Background: Biological functions of regulatory factor X1 (RFX1) are not clear.
Results: RFX1 decreases transforming growth factor β2 (TGFβ2) transcription and exogenous TGFβ2 reverses the inhibitory effects of RFX1 on cell proliferation.
Conclusion: RFX1 inhibits neuroblastoma cell proliferation through direct down-regulation of TGFβ2 transcription.
Significance: This might be the first evidence of RFX1 regulation of TGFβ2 transcription and cell proliferation.
Keywords: Cancer Biology, Cell Proliferation, Gene Expression, Medulloblastoma, Transcription Factors, Transforming Growth Factor β (TGFβ), Extracellular Signal-regulated Kinase
Abstract
Regulatory factor X (RFX) proteins are transcription factors. Seven mammalian RFX proteins have been identified. RFX1 is the prototype RFX. However, its biological functions are not known. Here, RFX1 overexpression reduced fetal bovine serum-stimulated proliferation of SH-SY5Y cells, a human neuroblastoma cell line. This inhibition is associated with decreased transforming growth factor β2 (TGFβ2) and phospho-extracellular signal-regulated kinase (ERK). Exogenous TGFβ2 increased cell proliferation and phospho-ERK in cells overexpressing RFX1. An anti-TGFβ2 antibody and PD98059, an ERK activation inhibitor, inhibited SH-SY5Y cell proliferation. TGFβ2 promoter activity was decreased in cells overexpressing RFX1. Chromosome immunoprecipitation assay showed that RFX1 bound the TGFβ2 promoter. RFX1 down-regulation increased TGFβ2 in SH-SY5Y and HCN-1A cells, a normal human neuronal cell line. More importantly, TGFβ2 concentrations were negatively correlated with RFX1 levels in human medulloblastoma tissues with a R2 of 0.464. These results suggest that RFX1 reduces cell proliferation through inhibiting the TGFβ2-ERK signaling pathway. RFX1 blocks TGFβ2 expression through its direct action on TGFβ2 transcription. This effect also appears in human brain tumor tissues. Because TGFβ is known to be involved in cancer development, our results provide initial evidence to suggest that RFX1 may play an important role in human tumor biology.
Introduction
Regulatory factor X (RFX)2 proteins are transcription factors that can bind X-box of the DNA sequence to regulate the expression of their target genes (1). Seven RFXs have been identified in mammals (2). Various functions are found for these proteins. For example, RFX5 participates in the regulation of the histocompatibility complex class II expression and is involved in immunity (3). However, the biological functions of RFX1, the prototype of RFX family, are not yet known.
We have found that RFX1 is expressed abundantly in the neurons of many brain regions (4, 5). It also is expressed in the microglial cells but is not expressed in the astrocytes (5). RFX1 up-regulates the expression of glutamate transporter type 3, a neuron-specific protein (4). RFX1 down-regulates fibroblast growth factor 1 (FGF1) expression in human glioblastoma cells (6). It also down-regulates the proto-oncogene c-myc (7). In addition, overexpression of RFX1 may reduce the proliferation of human glioma cells (8). These results suggest that RFX1 plays a role in brain tumor cell proliferation. However, the mechanisms for this effect have not been reported.
Transforming growth factor β (TGFβ) is secreted proteins that are known to regulate proliferation and differentiation of various cells (9, 10). Three TGFβs, i.e. TGFβ1, TGFβ2 and TGFβ3, have been identified (9, 10). We found that there is a consensus binding sequence for RFX1 in the TGFβ2 promoter region. Thus, we hypothesize that RFX1 regulates TGFβ2 expression, which contributes to the mechanisms for RFX1 inhibition of brain cell proliferation. To test this hypothesis, we overexpressed or down-regulated RFX1 expression in human brain cell lines. Cell proliferation, expression of TGFβ2, and the activation of extracellular signal-regulated kinase (ERK) and SMAD2/3, proteins that are downstream of TGFβ (9, 10), were analyzed. The regulation of TGFβ2 by RFX1 also was examined in human medulloblastoma tissue samples.
EXPERIMENTAL PROCEDURES
Cell Culture
The human neuroblastoma SH-SY5Y cells and normal neuron HCN-1A cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA). SH-SY5Y cells were cultured in a 1:1 mixture of ATCC-formulated Eagle's minimum essential medium and F-12 supplemented with 10% fetal bovine serum (FBS). HCN-1A cells were cultured in ATCC-formulated Dulbecco's modified Eagle's medium supplemented with 10% FBS. The 293FT cells were obtained from Invitrogen and maintained in Dulbecco's modified Eagle's medium supplemented with 10% FBS, 0.1 mm nonessential amino acids, 6 mm l-glutamine, 1 mm sodium pyruvate, and 500 μg/ml of geneticin.
Generation of SH-SY5Y Cells Stably Overexpressing RFX1
Full-length human RFX1 was obtained from pRFX1, which was provided by Dr. Patrick Hearing (State University of New York at Stony Brook, Stony Brook, NY) and cloned into pOZ-N. The pOZ-N, gag-pol plasmid, and env expression plasmid were provided by Dr. Yufei Xu (Harvard Medical School, Boston, MA). Retrovirus was generated by transfecting the pOZ-RFX1 or pOZ-N with the gag-pol plasmid and env expression plasmid into 293FT cells with the transfection reagent FuGENE (Promega Corp., Madison, WI). Packaged viruses were collected from the media and filtered through a 0.45-μm filter. SH-SY5Y cells were transduced by incubating them with the retrovirus in the presence of 4 μg/ml of Polybrene. Retrovirus produced with pOZ-N was used to generate the control cell line. Both of these transduced cell lines expressed interleukin-2 receptor subunit (IL-2R) as a selection marker. The transduced cells were sorted by magnetic Dynabeads M-450 (Invitrogen) coated with an IL-2R antibody (Millipore, Billerica, MA). After two rounds of positive sorting and one round of negative sorting, the cells without beads were used for experiments. These cells were maintained in complete medium containing 10% FBS.
Luciferase Activity Assay
Two fragments of the TGFβ2 5′ flanking region, which contained the RFX1-binding site but did not include the transcription start site, were obtained by PCR from the genomic DNA of the SH-SY5Y cells. The primers for one fragment were F2 (5′-CCCGGGCACCTCCTTCCTCCCTTACC-3′) and R2 (5′-AGATCTTCTCTGAACCACGTGTCTGC-3′). These primers contain the restriction sites for the endonucleases XmaI and BglII. The primers for the second fragment were F4 (5′-GGTACCAAATTCTGGGATGGCAACCT-3′) and R3 (5′-AGATCTGCTGCCAGCAGATAACATCA-3′). These primers contain the restriction sites for the endonucleases KpnI and BglII. These two fragments were first subcloned into Promega T vector (Promega Corp., Madison, WI) for sequencing. After sequence confirmation, the DNA fragments were digested with the corresponding endonucleases, and then subcloned into the pGL3-promoter-Luc vector (Promega Corp.) to generate pGL3-F2/R2 and pGL3-F4/R3. The third plasmid pGL3-MU containing a TGFβ2 5′-flanking fragment that had a sequence identical to the fragment in the pGL3-F2/R2 but without the RFX1 binding site was constructed as follows. First, the following DNA oligos were synthesized: MU-1 top, 5′-GGGCACCTCCTTCCTCCCTTACCCACAGCGGTCCTCATTTCCACACTCCCTCAACGGTTCGGGGAG-3′, MU-1 bottom, 5′-phoGAGCTCTCCCCGAACCGTTGAGGGAGTGTGGAAATGAGGACCGCTGTGGGTAAGGGAGGAAGGAGGTGCCC-3′; MU-2 top, 5′-phoAGCTCGAGAGGACTTCTGACTGTAATCCTAGCACGTCACTTTGTTGAAGGCAGACACGTGGTTCAGAA-3′ and MU-2 bottom, 5′-GATCTTCTGAACCACGTGTCTGCCTTCAACAAAGTGACGTGCTAGGATTACAGTCAGAAGTCCTCTC-3′. These oligos were annealed to form fragments MU-1 and MU-2, which were then ligated into pGL3-Luc digested with SmalI and BglII together. After sequence confirmation, pGL3-MU, pGL3-F2/R2, and pGL3-F4/R3 were used in the luciferase activity assay.
