Abstract
Intracellular membrane trafficking along endocytic and secretory transport pathways plays a critical role in diverse cellular functions including both developmental and pathological processes. Briefly, proteins and lipids destined for transport to distinct locations are collectively assembled into vesicles and delivered to their target site by vesicular fusion. SNARE (soluble N-ethylmaleimide-sensitive factor-attachment protein receptor) proteins are required for these events, during which v-SNAREs (vesicle SNAREs) interact with t-SNAREs (target SNAREs) to allow transfer of cargo from donor vesicle to target membrane. Recently, the t-SNARE family member, syntaxin-6, has been shown to play an important role in the transport of proteins that are key to diverse cellular dynamic processes. In this paper, we briefly discuss the specific role of SNAREs in various mammalian cell types and comprehensively review the various roles of the Golgi- and endosome-localized t-SNARE, syntaxin-6, in membrane trafficking during physiological as well as pathological conditions.
Keywords: cell dynamics, endosome, Golgi, membrane trafficking, syntaxin-6, target-soluble N-ethylmaleimide-sensitive factor-attachment protein receptor (t-SNARE)
Abbreviations: AD, Alzheimer's disease; CF, cystic fibrosis; CFTR, CF transmembrane conductance regulator; CNS, central nervous system; COG, conserved oligomeric Golgi; EC, endothelial cell; EE, early endosome; GG, gelatinase granule; Glut4, glucose transporter type 4; IRAP, insulin-responsive aminopeptidase; ISG, immature secretory granule; LE, late endosome; MARCH-II, membrane-associated RING-CH-II; MPR, mannose 6-phosphate receptor; MSG, mature secretory granule; NGF, nerve growth factor; SG, specific granule; SNARE, soluble N-ethylmaleimide-sensitive factor-attachment protein receptor; t-SNARE, target SNARE; v-SNARE, vesicle SNARE; SHIP164, syntaxin-6 Habc-interacting protein of 164 kDa; SNAP-23, 23-kDa synaptosome-associated protein; SNAP-25, 25-kDA synaptosome-associated protein; TGN, trans-Golgi network; TNFα, tumour necrosis factor α; VAMP, vesicle-associated membrane protein; VEGF, vascular endothelial growth factor; VEGFR2, VEGF receptor 2; VPS45, vacuolar protein sorting 45
THE ROLE OF SNARES (SOLUBLE N-ETHYLMALEIMIDE-SENSITIVE FACTOR-ATTACHMENT PROTEIN RECEPTORS) IN MEMBRANE TRAFFICKING
SNAREs are tail-anchored membrane proteins involved in the vesicle fusion process. There are 38 distinct SNAREs [25 t-SNAREs (target SNAREs), nine v-SNAREs (vesicle SNAREs) and four unclassified SNAREs], which have been identified in mammalian cells [1,2]. Each performs specific functions towards the delivery of cargo to specific destinations; together, these proteins ensure that recycling is efficient, i.e. that all the components necessary for successive rounds of transport are present [1,2]. SNARE family members are characterized by an evolutionarily conserved central coiled-coil SNARE motif that mediates the interaction of SNARE–SNARE proteins, which is the critical function of SNAREs. Functionally, SNAREs are classified as ‘v-SNAREs’ found on the vesicle membrane and ‘t-SNAREs’ found on the target membrane. Vesicular transport is driven by specific interactions between specified v-SNAREs and their cognate t-SNAREs. SNAREs are additionally classified on the basis of structure into ‘Q-’ or ‘R-SNAREs’ depending on the presence of a conserved glutamine or arginine residue respectively within the conserved SNARE domain. Based on sequence analysis, most of the v-SNAREs are classified as R-SNAREs, whereas syntaxins and SNAP-25 (25-kDA synaptosome-associated protein) are classified as Q-SNAREs (Figure 1a). The Q-SNAREs are further subdivided into ‘Qa-’, ‘Qb-’ and ‘Qc-SNAREs’, on the basis of the relative position of their SNARE motifs in the assembled trans-SNARE complex formed by union of the v- and t-SNARE during membrane fusion [1,3]. Most SNAREs are also transmembrane proteins with a hydrophobic C-terminal domain, with the exception of SNAP-25. SNAP-25 exists in two different forms, cytosolic and membrane bound, which are distinguished by the presence of the post-translational modification, palmitoylation. Newly synthesized, non-palmitoylated SNAP-25 remains soluble whereas mature, palmitoylated SNAP-25 accumulates on intracellular membranes [4]. Each cell type is known to express different combinations of SNARE family members selectively distributed on organelles and membrane domains. Depending on the physiological requirements of each cell type, the repertoire and function of its SNARE proteins can be as diverse as the transport of receptors to and from the cell surface, constitutive- and induced-secretion of immune and inflammatory mediators and the release of neurotransmitters [5–7]. Hormones are also released via SNARE-mediated exocytotic fusion events [8,9]. In the following section, we discuss the specific role of SNAREs in various cell types.
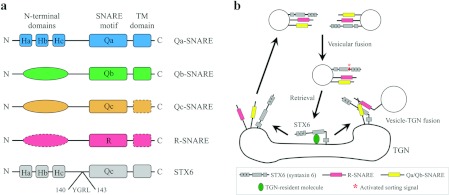
Figure 1. The domain structure of SNAREs and the itinerary of syntaxin 6 involvement in vesicle fusion events.
(a) The domain-based structures of the SNARE subfamilies and syntaxin-6. Some subfamily members skip certain domains, such as TM (transmembrane) and N-terminal domains, marked with dashed lines. Generally, three helix-bundles compose the N-terminal domain in Qa-SNAREs whereas Qb-, Qc- and R-SNAREs have various N-terminal domains, which are characterized by a basic oval shape. The primary structure of syntaxin-6 has a high homology to Qa-SNARE members, but the SNARE motif shares significant sequence homology with the SNARE motif of Qc-SNAREs. Syntaxin-6 also has one tyrosine-based sorting motif, YGRL at position 140–143, between N-terminal domain and SNARE motif, known to play a role in retrograde transport of syntaxin 6 to the TGN [2]. (b) The majority of syntaxin-6 is found at the TGN in steady state. The TGN-resident molecules bind to the SNARE motif of syntaxin-6 for retention in TGN. Once syntaxin-6 becomes a part of the intracellular membrane trafficking, it plays a role in various fusion events with different SNARE complexes until a sorting signal is activated and then syntaxin-6 is sorted back to the TGN.
Immune cells
The human immune system requires a highly dynamic and efficient intracellular transport system for the timely and targeted delivery of key immune molecules, including cytokines, chemokines and lysosomal enzymes, to their respective target sites [10,11]. Vesicles enclose these molecules; undergo budding, transport and tethering; and finally fuse with the plasma membrane to release their contents. A variety of immune cells circulate in the body and a large set of SNARE proteins has evolved to accommodate the protein transport needs of these distinct populations of immune cells. Each immune cell type may use a distinct subset of SNAREs depending on the cell's function within the immune system.
SNAP-23 (23-kDa synaptosome-associated protein) has been shown to be involved in the transport and secretion of immunoglobulins from human plasma cells which indicates that SNAREs have a function in the humoural immune response [12]. Platelets are rich in lysosome-containing granules whose contents are required for blood clotting and inflammation at the site of injury. Release of the granular contents requires SNARE complexes that consist of the t-SNAREs, syntaxin-4 or syntaxin-2; SNAP-23; and the v-SNAREs, VAMP3 (vesicle-associated membrane protein 3) and VAMP8 [13–16].
