-4159.1975.tb03657.x. [DOI] [PubMed] [Google Scholar]
- Erecińska M, Nelson D, Chance B. Depolarization-induced changes in cellular energy production. Proc Natl Acad Sci USA. 1991;88:7600–7604. doi: 10.1073/pnas.88.17.7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecińska M, Nelson D, Silver IA. Metabolic and energetic properties of isolated nerve ending particles (synaptosomes) Biochim Biophys Acta. 1996;1277:13–34. doi: 10.1016/s0005-2728(96)00103-x. [DOI] [PubMed] [Google Scholar]
- Erecińska M, Nelson D, Vanderkooi JM. Effects of NO-generating compounds on synaptosomal energy metabolism. J Neurochem. 1995;65:2699–2705. doi: 10.1046/j.1471-4159.1995.65062699.x. [DOI] [PubMed] [Google Scholar]
- Eriksson G, Peterson A, Iverfeldt K, Walum E. Sodium-dependent glutamate uptake as an activator of oxidative metabolism in primary astrocyte cultures from newborn rat. Glia. 1995;15:152–156. doi: 10.1002/glia.440150207. [DOI] [PubMed] [Google Scholar]
- Erlichman JS, Hewitt A, Damon TL, Hart M, Kurascz J, Li A, Leiter JC. Inhibition of monocarboxylate transporter 2 in the retrotrapezoid nucleus in rats: a test of the astrocyte-neuron lactate-shuttle hypothesis. J Neurosci. 2008;28:4888–4896. doi: 10.1523/JNEUROSCI.5430-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira JM, Burnett AL, Rameau GA. Activity-dependent regulation of surface glucose transporter-3. J Neurosci. 2011;31:1991–1999. doi: 10.1523/JNEUROSCI.1850-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Raichle ME. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci USA. 1986;83:1140–1144. doi: 10.1073/pnas.83.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Raichle ME, Mintun MA, Dence C. Nonoxidative glucose consumption during focal physiologic neural activation. Science. 1988;241:462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- Galeffi F, Foster KA, Sadgrove MP, Beaver CJ, Turner DA. Lactate uptake contributes to the NAD(P)H biphasic response and tissue oxygen response during synaptic stimulation in area CA1 of rat hippocampal slices. J Neurochem. 2007;103:2449–2461. doi: 10.1111/j.1471-4159.2007.04939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi GK, Ball KK, Cruz NF, Dienel GA. Hyperglycaemia and diabetes impair gap junctional communication among astrocytes. ASN Neuro. 2010;2:e00030. doi: 10.1042/AN20090048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi GK, Cruz NF, Ball KK, Dienel GA. Astrocytes are poised for lactate trafficking and release from activated brain and for supply of glucose to neurons. J Neurochem. 2009;111:522–536. doi: 10.1111/j.1471-4159.2009.06333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofalo O, Cox DW, Bachelard HS. Brain levels of NADH and NAD+ under hypoxic and hypoglycaemic conditions in vitro. J Neurochem. 1988;51:172–176. doi: 10.1111/j.1471-4159.1988.tb04851.x. [DOI] [PubMed] [Google Scholar]
- Gleichmann M, Collis LP, Smith PJ, Mattson MP. Simultaneous single neuron recording of O2 consumption, [Ca2+]i and mitochondrial membrane potential in glutamate toxicity. J Neurochem. 2009;109:644–655. doi: 10.1111/j.1471-4159.2009.05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleitz J, Beile A, Khan S, Wilffert B, Tegtmeier F. Anaerobic glycolysis and postanoxic recovery of respiration of rat cortical synaptosomes are reduced by synaptosomal sodium load. Brain Res. 1993;611:286–294. doi: 10.1016/0006-8993(93)90515-o. [DOI] [PubMed] [Google Scholar]
- Gordon GR, Choi HB, Rungta RL, Ellis-Davies GC, MacVicar BA. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456:745–749. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramsbergen JB, Leegsma-Vogt G, Venema K, Noraberg J, Korf J. Quantitative on-line monitoring of hippocampus glucose and lactate metabolism in organotypic cultures using biosensor technology. J Neurochem. 2003;85:399–408. doi: 10.1046/j.1471-4159.2003.01673.x. [DOI] [PubMed] [Google Scholar]
- Halestrap AP. The mitochondrial pyruvate carrier. Kinetics and specificity for substrates and inhibitors. Biochem J. 1975;148:85–96. doi: 10.1042/bj1480085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap AP, Denton RM. Specific inhibition of pyruvate transport in rat liver mitochondria and human erythrocytes by alpha-cyano-4-hydroxycinnamate. Biochem J. 1974;138:313–316. doi: 10.1042/bj1380313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap AP, Denton RM. The specificity and metabolic implications of the inhibition of pyruvate transport in isolated mitochondria and intact tissue preparations by alpha-cyano-4-hydroxycinnamate and related compounds. Biochem J. 1975;148:97–106. doi: 10.1042/bj1480097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves RJ, Planas AM, Cremer JE, Cunningham VJ. Studies on the relationship between cerebral glucose transport and phosphorylation using 2-deoxyglucose. J Cereb Blood Flow Metab. 1986;6:708–716. doi: 10.1038/jcbfm.1986.127. [DOI] [PubMed] [Google Scholar]