After being plated on 96-well plates for 24 h, cells stably overexpressing RFX1 or control cells were transiently transfected with pGL3-Luc (a positive control), pGL3-MU, pGL3-F2/R2, or pGL3-F4/R3. The Renilla luciferase expression vector was co-transfected as an internal control. At 24 h after transfection, luciferase activity was measured by a Dual-Glo Luciferase Assay System according to the protocol from Promega. After normalized by Renilla luciferase activity in each sample, the luciferase activity in cells transfected with pGL3-MU, pGL3-F2/R2, or pGL3-F4/R3 was normalized by that in cells transfected with pGL3-Luc.
Chromatin Immunoprecipitation (CHIP) Assay
The CHIP assay was performed according to the protocol of Magna ChIP G (Millipore). Briefly, after cross-linking with 1% formaldehyde, SH-SY5Y cells stably overexpressing RFX1 were washed with phosphate-buffered saline and sonicated in lysis buffer. The condition of sonication was modified to shear cross-linked DNA to about 200–500 base pairs. Approximately 5 × 106 cells were used per CHIP assay and the resulting DNA fragments were incubated with 2 μg of I-19 or E-16, RFX1 antibodies were generated from goat, or nonspecific goat IgG (Santa Cruz Biotechnology, Santa Cruz, CA). The sequences of the primers used in PCR amplification of the RFX1 fragment were as follows: F1, 5′-CACCTCCTTCCTCCCTTACC-3′ and R1, 5′-TCTCTGAACCACGTGTCTGC-3′. The human FGF1 gene, a known target gene for RFX1 (6), was used as the positive control.
Cell Proliferation Assay
As described previously (11), approximate 4000 or 8000 SH-SY5Y cells overexpressing RFX1 and control cells were plated in 96-well plates, deprived of FBS for 24 h and then treated with 10 or 20% FBS. Every 2 days, the numbers of cells were counted by the cell counting Kit-8 (Dojindo Molecular Technologies, Rockville, MD) according to the manufacturer's instructions. In another experiment, FBS-deprived cells were treated with 10% FBS and 2 or 20 ng/ml of human TGFβ2 (R&D Systems, Minneapolis, MN) for 2 days before cell counting. In the third experiment, FBS-deprived cells were incubated with 10% FBS and different concentrations of an anti-TGFβ2 antibody (R&D Systems, catalog number AF-302-NA) or PD98056 (R&D Systems), an ERK activation inhibitor, for 2 days before cell counting.
The Celltrace Violet (Invitrogen) cell proliferation assay was performed according to the manufacturer's protocol. Briefly, after being deprived of FBS for 24 h, control cells and cells overexpressing RFX1 were labeled with 2, 5, and 10 μm Celltrace Violet. Cells were then incubated in culture medium containing 20% FBS for 2 days and collected for flow cytometry analysis by a FACSCalibur (BD Biosciences). The data were analyzed by FlowJo software.
Cell Injury Assay
Cells after various treatments were resuspended in medium without serum. About 0.1 ml of 0.4% trypan blue solution (Sigma) was added to 0.5 ml of cell suspension. This incubation was for 3 min at room temperature. Cells that were positively stained or were not stained by trypan blue were counted by using a hemocytometer under a microscope. About 1000 cells per experimental condition were counted.
Total RNA Extraction and Real-time PCR
Real-time PCR was performed as described previously (12). The sequences of the primers were as follows: RFX1, forward, 5′-TCATCCGGCTGCTCTACGA-3′, reverse, 5′-TGGCCAGATTGGCGAACT-3′; TGFβ2, forward, 5′-TCCAACCCAGCGCTACATC-3′, reverse, 5′-GTGAAGCCATTCATGAACAGCAT-3′; TGFβ1, forward, 5′-TCGCCAGAGTGGTTATCTTTTG-3′, reverse, 5′-AGGAGCAGTGGGCGCTAAG-3′; TGFβ-R1, forward, 5′-GCAGAGCTGTGAAGCCTTGAG-3′, reverse, 5′-ATAATGTTTTCTTAATCCGCAATGC-3′; TGFβ-R2, forward, 5′-ACCACCAGGGCATCCAGAT-3′, reverse, 5′-CTGAAGCGTTCTGCCACACA-3′; and FGF1 (6), forward, 5′-ACAAGGGACAGGAGCGAC-3′, reverse, 5′-TCCAGCCTTTCCAGGAACA-3′. Amplifying PCR and monitoring the fluorescent emission in real-time were performed in the ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Carlsbad, CA). To verify that only a single PCR product was amplified per transcript, dissociation curve data were analyzed through 7900HT Sequence Detection Software. To account for possible differences in starting material, PCR of the housekeeping genes glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and actin also was carried out for each cDNA sample. The relative amount of mRNA in each sample was determined using the comparative threshold cycle method and then normalized to those of the housekeeping genes.
ELISA
Cells at a density of 0.5 × 106 cells/ml were plated. Four days later, the medium was collected and used for measuring TGFβ2 or FGF1 by an ELISA kit from the R&D Systems.
Western Blot
After various treatments including incubation with TGFβ2 in the presence of the FGF receptor inhibitor PD173074 (Sigma), cells were lysed with the M-PER Mammalian Protein Extraction Reagent (Promega Corp.) containing protease inhibitor mixture (Sigma) and PhosSTOP Phosphatase inhibitor (Roche Applied Science). Protein concentration was determined by a Bradford assay. About 30 μg of protein/lane were separated by SDS-PAGE and then transferred to nitrocellulose. After being blocked with the Protein-Free T20 Blocking Buffer (Thermo Scientific), membranes were incubated with each of the following primary antibodies: E-16 anti-RFX1 antibody (Santa Cruz Biotechnology), anti-phospho-ERK antibody (Cell Signaling Technology, Danvers, MA), anti-ERK antibody (Santa Cruz Biotechnology), anti-phospho-SMAD2 (Cell Signaling Technology), anti-phospho-SMAD3 (Cell Signaling Technology), anti-SMAD2/3 antibody (Cell Signaling Technology), anti-phospho-SMAD1/5/8 antibody (Cell Signaling Technology), and anti-GAPDH antibody (Sigma). Appropriate secondary antibodies were used. Proteins were visualized using a Genomic and Proteomic Gel Documentation (Gel Doc) System from Syngene (Frederick, MD). Protein band intensities were normalized by the corresponding band intensities of GAPDH from the same samples. The results under various experimental conditions were then normalized by those of the corresponding controls.
