Abstract
Since cancer is one of the leading causes of death worldwide, there is an urgent need to find better treatments. Currently, the use of chemotherapeutics remains the predominant option for cancer therapy. However, one of the major obstacles for successful cancer therapy using these chemotherapeutics is that patients often do not respond or eventually develop resistance after initial treatment. Therefore identification of genes involved in chemotherapeutic response is critical for predicting tumour response and treating drug-resistant cancer patients. A group of genes commonly lost or inactivated are tumour suppressor genes, which can promote the initiation and progression of cancer through regulation of various biological processes such as cell proliferation, cell death and cell migration/invasion. Recently, mounting evidence suggests that these tumour suppressor genes also play a very important role in the response of cancers to a variety of chemotherapeutic drugs. In the present review, we will provide a comprehensive overview on how major tumour suppressor genes [Rb (retinoblastoma), p53 family, cyclin-dependent kinase inhibitors, BRCA1 (breast-cancer susceptibility gene 1), PTEN (phosphatase and tensin homologue deleted on chromosome 10), Hippo pathway, etc.] are involved in chemotherapeutic drug response and discuss their applications in predicting the clinical outcome of chemotherapy for cancer patients. We also propose that tumour suppressor genes are critical chemotherapeutic targets for the successful treatment of drug-resistant cancer patients in future applications.
Keywords: cancer, chemoresistance, chemosensitivity, clinical prognosis, signal transduction, tumour suppressor gene (TSG)
Abbreviations: ATM, ataxia telangiectasia mutated; JNK, c-Jun N-terminal kinase; 5-FU, 5-fluorouracil; BRCA1−/−, breast-cancer susceptibility gene 1 knockout; CDK, cyclin-dependent kinase; CKI, cyclin-dependent kinase inhibitor; CTGF, connective tissue growth factor; EGFR, epidermal growth factor receptor; ESC, embryonic stem cell; HR, homologous recombination; INK4, inhibitor of CDK4; MAD2, myoadenylate deaminase 2; MDR1, multidrug resistance gene 1; MEF, mouse embryonic fibroblast; miR, microRNA; ΔNp63, N-terminal truncated p63; PI3K, phosphoinositide 3-kinase; PIP2, phosphatidylinositol, 4,5-bisphosphate; PIP3, phosphatidylinositol 3,4,5-trisphosphate; PTEN, phosphatase and tensin homologue deleted on chromosome 10; PUMA, p53 up-regulated modulator of apoptosis; Rb, retinoblastoma; siRNA, small interfering RNA; TA, transactivation; TAp63, full-length p63; TAZ, transcriptional co-activator with PDZ-binding motif; TSG, tumour suppressor gene; YAP, Yes kinase-associated protein
INTRODUCTION
Cancer is one of the leading causes of human death worldwide, accounting for 7.6 million deaths each year (World Health Organization, 2008). Various therapies have been developed to treat cancer patients such as radiotherapy, chemotherapy and targeted therapy, biological therapy (immunotherapy) and gene therapy. However, chemotherapy, which randomly kills rapidly growing cancer cells using chemotherapeutics and targeted therapy, which specifically kills cancer cells by targeting oncogenic molecules, are still the most commonly used treatment options for cancer patients. Clinically administered chemotherapeutic drugs are grouped into several families: DNA damaging agents [platinum compounds (cisplatin and carboplatin), anthracyclines (doxorubicin, epirubicin, etc.), alkylating agents (cyclophosphamide, temozolomide, carmustine, etc.) and topoisomerase inhibitors (irinotecan, etoposide, etc.), anti-metabolites [5-FU (5-fluorouracil), methotrexate, capecitabine, etc.], anti-microtubule agents [taxanes (paclitaxel/taxol, docetaxel, etc.) and the Vinca alkaloids (vinblastine, vincristine and vindesine)] and oncoprotein targeting agents [humanized monoclonal antibodies such as trastuzumab/herceptin for HER2, cetuximab for EGFR (epidermal growth factor receptor), etc., anti-hormone agents (tamoxifen, flutamide, etc.), and small molecule inhibitors (erlotinib/gefitinib for EGFR, apatinib for VEGFR (vascular endothelial growth factor), etc.)] [1–4]. Although these chemotherapeutics kill cancer cells and can sometimes effectively suppress tumour growth in cancer patients, a significant proportion of tumours either do not respond or later develop resistance to these chemotherapeutics after primary therapy. This leads to tumour recurrence, disease relapse and ultimately patient mortality, which remains a major challenge for successful cancer treatments [2,5–7]. Therefore the identification and characterization of cellular genes responsible for chemotherapeutic drug response is critical for successful prognosis and treatment of cancers. Although many cellular genes, including MDR1 (multidrug resistant gene 1) and c-Myc, have been shown to be involved in the resistance of specific cancer types to some chemotherapeutics [6,8,9], the molecular mechanisms underlying the resistance of distinct types of cancers to different groups of therapeutic drugs remain largely unknown. Most recently, a group of genes called TSGs (tumour suppressor genes) have emerged as important mediators of chemotherapeutic responses. TSGs are frequently dysregulated by mutations or epigenetic modifications in both hereditary cancer syndromes and/or somatically non-hereditary cancers and are also responsible for the initiation and progression of all types of cancers, thereby composing an essential class of signalling molecules within the cell. In this review, we will summarize for the first time the roles of these TSGs in predicting the sensitivity of cancer cells and patients to various chemotherapeutics and their underlying molecular mechanisms. We have also proposed the signalling pathways (Figure 1) illustrating how these TSGs co-ordinately regulate drug sensitivity in cancer cells.
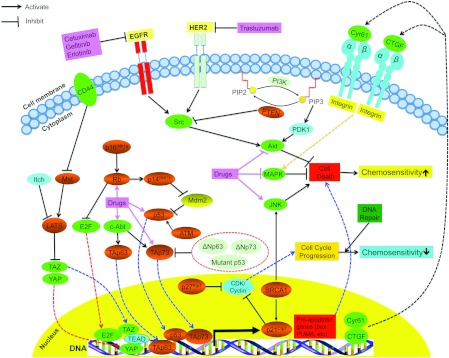
Figure 1. Signalling pathways mediating tumour suppressor function in chemotherapeutic drug response.
Tumour suppressors are shown in orange and chemotherapeutic drugs are shown in pink. JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase.
TSGs
Rb (retinoblastoma)
The Rb gene was the first TSG originally identified in retinoblastoma [10]. Later studies show that loss of heterozygosity, down-regulation and mutations of Rb have been detected in various human cancers [11–13]. Rb protects against tumorigenesis by regulating cell cycle progression, cellular senescence, differentiation, apoptosis and chromosomal integrity [11,14,15]. Importantly, mounting evidence suggests that Rb status is indicative of predicting chemotherapeutic response. In general, cell culture studies in MEFs (mouse embryonic fibroblasts), MAFs (mouse adult fibroblasts) and human cancer cells (e.g. breast, prostate, lung, etc.) have shown that various chemotherapeutic treatments activate Rb, resulting in cell cycle arrest and activation of DNA repair mechanisms, thereby rendering cells resistant to chemotherapeutics [16–21]. Alternatively, loss of Rb expression in these cell lines using RNAi (RNA interference) bypasses the Rb-induced checkpoint response, sensitizing cells to chemotherapeutic drug-induced apoptosis.
Several mechanisms have been proposed to explain how loss of Rb increases sensitivity to different chemotherapeutics. First, in the absence of Rb, cells continue to replicate unchecked. This continued replication of the damaged genome induced by DNA-damaging chemotherapeutics leads to the accumulation of double strand breaks and enhanced genomic instability [22]. When this DNA damage is irreparable, cells will trigger apoptosis to prevent the propagation of unstable cells. Secondly, it has also been shown that after DNA-damaging drug treatment, the E2F family of transcription factors, normally unrestrained in the absence of Rb, can induce apoptosis by transcriptionally activating pro-apoptotic genes such as the caspases, APAF1, and p73 [23–25]. In addition, DNA damage can also directly activate E2F through ATM (ataxia telangiectasia mutated)/ATR (ataxia telangiectasia mutated- and Rad3-related) and/or Chk2-mediated phosphorylation, thereby stimulating its pro-apoptotic activity [26,27].
Significantly, xenograft mouse models and clinical studies also support these cell line studies showing that loss of Rb increases the sensitivity of tumours to chemotherapeutics regardless of drug class or cancer type. For example, Zagorski et al. [28] demonstrated that tumours in mice xenografted with Rb knockdown lung cancer cell lines (H1299 and H520) regressed significantly when treated with the chemotherapeutics cisplatin, etoposide, or 5-FU compared with their Rb expressing tumours. Similarly, Rb-deficient xenograft mammary tumours responded favourably to cisplatin [19]. Furthermore, clinical studies extend these results demonstrating that Rb-deficient breast cancers treated with chemotherapy are associated with good clinical outcome compared with Rb-proficient breast cancers [20,29–31]. Therefore Rb status can serve as an important marker for predicting chemotherapeutic response.
However, it is worth noting that several studies present conflicting evidence where increased chemoresistance results from Rb deficiency. For example, several studies using sarcoma cell lines show that Rb-deficient cells are resistant to anti-metabolites, topoisomerase inhibitors (etoposide and camptothecin) and DNA damaging agents (doxorubicin and cisplatin) [32–34]. In addition, Rb deficiency in prostate cancer and hepatocellular carcinoma lines are resistant to cisplatin [18,33], whereas Rb-deficient glioblastoma cells are resistant to doxorubicin and etoposide [34].
p53 family (p53, p63 and p73)
p53
p53 is the most frequently mutated TSG in human cancer and is the founding member of the p53 family. In response to various cellular stresses, p53 regulates a variety of cellular functions including cell cycle progression, apoptosis, senescence, cell motility, DNA repair, genetic instability and cell metabolism by transcriptionally activating a variety of cellular genes [35–38]. Significantly, there is evidence from cell, animal and clinical studies that the status of p53 is also associated with cancer cell or patient sensitivity in response to various chemotherapeutics [39–41]. It has been well established that p53 not only induces apo-ptosis in response to chemotherapeutic drug-induced apoptosis, it can also induce cell cycle arrest, which protects tumour cells from further cytotoxic damage [38]. Despite this apparent discrepancy, in general, studies on various human cancer cell lines demonstrate that cells with mutant p53 are more resistant to drugs compared with those with wild-type p53 when treated with a wide variety of clinically used chemotherapeutic drugs [42–44]. Since p53 induces apoptosis by up-regulating pro-apoptotic genes such as PUMA (p53 up-regulated modulator of apoptosis), Bax, Bid and Noxa, it has been demonstrated that loss of p53 many cause drug resistance due to down-regulation of these genes [37–39,45–47].
However, preclinical and clinical studies also suggest that the relative contribution of p53 status to drug response varies depending on cellular context or the class of anticancer drugs used.
In one study, Vasey and Jones [48] showed that inactivation of p53 is associated with reduced sensitivity of ovarian cancer cells to cisplatin but not to the anti-microtubule drug paclitaxel. In addition, p53 disruption rendered colorectal cancer cells resistant to the anti-metabolite 5-FU but sensitized these cells to the DNA damaging drug doxorubicin [49]. Furthermore, the molecular mechanism underlying this distinct role of p53 in the response of different tumour cell types to various drug groups is not fully understood. However, previous studies suggest that diverse functions of p53-induced cell cycle arrest and apoptosis in response to different drugs may contribute to this variability. For example, in response to DNA damaging anthracycline-based therapy, p53 usually activates the apoptotic cascade rather than cell cycle arrest, resulting in tumour regression after drug treatment. However, in response to other drugs such as alkylating agents, p53 may induce cell cycle arrest, which allows the recovery of damaged cells and protect them from drug treatment, resulting in drug resistance and subsequent tumour growth [40]. In addition, p53 can induce cell cycle arrest in ovarian carcinomas, but apoptosis in other cancers after paclitaxel treatments. This may explain why the presence of p53 leads to resistance of ovarian cancer patients to some chemotherapeutic treatments [50]. Therefore the role of p53 in mediating a chemotherapeutic response is complex and depends on both cellular context and class of chemotherapeutics.
p63
p63 is another member of the p53 family. It contains all of the functional domains of p53: an acidic N-terminal TA (transactivation) domain, a highly conserved core DBD (DNA-binding domain) and a C-terminal oligomerization domain. p63 also contains a unique sterile α-motif domain implicated in protein–protein interaction. Besides the TAp63 (full-length p63), p63 can also be expressed as an N-terminal truncated isoform lacking the TA domain [ΔNp63 (N-terminal truncated p63)] transcribed from a second downstream promoter. This ΔNp63 isoform can act as a dominant-negative inhibitor of the full-length TAp63 and p53 [51] and have anti-apoptotic and pro-proliferative functions [52]. Unlike p53, mutations of the p63 gene are rarely detected in human cancer. Instead, down-regulation of p63 has been found to be associated with tumorigenesis and metastasis [53,54]. Most significantly, recent studies show that the level of p63 strongly correlates with the response of tumour cells to chemotherapeutics [55–57]. Chemotherapeutic agents induce TAp63 expression, which subsequently causes apoptosis by directly activating pro-apoptotic genes CD95, Bcl-2-family members such as bax and BCL2L11 as well as Apaf1 [55]. Consequently, inhibition of TAp63 function, which causes reduced apoptosis, leads to drug resistance in various cancers [55,58–60].
