Abstract
For more than a decade, Wnt signaling pathways have been the focus of intense research activity in bone biology laboratories because of their importance in skeletal development, bone mass maintenance, and therapeutic potential for regenerative medicine. It is evident that even subtle alterations in the intensity, amplitude, location, and duration of Wnt signaling pathways affects skeletal development, as well as bone remodeling, regeneration, and repair during a lifespan. Here we review recent advances and discrepancies in how Wnt/Lrp5 signaling regulates osteoblasts and osteocytes, introduce new players in Wnt signaling pathways that have important roles in bone development, discuss emerging areas such as the role of Wnt signaling in osteoclastogenesis, and summarize progress made in translating basic studies to clinical therapeutics and diagnostics centered around inhibiting Wnt pathway antagonists, such as sclerostin, Dkk1 and Sfrp1. Emphasis is placed on the plethora of genetic studies in mouse models and genome wide association studies that reveal the requirement for and crucial roles of Wnt pathway components during skeletal development and disease.
Keywords: Lrp5, Lrp6, Sclerostin, β-catenin, R-spondin, Bone mineral density, Polymorphisms
1. Introduction
Wnts are a large family of 19 secreted glycoproteins that trigger multiple signaling cascades essential for embryonic development and tissue regeneration. Proteins involved in the amplification and transduction of Wnt signals are often altered in cancer or lineage progenitor cells, leading to abnormal cell cycle control and/or altered cell fate decisions (MacDonald et al., 2009; Polakis, 2000). Mutations in several Wnt pathway components also contribute to human skeletal dysplasias. Most notably, mutations in the Wnt co-receptor LRP5 cause low or high bone mass depending on the nature of the alteration (Boyden et al., 2002; Gong et al., 2001; Little et al., 2002) and inactivation of the secreted Wnt antagonist Sclerostin produces high bone mass, sclerosteosis and van Buchem's disease (Balemans et al., 2001; Brunkow et al., 2001). A loss-of-function mutation in LRP6, another Wnt co-receptor, is linked to an inherited disorder characterized by osteoporosis, coronary artery disease, and metabolic syndrome (Mani et al., 2007). Less well known is that inactivating mutations in WTX, an intracellular regulator of β-catenin stability, cause osteopathia striata with cranial sclerosis (OCTS) (Jenkins et al., 2009) and FZD9, a Wnt co-receptor, is deleted in patients with Williams–Beuren syndrome, which is partially characterized by low bone density (Francke, 1999). During the last several years, polymorphisms in these and many more Wnt pathway components were linked to altered bone mineral density in genome wide association studies (Kiel et al., 2007b; Riancho et al., 2011; Rivadeneira et al., 2009; Sims et al., 2008; van Meurs et al., 2008). Thus, it has become clear that even subtle alterations in the intensity, amplitude, and duration of Wnt signaling pathways affects skeletal formation during development, as well as bone remodeling, regeneration, and repair during a lifespan. In this review, we provide an update to a 2004 review on Wnt signaling in osteoblasts and bone disease published in this journal (Westendorf et al., 2004). Emphasis is placed on new data from murine genetic studies assessing the requirement for and roles of Wnt pathway components during skeletal development and disease. These observations are discussed in context with current knowledge of molecular and physiological regulation of bone mass. Progress in translating these discoveries to treatments for altered bone mass conditions is also summarized.
1.1. Wnt signaling pathways
1.1.1. Wnt–β-catenin signaling
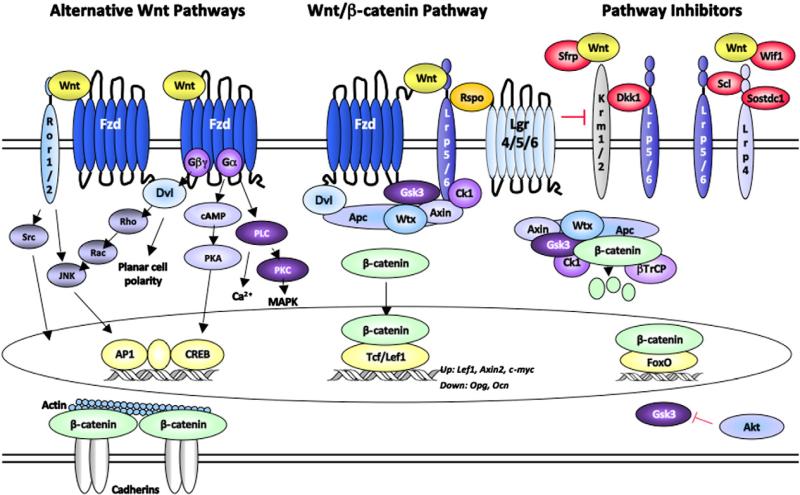
Wnts trigger several signaling cascades. The best known is the Wnt/β-catenin pathway (commonly called the canonical pathway), which features the stabilization and nuclear translocation of β-catenin as easily measurable outcomes. In the absence of Wnts, β-catenin associates with cadherins at the plasma membrane. Any excess β-catenin is quickly sequestered by a protein complex containing Axin1/2, Apc, casein kinase (Ck)1, glycogen synthase kinase (Gsk)3β, and Wtx and degraded by ubiquitin-mediated proteolysis (Fig. 1) (For more details see (Westendorf et al., 2004)). When certain Wnts (e.g., Wnt3a) are present, they crosslink cell surface molecules, Lrp5/6 and a Frizzled (Fzd), which mobilizes Gsk3β and Ck1 to the membrane where they phosphorylate serines on Lrp5/6, promote the formation of a signalosome, and recruit Disheveled (Dvl), Axin1/2, and caveolin (Bilic et al., 2007; MacDonald et al., 2009; Niehrs and Shen, 2010; Zeng et al., 2005). This releases β-catenin from the destruction complex, increases its levels, and allows it to enter the nucleus where it can displace co-repressors from transcription factors (e.g., Lef1, Tcf7) and regulate gene expression. Nuclear localization of β-catenin is often used as a metric of enhanced Wnt signaling. Expression levels of target genes (e.g., Axin2, Lef1) are also commonly measured to study Wnt signaling. Although β-catenin is activated by Wnts, it is important to remember that it is also mobilized by other signals (e.g., Igf and Akt activation) and is not exclusive to the canonical Wnt signaling cascade. This point is especially important in bone, as β-catenin deletion triggers bone loss via different mechanisms than Lrp5 inactivation (subsequent sections).
Fig. 1.
Wnt signaling pathways. The “canonical” Wnt–β-catenin signaling pathway is illustrated in the center of the diagram. Secreted and intracellular inhibitors of β-catenin are shown on the right side. Wnt signaling pathways that do not involve β-catenin are summarized on the left side.
The Wnt/β-catenin pathway stimulates cell proliferation and survival. Enhanced stimulation of the pathway is a feature of many cancers (Polakis, 2000). Under normal physiological settings, multiple proteins keep this cascade in check. In addition to intracellular inhibitors (Axin2), the canonical pathway is neutralized by extracellular factors (Fig. 1). Secreted frizzled related proteins (Sfrps) and Wnt inhibitory factors (Wifs) directly bind Wnts and prevent their interactions with receptors. Other secreted proteins including Dickkopfs (Dkk), Sclerostin (Scl), and Sostdc1 (Wise) bind to Lrp5/6 receptors, inducing receptor internalization and/or reducing their availability to Wnts. Thirdly, some Wnts (e.g., Wnt5a) trigger alternative signaling pathways by co-opting receptor components and thus competing with Wnts (e.g., Wnt3a) that induce β-catenin stabilization. For example, Wnt5a induces the formation of a complex consisting of Lrp5/6, Ror1/2, and Fzd2 (Sato et al., 2010).
1.1.2. Non-β-catenin Wnt signaling pathways
In some contexts, Wnts neither stabilize β-catenin nor interact with Lrp5/6. Rather, through Fzds and Dvl, Wnts can trigger alternative intracellular events (Fig. 1 and reviewed by (Gao and Chen, 2010)). Non-β-catenin cascades include the planar cell polarity (PCP) pathway, trimeric G-protein coupled receptor pathways including calcium ion (Ca2+) signaling, Rho family GTPase pathways, and the Jnk pathways. Dvl has multiple conserved domains that allow it to interact with many binding partners, which determines which downstream pathways are engaged (Gao and Chen, 2010). Furthermore, membrane-spanning receptors such Ror2 and Ryk can activate Dvl-independent signaling (Angers and Moon, 2009).
1.1.2.1. Planar cell polarity (PCP) pathway
The most extensively studied non-β-catenin Wnt signaling pathways is the PCP pathway, which enables cells to orient relative to an axis along the plane of a tissue (Henderson and Chaudhry, 2011). PCP signaling governs cell movement in the embryo via convergent extension (Sokol, 1996) and determines cell fates, enabling the creation of asymmetric and highly aligned structures such as hair follicles as well as orchestrating the polarized beating of motile cilia in numerous tissues (Devenport and Fuchs, 2008; Jones et al., 2008). The establishment of polarity in the plane of the epithelium provides directional information during development. Wnt binding to Fzd leads to Dvl-driven sorting of cellular components to either the proximal or distal regions of the cell and orients it within the tissue (Veeman et al., 2003). Thus far, little is known about PCP activation during bone remodeling.
1.1.2.2. Wnt and Rho/Rac GTPases
Dvl activation of the Rho GTPase family member Rac1 leads to Jnk activation and stimulation of the transcription factors c-Jun and ATF2 (Li et al., 1999; Ohkawara and Niehrs, 2011; Sato et al., 2010). Wnt3a causes chondrocyte de-differentiation by activating c-Jun/AP-1 and suppressing Sox-9 expression, supporting a role for a non-β-catenin/Wnt3a pathway in bone development (Hwang et al., 2005). Wnt binding to Fzd can also promote Dvl interactions with the adaptor protein disheveled-associated activator of morphogenesis (Daam)1, which activates the Rho guanine nuclear exchange factor WGEF (Wu and Herman, 2006). WGEF induces RhoA/ROCK pathway activation, which promotes cytoskeletal reorganization to control cell shape and adhesion (Gao and Chen, 2010). Dvl/Daam1 interactions can also cause cytoskeletal reorganization by influencing Profilin independent of RhoA activation (Gao and Chen, 2010).
1.1.2.3. Wnt and G-protein coupled receptor signaling
Evidence is mounting that Wnt activates trimeric G-protein signaling to control a number of downstream signaling pathways. G proteins are required for Wnt activity, but whether there are direct interactions between Fzd and G proteins remain unresolved (Katanaev and Tomlinson, 2006; Katanaev et al., 2005; Liu et al., 1999, 2005; Purvanov et al., 2010). Physical interactions between Fzd and G proteins were observed under physiological conditions (Koval and Katanaev, 2011). Thus, Wnt3a stimulated Gαs and Gαi/o, but not Gαq11 association with Fzd receptors in brain tissue. Wnt/Fzd induced cAMP accumulation and PKA activation though Gαs protein (Witze et al., 2008). In contrast, Gαi/o stimulated phospholipase C, intracellular Ca+2 release and direct PKC activation. G protein signaling, specifically, Gαq11 activation, was also required for nuclear localization of β-catenin following Wnt3a treatment (Tu et al., 2007). The βγ subunits of the trimeric G protein complex interact with Dvl in vertebrate cells. Fzd7 and G protein βγ subunits are required for Wnt11 to stimulate axis organization, indicating the βγ subunits as well as the α subunit are involved in non-β-catenin G protein-mediated signaling (Angers et al., 2006; Penzo-Mendez et al., 2003).
1.2. Osteoblast and osteoclast lineages: differentiation, maturation and coupling
Osteoblasts, osteocytes, and osteoclasts directly regulate bone mass. Osteoblasts originate from mesenchymal progenitor cells and are responsible for producing proteins, such as type 1 collagen, that form a mineralizable matrix. Runx2, Sp7 (osterix), Wnts, Lrp5, and β-catenin are among the crucial factors required for their specification from mesenchymal precursors and osteo-chondoprogenitors. Wnts and β-catenin subsequently contribute to proliferation and survival of osteoblasts (Westendorf et al., 2004). β-catenin also regulates the communication or coupling of osteoblasts with osteoclast precursors, which originate from hematopoietic stem cells, by controlling expression of osteoprotegerin (Opg), a competitive inhibitor of Rankl and Rank interaction, to affect bone resorption (Glass et al., 2005). Osteocytes are terminally differentiated osteoblasts embedded within the mineralized matrix that communicate changes in mechanical loading and the extracellular environment to osteoblasts and osteoclasts on the bone surface to stimulate fracture repair and influence bone remodeling (Bonewald, 2011).
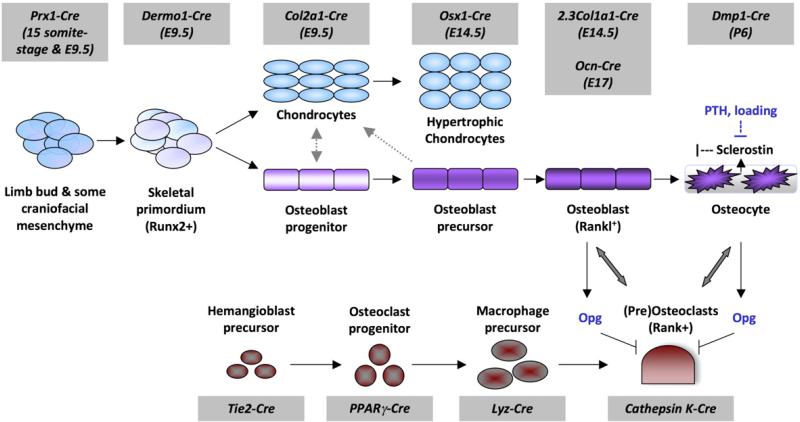
Wnts and Wnt pathway components are essential for many stages of osteoblast lineage development and maturation. Knowledge in this area has advanced in the last decade due to the availability and utilization of genetic approaches that test the requirement or role for certain molecules in bone development, biology, and disease. These models include germ-line knockout (KO), conditional knockout (CKO) or knock-in (CKI), and transgenic (Tg) expression. Table 1 summarizes bone phenotypes that result when Wnt pathway components are genetically altered in osteoblast lineage cells or the germline. Table 2 lists bone phenotypes of mice where β-catenin levels are altered in osteoclast lineage cells and their precursors. The CKO, CKI, and Tg strategies allow for tissue-specific and/or inducible expression. Several promoters drive expression of Cre recombinase (for CKO or CKI strategies) or transgenes in osteoblast and osteoclast lineage cells at different stages of maturation (Fig. 2) (Van Koevering and Williams, 2008). In the following sections, we review studies that utilized these technologies to advance our understanding of Wnt pathways in bone biology and disease.
Table 1.
Summary of bone phenotypes in mouse models of altered Wnt signaling in osteoblast lineage cells and the germline.
