Abstract
Platelets are most recognized for their vital role as the cellular mediator of thrombosis, but platelets also have important immune functions. Platelets initiate and sustain vascular inflammation in many disease conditions, including arthritis, atherosclerosis, transplant rejection and severe malaria. We now demonstrate that platelets express T cell co-stimulatory molecules, process and present antigen in MHC class I, and directly activate naïve T cells in a platelet MHCI dependent manner. Using an experimental cerebral malaria (ECM) mouse model we also demonstrate that platelets present pathogen derived antigen to promote T cell responses in vivo, and that platelets can be used in a cell based vaccine model to induce protective immune responses. Our study demonstrates a novel antigen presentation role for platelets.
Introduction
Platelets are numerous (~200,000/µL in humans), anucleate, megakaryocyte derived, multi-functional blood cells. They are the primary cellular mediator of hemostasis, but platelets also have important immune roles. When activated, platelets secrete pre-formed and synthesized immune mediators, and initiate interactions with quiescent leukocytes, making platelets the most numerous circulating cell with an immune function (1–4). Most of what is known about platelet-leukocyte interactions has focused on platelet interactions with monocytes and neutrophils, but platelets also have a role in T cell responses. In ischemia-reperfusion studies platelets augment the recruitment of T cells to liver sinusoids (5), and activated platelets enhance intrahepatic accumulation of CD8+ T cells in viral hepatitis (6). Our studies using the experimental cerebral malaria (ECM) mouse model have established that the platelet derived chemokine PF4 (CXCL4) increases T cell CXCR3 expression and T cell trafficking to the brain (7). Platelet and T cell interactions are also important in amplifying acquired immune responses. For example, platelets are a major source of CD154 (CD40L), and platelet derived CD154 assists in germinal center development, immunoglobulin isotype switching, and in driving allograft rejection (8–10).
We now report that platelets directly present antigen to T cells and stimulate naïve T cell responses in the context of MHC class I. Although platelets have not been previously associated with processing or presenting antigen, they are known to express many of the molecules necessary for these functions. Platelets have an active proteasome, an ER and Golgi, transporter of antigen peptide (TAP), calnexin, calreticulin, and ERp57 (11–15). Messenger RNA (mRNA) for MHCI subunits such as beta 2 microglobulin (β2M) and Human Leukocyte Antigens (HLA) are also highly expressed in platelets (15). In addition, T cell co-stimulatory and adhesion molecules associated with antigen presenting cells are also present on platelets, including CD40, CD44, ICAM-2, and DC-SIGN (16–19), further indicating a potential antigen presenting role for platelets.
Cerebral malaria (CM) is a severe complication of Plasmodium falciparum infection. More than 1 million people die each year from malaria and 90% of the deaths occur in children (20). The pathogenesis of CM is the result of a combination of vascular and immune system dysfunction, with autopsy specimens typified by multifocal cerebral capillary obstruction with infected RBCs (iRBCs), platelets and leukocytes (21). Our lab and others have demonstrated that platelets adversely impact the development of experimental cerebral malaria (ECM) by accelerating and supporting its disease course (7, 22–26). We have shown in mice infected with the cerebral malaria causing parasite P. berghei that platelet activation occurs in this disease model before any signs of illness, and that platelet derived inflammatory molecules (such as PF4) contribute significantly to the immune responses leading to ECM (7, 26). CD8+ T cells are necessary for the brain vascular damage seen in ECM (27, 28). Despite its fairly rapid clinical course, there is direct evidence using OVA transgenic P. berghei that blood-stage infection leads to antigen specific CD8+ T cell infiltration of the brain by day 5 post infection (29). Using the ECM model, we now have evidence that platelets directly activate naïve T cells through the ability of platelets to present antigen in the context of MHC class I.
Methods
Reagents
Antibodies against MHC class I and class II, CD40, CD86, CD80, ICOSL, MHCI-OVA complex, CD8, CD3, CD28 and CD69 and appropriate isotype controls were purchased from eBiosciences. Ova-pentamer was purchased from ProImmune. All ELISAs were purchased from R&D Systems. SIINFEKL peptide was purchased from Bachem. Platelet depleting and control IgG antibodies were purchased from Emfret Analytics. Ovalbumin, PGE2 and thrombin were purchased from Sigma and U46619 from Tocris. DQ-Ovalbumin was purchased from Invitrogen.
Procedures
All mice used were on a C57Bl6/J background and purchased from Jackson Labs. Platelets from mice and humans were isolated as we have described elsewhere (30–32) using University of Rochester School of Medicine approved animal and human protocols. For all experiments using mouse platelets, platelets were prepared as washed platelets as we have published (31) and resuspended in calcium and magnesium free Tyrodes solution. Human platelet rich plasma (PRP) was isolated and resuspended in Tyrodes solution at a 1:20 ratio as we have described (31, 32). Platelets were incubated with antibodies for 20–30 mins at room temperature and fixed with 2% formalin before flow cytometry using a BDFacs Canto instrument and analyzed using FlowJo software.
Mouse T cells from spleens and lymph nodes were isolated using a negative selection T cell enrichment kit from StemCell Technology. Plates were coated with anti-CD3 antibody prior to plating T cells and incubating in RPMI with 5% FBS and penicillin/streptomycin. T cells were incubated with washed platelets in an approximately physiologic ratio of 1:20. In vivo platelet T cell activation assay used washed mouse platelets injected intravenous via the retro-orbital plexus into recipient mice.
In P. berghei infections mice were injected intraperitoneal with approximately 500,000 infected mouse RBCs. Mice were platelet depleted by an intraperitoneal injection with 50 µg of platelet depleting antibody or control IgG. This antibody in our experience greatly depresses platelet counts for approximately 4 days (33). All mouse plasma was collected by bleeding into EDTA coated tubes and centrifuging at 2500 × g for 10 mins.
In platelet vaccine studies OVA peptide or control PBS was added to washed platelets, platelets were stimulated with thrombin and incubated for 2 hrs. Platelets were then washed in the presence of PGE2 and resuspended in Tyrodes buffer before 1 × 107 platelets in a volume of 100 µL were injected into WT mice via the retro-orbital plexus. Parasitemia was measured by isolating RBC and incubating with Sybr Green to label iRBC and the number determined by flow cytometry using described methods (34) and confirmed by Giemsa stained blood smears. The number of OVA specific T cells were determined by flow cytometry using splenocytes with antibody to CD8 and an Pentamer for OVA specific T cell receptors.
All statistics are shown as standard deviation and P value determined by a standard Student’s T-Test.