Apart from its roles in mediating a chemotherapeutic response, p63 also functions in the drug-induced side effect on fertility. It was recently shown that chemotherapeutic drugs may elicit the death of germ cells, particularly oocytes, through activation of TAp63 rather than p53, which can result in reproductive failure [61]. Most significantly, inhibition of TAp63 activation by blocking its activator c-Abl using an Abl-specific inhibitor imatinib protects mouse oocytes from chemotherapy-induced cell death [62]. Therefore, although inhibition of TAp63 may lead to a limited chemotherapeutic response, its inhibition may also protect from some of the side effect of this treatment, thereby improving the quality of life for patients.
p73
p73 is the third member of the p53 family. Similar to p63, it is also expressed as both a full-length TAp73 (or p73) and a dominant-negative truncated form ΔNp73. Although no mutations in p73 have been detected in human cancers, TAp73 and ΔNp73 are commonly dysregulated [63–67]. Several lines of evidence support a role for p73 in determining chemotherapeutic drug response in cancer treatment. First, TAp73 is induced by diverse groups of chemotherapeutics such as doxorubicin, etoposide, cisplatin and paclitaxel [68–72]. Secondly, p73 knockout (p73−/−) MEFs are more resistant to chemotherapeutics compared with their wild-type counterparts [73]. Thirdly, down-regulation of TAp73 caused by overexpression of its dominant-negative mutant ΔNp73 or siRNA (small interfering RNA) leads to enhanced resistance of human tumour cells to chemotherapeutics even when p53 is mutated, whereas overexpression of TAp73 enhances chemosensitivity [66,70,71,74–79]. Finally, these results are supported by in vivo clinical data showing that overexpression of the dominant-negative p73 isoform ΔNp73 contributes to drug resistance to platinum-based therapy [80].
Several proteins have been shown to regulate p73 activity in response to chemotherapeutic drug treatments. For example, it has been shown that endogenous p73 is activated in response to a variety of chemotherapeutic drugs in a c-Abl-dependent manner [68,74,81]. Specifically, following drug treatment the tyrosine kinase c-Abl phosphorylates p73 on the tyrosine residue at position 99 (Tyr99) and potentiates p73-mediated transactivation and apoptosis [68,74,81]. c-Abl can also phosphorylate the transcriptional co-activator YAP (Yes kinase-associated protein), which enhances its affinity to p73 and co-activates p73 pro-apoptotic target genes [82]. In addition, chemotherapeutic drug treatment can enhance the interaction between the prolyl isomerase Pin1 and p73 to promote p73 acetylation by acetyltransferase p300, which increases p73 stability and transcriptional activity [83]. Furthermore, it has also been shown both in vitro and in clinical cancers that mutant p53, ΔNp63 and ΔNp73 can directly bind and inhibit p73, resulting in reduced transcriptional activation of pro-apoptotic genes Bax, PUMA and p53AIP1, ultimately rendering cancers cells and patients resistant to chemotherapeutic drug treatment [52,70,71,84,85].
CKIs [CDK (cyclin-dependent kinase) inhibitors]
The CKI proteins primarily function as negative regulators of CDKs and cell cycle progression, although each member has also been associated with additional cellular functions, including apoptosis, senescence, transcription, or cell migration [86–88]. CKIs are divided into two families including the INK4 (inhibitor of CDK4) family (p16INK4a, p15INK4b, p18INK4c and p14INK4d) and the Cip/Kip family (p21Cip1, p27Kip1 and p57Kip2). INK4 family proteins inhibit CDK4 and CDK6 to prevent G1–S cell cycle progression, whereas the Cip/Kip family proteins inhibit all CDKs and modulate progression through each stage of the cell cycle [86]. Importantly, several of these CKIs including p16INK4a, p14INK4d, p21Cip1 and p27Kip1 are implicated in modulating chemotherapeutic sensitivity.
INK4 family
The INK4a-ARF (ADP-ribosylation factor) locus, encoding both p16INK4a and p14INK4d (p19Arf in mice) through alternative splicing, plays a prominent role in tumour development and is one of the most frequently inactivated TSGs in cancer through a combination of mutations and/or epigenetic silencing mechanisms [87,88]. Together p16INK4a and p14INK4d regulate Rb and p53 respectively [89,90]. Although p16INK4a and p14INK4d function through different signalling pathways, their response to various chemotherapeutics is quite similar.
In response to almost all chemotherapeutics and across all cell lines examined, expression of p16INK4a or p14INK4d increases apo-ptosis in cell lines with functional p53. For example, ectopic expression of p16INK4a expression in nasopharyngeal cell lines led to an increase in apoptosis in response to the anti-metabolite 5-FU or the DNA cross-linking agent cisplatin [91] and inducible p16INK4a expression in a melanoma cell line increased sensitivity to the DNA alkylating agent melphalan [92]. Similarly, ectopic expression of p14INK4d in breast carcinoma MCF-7 cells or osteosarcoma U2OS cells enhanced chemosensitivity to cisplatin or doxorubicin [93–95]. On the other hand, down-regulation of either p16INK4a or p14INK4d leads to drug resistance in wild-type p53 models. Specifically, in a mouse lymphoma model, Schmitt et al. [96,97] showed that knockout of either p16INK4a or p19Arf in MEFs leads to resistance to the alkylating agent cyclophosphamide. In addition, loss of p14INK4d in MEFs leads to resistance to doxorubicin [98]. Interestingly, in response to topoisomerase inhibitors, overexpression of either p16INK4a or p14INK4d had no effect on cell death [94,95], suggesting that p16INK4a and p14INK4d are specifically activated in response to certain chemotherapeutics.
Importantly these cell line studies correlate with in vivo patient clinical studies. Whereas high p16INK4a predicts a positive response to melphalan treatment in patients with melanoma [92] or 5-FU treatment in colorectal cancer patients [99], ovarian cancer patients with a p16INK4a deletion were less likely to respond positively to cisplatin-based chemotherapy after surgery [100]. In addition, loss of heterozygosity of p16INK4a in childhood B-cell precursor acute lymphoblastic leukaemia predicts for a slower response to induction therapy and poorer prognosis [101]. Thus, expression of p16Ink4a may be a useful marker for predicting chemosensitivity.
Cip/Kip family (p21Cip1 and p27Kip1)
Due to their role in regulating cell cycle progression in response to DNA damage signals or anti-mitogenic cues, a role for p21Cip1 and p27Kip2 in mediating a chemotherapeutic response became apparent. In general, increased expression of p21Cip1 or p27Kip1 leads to chemoresistance, whereas loss of p21Cip1 or p27Kip1 expression sensitizes cell lines to various chemotherapeutics [102–123]. Studies in patients with lung cancer or acute myelogenous leukaemia also show that high expression of p21Cip1 predicts for a less favourable response to their respective chemotherapy regimens [124,125]. However, exceptions to this rule occur. For example, several studies show that in ovarian cancer, head and neck carcinoma, or A549 lung cancer cells, ectopic expression of p21Cip1 and/or p27Kip1 enhanced cisplatin-induced apoptosis [126–128], although no mechanisms were proposed. In addition, low levels of p27Kip1 in ovarian cancer patients correlates with chemoresistance to paclitaxel/cisplatin-based therapy [129], and high levels of p27Kip1 were observed in 47% of breast cancer patients who were significantly more susceptible to doxorubicin treatment [130]. Thus, the roles of Cip/Kip in determining chemosensitivity may also depend on cell type and/or cellular localization, as well as the involvement of other signalling mechanisms.
Several mechanisms have been described for how p21Cip1 and p27Kip1 may inhibit drug-induced cell death. Expectedly, one potential mechanism relies on the primary role of p21Cip1 and p27Kip1 as CDK inhibitors. For example, in response to the microtubule inhibitor paclitaxel, p21Cip1 and p27Kip1 induce G2/M arrest [106,112,115,123]. In particular, paclitaxel treatment of breast cancer cells leads to transcriptional up-regulation of p21Cip1, which inhibits Cdc2 (cell division cycle 2)/cyclin B kinase activity, thereby delaying entry into mitosis where the microtubule inhibitor paclitaxel is most active [115,119,123]. Alternatively, when the S phase is perturbed due to DNA damaging agents such as cisplatin or doxorubicin, p21Cip1 halts the cell cycle and initiates a repair mechanism, thereby allowing cells to continue proliferating [110,114].
Besides a direct role in mediating cell cycle arrest, both p21Cip1 and p27Kip1 also participate in the mitochondrial or intrinsic apo-ptotic pathways. For example, loss of p21Cip1 increases p14INK4d and p53 expression. This leads to a reduction in the membrane mitochondrial potential, activation of caspase 9 and an increase in pro-apoptotic Bax with a decrease in the anti-apoptotic Bcl-2 protein [107]. In leukaemia cells, p21Cip1 prevents down-regulation of c-IAP1 (cellular inhibitor of apoptosis protein 1), an inhibitor of apoptosis [118], whereas overexpression of p27Kip1 inhibits activation of procaspase 3, mitochondrial potential changes and cytochrome c release in response to etoposide [104]. Thus, depending on cell type or drug treatment, expression and localization of p21Cip1 and p27Kip1 can mediate a variety of apoptotic effects leading to enhanced chemosensitivity.
PTEN (phosphatase and tensin homologue deleted from chromosome 10)
PTEN is a dual protein and lipid phosphatase that is commonly mutated in many human malignancies [131–133]. Loss of PTEN results in reduced dephosphorylation of PIP3 (phosphoinositide 3,4,5-trisphosphate), which allows PI3K (phosphoinositide 3-kinase) to phosphorylate PIP2 (phosphatidylinositol 4,5-bisphosphate) and enhance levels of PIP3. PIP3 induction causes increased cell proliferation and cell migration, cell survival and cell size through activation of downstream proteins such as Akt [134,135]. Recently, several reports have also shown that PTEN plays an important role in the response of human cancer cells to oncoprotein targeting agents. Nagata et al. [136] showed that treatment of HER2-overexpressing breast cancer cells with the HER2-targeting antibody, trastuzumab (herceptin), quickly increased PTEN membrane localization and phosphatase activity by reducing PTEN tyrosine phosphorylation via SRC kinase inhibition. On the other hand, down-regulation of PTEN in breast cancer cells results in trastuzumab-resistance both in vitro and in vivo. In addition, miR (microRNA)-21 can also cause drug resistance by down-regulation of PTEN [137]. Most importantly, clinical studies demonstrate that PTEN status can be used as a predictive marker for determining breast cancer patient response to trastuzumab. Patients with PTEN-deficient breast cancers had significantly poorer response to trastuzumab and shorter overall survival than those with PTEN expression [136,138]. Later studies demonstrated that PTEN down-regulation can be also used as a biomarker to predict low response to the EGFR inhibitors (cetuximab, gefitinib and erlotinib) for treatment of colorectal and lung cancers [138–140].
Several mechanisms have been proposed to explain the requirement of PTEN in response of cancers to oncoprotein targeting drug therapy. First, activation of PI3K pathway has been shown to be responsible for loss-of-PTEN-induced drug resistance [141,142]. In support of this notion, PI3K inhibitors can reverse loss-of-PTEN-induced trastuzumab resistance, whereas activation of the components of the PI3K pathway, namely PI3K3A and pAkt, caused trastuzumab resistance [136,138,141,143]. In addition, it has been shown that activation of PI3K induces drug resistance by inducing MRP1 (multidrug resistance protein-1) [144]. Secondly, a recent discovery has uncovered a new mechanism of PTEN in regulating trastuzumab resistance. Zhang et al. showed that the non-receptor tyrosine kinase SRC is a key modulator of trastuzumab response [145]. Activation of SRC by EGFR further activates Akt, which leads to both acquired and de novo trastuzumab resistance in breast cancer. Most significantly, they found that PTEN could dephosphorylate and inhibit SRC kinase activity. Therefore loss of PTEN leads to increased phosphorylation/activation of SRC and subsequent trastuzumab resistance, which can be reversed by inhibiting SRC with the SRC-specific inhibitor saracatinib [145].
BRCA1 (breast-cancer susceptibility gene 1)
BRCA1 is frequently mutated in inherited breast cancers [146,147]. Although somatic mutations of BRCA1 are rare in sporadic breast and ovarian cancers, epigenetic down-regulation of BRCA1 is a more frequent event [148,149]. BRCA1 functions as a tumour suppressor by regulating transcription, cell cycle checkpoint and DNA repair [150–152]. Most importantly, BRCA1 plays a significant role in the repair of DNA DSBs (double stranded breaks) through HR (homologous recombination) [153].