- Harvey SA, Booth RF, Clark JB. The effects in vitro of hypoglycaemia and recovery from anoxia on synaptosomal metabolism. Biochem J. 1982;206:433–439. doi: 10.1042/bj2060433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins RA, Miller AL, Nielsen RC, Veech RL. The acute action of ammonia on rat brain metabolism in vivo. Biochem J. 1973;134:1001–1008. doi: 10.1042/bj1341001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins RA, Williamson DH, Krebs HA. Ketone-body utilization by adult and suckling rat brain in vivo. Biochem J. 1971;122:13–18. doi: 10.1042/bj1220013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein TW, Xu W, Kuo L. Dilation of retinal arterioles in response to lactate: role of nitric oxide, guanylyl cyclase, and ATP-sensitive potassium channels. Invest Ophthalmol Vis Sci. 2006;47:693–699. doi: 10.1167/iovs.05-1224. [DOI] [PubMed] [Google Scholar]
- Herrero-Mendez A, Almeida A, Fernández E, Maestre C, Moncada S, Bolaños JP. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nat Cell Biol. 2009;11:747–752. doi: 10.1038/ncb1881. [DOI] [PubMed] [Google Scholar]
- Hertz L. The astrocyte-neuron lactate shuttle: a challenge of a challenge. J Cereb Blood Flow Metab. 2004;24:1241–1248. doi: 10.1097/00004647-200411000-00008. [DOI] [PubMed] [Google Scholar]
- Hertz L.2011Astrocytic energy metabolism and glutamate formation – relevance for (13)C-NMR spectroscopy and importance of cytosolic/mitochondrial trafficking Magn Reson ImagingEpub [ahead of print] [DOI] [PubMed]
- Hertz L, Drejer J, Schousboe A. Energy metabolism in glutamatergic neurons, GABAergic neurons and astrocytes in primary cultures. Neurochem Res. 1988;13:605–610. doi: 10.1007/BF00973275. [DOI] [PubMed] [Google Scholar]
- Hertz L, Swanson RA, Newman GC, Marrif H, Juurlink BH, Peng L. Can experimental conditions explain the discrepancy over glutamate stimulation of aerobic glycolysis. Dev Neurosci. 1998;20:339–347. doi: 10.1159/000017329. [DOI] [PubMed] [Google Scholar]
- Hertz L, Peng L, Dienel GA. Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J Cereb Blood Flow Metab. 2007;27:219–249. doi: 10.1038/sj.jcbfm.9600343. [DOI] [PubMed] [Google Scholar]
- Howse DC, Duffy TE. Control of the redox state of the pyridine nucleotides in the rat cerebral cortex. Effect of electroshock-induced seizures. J Neurochem. 1975;24:935–940. doi: 10.1111/j.1471-4159.1975.tb03658.x. [DOI] [PubMed] [Google Scholar]
- Hu Y, Wilson GS. Rapid changes in local extracellular rat brain glucose observed with an in vivo glucose sensor. J Neurochem. 1997a;68:1745–1752. doi: 10.1046/j.1471-4159.1997.68041745.x. [DOI] [PubMed] [Google Scholar]
- Hu Y, Wilson GS. A temporary local energy pool coupled to neuronal activity: fluctuations of extracellular lactate levels in rat brain monitored with rapid-response enzyme-based sensor. J Neurochem. 1997b;69:1484–1490. doi: 10.1046/j.1471-4159.1997.69041484.x. [DOI] [PubMed] [Google Scholar]
- Hyder F, Patel AB, Gjedde A, Rothman DL, Behar KL, Shulman RG. Neuronal-glial glucose oxidation and glutamatergic-GABAergic function. J Cereb Blood Flow Metab. 2006;26:865–877. doi: 10.1038/sj.jcbfm.9600263. [DOI] [PubMed] [Google Scholar]
- Ide K, Horn A, Secher NH. Cerebral metabolic response to submaximal exercise. J Appl Physiol. 1999;87:1604–1608. doi: 10.1152/jappl.1999.87.5.1604. [DOI] [PubMed] [Google Scholar]
- Ide K, Schmalbruch IK, Quistorff B, Horn A, Secher NH. Lactate, glucose and O2 uptake in human brain during recovery from maximal exercise. J Physiol. 2000;522:159–164. doi: 10.1111/j.1469-7793.2000.t01-2-00159.xm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide T, Steinke J, Cahill GF., Jr Metabolic interactions of glucose, lactate, and beta-hydroxybutyrate in rat brain slices. Am J Physiol. 1969;217:784–792. doi: 10.1152/ajplegacy.1969.217.3.784. [DOI] [PubMed] [Google Scholar]
- Ikemoto A, Bole DG, Ueda T. Glycolysis and glutamate accumulation into synaptic vesicles. Role of glyceraldehyde phosphate dehydrogenase and 3-phosphoglycerate kinase. J Biol Chem. 2003;278:5929–5940. doi: 10.1074/jbc.M211617200. [DOI] [PubMed] [Google Scholar]
- Ivannikov MV, Sugimori M, Llinás RR. Calcium clearance and its energy requirements in cerebellar neurons. Cell Calcium. 2010;47:507–513. doi: 10.1016/j.ceca.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson N, Olson J, Nguyen H, Holtzman D. Respiration in primary cultured cerebellar granule neurons and cerebral cortical neurons. J Neurochem. 1984;42:470–474. doi: 10.1111/j.1471-4159.1984.tb02701.x. [DOI] [PubMed] [Google Scholar]
- Jekabsons MB, Nicholls DG. In situ respiration and bioenergetic status of mitochondria in primary cerebellar granule neuronal cultures exposed continuously to glutamate. J Biol Chem. 2004;279:32989–33000. doi: 10.1074/jbc.M401540200. [DOI] [PubMed] [Google Scholar]