Results
Platelets Express Molecules for Antigen Presentation and T cell Co-Stimulation
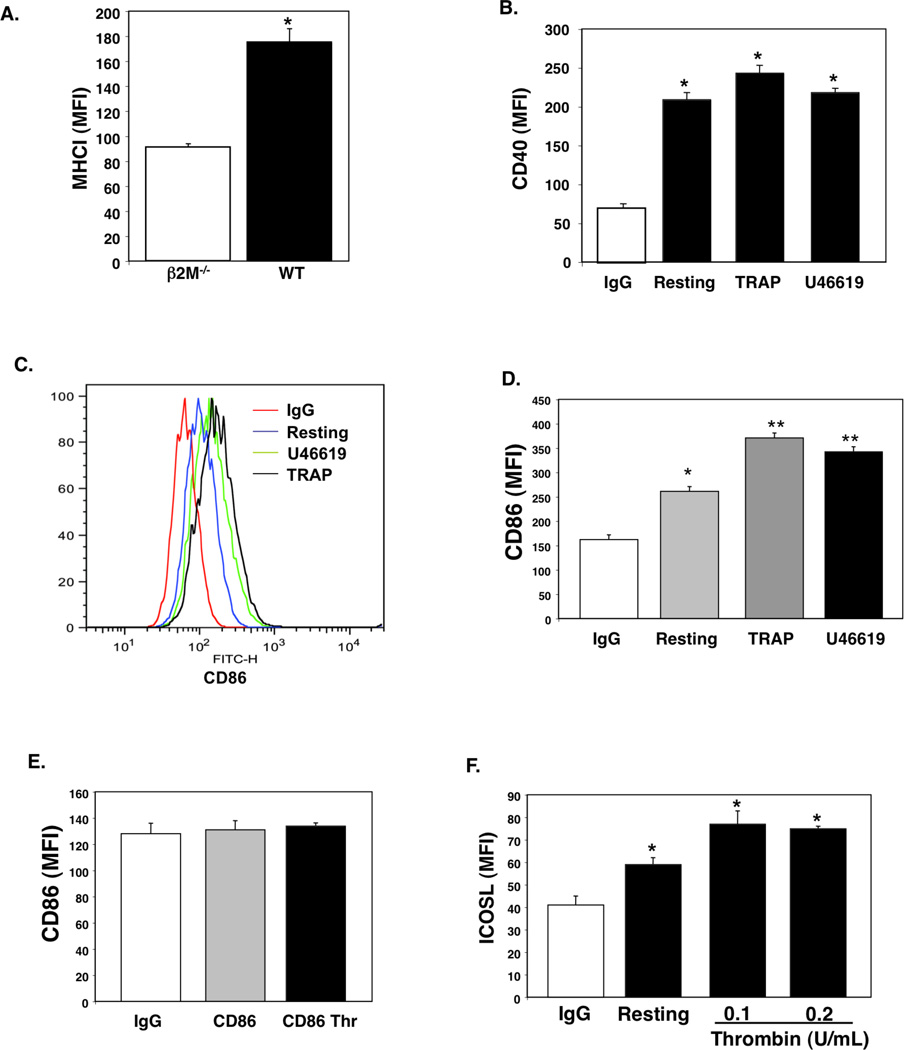
Platelets possess the molecular machinery needed to process and present antigen to CD8+ T cells. We first confirmed that platelets express MHC class I by flow cytometry comparing wild-type (WT) and MHCI deficient platelets from β2M−/− mice. WT platelets express MHCI (Fig. 1A). Although platelets have been noted to express MHC class II in some disease states (35, 36), under normal conditions platelets do not express MHC class II (Supplementary Fig. S1). Other molecules needed for MHCI antigen presentation, including TAP1 and ERp57, are also present in platelets (Supplementary Fig. S2). Not only do platelets express MHCI, they also express T cell co-stimulatory molecules. Others have described the presence of CD40 on platelets (37). We have confirmed its presence and found that CD40 expression does not increase significantly with platelet activation in response to thrombin receptor activating peptide (TRAP, 2 µM) or the thromboxane receptor agonist U46619 (2 µM) (Fig. 1B human platelets; mouse platelets Supplementary Fig. S3). CD86 (B7.2) is a ligand for T cell CD28. Human platelets express CD86 and CD86 surface expression increases slightly with platelet activation (Fig. 1C and D). However, mouse platelets do not express CD86 (Fig. 1E), and neither mouse nor human platelets express CD80 (B7.1, not shown). Other co-stimulatory molecules, such as ICOSL, are also expressed by both human and mouse platelets (Fig. 1F, mouse platelet example). Taken together these data demonstrate that platelets express the molecular machinery necessary to activate naive T cells.
Figure 1.
Platelets Express Antigen Presentation Molecules. A) MHCI expression. Platelets from β2M−/− and WT mice (MFI=Mean Fluorescent Intensity; n=4 ± S.D., *P<0.01 vs β2M−/−). B) CD40. Resting or activated human platelets (2 µM TRAP or U46619; n=4 ± S.D., *P<0.01 vs IgG). C) CD86. Human platelets express CD86, which is increased with stimulation. D) CD86 Quantification (n=4 ±S.D.,*P<0.05, **P<0.01 vs IgG). E) Mouse platelets do not express CD86 (n=4 ± S.D.). F). ICOSL. Mouse platelets were incubated with control PBS or activated with thrombin before addition of control IgG or anti-ICOSL antibody (n=4 ± S.D., *P<0.05).
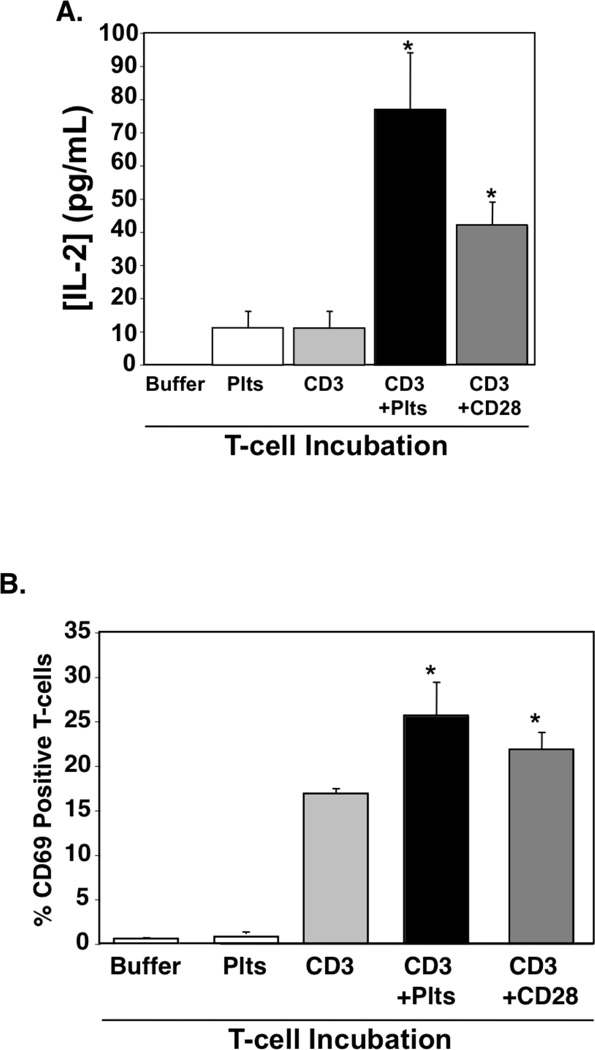
Because platelets express T cell co-stimulation molecules, we next determined whether platelets provide co-stimulation signals T cells (signal 2). Mouse T cells were incubated overnight with control buffer, platelets only, or anti-CD3 antibody only (signal 1) as negative controls. As a positive control T cells were incubated with both anti-CD3 and anti-CD28 antibody. T cells were also incubated with anti-CD3 antibody and platelets. As expected, T cells incubated with anti-CD3 and anti-CD28 produced IL-2 and had increased CD69 expression (Figs. 2A–B). T cells incubated with anti-CD3 and platelets also had significantly increased IL-2 production and CD69 expression as compared to CD3 alone (Fig. 2A–B). These data demonstrate that platelets are able to provide T cell co-stimulation.
Figure 2.
Platelets Provide T cell Co-Stimulation. T cells were incubated in anti-CD3 antibody or control PBS coated plates alone or with platelets or anti-CD28 antibody. A) IL-2 was measured by ELISA (n=4; ±S.D.,*P<0.01 vs CD3) and B) CD69 expression was measured by FACS (n=4; ±S.D.,*P<0.05 vs CD3).