The participation of BRCA1 in DNA repair provides a strong rationale for a role of BRCA1 in the response to DNA-damaging drugs. Early studies using BRCA1−/− (BRCA1 knockout) ESCs (embryonic stem cells) showed that BRCA1−/− ESCs were more sensitive to cisplatin, a DNA cross-linking platinum-based drug, compared with WT (wild-type) ESCs [154]. Later studies using BRCA1−/− MEF demonstrated that loss of BRCA1 leads to increased sensitivity to a number of DNA-damaging agents, including the anthracycline doxorubicin, the platinum compound carboplatin, and topoisomerase inhibitors irinotecan and etoposide [155]. Moreover, various other studies further confirmed that while reduction of BRCA1 expression by siRNA results in increased sensitivity, overexpression of BRCA1 causes resistance to platinum compounds and topoisomerase inhibitors [156–158]. Most significantly, clinical studies further establish BRCA1 as a biomarker in predicting the outcome of breast, lung and ovarian cancer treatment with DNA-damage-based therapy [159–163]. Together, data from both in vitro and in vivo studies suggest that low BRCA1 expression, which causes defects in DNA damage repair, represents tumours with high sensitivity to DNA damaging drugs such as cisplatin and PARP [poly(ADP-ribose) polymerase] inhibitors [156,157,159,164–166]. Interestingly, in contrast with DNA-damaging therapy, preclinical and clinical studies also show that loss of BRCA1 causes resistance of cancer cells to other chemotherapeutics including the anti-microtubule agents paclitaxel and docetaxel as well as targeting agents such as the ER (oestrogen receptor) antagonist tamoxifen [5,160,161,163,167,168,170]. Thus, depending on the type of chemotherapeutics, BRCA1 can have either a positive or negative role in mediating chemosensitivity.
Several studies have elucidated the molecular mechanism underlying the opposing effects of BRCA1 on the response of cancer cells to DNA damaging versus anti-microtubule agents. It has been shown that BRCA1 inhibits DNA damaging drug-induced apoptosis by either transcriptionally activating cell cycle checkpoint genes [p21Cip1 and GADD45 (growth-arrest and DNA-damage-inducible protein 45)] or facilitating DNA damage repair processes by interacting with proteins involved in HR (e.g. RAD50/MRE11/NBS1) [171]. Therefore when BRCA1 is inactivated by mutations, deletions or down-regulation, cancer cells will no longer be able to repair the drug-induced DNA damage and will therefore trigger apoptosis, which explains why loss of BRCA1 sensitizes cancer cells to DNA damaging agents. On the other hand, in response to microtubule damage induced by anti-microtubule agents such as paclitaxel or docetaxel, BRCA1 is activated to induce mitotic arrest and apoptosis by transcriptionally activating the spindle assembly checkpoint protein MAD2 (myoadenylate deaminase 2) [172] thereby activating the JNK (c-Jun N-terminal kinase) pathway via direct interaction with the JNK–MEKK3 {MEK [MAPK (mitogen-activated protein kinase)/ERK (extracellular-signal-regulated kinase) kinase] kinase 3} complex [167]. Therefore when BRCA1 is lost cancer cells will no longer activate the mitotic spindle checkpoint protein MAD2 and subsequent activation of the pro-apoptotic JNK pathway, resulting in resistance to anti-microtubule agents.
The Hippo tumour suppressor pathway
The emerging Hippo tumour suppressor pathway was originally identified in Drosophila and later in mammals [173–177]. In this pathway, the serine/threonine kinases Mst1/2 (Hippo in Drosophila) and LATS1/2 together with an adaptor protein hMOB1 are the core players, which transmit signals from upstream tumour suppressors (Fat4, RASSF1A, Kibra, Merlin, hEx, hWW45, etc.). This inhibits the transcriptional co-activators and oncoproteins YAP (Yes kinase-associated protein) and TAZ (transcriptional co-activator with PDZ-binding motif), resulting in reduced cell proliferation and enhanced cell death through modulation of downstream transcriptional targets [178]. In addition, we and others have also shown that the Hippo tumour suppressor pathway can be negatively regulated by several proteins such as the Itch ubiquitin ligase, HA (hyaluronan) receptor CD44 and p53 regulator ASPP1 [179–182]. Most significantly, recent studies show that the Hippo tumour suppressor pathway plays important roles not only in cancer but also in various biological processes such as organ size control, stem cell renewal and differentiation, tissue regeneration, neuronal dendrite growth and mechanotransduction [174,176,183].
Recently, mounting evidence strongly suggests that the Hippo tumour suppressor pathway may also regulate the response of cancer cells to chemotherapeutics. We have recently shown that knockdown of both LATS1 and its homologue LATS2 by siRNA causes resistance of HeLa cervical carcinoma cells to paclitaxel [184]. Consistent with our findings, in a screen of shRNAs (short hairpin RNAs) targeting TSGs, LATS1 was identified as one of the genes causing paclitaxel resistance upon knockdown in A549 lung cancer cells [185]. In addition, loss of LATS2 in leukaemic cells renders cells more resistant to the DNA-damaging agents doxorubicin and etoposide through promoting YAP and p73 interaction thereby inhibiting transcription of the pro-apoptotic gene PUMA [186]. Moreover, we have recently shown that enhanced levels of TAZ, a substrate and downstream target of LATS1/2 kinases (Figure 1), in breast cancer cells correlates with resistance to paclitaxel [187]. Specifically, overexpression of TAZ in TAZ-low MCF-10A immortalized mammary cells causes resistance to paclitaxel whereas knockdown of TAZ in TAZ-high paclitaxel-resistant MDA-MB231 breast cancer cells sensitizes those cells to paclitaxel [187]. This increased paclitaxel resistance results from TAZ activation of TEAD family of transcription factors, which induces transcription and expression of the extracellular matrix genes Cyr61 (cysteine-rich 61) and CTGF (connective tissue growth factor; Figure 1). Similarly, overexpression of the TAZ paralogue, YAP, has also been shown to cause paclitaxel and cisplatin resistance in mammary and ovarian cells [188,189]. Since LATS1/2 and YAP/TAZ have opposing effects on drug response and LATS1/2 can inhibit YAP/TAZ function through phosphorylation [190–192], we propose that YAP/TAZ-TEAD-Cyr61/CTGF is a novel signalling pathway mediating loss-of-LATS-induced drug resistance in the treatment of human cancers (Figure 1). It has also been shown that loss of other important TSGs in the Hippo pathway such as Mst1, hEx and RASSF1A also leads to drug resistance [193–196], whereas overexpression of CD44 or Itch, the negative regulators of the Hippo pathway, induces drug resistance [180,197]. Interestingly, it has also been shown that CD44 induces resistance of glioblastoma cells to the DNA damaging drugs temozolomide and carmustine by inactivating the Mst/LATS/YAP signalling pathway (Figure 1) [180]. Together, these findings clearly demonstrate that the Hippo pathway plays very important roles in the response of cancer cells to chemotherapeutics.
Other TSGs
Besides the TSGs mentioned above, many other TSGs have also been reported as critical regulators of cancer cells to chemotherapeutics. Loss of the TSGs including SMAD4 (response to paclitaxel), LZTS2, ST14 and VHL increases the cells' sensitivity to different chemotherapeutics [185,199], whereas loss of ARID1A, Caveolin-1, PDCD4, PCDH10, FBW7, SMAD4 (response to 5-FU), FUS1 and p33ING1b causes drug resistance [200–207]. In addition, many miRs functioning as TSGs have also been shown to regulate drug sensitivity [208]. For example, down-regulation of Let-7, miR-34, or miR-181 increases chemosensitivity, whereas down-regulation of miR-127 causes chemoresistance [209]. In addition, overexpression of miR-125a-5p increases sensitivity to drugs [210], whereas overexpression of miR-15-5p is associated drug resistance [211,212]. The growing list of tumour suppressor genes and their growing importance for mediating a chemotherapeutic response suggests their importance in regulating tumorigenesis and predicting overall survival.
Interaction of TSGs in chemotherapeutic response
Although different TSGs have distinct biological functions, they can co-operate co-ordinately in response to drugs in common signalling pathways as summarized in Figure 1. For example, p53 may interact with many other TSGs in the chemotherapeutic response [16,19,28,32,34]. First, p53 can modulate the TAp73/TAp63-mediated drug response by activating commonly regulated genes such as pro-apoptotic genes Bax and PUMA or the CKI p21Cip1 (Figure 1). In addition, p53 status can be used to predict the clinical outcome of breast cancer patients treated with adjuvant chemotherapy (5-FU, methotrexate and cyclophosphamide) only when Rb is active [213]. However, when Rb is deleted or down-regulated by methylation, p53 status has no predictive value for chemosensitivity in these patients. Furthermore, it has also been shown that the combined status of p53 and ATM are also important in predicting drug sensitivity. While suppression of ATM protects tumours from chemotherapeutic treatment in the presence of p53, suppression of ATM dramatically sensitizes tumours to DNA-damaging chemotherapy when p53 is inactivated (Figure 1) [214]. Moreover, p53 function also dictates how p16INK4a or p14INK4d mediate a chemotherapeutic response. In cell lines without functional p53 caused by mutation or down-regulation, increased expression of either p16INK4a or p14INK4d leads to resistance to different chemotherapeutics including anti-metabolites such as folate antagonists, the anti-microtubule paclitaxel, or the DNA intercalating agent cisplatin [116,215,216]. Since p14INK4d primarily functions as an inhibitor of Mdm2 (murine double minute 2), an inhibitor of p53 (Figure 1) [90], loss of p53 in this scenario provides a clear explanation for the inability of p14INK4d to enhance chemosensitivity [98]. The connection between p53 and p16INK4a is less clear and requires further study. Besides p53 and BRCA1 can also functionally interact with other TSGs such as the other p53 family members or PTEN through the modulation of p21Cip1 or JNK in response to chemotherapeutic drugs (Figure 1). All together, the complex interactions between TSGs suggest that modulation of TSGs will likely affect multiple signalling pathways and lead to a complex drug response.
CONCLUDING REMARKS
Through the use of mainly human cancer cell lines and mouse models, numerous studies have provided strong evidence that TSGs can either enhance or reduce the sensitivity of cancer cells to chemotherapeutic drugs depending on the cellular context or class of chemotherapeutics (Figure 1). Although some of the TSGs such as BRCA1 have been used as a biomarker in predicting the outcome of chemotherapeutic drug treatment in cancer patients, most of the findings from cell lines and mouse model remain to be further verified by large-scale screening of clinical cancer patients treated by chemotherapeutics. Furthermore, the complexity of cellular signalling programmes suggests that single gene biomarkers are insufficient for determining treatment outcome. Instead, understanding the contribution of each key player such Rb, p53, the CKIs, PTEN and the Hippo pathway in context with each other will provide a more reliable predictor for tumour response. We anticipate that in the next decade increasing numbers of TSGs will be established as important prognostic markers in predicting the chemotherapeutic response of cancer patients. This will lead to a promising therapeutic strategy whereby drug-resistant patients can be successfully treated through the modulation of tumour suppressor signalling pathways.
FUNDING
Our own work was supported by the CIHR (Canadian Institute of Health Research) [grant number MOP119325], CBCF (Canadian Breast Cancer Foundation) and CRS (Cancer Research Society) to X.Y. D.L. was supported by the CIHR/Terry Fox Foundation Training Program in Transdisciplinary Cancer Research and the Ontario Graduate Scholarship. S.V.G. was supported by the CIHR/Terry Fox Foundation Training Program in Transdisciplinary Cancer Research.