- Jolivet R, Allaman I, Pellerin L, Magistretti PJ, Weber B. Comment on recent modeling studies of astrocyte-neuron metabolic interactions. J Cereb Blood Flow Metab. 2010;30:1982–1986. doi: 10.1038/jcbfm.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce OJ, Farmer MK, Tipton KF, Porter RK. Oxidative phosphorylation by in situ synaptosomal mitochondria from whole brain of young and old rats. J Neurochem. 2003;86:1032–1041. doi: 10.1046/j.1471-4159.2003.01915.x. [DOI] [PubMed] [Google Scholar]
- Kasischke KA, Vishwasrao HD, Fisher PJ, Zipfel WR, Webb WW. Neural activity triggers neuronal oxidative metabolism followed by astrocytic glycolysis. Science. 2004;305:99–103. doi: 10.1126/science.1096485. [DOI] [PubMed] [Google Scholar]
- Kauppinen RA, Nicholls DG. Failure to maintain glycolysis in anoxic nerve terminals. J Neurochem. 1986a;47:1864–1869. doi: 10.1111/j.1471-4159.1986.tb13100.x. [DOI] [PubMed] [Google Scholar]
- Kauppinen RA, Nicholls DG. Synaptosomal bioenergetics. The role of glycolysis, pyruvate oxidation and responses to hypoglycaemia. Eur J Biochem. 1986b;158:159–165. doi: 10.1111/j.1432-1033.1986.tb09733.x. [DOI] [PubMed] [Google Scholar]
- Kauppinen RA, Taipale HT, Komulainen H. Interrelationships between glucose metabolism, energy state, and the cytosolic free calcium concentration in cortical synaptosomes from the guinea pig. J Neurochem. 1989;53:766–771. doi: 10.1111/j.1471-4159.1989.tb11771.x. [DOI] [PubMed] [Google Scholar]
- Kemppainen J, Aalto S, Fujimoto T, Kalliokoski KK, Långsjö J, Oikonen V, Rinne J, Nuutila P, Knuuti J. High intensity exercise decreases global brain glucose uptake in humans. J Physiol. 2005;568:323–332. doi: 10.1113/jphysiol.2005.091355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korf J, de Boer J. Lactography as an approach to monitor glucose metabolism on-line in brain and muscle. Int J Biochem. 1990;22:1371–1378. doi: 10.1016/0020-711x(90)90225-r. [DOI] [PubMed] [Google Scholar]
- Krebs HA. The Pasteur effect and the relations between respiration and fermentation. Essays Biochem. 1972;8:1–34. [PubMed] [Google Scholar]
- Krivanek J. Changes of brain glycogen in the spreading EEG-depression of Leao. J Neurochem. 1958;2:337–343. doi: 10.1111/j.1471-4159.1958.tb12383.x. [DOI] [PubMed] [Google Scholar]
- Krzanowski J, Matschinsky FM. Regulation of phosphofructokinase by phosphocreatine and phosphorylated glycolytic intermediates. Biochem Biophys Res Commun. 1969;34:816–823. doi: 10.1016/0006-291x(69)90253-8. [DOI] [PubMed] [Google Scholar]
- Ksiezak HJ, Gibson GE. Acetylcholine synthesis and CO2 production from variously labeled glucose in rat brain slices and synaptosomes. J Neurochem. 1981a;37:88–94. doi: 10.1111/j.1471-4159.1981.tb05294.x. [DOI] [PubMed] [Google Scholar]
- Ksiezak HJ, Gibson GE. Oxygen dependence of glucose and acetylcholine metabolism in slices and synaptosomes from rat brain. J Neurochem. 1981b;37:305–314. doi: 10.1111/j.1471-4159.1981.tb00456.x. [DOI] [PubMed] [Google Scholar]
- LaManna JC, Salem N, Puchowicz M, Erokwu B, Koppaka S, Flask C, Lee Z. Ketones suppress brain glucose consumption. Adv Exp Med Biol. 2009;645:301–306. doi: 10.1007/978-0-387-85998-9_45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavialle M, Aumann G, Anlauf E, Pröls F, Arpin M, Derouiche A. Structural plasticity of perisynaptic astrocyte processes involves ezrin and metabotropic glutamate receptors. Proc Natl Acad Sci USA. 2011;108:12915–12919. doi: 10.1073/pnas.1100957108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lear J. Glycolysis: link between PET and proton MR spectroscopic studies of the brain. Radiology. 1990;174:328–330. doi: 10.1148/radiology.174.2.2153309. [DOI] [PubMed] [Google Scholar]
- Lear J, Ackermann RF. Why the deoxyglucose method has proven so useful in cerebral activation studies: the unappreciated prevalence of stimulation-induced glycolysis. J Cereb Blood Flow Metab. 1989;9:911–913. doi: 10.1038/jcbfm.1989.128. [DOI] [PubMed] [Google Scholar]
- Lee DH, Chung MY, Lee JU, Kang DG, Paek YW. Changes of glucose transporters in the cerebral adaptation to hypoglycemia. Diabetes Res Clin Pract. 2000;47:15–23. doi: 10.1016/s0168-8227(99)00107-2. [DOI] [PubMed] [Google Scholar]
- Leino RL, Gerhart DZ, van Bueren AM, McCall AL, Drewes LR. Ultrastructural localization of GLUT 1 and GLUT 3 glucose transporters in rat brain. J Neurosci Res. 1997;49:617–626. doi: 10.1002/(SICI)1097-4547(19970901)49:5<617::AID-JNR12>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Liao SL, Chen CJ. L-glutamate decreases glucose utilization by rat cortical astrocytes. Neurosci Lett. 2003;348:81–84. doi: 10.1016/s0304-3940(03)00721-3. [DOI] [PubMed] [Google Scholar]
- Linde R, Schmalbruch IK, Paulson OB, Madsen PL. The Kety-Schmidt technique for repeated measurements of global cerebral blood flow and metabolism in the conscious rat. Acta Physiol Scand. 1999;165:395–401. doi: 10.1046/j.1365-201x.1999.00522.x. [DOI] [PubMed] [Google Scholar]