Neutrophils contain several types of granules containing inflammatory and antimicrobial products which require specific sets of SNARE proteins for their delivery to cell surface and to other organelles [11]. When neutrophils are activated, VAMP1- or VAMP2-containing granules [as well as the so-called SGs (specific granules) and GGs (gelatinase granules)] are translocated to the cell surface where their v-SNAREs associate with the plasma membrane-associated t-SNAREs, syntaxin-4 and SNAP-23, to form trans-SNARE complexes (such as syntaxin-4–SNAP-23–VAMP1 and syntaxin-4–SNAP-23–VAMP2) that modulate granule secretion [17–20]. Although present on all granules in neutrophils, VAMP7 preferentially functions in the release of contents from azurophilic granules. Similar to other v-SNAREs in neutrophils, VAMP7 on azurophilic granules couples with syntaxin-4 on the plasma membrane; however, SNAP-23 is not involved in the fusion and release of azurophilic granules and the alternative Q-SNARE for this role remains to be identified [19,21]. The v-SNARE, syntaxin-7, located in the major granules of neutrophils, functions in the exocytosis of azurophilic granules from activated neutrophils [22].
SNAREs expressed by eosinophils, whose granules contain substances that can contribute to the allergic inflammatory response, are similar in type to those expressed by neutrophils. Eosinophils induced to secrete the contents of their granules by IFN-γ (interferon γ) express syntaxin-4, SNAP-23 and SNAP-25 on their plasma membrane; binding of these t-SNAREs to granules expressing VAMP2 results in eosinophil degranulation and the subsequent release of the granule contents from the cell [23,24]. Mast cells, which play a major role in both allergic and non-allergic diseases, express SNAP-23, syntaxin-4, VAMP7 and VAMP8 which are known to be required for the release of stored histamine. These proteins are also involved in chemokine release, with syntaxin-3 and SNAP-23 crucial for the release of all chemokines and other SNAREs such as syntaxin-4, syntaxin-6 and VAMP8 participating in the release of only selected chemokines [25,26]. Mast cells also release cytokines in combination with non-canonical SNARE isoforms, such as complexin II and synaptotagmin [27,28]. Macrophages, which move towards the site of inflammation via chemotaxis, require the VAMP3–syntaxin-4–SNAP-23 SNARE complex to bring VAMP3-expressing recycling endosomes to the plasma membrane; their cargo is essential for expansion of the plasma membrane which is in turn required for macrophage adhesion and spreading [29]. These cells also require syntaxin-11 which mediates trafficking from late-endosomes to lysosomes by regulating the Vti1b-dependent SNARE complexes, syntaxin-6–syntaxin-7–Vti1b and syntaxin-7–syntaxin-8–Vti1b [30].
Neuronal cells
Components of SNARE complexes are also involved in the development and function of neuronal cells. Among the SNAREs studied (VAMP2, SNAP-25A and syntaxin 1A), VAMP2 promotes neurite elongation while SNAP-25A stimulates neurite sprouting while syntaxin 1A does not have any neuronal effects [31,32]. Synaptic vesicle fusion with the target membrane in neuronal cells is triggered by Ca2+ signalling and requires an interaction between syntaxin-1 and SNAP-25 located at the plasma membrane and the VAMP2 or synaptobrevin present on the synaptic vesicles [7,33]. In addition, synaptotagmins are also involved at the fusion step along with SNAREs during synaptic vesicle fusion [34]. Synaptotagmin-1 is the Ca2+ sensor for membrane fusion and its function is linked to the SNARE complex and is the only molecule besides the SNAREs that has been shown to have a direct effect on the kinetics of exocytosis [35]. In synaptotagmin-1, Ca2+ binding results in an electrostatic switch that changes the net charge from negative to positive and enables the Ca2+ binding domain to interact with and insert into the negatively charged membranes [36,37]. Furthermore, synaptotagmin-1 Ca2+ binding domains bind SNAREs in a Ca2+-regulated manner [38]. The Ca2+-dependent SNARE- and membrane-binding activities of synaptotagmin-1 are both essential for triggering exocytosis [39–41]. SM (Sec1/Munc18-like) proteins and complexin have also been identified that are required for Ca2+-dependent exocytosis at the synapse [42]. The oligodendrocytes that form the myelin sheath surrounding axons of the CNS (central nervous system) neurons require syntaxin-3, syntaxin-4, SNAP-23 and VAMP3 during vesicle fusion for the addition of proteins and lipids to growing myelin membrane [43]. Syntaxin-1, SNAP-25 (at the target or plasma membrane) and the v-SNARE, VAMP2, on secreting vesicles are required for Ca2+-triggered neuroexocytosis. SNAP-25 and syntaxin-1 are also involved in voltage-gated calcium channel function where they play a role in modulating steady-state inactivation of channel opening. In particular, SNAP-25 and the SNAP-25b isoform expressed in both mature glutamatergic and GABAergic (γ-aminobutyric acid) neurons serve as mediators of fast synaptic communication by regulating action potential-dependent neurotransmission in excitatory and inhibitory circuits required for brain function [33]. Syntaxin-12/13 is enriched in the brain and is localized in the recycling endosomes of the neuron, thus it has been shown to have a role in modulating the dynamics of neurotransmitter receptors. Syntaxin-12/13 interacts with NEEP21 (neuron-enriched endosomal protein of 21 kDa); this complex is also associated with the AMPA (α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid) receptor subunit, GluR2, and GRIP1 (glutamate receptor interacting protein 1), which plays an important role in receptor trafficking [44].
Given the central role that SNARE proteins play in the CNS, it is likely that their misregulation is involved in various CNS-related diseases. Consistent with this notion, SNARE proteins have been shown to be involved in γ-secretase function [which is impaired in AD (Alzheimer's disease)], schizophrenia and various other mental illnesses [45–49]. Although an unconventional mechanism (distinct from that used by the CNS) is utilized in ribbon synapses (in hair cells of cochlea) and specialized retinal synapses (between the photoreceptor and bipolar cells) for neurotransmitter release, these synapses still require a host of SNARE complex proteins for sustained release of neurotransmitters [50,51].
Endocrine tissues and epithelial cells
SNARE complexes are also critical for the release of secretory granules in a variety of epithelial cells located in endocrine tissues. For example, the SNARE proteins SNAP-23, SNAP-25, syntaxin-1 and VAMP1–3 are expressed in human parathyroid tissues. Among all normal and cancerous parathyroid gland samples studied, SNAP-23 and VAMP1–3 were expressed at similar levels whereas SNAP-25 and syntaxin-1 were not expressed in normal samples. Notably, in 20% of chief cell adenoma and 45% of parathyroid carcinoma samples, both SNAP-25 and syntaxin-1 were expressed [52]. These data suggest that the SNARE proteins SNAP-23 and VAMP1–3 play a role in stimulus-secretion coupling and exocytosis of parathyroid hormone under normal physiological conditions while SNAP-25 and syntaxin-1 play tumour-specific roles.
SNARE proteins are also involved in insulin secretion by β-cells of the pancreas [8,53]. Studies of insulin exocytosis in isolated pancreatic islets of Type 2 diabetic patients and non-diabetic controls revealed that the expression of SNARE proteins and SNARE-modulating proteins (including syntaxin-1A, SNAP-25, VAMP2, nSec1, Munc13-1 and synaptophysin) are decreased in samples from diabetic patients, possibly contributing to the impaired insulin secretion in patients with Type 2 diabetes [54]. This study illustrates the importance of SNARE protein function in pancreatic secretion of insulin under normal as well as diabetic conditions.
