Abstract
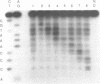
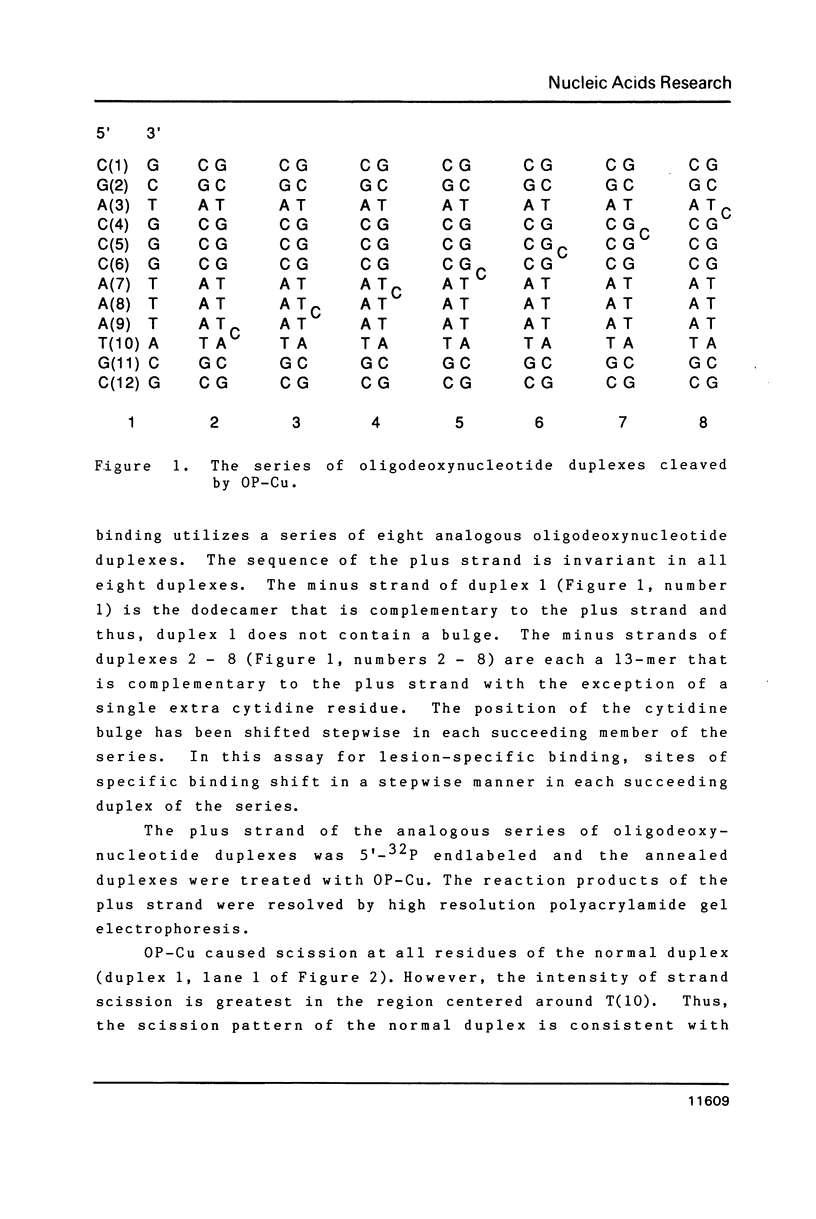
DNA intercalators are found to recognize a DNA lesion as a high affinity receptor site. This lesion-specific binding is observed when one strand of a DNA double helix contains an extra, unpaired nucleotide. Our assay for binding controls for the effects of sequence with a series of oligodeoxynucleotide duplexes which are identical except for the location of the lesion, an extra cytidine. Scission of the series of oligodeoxynucleotides by the cuprous complex of ortho-phenanthroline (OP-Cu) indicates that OP-Cu binds at the lesion-specific stable intercalation site, suggesting that OP-Cu intercalates into DNA. The dispersion of OP-Cu scission sites over three residues is consistent with scission via a diffusible intermediate. The location of the scission sites, directly on the 3' side of the lesion, is consistent with minor groove binding in B DNA.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Whitfield H. J., Jr Frameshift mutagenesis in Salmonella. Cold Spring Harb Symp Quant Biol. 1966;31:221–225. doi: 10.1101/sqb.1966.031.01.030. [DOI] [PubMed] [Google Scholar]
- BROCKMAN H. E., GOBEN W. MUTAGENICITY OF A MONOFUNCTIONAL ALKYLATING AGENT DERIVATIVE OF ACRIDINE IN NEUROSPORA. Science. 1965 Feb 12;147(3659):750–751. doi: 10.1126/science.147.3659.750. [DOI] [PubMed] [Google Scholar]
- Drew H. R., Travers A. A. DNA structural variations in the E. coli tyrT promoter. Cell. 1984 Jun;37(2):491–502. doi: 10.1016/0092-8674(84)90379-9. [DOI] [PubMed] [Google Scholar]
- Fresco J. R., Alberts B. M. THE ACCOMMODATION OF NONCOMPLEMENTARY BASES IN HELICAL POLYRIBONUCLEOTIDES AND DEOXYRIBONUCLEIC ACIDS. Proc Natl Acad Sci U S A. 1960 Mar;46(3):311–321. doi: 10.1073/pnas.46.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzberg R. P., Dervan P. B. Cleavage of DNA with methidiumpropyl-EDTA-iron(II): reaction conditions and product analyses. Biochemistry. 1984 Aug 14;23(17):3934–3945. doi: 10.1021/bi00312a022. [DOI] [PubMed] [Google Scholar]
- Kappen L. S., Goldberg I. H. Activation of neocarzinostatin chromophore and formation of nascent DNA damage do not require molecular oxygen. Nucleic Acids Res. 1985 Mar 11;13(5):1637–1648. doi: 10.1093/nar/13.5.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara M., Yoon C., Goyne T., Thederahn T., Sigman D. S. Nuclease activity of 1,10-phenanthroline-copper ion: reaction with CGCGAATTCGCG and its complexes with netropsin and EcoRI. Biochemistry. 1986 Nov 18;25(23):7401–7408. doi: 10.1021/bi00371a023. [DOI] [PubMed] [Google Scholar]
- Lee C. H., Tinoco I., Jr Mutagen--oligonucleotide complexes with a bulged base as models for frameshift mutation. Nature. 1978 Aug 10;274(5671):609–610. doi: 10.1038/274609a0. [DOI] [PubMed] [Google Scholar]