- Loaiza A, Porras OH, Barros LF. Glutamate triggers rapid glucose transport stimulation in astrocytes as evidenced by real-time confocal microscopy. J Neurosci. 2003;23:7337–7342. doi: 10.1523/JNEUROSCI.23-19-07337.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovatt D, Sonnewald U, Waagepetersen HS, Schousboe A, He W, Lin JH, Han X, Takano T, Wang S, Sim FJ, Goldman SA, Nedergaard M. The transcriptome and metabolic gene signature of protoplasmic astrocytes in the adult murine cortex. J Neurosci. 2007;27:12255–12266. doi: 10.1523/JNEUROSCI.3404-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Passonneau JV. Kinetic evidence for multiple binding sites on phosphofructokinase. J Biol Chem. 1966;241:2268–2279. [PubMed] [Google Scholar]
- Lowry OH, Passonneau JV, Hasselberger FX, Schulz DW. Effect of ischemia on known substrates and cofactors of the glycolytic pathway in brain. J Biol Chem. 1964;239:18–30. [PubMed] [Google Scholar]
- Madsen PL, Cruz NF, Sokoloff L, Dienel GA. Cerebral oxygen/glucose ratio is low during sensory stimulation and rises above normal during recovery: excess glucose consumption during stimulation is not accounted for by lactate efflux from or accumulation in brain tissue. J Cereb Blood Flow Metab. 1999;19:393–400. doi: 10.1097/00004647-199904000-00005. [DOI] [PubMed] [Google Scholar]
- Madsen PL, Hasselbalch SG, Hagemann LP, Olsen KS, Bülow J, Holm S, Wildschiødtz G, Paulson OB, Lassen NA. Persistent resetting of the cerebral oxygen/glucose uptake ratio by brain activation: evidence obtained with the Kety-Schmidt technique. J Cereb Blood Flow Metab. 1995;15:485–491. doi: 10.1038/jcbfm.1995.60. [DOI] [PubMed] [Google Scholar]
- Madsen PL, Linde R, Hasselbalch SG, Paulson OB, Lassen NA. Activation-induced resetting of cerebral oxygen and glucose uptake in the rat. J Cereb Blood Flow Metab. 1998;18:742–748. doi: 10.1097/00004647-199807000-00005. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ. Role of glutamate in neuron-glia metabolic coupling. Am J Clin Nutr. 2009;90:875S–880S. doi: 10.3945/ajcn.2009.27462CC. [DOI] [PubMed] [Google Scholar]
- Maher F, Simpson IA. Modulation of expression of glucose transporters GLUT3 and GLUT1 by potassium and N-methyl-D-aspartate in cultured cerebellar granule neurons. Mol Cell Neurosci. 1994;5:369–375. doi: 10.1006/mcne.1994.1044. [DOI] [PubMed] [Google Scholar]
- Malthankar-Phatak GH, Patel AB, Xia Y, Hong S, Chowdhury GM, Behar KL, Orina IA, Lai JC. Effects of continuous hypoxia on energy metabolism in cultured cerebro-cortical neurons. Brain Res. 2008;1229:147–154. doi: 10.1016/j.brainres.2008.06.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangia S, DiNuzzo M, Giove F, Carruthers A, Simpson IA, Vannucci SJ. Response to ‘comment on recent modeling studies of astrocyte-neuron metabolic interactions': much ado about nothing. J Cereb Blood Flow Metab. 2011;31:1346–1353. doi: 10.1038/jcbfm.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangia S, Giove F, Tkác I, Logothetis NK, Henry PG, Olman CA, Maraviglia B, Di Salle F, Uğurbil K. Metabolic and hemodynamic events after changes in neuronal activity: current hypotheses, theoretical predictions and in vivo NMR experimental findings. J Cereb Blood Flow Metab. 2009a;29:441–463. doi: 10.1038/jcbfm.2008.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangia S, Simpson IA, Vannucci SJ, Carruthers A. The in vivo neuron-to-astrocyte lactate shuttle in human brain: evidence from modeling of measured lactate levels during visual stimulation. J Neurochem. 2009b;109 (Suppl 1:55–62. doi: 10.1111/j.1471-4159.2009.06003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangia S, Tkác I, Logothetis NK, Gruetter R, Van de Moortele PF, Uğurbil K. Dynamics of lactate concentration and blood oxygen level-dependent effect in the human visual cortex during repeated identical stimuli. J Neurosci Res. 2007;85:3340–3346. doi: 10.1002/jnr.21371. [DOI] [PubMed] [Google Scholar]
- Mason GF, Rothman DL. Basic principles of metabolic modeling of NMR (13)C isotopic turnover to determine rates of brain metabolism in vivo. Metab Eng. 2004;6:75–84. doi: 10.1016/j.ymben.2003.10.003. [DOI] [PubMed] [Google Scholar]
- McKenna MC, Hopkins IB, Carey A. Alpha-cyano-4-hydroxycinnamate decreases both glucose and lactate metabolism in neurons and astrocytes: implications for lactate as an energy substrate for neurons. J Neurosci Res. 2001;66:747–754. doi: 10.1002/jnr.10084. [DOI] [PubMed] [Google Scholar]
- McKenna MC, Sonnewald U, Huang X, Stevenson J, Zielke HR. Exogenous glutamate concentration regulates the metabolic fate of glutamate in astrocytes. J Neurochem. 1996;66:386–393. doi: 10.1046/j.1471-4159.1996.66010386.x. [DOI] [PubMed] [Google Scholar]
- Medina MA, Deam AP, Stavinoha WB.1980Inactivation of brain tissue by microwave irradiationIn: Cerebral metabolism and neural function (Passonneau JV, Hawkins RA, Lust WD, Welsh FA, eds),Baltimore: Williams & Wilkins; 56–69. [Google Scholar]
- Meldrum BS, Nilsson B. Cerebral blood flow and metabolic rate early and late in prolonged epileptic seizures induced in rats by bicuculline. Brain. 1976;99:523–542. doi: 10.1093/brain/99.3.523. [DOI] [PubMed] [Google Scholar]