Mammary epithelial cells, which are responsible for producing milk after parturition, express a distinct class of SNARE proteins. In both whole mouse mammary glands and purified mouse mammary epithelial acini, t-SNAREs (SNAP-23, syntaxin-6, syntaxin-7 and syntaxin-12/13) and v-SNAREs (VAMP4 and VAMP8) coordinate the secretion of casein and milk fat globules into the mammary duct [55].
Thus, by facilitating the uptake, secretion and recycling of key biological molecules, SNARE proteins maintain cellular inventory and facilitate various dynamic cellular processes described above. In the next section, we focus on the SNARE family member syntaxin-6 and its roles in the context of additional cellular conditions.
SYNTAXIN-6 MEDIATED REGULATION OF MEMBRANE TRAFFICKING AT VARIOUS SUBCELLULAR LOCATIONS
Syntaxin-6 is composed of an N-terminal domain followed by a SNARE motif and a single C-terminal membrane anchor. Due to the primary structure and high homology to several syntaxin members in the SNARE motif, syntaxin-6 was first classified as a Qa-SNARE (Figure 1a). Syntaxin-6 also shares significant sequence homology with the C-terminal SNARE motif of SNAP-25, which led to the classification of syntaxin-6 as a member of the SNAP-25 family rather than the syntaxin family. As such, syntaxin-6 is known to interact with various SNAREs as well as components of other trafficking machinery [56]. In most cell types, syntaxin-6 is found in the TGN (trans-Golgi network) and EEs (early endosomes); however, in macrophages and neutrophils syntaxin-6 is also found in secretory vesicles and the plasma membrane respectively. The SNARE motif of syntaxin-6 leads to its localization to the TGN by retention via interaction with TGN-resident molecules. Syntaxin-6 also has one tyrosine-based sorting motif (YGRL at position 140–143 between the N-terminal domain and SNARE motif), which regulates retrograde transport of syntaxin-6 from the plasma membrane to the TGN (Figure 1b) [57]. Syntaxin-6 forms complexes with the v-SNARES, α-SNAP, VAMP2 and VPS45 (vacuolar protein sorting 45); interaction of VPS45 with the syntaxin-6-containing SNARE complex is mediated by the N-terminal short sequence of syntaxin-16 [58–60].
In human fibroblasts, syntaxin-6 regulates post-Golgi transport and delivery of the membrane microdomain components such as GM1 ganglioside (glycosphingolipid) and caveolin-1 to the plasma membrane [61]. Caveolae are flask-shaped endocytic structures that are enriched in membrane microdomain components and are abundant in fibroblasts, ECs (endothelial cells) and adipocytes [62]. Caveolar endocytosis has been shown to be dependent on microdomain composition at the plasma membrane [63]. Hence, alteration of surface membrane microdomain lipid and protein composition via inhibition of syntaxin-6 function was found to decrease the internalization of cargo molecules by caveolae [61]. In addition, retrograde transport of membrane microdomain-associated glycosphingolipids and LDL (low-density lipoprotein)-derived cholesterol to the TGN require syntaxin-6 [64,65].
Both endocrine and neuroendocrine cells contain MSGs (mature secretory granules), which are critical storage compartments for hormones and neuropeptides. These structures are generated by homotypic fusion among ISGs (immature secretory granules), an event that requires syntaxin-6 [66]. However, none of the syntaxin-6-cognate SNARE partners were involved in such ISG–ISG fusion processes [66]. Such homotypic fusion requires functional syntaxin-6 on donor as well as on acceptor membranes, suggesting that t–t-SNARE interactions, may drive fusion of ISG membranes. However, SNAREs that regulate MSG exocytosis – such as syntaxin-1, SNAP-25 and VAMP2 – do not contribute to this homotypic fusion. ISG-localized synaptotagmin IV interacts with syntaxin-6 and participates at different stages rather than acting synergistically at the same stage of ISG–ISG fusion [67].
EEs fuse homotypically as well as heterotypically with LEs (late endosomes) or lysosomes, for efficient recycling and degradation of cargo molecules. Syntaxin-6 along with other endosomal SNAREs, syntaxin-13, Vti1A and VAMP4 have been shown to promote liposome fusion in vitro [68]. Homotypic fusion between EEs requires the PI3P (phosphatidylinositol 3-phosphate)-binding protein, EEA1, an effector of Rab5. Syntaxin-6 and EEA1 were found to co-localize extensively on EEs, although syntaxin-6 is present in the TGN as well [64,69]. Syntaxin-6 interacts directly with the C-terminal-binding site of EEA1 which overlaps with that of Rab5 and participates in the in vitro fusion of EEs [64,70]. These studies indicate that SNAREs can interact directly with Rab effectors and participate in endocytic membrane trafficking. Indeed, depletion of syntaxin-6 in ECs resulted in inhibition of endocytic recycling of α5β1 integrin [69].
Chlamydia trachomatis is an obligate intracellular pathogen; syntaxin-6 has been shown to be involved in the formation of the parasitophorous vacuole, an inclusion that is required for replication of the pathogen. Host syntaxin-6 is recruited to the chlamydial inclusion membrane protein; although the exact function of syntaxin-6 at the inclusion membrane remains to be defined, it is believed that it may mediate specific vesicle fusion events required for maintaining the chlamydial inclusion [71]. Another intracellular pathogen Salmonella has been shown to acquire LAMP1 (lysosome-associated membrane protein 1) on phagosomes from the TGN via Salmonella effector protein-mediated recruitment of host syntaxin-6, probably to stabilize their niche in macrophages [72].
Syntaxin-6-mediated membrane trafficking appears to involve many proteins that are not members of the SNARE complex but bind to syntaxin-6 directly. For example, syntaxin-6 is directly bound by MARCH-II (membrane-associated RING-CH-II), a mammalian E3 ubiquitin ligase family member that localizes to endosomal vesicles and the plasma membrane [73]. MARCH-II regulates the cellular distribution of syntaxin-6 as well as protein trafficking between the TGN and endosomes. Syntaxin-6 is also regulated by SHIP164 (syntaxin-6 Habc-interacting protein of 164 kDa) and the COG (conserved oligomeric Golgi) complex. SHIP164 binds directly to the Habc domain of syntaxin-6 and thereby regulates trafficking through the early/recycling endosomal system to the TGN [74]. The COG complex also interacts with syntaxin-6 directly, via the Cog6 subunit and controls the steady-state levels of syntaxin-6 as well as those of the members of its cognate SNARE complex: syntaxin-16, Vti1a and VAMP4. The COG complex regulates syntaxin-6-dependent retrograde trafficking from the endosome to the TGN (Figure 2) [75].
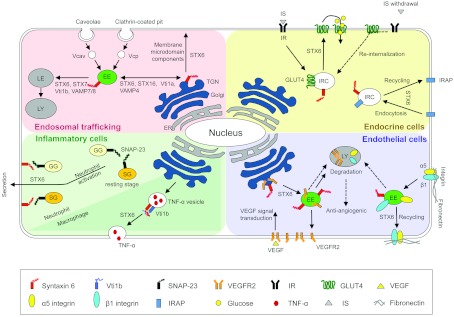
Figure 2. Syntaxin-6-mediated intracellular cargo transport in various cell types.