Platelets Can Present Antigen
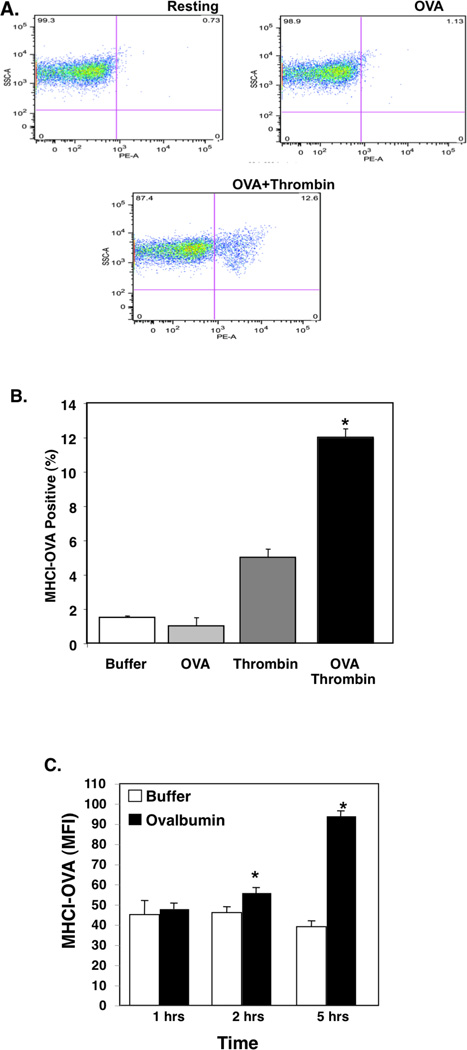
Platelets can be infected with viruses and bacteria including HIV, dengue virus, and Staphylococcus (38–40). Platelets have also been shown to be infected with intact Plasmodium and contain structures suggestive of degraded parasite material in electron microscopy (41, 42). Platelets are phagocytic, taking up molecules and proteins from their extracellular environment, and have active proteasomes (43–47). This raises the possibility that platelets may be able to acquire and present antigen to T cells. To determine whether platelets present antigen, we incubated platelets from C57Bl6/J (B6) mice with the ovalbumin octapeptide (SIINFEKL) presented by B6 mouse MHC class I haplotype H-2Kb. Resting and thrombin (0.1 U/mL) stimulated platelets were incubated with buffer or OVA peptide, and MHCI-SIINFEKL measured by flow cytometry using an antibody that recognizes this specific complex. Resting platelets did not have an increase in MHCI-SIINFEKL, but activated platelets incubated with OVA peptide had a large increase in OVA peptide presentation (Fig. 3A–B). Platelets were also mildly stimulated with thrombin in the presence of control buffer or full ovalbumin protein and OVA-MHCI determined. Platelets presented OVA-MHCI from ovalbumin protein (Fig. 3C) and this was greatly reduced by pre-treatment of platelets with brefeldin A (Supplementary Fig. S4), indicating that they can process and present antigen from full length protein. These data show that platelets may have the ability to take up antigenic proteins and present antigen in MHC class I.
Figure 3.
Platelets Process and Present Antigen. A) Platelets present antigen. Platelets were incubated with control PBS, OVA peptide only (200 µg/mL), thrombin, or OVA peptide with thrombin stimulation (Thr=thrombin, 0.2 U/mL). MHCI-OVA complex was measured by FACS. Representative plots. B) Quantification (n=4 ± S.D.*P<0.01 vs OVA). C) Full Ovalbumin. Platelets were incubated with control buffer or ovalbumin with thrombin and OVA antigen presentation in MHCI determined at multiple time points (n=4 ± S.D. *P<0.05 vs Control).
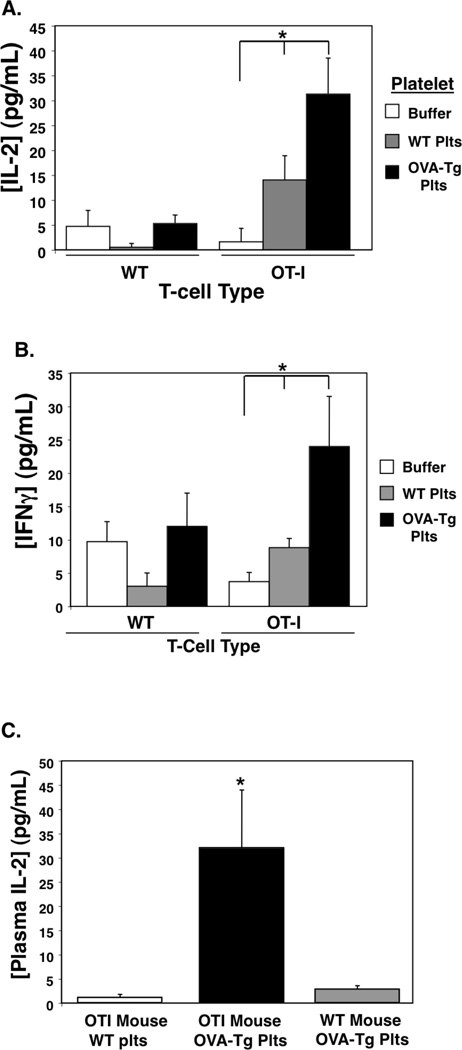
Ovalbumin transgenic mice (OVA-Tg) present OVA antigen complex in MHCI. If platelets can present antigen to T cells, platelets from OVA-Tg mice are predicted to activate T cells expressing receptors specific for OVA-MHCI. OT-I mice are transgenic for T cell receptors recognizing ovalbumin residues 257–264 in the context of B6 mouse MHCI. To provide an experimental proof of principle that platelets can present antigen to T cells, we incubated WT or OT-I T cells with buffer, platelets from wild-type (WT) mice, or with platelets from OVA-Tg mice. IL-2 and IFNγ production were measured 24 hrs and 48 hrs later respectively. OT-I T cells incubated with platelets from OVA-Tg mice produced greatly increased IL-2 and INF-γ compared to OT-I T cells and WT platelets (Fig. 4A–B). These data demonstrate that platelets present antigen and activate T cells in vitro.
Figure 4.
Platelets Present Antigen to T cells. A) In Vitro. WT or OT-I T cells were incubated with control buffer or platelets from WT or OVA-Tg mice. IL-2 was measured by ELISA (n=4 ± S.D., *P<0.01 vs WT). B) INFγ (n=4 ± S.D., *P<0.01 vs WT). C) In Vivo. WT or OT-I mice were given WT or OVA-Tg platelets i.v. and 48 hrs later plasma was isolated to measure IL-2 by ELISA. (n=4, ±S.D., *P<0.01 vs WT).
To determine whether platelets are a source of antigen in vivo, we intravenously (i.v.) injected OT-I mice with 1×108 WT or OVA-Tg platelets, and as a control OVA-Tg platelets were injected into WT mice. Plasma IL-2 was measured 48 hrs later. Only OT-I mice given OVA-Tg platelets had evidence of T cell stimulation in vivo (Fig. 4C). These data indicate that platelets present antigen to T cells in vitro and are a source of antigen in vivo.