| Gene | KO/CKO/Tg/CKI | Cre line | Bone phenotype(s) | References |
|---|---|---|---|---|
| APC | CKO | OCN-Cre | Early postnatal lethality, osteopetrosis, increased trabecular bone density, few osteoclasts, elevated Opg | Holmen et al., 2005 |
| CKO | Col2a1-Cre | Early postnatal lethality, reduced mineralization at E14.5, increased mineralization at E16.5 and in ribs | Miclea et al., 2009 | |
| Axin2 | KO | Germline | Craniosynostosis, increased BMD | Yu et al., 2005 |
| Ctnnb1 (β-Catenin) | CKO | Wnt1-Cre | Embryonic lethal, block in prechondrocyte condensation and craniofacial development | Brault et al., 2001 |
| CKO | Prx1-Cre | Lack of mineralization in head and distal skeletal elements, enhanced chondrogenesis, lower osteoblastogenesis | Hill et al., 2005 | |
| CKO | Dermo1-Cre | Shortened limbs, twisted body axis, diminished intramembranous and endochondral bone formation, ectopic cartilage | Day et al., 2005; Hu et al., 2005 | |
| CKO | Osx1-Cre | Lack of cranial ossification, increased chondrogenesis | Rodda et al., 2005 | |
| CKO | Col2a1-Cre | Ectopic cartilage in long bones, normal intramembranous bone | Day et al., 2005 | |
| CKO | 2.3Col1a1-Cre | Reduced bone mass, increased osteoclast numbers, decreased Opg | Glass et al., 2005 | |
| CKO | Ocn-Cre | Early postnatal lethality, reduced cortical and trabecular bone density, increased osteoclast numbers | Holmen et al., 2005 | |
| CKO | Dmp1-Cre | Premature postnatal lethality, impaired cortical and trabecular bone mass, increased osteoclast number and activity, decreased Opg levels | Kramer et al., 2010a | |
| CKI/GOF | Prx1-Cre: exon3 | Early postnatal lethality, no bone formation | Hill et al., 2005 | |
| CKI/GOF | Osx1-Cre: exon3 | Embryonic lethality, excessive premature ossification | Rodda et al., 2005 | |
| CKI/GOF | 2.3Col1a1-Cre: exon3 | Premature postnatal lethality, failed tooth eruption, increased ossification, decreased osteoclast numbers and function, normal osteoblast numbers, rib osteomata | Glass et al., 2005 | |
| Dkk1 | Het | Germline | High bone mass inversely proportional to Dkk1 concentration in hypomorphic animals | Morvan et al., 2006; MacDonald et al., 2004, 2007 |
| Tg | 2.3 and 3.6Col1a1 | Low bone mass, decreased osteoblast number, reduced serum osteocalcin levels, lower matrix mineralization | Li et al., 2006 | |
| Tg | 2.3Col1a1 | Osteopenia, reduced bone formation, normal PTH responsiveness | Fleming et al., 2008; Guo et al., 2010; Yao et al., 2011 | |
| Dkk1d | Hypomorphic mutation | Increased bone mass that is inversely proportional to Dkk1 expression, distal forelimb postaxial polysyndactyly | MacDonald et al., 2007 | |
| Dkk2 | KO | Germline | Low bone mass | Li et al., 2005 |
| Fzd9 | KO | Germline | Osteopenia, decreased bone formation | Albers et al., 2008 |
| Gsk3β | Het KO | Germline | Increased trabecular bone mass | Kugimiya et al., 2008; Noh et al., 2009 |
| Krm1/2 | DKO | Germline | Increased BMD | Ellwanger et al., 2008 |
| Krm2 | KO | Germline | High bone mass at 24 weeks, increased bone formation | Schulze et al., 2010 |
| Krm2 | Tg | 2.3Col1a1 | Osteoporosis, decreased bone formation, reduced cortical strength, reduced Opg expression, and increased bone resorption | Schulze et al., 2010 |
| Lef1 | Het KO | Germline | Reduced bone formation in females only | Noh et al., 2009 |
| KO | Germline | Reduced bone mass in all KO and Het mice | JJW et al., unpublished | |
| Lef1ΔN | Tg | 2.3Col1 | Increased trabecular bone mass | Hoeppner et al., 2010 |
| Lrp4 | Lrp4ECD | Hypomorph | Reduced BMD, increased bone turnover | Choi et al., 2009 |
| Lrp5 | KO | Germline | Decreased bone mass | Fujino et al., 2003; Kato et al., 2002 |
| CKO | 2.3Col1-Cre | Normal vertebral bone mass | Yadav et al., 2008 | |
| CKO | Dermo1-Cre | Normal vertebral bone mass | Yadav et al., 2010 | |
| CKO | Dmp1-Cre | Decreased trabecular bone mass and cortical strength | Cui et al., 2011 | |
| Tg | Rat 3.6Col1-HBM G171V | Increased bone mass and strength | Akhter et al., 2004; Babij et al., 2003 | |
| CKI/GOF | 2.3Col1-Cre: HBM G171V cDNA | Normal vertebral bone mass and bone formation rates | Yadav et al., 2008 | |
| CKI/GOF | Dmp1-Cre: HBM G171V or A214V | Increased trabecular bone mass, bone strength, and bone formation rates in distal femur and L5 | Cui et al., 2011 | |
| CKI/GOF | Prx1-Cre: HBM G171V | Increased bone mass in limbs, but not vertebrae | Cui et al., 2011 | |
| CKI/GOF | Villin-Cre: HBM G171V | Increased vertebral bone mass and bone formation rates | Yadav et al., 2008 | |
| CKO | Villin-Cre | Decreased vertebral bone mass, bone formation rates and osteoblast numbers | Yadav et al., 2008 | |
| CKI/GOF | Vil1-Cre: HBM G171V or A214V | Normal bone mass | Cui et al., 2011 | |
| CKO | Vil1-Cre | Normal bone mass | Cui et al., 2011 | |
| Lrp6 | Het KO | Germline | Decreased bone | Holmen et al., 2004 |
| Rs | Hypomorphic mutation | Decreased bone mineral density, no chance in osteoblast number, elevated Rankl expression, increased bone resorption | Kubota et al., 2009 | |
| Lrp5/6 | KO/Het | Germline | Decreased bone | Holmen et al., 2004 |
| Rspo2 | KO | Germline | Decreased ossification in distal phalanges and stunted fibula | Nam et al., 2007 |
| Sfrp1 | KO | Germline | Increased bone mass | Bodine et al., 2004 |
| Tg | Sfrp1 | Decreased bone mass | Yao et al., 2010 | |
| Sfrp4 | Tg | 2.3Col1a1 | Low bone mass, fewer osteoblasts; LiCl rescued these defects | Nakanishi et al., 2008 |
| Tg | Serum amyloid P | Decreased bone mass | Cho et al., 2010 | |
| Sclerostin (Scl, Sost) | KO | Germline | Increased bone mass | Balemans et al., 2003; Krause et al., 2010 |
| Tg | Ocn + APO E | Osteopenia | Winkler et al., 2003 | |
| Tg | SOST | Osteopenia | Loots et al., 2005 | |
| Sostdc1 (Wise) | KO | Germline | Supernumerary teeth, bone phenotype not determined | Ahn etal., 2010; Kassai et al., 2005; Murashima-Suginami et al., 2008 |
| Tcf7 (Tcf1) | KO | Germline | Lower bone mass (modest), increased bone resorption | Glass et al., 2005 |
| Wif1 | KO | Germline | Normal skeletal development, accelerated radiation-induced osteosarcoma formation | Kansara et al., 2009 |
| Tg | 2.3Col1a1 | Normal bone, depletion of hematopoietic stem cells | Schaniel et al., 2011 | |
| Wls (Gpr177) | CKO | Wnt1-Cre | Craniofacial defects, defective anterior–posterior axis formation | Carpenter et al., 2010; Fu et al., 2011 |
| Wnt3a | Het KO | Germline | Reduced BMD | Takada et al., 2007 |
| Wnt5a | Het KO | Germline | Reduced BMD, increased adipogenesis | Takada et al., 2007 |
| Wnt7b | KO | Germline | No defects in skeletal development | Rodda et al., 2005 |
| Wnt10b | KO | Germline | Reduced BMD, increased adipogenesis | Bennett et al., 2005, 2007; Stevens et al., 2010 |
| Tg | Fabp4 | Increased BMD | Bennett et al., 2005 | |
| Tg | Ocn | Increased BMD | Bennett et al., 2007 | |
| Wnt14 | Tg | Col2a1 | High expression blocked endochondral bone formation, lower transgene expression promoted chondrocyte maturation and enhanced endochondral bone formation | Day et al., 2005 |
| Wtx | KO | Germline | Sclerosis, increased osteoblastogenesis, but delayed mineralization | Moisan et al., 2011 |
| CKO | Prx1-Cre | Bone overgrowth, reduced marrow adiposity | ||
| CKO | Osx1-Cre | Increased cortical and trabecular bone mineralization | ||
| CKO | Col2a1 | Normal skeleton | ||
| CKO | Ocn-Cre | Normal skeleton |
BMD: bone mineral density; CKI: conditional knock-in; CKO: conditional knockout mouse; GOF, gain of function; HBM, high bone mass mutations; Het: heterozygous knockout mouse; KO: global knockout; DKO: double global knockout; Opg: osteoprotegerin; Rs: ringelschwanz hypomorphic Lrp6 mutation; Tg: transgenic.
Table 2.
Summary of bone phenotypes in mouse models of altered Wnt signaling in osteoclasts and precursors.
| Gene | CKO/CKI | Cre line | Bone phenotype(s) | References |
|---|---|---|---|---|
| Ctnnb1 (β-Catenin) | CKO | PPARγ-tTA: TRE-Cre: exon6 | Het mice: Osteoporosis, increased bone resorption, no change in bone formation; KO mice: Osteopetrosis, reduced bone resorption, reduced osteoclast precursor proliferation, no change in bone formation | Wei et al., 2011 |
| CKO | Tie2-Cre: exon6 | Het: Osteoporosis; KO: partial embryonic lethality, osteopetrosis | ||
| CKO | Lyz-Cre: exon6 | KO: Osteoporosis, increased bone resorption; Het: intermediate bone loss | ||
| CKO | Ctsk-Cre: exon 6 | Ibid | ||
| CKI/GOF | Tie2-Cre: exon3 | Embryonic lethality | ||
| CKI/GOF | PPARγ-tTA: TRE-Cre: exon3 | Osteopetrosis: more immature proliferating osteoclasts but fewer mature osteoclasts, reduced bone resorption, no change in bone formation | ||
| CKI/GOF | Lyz-Cre: exon3 | Ibid | ||
| CKI/GOF | Ctsk-Cre: exon 3 | Ibid |
BMD: bone mineral density; CKI: conditional knock-in; CKO: conditional knockout mouse; Het: heterozygous knockout mouse.
Fig. 2.
Cell lineages involved in bone development and homeostasis. Osteoblasts and chondrocytes are derived from mesenchymal progenitor cells, whereas osteoclasts are derived from hematopoietic precursors. Various promoters (indicated at the top and bottom of the diagram in gray boxes) drive transgene or Cre expression in these cells at various stages of their maturation. Mature osteoblasts and osteocytes stimulate osteoclast maturation through Rankl–Rank interactions, but also secrete the decoy receptor Opg to regulate the process. Osteocytes secrete Scl to inhibit Lrp5 activities. PTH and mechanical loading suppress Scl production by osteocytes to increase bone formation.
2. Wnts and Wntless
2.1. Wnts
Wnts are secreted, cysteine-rich glycoproteins involved in controlling cell proliferation, cell-fate specification, gene expression, and cell survival. Cells recognize Wnts with 10 Frizzled receptors (Fzd) and Lrp molecules (Lrp5/6 and potentially Lrp4). The large number of ligands and receptors creates great combinatorial diversity and contributes to widely variable cellular responses depending on the molecules present. Wnts were historically classified as either “canonical” or “non-canonical” based on their ability to activate β-catenin; however, in reality the distinction is not so clear because some Wnts stimulate both pathways depending on the cellular context. Understanding how Wnt molecules contribute to osteoblast function and overall bone homeostasis is crucial in developing treatments for the clinical intervention for various bone diseases, such as osteoporosis.
Expression analyses of the Wnt family members in various osteoblastic models provide insight into the possible function and physiological source of each Wnt. Witte and colleagues profiled all 19 Wnts during mouse limb development and cartilage differentiation (Witte et al., 2009). Each Wnt displayed a unique expression pattern and localization. Interestingly Wnt1, Wnt3a, Wnt8a, and Wnt8b were not detected at any developmental timepoint. Mak and colleagues found that expression levels of Wnt2, Wnt2b, Wnt4, Wnt5a, Wnt10b, and Wnt11 were higher in mature murine osteoblasts compared to their progenitors (Mak et al., 2009). These studies provide an important spatial and temporal context to begin to understand how the Wnt ligands affect bone biology.
Many Wnt ligands affect various aspects of bone biology in vitro, but their true importance in bone physiology will ultimately come from experimental observations in vivo. Currently available Wnt mouse models suggest that Wnt3a, Wnt5a, and Wnt10b are capable of regulating osteoblast function (Baksh et al., 2007; Boland et al., 2004; Etheridge et al., 2004; Hu et al., 2005), whereas Wnt14 contributes to endochondral bone formation (Day et al., 2005).
2.1.1. Wnt3a
Germline deletion of Wnt3a causes early embryonic lethality; however, heterozygotic Wnt3a males display bone loss, with decreases in bone mineral density and trabecular number (Takada et al., 2007). Recombinant Wnt3a is commercially available and is used in numerous in vitro assays to stimulate canonical Wnt signaling in osteoblasts where it induces cell proliferation and survival (Almeida et al., 2005). Wnt3a also induces the proliferation of mesenchymal precursor cells (Boland et al., 2004).
2.1.2. Wnt5a
Wnt5a heterozygote males also display bone loss, with decreases in bone mineral density and trabecular number and increases in adipocyte number (Takada et al., 2007). Recombinant Wnt5a is also commercially available and is often used to stimulate non-canonical (or non-β-catenin) signaling pathways. However, care needs to be used in interpreting results with rWnt5a as it activates or represses β-catenin/Tcf signaling depending on the receptor context (Mikels and Nusse, 2006). Thus, Wnt5a stabilizes β-catenin in the presence of Fzd4 but inhibits β-catenin if it binds to Ror2.
2.1.3. Wnt10b
Wnt10b levels are directly correlated with bone mineral density and indirectly related to marrow adiposity. Thus, transgenic overexpression of Wnt10b in either mature osteoblasts or marrow adipocytes increases bone formation (Bennett et al., 2005, 2007). Wnt10b also inhibits fat accumulation in genetically predisposed mouse models of obesity (Wright et al., 2007) and is important for the maintenance of mesenchymal progenitor cells (Stevens et al., 2010). The maintenance of a progenitor pool of preosteoblastic cells in the bone marrow possibly may be through activation of auxiliary pathways, such as Notch (Modder et al., 2011). The capability of Wnt10b to control osteoblastic lineage allocation could offer novel therapeutic interventions to osteoporotic- and/or obesity-related diseases.
2.1.4. Wnt14
Wnt14 is expressed in the tissue surrounding mesenchymal condensations and differentiating osteoblasts (Guo et al., 2004; Kato et al., 2002). It activates β-catenin and induces Lef1 expression (Day et al., 2005). High Wnt14 expression blocked endochondral bone formation; however, lower transgene levels promoted chondrocyte maturation and enhanced endochondral bone formation (Day et al., 2005). These data demonstrate that Wnt14 can contribute to bone formation.
2.2. Wntless (Wls, Evi, Gpr177)
The ability of Wnts to activate signaling cascades in either an autocrine or paracrine fashion requires that they be secreted from cells. Wntless (Wls) is a seven-pass transmembrane protein responsible for the processing and secretion of all Wnts (Banziger et al., 2006; Bartscherer et al., 2006; Goodman et al., 2006). Wls is expressed ubiquitously in human cells and rodent tissues (Jin et al., 2010; Yu et al., 2010), suggesting that Wnts are important in virtually all cell types, both developmentally and postnatally. Germline deletion of Wls causes embryonic lethality and Wnt protein accumulation in the Golgi (Fu et al., 2009). Conditional Wls knockouts with the Wnt1-Cre mouse strain caused craniofacial defects as well as defective anterior–posterior axis formation (Carpenter et al., 2010; Fu et al., 2011). Interestingly, the Wls gene itself is activated by β-catenin and Lef1/Tcf-dependent transcription, which then assists the cellular trafficking of Wnt proteins in a positive feedback mechanism (Fu et al., 2009). Several genome-wide association studies identified WLS as a gene linked to altered bone mineral density (Hsu et al., 2010; Kumar et al., 2011; Rivadeneira et al., 2009; Styrkarsdottir et al., 2010).
2.3. R-spondins
R-spondins (Rspo1–4) are secreted factors that synergize with Wnts (e.g., Wnt1, 3a and 7a, 11) to promote β-catenin stabilization (Kim et al., 2006). In this regard, Rspo2 and Rspo3 are more potent that Rspo1, whereas Rspo4 is a relatively weak activator (Kim et al., 2008). R-spondins interfere with Dkk1 binding to Krm2/Lrp6, thereby preventing Lrp6 internalization (Binnerts et al., 2007; Kim et al., 2008). R-spondins also bind to the leucine-rich repeat containing G protein-coupled receptor (Lgr)-4 and -5 with high affinity and enhance Lrp6 phosphorylation (Carmon et al., 2011; de Lau et al., 2011). Lgr4-null mice exhibit delayed osteoblast differentiation and mineralization during embryogenesis (Luo et al., 2009). Virtually all indices of bone formation were suppressed in both trabecular and cortical bone with concomitant downregulation of osteocalcin, bone siaoloprotein, and collagen transcripts in these animals.
R-spondins are required for development and reproduction (Aoki et al., 2007; Bell et al., 2008; Blaydon et al., 2006; Ishii et al., 2008; Parma et al., 2006), but little is known about their individual roles in skeletal development. Rspo2 is necessary for hind limb development, ossification of the most distal phalanges, and proper fibular growth (Nam et al., 2007). All four R-spondins appear to share similar mechanisms of action; therefore, it is possible that functional redundancy between R-spondins may account for the lack of a significant bone phenotype. Compound R-spondin mouse models may be needed to uncover the importance of Rspo function in bone during postnatal life.
A few studies examined the role of R-spondins in osteoblastic cell culture systems. In C2C12 and primary mouse calvarial cells, Rspo1 synergized with Wnt3a to induce osteoblast differentiation and Opg expression (Lu et al., 2008), suggesting suppression of osteoclastogenesis through upregulation of Opg may contribute to overall bone anabolism. Furthermore, in MC3T3-E1 mouse preosteoblasts, Wnt11 promoted osteoblast differentiation and mineralization through Rspo2 (Friedman et al., 2009). Together, these data identify R-spondins and Lgr4/5 as modulators of Wnt signaling. In accordance, Rspo1 protected arthritic mice from cartilage and bone damage in vivo (Kronke et al., 2010). Thus, R-spondins may represent a novel class of therapeutic agents to combat specific bone and cartilage diseases, although more research into the mechanism of R-spondins in bone and in vivo is necessary before any conclusions into the efficacy of these potential treatments can be drawn.
3. Wnt receptors
3.1. LDL receptor-related proteins
Low-density lipoprotein receptor-related proteins (Lrp) are evolutionarily conserved plasma membrane receptors with a variety of functions including lipid metabolism, cargo transport, and cellular signaling. Lrp5/6 are low affinity co-receptors for Wnts and high affinity receptors for soluble Wnt antagonists: Scl, Sost-dc1, and Dkk1. Lrp4 is also emerging as a regulator of bone mass density.
3.1.1. Lrp5
Lrp5 is one of the most interesting molecules in bone biology at the present time. Its story is one of remarkable achievements in translational research and like all intriguing tales is not without controversy. LRP5 was first implicated in bone biology by researchers interested in the genetic cause for osteoporosis pseudoglioma (OPPG) syndrome, a juvenile-onset autosomal recessive disease of low bone mass (Gong et al., 2001), and by physicians caring for patients with remarkably high bone mass (HBM) who appeared resistant to high impact fractures (such as from an automobile accident) and who anecdotally had trouble staying afloat while swimming (Boyden et al., 2002; Little et al., 2002). Molecular determinants for both of these conditions were mapped to the same region of chromosome 11, which was later identified as the LRP5 locus (Boyden et al., 2002; Gong et al., 1996; Little et al., 2002). Following the identification of mutations in LRP5 coding regions that led to loss-of-function (in OPPG patients) or gain-of-function (in HBM individuals), the conditions were reproduced in animal models. Thus, germline deletion of Lrp5 in all mouse tissues recapitulated the low bone density in OPPG patients (Fujino et al., 2003; Kato et al., 2002), while transgenic overexpression of LRP5-G171V (a gain-of-function mutation) with a relatively osteoblast-specific rat collagen 1 promoter produced high bone mass with increased mechanical strength (Akhter et al., 2004; Babij et al., 2003). Subsequent experiments indicated that Lrp5 was required for efficient Wnt signaling and β-catenin activation in osteoblasts, while the Lrp5 gain-of-function mutations prevented Lrp5 internalization and binding to other ligands such as Dkk1 and Scl (Boyden et al., 2002; Ellies et al., 2006; Zhang et al., 2004). The Lrp5 research path subsequently merged with several others focused on Dkk1 and Scl and has led to promising new anabolic therapies for low bone mass in less than two decades, making it an exciting example of translational research at its best.
The Lrp5 story is not yet complete though because the physiological mechanisms by which Lrp5 alterations regulate bone mass are not fully understood. Given that Lrp5 is expressed in osteoblast-lineage cells, it is possible that Lrp5 mutations directly alter the activities of bone-forming cells. However, the genetic mutations in the aforementioned patients and the Lrp5-deficient animal models are present in all cells and tissues, thus leaving the possibility that Lrp5 alterations indirectly affect bone formation. To determine if Lrp5 directly regulates osteoblast-lineage cells, two groups made Lrp5 CKO mice as well as Lrp5 conditional knock-in (CKI) mice containing a HBM gain-of-function mutation (e.g., G171V or A214V). Crossing these mice with ones expressing Cre under the control of various tissue-restricted promoters produced confounding results. In the first study, neither conditional deletion of Lrp5 in osteoblast progenitors (with Dermo1-Cre) or mature osteoblasts (with 2.3Col1a1-Cre), nor conditional knock-in of the Lrp5-G171V cDNA into mature osteoblasts (2.3Col1a1-Cre) affected vertebral bone volume density, osteoblast number, or bone formation rates as measured by static and dynamic histomorphometry (Yadav et al., 2008, 2010). Rather Lrp5 activity was inversely associated with serotonin synthesis in intestinal stem cells of the duodenum (Villin-Cre), which signaled back to osteoblasts to influence bone formation and regulate bone mass in an endocrine/hormonal fashion (Yadav et al., 2008). Elevated levels of circulating serotonin were also observed in OPPG patients (Yadav et al., 2010), whereas patients with high bone mass due to a gain-of-function LRP5 mutation had lower than normal serotonin plasma levels (Frost et al., 2010).