- Miller AL, Hawkins RA, Veech RL. The mitochondrial redox state of rat brain. J Neurochem. 1973;20:1393–1400. doi: 10.1111/j.1471-4159.1973.tb00251.x. [DOI] [PubMed] [Google Scholar]
- Minervini M, Atlante A, Gagliardi S, Ciotti MT, Marra E, Calissano P. Glutamate stimulates 2-deoxyglucose uptake in rat cerebellar granule cells. Brain Res. 1997;768:57–62. doi: 10.1016/s0006-8993(97)00547-7. [DOI] [PubMed] [Google Scholar]
- Nicholls DG. Bioenergetics and transmitter release in the isolated nerve terminal. Neurochem Res. 2003;28:1433–1441. doi: 10.1023/a:1025653805029. [DOI] [PubMed] [Google Scholar]
- Nicholls DG. Spare respiratory capacity, oxidative stress and excitotoxicity. Biochem Soc Trans. 2009;37:1385–1388. doi: 10.1042/BST0371385. [DOI] [PubMed] [Google Scholar]
- Nicholls DG. Mitochondrial ion circuits. Essays Biochem. 2010;47:25–35. doi: 10.1042/bse0470025. [DOI] [PubMed] [Google Scholar]
- Occhipinti R, Somersalo E, Calvetti D. Energetics of inhibition: insights with a computational model of the human GABAergic neuron-astrocyte cellular complex. J Cereb Blood Flow Metab. 2010;30:1834–1846. doi: 10.1038/jcbfm.2010.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Lipton P.2007Glucose, oxidative energy metabolism and neural function in brain slices–glycolysis plays a key role in neural activityIn: Brain energetics. Integration of molecular and cellular processes (Gibson GE, Dienel GA, eds),Berlin: Springer-Verlag; 17–39. [Google Scholar]
- O'Neal RM, Koeppe RE. Precursors in vivo of glutamate, aspartate and their derivatives of rat brain. J Neurochem. 1966;13:835–847. doi: 10.1111/j.1471-4159.1966.tb05879.x. [DOI] [PubMed] [Google Scholar]
- Orzi F, Lucignani G, Dow-Edwards D, Namba H, Nehlig A, Patlak CS, Pettigrew K, Schuier F, Sokoloff L. Local cerebral glucose utilization in controlled graded levels of hyperglycemia in the conscious rat. J Cereb Blood Flow Metab. 1988;8:346–356. doi: 10.1038/jcbfm.1988.70. [DOI] [PubMed] [Google Scholar]
- Owen OE, Morgan AP, Kemp HG, Sullivan JM, Herrera MG, Cahill GF., Jr Brain metabolism during fasting. J Clin Invest. 1967;46:1589–1595. doi: 10.1172/JCI105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öz G, Berkich DA, Henry PG, Xu Y, LaNoue K, Hutson SM, Gruetter R. Neuroglial metabolism in the awake rat brain: CO2 fixation increases with brain activity. J Neurosci. 2004;24:11273–11279. doi: 10.1523/JNEUROSCI.3564-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo B, Contreras L, Serrano A, Ramos M, Kobayashi K, Iijima M, Saheki T, Satrústegui J. Essential role of aralar in the transduction of small Ca2+ signals to neuronal mitochondria. J Biol Chem. 2006;281:1039–1047. doi: 10.1074/jbc.M507270200. [DOI] [PubMed] [Google Scholar]
- Pardo B, Rodrigues TB, Contreras L, Garzón M, Llorente-Folch I, Kobayashi K, Saheki T, Cerdan S, Satrústegui J. Brain glutamine synthesis requires neuronal-born aspartate as amino donor for glial glutamate formation. J Cereb Blood Flow Metab. 2011;31:90–101. doi: 10.1038/jcbfm.2010.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passonneau JV, Lowry OH. P-Fructokinase and the control of the citric acid cycle. Biochem Biophys Res Commun. 1963;13:372–379. [Google Scholar]
- Patel JR, Brewer GJ. Age-related changes in neuronal glucose uptake in response to glutamate and beta-amyloid. J Neurosci Res. 2003;72:527–536. doi: 10.1002/jnr.10602. [DOI] [PubMed] [Google Scholar]
- Pellerin L. Brain energetics (thought needs food) Curr Opin Clin Nutr Metab Care. 2008;11:701–705. doi: 10.1097/MCO.0b013e328312c368. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Bouzier-Sore AK, Aubert A, Serre S, Merle M, Costalat R, Magistretti PJ. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia. 2007;55:1251–1262. doi: 10.1002/glia.20528. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L, Hertz L. Amobarbital inhibits K(+)-stimulated glucose oxidation in cerebellar granule neurons by two mechanisms. Eur J Pharmacol. 2002;446:53–61. doi: 10.1016/s0014-2999(02)01794-6. [DOI] [PubMed] [Google Scholar]
- Peng L, Swanson RA, Hertz L. Effects of L-glutamate, D-aspartate, and monensin on glycolytic and oxidative glucose metabolism in mouse astrocyte cultures: further evidence that glutamate uptake is metabolically driven by oxidative metabolism. Neurochem Int. 2001;38:437–443. doi: 10.1016/s0197-0186(00)00104-2. [DOI] [PubMed] [Google Scholar]
- Peng L, Zhang X, Hertz L. High extracellular potassium concentrations stimulate oxidative metabolism in a glutamatergic neuronal culture and glycolysis in cultured astrocytes but have no stimulatory effect in a GABAergic neuronal culture. Brain Res. 1994;663:168–172. doi: 10.1016/0006-8993(94)90475-8. [DOI] [PubMed] [Google Scholar]