Platelets Promote T cell Activation in ECM
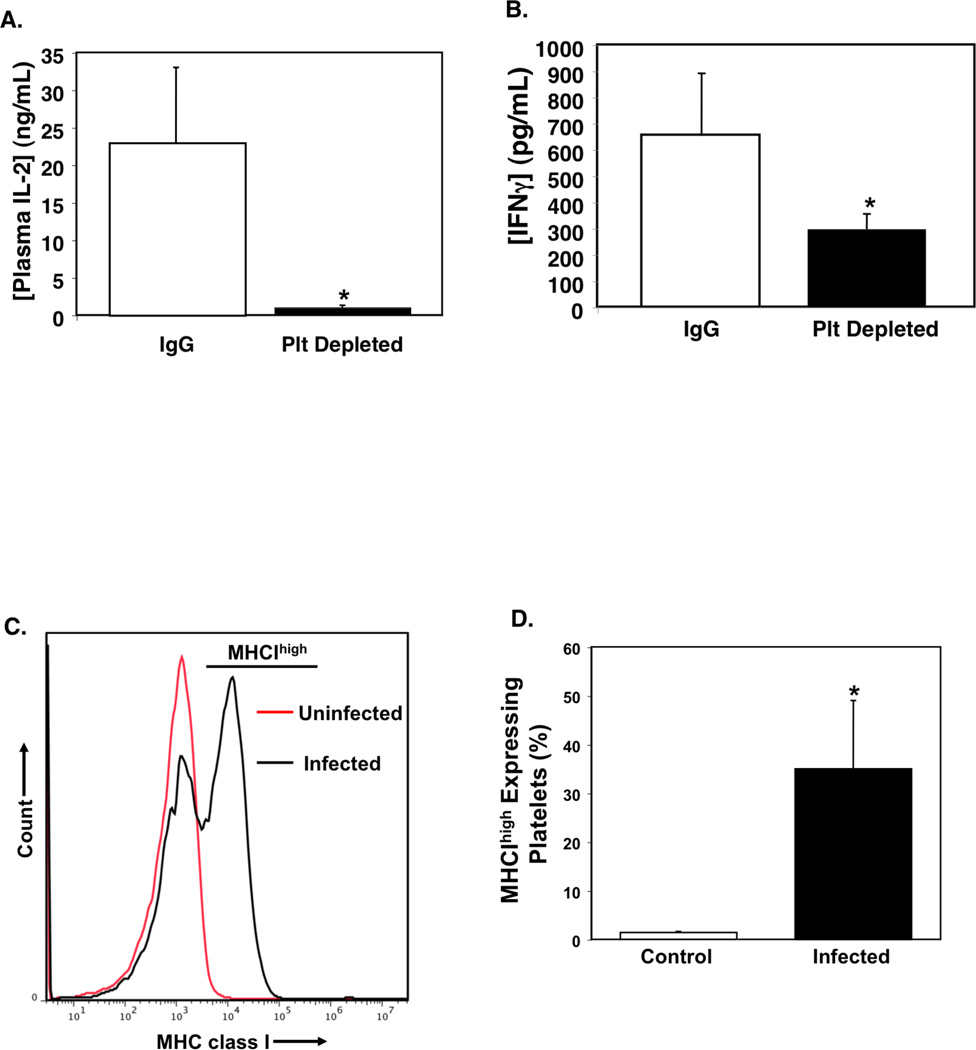
Cerebral malaria is a severe complication of infection with P. falciparum, and its pathogenesis is in part a CD8+ T cell dependent process (26, 28, 48, 49). Platelets also have an important role in initiating and sustaining the pathogenesis of ECM (21, 50). To determine whether platelets have a role in ECM associated T cell responses, we treated mice with a platelet-depleting antibody that greatly reduces platelet counts for about 4 days (33) or control IgG 24 hrs after P. berghei infection and measured plasma IL-2 and IFNγ on day 4 post infection. Platelet depleted mice had greatly reduced plasma IL-2 and IFNγ compared to control IgG treated mice (Fig. 5A and B). These data support our hypothesis that platelets have an in vivo role in P. berghei T cell responses.
Figure 5.
Platelets Increase T cell Responses to P. berghei. Platelet depleted P. berghei infected mice have A) lower plasma IL-2 compared to control IgG treated infected mice (day 4 post infection, n=5 ± S.D., *P<0.01 vs IgG), and B) decreased IFNγ (n=4 ± S.D., *P<0.05 vs IgG). C) Platelets increase MHCI expression in ECM. Platelets were isolated from infected mice on day 6 post infection and MHCI expression determined by FACS. D) Quantification of MHCIhigh expressing platelets (n=5 ± S.D., *P<0.01 vs Control Uninfected).
Non-professional antigen presenting cells typically increase MHCI expression to facilitate their role in antigen presentation. To determine whether platelets increase MHCI as part of an active role in ECM, we isolated platelets from uninfected control mice and P. berghei infected mice on days 4 and 6 post-infection and measured platelet MHCI expression by flow cytometry. Four days after infection MHCI expression was only slightly increased (not shown), however, by day 6 there was a population of platelets that expressed greatly increased levels of MHCI we have called MHCIhi platelets (Fig. 5C–D). These data demonstrate that platelets increase MHCI expression during infection.
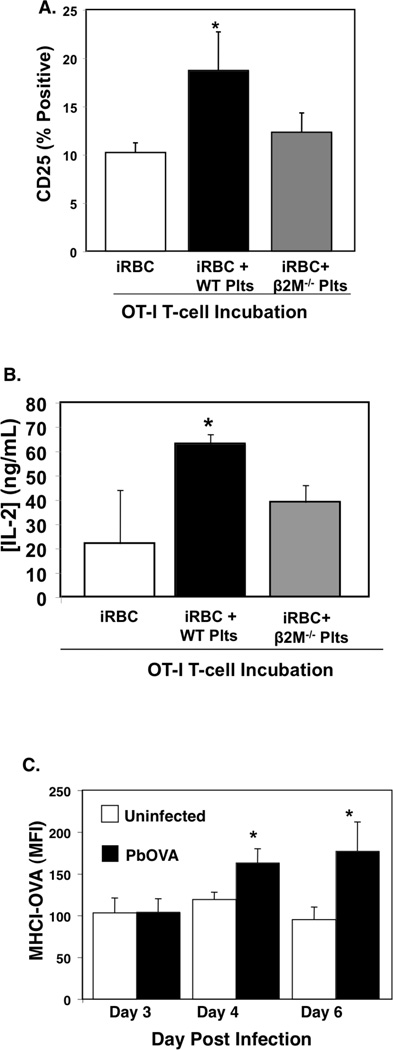
To more directly demonstrate a role for platelets in the presentation of Plasmodium antigen to T cells, we infected MHC class I deficient β2M−/− mice with a P. berghei parasite transgenic for the C-terminal amino acids 150–386 of ovalbumin (PbA-OVA) (51). On day 5 post infection RBC (iRBC) were isolated and incubated with OT-I T cells alone, with WT platelets, or with β2M−/− platelets (note: we used iRBC from β2M−/− PbA-OVA mice to eliminate the potential for contaminating antigen presenting leukocytes contributing to antigen presentation). T cell stimulation was determined 48 hrs later by measuring CD25 expression using flow cytometry and IL-2 production by ELISA. OT-I T cells incubated with iRBC and WT platelets, but not β2M−/− platelets, had significantly increased CD25 expression and IL-2 production (Fig. 6A–B). These data demonstrate that platelets can acquire and present parasite derived antigen to T cells in a platelet MHCI dependent manner.
Figure 6.