Syntaxin-6 (STX6) is present on the TGN, the EE, cargo-specialized vesicles and granules, and the plasma membrane and regulates the intracellular trafficking of cargo molecules. STX6 contributes to post-TGN transport and delivery of membrane microdomain components to the plasma membrane. The STX6-containing SNARE complex (STX6, STX16, Vti1a and VAMP4) participates in membrane trafficking between the EE and the TGN. Similarly, STX6 partners with STX7, Vti1b and VAMP7 or VAMP8 to regulate cargo transport to the LE in some cell types. Endocrine cells: in adipocytes, STX6 regulates the insulin responsive membrane proteins, GLUT4 and IRAP. Once the cells receive insulin, STX6 delivers GLUT4 to the plasma membrane, enabling glucose to enter the cell. Functional inhibition of STX6 leads to a reduction in the rate of Glut4 re-internalization after insulin withdrawal. IRAP, a known enhancer of GLUT4 function, is also dependent on STX6 for its endocytosis and recycling. Inflammatory cells: in granulocytes, STX6 contributes to the exocytosis of inflammatory granules and cytokines. In activated neutrophils, STX6 and SNAP-23 facilitate the secretion of GG and SG and in activated macrophages STX6 and the Vti1b complex accelerate the secretion of TNFα. Endothelial cells: STX6 also has a crucial role in endothelial dynamics. It regulates VEGFR2 trafficking from the TGN to the plasma membrane after cellular stimulation with VEGF. It also regulates recycling of the α5β1 integrin, which interacts with the extracellular matrix component fibronectin. The regulation of VEGFR2 and α5β1 integrin function depends on trafficking through TGN and EEs respectively and interference with syntaxin-6 function leads to degradation of these proteins and failure of angiogenesis. Vcav, vesicle derived from caveolae; Vcp, vesicle derived from clathrin-coated pit; dashed arrow, impaired trafficking by syntaxin-6 knockdown or inhibition.
Specialized functions of syntaxin-6 in highly differentiated cell types have also been identified. In pancreatic β-cells, for example, syntaxin-6 is involved in regulated secretion during granule maturation as well as in retrograde endosomal trafficking of MPRs (mannose 6-phosphate receptors) to the lysosome and the TGN. Syntaxin-6 on clathrin-coated buds on IGs (immature granules) co-localizes with the AP-1 (activator protein 1) adaptor and accelerates the delivery of MPR to endosomes during secretory granule maturation [76,77].
In adipocytes, syntaxin-6 is involved in insulin-responsive protein trafficking. It is expressed on both the TGN and vesicles that contain Glut4 (glucose transporter type 4), an insulin-regulated transporter. Upon exposure of the cells to insulin, syntaxin-6 facilitates the transport of Glut4-containing vesicles to the plasma membrane. Moreover, functional inhibition of syntaxin-6 significantly reduces the rate of Glut4 re-internalization after insulin withdrawal and perturbs endosomal sorting of Glut4 (Figure 2) [57,78]. In adipocytes and myocytes, syntaxin-6 is also required for endocytic trafficking of IRAP (insulin-responsive aminopeptidase), a known enhancer of Glut4 exocytosis. Specifically, it stimulates retrograde transport of this protein from the plasma membrane to an IRC (insulin-responsive compartment) (Figure 2) [79,80].
As illustrated above, exocytosis is critical to the function of immune cells during inflammation. Syntaxin-6 has also been found to play an important role in immune cell exocytosis. In resting human neutrophils, syntaxin-6 is localized mainly in the plasma membrane, whereas SNAP-23 is located primarily in the SGs and the GGs [18]. When neutrophils are activated, SNAP-23 is translocated to the plasma membrane and co-localizes with syntaxin-6 (Figure 2). Inhibiting SNAP-23 reduces Ca2+- and GTP-γ-S-induced exocytosis of CD67-enriched SGs, whereas the inhibition of syntaxin-6 prevents exocytosis of both CD67- and CD63-enriched granules. In activated macrophages, one of the major functions is to secrete pro-inflammatory cytokines such as TNFα (tumour necrosis factor α). Syntaxin-6 is up-regulated within a very short time period to meet the demand for TNFα trafficking and secretion. Since the up-regulation occurs rapidly, it is therefore likely that syntaxin-6 protein levels are regulated by post-translational or p53-mediated transcriptional mechanisms [81]. These two SNAREs are present on intracellular membranes, isolated Golgi membranes and Golgi-derived TNFα vesicles, where they contribute to TNFα trafficking and secretion (Figure 2).
Finally, in a melanoma cell line, syntaxin-7 was expressed at very high levels and its overexpression may be involved in the biogenesis of melanosomes. In this cell type, syntaxin-6 forms a SNARE complex with syntaxin-7, mVti1b and VAMP7 or VAMP8, thereby regulating fusion events within the late endosomal pathway. These data suggest that syntaxin-6 SNARE complex plays a critical role in melanogenesis in normal melanocytes (Figure 2) [82]. The specific roles of syntaxin-6 in a variety of cell types indicate that syntaxin-6 functions as an essential SNARE protein for maintenance of normal cellular homoeostasis and upon the activated physiological need of cells.
ROLE OF SYNTAXIN-6 IN ENDOTHELIAL-CELL DYNAMICS
ECs are specialized types of cells that line the interior surface of blood vessels and form the main barrier between the blood and the rest of the body. Unlike other cell types discussed above which require continuous active membrane transport events, the dynamic functions of ECs are activated only when angiogenesis is required. Angiogenesis is a physiological process that involves the growth of new blood vessels from pre-existing vessels, a process that requires dynamic cellular events such as cellular adhesion, proliferation and migration. These cellular events require an intracellular transport system that delivers crucial proteins involved in angiogenesis to the sites of their function. Impairment of the protein trafficking and delivery mechanisms causes endothelial dysfunction and is characteristic of many pathological and disease states such as atherosclerosis, diabetes, hypertension, inflammation and tumour metastasis [83,84]. Syntaxin-6 contributes to EC dynamics by regulating the trafficking of membrane-bound receptors in the EC that drives the physiological changes required for angiogenesis [69,85]. Recent reports from our group demonstrate that syntaxin-6 regulates angiogenesis by regulating the trafficking of VEGFR2 [VEGF(vascular endothelial growth factor) receptor 2] and the α5β1 integrin (Figure 2) [69,85]. Within the ECs, VEGFR2 is a key receptor tyrosine kinase present on the plasma membrane as well as on Golgi membranes whose function is regulated by transport from plasma membrane and secretory transport from the TGN. Studies by Manickam et al. [85] show that inhibition of syntaxin-6 in human ECs impairs the trafficking of the TGN pool of VEGFR2 and targets it to the lysosome for degradation. This work also demonstrated that the functional inhibition of syntaxin-6 reduced VEGF-dependent EC proliferation, migration and vascular tube formation [85]. In addition to its role in the trafficking of VEGFR2, syntaxin-6 has also been shown to affect the angiogenesis process by modulating endocytic recycling of the α5β1 integrin [69]. This integrin is one of the major adhesion molecules on ECs and plays an important role in adhesion and migration along the extracellular matrix during the angiogenic process. Tiwari et al. [69] demonstrate that the α5β1 integrin co-localizes with syntaxin-6 in EEA1-containing EEs and the inhibition of syntaxin-6 function causes misrouting of the α5β1 integrin to the degradation (against recycling) pathway via LEs and lysosomes (Figure 2). Furthermore, syntaxin-6 inhibition leads to a reduction in α5β1 integrin-dependent spreading on fibronectin and a reduction in Rac1 activation and altered Rac1 localization [69].
