Abstract
Tumor-associated mutations in the isocitrate dehydrogenase 1 and 2 (IDH1 and IDH2) genes result in the loss of normal catalytic activity, the production of α-ketoglutarate (α-KG), and gain of a new activity, the production of an oncometabolite, R-2-hydroxylglutarate (R-2-HG). New evidence supports previous findings that R-2-HG acts as an antagonist of α-KG to competitively inhibit the activity of multiple α-KG-dependent dioxygenases, including both histones and DNA demethylases involved in epigenetic control of gene expression and cell differentiation, and also reveals an intriguing new facet of R-2-HG in tumorigenesis.
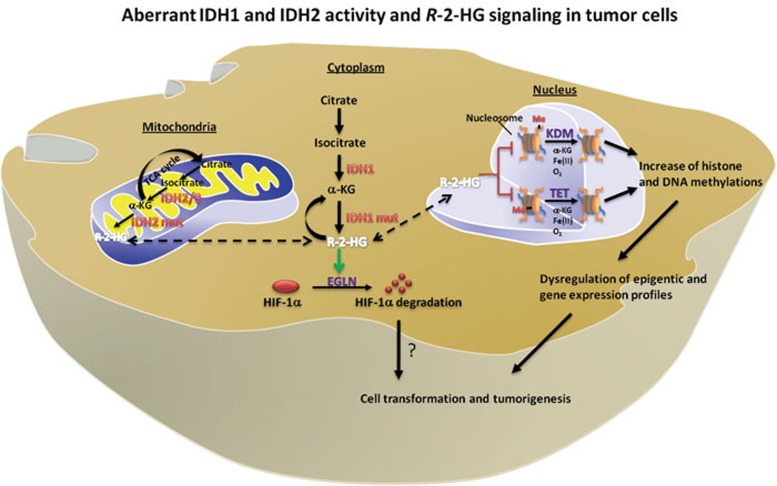
The NADP+-dependent isocitrate dehydrogenase IDH1 and IDH2 catalyze the oxidative decarboxylation of isocitrate to α-ketoglutarate (α-KG). IDH1 and IDH2 are localized in the cytoplasm and mitochondria, respectively, and represent by far the most frequently mutated metabolic enzymes in human cancer1. The tumor-derived mutants of both IDH1 and IDH2 lose their activity in producing α-KG2,3, and gain a surprising new catalytic activity, the production of R-2-hydroxyglutarate (R-2-HG) by reduction of α-KG4. Previous studies have shown that R-2-HG acts as an antagonist of α-KG to competitively inhibit a number of α-KG-dependent dioxygenases, including the JmjC domain-containing histone demethylases (KDMs) and the TET (ten-eleven translocation) family of DNA hydroxylases that catalyze the sequential oxidation of 5-methlycytosine (5mC) to 5-hydroxymethylcytosine (5hmC), 5-formylcytosine (5fC), and 5-carboxylcytosine (5caC), leading to eventual DNA demethylation (Figure 1)5,6. Three papers recently published in Nature provide additional evidence that α-KG-dependent dioxygenases are the pathophysiological targets of mutant IDH1/2, and further underscore the presumptive role of R-2-HG as the first oncometabolite in contributing to tumorigenesis after IDH1/2 mutations.
Figure 1.
Summarization of reported mechanisms linking IDH mutation to tumorigensis. Regulation of α-KG-dependent dioxygenases by R-2-HG is likely to play a major role in the pathophysiology of tumors with IDH mutation.
A subset of glioblastoma, known as the proneural subgroup, has previously found to display hypermethylation at a large number of loci and is enriched with IDH1 mutations7. In one of the three Nature papers, Turcan et al.8 determined whether IDH1 mutation alone is sufficient to cause the hypermethylation phenotype by ectopic expression of IDH1R132H mutant in immortalized primary human astrocytes, a cell type from which glioblastoma is believed to develop. The authors found that introduction of mutant IDH1 induced extensive DNA hypermethylation, altered the methylation of specific histones, and reshaped the methylome in a fashion that mirrors the changes observed in IDH1-mutated low-grade gliomas. The observed hypermethylation of DNA and histones can be explained by the direct inhibition of TET methylcytosine hydroxylases and JmjC family histone demethylases by R-2-HG, respectively. In keeping with the notion that TET hydroxylases directly regulate genomic DNA methylation levels and can be inhibited by the R-2-HG accumulated in IDH1/2-mutated cells, Turcan et al. also showed that ectopic expression of TET2 in cultured astrocytes decreased 5mC and increased 5hmC, and that both changes were inhibited by the co-expression of TET2 with mutant IDH1. These results are consistent with the findings made in acute myeloid leukemia (AML) in which IDH1/2 and TET2 genes are mutated in a mutually exclusive manner9. Moreover, Turcan et al. found that expression of wild-type IDH1 decreased the average DNA methylation level in the genome, supporting the notion that the concentration of α-KG may be a rate-limiting factor of TET-catalyzed DNA demethylation5.