RNA Interference
To silence RFX1 expression in cells, cells were transfected with siRNA duplexes HSS109204 (siRNA1) and HSS109206 (siRNA2) (Invitrogen). Their sequences were as follows: HSS109204 sense, 5′-GGGCAACUCCAAGUACCACUACUAU-3′, HSS109204 antisense, 5′-AUAGUAGUGGUACUUGGAGUUGCCC-3′; HSS109206 sense, 5′-UGGAAAUCCUCAUUCCCGACGUGCU-3′ and HSS109206 antisense, 5′-AGCACGUCGGGAAUGAGGAUUUCCA-3′. A medium GC duplex was used as the negative control and BLOCK-IT Alexa Fluor Red Fluorescent Oligo was used as positive control to assess and optimize transfection. For SH-SY5Y cells, the transfection reagent Lipofectamine RNAiMAX (Invitrogen) was used. The HCN-1A transfection reagent (Altogen Biosystems, Las Vegas, NV) was used for HCN-1A cells.
Assays with Brain Tumor Tissues
Frozen human medulloblastoma tissues were obtained from the Biorepository and Tissue Research Facility, University of Virginia, Charlottesville, VA. These tissues were diagnosed pathologically. Tumor stages were not a selecting criterion for being included in the analysis. A total of 13 of these tissues from different patients were identified in the Facility. Only 11 samples had enough tissue for our analysis. These tissues were sonicated on ice in a lysis buffer (200 mm mannitol, 80 mm HEPES, pH 7.4, and protease inhibitor mixture). After being centrifuged at 13,000 × g at 4 °C for 15 min, the supernatants were saved and used for Western blotting of RFX1 and ELISA for TGFβ2.
Genomic Sequence Analysis
The RFX1 binding site in the genomic sequences of human, mouse, and rat TGFβ2 was analyzed by CLUSTAL 2.1 multiple sequence alignment programs.
Statistical Analysis
The results were presented as mean ± S.D. (n ≥ 3). Statistical analysis was performed by one-way analysis of variance followed by the Tukey test after confirmation of normal distribution of the data, by Kruskal-Wallis analysis of variance on ranks followed by the Tukey test when the data were not normally distributed, or t test as appropriate. A p < 0.05 was considered significant.
RESULTS
RFX1 Overexpression Reduced Proliferation of SH-SY5Y Cells
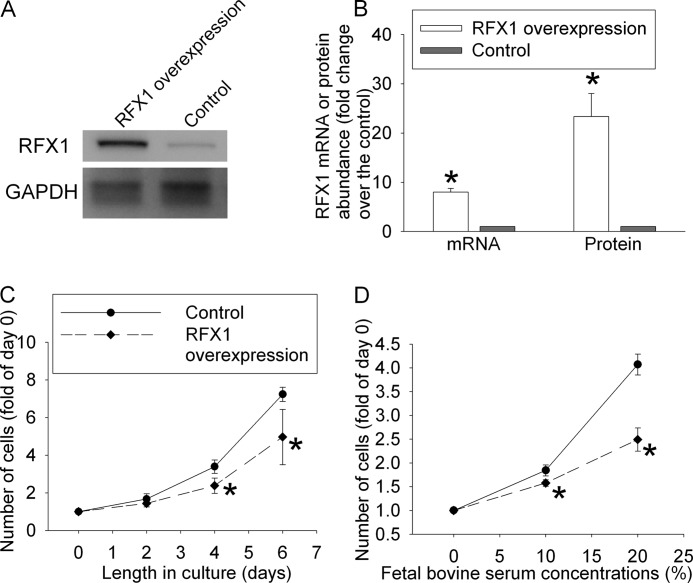
To study the effects of RFX1 on cell proliferation, human neuroblastoma SH-SY5Y cells stably overexpressing RFX1 were prepared by transducing the cells with a retrovirus carrying human RFX1 DNA coding sequence. Control cells were transduced with a retrovirus that did not carry the RFX1 DNA coding sequence. As shown in Fig. 1, A and B, the expression of RFX1 mRNA and protein was much higher in the cells transduced with virus containing the RFX1 DNA coding sequence than in cells transduced with control virus. The cells with RFX1 overexpression had a slower increase in cell number than the control cells, no matter whether the proliferation was stimulated by 10 or 20% FBS (Fig. 1, C and D). Cells stained positively by trypan blue accounted for 3.6 ± 0.4 and 3.7 ± 0.4% (n = 6, p > 0.05), respectively, of the total counted for control cells and cells overexpressing RFX1 after 6 days in culture with 10% FBS. This number was 2.9 ± 0.5 and 2.9 ± 0.6% (n = 6, p > 0.05), respectively, for control and cells overexpressing RFX1 after 2 days in culture medium containing 20% FBS. These results suggest that RFX1 overexpression does not cause cell injury. The slower increase in cell number in cells overexpressing RFX1 indicates that RFX1 inhibits FBS-stimulated proliferation of the SH-SY5Y cells.
FIGURE 1.
Reduction of cell proliferation after overexpression of RFX1. The human SH-SY5Y cells were transduced with retrovirus carrying the human RFX1 DNA coding sequence or control retrovirus to prepare cells stably overexpressing RFX1 or control cells. A, a representative Western blot image of RFX1 expression in the cells overexpressing RFX1 and control cells. B, quantification of RFX1 mRNA and protein expression. The RFX1 Western blot results were normalized by those of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The RFX1 real-time PCR results were normalized by those of GAPDH and actin. The RFX1 results from the RFX1 overexpressing cells then were normalized by those from control cells. C, cells were cultured in medium without fetal bovine serum for 24 h and then with 10% fetal bovine serum for various times up to 6 days. Cell numbers were measured and normalized by those at the beginning of the fetal bovine serum stimulation. D, cells were cultured in medium without fetal bovine serum for 24 h and then with 10 or 20% fetal bovine serum for 2 days. Cell numbers were then measured and normalized by those at the beginning of fetal bovine serum stimulation. Results are mean ± S.D. (n = 3–6). *, p < 0.05 compared with the corresponding control.
Role of TGFβ2 and ERK in RFX1 Overexpression-induced Inhibition of SH-SY5Y Cell Proliferation
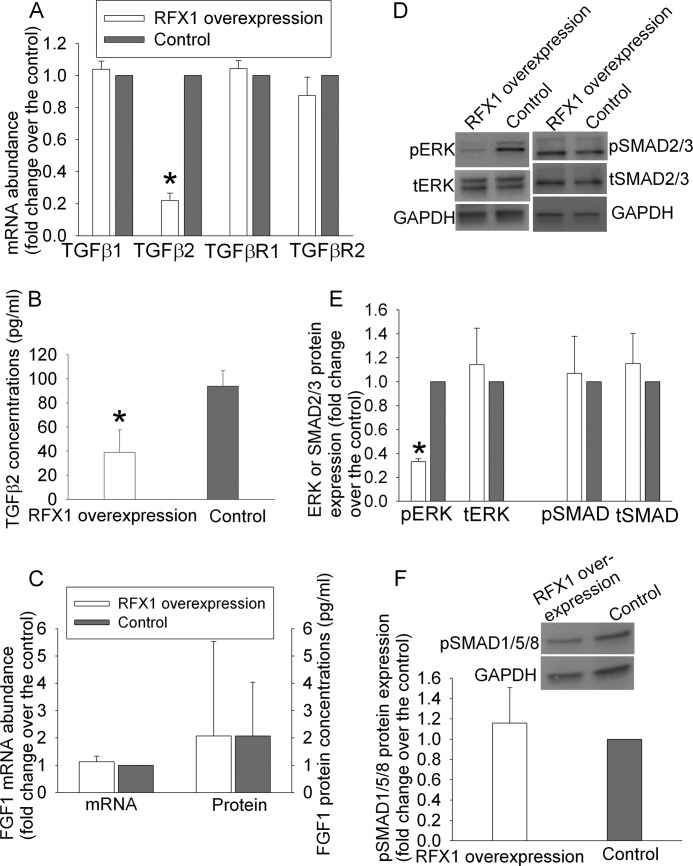
The cells with RFX1 overexpression also had decreased TGFβ2 mRNA. This effect was specific because mRNA expression of TGFβ1, TGFβ receptor 1, and TGFβ receptor 2 in these cells was not different from that in control cells (Fig. 2A). Consistent with the mRNA results, TGFβ2 protein concentrations in the culture medium of cells overexpressing RFX1 were significantly lower than those from control cells (Fig. 2B). However, the expression of FGF1 mRNA and protein was not affected by RFX1 overexpression in the SH-SY5Y cells (Fig. 2C). Although the total ERK was not changed, the phospho-ERK was decreased in cells overexpressing RFX1. These results suggest that RFX1 overexpression decreases activated ERK, a signaling molecule in the TGFβ signaling pathway (10). However, the expression of phospho-SMAD2/3, total SMAD2/3, and phospho-SMAD1/5/8 was not changed by RFX1 overexpression (Fig. 2, D–F). The SMAD system has been considered an important component to transmit the TGFβ signal into cells (9, 10).