- Pieper AA, Blackshaw S, Clements EE, Brat DJ, Krug DK, White AJ, Pinto-Garcia P, Favit A, Conover JR, Snyder SH, Verma A. Poly(ADP-ribosyl)ation basally activated by DNA strand breaks reflects glutamate-nitric oxide neurotransmission. Proc Natl Acad Sci USA. 2000;97:1845–1850. doi: 10.1073/pnas.97.4.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre K, Chatton JY, Parent A, Repond C, Gardoni F, Di Luca M, Pellerin L. Linking supply to demand: the neuronal monocarboxylate transporter MCT2 and the alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionic acid receptor GluR2/3 subunit are associated in a common trafficking process. Eur J Neurosci. 2009;29:1951–1963. doi: 10.1111/j.1460-9568.2009.06756.x. [DOI] [PubMed] [Google Scholar]
- Porras OH, Loaiza A, Barros LF. Glutamate mediates acute glucose transport inhibition in hippocampal neurons. J Neurosci. 2004;24:9669–9673. doi: 10.1523/JNEUROSCI.1882-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prebil M, Vardjan N, Jensen J, Zorec R, Kreft M. Dynamic monitoring of cytosolic glucose in single astrocytes. Glia. 2011;59:903–913. doi: 10.1002/glia.21161. [DOI] [PubMed] [Google Scholar]
- Qu H, Eloqayli H, Unsgard G, Sonnewald U. Glutamate decreases pyruvate carboxylase activity and spares glucose as energy substrate in cultured cerebellar astrocytes. J Neurosci Res. 2001;66:1127–1132. doi: 10.1002/jnr.10032. [DOI] [PubMed] [Google Scholar]
- Quistorff B, Grunnet N. High brain lactate is not caused by a shift in the lactate dehydrogenase A/B ratio. Proc Natl Acad Sci USA. 2011a;108:E21. doi: 10.1073/pnas.1017750108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quistorff B, Grunnet N. The isoenzyme pattern of LDH does not play a physiological role; except perhaps during fast transitions in energy metabolism. Aging (Albany NY) 2011b;3:457–460. doi: 10.18632/aging.100329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quistorff B, Secher NH, Van Lieshout JJ. Lactate fuels the human brain during exercise. FASEB J. 2008;22:3443–3449. doi: 10.1096/fj.08-106104. [DOI] [PubMed] [Google Scholar]
- Reagan LP, Magariños AM, Lucas LR, van Bueren A, McCall AL, McEwen BS. Regulation of GLUT-3 glucose transporter in the hippocampus of diabetic rats subjected to stress. Am J Physiol. 1999;276:E879–E886. doi: 10.1152/ajpendo.1999.276.5.E879. [DOI] [PubMed] [Google Scholar]
- Reichenbach A, Derouiche A, Kirchhoff F. Morphology and dynamics of perisynaptic glia. Brain Res Rev. 2010;63:11–25. doi: 10.1016/j.brainresrev.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Robinson AM, Williamson DH. Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol Rev. 1980;60:143–187. doi: 10.1152/physrev.1980.60.1.143. [DOI] [PubMed] [Google Scholar]
- Rodrigues TB, López-Larrubia P, Cerdán S. Redox dependence and compartmentation of [13C]pyruvate in the brain of deuterated rats bearing implanted C6 gliomas. J Neurochem. 2009;109 (Suppl 1:237–245. doi: 10.1111/j.1471-4159.2009.05935.x. [DOI] [PubMed] [Google Scholar]
- Rolleston FS, Newsholme EA. Effects of fatty acids, ketone bodies, lactate and pyruvate on glucose utilization by guinea-pig cerebral cortex slices. Biochem J. 1967;104:519–523. doi: 10.1042/bj1040519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderman NB, Ross PS, Berger M, Goodman MN. Regulation of glucose and ketone-body metabolism in brain of anaesthetized rats. Biochem J. 1974;138:1–10. doi: 10.1042/bj1380001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer WT, Olson MS. The regulation of pyruvate oxidation during membrane depolarization of rat brain synaptosomes. Biochem J. 1980;192:741–751. doi: 10.1042/bj1920741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalbruch IK, Linde R, Paulson OB, Madsen PL. Activation-induced resetting of cerebral metabolism and flow is abolished by beta-adrenergic blockade with propranolol. Stroke. 2002;33:251–255. doi: 10.1161/hs0102.101233. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Drejer J, Hansen GH, Meier E. Cultured neurons as model systems for biochemical and pharmacological studies on receptors for neurotransmitter amino acids. Dev Neurosci. 1985;7:252–262. doi: 10.1159/000112294. [DOI] [PubMed] [Google Scholar]
- Schurr A. Lactate: the ultimate cerebral oxidative energy substrate. J Cereb Blood Flow Metab. 2006;26:142–152. doi: 10.1038/sj.jcbfm.9600174. [DOI] [PubMed] [Google Scholar]
- Schurr A, West CA, Rigor BM. Lactate-supported synaptic function in the rat hippocampal slice preparation. Science. 1988;240:1326–1328. doi: 10.1126/science.3375817. [DOI] [PubMed] [Google Scholar]
- Scott ID, Nicholls DG. Energy transduction in intact synaptosomes. Influence of plasma-membrane depolarization on the respiration and membrane potential of internal mitochondria determined in situ. Biochem J. 1980;186:21–33. doi: 10.1042/bj1860021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher PK. Chronic hypoxia in neuronal cell culture: metabolic consequences. Brain Dev. 1990;12:293–300. doi: 10.1016/s0387-7604(12)80309-3. [DOI] [PubMed] [Google Scholar]