References
- 1.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer. 2007;7:573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 2.Coley H. M. Mechanisms and strategies to overcome chemotherapy resistance in metastatic breast cancer. Cancer Treat. Rev. 2008;34:378–390. doi: 10.1016/j.ctrv.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Andreetta C., Minisini A. M., Miscoria M., Puglisi F. First-line chemotherapy with or without biologic agents for metastatic breast cancer. Crit. Rev. Oncol. Hematol. 2010;76:99–111. doi: 10.1016/j.critrevonc.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Johnson K. A., Brown P. H. Drug development for cancer chemoprevention: focus on molecular targets. Semin. Oncol. 2010;37:345–358. doi: 10.1053/j.seminoncol.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 5.McGrogan B. T., Gilmartin B., Carney D. N., McCann A. Taxanes, microtubules and chemoresistant breast cancer. Biochim. Biophys. Acta. 2008;1785:96–132. doi: 10.1016/j.bbcan.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Fodale V., Pierobon M., Liotta L., Petricoin E. Mechanism of cell adaptation: When and how do cancer cells develop chemoresistance? Cancer J. 2011;17:89–95. doi: 10.1097/PPO.0b013e318212dd3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang A. Chemotherapy, chemoresistance and the changing treatment landscape for NSCLC. Lung Cancer. 2011;71:3–10. doi: 10.1016/j.lungcan.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 8.Indran I. R., Tufo G., Pervaiz S., Brenner C. Recent advances in apoptosis, mitochondria and drug resistance in cancer cells. Biochim. Biophys. Acta. 2011;1807:735–745. doi: 10.1016/j.bbabio.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Ganesan S. MYC, PARP1, and chemoresistance: BIN there, done that? Sci. Signaling. 2011;4:pe15. doi: 10.1126/scisignal.2001946. [DOI] [PubMed] [Google Scholar]
- 10.Friend S. H., Bernards R., Rogelj S., Weinberg R. A., Rapaport J. M., Albert D. M., Dryja T. P. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986;323:643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- 11.Burkhart D. L., Sage J. Cellular mechanisms of tumour suppression by the retinoblastoma gene. Nat. Rev. Cancer. 2008;8:671–682. doi: 10.1038/nrc2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knudsen E. S., Knudsen K. E. Tailoring to RB: tumour suppressor status and therapeutic response. Nat. Rev. Cancer. 2008;8:714–724. doi: 10.1038/nrc2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sherr C. J., McCormick F. The RB and p53 pathways in cancer. Cancer Cell. 2002;2:103–112. doi: 10.1016/s1535-6108(02)00102-2. [DOI] [PubMed] [Google Scholar]
- 14.Weinberg R. A. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 15.Du W., Searle J. S. The rb pathway and cancer therapeutics. Curr. Drug Targets. 2009;10:581–589. doi: 10.2174/138945009788680392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Almasan A., Yin Y., Kelly R. E., Lee E. Y., Bradley A., Li W., Bertino J. R., Wahl G. M. Deficiency of retinoblastoma protein leads to inappropriate S-phase entry, activation of E2F-responsive genes, and apoptosis. Proc. Natl. Acad. Sci. U.S.A. 1995;92:5436–5440. doi: 10.1073/pnas.92.12.5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knudsen K. E., Booth D., Naderi S., Sever-Chroneos Z., Fribourg A. F., Hunton I. C., Feramisco J. R., Wang J. Y., Knudsen E. S. RB-dependent S-phase response to DNA damage. Mol. Cell. Biol. 2000;20:7751–7763. doi: 10.1128/mcb.20.20.7751-7763.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma A., Comstock C. E., Knudsen E. S., Cao K. H., Hess-Wilson J. K., Morey L. M., Barrera J., Knudsen K. E. Retinoblastoma tumor suppressor status is a critical determinant of therapeutic response in prostate cancer cells. Cancer Res. 2007;67:6192–6203. doi: 10.1158/0008-5472.CAN-06-4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bosco E. E., Wang Y., Xu H., Zilfou J. T., Knudsen K. E., Aronow B. J., Lowe S. W., Knudsen E. S. The retinoblastoma tumor suppressor modifies the therapeutic response of breast cancer. J. Clin. Invest. 2007;117:218–228. doi: 10.1172/JCI28803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Derenzini M., Donati G., Mazzini G., Montanaro L., Vici M., Ceccarelli C., Santini D., Taffurelli M., Trere D. Loss of retinoblastoma tumor suppressor protein makes human breast cancer cells more sensitive to antimetabolite exposure. Clin. Cancer Res. 2008;14:2199–2209. doi: 10.1158/1078-0432.CCR-07-2065. [DOI] [PubMed] [Google Scholar]
- 21.Stengel K. R., Dean J. L., Seeley S. L., Mayhew C. N., Knudsen E. S. RB status governs differential sensitivity to cytotoxic and molecularly-targeted therapeutic agents. Cell Cycle. 2008;7:1095–1103. doi: 10.4161/cc.7.8.5737. [DOI] [PubMed] [Google Scholar]
- 22.Bosco E. E., Mayhew C. N., Hennigan R. F., Sage J., Jacks T., Knudsen E. S. RB signaling prevents replication-dependent DNA double-strand breaks following genotoxic insult. Nucleic Acids Res. 2004;32:25–34. doi: 10.1093/nar/gkg919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nahle Z., Polakoff J., Davuluri R. V., McCurrach M. E., Jacobson M. D., Narita M., Zhang M. Q., Lazebnik Y., Bar-Sagi D., Lowe S. W. Direct coupling of the cell cycle and cell death machinery by E2F. Nat. Cell Biol. 2002;4:859–864. doi: 10.1038/ncb868. [DOI] [PubMed] [Google Scholar]
- 24.Moroni M. C., Hickman E. S., Lazzerini Denchi E., Caprara G., Colli E., Cecconi F., Muller H., Helin K. Apaf-1 is a transcriptional target for E2F and p53. Nat. Cell Biol. 2001;3:552–558. doi: 10.1038/35078527. [DOI] [PubMed] [Google Scholar]
- 25.Irwin M., Marin M. C., Phillips A. C., Seelan R. S., Smith D. I., Liu W., Flores E. R., Tsai K. Y., Jacks T., Vousden K. H., Kaelin W. G., Jr Role for the p53 homologue p73 in E2F-1-induced apoptosis. Nature. 2000;407:645–648. doi: 10.1038/35036614. [DOI] [PubMed] [Google Scholar]
- 26.Lin W. C., Lin F. T., Nevins J. R. Selective induction of E2F1 in response to DNA damage, mediated by ATM-dependent phosphorylation. Genes Dev. 2001;15:1833–1844. [PMC free article] [PubMed] [Google Scholar]
- 27.Stevens C., Smith L., La Thangue N. B. Chk2 activates E2F-1 in response to DNA damage. Nat. Cell Biol. 2003;5:401–409. doi: 10.1038/ncb974. [DOI] [PubMed] [Google Scholar]
- 28.Zagorski W. A., Knudsen E. S., Reed M. F. Retinoblastoma deficiency increases chemosensitivity in lung cancer. Cancer Res. 2007;67:8264–8273. doi: 10.1158/0008-5472.CAN-06-4753. [DOI] [PubMed] [Google Scholar]
- 29.Trere D., Brighenti E., Donati G., Ceccarelli C., Santini D., Taffurelli M., Montanaro L., Derenzini M. High prevalence of retinoblastoma protein loss in triple-negative breast cancers and its association with a good prognosis in patients treated with adjuvant chemotherapy. Ann. Oncol. 2009;20:1818–1823. doi: 10.1093/annonc/mdp209. [DOI] [PubMed] [Google Scholar]
- 30.Ertel A., Dean J. L., Rui H., Liu C., Witkiewicz A. K., Knudsen K. E., Knudsen E. S. RB-pathway disruption in breast cancer: Differential association with disease subtypes, disease-specific prognosis and therapeutic response. Cell Cycle. 2010;9:4153–4163. doi: 10.4161/cc.9.20.13454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herschkowitz J. I., He X., Fan C., Perou C. M. The functional loss of the retinoblastoma tumour suppressor is a common event in basal-like and luminal B breast carcinomas. Breast Cancer Res. 2008;10:R75. doi: 10.1186/bcr2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li W., Fan J., Hochhauser D., Banerjee D., Zielinski Z., Almasan A., Yin Y., Kelly R., Wahl G. M., Bertino J. R. Lack of functional retinoblastoma protein mediates increased resistance to antimetabolites in human sarcoma cell lines. Proc. Natl. Acad. Sci. U.S.A. 1995;92:10436–10440. doi: 10.1073/pnas.92.22.10436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiu W., Wu J., Walsh E. M., Zhang Y., Chen C. Y., Fujita J., Xiao Z. X. Retinoblastoma protein modulates gankyrin-MDM2 in regulation of p53 stability and chemosensitivity in cancer cells. Oncogene. 2008;27:4034–4043. doi: 10.1038/onc.2008.43. [DOI] [PubMed] [Google Scholar]
- 34.Ianari A., Natale T., Calo E., Ferretti E., Alesse E., Screpanti I., Haigis K., Gulino A., Lees J. A. Proapoptotic function of the retinoblastoma tumor suppressor protein. Cancer Cell. 2009;15:184–194. doi: 10.1016/j.ccr.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vousden K. H. P53: Death star. Cell. 2000;103:691–694. doi: 10.1016/s0092-8674(00)00171-9. [DOI] [PubMed] [Google Scholar]
- 36.Harris S. L., Levine A. J. The p53 pathway: positive and negative feedback loops. Oncogene. 2005;24:2899–2908. doi: 10.1038/sj.onc.1208615. [DOI] [PubMed] [Google Scholar]
- 37.Chipuk J. E., Green D. R. Dissecting p53-dependent apoptosis. Cell Death Differ. 2006;13:994–1002. doi: 10.1038/sj.cdd.4401908. [DOI] [PubMed] [Google Scholar]
- 38.Vousden K. H., Prives C. Blinded by the light: the growing complexity of p53. Cell. 2009;137:413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 39.Sax J. K., El-Deiry W. S. P53 downstream targets and chemosensitivity. Cell Death Differ. 2003;10:413–417. doi: 10.1038/sj.cdd.4401227. [DOI] [PubMed] [Google Scholar]
- 40.Bertheau P., Espie M., Turpin E., Lehmann J., Plassa L. F., Varna M., Janin A., de The H. TP53 status and response to chemotherapy in breast cancer. Pathobiology. 2008;75:132–139. doi: 10.1159/000123851. [DOI] [PubMed] [Google Scholar]
- 41.Lu C., El-Deiry W. S. Targeting p53 for enhanced radio- and chemo-sensitivity. Apoptosis. 2009;14:597–606. doi: 10.1007/s10495-009-0330-1. [DOI] [PubMed] [Google Scholar]
- 42.Weinstein J. N., Myers T. G., O'Connor P. M., Friend S. H., Fornace A. J., Jr, Kohn K. W., Fojo T., Bates S. E., Rubinstein L. V., Anderson N. L., et al. An information-intensive approach to the molecular pharmacology of cancer. Science. 1997;275:343–349. doi: 10.1126/science.275.5298.343. [DOI] [PubMed] [Google Scholar]
- 43.O'Connor P. M., Jackman J., Bae I., Myers T. G., Fan S., Mutoh M., Scudiero D. A., Monks A., Sausville E. A., Weinstein J. N., et al. Characterization of the p53 tumor suppressor pathway in cell lines of the National Cancer Institute anticancer drug screen and correlations with the growth-inhibitory potency of 123 anticancer agents. Cancer Res. 1997;57:4285–4300. [PubMed] [Google Scholar]
- 44.Huang S., Benavente S., Armstrong E. A., Li C., Wheeler D. L., Harari P. M. p53 modulates acquired resistance to EGFR inhibitors and radiation. Cancer Res. 2011;71:7071–7079. doi: 10.1158/0008-5472.CAN-11-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sax J. K., Stoddard A., Murphy M. E., Chodosh L., El-Deiry W. S. Microarray expression profiling of p53-dependent transcriptional changes in an immortalized mouse embryo fibroblast cell line. Cancer. Biol. Ther. 2003;2:416–430. doi: 10.4161/cbt.2.4.477. [DOI] [PubMed] [Google Scholar]
- 46.Michalak E., Villunger A., Erlacher M., Strasser A. Death squads enlisted by the tumour suppressor p53. Biochem. Biophys. Res. Commun. 2005;331:786–798. doi: 10.1016/j.bbrc.2005.03.183. [DOI] [PubMed] [Google Scholar]
- 47.Yee K. S., Vousden K. H. Complicating the complexity of p53. Carcinogenesis. 2005;26:1317–1322. doi: 10.1093/carcin/bgi122. [DOI] [PubMed] [Google Scholar]