Using different animals strains, microcomputed tomography and DEXA scanning, another study showed that conditional Lrp5 deletion in pre-osteocytes and osteocytes (with Dmp1-Cre) reduced trabecular bone density in the distal femurs and L5 vertebra, and weakened cortical bone strength. However Lrp5 deletion in the intestinal stem cells (with a different villin promoter, Vil1-Cre) had no effect on bone mass (Cui et al., 2011). Accordingly, conditional expression of HBM alleles G171V or A214V (created by knocking in mutated exons 3 and 4 only, in contrast to the cDNA used in the other study (Yadav et al., 2008)) in osteocytes (with Dmp1-Cre) or the limb bud mesenchyme (with Prx1-Cre) increased bone mass and strength (Cui et al., 2011). No significant changes in serotonin levels were detected as a result of altering Lrp5 activity (Cui et al., 2011).
These seemingly contradictory results could be attributed to differences in the genetic constructs, mouse models, methods used to measure bone density, bones tested, mouse environments, and/or serotonin assay techniques. These possibilities are relatively easy to address and doing so may in fact reveal important insights into fundamental cellular and endocrine mechanisms of bone formation. Lrp5 is necessary for bone formation after loading (Akhter et al., 2004; Saxon et al., 2011) and PTH-induced high bone mass (O'Brien et al., 2008); results that best align with the need for Lrp5 signaling in osteocytes (Cui et al., 2011). The role of Lrp5 in less mature osteoblasts, which arise from multiple sources (e.g., pericytes, bone marrow derived mesenchymal progenitor cells, neural crest cells) could be less important if related Wnt/Dkk1/Scl (co-) receptors (e.g., Lrp6) or alternative growth and differentiation pathways compensate for altered Lrp5 activity. Thus, exactly how Lrp5 regulates bone mass is still unclear, but both direct regulation of osteoblast-lineage cells and indirect regulation via endocrine or paracrine signaling are viable options.
Beyond the existing controversy with the animal models, there is accumulating evidence that LRP5 polymorphisms affect bone mass and fracture risk in human populations (Kiel et al., 2007a, 2007b; Koay et al., 2004; Mizuguchi et al., 2004; Riancho et al., 2011; Rivadeneira et al., 2009; Sims et al., 2008; Urano et al., 2004; van Meurs et al., 2006, 2008). Several of these LRP5 variants alter canonical Wnt signaling (Kiel et al., 2007b), and a recent study showed that a lumbar spine bone mineral density-associated polymorphism, rs312009, affects Runx2 binding to the LRP5 promoter and alters gene transcription (Agueda et al., 2011).
3.1.2. Lrp6
Lrp6 is more than 70% identical to Lrp5 at the amino acid level and has many similar properties as it binds to Wnts, Scl, and Dkks. An inherited autosomal dominant LRP6 mutation that impairs Wnt signaling was found in a family with osteoporosis, coronary artery disease, and metabolic syndrome (Mani et al., 2007). Polymorphisms in LRP6 are associated with low bone mineral density and fracture risk in humans (Riancho et al., 2011; Sims et al., 2008; van Meurs et al., 2006, 2008). Lrp6 appears to have an earlier and perhaps broader role in development than Lrp5 as Lrp6 KO mice are not viable and show defective limb development (Pinson et al., 2000). However, Lrp6 heterozygous (+/–) mice have reduced total and trabecular bone mineral density (Holmen et al., 2004). Compound Lrp5–/–:Lrp6+/– mice have even lower bone mineral density than either the single or double heterozygotes as measured by DEXA; indicating that Lrp6 and Lrp5 genetically interact in skeletal development and have at least partially redundant functions in postnatal mice (Holmen et al., 2004). Mice carrying an Lrp6 hypomorphic mutation, ringelschwanz (rs), that prevents it from being chaperoned to the cell surface also have reduced bone mineral density (Kubota et al., 2008). Osteoblast number and mineralization were not impaired in these animals, but Rankl expression was elevated on osteoblasts and correlated with increased bone resorption. Lrp6 CKO mice have not yet been reported but are essential to determining its roles in osteoblast-lineage cells and perhaps unraveling clues to Lrp5's distinct functions.
Interesting biochemical studies revealed that Lrp6 contributes to optimal PTH signaling in osteoblasts. In response to PTH stimulation, Lrp6 binds the PTH receptor, PTHR1, and is phosphorylated by PKA (Wan et al., 2008, 2011). This recruits Axin, stabilizes β-catenin, and increases Tcf/Lef1-dependent gene transcription. The effects of PTH in Lrp6-insufficient animals were not determined; however, Lrp6 siRNAs efficiently blocked PTH stimulation of Tcf/Lef1 activity in rat osteosarcoma cells (Wan et al., 2008). Lrp5 was not tested in these assays because PTH stimulated bone formation in Lrp5-deficient mice to the same extent as it did in wildtype mice (Iwaniec et al., 2007; Sawakami et al., 2006); however, later studies showed that Lrp5 is necessary for increased bone formation, but not bone remodeling, in mice expressing a constitutively active (ca) PTHR1 in osteocytes (O'Brien et al., 2008). Decreased Scl expression in the caPTHR1 transgenic animals appeared to be was responsible for the anabolic effects in osteocytes. The specific roles of Lrp5 and Lrp6 in PTH responsiveness will certainly become clearer in the near future as tissue-specific Lrp6 CKO mice are studied.
3.1.3. Lrp4
Lrp4 (also known as Megf7) is an emerging regulator of bone mass. Lrp4-deficient mice have polysyndactyly due to defective limb development in the apical ectodermal ridge (AER) as early as embryonic day 9 (Johnson et al., 2005; Simon-Chazottes et al., 2006; Weatherbee et al., 2006). Mice containing a Lrp4 hypomorphic mutation, Lrp4ECD, exhibit impaired skeletal growth, reduced trabecular bone volume, and increased bone turnover (Choi et al., 2009). Lrp4 antagonizes canonical Wnt signaling and modulates several important development signaling pathways involving Wnts, Bmps, Fgfs, and Shh in skeletal and tooth development (Johnson et al., 2005). Lrp4 is expressed on human and rat osteoblasts and osteocytes (Leupin et al., 2011). It directly binds to Wnt antagonists, including Scl (Leupin et al., 2011) and Sost-dc1 (also called Wise) (Ohazama et al., 2008). Lrp4 suppression by RNA interference allowed for osteoblast mineralization in vitro, even in the presence of Scl (Leupin et al., 2011).
LRP4 appears to control bone density in humans as well. Like its cousins LRP5 and LRP6, LRP4 polymorphisms are associated with altered bone mineral density and lower fracture incidence in genome-wide association studies (Kumar et al., 2011; Rivadeneira et al., 2009; Styrkarsdottir et al., 2009). In addition, two mutations (R1170W and W1186S) in the extracellular region of LRP4 were found in patients exhibiting bone overgrowth (Leupin et al., 2011). These amino acid substitutions impaired LRP4 association with Scl, an inhibitor of bone formation.
3.2. Frizzleds
Fzds are highly versatile seven-pass membrane proteins that contribute to activation of both β-catenin and non-β-catenin signaling pathways by virtue of their interactions with Dvl and the existence of potential phosphorylation sites for cAMP-dependent PKA, PKC, and Ck2 in their intracellular domains. There is a paucity of information about the roles of specific Fzds in bone biology; however, data from Fzd9-deficient mice demonstrate that it contributes to optimal bone formation.
3.2.1. Fzd9
Patients with Williams–Beuren syndrome have low bone density and hemizygous deletion of a region on chromosome 7 that includes FZD9 (Francke, 1999). Fzd9 KO and heterozygote mice have reduced bone mineral density and low bone formation rates (Albers et al., 2011). β-catenin was not affected by Fzd9-deficiency, but Stat1 levels were reduced. This led to the reduction of interferon-stimulated genes, including Isg15, which encodes an ubiquitin-like molecule. Interestingly Isg15-deficient mice also have low bone density. Isg15 overexpression restored the ability of Fzd9-deficient osteoblasts to mineralize their extracellular matrix in vitro. Fzd9 expression was upregulated during osteoblast maturation. Thus, Fzd9 is a crucial regulator of late stages of bone mineralization.
4. Wnt antagonists
4.1. Secreted Wnt antagonists: Dkks, Sfrps, Wif1, Sost, and Sost-dc1
Secreted Wnt antagonists generally utilize two distinct mechanisms to inhibit Wnt signaling. Sfrps, Cerberus and Wif1 bind to Wnts and/or Fzds to directly interfere with association of the ligand with its receptor (Fig. 1). In contrast, Dkk, Sost and Sost-dc1 (Wise) bind to the Lrp5/6 co-receptor and inhibit Wnts from associating with the Fzd/Lrp receptor complex. Existing data suggest that some of these inhibitors are viable targets for new anabolic therapeutics.
4.1.1. Dickkopfs (Dkk)
The Dickkopf factors (Dkk1–4) have differing expression patterns during embryonic and postnatal development (Nie et al., 2005; Witte et al., 2009). Dkks bind and sequester the Lrp5/6 and Krm1/2 membrane complex to inhibit Wnt activity. Recent studies highlight the importance of Dkk1, Dkk2, and Dkk3 in osteoblastic function.
4.1.1.1. Dkk1
Dkk1 is a well-characterized secreted Wnt inhibitor that is active in many tissues (reviewed in (Pinzone et al., 2009)). Several lines of clinical evidence indicate that DKK1 regulates bone mass in humans. The first is that the gain-of-function mutations in LRP5 responsible high bone mass inhibit the ability of LRP5 to bind DKK1 (Ai et al., 2005; Boyden et al., 2002). Second, high DKK1 production by malignant plasma cells leads to osteolytic bone lesions in patients with multiple myeloma and blocks osteoblast differentiation (Tian et al., 2003). Data from various animal models confirm that Dkk1 suppresses Wnt signaling and inhibits bone formation. Dkk1–/– mice die shortly after birth with severe developmental abnormalities (del Barco Barrantes et al., 2003), but Dkk1+/– mice have increased bone formation and bone mass without a compensatory change in bone resorption (Morvan et al., 2006). Conversely, transgenic overexpression of Dkk1 using the rat collagen1α1 promoter specific to osteoblasts significantly decreased osteoblast number, bone formation rate, and serum osteocalcin levels (Fleming et al., 2008; Guo et al., 2010; Li et al., 2006; Yao et al., 2011). Finally, mice harboring the hypomorphic Dkk1d (doubleridge) allele, which display forelimb postaxial polysyndactyly, are informative models to study Dkk1 activity in bones (MacDonald et al., 2004). The trabecular and cortical bone density parameters of hypomorphic progeny of Dkk1+/– and Dkk1+/d mice are inversely proportional to the level of Dkk1 expression. (Macdonald et al., 2007). These studies demonstrate that Dkk1 is a negative regulator of bone in vivo.
In recent years, significant interest in DKK1 suppression as a treatment modality for various bone diseases has led to the development of an array of DKK1-neutralizing antibodies. In several murine multiple myeloma models, DKK1 antibodies significantly increased osteoblast numbers, serum osteocalcin levels, and trabecular bone volume (Diarra et al., 2007; Fulciniti et al., 2009; Heath et al., 2009). A Dkk1 antibody also increased bone formation at endosteal bone surfaces in a mouse model of ovariectomy-induced osteopenia (Glantschnig et al., 2011). Dkk1 is expressed in most tissues, thus using Dkk1 antibodies to treat a chronic and systemic disease like osteoporosis may produce unwanted effects. However, local delivery of Dkk1-neutralizing antibodies may be a treatment option for fractures non-union because Dkk1 inhibits fracture repair (Chen et al., 2007). Indeed, in a murine fracture repair model, anti-Dkk1 antibodies increased the callus area, bone mineral content/density, and biomechanical properties of the injured bone (Komatsu et al., 2010). Collectively, these reports suggest that Wnt pathway activation through suppression of Dkk1 may offer therapeutic treatments for select bone diseases and orthopedic conditions.
4.1.1.2. Dkk2
The molecular functions of Dkk2 vary with cellular context. Like Dkk1, Dkk2 effectively blocks Wnt1-dependent activation of Lef1/Tcf target genes and inhibits both the Wnt and osteogenic differentiation pathways in osteoarthritic osteoblasts (Chan et al., 2011b). However, Dkk2 also activates β-catenin in Xenopus embryos (Wu et al., 2000). These opposing effects may be modulated by Krm2, which converts Dkk2 from an agonist to an antagonist of Lrp6 (Mao and Niehrs, 2003). Dkk2-null mice are osteopenic with suppressed bone formation parameters (Li et al., 2002). Osteoblast cultures derived from bone marrow and calvaria of Dkk2–/– mice mineralize at a slower rate than wildtype cells. These data suggest that Dkk2 stimulates bone formation at least in early development, in stark contrast to the bone inhibitory functions of Dkk1. Interestingly, Wnt7b may facilitate Dkk2 induction of osteogenesis. Dkk2 inhibited bone formation in the absence of Wnt7b, but induced terminal osteoblast differentiation in the presence of high Wnt7b levels (Li et al., 2005a). Thus, the effects of Dkk2 on osteoblasts is critically dependent on cellular context, particularly Krm2 and Wnt7b levels.
4.1.1.3. Dkk3
Little information exists regarding Dkk3's activity in bone, but it was temporally co-expressed with osteogenic genes in Bmp2-producing C3H10T1/2 mesenchymal progenitor cells implanted into mice (Aslan et al., 2006). The Dkk3-expressing cell implants had decreased bone quality as measured by μCT and bioluminescence imaging. Further investigation is required to fully determine Dkk3's role in bone formation.
4.1.2. Secreted frizzled-related proteins (Sfrps)
Sfrp1-5 are secreted, cysteine-rich glycoproteins that share high homology to the Fzd receptors. Sfrps antagonize Wnts though direct binding, thereby preventing their functional association with Fzds on the cell surface (Kawano and Kypta, 2003). Several Sfrps are expressed in skeletal tissue and cells of the osteoblastic lineage. They have varying effects on bone development and osteoblast function.
4.1.2.1. Sfrp1
Sfrp1 action in bone has been extensively explored. Targeted disruption of Sfrp1 increased trabecular but not cortical bone mineral density to a similar extent as PTH (Bodine et al., 2004, 2007), whereas transgenic Sfrp1 overexpression decreased bone density and attenuated the bone anabolic effects of PTH (Yao et al., 2010). Sfrp1 expression is increased by dexamethasone and may be involved in glucocorticoid-induced osteoporosis (Wang et al., 2005). Mechanistically, Sfrp1 appears to affect cell viability and maturation as its deletion reduced osteoblast and osteocyte apoptosis in vivo, and cell proliferation and differentiation in vitro (Bodine et al., 2004). In an immortalized human osteoblast cell line, Sfrp1 potently suppressed Wnt signaling (Bodine et al., 2005). Similarly, Sfrp1 significantly increased osteoblast apoptosis with concomitant decreases in bone mineral density, trabecular bone volume, and cortical bone area in rat femurs (Wang et al., 2005). Sfrp1 also binds Rankl and blocks osteoblast-induced osteoclastogenesis (Hausler et al., 2004). Collectively, these data clearly demonstrate that Sfrp1 inhibits osteoblast viability and coupling to osteoclasts.
As with other secreted Wnt inhibitors, there is significant interest in isolating Sfrp1 antagonists to treat low bone mass conditions. A high throughput screen of potential small molecule inhibitors revealed a class of piperidinyl diphenylsulfonyl sulfonamide compounds that bind Sfrp1 and inhibit its activity (Bodine et al., 2009; Moore et al., 2010). These compounds blocked Sfrp1-mediated apoptosis of preosteoblasts and stimulated bone formation in vitro (Moore et al., 2009); however, the effectiveness of these compounds in vivo has not been reported. In a rodent model of periodontal bone loss, Sfrp1 polyclonal antibodies suppressed bone resorption and decreased pathogen-induced inflammation (Li and Amar, 2007). Like Dkk1 antagonists, Sfrp1-based therapeutics might be best suited for such localized conditions because of its broad expression pattern in multiple tissues.
4.1.2.2. Sfrp4
Sfrp4 is expressed in human mesenchymal stem cells and in areas of bone formation at E15.5 in the developing mouse limb (Etheridge et al., 2004; Witte et al., 2009). Transgenic overexpression of Sfrp4 using the osteoblast-directed rat 2.3 kb Col1a1 promoter suppressed osteoblast proliferation and decreased bone formation (Nakanishi et al., 2008). Moreover, transgenic mice overex-pressing Sfrp4 under the control of serum amyloid P promoter, which drives postnatal secretion of Sfrp1 from the liver and into serum, exhibited low bone mass (Cho et al., 2010). Recombinant Sfrp4 also inhibited osteoblast proliferation and partially suppressed the activity of Wnt3a in vitro (Nakanishi et al., 2006). Collectively, these data demonstrate that Sfrp4 negatively regulates bone formation and decreases bone mineral density through the inhibition of Wnt signaling.
SFRP4 polymorphisms were associated with altered hip and spine bone mineral density in numerous populations (Cho et al., 2009; Karasik et al., 2003; Styrkarsdottir et al., 2008). Furthermore, the Sfrp4 locus was associated with lower bone mineral density in the senescence-accelerated mouse P6 (Nakanishi et al., 2006).
4.1.3. Wnt inhibitory factor (Wif)-1
Wif1 is a secreted factor that inhibits Wnt signaling through direct interaction with Wnts (e.g., Wnt-3a, -4, -5a, -7a, -9a, -11) (Malinauskas et al., 2011; Surmann-Schmitt et al., 2009). Wif1 is expressed during Bmp2-induced osteoblast differentiation of C2C12 and MC3T3-E1 cells. Moreover, WIF1 was elevated in calvarial sutures of craniosynostosis patients (Coussens et al., 2007). These results suggest that Wif1 may be part of a negative feedback loop that controls osteoblast differentiation and maturation (Vaes et al., 2005). Wif1 KO mice have a normal skeleton but are more sensitive to radiation-induced osteosarcomas (Kansara et al., 2009). Similarly, mice with osteoblast-specific Wif1 overexpression display no overt bone phenotype, but have disrupted stem cell quiescence leading to a loss of self-renewal potential, suggesting an important role for Sfrp4 in regeneration of the progenitor cell niche (Schaniel et al., 2011).
4.1.4. Sclerostin/SOST
Inactivating mutations in the SOST gene, which encodes the protein Sclerostin (Scl), cause two rare bone sclerosing disorders, sclerosteosis and van Buchem disease. These diseases are characterized by endosteal hyperostosis, progressive generalized osteosclerosis, and high bone mass associated with increased osteoblastic activity and elevated bone formation markers (Wergedal et al., 2003). The mutations introduce premature transcriptional stop codons, interfere with splicing, or delete regulatory elements in SOST, thereby preventing osteocytes from secreting sufficient levels of fully functional Scl (Balemans et al., 2001, 2002; Brunkow et al., 2001; Staehling-Hampton et al., 2002). As is often true in medicine, identification of the genetic and molecular origin of these rare diseases has revealed an important mechanism in normal physiological processes and unleashed a flurry of activity to translate the information into therapy for more common disorders.