Platelets present Plasmodium derived antigen to T cells. A) Platelets acquire and present antigen. OT-I T cells were incubated alone, with iRBC, or with iRBC and WT or β2M−/− platelets. 48hrs later T cell CD25 expression was determined by FACS. WT platelets increased OT-I T cell CD25 expression (n=4 ± S.D., *P<0.02 vs iRBC) and B) increased IL-2 production (n=4 ± S.D., *P<0.03 vs iRBC). C) Platelets were isolated from control mice or mice infected with PbA-OVA and MHCI-OVA measured by FACS (n=5 ± S.D., *P<0.05 vs Control).
To explore whether platelets present Plasmodium derived antigen in vivo, platelets were isolated from uninfected mice and mice infected with PbA-OVA to determine platelet MHCI-OVA complex expression on days 3, 4, and 6 post infection. Beginning 4 days after infection platelets from PbA-OVA infected mice had increased MHCI-OVA complex expression (Fig. 6C).
Platelets Can Be Used to Induce Protective T cell Responses
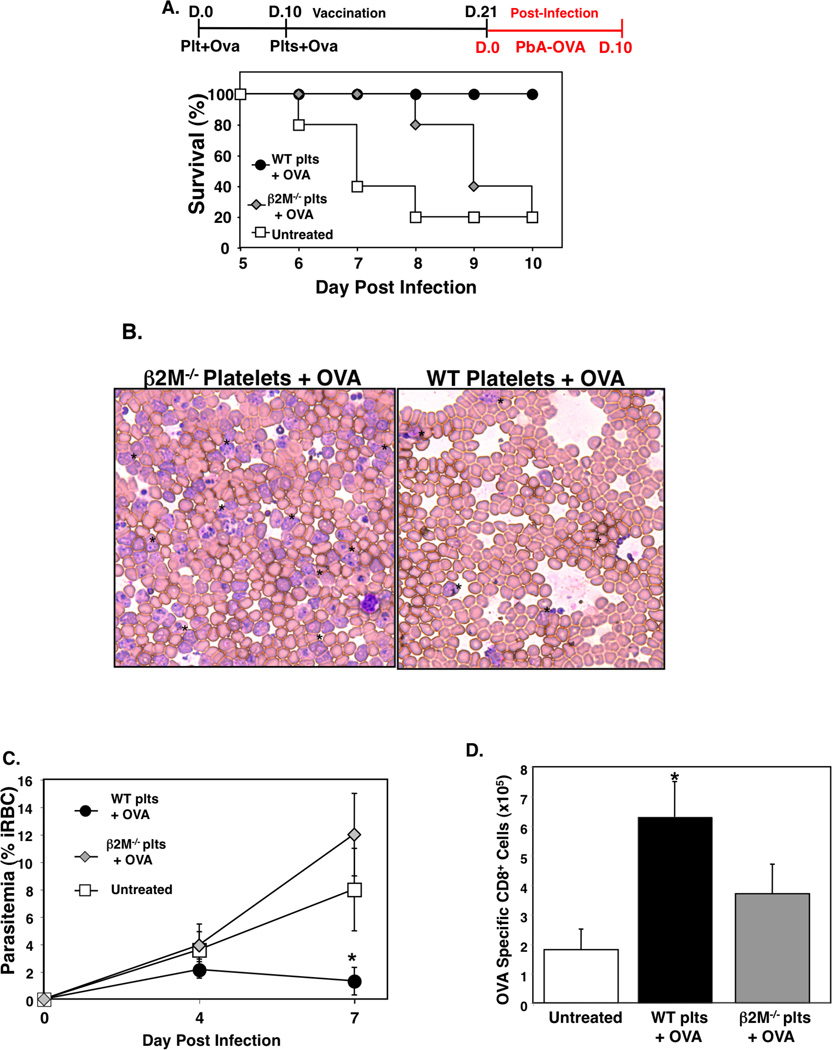
Antigen pulsed dendritic cells have been used in clinical trials to induce protective T cell responses for the treatment of cancer and HIV (52, 53). Because platelets can present antigen they may represent an alternative or adjunctive platform for cell based vaccines. To test this concept using the ECM model, we incubated washed WT platelets or β2M−/− platelets with OVA peptide (300 µg/mL), activated the platelets with mild thrombin stimulation (0.2 U/mL), and incubated for 2 hrs. Platelets were then washed, and 1×107 platelets injected i.v. into WT mice. This was repeated 10 days later and 21 days after the first platelet injection WT platelet/OVA, β2M−/− platelet/OVA, and untreated control mice were infected with PbA-OVA. WT platelet/OVA mice also had greatly improved survival compared to β2M−/− and control mice (Fig. 7A), indicating that the platelet vaccine strategy was protective against the development of ECM in a platelet MHCI dependent manner. Mice that received the WT platelet/OVA vaccine also had reduced parasite burdens (% iRBC) (Figs. 7B–C) indicating these mice were able to mount an immune response that minimized parasite expansion. A separate group of mice was sacrificed on day 4 post-infection and OVA specific CD8+ T cells in the spleen were quantified using an OVA MHC class I pentamer. As expected, PbA-OVA infected mice that received no platelet treatment (untreated) had few OVA specific T cells (Fig 7D). However, mice treated with the WT platelet/OVA regimen had a greatly increased number of OVA specific T cells compared to mice treated with the β2M−/− platelet OVA (Fig 7D), demonstrating that platelets induced a specific T cell response in a manner dependent on platelet MHCI. These data indicate that platelets can be used to present antigen to naïve T cells and induce protective T cell responses in a platelet MHCI dependent manner.
Figure 7.
Platelets Can Be Used as a Cell Based Vaccine. A) Platelets from WT and β2M−/− mice were incubated with OVA peptide SIINFEKL (300 µg/mL), thrombin stimulated (0.2 U/mL), washed, and 1×107 platelets injected i.v. into WT mice. This was repeated 10 days later, and platelet treated or control untreated mice were infected with PbA-OVA 21 days after the first platelet injection. Survival (n=5 ± S.D., *P<0.01 vs β2M−/−). B) Representative day 7 post infection blood smear. C) Parasitemia quantification (n=5 ± S.D., *P<0.05, **P<0.01 vs WT). D) OVA specific T cells. Mononuclear cells were isolated from the spleens of mice on day 4 post-infection and the number of OVA specific T cells quantified by flow cytometry (n=5 ± S.D., *P<0.03; vs β2M−/−).
Discussion
Our results demonstrate that platelets present antigen to T cells in a platelet MHCI dependent manner and that platelets acquire and present Plasmodium derived antigen to CD8+ T cells both in vitro and in vivo. This represents an important new concept; platelets not only support and promote acquired immune responses, but platelets may also directly participate in the initiation of acquired immune responses. A platelet antigen presentation role may be important in many blood borne infections and vascular inflammatory diseases. Thrombocytopenia and inflammation are associated with malaria, dengue and HIV amongst other infectious diseases. Immune mediated thrombocytopenia (ITP) can also be the result of cytoxic T cell responses to platelets presenting self antigen. The pathogenesis of infection related thrombocytopenias and ITP are likely multiple, but may in part be driven by platelets presenting antigen and being specifically targeted by CD8+ T cells.