ROLE OF SYNTAXIN-6 IN HUMAN DISEASES
Many studies that have revealed the importance of syntaxin-6 in vital cellular events have also brought to light the relevance of syntaxin-6-dependent trafficking of key human disease-associated proteins. VEGF promotes angiogenesis by inducing signalling through VEGFR2. Work from several laboratories, including our own, suggests that syntaxin-6-mediated intracellular trafficking of VEGFR2 regulates angiogenesis. Functional inhibition of syntaxin-6 in vivo via adenoviral gene transfer of the inhibitory form of syntaxin-6 reduced VEGF-A-mediated angiogenesis in a mouse ear model [85]. Taken together, these findings suggest that syntaxin-6 plays a critical role in regulating angiogenesis and could be a good target candidate for the development of anti-angiogenic therapies. The gene for syntaxin-6 has been shown to be a direct target of p53 and syntaxin-6 was found to promote cancer cell growth in a p53-dependent manner [86]. In cancer cells, expressing p53 at wild-type levels, syntaxin-6 knockdown inhibited cell proliferation and survival and led to cell cycle arrest and apoptosis; however, syntaxin-6 knockdown in the presence of simultaneous p53 knockdown had no effect on cell growth. Syntaxin-6 may also play a role in CF (cystic fibrosis) as it is a component of the CAL [CFTR (CF transmembrane conductance regulator)-associated ligand] complex, which mediates lysosomal trafficking and degradation of the wild-type CFTR and temperature-rescued ΔF508-CFTR. In this context, syntaxin-6 regulates the abundance and function of post-ER (endoplasmic reticulum) localized wild-type CFTR [87]. In addition, syntaxin-6 is found in the TGN of PC12 cells (derived from a transplantable rat pheochromocytoma) and moves to the distal tips of neurites upon stimulation with NGF (nerve growth factor). Thus, it may play a role in NGF-dependent neurite outgrowth, an important event in Parkinson's disease and AD [88]. Interestingly, a very recent study also showed that genetic variation in syntaxin-6 is relevant to tauopathy (progressive supranuclear palsy), a movement disorder characterized by prominent tau neuropathology, a pathology commonly observed in AD [89]. These studies emphasize the importance of syntaxin-6 not only in the maintenance of normal homoeostasis but also in pathological conditions.
PERSPECTIVE
Syntaxin-6 is involved in diverse cellular functions in a variety of cell types and has been shown to regulate many intracellular membrane trafficking events such as endocytosis, recycling and anterograde and retrograde trafficking. These in vitro studies in cells have enhanced the understanding of the role of syntaxin 6-containing SNARE complexes. However, future studies using animal models lacking syntaxin-6 will be needed to directly study the roles of this t-SNARE protein in various physiological and pathological processes.
ACKNOWLEDGEMENTS
We apologize to colleagues whose works have not been directly cited owing to space limitations.
FUNDING
Our own work was supported by the National Institutes of Health, National Heart, Lung and Blood Institute [grant number HL089599] and the Department of Anatomy and Cell Biology, University of Iowa.
References
- 1.Hong W. SNAREs and traffic. Biochim. Biophys. Acta. 2005;1744:120–144. doi: 10.1016/j.bbamcr.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Jahn R., Scheller R. H. SNAREs – engines for membrane fusion. Nat. Rev. Mol. Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 3.Ungermann C., Langosch D. Functions of SNAREs in intracellular membrane fusion and lipid bilayer mixing. J. Cell Sci. 2005;118:3819–3828. doi: 10.1242/jcs.02561. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalo S., Linder M. E. SNAP-25 palmitoylation and plasma membrane targeting require a functional secretory pathway. Mol. Biol. Cell. 1998;9:585–597. doi: 10.1091/mbc.9.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lacy P. The role of Rho GTPases and SNAREs in mediator release from granulocytes. Pharmacol. Ther. 2005;107:358–376. doi: 10.1016/j.pharmthera.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Logan M. R., Odemuyiwa S. O., Moqbel R. Understanding exocytosis in immune and inflammatory cells: the molecular basis of mediator secretion. J. Allergy Clin. Immunol. 2003;111:923–932. [PubMed] [Google Scholar]
- 7.Wang Y., Tang B. L. SNAREs in neurons–beyond synaptic vesicle exocytosis. Mol. Membr. Biol. 2006;23:377–384. doi: 10.1080/09687860600776734. [DOI] [PubMed] [Google Scholar]
- 8.Hou J. C., Min L., Pessin J. E. Insulin granule biogenesis, trafficking and exocytosis. Vitam. Horm. 2009;80:473–506. doi: 10.1016/S0083-6729(08)00616-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jewell J. L., Oh E., Thurmond D. C. Exocytosis mechanisms underlying insulin release and glucose uptake: conserved roles for Munc18c and syntaxin 4. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;298:R517–R531. doi: 10.1152/ajpregu.00597.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benado A., Nasagi-Atiya Y., Sagi-Eisenberg R. Protein trafficking in immune cells. Immunobiology. 2009;214:507–525. doi: 10.1016/j.imbio.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Stow J. L., Manderson A. P., Murray R. Z. SNAREing immunity: the role of SNAREs in the immune system. Nat. Rev. Immunol. 2006;6:919–929. doi: 10.1038/nri1980. [DOI] [PubMed] [Google Scholar]
- 12.Reales E., Mora-Lopez F., Rivas V., Garcia-Poley A., Brieva J. A., Campos-Caro A. Identification of soluble N-ethylmaleimide-sensitive factor attachment protein receptor exocytotic machinery in human plasma cells: SNAP-23 is essential for antibody secretion. J. Immunol. 2005;175:6686–6693. doi: 10.4049/jimmunol.175.10.6686. [DOI] [PubMed] [Google Scholar]
- 13.Chen D., Bernstein A. M., Lemons P. P., Whiteheart S. W. Molecular mechanisms of platelet exocytosis: role of SNAP-23 and syntaxin 2 in dense core granule release. Blood. 2000;95:921–929. [PubMed] [Google Scholar]
- 14.Chen D., Lemons P. P., Schraw T., Whiteheart S. W. Molecular mechanisms of platelet exocytosis: role of SNAP-23 and syntaxin 2 and 4 in lysosome release. Blood. 2000;96:1782–1788. [PubMed] [Google Scholar]
- 15.Feng D., Crane K., Rozenvayn N., Dvorak A. M., Flaumenhaft R. Subcellular distribution of 3 functional platelet SNARE proteins: human cellubrevin, SNAP-23, and syntaxin 2. Blood. 2002;99:4006–4014. doi: 10.1182/blood.v99.11.4006. [DOI] [PubMed] [Google Scholar]
- 16.Polgar J., Chung S. H., Reed G. L. Vesicle-associated membrane protein 3 (VAMP-3) and VAMP-8 are present in human platelets and are required for granule secretion. Blood. 2002;100:1081–1083. doi: 10.1182/blood.v100.3.1081. [DOI] [PubMed] [Google Scholar]