FIGURE 2.
Reduction of TGFβ2 and phospho-ERK expression by RFX1 in the SH-SY5Y cells. A, real-time PCR results of TGFβ1, TGFβ2, TGFβ receptor 1 (TGFβR1), and TGFβR2. These results were normalized by those of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and actin. The results from the RFX1 overexpressing cells were then normalized by those from control cells. B, TGFβ2 concentrations in the culture medium included with the cells for 4 days. C, real-time PCR results of fibroblast growth factor 1 (FGF1) and FGF1 protein concentrations in the culture medium included with the cells for 4 days. D, a representative Western blot image of phospho-ERK (pERK), total ERK (tERK), phospho-SMAD2/3, and total SMAD2/3 expression in the RFX1 overexpressing cells and control cells. E, quantification of protein expression of pERK, tERK, phospho-SMAD2/3 (pSMAD2/3), and total SMAD2/3 (tSMAD2/3). The ERK and SMAD Western blot results were normalized by those of GAPDH. The results from the RFX1 overexpressing cells were then normalized by those from control cells. F, protein expression of phospho-SMAD1/5/8 (pSMAD1/5/8). The normalization process was identical to that described for panel E. Results are mean ± S.D. (n = 3–6). *, p < 0.05 compared with the corresponding control.
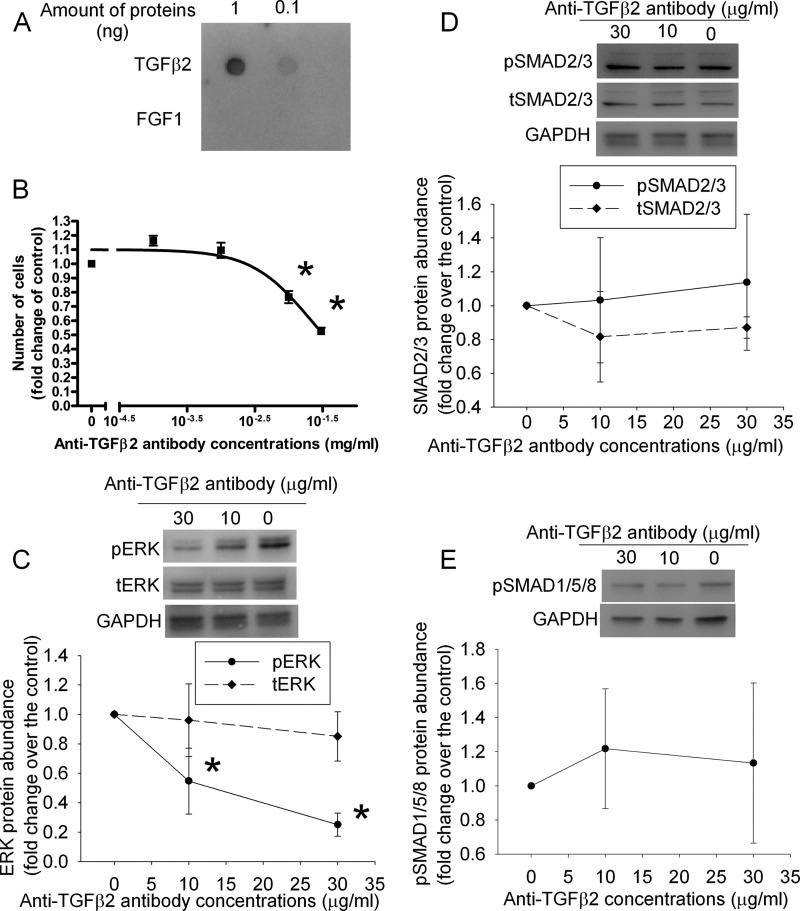
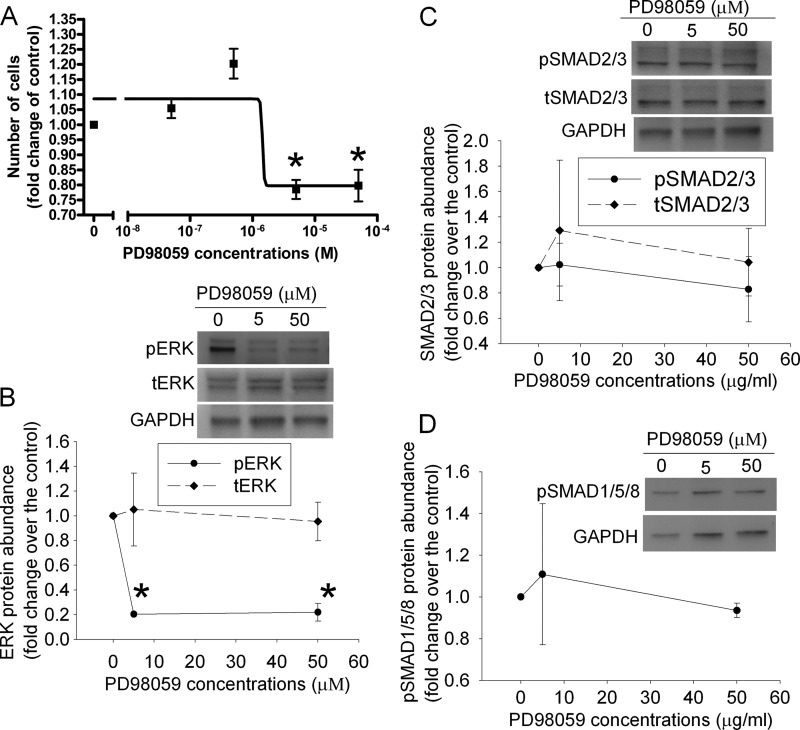
To determine whether decreased TGFβ2 and active ERK play a role in the reduced proliferation of cells overexpressing RFX1, control SH-SY5Y cells were incubated with various concentrations of an anti-TGFβ2 antibody or PD98059, an inhibitor of protein kinase upstream of ERK (13). The antibody specifically reacted with TGFβ2 but not with FGF1 (Fig. 3A). The anti-TGFβ2 antibody and PD98059 dose dependently reduced cell proliferation and phospho-ERK expression but did not affect the expression of total ERK, phospho-SMAD2/3, total SMAD2/3, and phospho-SMAD1/5/8 (Figs. 3 and 4). The anti-phospho-SMAD2/3 antibody and our Western blotting technique were sensitive to detect the differences in the amount of phospho-SMAD2/3 in the samples because the protein bands corresponding to phospho-SMAD2/3 had a 2.2-fold increase in our Western blot if the loading of a sample was increased from 15 to 30 μg/lane. The decreased cell number by the anti-TGFβ2 antibody and PD98059 could be due to cell injury caused by these agents. However, cells stained positively by trypan blue in the total counted cells was 3.4 ± 0.5 and 3.6 ± 0.6% (n = 6, p > 0.05), respectively, for control cells treated without or with 0.03 mg/ml of anti-TGFβ2 antibody, and 3.5 ± 0.4 and 3.6 ± 0.5% (n = 6, p > 0.05), respectively, for cells treated without and with 50 μm PD98059. These results suggest that the anti-TGFβ2 antibody and PD98059 do not cause cell injury.
