Abstract
Objective
To evaluate the immediate and longer-term effectiveness of Mindfulness-based Stress Reduction (MBSR) among treatment adherents on CD4+ T Lymphocyte Count (CD4 count) and medical and psychological symptoms among HIV+ patients in Tehran, Iran.
Methods
Using a randomized controlled trial design, data were analyzed from 173 HIV+ patients (CD4 Count > 250) not yet receiving antiretroviral therapy who participated in either an 8-week MBSR (n=87) or a brief education and support condition (ESC) (n=86) at the Imam Khomeini Hospital. Assessments included CD4 count, Symptom Checklist-90-Revised (SCL-90R), and Medical Symptom Checklist (MSCL) at baseline, immediate post-test, and 3, 6, 9 and 12-month follow-up periods.
Results
The treatment adherent sample had a mean age of 35.1(SD = 6.5) years and 69% were male. Linear mixed model estimates indicated mean CD4 count increased from baseline up to 9 months post-treatment, then returned to baseline level at 12 months. Improvements in mean SCL-90R (up to 6 months) and MSCL (up to 12 months) scores were observed for the MBSR condition while ESC scores remained the same over time; however, only MSCL improvements significantly differed between groups and these changes lasted up to the final assessment.
Conclusions
Findings suggest that among treatment adherent Iranian HIV+ patients not yet receiving antiretroviral drug treatment, MBSR appears to have the strongest potential to improve self-reported physical symptomatology.
Trial Registration
Iranian Registry of Clinical Trials IRCT201106084076N2.
Keywords: mindfulness-based, meditation, HIV/AIDS, CD4 count, mind-body, psychoneuroimmunology, medical symptoms
Introduction
HIV/AIDS remains a major public health challenge throughout the world. One main area of research has been the influence of psychological stressors on HIV/AIDS pathogenesis given the wide variation that exists in the rate of progression through the successive phases of HIV infection, including AIDS and AIDS-related morbidity and mortality. This may be at least partially attributable to the fact that patients who are HIV-positive (HIV+) encounter and respond differently to various stressors such as disease diagnosis and progression, disclosing HIV status to social networks, stigmatization, bereavement, loss of employment, discomfort and pain, loss of perceived control over health, and deteriorating health as well as other events (1–4), which can further suppress the immune system and its defense against HIV/AIDS progression (4,5).
Exposure to chronic and severe psychological stress is implicated both directly and indirectly in HIV/AIDS disease progression. For example, stress may be directly associated with endocrine response systems, specifically alterations in the hypothalamic-pituitary-adrenocortical axis (HPA) and sympathetic-adrenal-medullary (SAM) immune mechanisms, which are associated with diminished antiviral defenses and increased HIV replication (6–10). Moreover, stress-related factors such as depression can also function indirectly on disease progression via non-adherence to highly active antiretroviral therapy (ART) as well as unhealthy behavioral reactions including substance use and risky sex (6,8,11). Therefore, psychological stress and its related ailments are pertinent factors to consider in the maintenance of health among people living with HIV.
Findings from a literature review of longitudinal studies with follow-up periods ranging from 6 months to 9 years have suggested that stressful life events and trauma can negatively affect HIV progression (8). Some of the most compelling evidence noted in this review came from a study with the longest follow-up to date, which observed HIV-infected gay men every 6 months for up to 9 years (12). Findings from this study showed that the risk of AIDS diagnosis increased by 75% and the risk of a clinical AIDS condition tripled for every cumulative average increase of one severe stressor. Similarly, several other longitudinal studies have reported that the risk of AIDS is doubled for every cumulative average increase of one severe stressor (13–15). This relationship between psychological stress and HIV disease status is often accompanied by changes in specific HIV/AIDS-related immune markers.
CD4+ T lymphocyte count (CD4 count) is a main biomarker of HIV disease progression to AIDS, and has been used in the majority of research pertaining to the psychoneuroimmunology of HIV/AIDS (11). In a series of studies with various follow-up points (i.e., 5.5, 7.5 and 9 years), Leserman and colleagues reported that higher average cumulative stressful events were predictive of faster degradation in CD4 count to less than 200, the clinical cut-off for AIDS (12–14). Other studies have also reported severe stress to be associated with a greater decline in CD4 count (15), although not all studies have noted a significant association between stress and CD4 count (see review (9)). Accumulated evidence obtained from literature reviews shows that various hormones released by the adrenal glands during periods of stress such as cortisol and catecholeamines modulate effects of stress on CD4 count (16,17), suggesting the biological mechanisms linking stress and immune competence.
Attempts to reduce stress among people with HIV via cognitive-behavioral, relaxation, and other stress management interventions have been shown to improve perceived stress, cortisol level, and CD4 count (18–22). More recently, attention has been given to the influence of a mind-body stress management program called mindfulness-based stress reduction (MBSR) among people with HIV. MBSR is an 8-week intensive training in mindfulness meditation practices (i.e., sitting meditation, body scan, and light yoga) used to transform people’s relationship with their inner experience of psychological stress, including reactions to thoughts, difficult emotions, body sensations, and relationships with others, and thus reduce behavioral and physiological stress reactivity. Differing from other cognitive modalities, mindfulness practice is a metacognitive method of objectively and non-judgmentally observing the process of mental content rather than examining and explaining the content itself as is the case of conventional cognitive-based therapies (23).
