Abstract
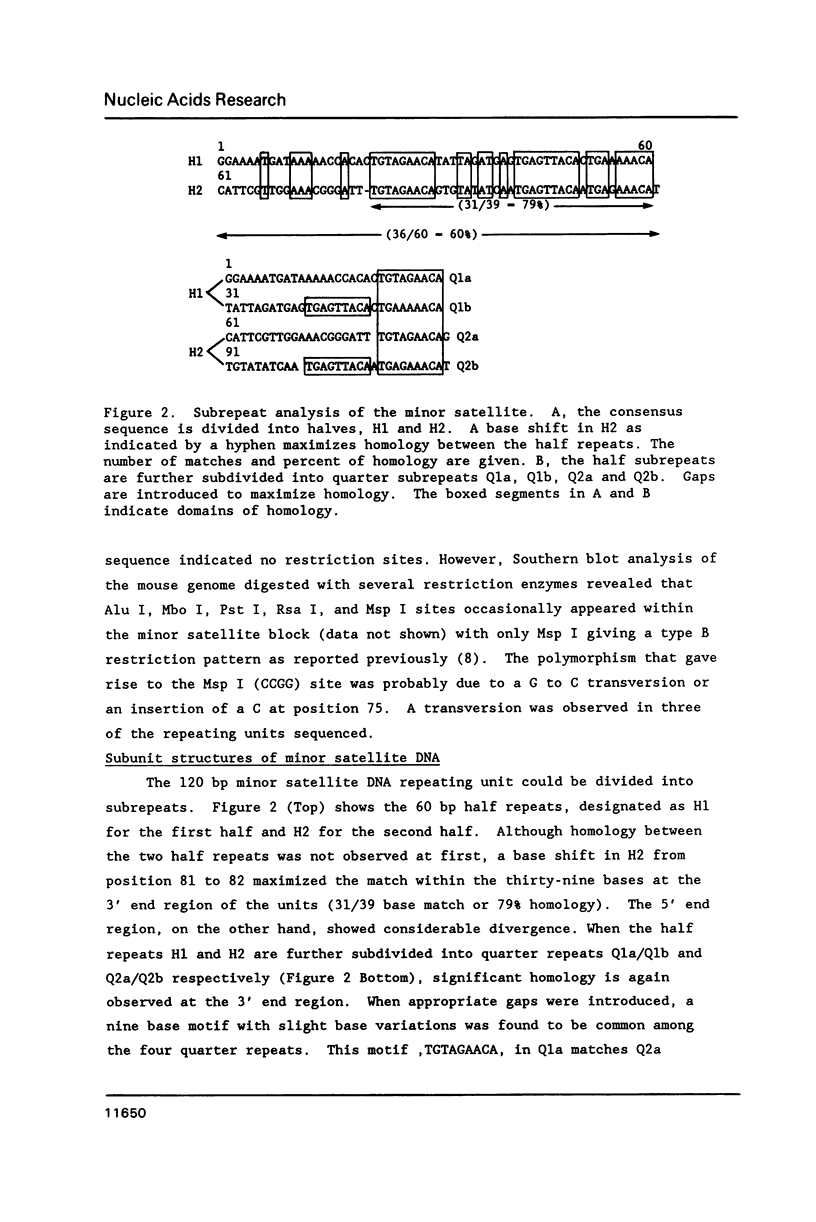
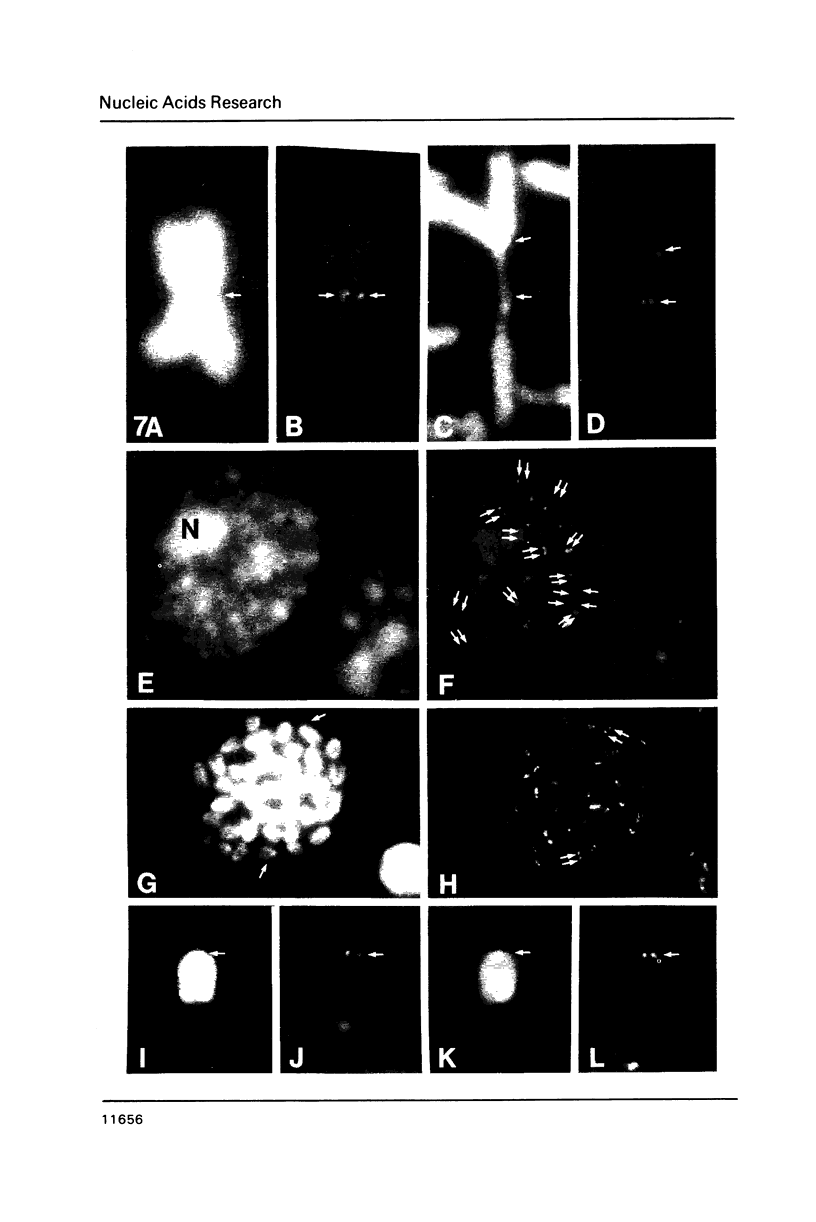
A complete 120 bp genomic consensus sequence for the mouse minor satellite has been determined from enriched L929 centromeric sequences. The extensive sequence homology existing between the major and minor satellite suggests an evolutionary relationship. Some sequences flanking the minor satellite has also been identified and they provide insight into centromeric DNA organization. Isotopic in situ hybridization analysis of the minor satellite to mouse L929 and Mus musculus metaphase spreads showed that this repetitive DNA class is localized specifically to centromeres of all chromosomes of the karyotype. With the use of high resolution non-isotopic fluorescence in situ hybridization the minor satellite is further localized to the outer surface of the centromere in a discrete region at or immediately adjacent to the kinetochore. Our cytological data suggests that the minor satellite might play a role in the organization of the kinetochore region rather than, as previously suggested, sites for general anchoring of the genome to the nuclear matrix.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett K. L., Hill R. E., Pietras D. F., Woodworth-Gutai M., Kane-Haas C., Houston J. M., Heath J. K., Hastie N. D. Most highly repeated dispersed DNA families in the mouse genome. Mol Cell Biol. 1984 Aug;4(8):1561–1571. doi: 10.1128/mcb.4.8.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke C. A., Heck M. M., Earnshaw W. C. The inner centromere protein (INCENP) antigens: movement from inner centromere to midbody during mitosis. J Cell Biol. 1987 Nov;105(5):2053–2067. doi: 10.1083/jcb.105.5.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw W. C., Halligan N., Cooke C., Rothfield N. The kinetochore is part of the metaphase chromosome scaffold. J Cell Biol. 1984 Jan;98(1):352–357. doi: 10.1083/jcb.98.1.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhard W., Meitinger T., Höchtl J., Zachau H. G. A new family of interspersed repetitive DNA sequences in the mouse genome. J Mol Biol. 1982 May 25;157(3):453–471. doi: 10.1016/0022-2836(82)90471-5. [DOI] [PubMed] [Google Scholar]