- 48.Vasey P. A., Jones N. A., Jenkins S., Dive C., Brown R. Cisplatin, camptothecin, and taxol sensitivities of cells with p53-associated multidrug resistance. Mol. Pharmacol. 1996;50:1536–1540. [PubMed] [Google Scholar]
- 49.Bunz F., Hwang P. M., Torrance C., Waldman T., Zhang Y., Dillehay L., Williams J., Lengauer C., Kinzler K. W., Vogelstein B. Disruption of p53 in human cancer cells alters the responses to therapeutic agents. J. Clin. Invest. 1999;104:263–269. doi: 10.1172/JCI6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moreno C. S., Matyunina L., Dickerson E. B., Schubert N., Bowen N. J., Logani S., Benigno B. B., McDonald J. F. Evidence that p53-mediated cell-cycle-arrest inhibits chemotherapeutic treatment of ovarian carcinomas. PLoS ONE. 2007;2:e441. doi: 10.1371/journal.pone.0000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Melino G., Lu X., Gasco M., Crook T., Knight R. A. Functional regulation of p73 and p63: Development and cancer. Trends Biochem. Sci. 2003;28:663–670. doi: 10.1016/j.tibs.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 52.Lunghi P., Costanzo A., Mazzera L., Rizzoli V., Levrero M., Bonati A. The p53 family protein p73 provides new insights into cancer chemosensitivity and targeting. Clin. Cancer Res. 2009;15:6495–6502. doi: 10.1158/1078-0432.CCR-09-1229. [DOI] [PubMed] [Google Scholar]
- 53.Urist M. J., Di Como C. J., Lu M. L., Charytonowicz E., Verbel D., Crum C. P., Ince T. A., McKeon F. D., Cordon-Cardo C. Loss of p63 expression is associated with tumor progression in bladder cancer. Am. J. Pathol. 2002;161:1199–1206. doi: 10.1016/S0002-9440(10)64396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Uramoto H., Yamada S., Hanagiri T. Immunohistochemical staining with ΔNp63 is useful for distinguishing the squamous cell component of adenosquamous cell carcinoma of the lung. Anticancer Res. 2010;30:4717–4720. [PubMed] [Google Scholar]
- 55.Gressner O., Schilling T., Lorenz K., Schulze Schleithoff E., Koch A., Schulze-Bergkamen H., Lena A. M., Candi E., Terrinoni A., Catani M. V., et al. TAp63α induces apoptosis by activating signaling via death receptors and mitochondria. EMBO J. 2005;24:2458–2471. doi: 10.1038/sj.emboj.7600708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zangen R., Ratovitski E., Sidransky D. ΔNp63α levels correlate with clinical tumor response to cisplatin. Cell Cycle. 2005;4:1313–1315. doi: 10.4161/cc.4.10.2066. [DOI] [PubMed] [Google Scholar]
- 57.Mundt H. M., Stremmel W., Melino G., Krammer P. H., Schilling T., Muller M. Dominant negative (DeltaN) p63α induces drug resistance in hepatocellular carcinoma by interference with apoptosis signaling pathways. Biochem. Biophys. Res. Commun. 2010;396:335–341. doi: 10.1016/j.bbrc.2010.04.093. [DOI] [PubMed] [Google Scholar]
- 58.Fomenkov A., Zangen R., Huang Y. P., Osada M., Guo Z., Fomenkov T., Trink B., Sidransky D., Ratovitski E. A. RACK1 and stratifin target ΔNp63α for a proteasome degradation in head and neck squamous cell carcinoma cells upon DNA damage. Cell Cycle. 2004;3:1285–1295. doi: 10.4161/cc.3.10.1155. [DOI] [PubMed] [Google Scholar]
- 59.Sun Q., Ming L., Thomas S. M., Wang Y., Chen Z. G., Ferris R. L., Grandis J. R., Zhang L., Yu J. PUMA mediates EGFR tyrosine kinase inhibitor-induced apoptosis in head and neck cancer cells. Oncogene. 2009;28:2348–2357. doi: 10.1038/onc.2009.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lu C., Lu S., Liang W., Li J., Dou X., Bian C., Shi D., Liao L., Zhao R. C. TAp63α mediates chemotherapeutic agent-induced apoptosis in human bone marrow mesenchymal stem cells. Stem Cells Dev. 2011;20:1319–1326. doi: 10.1089/scd.2010.0329. [DOI] [PubMed] [Google Scholar]
- 61.Suh E. K., Yang A., Kettenbach A., Bamberger C., Michaelis A. H., Zhu Z., Elvin J. A., Bronson R. T., Crum C. P., McKeon F. p63 protects the female germ line during meiotic arrest. Nature. 2006;444:624–628. doi: 10.1038/nature05337. [DOI] [PubMed] [Google Scholar]
- 62.Gonfloni S., Di Tella L., Caldarola S., Cannata S. M., Klinger F. G., Di Bartolomeo C., Mattei M., Candi E., De Felici M., Melino G., Cesareni G. Inhibition of the c-abl-TAp63 pathway protects mouse oocytes from chemotherapy-induced death. Nat. Med. 2009;15:1179–1185. doi: 10.1038/nm.2033. [DOI] [PubMed] [Google Scholar]
- 63.Dominguez G., Garcia J. M., Pena C., Silva J., Garcia V., Martinez L., Maximiano C., Gomez M. E., Rivera J. A., Garcia-Andrade C., Bonilla F. ΔTAp73 upregulation correlates with poor prognosis in human tumors: Putative in vivo network involving p73 isoforms, p53, and E2F-1. J. Clin. Oncol. 2006;24:805–815. doi: 10.1200/JCO.2005.02.2350. [DOI] [PubMed] [Google Scholar]
- 64.Dominguez G., Pena C., Silva J., Garcia J. M., Garcia V., Rodriguez R., Cantos B., Citores M. J., Espana P., Bonilla F. The presence of an intronic deletion in p73 and high levels of ZEB1 alter the TAp73/ΔTAp73 ratio in colorectal carcinomas. J. Pathol. 2006;210:390–397. doi: 10.1002/path.2066. [DOI] [PubMed] [Google Scholar]
- 65.Rizzo M. G., Giombini E., Diverio D., Vignetti M., Sacchi A., Testa U., Lo-Coco F., Blandino G. Analysis of p73 expression pattern in acute myeloid leukemias: Lack of ΔN-p73 expression is a frequent feature of acute promyelocytic leukemia. Leukemia. 2004;18:1804–1809. doi: 10.1038/sj.leu.2403483. [DOI] [PubMed] [Google Scholar]
- 66.Muller M., Schilling T., Sayan A. E., Kairat A., Lorenz K., Schulze-Bergkamen H., Oren M., Koch A., Tannapfel A., Stremmel W., et al. TAp73/ΔNp73 influences apoptotic response, chemosensitivity and prognosis in hepatocellular carcinoma. Cell Death Differ. 2005;12:1564–1577. doi: 10.1038/sj.cdd.4401774. [DOI] [PubMed] [Google Scholar]
- 67.Rufini A., Agostini M., Grespi F., Tomasini R., Sayan B. S., Niklison-Chirou M. V., Conforti F., Velletri T., Mastino A., Mak T. W., et al. p73 in cancer. Genes Cancer. 2011;2:491–502. doi: 10.1177/1947601911408890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gong J. G., Costanzo A., Yang H. Q., Melino G., Kaelin W. G., Jr, Levrero M., Wang J. Y. The tyrosine kinase c-abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature. 1999;399:806–809. doi: 10.1038/21690. [DOI] [PubMed] [Google Scholar]
- 69.Costanzo A., Merlo P., Pediconi N., Fulco M., Sartorelli V., Cole P. A., Fontemaggi G., Fanciulli M., Schiltz L., Blandino G., et al. DNA damage-dependent acetylation of p73 dictates the selective activation of apoptotic target genes. Mol. Cell. 2002;9:175–186. doi: 10.1016/s1097-2765(02)00431-8. [DOI] [PubMed] [Google Scholar]
- 70.Irwin M. S., Kondo K., Marin M. C., Cheng L. S., Hahn W. C., Kaelin W. G., Jr Chemosensitivity linked to p73 function. Cancer Cell. 2003;3:403–410. doi: 10.1016/s1535-6108(03)00078-3. [DOI] [PubMed] [Google Scholar]
- 71.Bergamaschi D., Gasco M., Hiller L., Sullivan A., Syed N., Trigiante G., Yulug I., Merlano M., Numico G., Comino A., et al. p53 polymorphism influences response in cancer chemotherapy via modulation of p73-dependent apoptosis. Cancer Cell. 2003;3:387–402. doi: 10.1016/s1535-6108(03)00079-5. [DOI] [PubMed] [Google Scholar]
- 72.Moll U. M., Slade N. p63 and p73: roles in development and tumor formation. Mol. Cancer Res. 2004;2:371–386. [PubMed] [Google Scholar]
- 73.Flores E. R., Tsai K. Y., Crowley D., Sengupta S., Yang A., McKeon F., Jacks T. p63 and p73 are required for p53-dependent apoptosis in response to DNA damage. Nature. 2002;416:560–564. doi: 10.1038/416560a. [DOI] [PubMed] [Google Scholar]
- 74.Yuan Z. M., Shioya H., Ishiko T., Sun X., Gu J., Huang Y. Y., Lu H., Kharbanda S., Weichselbaum R., Kufe D. p73 is regulated by tyrosine kinase c-abl in the apoptotic response to DNA damage. Nature. 1999;399:814–817. doi: 10.1038/21704. [DOI] [PubMed] [Google Scholar]
- 75.Ben-Yehoyada M., Ben-Dor I., Shaul Y. c-abl tyrosine kinase selectively regulates p73 nuclear matrix association. J. Biol. Chem. 2003;278:34475–34482. doi: 10.1074/jbc.M301051200. [DOI] [PubMed] [Google Scholar]
- 76.Muller M., Schleithoff E. S., Stremmel W., Melino G., Krammer P. H., Schilling T. One, two, three – p53, p63, p73 and chemosensitivity. Drug Resist. Updat. 2006;9:288–306. doi: 10.1016/j.drup.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 77.Vayssade M., Haddada H., Faridoni-Laurens L., Tourpin S., Valent A., Benard J., Ahomadegbe J. C. P73 functionally replaces p53 in adriamycin-treated, p53-deficient breast cancer cells. Int. J. Cancer. 2005;116:860–869. doi: 10.1002/ijc.21033. [DOI] [PubMed] [Google Scholar]
- 78.Tuve S., Racek T., Niemetz A., Schultz J., Soengas M. S., Putzer B. M. Adenovirus-mediated TA-p73β gene transfer increases chemosensitivity of human malignant melanomas. Apoptosis. 2006;11:235–243. doi: 10.1007/s10495-006-3407-0. [DOI] [PubMed] [Google Scholar]
- 79.Seitz S. J., Schleithoff E. S., Koch A., Schuster A., Teufel A., Staib F., Stremmel W., Melino G., Krammer P. H., Schilling T., Muller M. Chemotherapy-induced apoptosis in hepatocellular carcinoma involves the p53 family and is mediated via the extrinsic and the intrinsic pathway. Int. J. Cancer. 2010;126:2049–2066. doi: 10.1002/ijc.24861. [DOI] [PubMed] [Google Scholar]
- 80.Concin N., Hofstetter G., Berger A., Gehmacher A., Reimer D., Watrowski R., Tong D., Schuster E., Hefler L., Heim K., et al. Clinical relevance of dominant-negative p73 isoforms for responsiveness to chemotherapy and survival in ovarian cancer: eidence for a crucial p53-p73 cross-talk in vivo. Clin. Cancer Res. 2005;11:8372–8383. doi: 10.1158/1078-0432.CCR-05-0899. [DOI] [PubMed] [Google Scholar]
- 81.Agami R., Blandino G., Oren M., Shaul Y. Interaction of c-abl and p73α and their collaboration to induce apoptosis. Nature. 1999;399:809–813. doi: 10.1038/21697. [DOI] [PubMed] [Google Scholar]
- 82.Levy D., Adamovich Y., Reuven N., Shaul Y. Yap1 phosphorylation by c-abl is a critical step in selective activation of proapoptotic genes in response to DNA damage. Mol. Cell. 2008;29:350–361. doi: 10.1016/j.molcel.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 83.Mantovani F., Piazza S., Gostissa M., Strano S., Zacchi P., Mantovani R., Blandino G., Del Sal G. Pin1 links the activities of c-abl and p300 in regulating p73 function. Mol. Cell. 2004;14:625–636. doi: 10.1016/j.molcel.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 84.Meier M., den Boer M. L., Meijerink J. P., Broekhuis M. J., Passier M. M., van Wering E. R., Janka-Schaub G. E., Pieters R. Differential expression of p73 isoforms in relation to drug resistance in childhood T-lineage acute lymphoblastic leukaemia. Leukemia. 2006;20:1377–1384. doi: 10.1038/sj.leu.2404288. [DOI] [PubMed] [Google Scholar]
- 85.Leong C. O., Vidnovic N., DeYoung M. P., Sgroi D., Ellisen L. W. The p63/p73 network mediates chemosensitivity to cisplatin in a biologically defined subset of primary breast cancers. J. Clin. Invest. 2007;117:1370–1380. doi: 10.1172/JCI30866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Besson A., Dowdy S. F., Roberts J. M. CDK inhibitors: cell cycle regulators and beyond. Dev. Cell. 2008;14:159–169. doi: 10.1016/j.devcel.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 87.Li J., Poi M. J., Tsai M. D. Regulatory mechanisms of tumor suppressor P16INK4A and their relevance to cancer. Biochemistry. 2011;50:5566–5582. doi: 10.1021/bi200642e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Romagosa C., Simonetti S., Lopez-Vicente L., Mazo A., Lleonart M. E., Castellvi J., Ramon y Cajal S. p16Ink4a overexpression in cancer: A tumor suppressor gene associated with senescence and high-grade tumors. Oncogene. 2011;30:2087–2097. doi: 10.1038/onc.2010.614. [DOI] [PubMed] [Google Scholar]