Persuasive arguments for incorporating mindfulness meditation into treatment programs for individuals with HIV/AIDS have been presented (24,25). These arguments suggest that mindfulness meditation is an ideal complement to traditional treatment programs for HIV/AIDS given that these patients are subjected to a broad range of stressors across psychological, physical, social-cultural, and economic domains. Preliminary evidence garnered from studies examining the influence of MBSR on health maintenance among people with HIV is promising. For example, uncontrolled pilot studies have suggested that mindfulness training can improve psychological wellbeing and self-esteem among people with HIV (26,27). Moreover, the only uncontrolled pilot study conducted among HIV patients in Iran showed MBSR was associated with increased CD4 count from pretest to post-test (28). This study was limited by pre-post assessments and a small sample size (N=10). To date, only two controlled trials we know of have examined the impact of MBSR on the health of people with HIV. The first study used a non-randomized pre-post assessment design with a control condition, and found that MBSR participants displayed significant enhancement in immune competence (i.e., increased natural killer (NK) cell level and activity) compared to control participants who displayed an overall decrease in NK level and activity (29). Limitations to this study included pre-post assessment only and a high attrition rate (48%). The second study was a RCT that allocated HIV patients to either MBSR or a one-day stress reduction seminar (30). Results showed that those in the control seminar showed declines in CD4 count whereas CD4 count among MBSR participants remained stable from pretest to post-test. Treatment adherence to the mindfulness meditation program, as measured by class attendance, mediated the effects of mindfulness meditation training on CD4 count decline. Again this research was limited to pre-post assessment. No controlled trial to date has evaluated the longer-term effects of MBSR on health indices among people with HIV.
The Present Study
Considering the link between psychological stressors and HIV/AIDS progression, it is important to evaluate interventions that can reduce psychological stress and its harmful effects using experimental research trials with follow-up periods beyond a post-test assessment period. Therefore, the present study advances a new yet burgeoning area of research pertaining to the impact of mindfulness meditation practices and their associated improvements in biological and symptomology indices among people with HIV. MBSR has specific implications for treating HIV+ patients due to its ability to reduce psychological stress and its associated psychological sequelae ( see meta-analysis (31)), and therefore, perhaps reduce the impact of these factors on immune mechanisms. This appears to be the first study reporting the efficacy of MBSR among HIV+ patients living in Iran using RCT methodology. This RCT is also the first we know of to assess the longer-term effects of MBSR among people with HIV. Evaluation of MBSR (i.e., a group-based stress reduction program) is especially needed in Iran because people with HIV in this region face stigmatization resulting from socio-cultural norms (32), which further adds to the gamut of stressors associated with HIV diagnosis. To address the need for rigorous experimental research examining the biological and symptomological effects of MBSR over time, the present study hypothesized that MBSR would induce improvements in objective biomarkers of immune competence (CD4 count) as well as self-reported physical and mental health symptomatology compared to a brief control condition inclusive of education and social support.
Methods
Participants and Procedures
The study was conducted from August 2008 to March 2010. The Tehran University of Medical Sciences (TUMS) Institutional Review Board (IRB) approved all study procedures. Patients at the Imam Khomeini Hospital received initial counselling and testing at the Voluntary Counselling and Testing (VCT) centre. Patients diagnosed HIV+ were referred by the VCT to the Positive Club program, which provides free educational, counselling, and recreational services. Patients in the VCT and Positive Club programs were eligible to participate in the study if they were 18+ years and HIV+ confirmed by Western Blot. Table 1 presents the frequencies for education level and HIV transmission route for the total population of patients attending the Positive Club program at the time of the study. Based on medical record examination and/or psychiatrist interview guided by the DSM-IV clinical assessment, patients were excluded if they had current substance addiction, current psychosis, history of post-traumatic stress disorder, or if they had CD4 count under 250 (i.e., laboratory criteria for delivering ART to AIDS patients in Iran) or were clinically symptomatic (e.g., herpes zoster, oral candidiasis, shingles, significant weight loss).
Table 1.
Education and HIV transmission characteristics of the overall patient population attending the Positive Club program
| Variable | N | % |
|---|---|---|
| Education Level | ||
| Elementary | 65 | 17.3 |
| Middle School | 157 | 41.9 |
| High School | 113 | 30.1 |
| University | 30 | 8.0 |
| Unknown | 10 | 2.7 |
| HIV Transmission Routes | ||
| Males | ||
| Injection drug use | 273 | 92.9 |
| Sexual Contact | 10 | 3.4 |
| Blood transfusion | 6 | 2.0 |
| Mother to child | 3 | 1.0 |
| Unknown | 2 | 0.7 |
| Females | ||
| Sexual Contact | 71 | 87.6 |
| Injection drug use | 5 | 6.2 |
| Blood transfusion | 5 | 6.2 |
| Mother to child | 0 | 0 |
Notes. Values are for clinic-level data from which the sample was recruited in order to provide a context for the study as education level and transmission route data were not collected in our sample
No participants were on ART during the study interval because no CD4 count fell under 250. Participants were recruited via pamphlets about the study, which were posted in the Imam Khomeini Hospital. The MBSR instructor held informational sessions for patients showing interest in the program and assessed if the patient met the above mentioned criteria for study inclusion. Eligible patients provided written informed consent. All assessments and sessions were held at the Imam Khomeini Hospital in Tehran, Iran. Due to the intensive amount of time and effort required in MBSR, participants received $50 compensation in the MBSR condition at immediate post-test. A total of 245 HIV+ patients were enrolled in the study and then randomly assigned to condition. Participants were blinded to their treatment assignment. Randomization was conducted by assigning each name a unique number and each treatment condition a unique letter. A staff member blinded to condition mixed the participant numbers and assigned them into each condition in a 1:1 ratio. Research staff who administered the follow-up assessments were not blinded to treatment condition.
Treatment
Mindfulness Based Stress Reduction (MBSR)
MBSR is an eight-week group-based course in mindfulness meditation, which was informed by the original MBSR manual (33). The major program components of MBSR have been previously described in considerable detail (33,34), as have some of the more general features of mindfulness meditation practice and its applications in everyday life (35). MBSR is implemented in a class setting, in which sitting meditation, gentle mindful hatha yoga, a body scan meditation, and other approaches to cultivate mindfulness in everyday living are introduced. A psychologist who was formally trained in the MBSR program at the University of Massachusetts Center for Mindfulness in Medicine, Health Care, and Society delivered the classes. In brief, session 1 and 2 consisted of a mindfulness body scan practice. Session 3 and 4 consisted of awareness of body postures via light Hatha yoga practices. Sessions 5 through consisted of sitting mindfulness meditation. Session 8 consisted of a retreat lasting 6–7 hours, which incorporated all the practices learned in sessions 1 through 7. During the final session 8, a general discussion also took place regarding encountering stress and how to apply MBSR techniques in daily life.