FIGURE 3.
Inhibition of cell proliferation and phospho-ERK expression by an anti-TGFβ2 antibody in the SH-SY5Y cells. A, a dot blot produced by loading 0.1 or 1 ng of TGFβ2 or FGF1 on the membrane and visualizing the proteins by an anti-TGFβ2 antibody. B, cells were cultured in medium without fetal bovine serum for 24 h and then with 10% fetal bovine serum in the presence of various concentrations of an anti-TGFβ2 antibody for 2 days. Cell numbers then were measured and normalized by those at the beginning of fetal bovine serum stimulation. C–E, cells treated as described for panel B were harvested for Western blotting of phospho-ERK (pERK), total ERK (tERK), phospho-SMAD2/3 (pSMAD2/3), total SMAD2/3 (tSMAD2/3), and phospho-SMAD1/5/8 (pSMAD1/5/8). The ERK and SMAD Western blot results were normalized by those of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The results from cells incubated with the anti-TGFβ2 antibody were then normalized by those from control cells. Results are mean ± S.D. (n = 3–4). *, p < 0.05 compared with the corresponding results without the anti-TGFβ2 antibody.
FIGURE 4.
Inhibition of cell proliferation and phospho-ERK expression by an ERK activation inhibitor (PD98059) in the SH-SY5Y cells. A, cells were cultured in medium without fetal bovine serum for 24 h and then with 10% fetal bovine serum in the presence of various concentrations of PD98059 for 2 days. Cell numbers were then measured and normalized by those at the beginning of the fetal bovine serum stimulation. B–D, cells treated as described for panel A were harvested for Western blotting of phospho-ERK (pERK), total ERK (tERK), phospho-SMAD2/3 (pSMAD2/3), total SMAD2/3 (tSMAD2/3), and phospho-SMAD1/5/8 (pSMAD1/5/8). The ERK and SMAD Western blot results were normalized by those of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The results from cells incubated with PD98059 were then normalized by those from control cells. Results are mean ± S.D. (n = 3–4). *, p < 0.05 compared with the corresponding results without PD98059.
The role of TGFβ2 in SH-SY5Y cell proliferation was further tested by incubating the cells with various concentrations of exogenous TGFβ2. TGFβ2 dose-dependently increased the proliferation of cells overexpressing RFX1 but did not affect the proliferation of control cells (Fig. 5A). TGFβ2 did not affect the expression of RFX1 in control and RFX1 overexpressing cells (Fig. 5B). Consistent with cell proliferation results, TGFβ2 also increased the phospho-ERK in cells overexpressing RFX1 but did not change the phospho-ERK expression in the control cells. TGFβ2 did not affect the protein levels of RFX1, total ERK, phospho-SMAD2/3, total SMAD2/3, and phospho-SMAD1/5/8 in control cells and cells overexpressing RFX1 (Fig. 5, C–E). The TGFβ2-induced increase of phospho-ERK in cells overexpressing RFX1 was not affected by the FGF receptor inhibitor PD173074, suggesting that the FGF signaling pathway is not involved in TGFβ2-induced activation of ERK. Cells stained positively by trypan blue in the total counted cells was 3.0 ± 0.7 and 3.4 ± 0.8% (n = 6, p > 0.05), respectively, for control cells treated without or with 20 ng/ml of TGFβ2, and 3.2 ± 0.8 and 3.2 ± 0.8% (n = 6, p > 0.05), respectively, for RFX1 overexpressing cells treated without and with 20 ng/ml of TGFβ2, suggesting that TGFβ2 does not cause cell injury in the control and RFX1 overexpressing cells.
FIGURE 5.
Increase of cell proliferation and phospho-ERK expression by TGFβ2 in SH-SY5Y cells overexpressing RFX1. A, cells were cultured in medium without fetal bovine serum for 24 h and then with 20% fetal bovine serum in the presence of 2 or 20 ng/ml of TGFβ2 for 2 days. Cell numbers were then measured and normalized by those without exogenous TGFβ2. B–E, cells were treated identically as described for panel A and then harvested for Western blotting of RFX1, phospho-ERK (pERK), total ERK (tERK), phospho-SMAD2/3 (pSMAD2/3), total SMAD2/3 (tSMAD2/3), and phospho-SMAD1/5/8 (pSMAD1/5/8). The RFX1, ERK, and SMAD Western blot results were normalized by those of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The RFX1, ERK, and SMAD results from cells incubated with exogenous TGFβ2 were then normalized by those from cells without exogenous TGFβ2. F, cells overexpressing RFX1 were cultured in medium without fetal bovine serum for 24 h and then with 20% fetal bovine serum in the presence of 0.5 μm PD173074 with or without 20 ng/ml of TGFβ2 for 2 days. The expression of pERK and tERK in these cells was quantified by Western blotting. The normalization process was identical to that described for panels B–E. Results are mean ± S.D. (n = 3–4). *, p < 0.05 compared with the corresponding results without exogenous TGFβ2.
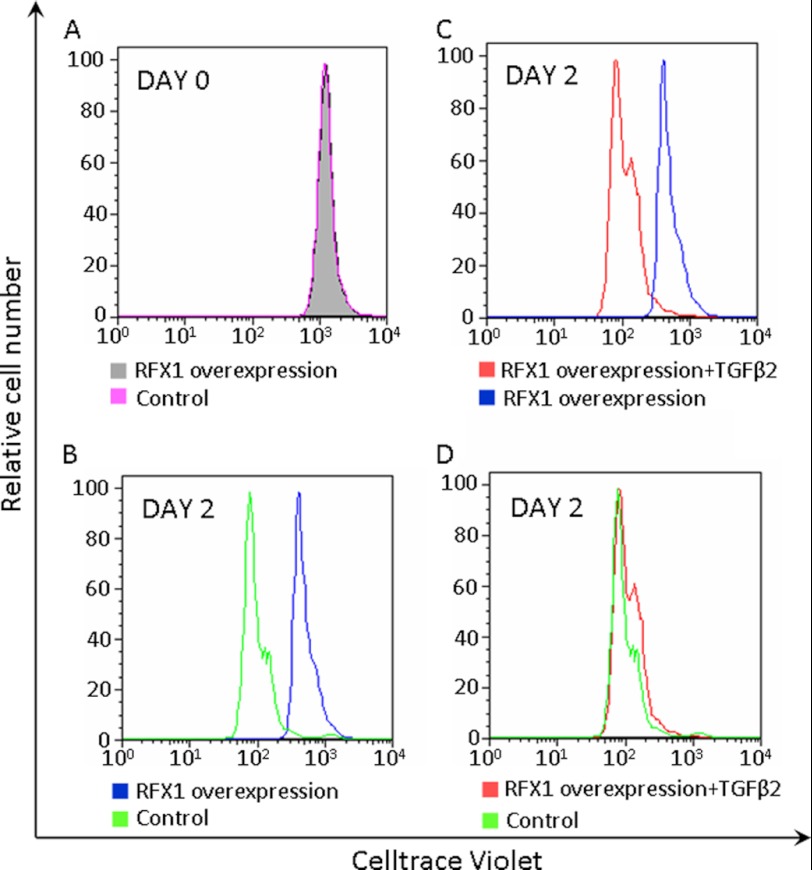
Consistent with the idea that RFX overexpression reduces cell proliferation, cells overexpressing RFX1 had a slower decay of Celltrace Violet fluorescence than control cells after a 2-day culture. This inhibitory effect was abolished by TGFβ2 (Fig. 6).