- 89.Sherr C. J. The INK4a/ARF network in tumour suppression. Nat. Rev. Mol. Cell Biol. 2001;2:731–737. doi: 10.1038/35096061. [DOI] [PubMed] [Google Scholar]
- 90.Lowe S. W., Sherr C. J. Tumor suppression by Ink4a-arf: Progress and puzzles. Curr. Opin. Genet. Dev. 2003;13:77–83. doi: 10.1016/s0959-437x(02)00013-8. [DOI] [PubMed] [Google Scholar]
- 91.Chow L. S., Wang X., Kwong D. L., Sham J. S., Tsao S. W., Nicholls J. M. Effect of p16INK4a on chemosensitivity in nasopharyngeal carcinoma cells. Int. J. Oncol. 2000;17:135–140. [PubMed] [Google Scholar]
- 92.Gallagher S. J., Thompson J. F., Indsto J., Scurr L. L., Lett M., Gao B. F., Dunleavey R., Mann G. J., Kefford R. F., Rizos H. p16INK4a expression and absence of activated B-RAF are independent predictors of chemosensitivity in melanoma tumors. Neoplasia. 2008;10:1231–1239. doi: 10.1593/neo.08702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Deng X., Kim M., Vandier D., Jung Y. J., Rikiyama T., Sgagias M. K., Goldsmith M., Cowan K. H. Recombinant adenovirus-mediated p14ARF overexpression sensitizes human breast cancer cells to cisplatin. Biochem. Biophys. Res. Commun. 2002;296:792–798. doi: 10.1016/s0006-291x(02)00948-8. [DOI] [PubMed] [Google Scholar]
- 94.Gallagher S., Kefford R. F., Rizos H. Enforced expression of p14ARF induces p53-dependent cell cycle arrest but not apoptosis. Cell Cycle. 2005;4:465–472. doi: 10.4161/cc.4.3.1526. [DOI] [PubMed] [Google Scholar]
- 95.Xie Q. C., Hu Y. D., Wang L. L., Chen Z. T., Diao X. W., Wang Z. X., Guan H. J., Zhu B., Sun J. G., Duan Y. Z., et al. The co-transfection of p16INK4a and p14ARF genes into human lung cancer cell line A549 and the effects on cell growth and chemosensitivity. Colloids Surf. B, Biointerfaces. 2005;46:188–196. doi: 10.1016/j.colsurfb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 96.Schmitt C. A., McCurrach M. E., de Stanchina E., Wallace-Brodeur R. R., Lowe S. W. INK4a/ARF mutations accelerate lymphomagenesis and promote chemoresistance by disabling p53. Genes Dev. 1999;13:2670–2677. doi: 10.1101/gad.13.20.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schmitt C. A., Fridman J. S., Yang M., Lee S., Baranov E., Hoffman R. M., Lowe S. W. A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell. 2002;109:335–346. doi: 10.1016/s0092-8674(02)00734-1. [DOI] [PubMed] [Google Scholar]
- 98.de Stanchina E., McCurrach M. E., Zindy F., Shieh S. Y., Ferbeyre G., Samuelson A. V., Prives C., Roussel M. F., Sherr C. J., Lowe S. W. E1A signaling to p53 involves the p19ARF tumor suppressor. Genes Dev. 1998;12:2434–2442. doi: 10.1101/gad.12.15.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kamoshida S., Matsuoka H., Shiogama K., Matsuyama A., Shimomura R., Inada K., Maruta M., Tsutsumi Y. Immunohistochemical analysis of thymidylate synthase, p16INK4a, cyclin-dependent kinase 4 and cyclin D1 in colorectal cancers receiving preoperative chemotherapy: Significance of p16INK4a-mediated cellular arrest as an indicator of chemosensitivity to 5-fluorouracil. Pathol. Int. 2004;54:564–575. doi: 10.1111/j.1440-1827.2004.01665.x. [DOI] [PubMed] [Google Scholar]
- 100.Kudoh K., Ichikawa Y., Yoshida S., Hirai M., Kikuchi Y., Nagata I., Miwa M., Uchida K. Inactivation of p16/CDKN2 and p15/MTS2 is associated with prognosis and response to chemotherapy in ovarian cancer. Int. J. Cancer. 2002;99:579–582. doi: 10.1002/ijc.10331. [DOI] [PubMed] [Google Scholar]
- 101.Tutor O., Diaz M. A., Ramirez M., Algara P., Madero L., Martinez P. Loss of heterozygosity of p16 correlates with minimal residual disease at the end of the induction therapy in non-high risk childhood B-cell precursor acute lymphoblastic leukemia. Leuk. Res. 2002;26:817–820. doi: 10.1016/s0145-2126(02)00020-6. [DOI] [PubMed] [Google Scholar]
- 102.Barboule N., Chadebech P., Baldin V., Vidal S., Valette A. Involvement of p21 in mitotic exit after paclitaxel treatment in MCF-7 breast adenocarcinoma cell line. Oncogene. 1997;15:2867–2875. doi: 10.1038/sj.onc.1201469. [DOI] [PubMed] [Google Scholar]
- 103.Chang B. D., Xuan Y., Broude E. V., Zhu H., Schott B., Fang J., Roninson I. B. Role of p53 and p21waf1/cip1 in senescence-like terminal proliferation arrest induced in human tumor cells by chemotherapeutic drugs. Oncogene. 1999;18:4808–4818. doi: 10.1038/sj.onc.1203078. [DOI] [PubMed] [Google Scholar]
- 104.Eymin B., Haugg M., Droin N., Sordet O., Dimanche-Boitrel M. T., Solary E. p27Kip1 induces drug resistance by preventing apoptosis upstream of cytochrome c release and procaspase-3 activation in leukemic cells. Oncogene. 1999;18:1411–1418. doi: 10.1038/sj.onc.1202437. [DOI] [PubMed] [Google Scholar]
- 105.Han Z., Wei W., Dunaway S., Darnowski J. W., Calabresi P., Sedivy J., Hendrickson E. A., Balan K. V., Pantazis P., Wyche J. H. Role of p21 in apoptosis and senescence of human colon cancer cells treated with camptothecin. J. Biol. Chem. 2002;277:17154–17160. doi: 10.1074/jbc.M112401200. [DOI] [PubMed] [Google Scholar]
- 106.Hawthorne V. S., Huang W. C., Neal C. L., Tseng L. M., Hung M. C., Yu D. ErbB2-mediated src and signal transducer and activator of transcription 3 activation leads to transcriptional up-regulation of p21Cip1 and chemoresistance in breast cancer cells. Mol. Cancer Res. 2009;7:592–600. doi: 10.1158/1541-7786.MCR-08-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Javelaud D., Besancon F. Inactivation of p21WAF1 sensitizes cells to apoptosis via an increase of both p14ARF and p53 levels and an alteration of the Bax/Bcl-2 ratio. J. Biol. Chem. 2002;277:37949–37954. doi: 10.1074/jbc.M204497200. [DOI] [PubMed] [Google Scholar]
- 108.Koster R., di Pietro A., Timmer-Bosscha H., Gibcus J. H., van den Berg A., Suurmeijer A. J., Bischoff R., Gietema J. A., de Jong S. Cytoplasmic p21 expression levels determine cisplatin resistance in human testicular cancer. J. Clin. Invest. 2010;120:3594–3605. doi: 10.1172/JCI41939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lazzarini R., Moretti S., Orecchia S., Betta P. G., Procopio A., Catalano A. Enhanced antitumor therapy by inhibition of p21waf1 in human malignant mesothelioma. Clin. Cancer Res. 2008;14:5099–5107. doi: 10.1158/1078-0432.CCR-08-0255. [DOI] [PubMed] [Google Scholar]
- 110.Le T. V., Seo Y., Ryu C. J., Lee H. R., Park H. J. Increased expression of p27 is associated with the cisplatin resistance in gastric cancer cell line YCC-3. Arch. Pharm. Res. 2010;33:1127–1132. doi: 10.1007/s12272-010-0720-5. [DOI] [PubMed] [Google Scholar]
- 111.Li Y., Dowbenko D., Lasky L. A. AKT/PKB phosphorylation of p21Cip/WAF1 enhances protein stability of p21Cip/WAF1 and promotes cell survival. J. Biol. Chem. 2002;277:11352–11361. doi: 10.1074/jbc.M109062200. [DOI] [PubMed] [Google Scholar]
- 112.Li W., Fan J., Banerjee D., Bertino J. R. Overexpression of p21waf1 decreases G2-M arrest and apoptosis induced by paclitaxel in human sarcoma cells lacking both p53 and functional rb protein. Mol. Pharmacol. 1999;55:1088–1093. doi: 10.1124/mol.55.6.1088. [DOI] [PubMed] [Google Scholar]
- 113.Mahyar-Roemer M., Roemer K. p21 Waf1/Cip1 can protect human colon carcinoma cells against p53-dependent and p53-independent apoptosis induced by natural chemopreventive and therapeutic agents. Oncogene. 2001;20:3387–3398. doi: 10.1038/sj.onc.1204440. [DOI] [PubMed] [Google Scholar]
- 114.Ruan S., Okcu M. F., Ren J. P., Chiao P., Andreeff M., Levin V., Zhang W. Overexpressed WAF1/Cip1 renders glioblastoma cells resistant to chemotherapy agents 1,3-bis(2-chloroethyl)-1-nitrosourea and cisplatin. Cancer Res. 1998;58:1538–1543. [PubMed] [Google Scholar]
- 115.Schmidt M., Lu Y., Liu B., Fang M., Mendelsohn J., Fan Z. Differential modulation of paclitaxel-mediated apoptosis by p21Waf1 and p27Kip1. Oncogene. 2000;19:2423–2429. doi: 10.1038/sj.onc.1203546. [DOI] [PubMed] [Google Scholar]
- 116.Schmidt M., Fan Z. Protection against chemotherapy-induced cytotoxicity by cyclin-dependent kinase inhibitors (CKI) in CKI-responsive cells compared with CKI-unresponsive cells. Oncogene. 2001;20:6164–6171. doi: 10.1038/sj.onc.1204814. [DOI] [PubMed] [Google Scholar]
- 117.St Croix B., Florenes V. A., Rak J. W., Flanagan M., Bhattacharya N., Slingerland J. M., Kerbel R. S. Impact of the cyclin-dependent kinase inhibitor p27Kip1 on resistance of tumor cells to anticancer agents. Nat. Med. 1996;2:1204–1210. doi: 10.1038/nm1196-1204. [DOI] [PubMed] [Google Scholar]
- 118.Steinman R. A., Johnson D. E. p21WAF1 prevents down-modulation of the apoptotic inhibitor protein c-IAP1 and inhibits leukemic apoptosis. Mol. Med. 2000;6:736–749. [PMC free article] [PubMed] [Google Scholar]
- 119.Stewart Z. A., Mays D., Pietenpol J. A. Defective G1-S cell cycle checkpoint function sensitizes cells to microtubule inhibitor-induced apoptosis. Cancer Res. 1999;59:3831–3837. [PubMed] [Google Scholar]
- 120.Vigneron A., Roninson I. B., Gamelin E., Coqueret O. Src inhibits adriamycin-induced senescence and G2 checkpoint arrest by blocking the induction of p21waf1. Cancer Res. 2005;65:8927–8935. doi: 10.1158/0008-5472.CAN-05-0461. [DOI] [PubMed] [Google Scholar]
- 121.Waldman T., Lengauer C., Kinzler K. W., Vogelstein B. Uncoupling of S phase and mitosis induced by anticancer agents in cells lacking p21. Nature. 1996;381:713–716. doi: 10.1038/381713a0. [DOI] [PubMed] [Google Scholar]
- 122.Wang Y., Blandino G., Givol D. Induced p21waf expression in H1299 cell line promotes cell senescence and protects against cytotoxic effect of radiation and doxorubicin. Oncogene. 1999;18:2643–2649. doi: 10.1038/sj.onc.1202632. [DOI] [PubMed] [Google Scholar]
- 123.Yu D., Jing T., Liu B., Yao J., Tan M., McDonnell T. J., Hung M. C. Overexpression of ErbB2 blocks taxol-induced apoptosis by upregulation of p21Cip1, which inhibits p34Cdc2 kinase. Mol. Cell. 1998;2:581–591. doi: 10.1016/s1097-2765(00)80157-4. [DOI] [PubMed] [Google Scholar]
- 124.Shih C. M., Chen K., Wang Y. C., Lee P. J., Wang Y. C. Elevated p53 and p21waf1 mRNA expression in blood lymphocytes from lung cancer patients with chemoresistance. Cancer Detect. Prev. 2007;31:366–370. doi: 10.1016/j.cdp.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 125.Zhang W., Kornblau S. M., Kobayashi T., Gambel A., Claxton D., Deisseroth A. B. High levels of constitutive WAF1/Cip1 protein are associated with chemoresistance in acute myelogenous leukemia. Clin. Cancer Res. 1995;1:1051–1057. [PubMed] [Google Scholar]
- 126.Lincet H., Poulain L., Remy J. S., Deslandes E., Duigou F., Gauduchon P., Staedel C. The p21cip1/waf1 cyclin-dependent kinase inhibitor enhances the cytotoxic effect of cisplatin in human ovarian carcinoma cells. Cancer Lett. 2000;161:17–26. doi: 10.1016/s0304-3835(00)00586-3. [DOI] [PubMed] [Google Scholar]
- 127.Taguchi T., Kato Y., Baba Y., Nishimura G., Tanigaki Y., Horiuchi C., Mochimatsu I., Tsukuda M. Protein levels of p21, p27, cyclin E and bax predict sensitivity to cisplatin and paclitaxel in head and neck squamous cell carcinomas. Oncol. Rep. 2004;11:421–426. [PubMed] [Google Scholar]