Education and Support (ESC)
Participants in ESC received their usual clinical attention and also met twice in small groups for a total of 2 hours during the MBSR intervention period. ESC was provided with educational information and pamphlets about living healthy with HIV/AIDS. Education content focused on the following topics: 1) routes of HIV transmission, 2) HIV prevention, 3) facts and expectations about HIV drug therapy, and 4) maintaining positive behavior and cognitions while living with HIV. A psychologist was also available for the ESC members during the intervention period to provide individual counselling. Therefore, ESC contained education, group support, and mental health support dimensions. Participants completed self-report questionnaires at pretest, immediate posttest, and 3, 6, 9 and 12 month post-intervention follow-up periods.
Flow of Participants in RCT
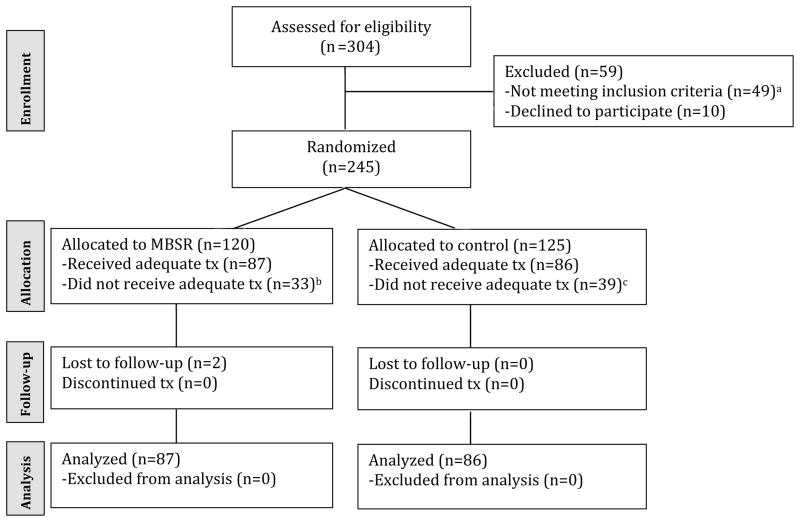
Figure 1 presents the flow of participants through each stage of the trial. A total of 304 HIV+ patients at the hospital completed an initial eligibility assessment. Of those assessed for eligibility, 59 patients were excluded from the study due to exclusion criteria (n=49) or declining to participate (n=10). The remaining 245 eligible participants were randomized to either MBSR (n=120) or ESC (n=125). Thirty-three participants in MBSR and 39 participants in ESC missed two or more program sessions. Of the 173 participants eligible for inclusion in the analysis, 171 (98.8%) completed all follow-up assessments (85 in MBSR; 86 in ESC).
Figure 1.
Flow of participants through study
Notes. aexcluded due to post-traumatic stress disorder (n=9), active substance addiction (n=14), comorbid post-traumatic stress disorder and active substance addiction (n=6), lack of time (n=9), and CD4 < 250 (n=11);bmissed >2 treatment sessions due to lack of time (n=25), and family commitments (n=8); cmissed single treatment session due to lack of time (n=27), family commitments (n=7), and illness (n=5)
Measures
CD4+ T Lymphocyte Count is a hallmark measure reflecting the status of immune competence and provides an indicator of disease progression from HIV to AIDS (36). Peripheral blood CD4+ T lymphocyte levels were assessed at baseline and all post-treatment follow-up assessments immediately following the self-report measures. CD4 count was determined by flow cytometry and complete blood count by a licensed clinical laboratory at the Imam Khomeini Hospital. Lower CD4 count corresponds to greater progression of disease status within the HIV/AIDS spectrum.
The Medical Symptom Checklist (MSCL (37)) is a 115-item self-report measure of physical symptomatology that quantifies the number of common medical symptoms occurring in the previous month. Respondents are asked to indicate whether they suffer from specific medical symptoms, and if so, to report the frequency of these symptoms. Responses are given on an 8-point scale ranging from never or almost never (0) to more than once a day (7). Responses are summed and higher scores indicated more medical symptoms. The English version of the MSCL was translated into Farsi by our research team; the translation was then confirmed by the epidemiology research group at TUMS. The general MSCL as well as MSCL Category B symptoms were assessed. Many of the items in category B are related to HIV pathogenesis (e.g., bacillary angiomatosis, thrush, vulvovaginal candidiasis, pelvic inflammatory disease, cervical dysplasia, hairy leukoplakia, oral herpes zoster, idiopathic thrombocytopenic purpura, constitutional symptoms, and peripheral neuropathy). MSCL Category C (AIDS-Indicator Conditions) was not assessed because participants did not have AIDS during the course of the study. Although the reliability and validity of the MSCL have not been published, the scale is highly used and several studies of MBSR have shown significant reductions in the MSCL associated with participation in the program (38–40).
The Symptom Checklist-90-Revised (SCL-90R (41)) includes a 90-item self-report of mental health measured on a 5-point rating scale to assess a broad range of psychological problems and symptoms of psychopathology including dimensions of somatization, obsessive-compulsive, interpersonal sensitivity, depression, anxiety, hostility, phobic anxiety, paranoid ideation, and psychoticism. The checklist is useful for measuring patient progress and treatment outcomes in clinical settings. Respondents are asked to indicate current psychological symptom status with reference to the past 7 days. Scores were summed and higher scores indicate more psychological symptoms. The English version of the SCL and was translated into Farsi by our research team; the translation was then confirmed by the epidemiology research group at TUMS. The SCL-90R has evidence for validity and reliability in samples from various countries and the measure is commonly used in medical research around the world (42–44).