- 17.Brumell J. H., Volchuk A., Sengelov H., Borregaard N., Cieutat A. M., Bainton D. F., Grinstein S., Klip A. Subcellular distribution of docking/fusion proteins in neutrophils, secretory cells with multiple exocytic compartments. J. Immunol. 1995;155:5750–5759. [PubMed] [Google Scholar]
- 18.Martin-Martin B., Nabokina S. M., Blasi J., Lazo P. A., Mollinedo F. Involvement of SNAP-23 and syntaxin 6 in human neutrophil exocytosis. Blood. 2000;96:2574–2583. [PubMed] [Google Scholar]
- 19.Mollinedo F., Calafat J., Janssen H., Martin-Martin B., Canchado J., Nabokina S. M., Gajate C. Combinatorial SNARE complexes modulate the secretion of cytoplasmic granules in human neutrophils. J. Immunol. 2006;177:2831–2841. doi: 10.4049/jimmunol.177.5.2831. [DOI] [PubMed] [Google Scholar]
- 20.Mollinedo F., Martin-Martin B., Calafat J., Nabokina S. M., Lazo P. A. Role of vesicle-associated membrane protein-2, through Q-soluble N-ethylmaleimide-sensitive factor attachment protein receptor/R-soluble N-ethylmaleimide-sensitive factor attachment protein receptor interaction, in the exocytosis of specific and tertiary granules of human neutrophils. J. Immunol. 2003;170:1034–1042. doi: 10.4049/jimmunol.170.2.1034. [DOI] [PubMed] [Google Scholar]
- 21.Logan M. R., Lacy P., Odemuyiwa S. O., Steward M., Davoine F., Kita H., Moqbel R. A critical role for vesicle-associated membrane protein-7 in exocytosis from human eosinophils and neutrophils. Allergy. 2006;61:777–784. doi: 10.1111/j.1398-9995.2006.01089.x. [DOI] [PubMed] [Google Scholar]
- 22.Xie L. X., Calafat J., Janssen H., de la Iglesia-Vicente J., Mollinedo F. Intracellular location of syntaxin 7 in human neutrophils. Immunol. Lett. 2010;129:72–77. doi: 10.1016/j.imlet.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Hoffmann H. J., Bjerke T., Karawajczyk M., Dahl R., Knepper M. A., Nielsen S. SNARE proteins are critical for regulated exocytosis of ECP from human eosinophils. Biochem. Biophys. Res. Commun. 2001;282:194–199. doi: 10.1006/bbrc.2001.4499. [DOI] [PubMed] [Google Scholar]
- 24.Logan M. R., Lacy P., Bablitz B., Moqbel R. Expression of eosinophil target SNAREs as potential cognate receptors for vesicle-associated membrane protein-2 in exocytosis. J. Allergy Clin. Immunol. 2002;109:299–306. doi: 10.1067/mai.2002.121453. [DOI] [PubMed] [Google Scholar]
- 25.Frank S. P., Thon K. P., Bischoff S. C., Lorentz A. SNAP-23 and syntaxin-3 are required for chemokine release by mature human mast cells. Mol. Immunol. 2011;49:353–358. doi: 10.1016/j.molimm.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 26.Sander L. E., Frank S. P., Bolat S., Blank U., Galli T., Bigalke H., Bischoff S. C., Lorentz A. Vesicle associated membrane protein (VAMP)-7 and VAMP-8, but not VAMP-2 or VAMP-3, are required for activation-induced degranulation of mature human mast cells. Eur. J. Immunol. 2008;38:855–863. doi: 10.1002/eji.200737634. [DOI] [PubMed] [Google Scholar]
- 27.Nagai Y., Tadokoro S., Sakiyama H., Hirashima N. Effects of synaptotagmin 2 on membrane fusion between liposomes that contain SNAREs involved in exocytosis in mast cells. Biochim. Biophys. Acta. 2011;1808:2435–2439. doi: 10.1016/j.bbamem.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Tadokoro S., Nakanishi M., Hirashima N. Complexin II regulates degranulation in RBL-2H3 cells by interacting with SNARE complex containing syntaxin-3. Cell Immunol. 2010;261:51–56. doi: 10.1016/j.cellimm.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 29.Veale K. J., Offenhauser C., Lei N., Stanley A. C., Stow J. L., Murray R. Z. VAMP3 regulates podosome organisation in macrophages and together with Stx4/SNAP23 mediates adhesion, cell spreading and persistent migration. Exp. Cell Res. 2011;317:1817–1829. doi: 10.1016/j.yexcr.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 30.Offenhauser C., Lei N., Roy S., Collins B. M., Stow J. L., Murray R. Z. Syntaxin 11 binds Vti1b and regulates late endosome to lysosome fusion in macrophages. Traffic. 2011;12:762–773. doi: 10.1111/j.1600-0854.2011.01189.x. [DOI] [PubMed] [Google Scholar]
- 31.Igarashi M., Tagaya M., Komiya Y. The soluble N-ethylmaleimide-sensitive factor attached protein receptor complex in growth cones: molecular aspects of the axon terminal development. J. Neurosci. 1997;17:1460–1470. doi: 10.1523/JNEUROSCI.17-04-01460.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kimura K., Mizoguchi A., Ide C. Regulation of growth cone extension by SNARE proteins. J. Histochem. Cytochem. 2003;51:429–433. doi: 10.1177/002215540305100404. [DOI] [PubMed] [Google Scholar]
- 33.Tafoya L. C., Shuttleworth C. W., Yanagawa Y., Obata K., Wilson M. C. The role of the t-SNARE SNAP-25 in action potential-dependent calcium signaling and expression in GABAergic and glutamatergic neurons. BMC Neurosci. 2008;9:105. doi: 10.1186/1471-2202-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sorensen J. B. Formation, stabilisation and fusion of the readily releasable pool of secretory vesicles. Pflugers Arch. 2004;448:347–362. doi: 10.1007/s00424-004-1247-8. [DOI] [PubMed] [Google Scholar]
- 35.Nagy G., Kim J. H., Pang Z. P., Matti U., Rettig J., Sudhof T. C., Sorensen J. B. Different effects on fast exocytosis induced by synaptotagmin 1 and 2 isoforms and abundance but not by phosphorylation. J. Neurosci. 2006;26:632–643. doi: 10.1523/JNEUROSCI.2589-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herrick D. Z., Sterbling S., Rasch K. A., Hinderliter A., Cafiso D. S. Position of synaptotagmin I at the membrane interface: cooperative interactions of tandem C2 domains. Biochemistry. 2006;45:9668–9674. doi: 10.1021/bi060874j. [DOI] [PubMed] [Google Scholar]
- 37.Hui E., Bai J., Chapman E. R. Ca2+-triggered simultaneous membrane penetration of the tandem C2-domains of synaptotagmin I. Biophys. J. 2006;91:1767–1777. doi: 10.1529/biophysj.105.080325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Markin V. S., Albanesi J. P. Membrane fusion: stalk model revisited. Biophys. J. 2002;82:693–712. doi: 10.1016/S0006-3495(02)75432-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernandez-Chacon R., Konigstorfer A., Gerber S. H., Garcia J., Matos M. F., Stevens C. F., Brose N., Rizo J., Rosenmund C., Sudhof T. C. Synaptotagmin I functions as a calcium regulator of release probability. Nature. 2001;410:41–49. doi: 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- 40.Rhee J. S., Li L. Y., Shin O. H., Rah J. C., Rizo J., Sudhof T. C., Rosenmund C. Augmenting neurotransmitter release by enhancing the apparent Ca2+ affinity of synaptotagmin 1. Proc. Natl. Acad. Sci. U.S.A. 2005;102:18664–18669. doi: 10.1073/pnas.0509153102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pang Z. P., Shin O. H., Meyer A. C., Rosenmund C., Sudhof T. C. A gain-of-function mutation in synaptotagmin-1 reveals a critical role of Ca2+-dependent soluble N-ethylmaleimide-sensitive factor attachment protein receptor complex binding in synaptic exocytosis. J. Neurosci. 2006;26:12556–12565. doi: 10.1523/JNEUROSCI.3804-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sudhof T. C., Rothman J. E. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feldmann A., Winterstein C., White R., Trotter J., Kramer-Albers E. M. Comprehensive analysis of expression, subcellular localization, and cognate pairing of SNARE proteins in oligodendrocytes. J. Neurosci. Res. 2009;87:1760–1772. doi: 10.1002/jnr.22020. [DOI] [PubMed] [Google Scholar]