- Goldberg G. I., Collier I., Cassel A. Specific DNA sequences associated with the nuclear matrix in synchronized mouse 3T3 cells. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6887–6891. doi: 10.1073/pnas.80.22.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilwig I., Gropp A. Decondensation of constitutive heterochromatin in L cell chromosomes by a benzimidazole compound ("33258 Hoechst"). Exp Cell Res. 1973 Oct;81(2):474–477. doi: 10.1016/0014-4827(73)90537-5. [DOI] [PubMed] [Google Scholar]
- Hsieh T., Brutlag D. Sequence and sequence variation within the 1.688 g/cm3 satellite DNA of Drosophila melanogaster. J Mol Biol. 1979 Dec 5;135(2):465–481. doi: 10.1016/0022-2836(79)90447-9. [DOI] [PubMed] [Google Scholar]
- Hörz W., Altenburger W. Nucleotide sequence of mouse satellite DNA. Nucleic Acids Res. 1981 Feb 11;9(3):683–696. doi: 10.1093/nar/9.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingwell B., Rattner J. B. Mammalian kinetochore/centromere composition: a 50 kDa antigen is present in the mammalian kinetochore/centromere. Chromosoma. 1987;95(6):403–407. doi: 10.1007/BF00333991. [DOI] [PubMed] [Google Scholar]
- Krauth W., Werner D. Analysis of the most tightly bound proteins in eukaryotic DNA. Biochim Biophys Acta. 1979 Oct 25;564(3):390–401. doi: 10.1016/0005-2787(79)90030-3. [DOI] [PubMed] [Google Scholar]
- Lica L., Hamkalo B. Preparation of centromeric heterochromatin by restriction endonuclease digestion of mouse L929 cells. Chromosoma. 1983;88(1):42–49. doi: 10.1007/BF00329502. [DOI] [PubMed] [Google Scholar]
- Loeb D. D., Padgett R. W., Hardies S. C., Shehee W. R., Comer M. B., Edgell M. H., Hutchison C. A., 3rd The sequence of a large L1Md element reveals a tandemly repeated 5' end and several features found in retrotransposons. Mol Cell Biol. 1986 Jan;6(1):168–182. doi: 10.1128/mcb.6.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuer-Nitsche B., Lu X. N., Werner D. Functional role of a highly repetitive DNA sequence in anchorage of the mouse genome. Nucleic Acids Res. 1988 Sep 12;16(17):8351–8360. doi: 10.1093/nar/16.17.8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuer B., Plagens U., Werner D. Phosphodiester bonds between polypeptides and chromosomal DNA. J Mol Biol. 1983 Feb 25;164(2):213–235. doi: 10.1016/0022-2836(83)90076-1. [DOI] [PubMed] [Google Scholar]