Data Analyses
Descriptive statistics and differences between treatment conditions on all baseline variables were generated in SPSS version 20. Two-tailed statistical tests were used with significance criteria of p < .05. Treatment (MBSR vs. ESC), time (pretest, post-test, and 3, 6, 9, 12 month follow-up), and treatment by time effects among treatment adherent participants (i.e., must attend 6 or more MBSR sessions or 2 ESC sessions) were analyzed using a linear mixed-effects model (MIXED) procedure that included all available data from participants, adjusted for age and gender. Our criteria for treatment adherence to MBSR (i.e., attend 75% of sessions) has been reported on previously (45). Model fit statistics including −2 log likelihood (−2LL) and Akaike’s Information Criterion (AIC) are provided. A −2LL ratio test indicated that a curvilinear model did not fit the data significantly better than a linear model for all outcomes. CD4 count, SCL-90R, and MSCL outcome variables were modelled separately. Within-group paired sample t-tests were used to examine observed mean differences between scores on outcome variables between pre-test and each subsequent follow-up period using equation eight from (46) to account for dependence among means. Cohen’s d was calculated to determine differences in effect sizes between treatment groups at each time point.
Results
Demographics
Table 1 presents frequencies for education and HIV transmission characteristics for the total Positive Club program (these data were not collected from participants in our sample). The vast majority of participants were educated at the high school level or below (89.3%), and the main source of HIV transmission was injection drug use for males (92.9%) and sexual contact (87.6%) for females. Table 2 presents the baseline characteristics of the participants in each treatment condition. Mean scores for all demographic covariates and outcome variables were equivalent except for CD4 count. Mean CD4 count was significantly lower in the MBSR condition (CD4 count = 479.1) than in the control condition (CD4 count = 579.7, p<.001).
Table 2.
Baseline characteristics of analysis sample by treatment condition, N = 173
| Variable | MBSR (n=85) | ESC (n=86) | Total (N=171) | pa |
|---|---|---|---|---|
| Age mean (SD) | 34.7 (6.1) | 35.6 (6.9) | 35.1 (6.5) | .45 |
| Gender N (%) | .25 | |||
| Male | 55 (64.7%) | 63 (73.3%) | 118 (69.0%) | |
| Female | 30 (35.3%) | 23 (26.7%) | 53 (31.0%) | |
| CD4 mean (SD) | 479.1 (180.9) | 579.7 (188.7) | 529.65 (191.1) | <.001 |
| MSCL mean (SD) | 24.9 (16.8) | 25.5 (14.6) | 25.2 (15.7) | .80 |
| SCL-90R mean (SD) | 109.3 (64.8) | 109.2 (59.2) | 109.3 (61.8) | .95 |
Notes.
T-test or chi-square test for significant differences between conditions; SCL-90R = Symptom Checklist-90-Revised; MSCL = Medical Symptom Checklist; CD4 = CD4+ T Lymphocyte Count
Effect of Treatment on CD4 Count
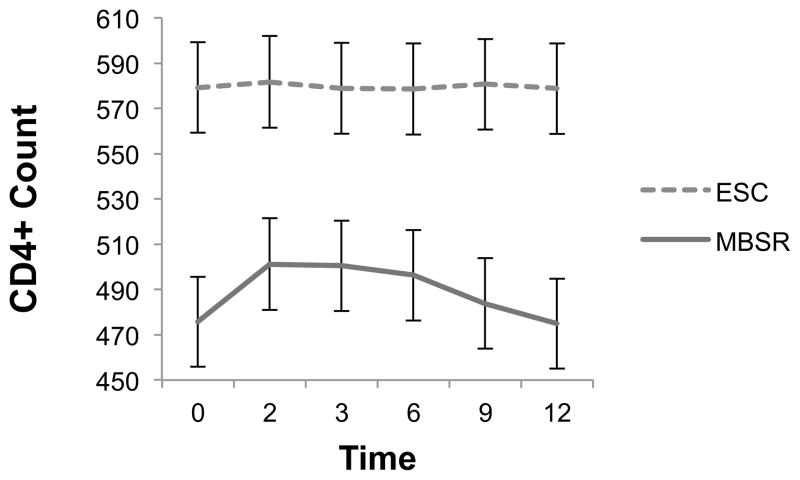
Figure 2 presents the estimated marginal means with corresponding standard errors for CD4 count by treatment condition across time. The covariate adjusted linear mixed-effects model showed significant treatment, F(1, 169.05) = 10.35, p = .002; time, F(5, 169.02) = 7.58, p < .001; and treatment by time, F(5, 169.02) = 6.72, p < .001 effects (model fit: −2LL = 9932.77; AIC = 9974.77). The trajectory of CD4 count in the MBSR condition appears curvilinear, indicating an immediate post-MBSR improvement in CD4 count lasting until 9-month follow-up, which then declined back to baseline level at 12-month follow-up. Cohen’s d indicated effect sizes for between group differences were of medium size up to 6-month (d range = .56–.66) and of small size up to 9-month follow-up (d = .26; see Table 3).
Figure 2.
Estimated marginal means(SE) for CD4+ count across time by treatment condition
Notes. SE = standard error; Time 0 = baseline, 2 = immediate posttest, 3 = 3 months post-intervention, 6 = 6 months post-intervention, 9 = 9 months post-intervention, 12 = 12 months
Table 3.