Unlike Dkks, Sfrps, and Wise, Scl is produced primarily by bone cells and is abundant in the osteocytic canalicular system (van Bezooijen et al., 2004; Winkler et al., 2003) but has also been detected in cementocytes in teeth, mineralized hypertrophic chondrocytes in the growth plate, and osteoarthritic cartilage (Chan et al., 2011a; van Bezooijen et al., 2009). Scl binds Lrp5/6 and inhibits their association with Fzd and Wnts (Li et al., 2005b; Semenov et al., 2005). Scl inhibits proliferation and differentiation and stimulates apoptosis of osteogenic cultures (Sutherland et al., 2004; van Bezooijen et al., 2004; Winkler et al., 2003). In support of the Wnt inhibitory function of Scl in vivo, canonical Wnt signaling, bone density, and bone mechanical strength are elevated in Sost knockout mice (Krause et al., 2010; Li et al., 2008); whereas transgenic overexpression of Sost induced osteopenia (Loots et al., 2005; Winkler et al., 2003). Collectively, these data clearly demonstrate that Scl is an important negative regulator of bone formation.
By virtue of its relatively exclusive expression in bone and its role in repressing bone formation from the extracellular space, Scl is an attractive target for anabolic therapeutics. Scl neutralizing antibodies have shown efficacy in multiple pre-clinical models (e.g., rodents, non-human primates) and more recently in clinical trials for osteoporosis (reviewed in (Rachner et al., 2011)). For example, Scl antibodies increased bone mass and prevented bone loss associated with estrogen deficiency in ovariectomized rats (Li et al., 2009). In phase-2 clinical studies, a fully humanized Scl neutralizing antibody increased bone formation parameters in post-menopausal osteoporotic women (Padhi et al., 2011). These studies and others indicate that Scl inhibitors may provide skeletal benefits for patients with osteoporosis and other diseases of low bone mass. Scl antibodies may also improve outcomes of orthopedic stabilization and fixation procedures that are complicated by low bone volumes.
Scl has quickly emerged as an important modulator of anabolic signaling pathways in bone, particularly PTH stimulation and mechanical loading. Intermittent PTH stimulates bone formation; however, the molecular mechanisms underlying this response are not fully understood (reviewed in (Kramer et al., 2010b)). PTH suppresses Scl expression both in vitro and in vivo (Bellido et al., 2005; Keller and Kneissel, 2005) by inhibiting myocyte enhancer factor 2, which normally activates Sost transcription through a specific enhancer element (Leupin et al., 2007). PTH-dependent bone anabolism is suppressed in mice where Sost is overexpressed, indicating that Scl levels can modulate PTH-induced bone formation (Kramer et al., 2010c; O'Brien et al., 2008). Scl expression is also suppressed in osteocytes by mechanical loading in vivo (Robling et al., 2006). This may contribute to high Wnt/β-catenin signaling that occurs after mechanical loading (Robinson et al., 2006).
4.1.5. Sost-dc1 (Ectodin, Wise, Usag1)
Sost-dc1 (sclerostin domain containing 1) is a secreted factor that belongs to the Dan/Cerberus family of proteins. It binds to Bmps to neutralize their activity (Itasaki et al., 2003; Kassai et al., 2005; Laurikkala et al., 2003; Yanagita et al., 2004). It also blocks Wnt1, Wnt3a, and Wnt10b activities in various cellular models (Beaudoin et al., 2005; Blish et al., 2008; Lintern et al., 2009). Sost-dc1 inhibits Wnt activity by binding Lrp6 (Itasaki et al., 2003; Lintern et al., 2009) and possibly Lrp4 (Ohazama et al., 2010). Sost-dc1-deficient mice have extra teeth due to excessive Bmp signaling and reduced apoptosis of developing odontogenic mesenchymal cells (Ahn et al., 2010; Kassai et al., 2005; Munne et al., 2009; Murashima-Suginami et al., 2008). The bone phenotype of the Sost-dc1-null models has not been characterized; however, several lines of evidence suggest a role in the skeleton. SOST-DC1 polymorphisms were associated with attainment and maintenance of peak bone mass in Chinese women (He et al., 2011). Furthermore, Wnt10b suppressed SOST-DC1 expression in a human osteosarcoma cell model (Modder et al., 2011). Since osteoblast function is critically dependent on both Bmp and Wnt signals, a potential role of Sost-dc1 in osteoblasts is intriguing, although further research is needed to clarify its role in bone.
4.2. Transmembrane modulators of Wnt signaling
Several transmembrane proteins modulate Wnt signaling pathways by binding to Wnts or the secreted antagonists discussed above. These molecules include Kremen (Krm) 1, Krm 2, and the receptor tyrosine kinases, Ror2 and Ryk.
4.2.1. Kremen1/2
Krm1 and Krm2 are single-pass transmembrane co-receptors for Dkk1. Krms and Dkk1 form a ternary complex with Lrp6, which is rapidly endocytosed within 5 min to reduce Wnt/β-catenin signaling (Mao et al., 2002). Krms are expressed in developing limb buds. Double mutant Krm1–/–:Krm2–/– mice have elevated Wnt signaling, expanded AERs and ectopic postaxial forelimb digits (Ellwanger et al., 2008). Ectopic growth of digits is enhanced in triple mutant Krm1–/–:Krm2–/–:Dkk1+/– mice, demonstrating a genetic interaction between Krms and Dkk1 in limb development. Double mutant Krm1–/–:Krm2–/– mice had increased bone volume and bone formation rates at 12 weeks of age (Ellwanger et al., 2008). Single mutant Krm1–/– and Krm2–/– mice had normal bone volume and bone formation rates at this age, but the Krm2–/– mice developed high bone mass associated with increased bone formation 12 weeks later, at 24 weeks of age (Schulze et al., 2010). Transgenic expression of Krm2 in mature osteoblasts under control of the 2.3Col1a1 promoter suppressed osteoblast maturation and Opg production (Schulze et al., 2010). Cortical strength was reduced and osteoclast activity was elevated. Krm2 is predominantly expressed in bones of 6 week-old mice, whereas as Krm1 is expressed in bone as well as other tissues (Schulze et al., 2010). These data suggest that Krm2 is a potential bone-specific target for future for anabolic agents.
4.2.2. Ror2
The Ror family of membrane-spanning tyrosine kinases bind certain Wnts either alone or as Fzd co-receptors to activate non-β-catenin signaling in mammalian tissues (Grumolato et al., 2010; Minami et al., 2010). Wnt5a, for example, induces the formation of a complex consisting of Lrp5/6, Ror1/2, and Fzd2 (Sato et al., 2010). Via Ror2, Wnt5a blocks Wnt3a-mediated β-catenin activation (Mikels and Nusse, 2006). ROR2 mutations are linked to several skeletal disorders (e.g., dominant brachydactyly type B and recessive Robinow syndrome), further supporting a role for this pathway in endochondral bone formation (Afzal et al., 2000; Angers and Moon, 2009; DeChiara et al., 2000; Oldridge et al., 2000).
4.2.3. Ryk
Ryk is an atypical tyrosine kinase receptor that is predicted to lack intrinsic enzymatic activity, but may associate with Src kinases (Hovens et al., 1992; Wouda et al., 2008). Ryk's fly homolog, Drl, binds Wnt5a in the absence of Fzd or Dvl to regulate growth cone guidance (Bonkowsky et al., 1999). In HEK293T cells, RYK can be co-immunoprecipated with Wnt1, Wnt3a, Fzd and Dvl, and is required for Wnt-mediated β-catenin activation (Lu et al., 2004). Ryk activities in bone cells have not been reported.
5. β-catenin and associated intracellular proteins
5.1. Ctnnb1 (β-catenin)
β-catenin is a cytoplasmic and nuclear protein encoded by the Ctnnb1 gene. It is a key link in numerous signaling cascades, including the “canonical Wnt pathway”, is essential for embryonic development, and is hyperactivated by mutations in many cancers. Wnt ligation of Lrp5/6 and Frizzled receptors inactivates the β-catenin destruction complex consisting of Apc, Axin, Ck1, Gsk3, Wtx, and the E2 ubiquitin ligase, βTrCP. As β-catenin accumulates, some is transported to the nucleus where it interacts with Lef1/Tcf transcription factors to regulate numerous genes, including Axin2, which in turn can provide feedback inhibition (Jho et al., 2002). β-catenin proteolysis is triggered by phosphorylation of several serine residues in its N-terminus by Ck1 and Gsk3β. Deletion of exon 3 in Ctnnb1, removes these residues and produces a stable protein that acts as a gain-of-function mutation (Harada et al., 1999). Over the last decade, numerous genetic studies were performed using mice in which exon 3 (to activate β-catenin) or exons 6–10 (to eliminate β-catenin) of Ctnnb1 is flanked by loxP sequences to dissect β-catenin's role(s) in skeletal development through gain- and loss-of function, respectively.
β-catenin is essential for controlling mesenchymal cell fate decisions and linking bone formation to bone resorption. Ctnnb1-deletion in mesenchymal progenitors (as early as E9.5) caused severe defects in skeletal formation, characterized by reduced mineralization, defective osteoblastogenesis, and ectopic chondrogenesis (Brault et al., 2001; Day et al., 2005; Hill et al., 2005; Hu et al., 2005; Rodda and McMahon, 2006). Targeted Ctnnb1-deletion in committed osteoblast-lineage cells at a later stage in development (E14.5) also reduced bone mass, but surprisingly osteoblast numbers and bone formation rates were normal (Glass et al., 2005; Holmen et al., 2005; Kramer et al., 2010a). These Ctnnb1-deficient osteoblasts and osteocytes produced less Opg, which allowed for more interactions between Rankl-positive osteoblasts and Rank-expressing osteoclasts and promoted bone resorption.
Increasing β-catenin activities through deletion of exon 3 produced nearly opposite phenotypes as the knockout mutations and early lethality. Thus, Ctnnb1 gain-of-function mutations in mature osteoblasts and osteocytes caused premature and excessive ossification by reducing osteoclast numbers without changing osteoblast numbers (Glass et al., 2005; Rodda and McMahon, 2006). In animals where β-catenin was activated in premature limb bud and craniofacial mesenchyme (with Prx1-Cre), appendicular and skull bone elements were absent, suggesting that β-catenin stabilization negatively impacts this early stage of differentiation (Hill et al., 2005).
Because of the different phenotypes of the Ctnnb1 and Lrp5-deficient mice, it is crucial to discuss β-catenin's roles outside of the Wnt signaling pathway. Notably, β-catenin associates with cadherins to regulate epithelial cell growth, cell adhesions and migration. N-cadherin overexpression inhibits osteoblast proliferation and survival by blocking Wnt3a, PI3K/Akt and Erk signaling (Hay et al., 2009). β-catenin also links the membrane to the actincytoskeleton, which may transmit signals responsible for contact-mediated inhibition of cell growth, or in the case of bone homeostasis, signals from mechanical strains. Several reports demonstrated that mechanical loading activated a Lef/Tcf reporter and promoted nuclear β-catenin localization in primary calvarial cells (Armstrong et al., 2007; Hens et al., 2005). In murine calvarial osteoblasts, mechanical loading by biaxial strain increased nuclear β-catenin levels through the activation of Akt and consequent inactivation of Gsk3β (Case et al., 2008). Gsk3β inhibition by mechanical strain stimulated Nfatc1 as well as β-catenin signaling to induce osteogenesis and inhibit adipo-genesis of multipotent mesenchymal cells (Sen et al., 2008). In osteocytes, the mechanosensory cells of bone, fluid flow sheer stress indirectly stabilized and stimulated nuclear translocation of β-catenin through prostaglandin E2 (PGE2) and EP2/4 synthesis, PI3K/Akt and cAMP/PKA signaling, and Gsk3β inactivation to protect osteocytes from glucocorticoid apoptosis and stimulate gap junctions (Bonewald and Johnson, 2008; Kamel et al., 2010; Kitase et al., 2010; Xia et al., 2010). Together these data indicate that mechanical strain activates multiple pathways, many of which converge on β-catenin to control cell fate and promote bone formation.
Recently, the effects of altering β-catenin levels in osteoclast lineage cells were reported (Wei et al., 2011). Using a variety of Cre drivers, it was determined that β-catenin regulates osteoclastogenesis in a dosage-dependent manner (Table 2). A minimum amount of β-catenin was required to induce the proliferation of osteoclast progenitors as complete Cttnb1 deletion caused osteopetrosis. In contrast, Cttnb1 haploinsufficiency accelerated osteoclastogenesis and produced an osteoporotic phenotype. High levels of constitutively active β-catenin inhibited osteoclast maturation and bone resorption to cause osteopetrosis. Wnt3a and two Gsk3β inhibitors attenuated osteoclast differentiation (Wei et al., 2011). Moreover, Wnt3a stabilized β-catenin in human osteoclast precursor cells from multiple myeloma patients in vitro to suppress osteoclast differentiation (Qiang et al., 2010). Rankl treatment suppressed β-catenin expression in osteoclasts to suppress proliferation and induce terminal differentiation programs (Wei et al., 2011). Further analysis showed that β-catenin promotes osteoclast precursor proliferation in response to M-CSF by inducing expression of Gata2 and Evi1, but blocks Rankl-induced osteoclast maturation by impairing c-Jun activity. These data suggest that therapeutic strategies designed to increase bone mass by activating the canonical Wnt pathway may confer both anabolic and anti-resorptive effects.
Alterations in β-catenin sequence and/or activity contribute to numerous diseases in humans. More than half of human bone and soft tissue sarcomas have excess β-catenin activity (Iwao et al., 1999; Vijayakumar et al., 2011). Tumors have not yet been reported in mice expressing gain-of-function β-catenin mutations; however, benign rib osteomata were found in 80% of animals (Glass et al., 2005), suggesting that sustained β-catenin activation combined with other genetic or epigenetic events may promote carcinogenesis. CTNNB1 polymorphisms were linked to altered bone mineral density in some human population studies (Rivadeneira et al., 2009), but the molecular consequences of these variants have not been elucidated.
5.2. Adenomatous polyposis coli (Apc)
Apc is a tumor suppressor and β-catenin binding protein. Defects in APC cause familial adenomatous polyposis, an autosomal dominant pre-malignant condition that usually progresses to colon cancer. Apc's major function in the cell is to inhibit β-catenin activity. Apc is a scaffold for other components of the β-catenin destruction complex in the cytoplasm. Apc also associates with β-catenin in the nucleus where it prevents β-catenin from associating with Lef/Tcf transcription factors (Neufeld and White, 1997; Neufeld et al., 2000a, 2000b). Although not as thoroughly studied as β-catenin or Lrp5 at the genetic level, the phenotypes of Apc CKO mice are consistent with its role as a negative regulator of β-catenin activity, as well as with β-catenin being a crucial regulator of bone resorption. In both studies, conditional deletion of Apc in either chondrocytes (with Col2a1-Cre) or osteoblasts (with OCN-Cre) elevated β-catenin levels and produced early postnatal lethality as all mice died within 5 weeks (Holmen et al., 2005; Miclea et al., 2009). Similar to the Cttnb1 CKI models, Apc deletion in mature osteoblasts increased trabecular bone volumes, but its deletion in progenitors cells caused severe delays and skeletal malformations. In the OCN-Cre driven Apc CKO mice, no defects in osteoblast development were detected in vitro or in vivo; however, osteoclasts were not detected in histological sections. Opg levels were elevated in these Apc CKO mice, whereas Rankl mRNA levels were reduced in osteoblasts. Cttnb1 CKO mice made by the same group with the same OCN-Cre driver had nearly the opposite phenotype. Moreover, mice carrying osteoblast-specific deletions of both Apc and Cttnb1 had phenotypes resembling the Cttnb1 CKO animals. Together, these data support the conclusion that Apc is a negative regulator of β-catenin in skeletal progenitors and mature osteoblasts. SNPs in APC were found associated with altered trabecular volumetric bone mineral density in several human population studies (Miclea et al., 2010; Yerges et al., 2009).
5.3. Axin1/2
Axin1 and Axin2 are functionally equivalent scaffolding proteins required for the assembly of the β-catenin destruction complex that includes Gsk3β, Dvd, Apc, and Wtx (Chia and Costantini, 2005). In the presence of Wnt ligands, Axin and other components of the destruction complex are recruited to Lrp5/6 at the cell membrane where they facilitate downstream β-catenin signaling (Bilic et al., 2007; MacDonald et al., 2009; Niehrs and Shen, 2010; Zeng et al., 2005). Axin1 is widely expressed and Axin1-deficient mice do not survive past E9.5 due to forebrain and neural tube defects (Zeng et al., 1997). In contrast, Axin2 exhibits a more restricted expression pattern, and is upregulated by Wnt/β-catenin/Tcf signaling. Thus, Axin2 is a negative feedback inhibitor of the Wnt/β-catenin pathway (Jho et al., 2002). Axin2 KO mice are born with no noticeable morphologic abnormalities; however, skull doming was evident by postnatal day 28 (Yu et al., 2005). Further analysis revealed that nuclear β-catenin expression is elevated in cranial bones by seven days of age, leading to increased osteoblast progenitor proliferation, increased osteoblast differentiation, and craniosynostosis characterized by premature fusion of the frontal/metopic suture (Liu et al., 2007a). Bone mass and strength of the axial skeleton was increased at six months of age in Axin2 KO mice due to increased osteoblast differentiation, enhanced osteoblast function, and decreased osteoclast formation (Yan et al., 2009). Introducing Ctnnb1-deficiency onto the Axin2 KO background attenuated the increased osteoblast activity and craniosynostosis phenotype in these mice (Liu et al., 2007a; Yan et al., 2009), whereas conditional activation of β-catenin recapitulated many aspects of the Axin2 KO skeletal phenotype (Mirando et al., 2010), confirming the role of β-catenin signaling in this model.
Interestingly, Axin2–/– mice displayed a runted phenotype compared to wildtype littermates. This disrupted growth was compounded in double mutant Axin2–/–:Axin1+/– mice (Dao et al., 2010). The runted phenotype appears to be due to Axin2's role in chondrocyte maturation. Axin2–/– mice have shorter hypertrophic zones in the growth plate and enhanced expression of type 10 collagen, a marker of mature chondrocytes (Dao et al., 2010). Thus, under normal circumstances Axin2 expression inhibits late chondrocyte differentiation, as it does in osteoblasts. Because of its key role in osteoblast and chondrocyte development, Axin2 may also contribute to musculoskeletal repair. Indeed, Axin2-deficient mice demonstrated rapid healing in fracture models (Minear et al., 2010).
5.4. Gsk3β
Glycogen synthase kinases (Gsk) 3 alpha and beta are highly conserved and ubiquitous serine/threonine enzymes that participate in multiple signaling pathways, including both canonical and non-canonical Wnt signaling. It has long been known that Gsk3 phosphorylates multiple components of Wnt pathways, including β-catenin, Axin, and Apc (reviewed previously in (Westendorf et al., 2004)). Gsk3 phosphorylation of the N-terminus of β-catenin promotes its degradation by the 26S proteosome. Seemingly paradoxically, Gsk3 also has a positive role in promoting Wnt signaling. In response to ligands, Gsk3 and Axin move to the membrane where Gsk3 phosphorylates Wnt receptors, Lrp5/6 (Bilic et al., 2007; MacDonald et al., 2009; Niehrs and Shen, 2010; Zeng et al., 2005). Lrp5/6 phosphorylation results in the formation of a large multi-protein signalosome, which consequently sequesters Gsk3 and facilitates β-catenin accumulation and enhanced gene transcription (reviewed by (Niehrs and Shen, 2010)).