FIGURE 6.
Decreased proliferation of the SH-SY5Y cells overexpressing RFX1. A, flow cytometry results on day 0 after cells were labeled with 10 μm Celltrace Violet. B–D, after labeling with 10 μm Celltrace Violet, cells were cultured in medium containing 20% fetal bovine serum for 2 days in the presence or absence of 20 ng/ml of TGFβ2. Similar results were obtained with cells labeled with 2 or 5 μm Celltrace Violet.
Regulation of TGFβ2 Expression by RFX1
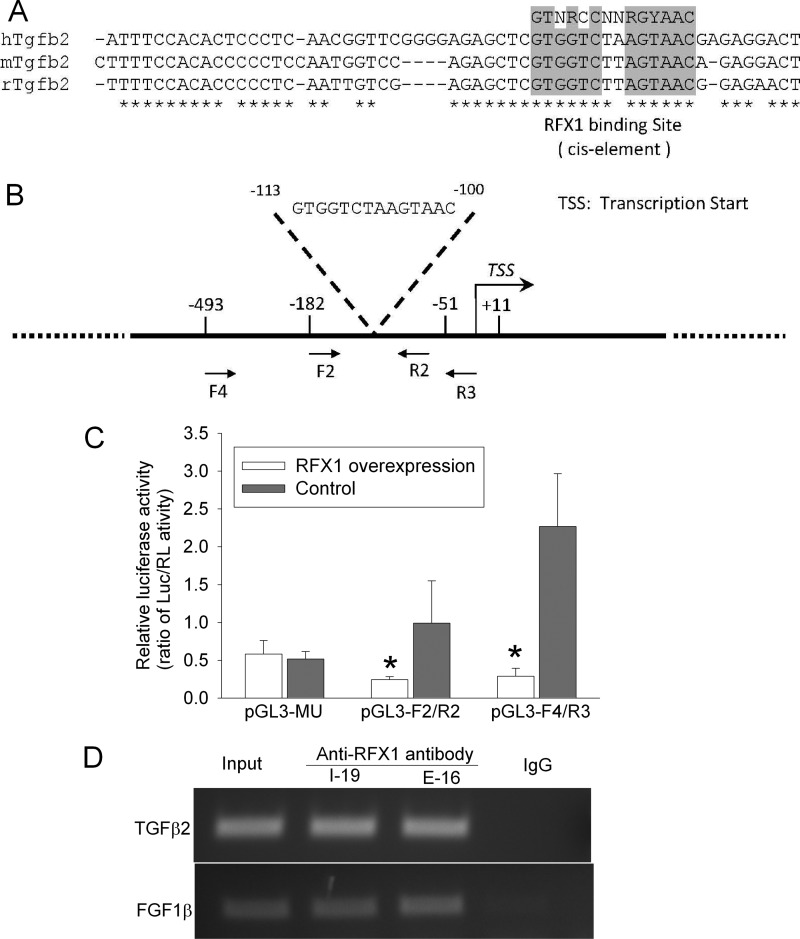
Because the above results have indicated a role of TGFβ2 in mediating the effects of RFX1 on cell proliferation, it is important to determine how RFX1 can regulate TGFβ2 expression. The human, mouse, and rat TGFβ2 genes contain a consensus binding sequence for RFX1 (Fig. 7A). This sequence was at −113 to −100 from the transcription start site in the human TGFβ2 gene (Fig. 7B). The activity of human TGFβ2 promoter fragments that contained the RFX1 binding sequence was significantly decreased in cells overexpressing RFX1. However, RFX1 overexpression did not change the activity of the promoter fragment that did not contain the RFX1 binding sequence (Fig. 7C). Also, TGFβ2 DNA fragments prepared from cells overexpressing RFX1 were precipitated by 2 different anti-RFX1 antibodies from goat. As a positive control, the DNA fragments of FGF1β, a known RFX1 target gene (6), was also precipitated by the anti-RFX1 antibodies in our study. This effect was specific because nonspecific goat IgG did not precipitate the DNA fragments of either TGFβ2 or FGF1β (Fig. 7D). These results suggest that RFX1 can directly regulate TGFβ2 transcription.
FIGURE 7.
Direct regulation of TGFβ2 expression by RFX1 in the SH-SY5Y cells. A, diagram of the consensus binding sequence for RFX1 in the human, mouse, and rat TGFβ2 genes. B, location of the consensus binding sequence for RFX1 in the human TGFβ2 gene. C, control cells and cells overexpressing RFX1 were transfected with plasmids containing the coding sequence for luciferase whose expression was controlled by fragments of the TGFβ2 promoter (F2/R2 and F4/R3 as shown in panel B and a fragment (MU) that had a sequence identical to F2/R2 but without the RFX1 binding sequence). The luciferase (Luc) results were normalized by the corresponding Renilla luciferase (RL) data. Results are mean ± S.D. (n = 4–6). *, p < 0.05 compared with the corresponding control. D, chromosome immunoprecipitation results prepared using two anti-RFX1 antibodies (I-19 and E-16) and the DNA samples from the SH-SY5Y cells overexpressing RFX1. The precipitates were amplified by PCR with a pair of primers, F2 and R2. Input was a DNA sample before immunoprecipitation. FGF1, fibroblast growth factor 1.
This RFX1 regulation was very effective because RFX1 overexpression decreased TGFβ2 transcription (Fig. 2, A and B). In addition, RFX1 down-regulation significantly increased TGFβ2 mRNA expression in SH-SY5Y (Fig. 8, A and B) and HCN-1A cells (Fig. 8C), a normal human neuronal cell line. More importantly, there was a significant negative relationship between the RFX1 protein levels and TGFβ2 protein concentrations in human medulloblastoma tissues with a R2 at 0.464 (Fig. 8D). These results suggest that RFX1 is a powerful regulator of TGFβ2 in human cell cultures and brain tumor tissues under in vivo conditions.
FIGURE 8.