Observed mean scores and effect sizes for outcome measures over time by treatment condition, N=171–173
| Time/TX Condition | CD4 Count | SCL-90R | MSCL | ||||||
|---|---|---|---|---|---|---|---|---|---|
| M (SD) | a M Δ | d | M (SD) | a M Δ | d | M (SD) | a M Δ | d | |
| Pre-test | |||||||||
| MBSR | 479.05 (180.88) | 109.32 (64.81) | 24.92 (16.75) | ||||||
| ESC | 579.67 (188.67) | 109.23 (59.16) | 25.58 (14.49) | ||||||
| Post-test | |||||||||
| MBSR | 504.48 (186.45) | +25.44*** | .62 | 97.46 (60.68) | −11.86*** | −1.34 | 18.49 (13.85) | −6.42*** | −1.26 |
| ESC | 582.20 (189.72) | +2.52 | .08 | 109.38 (59.12) | +0.15 | <.01 | 25.73 (14.59) | +0.15 | .07 |
| Diff. in d | .54 | 1.34 | 1.33 | ||||||
| 3-month | |||||||||
| MBSR | 503.71 (182.07) | +24.66*** | .65 | 101.74 (60.84) | −7.58*** | −.85 | 18.91 (13.82) | −6.01*** | −1.16 |
| ESC | 579.33 (188.57) | −0.35 | −.01 | 109.44 (60.14) | +0.21 | .02 | 25.69 (14.61) | +0.11 | .09 |
| Diff. in d | .66 | .87 | 1.25 | ||||||
| 6-month | |||||||||
| MBSR | 499.56 (183.47) | +20.52*** | .54 | 106.32 (61.77) | −3.00** | −.35 | 18.88 (13.75) | −6.04*** | −1.15 |
| ESC | 579.00 (188.64) | −0.67 | −.02 | 109.70 (60.76) | +0.47 | .02 | 25.72 (14.63) | +0.14 | .06 |
| Diff. in d | .56 | .37 | 1.21 | ||||||
| 9-month | |||||||||
| MBSR | 487.09 (183.62) | +8.05* | .29 | 110.68 (62.46) | +1.36 | .16 | 19.54 (14.21) | −5.38*** | −1.10 |
| ESC | 581.10 (187.12) | +1.43 | .03 | 110.10 (59.71) | +0.87 | .04 | 25.73 (14.63) | −0.15 | −.02 |
| Diff. in d | .26 | .12 | 1.08 | ||||||
| 12-month | |||||||||
| MBSR | 478.25 (182.93) | −0.80 | −.10 | 116.11 (64.13) | +6.79*** | .79 | 19.82 (14.37) | −5.09*** | −1.09 |
| ESC | 579.21 (186.79) | −0.47 | −.02 | 110.62 (60.15) | +1.39 | .05 | 25.62 (14.58) | −0.04 | −.01 |
| Diff. in d | .08 | .74 | 1.08 |
Notes. d = Cohen’s d; MBSR = mindfulness-based stress reduction; ESC = education and support condition;
p < 0.001;
p < 0.01;
p < 0.05;
= difference between pre-test and follow-up period;
Paired sample t-test for differences between pretest mean score and each respective follow-up mean score;
Difference in d between treatment conditions
Effect of Treatment on SCL-90R
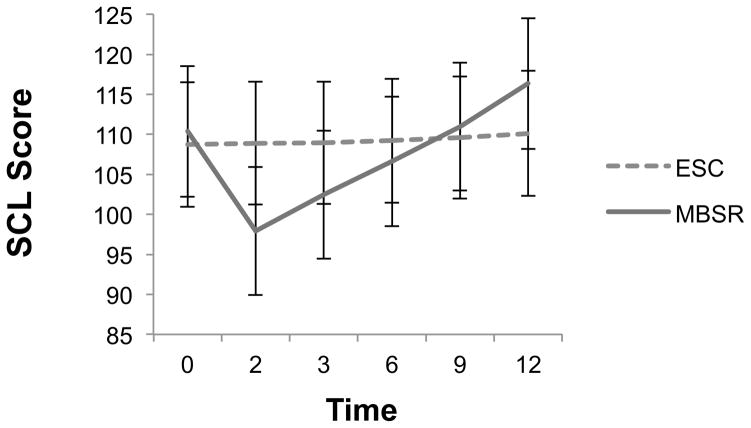
Figure 3 presents estimated marginal means with corresponding standard errors for SCL-90R scores by treatment condition across time. The covariate adjusted linear mixed-effects model showed non-significant treatment, F(1, 169.41) = .04, p = .84; significant time, F(5, 168.17) = 40.31, p < .001; and significant treatment by time, F(5, 168.17) = 33.75, p < .001 effects(model fit: −2LL = 8378.70; AIC = 8420.70). Table 3 indicates that SCL-90R scores were significantly lower (indicating less psychological symptoms) up to 6-month follow-up as compared to pre-test levels. In ESC, results indicated no changes in SCL-90R count between pre-test and any other follow-up period. Cohen’s d indicated effect sizes for between group differences were of large size up to 3-month (d range = .87–1.34) and of small size up to 6-month follow-up (d = .37; see Table 3). After 6 months a rebound effect is evident wherein MBSR compared to ESC participants gradually reported more psychological symptoms persisting up to 12-month follow-up.
Figure 3.
Estimated marginal means(SE) for SCL scores across time by treatment condition.
Notes. SE = standard error; Time 0 = baseline, 2 = immediate posttest, 3 = 3 months post-intervention, 6 = 6 months post-intervention, 9 = 9 months post-intervention, 12 = 12 months
Effect of Treatment on MSCL
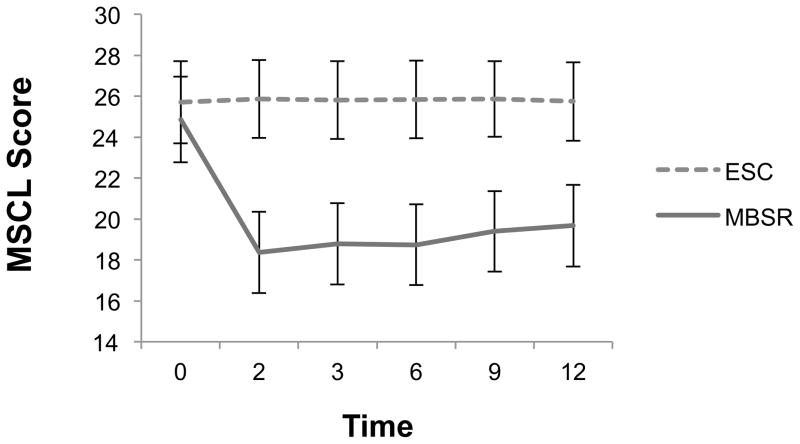
Figure 4 presents estimated marginal mean MSCL scores by treatment condition across time. The covariate adjusted linear mixed-effects model showed significant treatment, F(1, 169.69) = 7.30, p = .008; time, F(5, 169.81) = 22.40, p < .001; and treatment by time effects, F(5, 169.82) = 24.19, p < .001 (model fit: −2LL = 5079.95; AIC = 5121.95). In the MBSR arm, results from paired sample t-tests between pre-test and each subsequent follow-up period indicated that mean MSCL scores were significantly lower (indicating improved physical symptomatology) up to 12-month follow-up as compared to pre-test. Cohen’s d indicated that effect size differences between treatments groups were of large size for the entire duration of follow-up (d range = 1.08–1.33), with improvements consistently favouring MBSR.