There is much evidence that Gsk3 inhibition promotes bone formation in vivo. Gsk3β suppression by genetic deletion or pharmacological inhibition enhances bone density. Gsk3β KO mice do not survive embryogenesis; however, Gsk3β +/– mice have higher trabecular bone volume density, more osteoblasts per bone surface and increased bone formation rates (Kugimiya et al., 2007; Noh et al., 2009). The numbers of osteoclasts per bone perimeter and eroded surface areas were also elevated, indicating that increased bone formation was coupled to increased resorption (Kugimiya et al., 2007). Osteoblasts from Gsk3β+/– mice had more Runx2 activity because the phosphorylation of an inhibitory residue in Runx2 was suppressed in the absence of adequate Gsk3β levels. Interestingly, Gsk3β haploinsufficiency or lithium chloride treatment rescued the cleidocranial dysplasia in Runx2+/– animals (Kugimiya et al., 2007). Lithium chloride also rescued the low bone mass phenotypes of Lrp5–/– and SAMP6 mice, and increased bone mass in wildtype mice (Clement-Lacroix et al., 2005). Other small molecule Gsk3β inhibitors also increase bone mass in wildtype and ovariectimized animals and improve vertebral strength (Clement-Lacroix et al., 2005; Kulkarni et al., 2006). Reductions in bone marrow adiposity and enhancements in osteocytic responses to mechanical strain were also observed, suggesting that Gsk3β influences mesenchymal cell fate and osteocyte responses to loading (Case et al., 2008; Sen et al., 2008). These positive effects on early progenitors and terminal osteocytes may be the primary reasons why bone formation is enhanced as a result of Gsk3β inhibition, despite increased bone resorption.
Oral lithium chloride has been a treatment option for bipolar disease for more than a half-century. Some studies indicate that patients taking lithium have lower fracture rates and less bone turnover than normal individuals, while other surveys did not observe any differences (Vestergaard et al., 2005; Wilting et al., 2007). Thus, lithium and other Gsk3 inhibitors may be safe for short-term anabolic uses in some patients; however, long-term use and effectiveness remains to be proven.
In summary, Gsk3 is an important contributor to bone formation in vivo and osteoblast maturation in vitro. Existing in vivo models are insufficient to determine whether enhanced bone formation is solely due to osteoblastic responses. Future studies in which Gsk3β is conditionally deleted at different stages of osteoblast and osteoclast development may unravel some of the complexities observed in vitro, with the germline knockout/heterozygote mice, and with lithium chloride response in patients. Finally, Gsk3 proteins participate in multiple signaling pathways besides Wnt, Lrp5/6, Ror1/2, and β-catenin. Most notable are their roles in G protein coupled receptor, Akt, and Bmp2 signaling pathways. Untangling these pathways will require information on molecular structures and posttranslational modifications of relevant proteins.
5.5. Tcf7 and Lef1 transcription factors
T cell factors 7 (Tcf7) and lymphoid enhancer binding factor (Lef1) are nuclear proteins that link Wnt signaling and β-catenin to the genome. They bind to the DNA sequence YCTTTGWW via a C-terminal DNA binding domain and to β-catenin via N-terminal sequences. Central regions of Tcf7s and Lef1 interact weakly with β-catenin and strongly with co-repressors, including Hdacs and Tle proteins. Temporal and spatial expression patterns, alternative splicing, and differential promoter usage of Lef1 and the three Tcf7 genes, Tcf7 (Tcf1), Tcf7L1 (Tcf3), and Tcf7L2 (Tcf4), affect their activities during skeletal development (Glass et al., 2005; Waterman, 2004; Westendorf et al., 2004). In adults, their expression is typically restricted to regenerating tissues. Thus far, no polymorphisms in TCF7 or LEF1 have been associated with altered BMD; however, Tcf7 (Tcf1) or Lef1 KO mice indicate that they play important roles in bone turnover.
5.5.1. Tcf7 (Tcf1)
Tcf1–/– mice were originally described as being of normal size (Verbeek et al., 1995; Roose et al., 1999); however, more careful analysis of the skeleton revealed modest decreases in bone mineral density at one month of age (Glass et al., 2005). These bone mass reductions were attributed to increased bone resorption as osteoclast numbers and activity were elevated, Opg levels were reduced, and no changes were noted in osteoblast numbers or function. The results were consistent with the Ctnnb1 CKO animals reported in the same study. Mice heterozygous for Ctnnb1 and Tcf7 (Tcf1) also had reduced bone density as a result of increased osteoclast activity, demonstrating a genetic link between the molecules (Glass et al., 2005). Tcf7 (Tcf1), Tcf7L2 (Tcf4), and β-catenin associated with the Opg promoter and Lef1 cooperated with β-catenin to activate Opg promoter activity in vitro (Glass et al., 2005). Tcf1 also binds to the Runx2 promoter, which was activated by canonical Wnt signaling (Gaur et al., 2005). Together these data demonstrate that Tcf7 is a crucial mediator of β-catenin signaling in mature osteoblasts and indirectly regulates osteoclast activation.
5.5.2. Lef1
Lef1-deficient mice die within a few days of birth with multi-organ defects due to impaired epithelial and mesenchymal cell interactions (van Genderen et al., 1994). Bone mass was not measured in these mice because of the early postnatal lethality (Noh et al., 2009; van Genderen et al., 1994); however, young female heterozygotes in this Lef1 strain had low trabecular bone mass with reduced osteoblast activity (Noh et al., 2009). This phenotype was temporal and normalized as the animals aged. Male Lef1+/– animals did not have low bone mass unless the androgen receptor was inactivated (Noh et al., 2009). Thus, Lef1 may have an age- and gender-related role in bone homeostasis. A new Lef1 KO model in which a 5’-exon that encodes the β-catenin binding domain was targeted has a similar gross phenotype as the original Lef1 KO mice (JJW, unpublished). Bone structures were measurable by microcomputed tomography in a few animals of this strain that lived to three weeks of age. These Lef1 KO mice have dramatically lower trabecular bone mass and fewer trabeculae; however, no changes were observed in heterozygotes.
The different bone phenotypes of the heterozygotes in the two Lef1 mutant strains of mice may provide important information and insight into the functions of Lef1 isoforms. Lef1 contains two promoters that drive expression of a full-length protein (Lef1) and an N-terminally truncated isoform (Lef1ΔN). The original Lef1-deficient mouse (van Genderen et al., 1994) would eliminate both isoforms, whereas the newer Lef1-deficient mouse only inactivates the product of the first promoter (JJW, unpublished). Lef1 overexpression inhibited osteoblast maturation and Runx2 activity on the osteocalcin promoter in vitro (Kahler and Westendorf, 2003; Kahler et al., 2006), but Lef1ΔN had the opposite effect (Hoeppner et al., 2009). Lef1ΔN is present in mature osteoblasts and is induced by Bmp2, but repressed by Wnt3a (Hoeppner et al., 2009). Despite the absence of the N-terminal high affinity β-catenin binding domain, Lef1ΔN retains the ability to weakly interact with β-catenin via second domain (Hoeppner et al., 2011). Thus, Lef1ΔN can facilitate β-catenin activity, although in other scenarios it acts as a competitive inhibitor of Wnt activity (Hovanes et al., 2001). Transgenic mice expressing Lef1ΔN in mature osteoblasts with the 2.3Col1a1 promoter have a modestly higher trabecular bone mass, increased bone formation rates, and elevated serum osteocalcin levels (Hoeppner et al., 2011). Together these data demonstrate that alternative isoforms of Lef1 temporally and spatially regulate osteoblast function and trabecular bone mass. Lef1 or Tcf7 CKO mice have not yet been made but would be useful for dissecting their specific roles in bone.
5.6. Wtx (FAM123B)
WTX (also called FAM123B, OSCS or AMER1) is a tumor suppressor encoded on the X chromosome that contains somatic mutations in approximately 30% of Wilms tumors. Germline loss-of-function mutations in WTX cause osteopathia striata with cranial sclerosis (OSCS), a disorder characterized by osteosclerosis in females and high incidence of lethality in male fetuses (Jenkins et al., 2009). WTX directly binds to β-catenin, Apc, and Axin1/2 and is a component of the β-catenin destruction complex (Major et al., 2007); thus, loss-of-function mutations in WTX enhance β-catenin stability. Wtx is expressed in the developing mouse skeleton and skull at E14.5 and contributes to both intramembranous and endochondral bone formation (Jenkins et al., 2009). Germline deletion of Wtx caused somatic overgrowth and developmental defects in mesenchymal tissues, including bone. Wtx deficient animals died within one day of birth but had enlarged cranial vaults, bowed long bones, and increased cortical bone mineral densities (Moisan et al., 2011). Heterozygous animals also had higher bone mineral densities. In contrast, adipogenesis was inhibited and Wtx-deficient mice had less white and brown fat. Targeted deletion of Wtx with a variety of Cre lines demonstrated that Wtx is active in early mesenchymal progenitors as conditional deletion in mice expressing Prx1- and Osx-Cre displayed bone overgrowth; whereas deletion in committed chondrocytes (Col2a1-Cre) or osteoblasts (Ocn-Cre) did not alter the skeleton (Moisan et al., 2011). Runx2 and Osterix levels were higher in Wtx-deficient embryos and β-catenin activity was increased in progenitor cells. Interestingly, older Wtx-deficient mice had persistent defects in matrix mineralization. Together these data indicate that Wtx is a negative regulator of mesenchymal and osteoblastic progenitors, but a positive regulator of osteoblast maturation into osteocytes. These studies did not examine how Wtx-deletion affects osteoclastogenesis.
6. Emerging areas for Wnts in bone biology
The remarkable advancements in our understanding of the molecular underpinnings of rare bone diseases and in how Wnts control bone formation and osteoblast proliferation, differentiation, and survival have quickly led to the development of multiple therapies for more common diseases of altered bone mass, such as osteoporosis (Rachner et al., 2011). To fully understand the effects of these drugs, it will be crucial to also study how Wnts and Wnt antagonists affect other cells in the bone marrow and to examine their effectiveness during aging.
6.1. Wnt signaling and osteoclasts
In contrast to the plethora of data about Wnt signaling in osteo-blast lineage cells, there is a paucity of information about the cell autonomous influences of Wnts on osteoclasts. As has been discussed, β-catenin indirectly regulates osteoclastogenesis by raising the Opg to Rankl ratio in osteoblasts (Glass et al., 2005) and there is a threshold tolerance for β-catenin expression during osteoclastogenesis (Wei et al., 2011). Other Wnt pathway components, including Wnts, Fzds, Lrps, and Tcf family members, are also expressed in osteoclast lineage cells (Qiang et al., 2010). Thus, Wnts/β-catenin signaling appears to reduce bone resorption. This area is clearly in need of further investigation to fully resolve the scope of Wnt influences on bone metabolism and to understand the effectiveness of Wnt-based therapies on bone structure and function.
6.2. Aging
Work in non-bone cells provides evidence for beneficial effects of Wnts on aging but also raise some concerns about therapies to promote Wnt signaling to prevent age-related bone loss. For example, Wnt signaling is required for T cell development and protects thymic epithelial cells against senescence (Pongracz et al., 2003; Talaber et al., 2011). Wnt/Tcf signaling also keeps preadipocytes in an undifferentiated proliferative stage to inhibit adipogenesis (Bilkovski et al., 2011). In contrast, Wnts are activated in several models of accelerated aging and increase fibrosis of aged muscle progenitor cells (Brack et al., 2007; Liu et al., 2007b). β-catenin-mediated Wnt signaling also induces mesenchymal stem cell aging and DNA damage through the p53/p21 pathway and ROS generation, promotes cardiovascular cell aging, and induces mitochondrial biogenesis to cause cell senescence (Naito et al., 2010; Yoon et al., 2010; Zhang et al., 2011).
6.3. Cancer
It has been extensively documented that Wnt/β-catenin signaling effectively promotes tumor development and progression in a number of cancers including breast, multiple myeloma, endometrial, and lung (Polakis, 2000). Benign osteomas developed in mice expressing constitutively active β-catenin in osteoblasts (Glass et al., 2005) and Wif1 depletion made animals more susceptible to radiation-induced osteosarcomas (Kansara et al., 2009); however, there is no increased incidence in cancer in families carrying LRP5 gain-of-function mutations, nor are there any reports of increased tumors in Sost- or Dkk1-deficient animals. Interestingly, anti-Wnt therapy is under development to as means to treat a number of cancers. How these therapies will influence bone metabolism will need to be examined, particularly in cancers that cause osteolysis such as multiple myeloma and breast cancer.
7. Summary and conclusions
In conclusion, much has been learned about the roles of Wnt pathway components in bone development, remodeling, and repair during the last decade though the use of genetic animal models. These studies were fueled by the desire to understand the molecular underpinnings for rare bone diseases and have quickly led to the development of multiple therapies for common diseases of altered bone mass (e.g., postmenopausal osteoporosis) and for regenerative medicine. Despite these rapid and measurable accomplishments, much remains to be learned about the effects of Wnts and Wnt antagonists on skeletal physiology and regeneration. Undoubtedly, new components of Wnt pathways will be identified, receptor–ligand specificities will be defined, and a deeper understanding of how Wnt pathways interact with other signaling cascades in a variety of cell types will be achieved in the next decade. Meanwhile, clinical trials will test the effectiveness of current Wnt pathway drugs on a variety of endocrine and orthopedic conditions and advanced genome sequencing technologies will point us in new directions. Thus, continuing the bedside-to-bench exchange of information that has made this story so successful and compelling.
Acknowledgments
The authors are supported by several NIH grants (AG04875, AR48147, AR60140, DE020194, DK91145).