- 44.Steiner P., Alberi S., Kulangara K., Yersin A., Sarria J. C., Regulier E., Kasas S., Dietler G., Muller D., Catsicas S., Hirling H. Interactions between NEEP21, GRIP1 and GluR2 regulate sorting and recycling of the glutamate receptor subunit GluR2. EMBO J. 2005;24:2873–2884. doi: 10.1038/sj.emboj.7600755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barakauskas V. E., Beasley C. L., Barr A. M., Ypsilanti A. R., Li H. Y., Thornton A. E., Wong H., Rosokilja G., Mann J. J., Mancevski B., et al. A novel mechanism and treatment target for presynaptic abnormalities in specific striatal regions in schizophrenia. Neuropsychopharmacology. 2010;35:1226–1238. doi: 10.1038/npp.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chana G., Lucero G., Salaria S., Lozach J., Du P., Woelk C., Everall I. Upregulation of NRG-1 and VAMP-1 in human brain aggregates exposed to clozapine. Schizophr. Res. 2009;113:273–276. doi: 10.1016/j.schres.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gray L. J., Dean B., Kronsbein H. C., Robinson P. J., Scarr E. Region and diagnosis-specific changes in synaptic proteins in schizophrenia and bipolar I disorder. Psychiatry Res. 2010;178:374–380. doi: 10.1016/j.psychres.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 48.Honer W. G., Falkai P., Bayer T. A., Xie J., Hu L., Li H. Y., Arango V., Mann J. J., Dwork A. J., Trimble W. S. Abnormalities of SNARE mechanism proteins in anterior frontal cortex in severe mental illness. Cereb. Cortex. 2002;12:349–356. doi: 10.1093/cercor/12.4.349. [DOI] [PubMed] [Google Scholar]
- 49.Vetrivel K. S., Cheng H., Lin W., Sakurai T., Li T., Nukina N., Wong P. C., Xu H., Thinakaran G. Association of gamma-secretase with lipid rafts in post-Golgi and endosome membranes. J. Biol. Chem. 2004;279:44945–44954. doi: 10.1074/jbc.M407986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Curtis L., Datta P., Liu X., Bogdanova N., Heidelberger R., Janz R. Syntaxin 3B is essential for the exocytosis of synaptic vesicles in ribbon synapses of the retina. Neuroscience. 2010;166:832–841. doi: 10.1016/j.neuroscience.2009.12.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Safieddine S., Wenthold R. J. SNARE complex at the ribbon synapses of cochlear hair cells: analysis of synaptic vesicle- and synaptic membrane-associated proteins. Eur. J. Neurosci. 1999;11:803–812. doi: 10.1046/j.1460-9568.1999.00487.x. [DOI] [PubMed] [Google Scholar]
- 52.Lu M., Forsberg L., Hoog A., Juhlin C. C., Vukojevic V., Larsson C., Conigrave A. D., Delbridge L. W., Gill A., Bark C., et al. Heterogeneous expression of SNARE proteins SNAP-23, SNAP-25, Syntaxin1 and VAMP in human parathyroid tissue. Mol. Cell. Endocrinol. 2008;287:72–80. doi: 10.1016/j.mce.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 53.Wang Z., Thurmond D. C. Mechanisms of biphasic insulin-granule exocytosis–roles of the cytoskeleton, small GTPases and SNARE proteins. J. Cell Sci. 2009;122:893–903. doi: 10.1242/jcs.034355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ostenson C. G., Gaisano H., Sheu L., Tibell A., Bartfai T. Impaired gene and protein expression of exocytotic soluble N-ethylmaleimide attachment protein receptor complex proteins in pancreatic islets of type 2 diabetic patients. Diabetes. 2006;55:435–440. doi: 10.2337/diabetes.55.02.06.db04-1575. [DOI] [PubMed] [Google Scholar]
- 55.Chat S., Layani S., Mahaut C., Henry C., Chanat E., Truchet S. Characterisation of the potential SNARE proteins relevant to milk product release by mouse mammary epithelial cells. Eur. J. Cell Biol. 2011;90:401–413. doi: 10.1016/j.ejcb.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 56.Bock J. B., Lin R. C., Scheller R. H. A new syntaxin family member implicated in targeting of intracellular transport vesicles. J. Biol. Chem. 1996;271:17961–17965. doi: 10.1074/jbc.271.30.17961. [DOI] [PubMed] [Google Scholar]
- 57.Watson R. T., Pessin J. E. Functional cooperation of two independent targeting domains in syntaxin 6 is required for its efficient localization in the trans-Golgi network of 3T3L1 adipocytes. J. Biol. Chem. 2000;275:1261–1268. doi: 10.1074/jbc.275.2.1261. [DOI] [PubMed] [Google Scholar]
- 58.Bock J. B., Klumperman J., Davanger S., Scheller R. H. Syntaxin 6 functions in trans-Golgi network vesicle trafficking. Mol. Biol. Cell. 1997;8:1261–1271. doi: 10.1091/mbc.8.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burkhardt P., Hattendorf D. A., Weis W. I., Fasshauer D. Munc18a controls SNARE assembly through its interaction with the syntaxin N-peptide. EMBO J. 2008;27:923–933. doi: 10.1038/emboj.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dulubova I., Yamaguchi T., Gao Y., Min S. W., Huryeva I., Sudhof T. C., Rizo J. How Tlg2p/syntaxin 16 ‘snares’ Vps45. EMBO J. 2002;21:3620–3631. doi: 10.1093/emboj/cdf381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Choudhury A., Marks D. L., Proctor K. M., Gould G. W., Pagano R. E. Regulation of caveolar endocytosis by syntaxin 6-dependent delivery of membrane components to the cell surface. Nat. Cell Biol. 2006;8:317–328. doi: 10.1038/ncb1380. [DOI] [PubMed] [Google Scholar]
- 62.Parton R. G., Simons K. The multiple faces of caveolae. Nat. Rev. Mol. Cell Biol. 2007;8:185–194. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- 63.Sharma D. K., Brown J. C., Choudhury A., Peterson T. E., Holicky E., Marks D. L., Simari R., Parton R. G., Pagano R. E. Selective stimulation of caveolar endocytosis by glycosphingolipids and cholesterol. Mol. Biol. Cell. 2004;15:3114–3122. doi: 10.1091/mbc.E04-03-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Simonsen A., Gaullier J. M., D'Arrigo A., Stenmark H. The Rab5 effector EEA1 interacts directly with syntaxin-6. J. Biol. Chem. 1999;274:28857–28860. doi: 10.1074/jbc.274.41.28857. [DOI] [PubMed] [Google Scholar]