Figure 4.
Estimated marginal means(SE) for MSCL scores across time by treatment condition
Notes. SE = standard error; Time 0 = baseline, 2 = immediate posttest, 3 = 3 months post-intervention, 6 = 6 months post-intervention, 9 = 9 months post-intervention, 12 = 12 months
Discussion
This study appears to be the first RCT to examine the impact of MBSR on immune competence and medical symptomatology among people with HIV living in Iran. Furthermore, this is the first trial in this field of study to evaluate MBSR using multiple follow-up assessments. Our findings suggest that HIV+ patients who engage in MBSR training in a clinic-based setting show signs of improvement in biological indices of immune competence (i.e., CD4 count) and self-reported indices of physical and mental health symptomatology (MSCL; SCL-90R) relative to a brief education and support control. These findings indicate that in a region such as Iran where the stress of HIV diagnosis may be multiplied due to the stigma resulting from socio-cultural norms (32), MBSR may be one adjunctive treatment to help this population manage stressors and related disease pathology/symptomatology more effectively.
CD4 depletion is a hallmark feature of HIV pathogenesis and progression to AIDS. Although the trajectory of CD4 in the MBSR condition indicated improvement in our study, the difference in CD4 count between treatments over time requires careful interpretation given that randomization failed to equalize CD4 at baseline. This failure of randomization is most likely due to chance given that all other outcome measures were successfully randomized between groups. Therefore, we believe a close examination of the linear trajectories of each group provides better insight into the observed program effects on CD4. For example, our results showed CD4 remained constant in ESC (flat trajectory) while CD4 count in MBSR showed an evident increase in CD4 that lasted to 6 months post-MBSR and then gradually declined back to baseline by 12 months post-MBSR (curvilinear trajectory). Therefore, a main challenge to the validity of these findings--regression to the mean--does not appear relevant given a return of CD4 to baseline after an immediate increase post-MBSR. However, research obtaining baseline equivalence between groups is needed to re-test our hypothesis in order to gain a clearer understanding of the impact of MBSR on CD4 count among HIV+ patients.
Contrary to CD4, mental and physical health symptomatology scores were equally assigned between treatment groups. Our strongest and most consistent findings were observed for MSCL. We found participants in the MBSR condition reported immediate and significant reductions in MSCL scores relative to controls and these effects lasted across all assessment periods. Based on our estimated model, MBSR participants as compared to ESC participants reported a mean of >6 fewer symptoms at each follow-up assessment. Thus, our findings indicate MBSR induces lasting improvements in physical health symptomatology among people with HIV. Given that a wide range a HIV symptoms were assessed with the MSCL, it is possible that the observed improvements in immune competence (CD4) induced by MBSR contributed, at least in part, to reductions in physical symptomatology. Possible mechanisms for change in CD4 count include MBSR-induced effects on T-cell redistribution dynamics, T-cell turnover in lymphoid tissue, and haematopoiesis (46). Other researchers have also suggested that mindfulness training may reduce HIV RNA levels (30), which is a suggestion based on evidence showing a strong link between stress and HIV viral replication (48,49) and other studies showing stress management programs can induce reductions in HIV RNA levels(50,51).
Results observed for psychological symptoms (SCL-90R) were less consistent. Although not statistically significant, our findings indicate that SCL-90R scores improved in favor of the MBSR condition up to 6 months post MBSR intervention; however, we observed a rebound effect occurring specifically at the 12-month post intervention assessment period in MSBR. It is unclear why this rebound effect occurred, but it is possible that more difficult psychological states arise when mindful practice progresses. For example, it is commonly discussed among mindfulness teachers that basic mindfulness skills (e.g., attention to the breath and awareness of sensations) are cultivated early in mindfulness training while the processing and acceptance of difficult emotions (i.e., emotion regulation) arises with more advanced training as the mind becomes more finely attuned to its judgments, habits, and aversions. Therefore, it may be worthwhile for future research to explore if psychological symptoms can be better managed with additional guidance from a trained MBSR teacher in order to cultivate the skills required for more advanced stages of meditation practice and personal insight.
Overall, the present study advances a new yet burgeoning area of research pertaining to the impact of mindfulness meditation practices and their influence on health maintenance among people with HIV. Our findings lend empirical evidence to suggest MBSR can be an effective adjunctive therapy to conventional HIV/AIDS treatment (24,25). Of the three outcomes assessed in the present study, MBSR appears most beneficial for addressing physical symptomatology among HIV patients with lasting effects (i.e., at least 12 months), which extends preliminary evidence accumulated from uncontrolled pilot studies (26–28). Our findings also corroborate a very small pool of evidence from controlled trials that suggest MBSR induces improvement in biomarkers of immune competence (29,30). Although our CD4 findings are not conclusive, they extend this previous pool of work that has been limited by small sample size and high attrition by the present study suggesting that MBSR can induce improvements in CD4 count lasting up to 9 months post MBSR. The impact of this area of research would be greatly enhanced if future work targeted HIV patients with CD4 counts just above the threshold for initiating ART and determined the cost-effectiveness of using MBSR to delay the need for ART administration.
There are several limitations to this study. The MSCL and SCL-90R measures were based on self-report, which is a method that can be sensitive to bias, such as social desirability bias and other respondent biases. However, we informed respondents to report accurately and assured them about the confidentiality of their responses. Staff who administered the follow-up assessments were not blinded to treatment condition, and although they were informed to treat all participants equally, there is a risk of eliciting positive response bias. It was unanticipated that our randomization procedures did not distribute CD4 count equally across treatment groups at baseline. Although this might allow for greater improvement in the MBSR group relative to the control group, we don’t believe the trajectory of the MBSR condition was a phenomenon induced by regression to the mean. Rather, the MBSR group showed expected effects by improving post intervention and continually up to 6 months post intervention and then gradually returning to baseline over time. Our brief ESC condition did not match the time and attention of the MBSR condition. Although our condition is more adequate than wait-list alone, the validity of our findings are challenged by lack of adequate control for the intensive MBSR program and the increased amount of time spent with a group in the MBSR condition, which may provide additional social support effects. Future research will benefit from using an educational control group matched for time and attention across the entire 8-week MBSR period. Results from the study are based on treatment adherent participants (i.e., attending 6 or more sessions in an 8-week MBSR program). Lacking data from non-adherent participants limited our ability to conduct intention-to-treat analyses. Finally, participant data on education, sexual orientation, years since HIV diagnosis, and transmission route was not collected, which limits the generalizability of our sample. However, we did provide clinic-level characteristics to indicate from which population the sample was drawn.