- 128.Wei J., Zhao J., Long M., Han Y., Wang X., Lin F., Ren J., He T., Zhang H. p21WAF1/CIP1 gene transcriptional activation exerts cell growth inhibition and enhances chemosensitivity to cisplatin in lung carcinoma cell. BMC Cancer. 2010;10:632. doi: 10.1186/1471-2407-10-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Goff B. A., Paley P. J., Greer B. E., Gown A. M. Evaluation of chemoresistance markers in women with epithelial ovarian carcinoma. Gynecol. Oncol. 2001;81:18–24. doi: 10.1006/gyno.2000.6105. [DOI] [PubMed] [Google Scholar]
- 130.Yang Q., Sakurai T., Yoshimura G., Takashi Y., Suzuma T., Tamaki T., Umemura T., Nakamura Y., Nakamura M., Utsunomiya H., et al. Overexpression of p27 protein in human breast cancer correlates with in vitro resistance to doxorubicin and mitomycin C. Anticancer Res. 2000;20:4319–4322. [PubMed] [Google Scholar]
- 131.Hollander M. C., Blumenthal G. M., Dennis P. A. PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nat. Rev. Cancer. 2011;11:289–301. doi: 10.1038/nrc3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li D. M., Sun H. TEP1, encoded by a candidate tumor suppressor locus, is a novel protein tyrosine phosphatase regulated by transforming growth factor β. Cancer Res. 1997;57:2124–2129. [PubMed] [Google Scholar]
- 133.Myers M. P., Pass I., Batty I. H., Van der Kaay J., Stolarov J. P., Hemmings B. A., Wigler M. H., Downes C. P., Tonks N. K. The lipid phosphatase activity of PTEN is critical for its tumor supressor function. Proc. Natl. Acad. Sci. U.S.A. 1998;95:13513–13518. doi: 10.1073/pnas.95.23.13513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cantley L. C., Neel B. G. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc. Natl. Acad. Sci. U.S.A. 1999;96:4240–4245. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cully M., You H., Levine A. J., Mak T. W. Beyond PTEN mutations: The PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat. Rev. Cancer. 2006;6:184–192. doi: 10.1038/nrc1819. [DOI] [PubMed] [Google Scholar]
- 136.Nagata Y., Lan K. H., Zhou X., Tan M., Esteva F. J., Sahin A. A., Klos K. S., Li P., Monia B. P., Nguyen N. T., et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117–127. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 137.Wang Z. X., Lu B. B., Wang H., Cheng Z. X., Yin Y. M. MicroRNA-21 modulates chemosensitivity of breast cancer cells to doxorubicin by targeting PTEN. Arch. Med. Res. 2011;42:281–290. doi: 10.1016/j.arcmed.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 138.Esteva F. J., Guo H., Zhang S., Santa-Maria C., Stone S., Lanchbury J. S., Sahin A. A., Hortobagyi G. N., Yu D. PTEN, PIK3CA, p-AKT, and p-p70S6K status: association with trastuzumab response and survival in patients with HER2-positive metastatic breast cancer. Am. J. Pathol. 2010;177:1647–1656. doi: 10.2353/ajpath.2010.090885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kokubo Y., Gemma A., Noro R., Seike M., Kataoka K., Matsuda K., Okano T., Minegishi Y., Yoshimura A., Shibuya M., Kudoh S. Reduction of PTEN protein and loss of epidermal growth factor receptor gene mutation in lung cancer with natural resistance to gefitinib (IRESSA) Br. J. Cancer. 2005;92:1711–1719. doi: 10.1038/sj.bjc.6602559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Frattini M., Saletti P., Romagnani E., Martin V., Molinari F., Ghisletta M., Camponovo A., Etienne L. L., Cavalli F., Mazzucchelli L. PTEN loss of expression predicts cetuximab efficacy in metastatic colorectal cancer patients. Br. J. Cancer. 2007;97:1139–1145. doi: 10.1038/sj.bjc.6604009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Berns K., Horlings H. M., Hennessy B. T., Madiredjo M., Hijmans E. M., Beelen K., Linn S. C., Gonzalez-Angulo A. M., Stemke-Hale K., Hauptmann M., et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 142.Jhawer M., Goel S., Wilson A. J., Montagna C., Ling Y. H., Byun D. S., Nasser S., Arango D., Shin J., Klampfer L., Augenlicht L. H., et al. PIK3CA mutation/PTEN expression status predicts response of colon cancer cells to the epidermal growth factor receptor inhibitor cetuximab. Cancer Res. 2008;68:1953–1961. doi: 10.1158/0008-5472.CAN-07-5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kim D., Dan H. C., Park S., Yang L., Liu Q., Kaneko S., Ning J., He L., Yang H., Sun M., et al. AKT/PKB signaling mechanisms in cancer and chemoresistance. Front. Biosci. 2005;10:975–987. doi: 10.2741/1592. [DOI] [PubMed] [Google Scholar]
- 144.Lee J. T., Jr, Steelman L. S., McCubrey J. A. Phosphatidylinositol 3′-kinase activation leads to multidrug resistance protein-1 expression and subsequent chemoresistance in advanced prostate cancer cells. Cancer Res. 2004;64:8397–8404. doi: 10.1158/0008-5472.CAN-04-1612. [DOI] [PubMed] [Google Scholar]
- 145.Zhang S., Huang W. C., Li P., Guo H., Poh S. B., Brady S. W., Xiong Y., Tseng L. M., Li S. H., Ding Z., et al. Combating trastuzumab resistance by targeting SRC, a common node downstream of multiple resistance pathways. Nat. Med. 2011;17:461–469. doi: 10.1038/nm.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.King M. C., Marks J. H., Mandell J. B., Cancer Study Group, New York Breast Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302:643–646. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- 147.Linger R. J., Kruk P. A. BRCA1 16 years later: Risk-associated BRCA1 mutations and their functional implications. FEBS J. 2010;277:3086–3096. doi: 10.1111/j.1742-4658.2010.07735.x. [DOI] [PubMed] [Google Scholar]
- 148.Kennedy R. D., Quinn J. E., Johnston P. G., Harkin D. P. BRCA1: Mechanisms of inactivation and implications for management of patients. Lancet. 2002;360:1007–1014. doi: 10.1016/S0140-6736(02)11087-7. [DOI] [PubMed] [Google Scholar]
- 149.Mueller C. R., Roskelley C. D. Regulation of BRCA1 expression and its relationship to sporadic breast cancer. Breast Cancer Res. 2003;5:45–52. doi: 10.1186/bcr557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Narod S. A., Foulkes W. D. BRCA1 and BRCA2: 1994 and beyond. Nat. Rev. Cancer. 2004;4:665–676. doi: 10.1038/nrc1431. [DOI] [PubMed] [Google Scholar]
- 151.Dapic V., Monteiro A. N. Functional implications of BRCA1 for early detection, prevention, and treatment of breast cancer. Crit. Rev. Eukaryot. Gene Expr. 2006;16:233–252. doi: 10.1615/critreveukargeneexpr.v16.i3.30. [DOI] [PubMed] [Google Scholar]
- 152.Deng C. X. BRCA1: cell cycle checkpoint, genetic instability, DNA damage response and cancer evolution. Nucleic Acids Res. 2006;34:1416–1426. doi: 10.1093/nar/gkl010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Durant S. T., Nickoloff J. A. Good timing in the cell cycle for precise DNA repair by BRCA1. Cell Cycle. 2005;4:1216–1222. doi: 10.4161/cc.4.9.2027. [DOI] [PubMed] [Google Scholar]
- 154.Bhattacharyya A., Ear U. S., Koller B. H., Weichselbaum R. R., Bishop D. K. The breast cancer susceptibility gene BRCA1 is required for subnuclear assembly of Rad51 and survival following treatment with the DNA cross-linking agent cisplatin. J. Biol. Chem. 2000;275:23899–23903. doi: 10.1074/jbc.C000276200. [DOI] [PubMed] [Google Scholar]
- 155.Fedier A., Steiner R. A., Schwarz V. A., Lenherr L., Haller U., Fink D. The effect of loss of Brca1 on the sensitivity to anticancer agents in p53-deficient cells. Int. J. Oncol. 2003;22:1169–1173. [PubMed] [Google Scholar]
- 156.Husain A., He G., Venkatraman E. S., Spriggs D. R. BRCA1 up-regulation is associated with repair-mediated resistance to cis-diamminedichloroplatinum(II) Cancer Res. 1998;58:1120–1123. [PubMed] [Google Scholar]
- 157.Quinn J. E., Kennedy R. D., Mullan P. B., Gilmore P. M., Carty M., Johnston P. G., Harkin D. P. BRCA1 functions as a differential modulator of chemotherapy-induced apoptosis. Cancer Res. 2003;63:6221–6228. [PubMed] [Google Scholar]
- 158.Tassone P., Tagliaferri P., Perricelli A., Blotta S., Quaresima B., Martelli M. L., Goel A., Barbieri V., Costanzo F., Boland C. R., Venuta S. BRCA1 expression modulates chemosensitivity of BRCA1-defective HCC1937 human breast cancer cells. Br. J. Cancer. 2003;88:1285–1291. doi: 10.1038/sj.bjc.6600859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Quinn J. E., James C. R., Stewart G. E., Mulligan J. M., White P., Chang G. K., Mullan P. B., Johnston P. G., Wilson R. H., Harkin D. P. BRCA1 mRNA expression levels predict for overall survival in ovarian cancer after chemotherapy. Clin. Cancer Res. 2007;13:7413–7420. doi: 10.1158/1078-0432.CCR-07-1083. [DOI] [PubMed] [Google Scholar]
- 160.Wysocki P. J., Korski K., Lamperska K., Zaluski J., Mackiewicz A. Primary resistance to docetaxel-based chemotherapy in metastatic breast cancer patients correlates with a high frequency of BRCA1 mutations. Med. Sci. Monit. 2008;14:SC7–SC10. [PubMed] [Google Scholar]
- 161.Stordal B., Davey R. A systematic review of genes involved in the inverse resistance relationship between cisplatin and paclitaxel chemotherapy: role of BRCA1. Curr. Cancer Drug Targets. 2009;9:354–365. doi: 10.2174/156800909788166592. [DOI] [PubMed] [Google Scholar]
- 162.Yang D., Khan S., Sun Y., Hess K., Shmulevich I., Sood A. K., Zhang W. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA, J. Am. Med. Assoc. 2011;306:1557–1565. doi: 10.1001/jama.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Papadaki C., Tsaroucha E., Kaklamanis L., Lagoudaki E., Trypaki M., Tryfonidis K., Mavroudis D., Stathopoulos E., Georgoulias V., Souglakos J. Correlation of BRCA1, TXR1 and TSP1 mRNA expression with treatment outcome to docetaxel-based first-line chemotherapy in patients with advanced/metastatic non-small-cell lung cancer. Br. J. Cancer. 2011;104:316–323. doi: 10.1038/sj.bjc.6606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lafarge S., Sylvain V., Ferrara M., Bignon Y. J. Inhibition of BRCA1 leads to increased chemoresistance to microtubule-interfering agents, an effect that involves the JNK pathway. Oncogene. 2001;20:6597–6606. doi: 10.1038/sj.onc.1204812. [DOI] [PubMed] [Google Scholar]
- 165.Rottenberg S., Jaspers J. E., Kersbergen A., van der Burg E., Nygren A. O., Zander S. A., Derksen P. W., de Bruin M., Zevenhoven J., Lau A., et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc. Natl. Acad. Sci. U.S.A. 2008;105:17079–17084. doi: 10.1073/pnas.0806092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Michalak E. M., Jonkers J. Studying therapy response and resistance in mouse models for BRCA1-deficient breast cancer. J. Mammary Gland Biol. Neoplasia. 2011;16:41–50. doi: 10.1007/s10911-011-9199-z. [DOI] [PubMed] [Google Scholar]
- 167.Gilmore P. M., McCabe N., Quinn J. E., Kennedy R. D., Gorski J. J., Andrews H. N., McWilliams S., Carty M., Mullan P. B., Duprex W. P., et al. BRCA1 interacts with and is required for paclitaxel-induced activation of mitogen-activated protein kinase kinase kinase 3. Cancer Res. 2004;64:4148–4154. doi: 10.1158/0008-5472.CAN-03-4080. [DOI] [PubMed] [Google Scholar]
- 168.Chabalier C., Lamare C., Racca C., Privat M., Valette A., Larminat F. BRCA1 downregulation leads to premature inactivation of spindle checkpoint and confers paclitaxel resistance. Cell Cycle. 2006;5:1001–1007. doi: 10.4161/cc.5.9.2726. [DOI] [PubMed] [Google Scholar]
- 169. Reference deleted.