- 65.Urano Y., Watanabe H., Murphy S. R., Shibuya Y., Geng Y., Peden A. A., Chang C. C., Chang T. Y. Transport of LDL-derived cholesterol from the NPC1 compartment to the ER involves the trans-Golgi network and the SNARE protein complex. Proc. Natl. Acad. Sci. U.S.A. 2008;105:16513–16518. doi: 10.1073/pnas.0807450105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wendler F., Page L., Urbe S., Tooze S. A. Homotypic fusion of immature secretory granules during maturation requires syntaxin 6. Mol. Biol. Cell. 2001;12:1699–1709. doi: 10.1091/mbc.12.6.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ahras M., Otto G. P., Tooze S. A. Synaptotagmin IV is necessary for the maturation of secretory granules in PC12 cells. J. Cell Biol. 2006;173:241–251. doi: 10.1083/jcb.200506163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zwilling D., Cypionka A., Pohl W. H., Fasshauer D., Walla P. J., Wahl M. C., Jahn R. Early endosomal SNAREs form a structurally conserved SNARE complex and fuse liposomes with multiple topologies. EMBO J. 2007;26:9–18. doi: 10.1038/sj.emboj.7601467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tiwari A., Jung J. J., Inamdar S. M., Brown C. O., Goel A., Choudhury A. Endothelial cell migration on fibronectin is regulated by syntaxin 6-mediated {alpha}5{beta}1 integrin recycling. J. Biol. Chem. 2011;286:36749–36761. doi: 10.1074/jbc.M111.260828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mills I. G., Urbe S., Clague M. J. Relationships between EEA1 binding partners and their role in endosome fusion. J. Cell. Sci. 2001;114:1959–1965. doi: 10.1242/jcs.114.10.1959. [DOI] [PubMed] [Google Scholar]
- 71.Moore E. R., Mead D. J., Dooley C. A., Sager J., Hackstadt T. The trans-Golgi SNARE syntaxin 6 is recruited to the chlamydial inclusion membrane. Microbiology. 2011;157:830–838. doi: 10.1099/mic.0.045856-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Madan R., Rastogi R., Parashuraman S., Mukhopadhyay A. Salmonella acquires lysosome-associated membrane protein 1 (LAMP1) on phagosomes from Golgi via SipC pProtein-mediated recruitment of host syntaxin 6. J. Biol. Chem. 2012;287:5574–5587. doi: 10.1074/jbc.M111.286120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nakamura N., Fukuda H., Kato A., Hirose S. MARCH-II is a syntaxin-6-binding protein involved in endosomal trafficking. Mol. Biol. Cell. 2005;16:1696–1710. doi: 10.1091/mbc.E04-03-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Otto G. P., Razi M., Morvan J., Stenner F., Tooze S. A. A novel syntaxin 6-interacting protein, SHIP164, regulates syntaxin 6-dependent sorting from early endosomes. Traffic. 2010;11:688–705. doi: 10.1111/j.1600-0854.2010.01049.x. [DOI] [PubMed] [Google Scholar]
- 75.Laufman O., Hong W., Lev S. The COG complex interacts directly with Syntaxin 6 and positively regulates endosome-to-TGN retrograde transport. J. Cell Biol. 2011;194:459–472. doi: 10.1083/jcb.201102045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Klumperman J., Kuliawat R., Griffith J. M., Geuze H. J., Arvan P. Mannose 6-phosphate receptors are sorted from immature secretory granules via adaptor protein AP-1, clathrin, and syntaxin 6-positive vesicles. J. Cell Biol. 1998;141:359–371. doi: 10.1083/jcb.141.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kuliawat R., Kalinina E., Bock J., Fricker L., McGraw T. E., Kim S. R., Zhong J., Scheller R., Arvan P. Syntaxin-6 SNARE involvement in secretory and endocytic pathways of cultured pancreatic beta-cells. Mol. Biol. Cell. 2004;15:1690–1701. doi: 10.1091/mbc.E03-08-0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Perera H. K., Clarke M., Morris N. J., Hong W., Chamberlain L. H., Gould G. W. Syntaxin 6 regulates Glut4 trafficking in 3T3-L1 adipocytes. Mol. Biol. Cell. 2003;14:2946–2958. doi: 10.1091/mbc.E02-11-0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Watson R. T., Hou J. C., Pessin J. E. Recycling of IRAP from the plasma membrane back to the insulin-responsive compartment requires the Q-SNARE syntaxin 6 but not the GGA clathrin adaptors. J. Cell Sci. 2008;121:1243–1251. doi: 10.1242/jcs.017517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yeh T. Y., Sbodio J. I., Tsun Z. Y., Luo B., Chi N. W. Insulin-stimulated exocytosis of GLUT4 is enhanced by IRAP and its partner tankyrase. Biochem. J. 2007;402:279–290. doi: 10.1042/BJ20060793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Murray R. Z., Wylie F. G., Khromykh T., Hume D. A., Stow J. L. Syntaxin 6 and Vti1b form a novel SNARE complex, which is up-regulated in activated macrophages to facilitate exocytosis of tumor necrosis factor-alpha. J. Biol. Chem. 2005;280:10478–10483. doi: 10.1074/jbc.M414420200. [DOI] [PubMed] [Google Scholar]
- 82.Wade N., Bryant N. J., Connolly L. M., Simpson R. J., Luzio J. P., Piper R. C., James D. E. Syntaxin 7 complexes with mouse Vps10p tail interactor 1b, syntaxin 6, vesicle-associated membrane protein (VAMP)8, and VAMP7 in b16 melanoma cells. J. Biol. Chem. 2001;276:19820–19827. doi: 10.1074/jbc.M010838200. [DOI] [PubMed] [Google Scholar]
- 83.Dejana E., Tournier-Lasserve E., Weinstein B. M. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev. Cell. 2009;16:209–221. doi: 10.1016/j.devcel.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 84.Weis S. M., Cheresh D. A. Pathophysiological consequences of VEGF-induced vascular permeability. Nature. 2005;437:497–504. doi: 10.1038/nature03987. [DOI] [PubMed] [Google Scholar]
- 85.Manickam V., Tiwari A., Jung J. J., Bhattacharya R., Goel A., Mukhopadhyay D., Choudhury A. Regulation of vascular endothelial growth factor receptor 2 trafficking and angiogenesis by Golgi localized t-SNARE syntaxin 6. Blood. 2011;117:1425–1435. doi: 10.1182/blood-2010-06-291690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang Y., Shu L., Chen X. Syntaxin 6, a regulator of the protein trafficking machinery and a target of the p53 family, is required for cell adhesion and survival. J. Biol. Chem. 2008;283:30689–30698. doi: 10.1074/jbc.M801711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cheng J., Cebotaru V., Cebotaru L., Guggino W. B. Syntaxin 6 and CAL mediate the degradation of the cystic fibrosis transmembrane conductance regulator. Mol. Biol. Cell. 2010;21:1178–1187. doi: 10.1091/mbc.E09-03-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kabayama H., Tokushige N., Takeuchi M., Mikoshiba K. Syntaxin 6 regulates nerve growth factor-dependent neurite outgrowth. Neurosci. Lett. 2008;436:340–344. doi: 10.1016/j.neulet.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 89.Hoglinger G. U., Melhem N. M., Dickson D. W., Sleiman P. M., Wang L. S., Klei L., Rademakers R., de Silva R., Litvan I., Riley D. E., et al. Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nat. Genet. 2011;43:699–705. doi: 10.1038/ng.859. [DOI] [PMC free article] [PubMed] [Google Scholar]