Notwithstanding these limitations, the present study offers the most compelling evidence to date regarding the efficacy of MBSR in a clinical context among HIV+ patients given our multiple follow-up periods and low attrition rate. This study informs future research on MBSR and health maintenance among people with HIV/AIDS. First, the progression of HIV to AIDS infection occurs slowly, and therefore studies done over short periods have little opportunity to capture changes in disease status (see (8)). The present study has the longest follow-up design to date (i.e., 12 months) among studies examining MBSR in the context of HIV/AIDS, and as observed in the present study, CD4 count remained relatively high in both treatment conditions (mean CD4 count remained > 478). Thus, one fruitful line of research is to evaluate MBSR using longer follow-up periods to assess CD4 and associated immune measures and to incorporate MBSR booster sessions during follow-up periods. A second opportunity for research is to implement MBSR training among people whose CD4 count is approaching the clinical criteria for AIDS. This type of research would provide clearer evidence as to how MBSR may impact health maintenance at crucial stages of disease progression and how it might be cost-effective by delaying antiretroviral drug therapy.
In conclusion, it remains essential to evaluate interventions that can reduce psychological stress among people with HIV/AIDS in order to maintain their physical and psychological health and to prevent disease progression. This is especially important in regions of the world where stigma from socio-cultural norms add a significant amount of psychological stress to the gamut of stressors faced when diagnosed with HIV. It appears that training in mindfulness via MBSR might have at least some beneficial effects on immune competence and medical symptomatology and these benefits might have the potential to delay the progression from HIV to AIDS, yet much future research is needed to address this notion. Indeed, the formalized MBSR program appears to show promise as an adjunctive therapy in conventional treatment to help address the public health challenges of HIV/AIDS faced throughout the world.
Acknowledgments
Funding: This work was made possible by grant support from the Tehran University of Medical Sciences [86-04-55-6503 to M.M.] and two research training fellowships [2T32CA9492-26 & 5T32MH019925-13 to D.S.B.].
Glossary
- MBSR
Mindfulness-Based Stress Reduction
- ESC
Education and Support Condition
- SCL-90R
Symptom Checklist-90-Revised
- MSCL
Medical Symptom Checklist
- CD4 count
CD4+ T Lymphocyte Count
- HPA
hypothalamic-pituitary-adrenocortical axis
- SAM
sympathetic-adrenal-medullary
- HIV+
HIV-positive
- ART
antiretroviral therapy
References
- 1.Ostrow DG, Joseph JG, Kessler R, Soucy J, Tal M, Eller M, Chmiel J, Phair JP. Disclosure of HIV antibody status: behavioral and mental health correlates. AIDS Education and Prevention. 1989;1(1):1–11. [PubMed] [Google Scholar]
- 2.Jacobsen PB, Perry SW, Hirsch DA, Scavuzzo D, Roberts RB. Psychological reactions of individuals at risk for AIDS during an experimental drug trial. Psychosomatics. 1988;29(2):182. doi: 10.1016/S0033-3182(88)72395-6. [DOI] [PubMed] [Google Scholar]
- 3.Kalichman SC, DiMarco M, Austin J, Luke W, DiFonzo K. Stress, social support, and HIV-status disclosure to family and friends among HIV-positive men and women. Journal of Behavioral Medicine. 2003;26(4):315–332. doi: 10.1023/a:1024252926930. [DOI] [PubMed] [Google Scholar]
- 4.Ironson G, LaPerriere A, Antoni M, O’Hearn P, Schneiderman N, Klimas N, Fletcher MA. Changes in immune and psychological measures as a function of anticipation and reaction to news of HIV-1 antibody status. Psychosomatic Medicine. 1990;52(3):247–70. doi: 10.1097/00006842-199005000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Evans DL, Leserman J, Pedersen CA, Golden RN, Lewis MH, Folds JA, Ozer H. Immune correlates of stress and depression. Psychopharmacology Bulletin. 1989;25(3):319–24. [PubMed] [Google Scholar]
- 6.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298(14):1685–87. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 7.Leserman J, Petitto JM, Perkins DO, Folds JD, Golden RN, Evans DL. Severe stress, depressive symptoms, and changes in lymphocyte subsets in human immunodeficiency virus-infected men. A 2-year follow-up study. Archives of General Psychiatry. 1997;54(3):279–85. doi: 10.1001/archpsyc.1997.01830150105015. [DOI] [PubMed] [Google Scholar]
- 8.Leserman J. HIV disease progression: depression, stress, and possible mechanisms. Biological Psychiatry. 2003;54(3):295–306. doi: 10.1016/s0006-3223(03)00323-8. [DOI] [PubMed] [Google Scholar]
- 9.Leserman J. Role of depression, stress, and trauma in HIV disease progression. Psychosomatic Medicine. 2008;70(5):539–45. doi: 10.1097/PSY.0b013e3181777a5f. [DOI] [PubMed] [Google Scholar]
- 10.Ickovics JR, Hamburger ME, Vlahov D, Schoenbaum EE, Schuman P, Boland RJ, Moore J. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. JAMA. 2001;285(11):1466–74. doi: 10.1001/jama.285.11.1466. [DOI] [PubMed] [Google Scholar]