- 170.Wen J., Li R., Lu Y., Shupnik M. A. Decreased BRCA1 confers tamoxifen resistance in breast cancer cells by altering estrogen receptor-coregulator interactions. Oncogene. 2009;28:575–586. doi: 10.1038/onc.2008.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhang J., Willers H., Feng Z., Ghosh J. C., Kim S., Weaver D. T., Chung J. H., Powell S. N., Xia F. Chk2 phosphorylation of BRCA1 regulates DNA double-strand break repair. Mol. Cell. Biol. 2004;24:708–718. doi: 10.1128/MCB.24.2.708-718.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wang R. H., Yu H., Deng C. X. A requirement for breast-cancer-associated gene 1 (BRCA1) in the spindle checkpoint. Proc. Natl. Acad. Sci. U.S.A. 2004;101:17108–17113. doi: 10.1073/pnas.0407585101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Grusche F. A., Richardson H. E., Harvey K. F. Upstream regulation of the hippo size control pathway. Curr. Biol. 2010;20:R574–R582. doi: 10.1016/j.cub.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 174.Halder G., Johnson R. L. Hippo signaling: growth control and beyond. Development. 2011;138:9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhao B., Li L., Guan K. L. Hippo signaling at a glance. J. Cell Sci. 2010;123:4001–4006. doi: 10.1242/jcs.069070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhao B., Tumaneng K., Guan K. L. The hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat. Cell Biol. 2011;13:877–883. doi: 10.1038/ncb2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chan S. W., Lim C. J., Chen L., Chong Y. F., Huang C., Song H., Hong W. The hippo pathway in biological control and cancer development. J. Cell. Physiol. 2011;226:928–939. doi: 10.1002/jcp.22435. [DOI] [PubMed] [Google Scholar]
- 178.Badouel C., McNeill H. SnapShot: The hippo signaling pathway. Cell. 2011;145:484–484. doi: 10.1016/j.cell.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 179.Vigneron A. M., Ludwig R. L., Vousden K. H. Cytoplasmic ASPP1 inhibits apoptosis through the control of YAP. Genes Dev. 2010;24:2430–2439. doi: 10.1101/gad.1954310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Xu Y., Stamenkovic I., Yu Q. CD44 attenuates activation of the hippo signaling pathway and is a prime therapeutic target for glioblastoma. Cancer Res. 2010;70:2455–2464. doi: 10.1158/0008-5472.CAN-09-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Salah Z., Melino G., Aqeilan R. I. Negative regulation of the hippo pathway by E3 ubiquitin ligase ITCH is sufficient to promote tumorigenicity. Cancer Res. 2011;71:2010–2020. doi: 10.1158/0008-5472.CAN-10-3516. [DOI] [PubMed] [Google Scholar]
- 182.Ho K. C., Zhou Z., She Y. M., Chun A., Cyr T. D., Yang X. Itch E3 ubiquitin ligase regulates large tumor suppressor 1 stability. Proc. Natl. Acad. Sci. U.S.A. 2011;108:4870–4875. doi: 10.1073/pnas.1101273108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wrighton K. H. Mechanotransduction: YAP and TAZ feel the force. Nat. Rev. Mol. Cell Biol. 2011;12:404. doi: 10.1038/nrm3136. [DOI] [PubMed] [Google Scholar]
- 184.Visser S., Yang X. Identification of LATS transcriptional targets in HeLa cells using whole human genome oligonucleotide microarray. Gene. 2010;449:22–29. doi: 10.1016/j.gene.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 185.Ji D., Deeds S. L., Weinstein E. J. A screen of shRNAs targeting tumor suppressor genes to identify factors involved in A549 paclitaxel sensitivity. Oncol. Rep. 2007;18:1499–1505. [PubMed] [Google Scholar]
- 186.Kawahara M., Hori T., Chonabayashi K., Oka T., Sudol M., Uchiyama T. Kpm/Lats2 is linked to chemosensitivity of leukemic cells through the stabilization of p73. Blood. 2008;112:3856–3866. doi: 10.1182/blood-2007-09-111773. [DOI] [PubMed] [Google Scholar]
- 187.Lai D., Ho K. C., Hao Y., Yang X. Taxol resistance in breast cancer cells is mediated by the hippo pathway component TAZ and its downstream transcriptional targets Cyr61 and CTGF. Cancer Res. 2011;71:2728–2738. doi: 10.1158/0008-5472.CAN-10-2711. [DOI] [PubMed] [Google Scholar]
- 188.Overholtzer M., Zhang J., Smolen G. A., Muir B., Li W., Sgroi D. C., Deng C. X., Brugge J. S., Haber D. A. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc. Natl. Acad. Sci. U.S.A. 2006;103:12405–12410. doi: 10.1073/pnas.0605579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zhang X., George J., Deb S., Degoutin J. L., Takano E. A., Fox S. B., Bowtell D. D., Harvey K. F., group, AOCS Study The hippo pathway transcriptional co-activator, YAP, is an ovarian cancer oncogene. Oncogene. 2011;30:2810–2822. doi: 10.1038/onc.2011.8. [DOI] [PubMed] [Google Scholar]
- 190.Zhao B., Wei X., Li W., Udan R. S., Yang Q., Kim J., Xie J., Ikenoue T., Yu J., Li L., et al. Inactivation of YAP oncoprotein by the hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hao Y., Chun A., Cheung K., Rashidi B., Yang X. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J. Biol. Chem. 2008;283:5496–5509. doi: 10.1074/jbc.M709037200. [DOI] [PubMed] [Google Scholar]
- 192.Lei Q. Y., Zhang H., Zhao B., Zha Z. Y., Bai F., Pei X. H., Zhao S., Xiong Y., Guan K. L. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol. Cell. Biol. 2008;28:2426–2436. doi: 10.1128/MCB.01874-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ren A., Yan G., You B., Sun J. Down-regulation of mammalian sterile 20-like kinase 1 by heat shock protein 70 mediates cisplatin resistance in prostate cancer cells. Cancer Res. 2008;68:2266–2274. doi: 10.1158/0008-5472.CAN-07-6248. [DOI] [PubMed] [Google Scholar]
- 194.Visser-Grieve S., Hao Y., Yang X. Human homolog of Drosophila expanded, hEx, functions as a putative tumor suppressor in human cancer cell lines independently of the hippo pathway. Oncogene. 2011;21:1513–1516. doi: 10.1038/onc.2011.318. [DOI] [PubMed] [Google Scholar]
- 195.Zhao Q. Z., Dou K. F. Methylation of ras association domain family protein 1, isoform A correlated with proliferation and drug resistance in hepatocellular carcinoma cell line SMMC-7721. J. Gastroenterol. Hepatol. 2007;22:683–689. doi: 10.1111/j.1440-1746.2006.04676.x. [DOI] [PubMed] [Google Scholar]
- 196.Teng I. W., Hou P. C., Lee K. D., Chu P. Y., Yeh K. T., Jin V. X., Tseng M. J., Tsai S. J., Chang Y. S., Wu C. S., et al. Targeted methylation of two tumor suppressor genes is sufficient to transform mesenchymal stem cells into cancer stem/initiating cells. Cancer Res. 2011;71:4653–4663. doi: 10.1158/0008-5472.CAN-10-3418. [DOI] [PubMed] [Google Scholar]
- 197.Hansen T. M., Rossi M., Roperch J. P., Ansell K., Simpson K., Taylor D., Mathon N., Knight R. A., Melino G. Itch inhibition regulates chemosensitivity in vitro. Biochem. Biophys. Res. Commun. 2007;361:33–36. doi: 10.1016/j.bbrc.2007.06.104. [DOI] [PubMed] [Google Scholar]
- 198. Reference deleted.
- 199.Suwaki N., Vanhecke E., Atkins K. M., Graf M., Swabey K., Huang P., Schraml P., Moch H., Cassidy A. M., Brewer D., et al. A HIF-regulated VHL-PTP1B-src signaling axis identifies a therapeutic target in renal cell carcinoma. Sci. Transl. Med. 2011;3:85ra47. doi: 10.1126/scitranslmed.3002004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhu J. J., Li F. B., Zhu X. F., Liao W. M. The p33ING1b tumor suppressor cooperates with p53 to induce apoptosis in response to etoposide in human osteosarcoma cells. Life Sci. 2006;78:1469–1477. doi: 10.1016/j.lfs.2005.07.044. [DOI] [PubMed] [Google Scholar]
- 201.Deng W. G., Wu G., Ueda K., Xu K., Roth J. A., Ji L. Enhancement of antitumor activity of cisplatin in human lung cancer cells by tumor suppressor FUS1. Cancer Gene Ther. 2008;15:29–39. doi: 10.1038/sj.cgt.7701094. [DOI] [PubMed] [Google Scholar]
- 202.Zhang X., Wang X., Song X., Liu C., Shi Y., Wang Y., Afonja O., Ma C., Chen Y. H., Zhang L. Programmed cell death 4 enhances chemosensitivity of ovarian cancer cells by activating death receptor pathway in vitro and in vivo. Cancer Sci. 2010;101:2163–2170. doi: 10.1111/j.1349-7006.2010.01664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wertz I. E., Kusam S., Lam C., Okamoto T., Sandoval W., Anderson D. J., Helgason E., Ernst J. A., Eby M., Liu J., et al. Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7. Nature. 2011;471:110–114. doi: 10.1038/nature09779. [DOI] [PubMed] [Google Scholar]
- 204.Hehlgans S., Cordes N. Caveolin-1: an essential modulator of cancer cell radio-and chemoresistance. Am. J. Cancer. Res. 2011;1:521–530. [PMC free article] [PubMed] [Google Scholar]
- 205.Narayan G., Freddy A. J., Xie D., Liyanage H., Clark L., Kisselev S., Un Kang J., Nandula S. V., McGuinn C., Subramaniyam S., et al. Promoter methylation-mediated inactivation of PCDH10 in acute lymphoblastic leukemia contributes to chemotherapy resistance. Genes Chromosomes Cancer. 2011;50:1043–1053. doi: 10.1002/gcc.20922. [DOI] [PubMed] [Google Scholar]
- 206.Papageorgis P., Cheng K., Ozturk S., Gong Y., Lambert A. W., Abdolmaleky H. M., Zhou J. R., Thiagalingam S. Smad4 inactivation promotes malignancy and drug resistance of colon cancer. Cancer Res. 2011;71:998–1008. doi: 10.1158/0008-5472.CAN-09-3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Katagiri A., Nakayama K., Rahman M. T., Rahman M., Katagiri H., Nakayama N., Ishikawa M., Ishibashi T., Iida K., Kobayashi H., et al. Loss of ARID1A expression is related to shorter progression-free survival and chemoresistance in ovarian clear cell carcinoma. Mod. Pathol. 2012;25:282–288. doi: 10.1038/modpathol.2011.161. [DOI] [PubMed] [Google Scholar]
- 208.Hummel R., Hussey D. J., Haier J. MicroRNAs: Predictors and modifiers of chemo- and radiotherapy in different tumour types. Eur. J. Cancer. 2010;46:298–311. doi: 10.1016/j.ejca.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 209.Nakajima G., Hayashi K., Xi Y., Kudo K., Uchida K., Takasaki K., Yamamoto M., Ju J. Non-coding microRNAs hsa-let-7g and hsa-miR-181b are associated with chemoresponse to S-1 in colon cancer. Cancer Genomics Proteomics. 2006;3:317–324. [PMC free article] [PubMed] [Google Scholar]
- 210.Nishida N., Mimori K., Fabbri M., Yokobori T., Sudo T., Tanaka F., Shibata K., Ishii H., Doki Y., Mori M. MicroRNA-125a-5p is an independent prognostic factor in gastric cancer and inhibits the proliferation of human gastric cancer cells in combination with trastuzumab. Clin. Cancer Res. 2011;17:2725–2733. doi: 10.1158/1078-0432.CCR-10-2132. [DOI] [PubMed] [Google Scholar]
- 211.Fontana L., Fiori M. E., Albini S., Cifaldi L., Giovinazzi S., Forloni M., Boldrini R., Donfrancesco A., Federici V., Giacomini P., et al. Antagomir-17-5p abolishes the growth of therapy-resistant neuroblastoma through p21 and BIM. PLoS ONE. 2008;3:e2236. doi: 10.1371/journal.pone.0002236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Schetter A. J., Leung S. Y., Sohn J. J., Zanetti K. A., Bowman E. D., Yanaihara N., Yuen S. T., Chan T. L., Kwong D. L., Au G. K., et al. MicroRNA expression profiles associated with prognosis and therapeutic outcome in colon adenocarcinoma. JAMA, J. Am. Med. Assoc. 2008;299:425–436. doi: 10.1001/jama.299.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Derenzini M., Brighenti E., Donati G., Vici M., Ceccarelli C., Santini D., Taffurelli M., Montanaro L., Trere D. The p53-mediated sensitivity of cancer cells to chemotherapeutic agents is conditioned by the status of the retinoblastoma protein. J. Pathol. 2009;219:373–382. doi: 10.1002/path.2612. [DOI] [PubMed] [Google Scholar]
- 214.Jiang H., Reinhardt H. C., Bartkova J., Tommiska J., Blomqvist C., Nevanlinna H., Bartek J., Yaffe M. B., Hemann M. T. The combined status of ATM and p53 link tumor development with therapeutic response. Genes Dev. 2009;23:1895–1909. doi: 10.1101/gad.1815309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Fueyo J., Gomez-Manzano C., Puduvalli V. K., Martin-Duque P., Perez-Soler R., Levin V. A., Yung W. K., Kyritsis A. P. Adenovirus-mediated p16 transfer to glioma cells induces G1 arrest and protects from paclitaxel and topotecan: implications for therapy. Int. J. Oncol. 1998;12:665–669. doi: 10.3892/ijo.12.3.665. [DOI] [PubMed] [Google Scholar]
- 216.Magro P. G., Russo A. J., Li W. W., Banerjee D., Bertino J. R. p14ARF expression increases dihydrofolate reductase degradation and paradoxically results in resistance to folate antagonists in cells with nonfunctional p53. Cancer Res. 2004;64:4338–4345. doi: 10.1158/0008-5472.CAN-03-1045. [DOI] [PubMed] [Google Scholar]