Initiation of innate and acquired immune responses by platelets is a potentially important mechanism to combat infectious agents. Following a breach in the skin for example, platelets limit bleeding, but they also simultaneously recruit and activate ‘professional’ immune cells to limit skin pathogen dissemination. Platelet presentation of foreign antigen derived from the site of the skin lesion may provide an early means to respond to subsequent antigen exposure. Because platelets are much more numerous than leukocytes and tissue restricted professional antigen presenting cells, an antigen presentation function may also provide early blood surveillance to begin antigen specific responses before more specialized cells become involved. Platelets can be activated by and phagocytose bacteria, and can be infected by numerous types of viruses, perhaps indicating a more active role in antigen presentation than previously considered. There is also growing evidence that platelets may traffic in a regulated manner. Platelets can access lymph nodes and appear to have the ability to undergo regulated diapedesis supporting our evidence that they may have specific T cell interactions (54, 55).
It has been suggested that more platelet MHCI is absorbed from the plasma than is derived from the platelet itself (56). Our results indicate that platelets express significant amounts of MHCI and that during infection a population of platelets emerges that express greatly increased MHCI. MHC peptide loading takes place in the endoplasmic reticulum (ER) and traffics through the Golgi to the membrane surface via a secretory pathway. As noted above, platelets have an ER and Golgi (11) as well as other components necessary for MHCI peptide loading including TAP, calnexin, calreticulin, and ERp57 (12–14), further supporting the concept that platelets process antigen and regulate MHCI expression. Platelet mRNA expression data has shown that compared to other transcripts, platelets have large amounts of antigen presentation related mRNA, in particular β2M and HLA mRNA are highly abundant in platelets (15). Our data, the high mRNA expression, and ability of platelets to upregulate protein expression in the periphery (57) may further indicate the importance of regulating MHCI expression and antigen presentation in platelets. Whether platelets present antigen as a result of platelet infection, or whether platelets present antigen they have taken up by phagocytosis is unclear from our studies and requires much further work to clarify.
Because dendritic cells (DC) induce robust T cell responses, cell-based vaccine research has focused on DCs, with cancer and HIV the diseases most often targeted. Mature DCs provide optimal immune stimulation, but immature DCs may tolerize T cells. This means that DCs need to be significantly manipulated in culture prior to in vivo use. On an individual cell basis, platelets may not induce as robust an immune response as professional antigen presenting cells, and platelets are limited by their expression of only MHC class I. But platelets may offer many other unique advantages for cell based vaccine development, including platelets are easy to isolate in large numbers and do not require extensive manipulation and differentiation. Much further study is needed to determine whether platelets can be effective in presenting Plasmodium restricted antigens for vaccine development, but our data does demonstrate the ability of platelets presenting antigen to induce T cell activation in vivo. The ‘WT platelet/OVA vaccine’ treated mice eventually developed an increasing parasite burden after about day 15 post-infection indicating that the mice were protected from the ECM phase, but the parasite eventually escapes the protection offered by the platelet vaccine. CM protection is an important therapeutic goal and these results point out the complexity of malaria immune responses and disease pathogenesis. Our data also demonstrates that although CD8+ T cells are deleterious in the late stages of ECM and drive ECM pathogenesis (51, 58, 59), an early, robust CD8 response can be protective. Although our data represents an important novel proof of principle, there is of course much work that must be done to better understand the long-term therapeutic potential of our results, and any potential adverse side effects. Because platelets are pro-inflammatory and the cellular mediator of thrombosis unintended side effects must also be given great study and consideration.
Platelets are dynamic cells and represent an early link between the innate and acquired immune responses in many vascular inflammatory processes, including cerebral malaria. Thrombosis and vascular inflammation represent a continuum of each other; platelet activation incites an immune response and vascular inflammation leads to platelet activation. Our data extends this important paradigm to platelet antigen presentation in the context of MHCI.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants R01HL093179, R01HL093179-02S109, and R01HL094547 to CNM. WMB is supported by National Institutes of Health Grant P01AI087586.
Footnotes
Disclosures
The authors have not financial conflicts of interest.
References
- 1.Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J Clin Invest. 2005;115:3378–3384. doi: 10.1172/JCI27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Massberg S, Konrad I, Schurzinger K, Lorenz M, Schneider S, Zohlnhoefer D, Hoppe K, Schiemann M, Kennerknecht E, Sauer S, Schulz C, Kerstan S, Rudelius M, Seidl S, Sorge F, Langer H, Peluso M, Goyal P, Vestweber D, Emambokus NR, Busch DH, Frampton J, Gawaz M. Platelets secrete stromal cell-derived factor 1alpha and recruit bone marrow-derived progenitor cells to arterial thrombi in vivo. J Exp Med. 2006;203:1221–1233. doi: 10.1084/jem.20051772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gawaz M. Role of platelets in coronary thrombosis and reperfusion of ischemic myocardium. Cardiovasc Res. 2004;61:498–511. doi: 10.1016/j.cardiores.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 4.Langer H, May AE, Daub K, Heinzmann U, Lang P, Schumm M, Vestweber D, Massberg S, Schonberger T, Pfisterer I, Hatzopoulos AK, Gawaz M. Adherent platelets recruit and induce differentiation of murine embryonic endothelial progenitor cells to mature endothelial cells in vitro. Circ Res. 2006;98:e2–e10. doi: 10.1161/01.RES.0000201285.87524.9e. [DOI] [PubMed] [Google Scholar]
- 5.Khandoga A, Hanschen M, Kessler JS, Krombach F. CD4+ T cells contribute to postischemic liver injury in mice by interacting with sinusoidal endothelium and platelets. Hepatology. 2006;43:306–315. doi: 10.1002/hep.21017. [DOI] [PubMed] [Google Scholar]