Increased TGFβ2 expression after RFX1 silencing. A, SH-SY5Y cells were incubated with two small interference RNA (siRNA) for RFX1 or a control small RNA for 72 h. Cells were harvested for real-time PCR of RFX1 and TGFβ2. The RFX1 and TGFβ2 results were normalized by those of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and actin. The results from RFX1 siRNA were then normalized by those from control small RNA. B, cells were treated identically as described for panel A and then harvested for Western blotting of RFX1. RFX1 results were normalized by those of GAPDH and then normalized by those from control small RNA. C, identical treatment and analysis were performed in HCN-1A cells, a normal human cell line, as described for panel A. Results in panels A–C are mean ± S.D. (n = 3–6). *, p < 0.05 compared with the results from control small RNA. D, linear correlation analysis of RFX1 protein levels and TGFβ2 protein concentrations in human medulloblastoma tissues.
DISCUSSION
Our results strongly suggest the regulation of TGFβ2 by RFX1 because RFX1 overexpression reduced TGFβ2 expression and silencing RFX1 increased TGFβ2. This regulation occurred in a human neuroblastoma cell line and a normal human neuronal cell line and may be through direct effects of RFX1 on the TGFβ2 gene. This suggestion is supported by three lines of evidence. First, there is a consensus binding sequence for RFX1 in the promoter region of the human TGFβ2 gene. Second, the activity of TGFβ2 promoter fragments containing the RFX1 binding sequence was decreased in cells overexpressing RFX1; whereas RFX1 overexpression did not affect the activity of the TGFβ2 promoter fragment that did not include the RFX1 binding sequence. Finally, DNA fragments of the TGFβ2 gene were precipitated by anti-RFX1 antibodies, suggesting that RFX1 proteins bind directly to the TGFβ2 gene. Two anti-RFX1 antibodies and a nonspecific IgG were used in the chromosome immunoprecipitation to increase the specificity of the findings.
Our results showed that RFX1 inhibited proliferation of human neuroblastoma cell cultures because RFX1 overexpression attenuated the rate of increase in cell numbers, delayed the decay of Celltrace Violet fluorescence, and did not cause cell injury while these cells were in culture. Consistent with our results, previous studies have shown that RFX1 reduces the proliferation of human glioma cell cultures (6, 8). Our results suggest that the effects of RFX1 on cell proliferation are mediated by TGFβ2 because RFX1 overexpression reduced TGFβ2 expression, an anti-TGFβ2 antibody inhibited the proliferation of SH-SY5Y cells and exogenous TGFβ2 increased the proliferation of SH-SY5Y cells overexpressing RFX1. In supporting our findings, it is known that TGFβ2 is involved in cell proliferation and differentiation (9, 10).
ERK may be a molecule downstream of TGFβ2 that mediates the RFX1 effects on cell proliferation. ERK is a signaling molecule that can be activated after TGFβ binds to its receptors (10). ERK is known to be involved in various functions including cell survival and proliferation (14, 15). In our study, RFX1 overexpression reduced the activated ERK, an anti-TGFβ2 antibody reduced cell proliferation and activated ERK, and the ERK activation inhibitor PD98059 inhibited cell proliferation. Also, exogenous TGFβ2 increased cell proliferation and activated ERK in SH-SY5Y cells overexpressing RFX1. These results strongly suggest that reduced ERK activation is an event downstream of TGFβ2 for RFX1 to inhibit the proliferation of human neuroblastoma cells.
In addition to activating ERK, binding of TGFβ to its receptors can activate the SMAD system (9, 10). This starts with phosphorylation/activation of SMAD2/3, the receptor-regulated SMADs, which then leads the TGFβ signal to the nucleus to regulate gene expression. TGFβ also can activate SMAD1/5/8 (16). The SMAD system is known to be involved in regulating cell proliferation and remodeling (17). However, our results suggest that the RFX1 effects on cell proliferation are not mediated by the SMAD system because RFX1 overexpression reduced cell proliferation but did not increase the activated SMAD2/3 and SMAD1/5/8. In addition, the activated SMAD2/3 and SMAD1/5/8 were not changed by an anti-TGFβ2 antibody or exogenous TGFβ2, both of which affected cell proliferation. These results indicate that TGFβ2 may not activate the SMAD system in the SY-SH5Y cells.
Our results suggest involvement of the TGFβ2-ERK pathway in RFX1 regulation of cell proliferation. Other mechanisms may participate in this RFX1 effect. For example, RFX1 can inhibit the expression of the proliferating cell nuclear antigen promoter in HeLa cells (18). This promoter is known to enhance the process of DNA synthesis (19, 20). Thus, inhibition of proliferating cell nuclear antigen promoter expression by RFX1 may result in decreased cell proliferation. However, cell proliferation was not measured in the study. RFX1 has also been shown to inhibit FGF1 expression in human glioblastoma cells (6). FGF1 is a broadly distributed growth factor that has been shown to participate in cell proliferation and neurogenesis (21, 22). The inhibition of FGF1 expression was considered to be the mechanism for RFX1 overexpression-induced reduction of neurosphere formation from glioblastoma cells (6), an indicator for self-renewal capacity of the glioblastoma stem cells. However, overall cell proliferation was not assayed in the previous study. Nevertheless, FGF1 may not play a role in mediating RFX1-induced inhibition of SH-SY5Y cell proliferation because RFX1 overexpression did not significantly reduce FGF1 expression in these cells and FGF signaling was not involved in TGFβ2-induced ERK activation, a critical step that was inhibited by RFX1 for its decrease of SH-SY5Y cell proliferation. Our results that RFX1 does not inhibit FGF1 expression seems to be different from those in a previous study (6). Our study showed that SH-SY5Y cells expressed a very low amount of FGF1. Thus, a further decrease of FGF1 expression by RFX1 in these cells may not be efficient.
The expression of RFX1 in cells stably overexpressing RFX1 was more than 8-fold of that in the control cells. However, the increase in cell number at the end of our experiments in control cells was about 1.5- to 2-fold of that in cells overexpressing RFX1. These results do not seem to be consistent with each other in the magnitude. The doubling time for SH-SY5Y cells is 48 h. We determined cell number after the cells were in various experimental conditions for 2 days in most experiments. Thus, the 1.5–2-fold difference in the cell number increase between the control cells and cells overexpressing RFX1 at this time point reflect a robust effect of RFX1 on cell proliferation. Also, the magnitude of RFX1 expression change is not expected to be similar to the magnitude in cell proliferation change because cell proliferation is controlled by multiple factors and mechanisms.
Exogenous TGFβ2 increased the proliferation of SH-SY5Y cells overexpressing RFX1 but did not promote the proliferation of controls cells. The reasons for this ineffectiveness of TGFβ2 in control cells are not known. The SH-SY5Y cells expressed significant levels of TGFβ2 under control conditions. These levels may have maximized its effect in promoting cell proliferation.
Our results on RFX1 regulation of TGFβ2 expression in cell cultures may also be applicable to human medulloblastoma tissues. The linear regression assay results suggest that RFX1 is a powerful regulator of TGFβ2 expression in brain tumor tissues under in vivo conditions. TGFβ2 is known to increase cell death via apoptosis (9, 10). This effect is often mediated by the SMAD system and can result in inhibition of tumor growth and tumorigenesis (9, 10). However, alteration in cell responses can eliminate this anti-tumor effect. For example, TGFβ2 does not activate the SMAD system in human neuroblastoma cells in our current study. On the other hand, TGFβ enhances angiogenesis and inhibits inflammation and immunity, which can facilitate tumorigenesis and tumor invasion (9, 10). Thus, RFX1, via its powerful regulating effects on TGFβ2 expression, may play an important role in tumor biology. Consistent with this idea, a recent study showed that RFX1 expression in the esophageal epithelium was decreased gradually with the pathology changed from normal esophageal epithelium to esophageal adenocarcinoma (23). In addition, RFX1 has been shown to inhibit c-myc, a well known proto-oncogene (7). These previous results and our current findings have begun to indicate that RFX1 is an important molecule participating in regulating human cancer biology.