- Marshall L. E., Graham D. R., Reich K. A., Sigman D. S. Cleavage of deoxyribonucleic acid by the 1,10-phenanthroline-cuprous complex. Hydrogen peroxide requirement and primary and secondary structure specificity. Biochemistry. 1981 Jan 20;20(2):244–250. doi: 10.1021/bi00505a003. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Moser H. E., Dervan P. B. Sequence-specific cleavage of double helical DNA by triple helix formation. Science. 1987 Oct 30;238(4827):645–650. doi: 10.1126/science.3118463. [DOI] [PubMed] [Google Scholar]
- Nelson J. W., Tinoco I., Jr Ethidium ion binds more strongly to a DNA double helix with a bulged cytosine than to a regular double helix. Biochemistry. 1985 Nov 5;24(23):6416–6421. doi: 10.1021/bi00344a016. [DOI] [PubMed] [Google Scholar]
- Schultz P. G., Dervan P. B. Sequence-specific double-strand cleavage of DNA by penta-N-methylpyrrolecarboxamide-EDTA X Fe(II). Proc Natl Acad Sci U S A. 1983 Nov;80(22):6834–6837. doi: 10.1073/pnas.80.22.6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigman D. S., Graham D. R., D'Aurora V., Stern A. M. Oxygen-dependent cleavage of DNA by the 1,10-phenanthroline . cuprous complex. Inhibition of Escherichia coli DNA polymerase I. J Biol Chem. 1979 Dec 25;254(24):12269–12272. [PubMed] [Google Scholar]
- Sigman D. S., Spassky A., Rimsky S., Buc H. Conformational analysis of lac promoters using the nuclease activity of 1,10-phenanthroline-copper ion. Biopolymers. 1985 Jan;24(1):183–197. doi: 10.1002/bip.360240115. [DOI] [PubMed] [Google Scholar]
- Streisinger G., Okada Y., Emrich J., Newton J., Tsugita A., Terzaghi E., Inouye M. Frameshift mutations and the genetic code. This paper is dedicated to Professor Theodosius Dobzhansky on the occasion of his 66th birthday. Cold Spring Harb Symp Quant Biol. 1966;31:77–84. doi: 10.1101/sqb.1966.031.01.014. [DOI] [PubMed] [Google Scholar]
- Takeshita M., Grollman A. P., Ohtsubo E., Ohtsubo H. Interaction of bleomycin with DNA. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5983–5987. doi: 10.1073/pnas.75.12.5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veal J. M., Rill R. L. Sequence specificity of DNA cleavage by bis(1,10-phenanthroline)copper(I). Biochemistry. 1988 Mar 22;27(6):1822–1827. doi: 10.1021/bi00406a004. [DOI] [PubMed] [Google Scholar]
- White S. A., Draper D. E. Single base bulges in small RNA hairpins enhance ethidium binding and promote an allosteric transition. Nucleic Acids Res. 1987 May 26;15(10):4049–4064. doi: 10.1093/nar/15.10.4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L. D., Goldberg I. H. Selective strand scission by intercalating drugs at DNA bulges. Biochemistry. 1988 Apr 19;27(8):3004–3011. doi: 10.1021/bi00408a051. [DOI] [PubMed] [Google Scholar]
- Wu J. C., Kozarich J. W., Stubbe J. Mechanism of bleomycin: evidence for a rate-determining 4'-hydrogen abstraction from poly(dA-dU) associated with the formation of both free base and base propenal. Biochemistry. 1985 Dec 17;24(26):7562–7568. doi: 10.1021/bi00347a009. [DOI] [PubMed] [Google Scholar]