Abbreviations
- AER
apical ectodermal ridge
- Apc
adenomatous polyposis coli
- Bmp
bone morphogenic protein
- Ck1
casein kinase 1
- CKI
conditional knock-in
- CKO
conditional knock-out
- Ctnnb1
catenin (cadherin-associated protein), beta 1
- Daam1
disheveled-associated activator of morphogenesis 1
- Dkk
Dickkopf
- Dmp1
Dentin matrix acidic phosphoprotein 1
- Dvl
Disheveled
- Fgf
fibroblast growth factor
- Fzd
Frizzled
- Gsk
glycogen synthase kinase
- GWAS
genome-wide association studies
- HBM
high bone mass
- Jnk
c-Jun N-terminal kinase
- KO
knockout
- Krm
Kremen
- Lef1
Lymphoid enhancer binding factor
- LiCl
lithium chloride
- Lrg
leucine-rich repeat containing G protein-coupled receptor
- Lrp
low-density lipoprotein receptor-related protein
- Ocn
Osteocalcin
- Opg
osteoprotegerin
- OPPG
osteoporosis-pseudoglioma syndrome
- Osx
Osterix
- OSCS
osteopathia striata with cranial sclerosis
- PCP
planar cell polarity
- PKA
protein kinase A
- PKC
protein kinase C
- PTH
parathyroid hormone
- Rankl
receptor activator of NF-κB ligand
- Scl
sclerostin
- Sfrp
secreted Frizzled-related protein
- Shh
sonic hedgehog
- SNP
single nucleotide polymorphism
- Tcf
T cell factor
- Tg
transgenic
- Wif
Wnt inhibitory factor
- Wise
Wnt modulator in surface ectoderm
- Wls
Wntless
- Wnt
Wingless-type MMTV integration site
- Wtx
Wilm's tumor genes on chromosome X
References
- Afzal AR, et al. Recessive Robinow syndrome, allelic to dominant brachydactyly type B, is caused by mutation of ROR2. Nat. Genet. 2000;25:419–422. doi: 10.1038/78107. [DOI] [PubMed] [Google Scholar]
- Agueda L, et al. Functional relevance of the BMD-associated polymorphism rs312009: novel involvement of RUNX2 in LRP5 transcriptional regulation. J. Bone Miner. Res. 2011;26:1133–1144. doi: 10.1002/jbmr.293. [DOI] [PubMed] [Google Scholar]
- Ahn Y, Sanderson BW, Klein OD, Krumlauf R. Inhibition of Wnt signaling by Wise (Sostdc1) and negative feedback from Shh controls tooth number and patterning. Development. 2010;137:3221–3231. doi: 10.1242/dev.054668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai M, Holmen SL, Van Hul W, Williams BO, Warman ML. Reduced affinity to and inhibition by DKK1 form a common mechanism by which high bone mass-associated missense mutations in LRP5 affect canonical Wnt signaling. Mol. Cell. Biol. 2005;25:4946–4955. doi: 10.1128/MCB.25.12.4946-4955.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhter MP, et al. Bone biomechanical properties in LRP5 mutant mice. Bone. 2004;35:162–169. doi: 10.1016/j.bone.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Albers J, et al. Control of bone formation by the serpentine receptor Frizzled-9. J. Cell Biol. 2011;192:1057–1072. doi: 10.1083/jcb.201008012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida M, Han L, Bellido T, Manolagas SC, Kousteni S. Wnt proteins prevent apoptosis of both uncommitted osteoblast progenitors and differentiated osteoblasts by beta-catenin-dependent and -independent signaling cascades involving Src/ERK and phosphatidylinositol 3-kinase/AKT. J. Biol. Chem. 2005;280:41342–41351. doi: 10.1074/jbc.M502168200. [DOI] [PubMed] [Google Scholar]
- Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat. Rev. Mol. Cell Biol. 2009;10:468–477. doi: 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- Angers S, et al. The KLHL12-Cullin-3 ubiquitin ligase negatively regulates the Wnt-beta-catenin pathway by targeting Dishevelled for degradation. Nat. Cell Biol. 2006;8:348–357. doi: 10.1038/ncb1381. [DOI] [PubMed] [Google Scholar]
- Aoki M, Mieda M, Ikeda T, Hamada Y, Nakamura H, Okamoto H. R-spondin3 is required for mouse placental development. Dev. Biol. 2007;301:218–226. doi: 10.1016/j.ydbio.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Armstrong VJ, et al. Wnt/beta-catenin signaling is a component of osteoblastic bone cell early responses to load-bearing and requires estrogen receptor alpha. J. Biol. Chem. 2007;282:20715–20727. doi: 10.1074/jbc.M703224200. [DOI] [PubMed] [Google Scholar]
- Aslan H, et al. Advanced molecular profiling in vivo detects novel function of dickkopf-3 in the regulation of bone formation. J. Bone Miner. Res. 2006;21:1935–1945. doi: 10.1359/jbmr.060819. [DOI] [PubMed] [Google Scholar]
- Babij P, et al. High bone mass in mice expressing a mutant LRP5 gene. J. Bone Miner. Res. 2003;18:960–974. doi: 10.1359/jbmr.2003.18.6.960. [DOI] [PubMed] [Google Scholar]
- Baksh D, Boland GM, Tuan RS. Cross-talk between Wnt signaling pathways in human mesenchymal stem cells leads to functional antagonism during osteogenic differentiation. J. Cell. Biochem. 2007;101:1109–1124. doi: 10.1002/jcb.21097. [DOI] [PubMed] [Google Scholar]
- Balemans W, et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum. Mol. Genet. 2001;10:537–543. doi: 10.1093/hmg/10.5.537. [DOI] [PubMed] [Google Scholar]
- Balemans W, et al. Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J. Med. Genet. 2002;39:91–97. doi: 10.1136/jmg.39.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banziger C, Soldini D, Schutt C, Zipperlen P, Hausmann G, Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125:509–522. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- Bartscherer K, Pelte N, Ingelfinger D, Boutros M. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell. 2006;125:523–533. doi: 10.1016/j.cell.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Beaudoin GM, III, Sisk JM, Coulombe PA, Thompson CC. Hairless triggers reactivation of hair growth by promoting Wnt signaling. Proc. Natl. Acad. Sci. U. S. A. 2005;102:14653–14658. doi: 10.1073/pnas.0507609102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell SM, Schreiner CM, Wert SE, Mucenski ML, Scott WJ, Whitsett JA. R-spondin 2 is required for normal laryngeal-tracheal, lung and limb morphogenesis. Development. 2008;135:1049–1058. doi: 10.1242/dev.013359. [DOI] [PubMed] [Google Scholar]
- Bellido T, et al. Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology. 2005;146:4577–4583. doi: 10.1210/en.2005-0239. [DOI] [PubMed] [Google Scholar]
- Bennett CN, et al. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc. Natl. Acad. Sci. U. S. A. 2005;102:3324–3329. doi: 10.1073/pnas.0408742102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett CN, et al. Wnt10b increases postnatal bone formation by enhancing osteoblast differentiation. J. Bone Miner. Res. 2007;22:1924–1932. doi: 10.1359/jbmr.070810. [DOI] [PubMed] [Google Scholar]
- Bilic J, et al. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science. 2007;316:1619–1622. doi: 10.1126/science.1137065. [DOI] [PubMed] [Google Scholar]
- Bilkovski R, et al. Adipose tissue macrophages inhibit adipogenesis of mesenchymal precursor cells via wnt-5a in humans. Int. J. Obes. 2011 doi: 10.1038/ijo.2011.6. [DOI] [PubMed] [Google Scholar]
- Binnerts ME, et al. R-Spondin1 regulates Wnt signaling by inhibiting internalization of LRP6. Proc. Natl. Acad. Sci. U. S. A. 2007;104:14700–14705. doi: 10.1073/pnas.0702305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaydon DC, et al. The gene encoding R-spondin 4 (RSPO4), a secreted protein implicated in Wnt signaling, is mutated in inherited anonychia. Nat. Genet. 2006;38:1245–1247. doi: 10.1038/ng1883. [DOI] [PubMed] [Google Scholar]
- Blish KR, et al. A human bone morphogenetic protein antagonist is down-regulated in renal cancer. Mol. Biol. Cell. 2008;19:457–464. doi: 10.1091/mbc.E07-05-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodine PV, et al. The Wnt Antagonist Secreted Frizzled-Related Protein-1 is a Negative Regulator of Trabecular Bone Formation in Adult Mice. Mol. Endocrinol. 2004;18:1222–1237. doi: 10.1210/me.2003-0498. [DOI] [PubMed] [Google Scholar]
- Bodine PV, et al. The Wnt antagonist secreted frizzled-related protein-1 controls osteoblast and osteocyte apoptosis. J. Cell. Biochem. 2005;96:1212–1230. doi: 10.1002/jcb.20599. [DOI] [PubMed] [Google Scholar]
- Bodine PV, Seestaller-Wehr L, Kharode YP, Bex FJ, Komm BS. Bone anabolic effects of parathyroid hormone are blunted by deletion of the Wnt antagonist secreted frizzled-related protein-1. J. Cell. Physiol. 2007;210:352–357. doi: 10.1002/jcp.20834. [DOI] [PubMed] [Google Scholar]
- Bodine PV, et al. A small molecule inhibitor of the Wnt antagonist secreted frizzled-related protein-1 stimulates bone formation. Bone. 2009;44:1063–1068. doi: 10.1016/j.bone.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Boland GM, Perkins G, Hall DJ, Tuan RS. Wnt 3a promotes proliferation and suppresses osteogenic differentiation of adult human mesenchymal stem cells. J. Cell. Biochem. 2004;93:1210–1230. doi: 10.1002/jcb.20284. [DOI] [PubMed] [Google Scholar]
- Bonewald LF. The amazing osteocyte. J. Bone Miner. Res. 2011;26:229–238. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42:606–615. doi: 10.1016/j.bone.2007.12.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkowsky JL, Yoshikawa S, O'Keefe DD, Scully AL, Thomas JB. Axon routing across the midline controlled by the Drosophila Derailed receptor. Nature. 1999;402:540–544. doi: 10.1038/990122. [DOI] [PubMed] [Google Scholar]
- Boyden LM, et al. High bone density due to a mutation in LDL-receptor-related protein 5. N. Engl. J. Med. 2002;346:1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- Brack AS, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- Brault V, et al. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- Brunkow ME, et al. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am. J. Hum. Genet. 2001;68:577–589. doi: 10.1086/318811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmon KS, Gong X, Lin Q, Thomas A, Liu Q. R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/{beta}-catenin signaling. Proc. Natl. Acad. Sci. U. S. A. 2011;108:11452–11457. doi: 10.1073/pnas.1106083108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter AC, Rao S, Wells JM, Campbell K, Lang RA. Generation of mice with a conditional null allele for Wntless. Genesis. 2010;48:554–558. doi: 10.1002/dvg.20651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case N, Ma M, Sen B, Xie Z, Gross TS, Rubin J. Beta-catenin levels influence rapid mechanical responses in osteoblasts. J. Biol. Chem. 2008;283:29196–29205. doi: 10.1074/jbc.M801907200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan BY, et al. Increased chondrocyte sclerostin may protect against cartilage degradation in osteoarthritis. Osteoarthritis Cartilage. 2011a;19:874–885. doi: 10.1016/j.joca.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Chan TF, Couchourel D, Abed E, Delalandre A, Duval N, Lajeunesse D. Elevated Dickkopf-2 levels contribute to the abnormal phenotype of human osteoarthritic osteoblasts. J. Bone Miner. Res. 2011b;26:1399–1410. doi: 10.1002/jbmr.358. [DOI] [PubMed] [Google Scholar]
- Chen Y, et al. Beta-Catenin Signaling Plays a Disparate Role in Different Phases of Fracture Repair: Implications for Therapy to Improve Bone Healing. PLoS Med. 2007;4:e249. doi: 10.1371/journal.pmed.0040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia IV, Costantini F. Mouse axin and axin2/conductin proteins are functionally equivalent in vivo. Mol. Cell. Biol. 2005;25:4371–4376. doi: 10.1128/MCB.25.11.4371-4376.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YS, et al. A large-scale genome-wide association study of Asian populations uncovers genetic factors influencing eight quantitative traits. Nat. Genet. 2009;41:527–534. doi: 10.1038/ng.357. [DOI] [PubMed] [Google Scholar]
- Cho HY, et al. Transgenic mice overexpressing secreted frizzled-related proteins (sFRP)4 under the control of serum amyloid P promoter exhibit low bone mass but did not result in disturbed phosphate homeostasis. Bone. 2010;47:263–271. doi: 10.1016/j.bone.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Choi HY, Dieckmann M, Herz J, Niemeier A. Lrp4, a novel receptor for Dickkopf 1 and sclerostin, is expressed by osteoblasts and regulates bone growth and turnover in vivo. PLoS One. 2009;4:e7930. doi: 10.1371/journal.pone.0007930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement-Lacroix P, et al. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc. Natl. Acad. Sci. U. S. A. 2005;102:17406–17411. doi: 10.1073/pnas.0505259102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens AK, et al. Unravelling the molecular control of calvarial suture fusion in children with craniosynostosis. BMC Genomics. 2007;8:458. doi: 10.1186/1471-2164-8-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, et al. Lrp5 functions in bone to regulate bone mass. Nat. Med. 2011;17:684–691. doi: 10.1038/nm.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao DY, Yang X, Flick LM, Chen D, Hilton MJ, O'Keefe RJ. Axin2 regulates chondrocyte maturation and axial skeletal development. J. Orthop. Res. 2010;28:89–95. doi: 10.1002/jor.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev. Cell. 2005;8:739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- de Lau W, et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature. 2011;476:293–297. doi: 10.1038/nature10337. [DOI] [PubMed] [Google Scholar]
- DeChiara TM, et al. Ror2, encoding a receptor-like tyrosine kinase, is required for cartilage and growth plate development. Nat. Genet. 2000;24:271–274. doi: 10.1038/73488. [DOI] [PubMed] [Google Scholar]
- del Barco Barrantes I, Davidson G, Grone HJ, Westphal H, Niehrs C. Dkk1 and noggin cooperate in mammalian head induction. Genes Dev. 2003;17:2239–2244. doi: 10.1101/gad.269103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenport D, Fuchs E. Planar polarization in embryonic epidermis orchestrates global asymmetric morphogenesis of hair follicles. Nat. Cell Biol. 2008;10:1257–1268. doi: 10.1038/ncb1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diarra D, et al. Dickkopf-1 is a master regulator of joint remodeling. Nat. Med. 2007;13:156–163. doi: 10.1038/nm1538. [DOI] [PubMed] [Google Scholar]
- Ellies DL, et al. Bone density ligand, Sclerostin, directly interacts with LRP5 but not LRP5G171V to modulate Wnt activity. J. Bone Miner. Res. 2006;21:1738–1749. doi: 10.1359/jbmr.060810. [DOI] [PubMed] [Google Scholar]
- Ellwanger K, et al. Targeted disruption of the Wnt regulator Kremen induces limb defects and high bone density. Mol. Cell. Biol. 2008;28:4875–4882. doi: 10.1128/MCB.00222-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etheridge SL, Spencer GJ, Heath DJ, Genever PG. Expression profiling and functional analysis of wnt signaling mechanisms in mesenchymal stem cells. Stem Cells. 2004;22:849–860. doi: 10.1634/stemcells.22-5-849. [DOI] [PubMed] [Google Scholar]
- Fleming HE, et al. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. 2008;2:274–283. doi: 10.1016/j.stem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francke U. Williams-Beuren syndrome: genes and mechanisms. Hum. Mol. Genet. 1999;8:1947–1954. doi: 10.1093/hmg/8.10.1947. [DOI] [PubMed] [Google Scholar]
- Friedman MS, Oyserman SM, Hankenson KD. Wnt11 promotes osteoblast maturation and mineralization through R-spondin 2. J. Biol. Chem. 2009;284:14117–14125. doi: 10.1074/jbc.M808337200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost M, Andersen TE, Yadav V, Brixen K, Karsenty G, Kassem M. Patients with high-bone-mass phenotype owing to Lrp5-T253I mutation have low plasma levels of serotonin. J. Bone Miner. Res. 2010;25:673–675. doi: 10.1002/jbmr.44. [DOI] [PubMed] [Google Scholar]
- Fu J, Jiang M, Mirando AJ, Yu HM, Hsu W. Reciprocal regulation of Wnt and Gpr177/mouse Wntless is required for embryonic axis formation. Proc. Natl. Acad. Sci. U. S. A. 2009;106:18598–18603. doi: 10.1073/pnas.0904894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Ivy Yu HM, Maruyama T, Mirando AJ, Hsu W. Gpr177/mouse Wntless is essential for Wnt-mediated craniofacial and brain development. Dev. Dyn. 2011;240:365–371. doi: 10.1002/dvdy.22541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino T, et al. Low-density lipoprotein receptor-related protein 5 (LRP5) is essential for normal cholesterol metabolism and glucose-induced insulin secretion. Proc. Natl. Acad. Sci. U. S. A. 2003;100:229–234. doi: 10.1073/pnas.0133792100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulciniti M, et al. Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood. 2009;114:371–379. doi: 10.1182/blood-2008-11-191577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Chen YG. Dishevelled: The hub of Wnt signaling. Cell. Signal. 2010;22:717–727. doi: 10.1016/j.cellsig.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Gaur T, et al. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J. Biol. Chem. 2005;280:33132–33140. doi: 10.1074/jbc.M500608200. [DOI] [PubMed] [Google Scholar]
- Glantschnig H, et al. A rate-limiting role for dickkopf-1 in bone formation and the remediation of bone loss in mouse and primate models of postmenopausal osteoporosis by an experimental therapeutic antibody. J. Pharmacol. Exp. Ther. 2011;338:568–578. doi: 10.1124/jpet.111.181404. [DOI] [PubMed] [Google Scholar]
- Glass DA, II, et al. Canonical wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev. Cell. 2005;8:751–764. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Gong Y, et al. Osteoporosis-pseudoglioma syndrome, a disorder affecting skeletal strength and vision, is assigned to chromosome region 11q12-13. Am. J. Hum. Genet. 1996;59:146–151. [PMC free article] [PubMed] [Google Scholar]
- Gong Y, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- Goodman RM, et al. Sprinter: a novel transmembrane protein required for Wg secretion and signaling. Development. 2006;133:4901–4911. doi: 10.1242/dev.02674. [DOI] [PubMed] [Google Scholar]
- Grumolato L, et al. Canonical and noncanonical Wnts use a common mechanism to activate completely unrelated coreceptors. Genes Dev. 2010;24:2517–2530. doi: 10.1101/gad.1957710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Day TF, Jiang X, Garrett-Beal L, Topol L, Yang Y. Wnt/beta-catenin signaling is sufficient and necessary for synovial joint formation. Genes Dev. 2004;18:2404–2417. doi: 10.1101/gad.1230704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, et al. Suppression of Wnt signaling by Dkk1 attenuates PTH-mediated stromal cell response and new bone formation. Cell Metab. 2010;11:161–171. doi: 10.1016/j.cmet.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada N, et al. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausler KD, et al. Secreted frizzled-related protein-1 inhibits RANKL-dependent osteoclast formation. J. Bone Miner. Res. 2004;19:1873–1881. doi: 10.1359/JBMR.040807. [DOI] [PubMed] [Google Scholar]
- Hay E, Nouraud A, Marie PJ. N-cadherin negatively regulates osteoblast proliferation and survival by antagonizing Wnt, ERK and PI3K/Akt signalling. PLoS One. 2009;4:e8284. doi: 10.1371/journal.pone.0008284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He JW, Yue H, Hu WW, Hu YQ, Zhang ZL. Contribution of the sclerostin domain-containing protein 1 (SOSTDC1) gene to normal variation of peak bone mineral density in Chinese women and men. J. Bone Miner. Metab. 2011;29:571–581. doi: 10.1007/s00774-010-0253-5. [DOI] [PubMed] [Google Scholar]
- Heath DJ, et al. Inhibiting Dickkopf-1 (Dkk1) removes suppression of bone formation and prevents the development of osteolytic bone disease in multiple myeloma. J. Bone Miner. Res. 2009;24:425–436. doi: 10.1359/jbmr.081104. [DOI] [PubMed] [Google Scholar]
- Henderson DJ, Chaudhry B. Getting to the heart of planar cell polarity signaling. Birth Defects Res. A Clin. Mol. Teratol. 2011;91:460–467. doi: 10.1002/bdra.20792. [DOI] [PubMed] [Google Scholar]
- Hens JR, Wilson KM, Dann P, Chen X, Horowitz MC, Wysolmerski JJ. TOPGAL mice show that the canonical Wnt signaling pathway is active during bone development and growth and is activated by mechanical loading in vitro. J. Bone Miner. Res. 2005;20:1103–1113. doi: 10.1359/JBMR.050210. [DOI] [PubMed] [Google Scholar]