- 6.Iannacone M, Sitia G, Isogawa M, Marchese P, Castro MG, Lowenstein PR, Chisari FV, Ruggeri ZM, Guidotti LG. Platelets mediate cytotoxic T lymphocyte-induced liver damage. Nat Med. 2005;11:1167–1169. doi: 10.1038/nm1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srivastava K, Cockburn IA, Swaim A, Thompson LE, Tripathi A, Fletcher CA, Shirk EM, Sun H, Kowalska MA, Fox-Talbot K, Sullivan D, Zavala F, Morrell CN. Platelet factor 4 mediates inflammation in experimental cerebral malaria. Cell Host Microbe. 2008;4:179–187. doi: 10.1016/j.chom.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu H, Zhang X, Mannon RB, Kirk AD. Platelet-derived or soluble CD154 induces vascularized allograft rejection independent of cell-bound CD154. J Clin Invest. 2006;116:769–774. doi: 10.1172/JCI27155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elzey DB, Sprague DL, Ratliff TL. The emerging role of platelets in adaptive immunity. Cell Immunol. 2005;238:1–9. doi: 10.1016/j.cellimm.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Kirk AD, Morrell CN, Baldwin WM., 3rd Platelets influence vascularized organ transplants from start to finish. Am J Transplant. 2009;9:14–22. doi: 10.1111/j.1600-6143.2008.02473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han SS, Baker BL. The Ultrastructure of Megakaryocytes and Blood Platelets in the Rat Spleen. Anat Rec. 1964;149:251–267. doi: 10.1002/ar.1091490208. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell WB, Li J, French DL, Coller BS. alphaIIbbeta3 biogenesis is controlled by engagement of alphaIIb in the calnexin cycle via the N15-linked glycan. Blood. 2006;107:2713–2719. doi: 10.1182/blood-2005-07-2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elton CM, Smethurst PA, Eggleton P, Farndale RW. Physical and functional interaction between cell-surface calreticulin and the collagen receptors integrin alpha2beta1 and glycoprotein VI in human platelets. Thromb Haemost. 2002;88:648–654. [PubMed] [Google Scholar]
- 14.Schulz C, Leuschen NV, Frohlich T, Lorenz M, Pfeiler S, Gleissner CA, Kremmer E, Kessler M, Khandoga AG, Engelmann B, Ley K, Massberg S, Arnold GJ. Identification of novel downstream targets of platelet glycoprotein VI activation by differential proteome analysis: implications for thrombus formation. Blood. 2010;115:4102–4110. doi: 10.1182/blood-2009-07-230268. [DOI] [PubMed] [Google Scholar]
- 15.Rowley JW, Oler A, Tolley ND, Hunter B, Low EN, Nix DA, Yost CC, Zimmerman GA, Weyrich AS. Genome wide RNA-seq analysis of human and mouse platelet transcriptomes. Blood. 2011 doi: 10.1182/blood-2011-03-339705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koshiishi I, Shizari M, Underhill CB. CD44 can mediate the adhesion of platelets to hyaluronan. Blood. 1994;84:390–396. [PubMed] [Google Scholar]
- 17.Weber KS, Alon R, Klickstein LB. Sialylation of ICAM-2 on platelets impairs adhesion of leukocytes via LFA-1 and DC-SIGN. Inflammation. 2004;28:177–188. doi: 10.1023/b:ifla.0000049042.73926.eb. [DOI] [PubMed] [Google Scholar]
- 18.Chaipan C, Soilleux EJ, Simpson P, Hofmann H, Gramberg T, Marzi A, Geier M, Stewart EA, Eisemann J, Steinkasserer A, Suzuki-Inoue K, Fuller GL, Pearce AC, Watson SP, Hoxie JA, Baribaud F, Pohlmann S. DC-SIGN and CLEC-2 mediate human immunodeficiency virus type 1 capture by platelets. J Virol. 2006;80:8951–8960. doi: 10.1128/JVI.00136-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davi G, Ferroni P. CD40-CD40L interactions in platelet activation. Thromb Haemost. 2005;93:1011–1012. doi: 10.1160/TH05-04-0270. [DOI] [PubMed] [Google Scholar]
- 20.Idro R, Ndiritu M, Ogutu B, Mithwani S, Maitland K, Berkley J, Crawley J, Fegan G, Bauni E, Peshu N, Marsh K, Neville B, Newton C. Burden, features, and outcome of neurological involvement in acute falciparum malaria in Kenyan children. Jama. 2007;297:2232–2240. doi: 10.1001/jama.297.20.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Heyde HC, Nolan J, Combes V, Gramaglia I, Grau GE. A unified hypothesis for the genesis of cerebral malaria: sequestration, inflammation and hemostasis leading to microcirculatory dysfunction. Trends Parasitol. 2006;22:503–508. doi: 10.1016/j.pt.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Wassmer SC, Combes V, Candal FJ, Juhan-Vague I, Grau GE. Platelets potentiate brain endothelial alterations induced by Plasmodium falciparum. Infect Immun. 2006;74:645–653. doi: 10.1128/IAI.74.1.645-653.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grau GE, Mackenzie CD, Carr RA, Redard M, Pizzolato G, Allasia C, Cataldo C, Taylor TE, Molyneux ME. Platelet accumulation in brain microvessels in fatal pediatric cerebral malaria. J Infect Dis. 2003;187:461–466. doi: 10.1086/367960. [DOI] [PubMed] [Google Scholar]
- 24.Lou J, Donati YR, Juillard P, Giroud C, Vesin C, Mili N, Grau GE. Platelets play an important role in TNF-induced microvascular endothelial cell pathology. Am J Pathol. 1997;151:1397–1405. [PMC free article] [PubMed] [Google Scholar]
- 25.Mannel DN, Grau GE. Role of platelet adhesion in homeostasis and immunopathology. Mol Pathol. 1997;50:175–185. doi: 10.1136/mp.50.4.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Srivastava K, Field DJ, Aggrey A, Yamakuchi M, Morrell CN. Platelet factor 4 regulation of monocyte KLF4 in experimental cerebral malaria. PLoS One. 2010;5 doi: 10.1371/journal.pone.0010413. e10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belnoue E, Potter SM, Rosa DS, Mauduit M, Gruner AC, Kayibanda M, Mitchell AJ, Hunt NH, Renia L. Control of pathogenic CD8+ T cell migration to the brain by IFN-gamma during experimental cerebral malaria. Parasite Immunol. 2008;30:544–553. doi: 10.1111/j.1365-3024.2008.01053.x. [DOI] [PubMed] [Google Scholar]
- 28.Hunt NH, Golenser J, Chan-Ling T, Parekh S, Rae C, Potter S, Medana IM, Miu J, Ball HJ. Immunopathogenesis of cerebral malaria. Int J Parasitol. 2006;36:569–582. doi: 10.1016/j.ijpara.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 29.Lundie RJ, de Koning-Ward TF, Davey GM, Nie CQ, Hansen DS, Lau LS, Mintern JD, Belz GT, Schofield L, Carbone FR, Villadangos JA, Crabb BS, Heath WR. Blood-stage Plasmodium infection induces CD8+ T lymphocytes to parasite-expressed antigens, largely regulated by CD8alpha+ dendritic cells. Proc Natl Acad Sci U S A. 2008;105:14509–14514. doi: 10.1073/pnas.0806727105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsushita K, Morrell CN, Cambien B, Yang SX, Yamakuchi M, Bao C, Hara MR, Quick RA, Cao W, O'Rourke B, Lowenstein JM, Pevsner J, Wagner DD, Lowenstein CJ. Nitric oxide regulates exocytosis by S-nitrosylation of N-ethylmaleimide-sensitive factor. Cell. 2003;115:139–150. doi: 10.1016/s0092-8674(03)00803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morrell CN, Matsushita K, Chiles K, Scharpf RB, Yamakuchi M, Mason RJ, Bergmeier W, Mankowski JL, Baldwin WM, 3rd, Faraday N, Lowenstein CJ. Regulation of platelet granule exocytosis by S-nitrosylation. Proc Natl Acad Sci U S A. 2005;102:3782–3787. doi: 10.1073/pnas.0408310102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morrell CN, Sun H, Ikeda M, Beique JC, Swaim AM, Mason E, Martin TV, Thompson LE, Gozen O, Ampagoomian D, Sprengel R, Rothstein J, Faraday N, Huganir R, Lowenstein CJ. Glutamate mediates platelet activation through the AMPA receptor. J Exp Med. 2008 doi: 10.1084/jem.20071474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swaim AF, Field DJ, Fox-Talbot K, Baldwin WM, 3rd, Morrell CN. Platelets contribute to allograft rejection through glutamate receptor signaling. J Immunol. 2010;185:6999–7006. doi: 10.4049/jimmunol.1000929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karl S, Wong RP, St Pierre TG, Davis TM. A comparative study of a flow-cytometry-based assessment of in vitro Plasmodium falciparum drug sensitivity. Malar J. 2009;8:294. doi: 10.1186/1475-2875-8-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Semple JW, Milev Y, Cosgrave D, Mody M, Hornstein A, Blanchette V, Freedman J. Differences in serum cytokine levels in acute and chronic autoimmune thrombocytopenic purpura: relationship to platelet phenotype and antiplatelet T-cell reactivity. Blood. 1996;87:4245–4254. [PubMed] [Google Scholar]