In the second paper, Lu et al.10 reported that ectopic expression of tumor-derived mutant IDH1/2 or feeding cells with cell-permeable R-2-HG increases histone demethylation and results in blockade of the differentiation of 3T3-L1 adipoblasts to adipocytes. These results indicate that mutation of IDH1/2 and accumulation of R-2-HG can broadly impair cell differentiation beyond the cell types in which IDH1/2 mutations are found to associate with tumorigenesis. The authors further confirmed that IDH1-mutated gliomas have elevated levels of histone methylation compared with gliomas retaining the wild-type IDH15,6. As previously reported5,6, multiple KDMs that are inhibited by 2-HG, including KDM4C/JMJD2C, which causes repressive histone H3K9 di- and trimethylation and, when suppressed by RNA interference, blocks the 3T3-L1 adipogenesis. It remains to be determined whether collective inhibition of multiple KDMs or a few individual ones, such as KDM4C, is responsible for altering cell differentiation in IDH1/2-mutated cells. The authors also noted that expression of mutant IDH1 increased histone methylation prior to the increase of DNA methylation, raising an intriguing possibility that histone methylation status may affect DNA methylation.
In the third paper, Koivunen et al.11 proposed an enantiomer-specific mechanism of 2-HG in tumorigenesis. The authors reported two surprising findings. They showed first that immortalized human astrocytes stably expressing tumor-derived IDH1R132H mutant proliferate faster during late passages than those expressing either wild-type IDH1 or IDH1R132H/3DN mutant that lacks 2-HG-producing activity. Ectopic expression of R132H mutant IDH1 has previously been reported to decrease the growth of D54 glioblastoma cells12, raising an intriguing possibility that the mutation of IDH1/2 may exhibit different effects on cell growth in a cell context-dependent manner. More surprisingly, they found that R-2-HG, but not its enantiomer S-2-HG, substitutes for α-KG as a co-substrate, as opposed to an inhibitor, of EGLN, an α-KG-dependent prolylhydroxylase responsible for promoting the degradation of hypoxia inducible factor 1α (HIF-1α) (Figure 1). As the result of stimulating EGLN, accumulation of R-2-HG was found to associate with diminished, instead of increased, HIF-1α levels in cells expressing mutant IDH1/2. At first glance, these observations appear to be at odds with the generally accepted role of both enantiomers of 2-HG as inhibitors of α-KG-dependent dioxygenases, and HIF-1α as an oncogene in tumorigenesis, but may at least in part explain the apparent selection for IDH mutations to produce R-, but not S-2-HG in cancer. This data, also for the first time, reveals a qualitatively different property of two 2-HG enantiomers with respect to α-KG-dependent dioxygenases. It will be interesting to determine the strutural basis of this enantimoer-specific effect of 2-HG toward different α-KG-dependent dixoygenases. The observation that ectopic increase of R-2-HG reduces HIF-1α suggests that endogenous α-KG is limiting for HIF-1α hydroxylation by EGLN. The study by Koivunen et al. also suggests the complexity of EGLN regulation by R-2-HG and subsequent downregulation of HIF-1α. It remains to be determined genetically whether a reduction or fluctuation of HIF-1α levels contributes to gliomagenesis in IDH1/2-mutated cells, because elevated HIF-1α generally contributes to cancer development. The only piece of genetic evidece—IDH1/2 mutation occurs in a mutually exclusive manner with TET2 mutation in AML—supports the notion that epigenetic alteration plays a direct and perhaps a key role in IDH1/2 mutation-associated tumorigenesis.
IDH1/2 mutation has rapidly emerged as a favorable diagnostic and prognostic marker for certain tumors, such as low-grade gliomas and benign cartilaginous tumors. While the full mechanism linking IDH mutation to tumorigenesis is incompletely understood, regulation of α-KG-dependent dioxygenases by 2-HG is likely to play a major role in the pathophysiology of tumors with IDH mutation. These recent reports also highlight the impact of altered metabolism and metabolites on the epigenetic modification of cell differentiation and tumorigenesis.
Acknowledgments
We thank previous and current members of Fudan MCB lab for the discussion and Eric Oermann for reading the manuscript.
References
- Oermann EK, Wu J, Guan KL, Xiong Y. Alterations of metabolic genes and metabolites in cancer. Semin Cell Dev Biol. 2012;23:370–380. doi: 10.1016/j.semcdb.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Lin Y, Xu W, et al. Glioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1alpha. Science. 2009;324:261–265. doi: 10.1126/science.1170944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Yang H, Liu Y, et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell. 2011;19:17–30. doi: 10.1016/j.ccr.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury R, Yeoh KK, Tian YM, et al. The oncometabolite 2-hydroxyglutarate inhibits histone lysine demethylases. EMBO Rep. 2011;12:463–469. doi: 10.1038/embor.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noushmehr H, Weisenberger DJ, Diefes K, et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell. 2010;17:510–522. doi: 10.1016/j.ccr.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcan S, Rohle D, Goenka A, et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature. 2012;483:479–483. doi: 10.1038/nature10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa ME, Abdel-Wahab O, Lu C, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18:553–567. doi: 10.1016/j.ccr.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Ward PS, Kapoor GS, et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature. 2012;483:474–478. doi: 10.1038/nature10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivunen P, Lee S, Duncan CG, et al. Transformation by the (R)-enantiomer of 2-hydroxyglutarate linked to EGLN activation. Nature. 2012;483:484–488. doi: 10.1038/nature10898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer MJ, Bennett BD, Joshi AD, et al. Inhibition of glutaminase preferentially slows growth of glioma cells with mutant IDH1. Cancer Res. 2010;70:8981–8987. doi: 10.1158/0008-5472.CAN-10-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]