- Hill TP, Spater D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev. Cell. 2005;8:727–738. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Hoeppner LH, Secreto F, Jensen ED, Li X, Kahler RA, Westendorf JJ. Runx2 and bone morphogenic protein 2 regulate the expression of an alternative Lef1 transcript during osteoblast maturation. J. Cell. Physiol. 2009;221:480–489. doi: 10.1002/jcp.21879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeppner LH, Secreto FJ, Razidlo DF, Whitney TJ, Westendorf JJ. Lef1DeltaN binds beta-catenin and increases osteoblast activity and trabecular bone mass. J. Biol. Chem. 2011;286:10950–10959. doi: 10.1074/jbc.M110.165100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmen SL, et al. Decreased BMD and limb deformities in mice carrying mutations in both Lrp5 and Lrp6. J. Bone Miner. Res. 2004;19:2033–2040. doi: 10.1359/JBMR.040907. [DOI] [PubMed] [Google Scholar]
- Holmen SL, et al. Essential role of beta-catenin in postnatal bone acquisition. J. Biol. Chem. 2005;280:21162–21168. doi: 10.1074/jbc.M501900200. [DOI] [PubMed] [Google Scholar]
- Hovanes K, et al. Beta-catenin-sensitive isoforms of lymphoid enhancer factor-1 are selectively expressed in colon cancer. Nat. Genet. 2001;28:53–57. doi: 10.1038/ng0501-53. [DOI] [PubMed] [Google Scholar]
- Hovens CM, Stacker SA, Andres AC, Harpur AG, Ziemiecki A, Wilks AF. RYK, a receptor tyrosine kinase-related molecule with unusual kinase domain motifs. Proc. Natl. Acad. Sci. U. S. A. 1992;89:11818–11822. doi: 10.1073/pnas.89.24.11818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YH, et al. An integration of genome-wide association study and gene expression profiling to prioritize the discovery of novel susceptibility Loci for osteoporosis-related traits. PLoS Genet. 2010;6:e1000977. doi: 10.1371/journal.pgen.1000977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Hilton MJ, Tu X, Yu K, Ornitz DM, Long F. Sequential roles of Hedgehog and Wnt signaling in osteoblast development. Development. 2005;132:49–60. doi: 10.1242/dev.01564. [DOI] [PubMed] [Google Scholar]
- Hwang SG, Yu SS, Lee SW, Chun JS. Wnt-3a regulates chondrocyte differentiation via c-Jun/AP-1 pathway. FEBS Lett. 2005;579:4837–4842. doi: 10.1016/j.febslet.2005.07.067. [DOI] [PubMed] [Google Scholar]
- Ishii Y, et al. Mutations in R-spondin 4 (RSPO4) underlie inherited anonychia. J. Invest. Dermatol. 2008;128:867–870. doi: 10.1038/sj.jid.5701078. [DOI] [PubMed] [Google Scholar]
- Itasaki N, et al. Wise, a context-dependent activator and inhibitor of Wnt signalling. Development. 2003;130:4295–4305. doi: 10.1242/dev.00674. [DOI] [PubMed] [Google Scholar]
- Iwaniec UT, et al. PTH stimulates bone formation in mice deficient in Lrp5. J. Bone Miner. Res. 2007;22:394–402. doi: 10.1359/jbmr.061118. [DOI] [PubMed] [Google Scholar]
- Iwao K, Miyoshi Y, Nawa G, Yoshikawa H, Ochi T, Nakamura Y. Frequent beta-catenin abnormalities in bone and soft-tissue tumors. Jpn. J. Cancer Res. 1999;90:205–209. doi: 10.1111/j.1349-7006.1999.tb00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins ZA, et al. Germline mutations in WTX cause a sclerosing skeletal dysplasia but do not predispose to tumorigenesis. Nat. Genet. 2009;41:95–100. doi: 10.1038/ng.270. [DOI] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol. Cell. Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Morse M, Frey C, Petko J, Levenson R. Expression of GPR177 (Wntless/Evi/Sprinter), a highly conserved Wnt-transport protein, in rat tissues, zebrafish embryos, and cultured human cells. Dev. Dyn. 2010;239:2426–2434. doi: 10.1002/dvdy.22369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EB, Hammer RE, Herz J. Abnormal development of the apical ectodermal ridge and polysyndactyly in Megf7-deficient mice. Hum. Mol. Genet. 2005;14:3523–3538. doi: 10.1093/hmg/ddi381. [DOI] [PubMed] [Google Scholar]
- Jones C, et al. Ciliary proteins link basal body polarization to planar cell polarity regulation. Nat. Genet. 2008;40:69–77. doi: 10.1038/ng.2007.54. [DOI] [PubMed] [Google Scholar]
- Kahler RA, Westendorf JJ. Lymphoid enhancer factor-1 and beta-catenin inhibit Runx2-dependent transcriptional activation of the osteocalcin promoter. J. Biol. Chem. 2003;278:11937–11944. doi: 10.1074/jbc.M211443200. [DOI] [PubMed] [Google Scholar]
- Kahler RA, Galindo M, Lian J, Stein GS, van Wijnen AJ, Westendorf JJ. Lymphocyte enhancer-binding factor 1 (Lef1) inhibits terminal differentiation of osteoblasts. J. Cell. Biochem. 2006;97:969–983. doi: 10.1002/jcb.20702. [DOI] [PubMed] [Google Scholar]
- Kamel MA, Picconi JL, Lara-Castillo N, Johnson ML. Activation of beta-catenin signaling in MLO-Y4 osteocytic cells versus 2T3 osteoblastic cells by fluid flow shear stress and PGE2: Implications for the study of mechanosensation in bone. Bone. 2010;47:872–881. doi: 10.1016/j.bone.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansara M, et al. Wnt inhibitory factor 1 is epigenetically silenced in human osteosarcoma, and targeted disruption accelerates osteosarcomagenesis in mice. J. Clin. Invest. 2009;119:837–851. doi: 10.1172/JCI37175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasik D, Cupples LA, Hannan MT, Kiel DP. Age, gender, and body mass effects on quantitative trait loci for bone mineral density: the Framingham Study. Bone. 2003;33:308–316. doi: 10.1016/s8756-3282(03)00173-x. [DOI] [PubMed] [Google Scholar]
- Kassai Y, et al. Regulation of mammalian tooth cusp patterning by ectodin. Science. 2005;309:2067–2070. doi: 10.1126/science.1116848. [DOI] [PubMed] [Google Scholar]
- Katanaev VL, Tomlinson A. Dual roles for the trimeric G protein Go in asymmetric cell division in Drosophila. Proc. Natl. Acad. Sci. U. S. A. 2006;103:6524–6529. doi: 10.1073/pnas.0601853103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katanaev VL, Ponzielli R, Semeriva M, Tomlinson A. Trimeric G protein-dependent frizzled signaling in Drosophila. Cell. 2005;120:111–122. doi: 10.1016/j.cell.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Kato M, et al. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J. Cell Biol. 2002;157:303–314. doi: 10.1083/jcb.200201089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J. Cell Sci. 2003;116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- Keller H, Kneissel M. SOST is a target gene for PTH in bone. Bone. 2005;37:148–158. doi: 10.1016/j.bone.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Kiel DP, Demissie S, Dupuis J, Lunetta KL, Murabito JM, Karasik D. Genome-wide association with bone mass and geometry in the Framingham Heart Study. BMC Med. Genet. 2007a;8(Suppl. 1):S14. doi: 10.1186/1471-2350-8-S1-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel DP, et al. Genetic variation at the low-density lipoprotein receptor-related protein 5 (LRP5) locus modulates Wnt signaling and the relationship of physical activity with bone mineral density in men. Bone. 2007b;40:587–596. doi: 10.1016/j.bone.2006.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KA, et al. R-Spondin proteins: a novel link to beta-catenin activation. Cell Cycle. 2006;5:23–26. doi: 10.4161/cc.5.1.2305. [DOI] [PubMed] [Google Scholar]
- Kim KA, et al. R-Spondin family members regulate the Wnt pathway by a common mechanism. Mol. Biol. Cell. 2008;19:2588–2596. doi: 10.1091/mbc.E08-02-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitase Y, et al. Mechanical induction of PGE2 in osteocytes blocks glucocorticoid-induced apoptosis through both the beta-catenin and PKA pathways. J. Bone Miner. Res. 2010;25:2657–2668. doi: 10.1002/jbmr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koay MA, et al. Influence of LRP5 polymorphisms on normal variation in BMD. J. Bone Miner. Res. 2004;19:1619–1627. doi: 10.1359/JBMR.040704. [DOI] [PubMed] [Google Scholar]
- Komatsu DE, Mary MN, Schroeder RJ, Robling AG, Turner CH, Warden SJ. Modulation of Wnt signaling influences fracture repair. J. Orthop. Res. 2010;28:928–936. doi: 10.1002/jor.21078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koval A, Katanaev VL. Wnt3a stimulation elicits G-protein-coupled receptor properties of mammalian Frizzled proteins. Biochem. J. 2011;433:435–440. doi: 10.1042/BJ20101878. [DOI] [PubMed] [Google Scholar]
- Kramer I, et al. Osteocyte Wnt/beta-catenin signaling is required for normal bone homeostasis. Mol. Cell. Biol. 2010a;30:3071–3085. doi: 10.1128/MCB.01428-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer I, Keller H, Leupin O, Kneissel M. Does osteocytic SOST suppression mediate PTH bone anabolism? Trends Endocrinol. Metab. 2010b;21:237–244. doi: 10.1016/j.tem.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Kramer I, Loots GG, Studer A, Keller H, Kneissel M. Parathyroid hormone (PTH)-induced bone gain is blunted in SOST overexpressing and deficient mice. J. Bone Miner. Res. 2010c;25:178–189. doi: 10.1359/jbmr.090730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause C, et al. Distinct modes of inhibition by sclerostin on bone morphogenetic protein and Wnt signaling pathways. J. Biol. Chem. 2010;285:41614–41626. doi: 10.1074/jbc.M110.153890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronke G, et al. R-spondin 1 protects against inflammatory bone damage during murine arthritis by modulating the Wnt pathway. Arthritis Rheum. 2010;62:2303–2312. doi: 10.1002/art.27496. [DOI] [PubMed] [Google Scholar]
- Kubota T, et al. Lrp6 hypomorphic mutation affects bone mass through bone resorption in mice and impairs interaction with Mesd. J. Bone Miner. Res. 2008;23:1661–1671. doi: 10.1359/jbmr.080512. [DOI] [PubMed] [Google Scholar]
- Kugimiya F, et al. GSK-3beta controls osteogenesis through regulating Runx2 activity. PLoS One. 2007;2:e837. doi: 10.1371/journal.pone.0000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni NH, et al. Orally bioavailable GSK-3alpha/beta dual inhibitor increases markers of cellular differentiation in vitro and bone mass in vivo. J. Bone Miner. Res. 2006;21:910–920. doi: 10.1359/jbmr.060316. [DOI] [PubMed] [Google Scholar]
- Kumar J, Swanberg M, McGuigan F, Callreus M, Gerdhem P, Akesson K. LRP4 association to bone properties and fracture and interaction with genes in the Wnt- and BMP signaling pathways. Bone. 2011;49:343–348. doi: 10.1016/j.bone.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Laurikkala J, Kassai Y, Pakkasjarvi L, Thesleff I, Itoh N. Identification of a secreted BMP antagonist, ectodin, integrating BMP, FGF, and SHH signals from the tooth enamel knot. Dev. Biol. 2003;264:91–105. doi: 10.1016/j.ydbio.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Leupin O, et al. Control of the SOST bone enhancer by PTH using MEF2 transcription factors. J. Bone Miner. Res. 2007;22:1957–1967. doi: 10.1359/jbmr.070804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leupin O, et al. Bone Overgrowth-associated Mutations in the LRP4 Gene Impair Sclerostin Facilitator Function. J. Biol. Chem. 2011;286:19489–19500. doi: 10.1074/jbc.M110.190330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CH, Amar S. Inhibition of SFRP1 reduces severity of periodontitis. J. Dent. Res. 2007;86:873–877. doi: 10.1177/154405910708600913. [DOI] [PubMed] [Google Scholar]
- Li L, et al. Dishevelled proteins lead to two signaling pathways. Regulation of LEF-1 and c-Jun N-terminal kinase in mammalian cells. J. Biol. Chem. 1999;274:129–134. doi: 10.1074/jbc.274.1.129. [DOI] [PubMed] [Google Scholar]
- Li L, Mao J, Sun L, Liu W, Wu D. Second cysteine-rich domain of Dickkopf-2 activates canonical Wnt signaling pathway via LRP-6 independently of dishevelled. J. Biol. Chem. 2002;277:5977–5981. doi: 10.1074/jbc.M111131200. [DOI] [PubMed] [Google Scholar]
- Li X, et al. Dkk2 has a role in terminal osteoblast differentiation and mineralized matrix formation. Nat. Genet. 2005a;37:945–952. doi: 10.1038/ng1614. [DOI] [PubMed] [Google Scholar]
- Li X, et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J. Biol. Chem. 2005b;280:19883–19887. doi: 10.1074/jbc.M413274200. [DOI] [PubMed] [Google Scholar]
- Li J, et al. Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone. 2006;39:754–766. doi: 10.1016/j.bone.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Li X, et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J. Bone Miner. Res. 2008;23:860–869. doi: 10.1359/jbmr.080216. [DOI] [PubMed] [Google Scholar]
- Li X, et al. Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J. Bone Miner. Res. 2009;24:578–588. doi: 10.1359/jbmr.081206. [DOI] [PubMed] [Google Scholar]
- Lintern KB, Guidato S, Rowe A, Saldanha JW, Itasaki N. Characterization of wise protein and its molecular mechanism to interact with both Wnt and BMP signals. J. Biol. Chem. 2009;284:23159–23168. doi: 10.1074/jbc.M109.025478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little RD, et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am. J. Hum. Genet. 2002;70:11–19. doi: 10.1086/338450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Liu X, Wang H, Moon RT, Malbon CC. Activation of rat frizzled-1 promotes Wnt signaling and differentiation of mouse F9 teratocarcinoma cells via pathways that require Galpha(q) and Galpha(o) function. J. Biol. Chem. 1999;274:33539–33544. doi: 10.1074/jbc.274.47.33539. [DOI] [PubMed] [Google Scholar]
- Liu X, Rubin JS, Kimmel AR. Rapid, Wnt-induced changes in GSK3beta associations that regulate beta-catenin stabilization are mediated by Galpha proteins. Curr. Biol. 2005;15:1989–1997. doi: 10.1016/j.cub.2005.10.050. [DOI] [PubMed] [Google Scholar]
- Liu B, Yu HM, Hsu W. Craniosynostosis caused by Axin2 deficiency is mediated through distinct functions of beta-catenin in proliferation and differentiation. Dev. Biol. 2007a;301:298–308. doi: 10.1016/j.physletb.2003.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007b;317:803–806. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- Loots GG, et al. Genomic deletion of a long-range bone enhancer misregulates sclerostin in Van Buchem disease. Genome Res. 2005;15:928–935. doi: 10.1101/gr.3437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Yamamoto V, Ortega B, Baltimore D. Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell. 2004;119:97–108. doi: 10.1016/j.cell.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Lu W, et al. R-spondin1 synergizes with Wnt3A in inducing osteoblast differentiation and osteoprotegerin expression. FEBS Lett. 2008;582:643–650. doi: 10.1016/j.febslet.2008.01.035. [DOI] [PubMed] [Google Scholar]
- Luo J, et al. Regulation of bone formation and remodeling by G-protein-coupled receptor 48. Development. 2009;136:2747–2756. doi: 10.1242/dev.033571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald BT, Adamska M, Meisler MH. Hypomorphic expression of Dkk1 in the doubleridge mouse: dose dependence and compensatory interactions with Lrp6. Development. 2004;131:2543–2552. doi: 10.1242/dev.01126. [DOI] [PubMed] [Google Scholar]
- Macdonald BT, et al. Bone mass is inversely proportional to Dkk1 levels in mice. Bone. 2007;41:331–339. doi: 10.1016/j.bone.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev. Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major MB, et al. Wilms tumor suppressor WTX negatively regulates WNT/beta-catenin signaling. Science. 2007;316:1043–1046. doi: 10.1126/science/1141515. [DOI] [PubMed] [Google Scholar]
- Mak W, Shao X, Dunstan CR, Seibel MJ, Zhou H. Biphasic glucocorticoid-dependent regulation of Wnt expression and its inhibitors in mature osteoblastic cells. Calcif. Tissue Int. 2009;85:538–545. doi: 10.1007/s00223-009-9303-1. [DOI] [PubMed] [Google Scholar]
- Malinauskas T, Aricescu AR, Lu W, Siebold C, Jones EY. Modular mechanism of Wnt signaling inhibition by Wnt inhibitory factor 1. Nat. Struct. Mol. Biol. 2011;18:886–893. doi: 10.1038/nsmb.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani A, et al. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science. 2007;315:1278–1282. doi: 10.1126/science.1136370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao B, Niehrs C. Kremen2 modulates Dickkopf2 activity during Wnt/LRP6 signaling. Gene. 2003;302:179–183. doi: 10.1016/s0378-1119(02)01106-x. [DOI] [PubMed] [Google Scholar]
- Mao B, et al. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature. 2002;417:664–667. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- Miclea RL, et al. Adenomatous polyposis coli-mediated control of beta-catenin is essential for both chondrogenic and osteogenic differentiation of skeletal precursors. BMC Dev. Biol. 2009;9:26. doi: 10.1186/1471-213X-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miclea RL, et al. APC mutations are associated with increased bone mineral density in patients with familial adenomatous polyposis. J. Bone Miner. Res. 2010;25:2624–2632. doi: 10.1002/jbmr.153. [DOI] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami Y, Oishi I, Endo M, Nishita M. Ror-family receptor tyrosine kinases in noncanonical Wnt signaling: their implications in developmental morphogenesis and human diseases. Dev. Dyn. 2010;239:1–15. doi: 10.1002/dvdy.21991. [DOI] [PubMed] [Google Scholar]
- Minear S, et al. Wnt proteins promote bone regeneration. Sci. Transl. Med. 2010;2:29ra30. doi: 10.1126/scitranslmed.3000231. [DOI] [PubMed] [Google Scholar]
- Mirando AJ, Maruyama T, Fu J, Yu HM, Hsu W. beta-catenin/cyclin D1 mediated development of suture mesenchyme in calvarial morphogenesis. BMC Dev. Biol. 2010;10:116. doi: 10.1186/1471-213X-10-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi T, et al. LRP5, low-density-lipoprotein-receptor-related protein 5, is a determinant for bone mineral density. J. Hum. Genet. 2004;49:80–86. doi: 10.1007/s10038-003-0111-6. [DOI] [PubMed] [Google Scholar]
- Modder UI, Oursler MJ, Khosla S, Monroe DG. Wnt10b activates the Wnt, notch, and NFkappaB pathways in U2OS osteosarcoma cells. J. Cell. Biochem. 2011;112:1392–1402. doi: 10.1002/jcb.23048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisan A, et al. The WTX tumor suppressor regulates mesenchymal progenitor cell fate specification. Dev. Cell. 2011;20:583–596. doi: 10.1016/j.devcel.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore WJ, et al. Modulation of Wnt signaling through inhibition of secreted frizzled-related protein I (sFRP-1) with N-substituted piperidinyl diphenylsulfonyl sulfonamides. J. Med. Chem. 2009;52:105–116. doi: 10.1021/jm801144h. [DOI] [PubMed] [Google Scholar]
- Moore WJ, et al. Modulation of Wnt signaling through inhibition of secreted frizzled-related protein I (sFRP-1) with N-substituted piperidinyl diphenylsulfonyl sulfonamides: part II. Bioorg. Med. Chem. 2010;18:190–201. doi: 10.1016/j.bmc.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Morvan F, et al. Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J. Bone Miner. Res. 2006;21:934–945. doi: 10.1359/jbmr.060311. [DOI] [PubMed] [Google Scholar]
- Munne PM, Felszeghy S, Jussila M, Suomalainen M, Thesleff I, Jernvall J. Splitting placodes: effects of bone morphogenetic protein and Activin on the patterning and identity of mouse incisors. Evol. Dev. 2009;12:383–392. doi: 10.1111/j.1525-142X.2010.00425.x. [DOI] [PubMed] [Google Scholar]
- Murashima-Suginami A, et al. Enhanced BMP signaling results in supernumerary tooth formation in USAG-1 deficient mouse. Biochem. Biophys. Res. Commun. 2008;369:1012–1016. doi: 10.1016/j.bbrc.2008.02.135. [DOI] [PubMed] [Google Scholar]
- Naito AT, Shiojima I, Komuro I. Wnt signaling and aging-related heart disorders. Circ. Res. 2010;107:1295–1303. doi: 10.1161/CIRCRESAHA.110.223776. [DOI] [PubMed] [Google Scholar]
- Nakanishi R, et al. Secreted frizzled-related protein 4 is a negative regulator of peak BMD in SAMP6 mice. J. Bone Miner. Res. 2006;21:1713–1721. doi: 10.1359/jbmr.060719. [DOI] [PubMed] [Google Scholar]