- Shestov AA, Emir UE, Kumar A, Henry PG, Seaquist ER, Oz G. Simultaneous measurement of glucose transport and utilization in the human brain. Am J Physiol Endocrinol Metab. 2011;301:E1040–E1049. doi: 10.1152/ajpendo.00110.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth CW. Use of NAD(P)H and flavoprotein autofluorescence transients to probe neuron and astrocyte responses to synaptic activation. Neurochem Int. 2010;56:379–386. doi: 10.1016/j.neuint.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth CW, Brennan AM, Connor JA. NAD(P)H fluorescence imaging of postsynaptic neuronal activation in murine hippocampal slices. J Neurosci. 2003;23:3196–3208. doi: 10.1523/JNEUROSCI.23-08-03196.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siesjö BK. Brain Energy Metabolism. Chichester: John Wiley & Sons; 1978. [Google Scholar]
- Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab. 2007;27:1766–1791. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D, Pernet A, Hallett WA, Bingham E, Marsden PK, Amiel SA. Lactate: a preferred fuel for human brain metabolism in vivo. J Cereb Blood Flow Metab. 2003;23:658–664. doi: 10.1097/01.WCB.0000063991.19746.11. [DOI] [PubMed] [Google Scholar]
- Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Sonnewald U, Müller TB, Westergaard N, Unsgård G, Petersen SB, Schousboe A. NMR spectroscopic study of cell cultures of astrocytes and neurons exposed to hypoxia: compartmentation of astrocyte metabolism. Neurochem Int. 1994;24:473–483. doi: 10.1016/0197-0186(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Soucek T, Cumming R, Dargusch R, Maher P, Schubert D. The regulation of glucose metabolism by HIF-1 mediates a neuroprotective response to amyloid beta peptide. Neuron. 2003;39:43–56. doi: 10.1016/s0896-6273(03)00367-2. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Benington JH. Astrocyte glucose metabolism under normal and pathological conditions in vitro. Dev Neurosci. 1996;18:515–521. doi: 10.1159/000111448. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Yu AC, Chan PH, Sharp FR. Glutamate increases glycogen content and reduces glucose utilization in primary astrocyte culture. J Neurochem. 1990;54:490–496. doi: 10.1111/j.1471-4159.1990.tb01898.x. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Driscoll BF, Law MJ, Sokoloff L. Role of sodium and potassium ions in regulation of glucose metabolism in cultured astroglia. Proc Natl Acad Sci USA. 1995;92:4616–4620. doi: 10.1073/pnas.92.10.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrian DM, Johnston D, Claiborne BJ, Ansah-Yiadom R, Strittmatter WJ, Rea MA. Glutamate and dynorphin release from a subcellular fraction enriched in hippocampal mossy fiber synaptosomes. Brain Res Bull. 1988;21:343–351. doi: 10.1016/0361-9230(88)90146-3. [DOI] [PubMed] [Google Scholar]
- Vaishnavi SN, Vlassenko AG, Rundle MM, Snyder AZ, Mintun MA, Raichle ME. Regional aerobic glycolysis in the human brain. Proc Natl Acad Sci USA. 2010;107:17757–17762. doi: 10.1073/pnas.1010459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hall G, Strømstad M, Rasmussen P, Jans O, Zaar M, Gam C, Quistorff B, Secher NH, Nielsen HB. Blood lactate is an important energy source for the human brain. J Cereb Blood Flow Metab. 2009;29:1121–1129. doi: 10.1038/jcbfm.2009.35. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Maher F, Koehler E, Simpson IA. Altered expression of GLUT-1 and GLUT-3 glucose transporters in neurohypophysis of water-deprived or diabetic rats. Am J Physiol. 1994;267:E605–E611. doi: 10.1152/ajpendo.1994.267.4.E605. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Simpson IA. Developmental switch in brain nutrient transporter expression in the rat. Am J Physio Endocrinol Metab. 2003;285:E1127–E1134. doi: 10.1152/ajpendo.00187.2003. [DOI] [PubMed] [Google Scholar]
- Veech RL. The metabolism of lactate. NMR Biomed. 1991;4:53–58. doi: 10.1002/nbm.1940040204. [DOI] [PubMed] [Google Scholar]
- Veech RL, Harris RL, Veloso D, Veech EH. Freeze-blowing: a new technique for the study of brain in vivo. J Neurochem. 1973;20:183–188. doi: 10.1111/j.1471-4159.1973.tb12115.x. [DOI] [PubMed] [Google Scholar]
- Vissing J, Andersen M, Diemer NH. Exercise-induced changes in local cerebral glucose utilization in the rat. J Cereb Blood Flow Metab. 1996;16:729–736. doi: 10.1097/00004647-199607000-00025. [DOI] [PubMed] [Google Scholar]
- Waagepetersen HS, Sonnewald U, Larsson OM, Schousboe A. Compartmentation of TCA cycle metabolism in cultured neocortical neurons revealed by 13C MR spectroscopy. Neurochem Int. 2000;36:349–358. doi: 10.1016/s0197-0186(99)00143-6. [DOI] [PubMed] [Google Scholar]
- Walz W, Mukerji S. Lactate release from cultured astrocytes and neurons: a comparison. Glia. 1988;1:366–370. doi: 10.1002/glia.440010603. [DOI] [PubMed] [Google Scholar]
- Wang L, Bill A. Effects of constant and flickering light on retinal metabolism in rabbits. Acta Ophthalmol Scand. 1997;75:227–231. doi: 10.1111/j.1600-0420.1997.tb00761.x. [DOI] [PubMed] [Google Scholar]
- Wang L, Kondo M, Bill A. Glucose metabolism in cat outer retina. Effects of light and hyperoxia. Invest Ophthalmol Vis Sci. 1997a;38:48–55. [PubMed] [Google Scholar]