- Pardue M. L., Gall J. G. Chromosomal localization of mouse satellite DNA. Science. 1970 Jun 12;168(3937):1356–1358. doi: 10.1126/science.168.3937.1356. [DOI] [PubMed] [Google Scholar]
- Pietras D. F., Bennett K. L., Siracusa L. D., Woodworth-Gutai M., Chapman V. M., Gross K. W., Kane-Haas C., Hastie N. D. Construction of a small Mus musculus repetitive DNA library: identification of a new satellite sequence in Mus musculus. Nucleic Acids Res. 1983 Oct 25;11(20):6965–6983. doi: 10.1093/nar/11.20.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkel D., Straume T., Gray J. W. Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proc Natl Acad Sci U S A. 1986 May;83(9):2934–2938. doi: 10.1073/pnas.83.9.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser J., Frommer M., Paul C., Vincent P. C. Sequence relationships of three human satellite DNAs. J Mol Biol. 1986 Jan 20;187(2):145–155. doi: 10.1016/0022-2836(86)90224-x. [DOI] [PubMed] [Google Scholar]
- Radic M. Z., Lundgren K., Hamkalo B. A. Curvature of mouse satellite DNA and condensation of heterochromatin. Cell. 1987 Sep 25;50(7):1101–1108. doi: 10.1016/0092-8674(87)90176-0. [DOI] [PubMed] [Google Scholar]
- Rattner J. B., Kingwell B. G., Fritzler M. J. Detection of distinct structural domains within the primary constriction using autoantibodies. Chromosoma. 1988;96(5):360–367. doi: 10.1007/BF00330702. [DOI] [PubMed] [Google Scholar]
- Rattner J. B., Krystal G., Hamkalo B. A. Selective digestion of mouse metaphase chromosomes. Chromosoma. 1978 Apr 25;66(3):259–268. doi: 10.1007/BF00330554. [DOI] [PubMed] [Google Scholar]
- Rattner J. B. Organization within the mammalian kinetochore. Chromosoma. 1986;93(6):515–520. doi: 10.1007/BF00386793. [DOI] [PubMed] [Google Scholar]
- Rieder C. L. The formation, structure, and composition of the mammalian kinetochore and kinetochore fiber. Int Rev Cytol. 1982;79:1–58. doi: 10.1016/s0074-7696(08)61672-1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunis J. J., Yasmineh W. G. Heterochromatin, satellite DNA, and cell function. Structural DNA of eucaryotes may support and protect genes and aid in speciation. Science. 1971 Dec 17;174(4015):1200–1209. doi: 10.1126/science.174.4015.1200. [DOI] [PubMed] [Google Scholar]