- Nakanishi R, et al. Osteoblast-targeted expression of sfrp4 in mice results in low bone mass. J. Bone Miner. Res. 2008;23:271–277. doi: 10.1359/jbmr.071007. [DOI] [PubMed] [Google Scholar]
- Nam JS, Turcotte TJ, Yoon JK. Dynamic expression of R-spondin family genes in mouse development. Gene Expr. Patterns. 2007;7:306–312. doi: 10.1016/j.modgep.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Neufeld KL, White RL. Nuclear and cytoplasmic localizations of the adenomatous polyposis coli protein. Proc. Natl. Acad. Sci. U. S. A. 1997;94:3034–3039. doi: 10.1073/pnas.94.7.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld KL, et al. Adenomatous polyposis coli protein contains two nuclear export signals and shuttles between the nucleus and cytoplasm. Proc. Natl. Acad. Sci. U. S. A. 2000a;97:12085–12090. doi: 10.1073/pnas.220401797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld KL, Zhang F, Cullen BR, White RL. APC-mediated downregulation of beta-catenin activity involves nuclear sequestration and nuclear export. EMBO Rep. 2000b;1:519–523. doi: 10.1093/embo-reports/kvd117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie X, Luukko K, Fjeld K, Kvinnsland IH, Kettunen P. Developmental expression of Dkk1-3 and Mmp9 and apoptosis in cranial base of mice. J. Mol. Histol. 2005;36:419–426. doi: 10.1007/s10735-005-9014-5. [DOI] [PubMed] [Google Scholar]
- Niehrs C, Shen J. Regulation of Lrp6 phosphorylation. Cell. Mol. Life Sci. 2010;67:2551–2562. doi: 10.1007/s00018-010-0329-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh T, et al. Lef1 haploinsufficient mice display a low turnover and low bone mass phenotype in a gender- and age-specific manner. PLoS One. 2009;4:e5438. doi: 10.1371/journal.pone.0005438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien CA, et al. Control of bone mass and remodeling by PTH receptor signaling in osteocytes. PLoS One. 2008;3:e2942. doi: 10.1371/journal.pone.0002942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohazama A, et al. Lrp4 modulates extracellular integration of cell signaling pathways in development. PLoS One. 2008;3:e4092. doi: 10.1371/journal.pone.0004092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohazama A, Porntaveetus T, Ota MS, Herz J, Sharpe PT. Lrp4: A novel modulator of extracellular signaling in craniofacial organogenesis. Am. J. Med. Genet. A. 2010;152A:2974–2983. doi: 10.1002/ajmg.a.33372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawara B, Niehrs C. An ATF2-based luciferase reporter to monitor non-canonical Wnt signaling in Xenopus embryos. Dev. Dyn. 2011;240:188–194. doi: 10.1002/dvdy.22500. [DOI] [PubMed] [Google Scholar]
- Oldridge M, et al. Dominant mutations in ROR2, encoding an orphan receptor tyrosine kinase, cause brachydactyly type B. Nat. Genet. 2000;24:275–278. doi: 10.1038/73495. [DOI] [PubMed] [Google Scholar]
- Padhi D, Jang G, Stouch B, Fang L, Posvar E. Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J. Bone Miner. Res. 2011;26:19–26. doi: 10.1002/jbmr.173. [DOI] [PubMed] [Google Scholar]
- Parma P, et al. R-spondin1 is essential in sex determination, skin differentiation and malignancy. Nat. Genet. 2006;38:1304–1309. doi: 10.1038/ng1907. [DOI] [PubMed] [Google Scholar]
- Penzo-Mendez A, Umbhauer M, Djiane A, Boucaut JC, Riou JF. Activation of Gbetagamma signaling downstream of Wnt-11/Xfz7 regulates Cdc42 activity during Xenopus gastrulation. Dev. Biol. 2003;257:302–314. doi: 10.1016/s0012-1606(03)00067-8. [DOI] [PubMed] [Google Scholar]
- Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407:535–538. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- Pinzone JJ, et al. The role of Dickkopf-1 in bone development, homeostasis, and disease. Blood. 2009;113:517–525. doi: 10.1182/blood-2008-03-145169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- Pongracz J, Hare K, Harman B, Anderson G, Jenkinson EJ. Thymic epithelial cells provide WNT signals to developing thymocytes. Eur. J. Immunol. 2003;33:1949–1956. doi: 10.1002/eji.200323564. [DOI] [PubMed] [Google Scholar]
- Purvanov V, Koval A, Katanaev VL. A direct and functional interaction between Go and Rab5 during G protein-coupled receptor signaling. Sci. Signal. 2010;3:ra65. doi: 10.1126/scisignal.2000877. [DOI] [PubMed] [Google Scholar]
- Qiang YW, et al. Characterization of Wnt/beta-catenin signalling in osteoclasts in multiple myeloma. Br. J. Haematol. 2010;148:726–738. doi: 10.1111/j.1365-2141.2009.08009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377:1276–1287. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riancho JA, et al. Wnt receptors, bone mass, and fractures: gene-wide association analysis of LRP5 and LRP6 polymorphisms with replication. Eur. J. Endocrinol. 2011;164:123–131. doi: 10.1530/EJE-10-0582. [DOI] [PubMed] [Google Scholar]
- Rivadeneira F, et al. Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nat. Genet. 2009;41:1199–1206. doi: 10.1038/ng.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JA, et al. Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J. Biol. Chem. 2006;281:31720–31728. doi: 10.1074/jbc.M602308200. [DOI] [PubMed] [Google Scholar]
- Robling AG, Bellido T, Turner CH. Mechanical stimulation in vivo reduces osteocyte expression of sclerostin. J. Musculoskelet. Neuronal Interact. 2006;6:354. [PubMed] [Google Scholar]
- Rodda SJ, McMahon AP. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development. 2006;133:3231–3244. doi: 10.1242/dev.02480. [DOI] [PubMed] [Google Scholar]
- Roose J, et al. Synergy between tumor suppressor APC and the beta-catenin-Tcf4 target Tcf1. Science. 1999;285:1923–1926. doi: 10.1126/science.285.5435.1923. [DOI] [PubMed] [Google Scholar]
- Sato A, Yamamoto H, Sakane H, Koyama H, Kikuchi A. Wnt5a regulates distinct signalling pathways by binding to Frizzled2. EMBO J. 2010;29:41–54. doi: 10.1038/emboj.2009.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawakami K, et al. The Wnt co-receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment. J. Biol. Chem. 2006;281:23698–23711. doi: 10.1074/jbc.M601000200. [DOI] [PubMed] [Google Scholar]
- Saxon LK, Jackson BF, Sugiyama T, Lanyon LE, Price JS. Analysis of multiple bone responses to graded strains above functional levels, and to disuse, in mice in vivo show that the human Lrp5 G171V High Bone Mass mutation increases the osteogenic response to loading but that lack of Lrp5 activity reduces it. Bone. 2011;49:184–193. doi: 10.1016/j.bone.2011.03.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaniel C, Sirabella D, Qiu J, Niu X, Lemischka IR, Moore KA. Wnt-inhibitory factor 1 dysregulation of the bone marrow niche exhausts hematopoietic stem cells. Blood. 2011;118:2420–2429. doi: 10.1182/blood-2010-09-305664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze J, et al. Negative regulation of bone formation by the transmembrane Wnt antagonist Kremen-2. PLoS One. 2010;5:e10309. doi: 10.1371/journal.pone.0010309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenov M, Tamai K, He X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J. Biol. Chem. 2005;280:26770–26775. doi: 10.1074/jbc.M504308200. [DOI] [PubMed] [Google Scholar]
- Sen B, Xie Z, Case N, Ma M, Rubin C, Rubin J. Mechanical strain inhibits adipogenesis in mesenchymal stem cells by stimulating a durable beta-catenin signal. Endocrinology. 2008;149:6065–6075. doi: 10.1210/en.2008-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon-Chazottes D, et al. Mutations in the gene encoding the low-density lipoprotein receptor LRP4 cause abnormal limb development in the mouse. Genomics. 2006;87:673–677. doi: 10.1016/j.ygeno.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Sims AM, et al. Genetic analyses in a sample of individuals with high or low BMD shows association with multiple Wnt pathway genes. J. Bone Miner. Res. 2008;23:499–506. doi: 10.1359/jbmr.071113. [DOI] [PubMed] [Google Scholar]
- Sokol SY. Analysis of Dishevelled signalling pathways during Xenopus development. Curr. Biol. 1996;6:1456–1467. doi: 10.1016/s0960-9822(96)00750-6. [DOI] [PubMed] [Google Scholar]
- Staehling-Hampton K, et al. A 52-kb deletion in the SOST-MEOX1 intergenic region on 17q12-q21 is associated with van Buchem disease in the Dutch population. Am. J. Med. Genet. 2002;110:144–152. doi: 10.1002/ajmg.10401. [DOI] [PubMed] [Google Scholar]
- Stevens JR, Miranda-Carboni GA, Singer MA, Brugger SM, Lyons KM, Lane TF. Wnt10b deficiency results in age-dependent loss of bone mass and progressive reduction of mesenchymal progenitor cells. J. Bone Miner. Res. 2010;25:2138–2147. doi: 10.1002/jbmr.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styrkarsdottir U, et al. Multiple genetic loci for bone mineral density and fractures. N. Engl. J. Med. 2008;358:2355–2365. doi: 10.1056/NEJMoa0801197. [DOI] [PubMed] [Google Scholar]
- Styrkarsdottir U, et al. New sequence variants associated with bone mineral density. Nat. Genet. 2009;41:15–17. doi: 10.1038/ng.284. [DOI] [PubMed] [Google Scholar]
- Styrkarsdottir U, et al. European bone mineral density loci are also associated with BMD in East-Asian populations. PLoS One. 2010;5:e13217. doi: 10.1371/journal.pone.0013217. [Electronic Resource] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmann-Schmitt C, et al. Wif-1 is expressed at cartilage-mesenchyme interfaces and impedes Wnt3a-mediated inhibition of chondrogenesis. J. Cell Sci. 2009;122:3627–3637. doi: 10.1242/jcs.048926. [DOI] [PubMed] [Google Scholar]
- Sutherland MK, et al. Sclerostin promotes the apoptosis of human osteoblastic cells: a novel regulation of bone formation. Bone. 2004;35:828–835. doi: 10.1016/j.bone.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Takada I, et al. A histone lysine methyltransferase activated by non-canonical Wnt signalling suppresses PPAR-gamma transactivation. Nat. Cell Biol. 2007;9:1273–1285. doi: 10.1038/ncb1647. [DOI] [PubMed] [Google Scholar]
- Talaber G, et al. Wnt-4 protects thymic epithelial cells against dexamethasone-induced senescence. Rejuvenation Res. 2011;14:241–248. doi: 10.1089/rej.2010.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian E, et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N. Engl. J. Med. 2003;349:2483–2494. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- Tu X, et al. Noncanonical Wnt signaling through G protein-linked PKCdelta activation promotes bone formation. Dev. Cell. 2007;12:113–127. doi: 10.1016/j.devcel.2006.11.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urano T, et al. Association of a single-nucleotide polymorphism in low-density lipoprotein receptor-related protein 5 gene with bone mineral density. J. Bone Miner. Metab. 2004;22:341–345. doi: 10.1007/s00774-003-0492-9. [DOI] [PubMed] [Google Scholar]
- Vaes BL, et al. Microarray analysis reveals expression regulation of Wnt antagonists in differentiating osteoblasts. Bone. 2005;36:803–811. doi: 10.1016/j.bone.2005.02.001. [DOI] [PubMed] [Google Scholar]
- van Bezooijen RL, et al. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J. Exp. Med. 2004;199:805–814. doi: 10.1084/jem.20031454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bezooijen RL, et al. Sclerostin in mineralized matrices and van Buchem disease. J. Dent. Res. 2009;88:569–574. doi: 10.1177/0022034509338340. [DOI] [PubMed] [Google Scholar]
- van Genderen C, et al. Development of several organs that require inductive epithelial- mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev. 1994;8:2691–2703. doi: 10.1101/gad.8.22.2691. [DOI] [PubMed] [Google Scholar]
- Van Koevering KK, Williams BO. Transgenic Mouse Strains for Conditional Gene Deletion During Skeletal Development. 2008. pp. 151–170.
- van Meurs JB, et al. Common genetic variation of the low-density lipoprotein receptor-related protein 5 and 6 genes determines fracture risk in elderly white men. J. Bone Miner. Res. 2006;21:141–150. doi: 10.1359/JBMR.050904. [DOI] [PubMed] [Google Scholar]
- van Meurs JB, et al. Large-scale analysis of association between LRP5 and LRP6 variants and osteoporosis. JAMA. 2008;299:1277–1290. doi: 10.1001/jama.299.11.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev. Cell. 2003;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- Verbeek S, et al. An HMG-box-containing T-cell factor required for thymocyte differentiation. Nature. 1995;374:70–74. doi: 10.1038/374070a0. [DOI] [PubMed] [Google Scholar]
- Vestergaard P, Rejnmark L, Mosekilde L. Reduced relative risk of fractures among users of lithium. Calcif. Tissue Int. 2005;77:1–8. doi: 10.1007/s00223-004-0258-y. [DOI] [PubMed] [Google Scholar]
- Vijayakumar S, et al. High-frequency canonical Wnt activation in multiple sarcoma subtypes drives proliferation through a TCF/beta-catenin target gene, CDC25A. Cancer Cell. 2011;19:601–612. doi: 10.1016/j.ccr.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan M, et al. Parathyroid hormone signaling through low-density lipoprotein-related protein 6. Genes Dev. 2008;22:2968–2979. doi: 10.1101/gad.1702708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan M, et al. LRP6 mediates cAMP generation by G protein-coupled receptors through regulating the membrane targeting of Galpha(s). Sci. Signal. 2011;4:ra15. doi: 10.1126/scisignal.2001464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang FS, et al. Secreted frizzled-related protein 1 modulates glucocorticoid attenuation of osteogenic activities and bone mass. Endocrinology. 2005;146:2415–2423. doi: 10.1210/en.2004-1050. [DOI] [PubMed] [Google Scholar]
- Waterman ML. Lymphoid enhancer factor/T cell factor expression in colorectal cancer. Cancer Metastasis Rev. 2004;23:41–52. doi: 10.1023/a:1025858928620. [DOI] [PubMed] [Google Scholar]
- Weatherbee SD, Anderson KV, Niswander LA. LDL-receptor-related protein 4 is crucial for formation of the neuromuscular junction. Development. 2006;133:4993–5000. doi: 10.1242/dev.02696. [DOI] [PubMed] [Google Scholar]
- Wei W, Zeve D, Suh JM, Wang X, Du Y, Zerwekh JE, Dechow PC, Graff JM, Wan Y. Biphasic and Dosage-Dependent Regulation of Osteoclastogenesis by {beta}-Catenin. Mol Cell Biol. 2011;31:4706–4719. doi: 10.1128/MCB.05980-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wergedal JE, et al. Patients with Van Buchem disease, an osteosclerotic genetic disease, have elevated bone formation markers, higher bone density, and greater derived polar moment of inertia than normal. J. Clin. Endocrinol. Metab. 2003;88:5778–5783. doi: 10.1210/jc.2003-030201. [DOI] [PubMed] [Google Scholar]
- Westendorf JJ, Kahler RA, Schroeder TM. Wnt signaling in osteoblasts and bone diseases. Gene. 2004;341:19–39. doi: 10.1016/j.gene.2004.06.044. [DOI] [PubMed] [Google Scholar]
- Wilting I, et al. Lithium use and the risk of fractures. Bone. 2007;40:1252–1258. doi: 10.1016/j.bone.2006.12.055. [DOI] [PubMed] [Google Scholar]
- Winkler DG, et al. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003;22:6267–6276. doi: 10.1093/emboj/cdg599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte F, Dokas J, Neuendorf F, Mundlos S, Stricker S. Comprehensive expression analysis of all Wnt genes and their major secreted antagonists during mouse limb development and cartilage differentiation. Gene Expr. Patterns. 2009;9:215–223. doi: 10.1016/j.gep.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Witze ES, Litman ES, Argast GM, Moon RT, Ahn NG. Wnt5a control of cell polarity and directional movement by polarized redistribution of adhesion receptors. Science. 2008;320:365–369. doi: 10.1126/science.1151250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouda RR, Bansraj MR, de Jong AW, Noordermeer JN, Fradkin LG. Src family kinases are required for WNT5 signaling through the Derailed/RYK receptor in the Drosophila embryonic central nervous system. Development. 2008;135:2277–2287. doi: 10.1242/dev.017319. [DOI] [PubMed] [Google Scholar]
- Wright WS, et al. Wnt10b inhibits obesity in ob/ob and agouti mice. Diabetes. 2007;56:295–303. doi: 10.2337/db06-1339. [DOI] [PubMed] [Google Scholar]
- Wu M, Herman MA. A novel noncanonical Wnt pathway is involved in the regulation of the asymmetric B cell division in C. elegans. Dev. Biol. 2006;293:316–329. doi: 10.1016/j.ydbio.2005.12.024. [DOI] [PubMed] [Google Scholar]
- Wu W, Glinka A, Delius H, Niehrs C. Mutual antagonism between dickkopf1 and dickkopf2 regulates Wnt/beta-catenin signalling. Curr. Biol. 2000;10:1611–1614. doi: 10.1016/s0960-9822(00)00868-x. [DOI] [PubMed] [Google Scholar]
- Xia X, Batra N, Shi Q, Bonewald LF, Sprague E, Jiang JX. Prostaglandin promotion of osteocyte gap junction function through transcriptional regulation of connexin 43 by glycogen synthase kinase 3/beta-catenin signaling. Mol. Cell. Biol. 2010;30:206–219. doi: 10.1128/MCB.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav VK, et al. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 2008;135:825–837. doi: 10.1016/j.cell.2008.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav VK, Arantes HP, Barros ER, Lazaretti-Castro M, Ducy P. Genetic analysis of Lrp5 function in osteoblast progenitors. Calcif. Tissue Int. 2010;86:382–388. doi: 10.1007/s00223-010-9350-7. [DOI] [PubMed] [Google Scholar]
- Yan Y, et al. Axin2 controls bone remodeling through the beta-catenin-BMP signaling pathway in adult mice. J. Cell Sci. 2009;122:3566–3578. doi: 10.1242/jcs.051904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagita M, et al. USAG-1: a bone morphogenetic protein antagonist abundantly expressed in the kidney. Biochem. Biophys. Res. Commun. 2004;316:490–500. doi: 10.1016/j.bbrc.2004.02.075. [DOI] [PubMed] [Google Scholar]
- Yao W, Cheng Z, Shahnazari M, Dai W, Johnson ML, Lane NE. Overexpression of secreted frizzled-related protein 1 inhibits bone formation and attenuates parathyroid hormone bone anabolic effects. J. Bone Miner. Res. 2010;25:190–199. doi: 10.1359/jbmr.090719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao GQ, Wu JJ, Troiano N, Insogna K. Targeted overexpression of Dkk1 in osteoblasts reduces bone mass but does not impair the anabolic response to intermittent PTH treatment in mice. J. Bone Miner. Metab. 2011;29:141–148. doi: 10.1007/s00774-010-0202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerges LM, et al. High-density association study of 383 candidate genes for volumetric BMD at the femoral neck and lumbar spine among older men. J. Bone Miner. Res. 2009;24:2039–2049. doi: 10.1359/JBMR.090524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JC, Ng A, Kim BH, Bianco A, Xavier RJ, Elledge SJ. Wnt signaling regulates mitochondrial physiology and insulin sensitivity. Genes Dev. 2010;24:1507–1518. doi: 10.1101/gad.1924910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HM, et al. The role of Axin2 in calvarial morphogenesis and craniosynostosis. Development. 2005;132:1995–2005. doi: 10.1242/dev.01786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HM, Jin Y, Fu J, Hsu W. Expression of Gpr177, a Wnt trafficking regulator, in mouse embryogenesis. Dev. Dyn. 2010;239:2102–2109. doi: 10.1002/dvdy.22336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L, et al. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90:181–192. doi: 10.1016/s0092-8674(00)80324-4. [DOI] [PubMed] [Google Scholar]
- Zeng X, et al. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438:873–877. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, et al. The LRP5 High-Bone-Mass G171V Mutation Disrupts LRP5 Interaction with Mesd. Mol. Cell. Biol. 2004;24:4677–4684. doi: 10.1128/MCB.24.11.4677-4684.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DY, Wang HJ, Tan YZ. Wnt/beta-Catenin Signaling Induces the Aging of Mesenchymal Stem Cells through the DNA Damage Response and the p53/p21 Pathway. PLoS One. 2011;6:e21397. doi: 10.1371/journal.pone.0021397. [DOI] [PMC free article] [PubMed] [Google Scholar]