In conclusion, our study has shown that RFX1 overexpression inhibits proliferation of human neuroblastoma cell cultures. This effect is mediated by the inhibition of the TGFβ2-ERK signaling pathway. RFX1 is a powerful regulator of TGFβ2 expression in both human neuroblastoma cell cultures and medulloblastoma tissues in vivo. This regulation is through direct effects of RFX1 on the TGFβ2 gene.
Acknowledgments
We thank the Biorepository and Tissue Research Facility, University of Virginia, Charlottesville, VA, for providing the human medulloblastoma tissues for this study.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 GM065211 (to Z. Z.), a International Anesthesia Research Society (2007 Frontiers in Anesthesia Research Award, Cleveland, OH (to Z. Z.), Grant-in-Aid 10GRNT3900019 from the American Heart Association Mid-Atlantic Affiliate, Baltimore, MD (to Z. Z.), and the Robert M. Epstein Professorship endowment, University of Virginia, Charlottesville, VA.
- RFX
- regulatory factor X
- ERK
- extracellular signal-regulated kinase
- FBS
- fetal bovine serum
- FGF1
- fibroblast growth factor 1
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- TGFβ2
- transforming growth factor β2.
REFERENCES
- 1. Emery P., Durand B., Mach B., Reith W. (1996) RFX proteins, a novel family of DNA-binding proteins conserved in the eukaryotic kingdom. Nucleic Acids Res. 24, 803–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aftab S., Semenec L., Chu J. S., Chen N. (2008) Identification and characterization of novel human tissue-specific RFX transcription factors. BMC Evol. Biol. 8, 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clausen B. E., Waldburger J. M., Schwenk F., Barras E., Mach B., Rajewsky K., Förster I., Reith W. (1998) Residual MHC class II expression on mature dendritic cells and activated B cells in RFX5-deficient mice. Immunity 8, 143–155 [DOI] [PubMed] [Google Scholar]
- 4. Ma K., Zheng S., Zuo Z. (2006) The transcription factor regulatory factor X1 increases the expression of neuronal glutamate transporter type 3. J. Biol. Chem. 281, 21250–21255 [DOI] [PubMed] [Google Scholar]
- 5. Feng C., Li J., Zuo Z. (2011) Monoallelic expression of regulatory factor X1 in mouse brain. Folia Histochem. Cytobiol. 49, 344–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hsu Y. C., Liao W. C., Kao C. Y., Chiu I. M. (2010) Regulation of FGF1 gene promoter through transcription factor RFX1. J. Biol. Chem. 285, 13885–13895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen L., Smith L., Johnson M. R., Wang K., Diasio R. B., Smith J. B. (2000) Activation of protein kinase C induces nuclear translocation of RFX1 and down-regulates c-myc via an intron 1X box in undifferentiated leukemia HL-60 cells. J. Biol. Chem. 275, 32227–32233 [DOI] [PubMed] [Google Scholar]
- 8. Ohashi Y., Ueda M., Kawase T., Kawakami Y., Toda M. (2004) Identification of an epigenetically silenced gene, RFX1, in human glioma cells using restriction landmark genomic scanning. Oncogene 23, 7772–7779 [DOI] [PubMed] [Google Scholar]
- 9. Blobe G. C., Schiemann W. P., Lodish H. F. (2000) Role of transforming growth factor β in human disease. N. Engl. J. Med. 342, 1350–1358 [DOI] [PubMed] [Google Scholar]
- 10. Massagué J. (2008) TGFβ in cancer. Cell 134, 215–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shah C. A., Wang H., Bei L., Platanias L. C., Eklund E. A. (2011) HoxA10 regulates transcription of the gene encoding transforming growth factor β2 (TGFβ2) in myeloid cells. J. Biol. Chem. 286, 3161–3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Feng C., Cao L., Zuo Z. (2011) RNA interference-produced autoregulation of inducible nitric-oxide synthase expression. FEBS Lett. 585, 2488–2492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Crews C. M., Alessandrini A., Erikson R. L. (1992) The primary structure of MEK, a protein kinase that phosphorylates the ERK gene product. Science 258, 478–480 [DOI] [PubMed] [Google Scholar]
- 14. Bickler P. E., Fahlman C. S. (2006) The inhaled anesthetic, isoflurane, enhances Ca2+-dependent survival signaling in cortical neurons and modulates MAP kinases, apoptosis proteins and transcription factors during hypoxia. Anesth. Analg. 103, 419–429 [DOI] [PubMed] [Google Scholar]
- 15. Yao Y., Li W., Wu J., Germann U. A., Su M. S., Kuida K., Boucher D. M. (2003) Extracellular signal-regulated kinase 2 is necessary for mesoderm differentiation. Proc. Natl. Acad. Sci. U.S.A. 100, 12759–12764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu I. M., Schilling S. H., Knouse K. A., Choy L., Derynck R., Wang X. F. (2009) TGFβ-stimulated Smad1/5 phosphorylation requires the ALK5 L45 loop and mediates the pro-migratory TGFβ switch. EMBO J. 28, 88–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhai Y., Gao X., Wu Q., Peng L., Lin J., Zuo Z. (2008) Fluvastatin decreases cardiac fibrosis possibly through regulation of TGF-β1/Smad 7 expression in the spontaneously hypertensive rats. Eur. J. Pharmacol. 587, 196–203 [DOI] [PubMed] [Google Scholar]
- 18. Liu M., Lee B. H., Mathews M. B. (1999) Involvement of RFX1 protein in the regulation of the human proliferating cell nuclear antigen promoter. J. Biol. Chem. 274, 15433–15439 [DOI] [PubMed] [Google Scholar]
- 19. Bravo R., Frank R., Blundell P. A., Macdonald-Bravo H. (1987) Cyclin/PCNA is the auxiliary protein of DNA polymerase-δ. Nature 326, 515–517 [DOI] [PubMed] [Google Scholar]
- 20. Prelich G., Kostura M., Marshak D. R., Mathews M. B., Stillman B. (1987) The cell cycle-regulated proliferating cell nuclear antigen is required for SV40 DNA replication in vitro. Nature 326, 471–475 [DOI] [PubMed] [Google Scholar]
- 21. Eckenstein F. P., Andersson C., Kuzis K., Woodward W. R. (1994) Distribution of acidic and basic fibroblast growth factors in the mature, injured and developing rat nervous system. Prog. Brain Res. 103, 55–64 [DOI] [PubMed] [Google Scholar]
- 22. Nurcombe V., Ford M. D., Wildschut J. A., Bartlett P. F. (1993) Developmental regulation of neural response to FGF-1 and FGF-2 by heparan sulfate proteoglycan. Science 260, 103–106 [DOI] [PubMed] [Google Scholar]
- 23. Watts J. A., Zhang C., Klein-Szanto A. J., Kormish J. D., Fu J., Zhang M. Q., Zaret K. S. (2011) Study of FoxA pioneer factor at silent genes reveals Rfx-repressed enhancer at Cdx2 and a potential indicator of esophageal adenocarcinoma development. PLoS Genet. 7, e1002277. [DOI] [PMC free article] [PubMed] [Google Scholar]