- Wang L, Tornquist P, Bill A. Glucose metabolism of the inner retina in pigs in darkness and light. Acta Physiol Scand. 1997b;160:71–74. doi: 10.1046/j.1365-201X.1997.00131.x. [DOI] [PubMed] [Google Scholar]
- Wang L, Tornquist P, Bill A. Glucose metabolism in pig outer retina in light and darkness. Acta Physiol Scand. 1997c;160:75–81. doi: 10.1046/j.1365-201X.1997.00030.x. [DOI] [PubMed] [Google Scholar]
- Ward MW, Huber HJ, Weisová P, Düssmann H, Nicholls DG, Prehn JH. Mitochondrial and plasma membrane potential of cultured cerebellar neurons during glutamate-induced necrosis, apoptosis, and tolerance. J Neurosci. 2007;27:8238–8249. doi: 10.1523/JNEUROSCI.1984-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisová P, Concannon CG, Devocelle M, Prehn JH, Ward MW. Regulation of glucose transporter 3 surface expression by the AMP-activated protein kinase mediates tolerance to glutamate excitation in neurons. J Neurosci. 2009;29:2997–3008. doi: 10.1523/JNEUROSCI.0354-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh FA.1980In situ freezing of cat brainIn: Cerebral metabolism and neural function (Passonneau JV, Hawkins RA, Lust WD, Welsh FA, eds),Baltimore: Williams & Wilkins; 28–33. [Google Scholar]
- White EJ, Juchniewicz HJ, Clark JB. Effects of lactic acidosis on the function of cerebral cortical synaptosomes. J Neurochem. 1989;52:154–161. doi: 10.1111/j.1471-4159.1989.tb10910.x. [DOI] [PubMed] [Google Scholar]
- Wree A, Erselius R, Tønder N, Beck T. Time course of hippocampal glucose utilization and persistence of parvalbumin immunoreactive neurons after ibotenic acid-induced lesions of the rat dentate area. J Cereb Blood Flow Metab. 1993;13:998–1005. doi: 10.1038/jcbfm.1993.125. [DOI] [PubMed] [Google Scholar]
- Wu K, Aoki C, Elste A, Rogalski-Wilk AA, Siekevitz P. The synthesis of ATP by glycolytic enzymes in the postsynaptic density and the effect of endogenously generated nitric oxide. Proc Natl Acad Sci USA. 1997;94:13273–13278. doi: 10.1073/pnas.94.24.13273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss MT, Jolivet R, Buck A, Magistretti PJ, Weber B. In vivo evidence for lactate as a neuronal energy source. J Neurosci. 2011;31:7477–7485. doi: 10.1523/JNEUROSCI.0415-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss MT, Weber B, Treyer V, Heer S, Pellerin L, Magistretti PJ, Buck A. Stimulation-induced increases of astrocytic oxidative metabolism in rats and humans investigated with 1-11C-acetate. J Cereb Blood Flow Metab. 2009;29:44–56. doi: 10.1038/jcbfm.2008.86. [DOI] [PubMed] [Google Scholar]
- Yadava N, Nicholls DG. Spare respiratory capacity rather than oxidative stress regulates glutamate excitotoxicity after partial respiratory inhibition of mitochondrial complex I with rotenone. J Neurosci. 2007;27:7310–7317. doi: 10.1523/JNEUROSCI.0212-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanishi S, Katsumura K, Kobayashi T, Puro DG. Extracellular lactate as a dynamic vasoactive signal in the rat retinal microvasculature. Am J Physiol Heart Circ Physiol. 2006;290:H925–H934. doi: 10.1152/ajpheart.01012.2005. [DOI] [PubMed] [Google Scholar]
- Yu AC, Hertz E, Hertz L. Alterations in uptake and release rates for GABA, glutamate, and glutamine during biochemical maturation of highly purified cultures of cerebral cortical neurons, a GABAergic preparation. J Neurochem. 1984;42:951–960. doi: 10.1111/j.1471-4159.1984.tb12696.x. [DOI] [PubMed] [Google Scholar]
- Yu S, Zhao T, Guo M, Fang H, Ma J, Ding A, Wang F, Chan P, Fan M. Hypoxic preconditioning up-regulates glucose transport activity and glucose transporter (GLUT1 and GLUT3) gene expression after acute anoxic exposure in the cultured rat hippocampal neurons and astrocytes. Brain Res. 2008;1211:22–29. doi: 10.1016/j.brainres.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Zeng J, Yang GY, Ying W, Kelly M, Hirai K, James TL, Swanson RA, Litt L. Pyruvate improves recovery after PARP-1-associated energy failure induced by oxidative stress in neonatal rat cerebrocortical slices. J Cereb Blood Flow Metab. 2007;27:304–315. doi: 10.1038/sj.jcbfm.9600335. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Fung C, Shin D, Shin BC, Thamotharan S, Sankar R, Ehninger D, Silva A, Devaskar SU. Neuronal glucose transporter isoform 3 deficient mice demonstrate features of autism spectrum disorders. Mol Psychiatry. 2010;15:286–299. doi: 10.1038/mp.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielke HR, Zielke CL, Baab PJ, Tildon JT. Effect of fluorocitrate on cerebral oxidation of lactate and glucose in freely moving rats. J Neurochem. 2007;101:9–16. doi: 10.1111/j.1471-4159.2006.04335.x. [DOI] [PubMed] [Google Scholar]
- Zielke HR, Zielke CL, Baab PJ. Direct measurement of oxidative metabolism in the living brain by microdialysis: a review. J Neurochem. 2009;109 (Suppl 1:24–29. doi: 10.1111/j.1471-4159.2009.05941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]