- 36.Boshkov LK, Kelton JG, Halloran PF. HLA-DR expression by platelets in acute idiopathic thrombocytopenic purpura. Br J Haematol. 1992;81:552–557. doi: 10.1111/j.1365-2141.1992.tb02991.x. [DOI] [PubMed] [Google Scholar]
- 37.Inwald DP, McDowall A, Peters MJ, Callard RE, Klein NJ. CD40 is constitutively expressed on platelets and provides a novel mechanism for platelet activation. Circ Res. 2003;92:1041–1048. doi: 10.1161/01.RES.0000070111.98158.6C. [DOI] [PubMed] [Google Scholar]
- 38.Lee TH, Stromberg RR, Henrard D, Busch MP. Effect of platelet-associated virus on assays of HIV-1 in plasma. Science. 1993;262:1585–1586. doi: 10.1126/science.8248811. [DOI] [PubMed] [Google Scholar]
- 39.Youssefian T, Drouin A, Masse JM, Guichard J, Cramer EM. Host defense role of platelets: engulfment of HIV and Staphylococcus aureus occurs in a specific subcellular compartment and is enhanced by platelet activation. Blood. 2002;99:4021–4029. doi: 10.1182/blood-2001-12-0191. [DOI] [PubMed] [Google Scholar]
- 40.Wiwanitkit V. Platelet CD61 might have an important role in causing hemorrhagic complication in dengue infection. Clin Appl Thromb Hemost. 2005;11:112. doi: 10.1177/107602960501100116. [DOI] [PubMed] [Google Scholar]
- 41.Fajardo LF. Letter: Malarial parasites in mammalian platelets. Nature. 1973;243:298–299. doi: 10.1038/243298a0. [DOI] [PubMed] [Google Scholar]
- 42.Perkash A, Kelly NI, Fajardo LF. Enhanced parasitization of platelets by Plasmodium berghei yoelii. Trans R Soc Trop Med Hyg. 1984;78:451–455. doi: 10.1016/0035-9203(84)90058-0. [DOI] [PubMed] [Google Scholar]
- 43.Lewis JC, Maldonado JE, Mann KG. Phagocytosis in human platelets: localization of acid phosphatase-positive phagosomes following latex uptake. Blood. 1976;47:833–840. [PubMed] [Google Scholar]
- 44.Male R, Vannier WE, Baldeschwieler JD. Phagocytosis of liposomes by human platelets. Proc Natl Acad Sci U S A. 1992;89:9191–9195. doi: 10.1073/pnas.89.19.9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ostrowska H, Ostrowska JK, Worowski K, Radziwon P. Human platelet 20S proteasome: inhibition of its chymotrypsin-like activity and identification of the proteasome activator PA28. A preliminary report. Platelets. 2003;14:151–157. doi: 10.1080/0953710031000092802. [DOI] [PubMed] [Google Scholar]
- 46.Ostrowska H, Wojcik C, Omura S, Worowski K. Lactacystin, a specific inhibitor of the proteasome, inhibits human platelet lysosomal cathepsin A-like enzyme. Biochem Biophys Res Commun. 1997;234:729–732. doi: 10.1006/bbrc.1997.6434. [DOI] [PubMed] [Google Scholar]
- 47.Bessler H, Agam G, Djaldetti M. Increased protein synthesis by human platelets during phagocytosis of latex particles in vitro. Thromb Haemost. 1976;35:350–357. [PubMed] [Google Scholar]
- 48.Renia L, Potter SM, Mauduit M, Rosa DS, Kayibanda M, Deschemin JC, Snounou G, Gruner AC. Pathogenic T cells in cerebral malaria. Int J Parasitol. 2006;36:547–554. doi: 10.1016/j.ijpara.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 49.Hafalla JC, Cockburn IA, Zavala F. Protective and pathogenic roles of CD8+ T cells during malaria infection. Parasite Immunol. 2006;28:15–24. doi: 10.1111/j.1365-3024.2006.00777.x. [DOI] [PubMed] [Google Scholar]
- 50.van der Heyde HC, Gramaglia I, Sun G, Woods C. Platelet depletion by anti-CD41 (alphaIIb) mAb injection early but not late in the course of disease protects against Plasmodium berghei pathogenesis by altering the levels of pathogenic cytokines. Blood. 2005;105:1956–1963. doi: 10.1182/blood-2004-06-2206. [DOI] [PubMed] [Google Scholar]
- 51.Miyakoda M, Kimura D, Yuda M, Chinzei Y, Shibata Y, Honma K, Yui K. Malaria-specific and nonspecific activation of CD8+ T cells during blood stage of Plasmodium berghei infection. J Immunol. 2008;181:1420–1428. doi: 10.4049/jimmunol.181.2.1420. [DOI] [PubMed] [Google Scholar]
- 52.Murphy G, Tjoa B, Ragde H, Kenny G, Boynton A. Phase I clinical trial: T-cell therapy for prostate cancer using autologous dendritic cells pulsed with HLA-A0201-specific peptides from prostate-specific membrane antigen. Prostate. 1996;29:371–380. doi: 10.1002/(SICI)1097-0045(199612)29:6<371::AID-PROS5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 53.Kundu SK, Engleman E, Benike C, Shapero MH, Dupuis M, van Schooten WC, Eibl M, Merigan TC. A pilot clinical trial of HIV antigen-pulsed allogeneic and autologous dendritic cell therapy in HIV-infected patients. AIDS Res Hum Retroviruses. 1998;14:551–560. doi: 10.1089/aid.1998.14.551. [DOI] [PubMed] [Google Scholar]
- 54.Kraemer BF, Borst O, Gehring EM, Schoenberger T, Urban B, Ninci E, Seizer P, Schmidt C, Bigalke B, Koch M, Martinovic I, Daub K, Merz T, Schwanitz L, Stellos K, Fiesel F, Schaller M, Lang F, Gawaz M, Lindemann S. PI3 kinase-dependent stimulation of platelet migration by stromal cell-derived factor 1 (SDF-1) J Mol Med (Berl) 2010;88:1277–1288. doi: 10.1007/s00109-010-0680-8. [DOI] [PubMed] [Google Scholar]
- 55.Diacovo TG, Puri KD, Warnock RA, Springer TA, von Andrian UH. Platelet-mediated lymphocyte delivery to high endothelial venules. Science. 1996;273:252–255. doi: 10.1126/science.273.5272.252. [DOI] [PubMed] [Google Scholar]
- 56.Semple JW, Italiano JE, Jr, Freedman J. Platelets and the immune continuum. Nat Rev Immunol. 2011;11:264–274. doi: 10.1038/nri2956. [DOI] [PubMed] [Google Scholar]
- 57.Denis MM, Tolley ND, Bunting M, Schwertz H, Jiang H, Lindemann S, Yost CC, Rubner FJ, Albertine KH, Swoboda KJ, Fratto CM, Tolley E, Kraiss LW, McIntyre TM, Zimmerman GA, Weyrich AS. Escaping the nuclear confines: signal-dependent pre-mRNA splicing in anucleate platelets. Cell. 2005;122:379–391. doi: 10.1016/j.cell.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Claser C, Malleret B, Gun SY, Wong AY, Chang ZW, Teo P, See PC, Howland SW, Ginhoux F, Renia L. CD8+ T cells and IFN-gamma mediate the time-dependent accumulation of infected red blood cells in deep organs during experimental cerebral malaria. PLoS One. 2011;6 doi: 10.1371/journal.pone.0018720. e18720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nitcheu J, Bonduelle O, Combadiere C, Tefit M, Seilhean D, Mazier D, Combadiere B. Perforin-dependent brain-infiltrating cytotoxic CD8+ T lymphocytes mediate experimental cerebral malaria pathogenesis. J Immunol. 2003;170:2221–2228. doi: 10.4049/jimmunol.170.4.2221. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.