- 11.Balbin EG, Ironson GH, Solomon GF. Stress and coping: the psychoneuroimmunology of HIV/AIDS. Baillière’s best practice & research. Clinical Endocrinology & Metabolism. 1999;13(4):615–33. doi: 10.1053/beem.1999.0047. [DOI] [PubMed] [Google Scholar]
- 12.Leserman J, Petitto JM, Gu H, Gaynes BN, Barroso J, Golden RN, Perkins DO, Folds JD, Evans DL. Progression to AIDS, a clinical AIDS condition and mortality: psychosocial and physiological predictors. Psychological Medicine. 2002;32:1059–73. doi: 10.1017/s0033291702005949. [DOI] [PubMed] [Google Scholar]
- 13.Leserman J, Petitto JM, Golden RN, Gaynes BN, Gu H, Perkins DO, Silva SG, Folds JD, Evans DL. Impact of stressful life events, depression, social support, coping, and cortisol on progression to AIDS. The American Journal of Psychiatry. 2000;157:1221–8. doi: 10.1176/appi.ajp.157.8.1221. [DOI] [PubMed] [Google Scholar]
- 14.Leserman J, Jackson ED, Petitto JM, Golden RN, Silva SG, Perkins DO, Cal J, Folds JD, Evans DL. Progression to AIDS: the effects of stress, depressive symptoms, and social support. Psychosomatic Medicine. 1999;61:397–406. doi: 10.1097/00006842-199905000-00021. [DOI] [PubMed] [Google Scholar]
- 15.Evans DL, Leserman J, Perkins DO, Stern RA, Murphy C, Zheng B, Gettes D, Longmate JA, Silva SG, van der Horst CM, Hall CD, Folds JD, Golden RN, Petitto JM. Severe life stress as a predictor of early disease progression in HIV infection. The American Journal of Psychiatry. 1997;154:630–4. doi: 10.1176/ajp.154.5.630. [DOI] [PubMed] [Google Scholar]
- 16.Christeff N, Gherbi N, Mammes O, Dalle MT, Gharakhanian S, Lortholary O, Melchior JC, Nunez EA. Serum cortisol and DHEA concentrations during HIV infection. Psychoneuroendocrinology. 1997;22(1):S11–18. doi: 10.1016/s0306-4530(97)00015-2. [DOI] [PubMed] [Google Scholar]
- 17.Cole SW. Psychosocial influences on HIV-1 disease progression: neural, endocrine, and virologic mechanisms. Psychosomatic Medicine. 2008;70(5):562. doi: 10.1097/PSY.0b013e3181773bbd. [DOI] [PubMed] [Google Scholar]
- 18.Antoni MH, Cruess DG, Klimas N, Maher K, Cruess S, Kumar M, Lutgendorf S, Ironson G, Schneiderman N, Fletcher MA. Stress management and immune system reconstitution in symptomatic HIV-infected gay men over time: Effects on transitional naive T cells (CD4+ CD45RA+ CD29+) Am J Psychiatry. 2002;159:143–5. doi: 10.1176/appi.ajp.159.1.143. [DOI] [PubMed] [Google Scholar]
- 19.Antoni MH, Cruess S, Cruess DG, Kumar M, Lutgendorf S, Ironson G, Dettmer E, Williams J, Klimas N, Fletcher MA, Schneiderman N. Cognitive-behavioral stress management reduces distress and 24-hour urinary free cortisol output among symptomatic HIV-infected gay men. Annals of Behavioral Medicine. 2000;22:29–37. doi: 10.1007/BF02895165. [DOI] [PubMed] [Google Scholar]
- 20.Antoni MH, Cruess DG, Klimas N, Carrico AW, Maher K, Cruess S, Lechner SC, Kumar M, Lutgendorf S, Ironson G, Fletcher MA, Schneiderman N. Increases in a marker of immune system reconstitution are predated by decreases in 24-h urinary cortisol output and depressed mood during a 10-week stress management intervention in symptomatic HIV-infected men. Journal of Psychosomatic Research. 2005;58:3–13. doi: 10.1016/j.jpsychores.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Leserman J. The effects of stressful life events, coping, and cortisol on HIV infection. CNS Spectrums. 2003;8(1):25–30. doi: 10.1017/s1092852900023439. [DOI] [PubMed] [Google Scholar]
- 22.McCain NL, Gray DP, Elswick RK, Robins JW, Tuck I, Walter JM, Rausch SM, Ketchum JM. A randomized clinical trial of alternative stress management interventions in persons with HIV infection. Journal of Consulting and Clinical Psychology. 2008;76:431–41. doi: 10.1037/0022-006X.76.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teasdale JD. Metacognition, mindfulness and the modification of mood disorders. Clinical Psychology & Psychotherapy. 1999;6(2):146–55. [Google Scholar]
- 24.Logsdon-Conradsen S. Using mindfulness meditation to promote holistic health in individuals with HIV/AIDS. Cognitive and Behavioral Practice. 2002;9(1):67–72. [Google Scholar]
- 25.Barrows K. The application of mindfulness to HIV. Focus. 2006;21(8):1–8. [PubMed] [Google Scholar]
- 26.Ampunsiriratana A, Triamchaisri S, Nontasorn T, Chuaprapaisilp A, Sangkard K. A palliated-suffering model for HIV-infected patients: a combination of the foundations of mindfulness meditation and Watson’s caring. Thai Journal of Nursing Research. 2005;9(4):268–280. [Google Scholar]
- 27.Sibinga EM, Stewart M, Magyari T, Welsh CK, Hutton N, Ellen JM. Mindfulness-based stress reduction for HIV-infected youth: a pilot study. Explore. 2008;4(1):36–37. doi: 10.1016/j.explore.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Jam S, Imani AH, Foroughi M, SeyedAlinaghi SA, Koochak HE, Mohraz M. The Effects of Mindfulness-Based Stress Reduction (MBSR) Program in Iranian HIV/AIDS Patients: A Pilot Study. Acta Medica Iranica. 2010;48(2):101–106. [PubMed] [Google Scholar]
- 29.Robinson FP, Mathews HL, Witek-Janusek L. Psycho-endocrine-immune response to mindfulness-based stress reduction in individuals infected with the human immunodeficiency virus: a quasiexperimental study. The Journal of Alternative & Complementary Medicine. 2003;9(5):683–694. doi: 10.1089/107555303322524535. [DOI] [PubMed] [Google Scholar]
- 30.Creswell JD, Myers HF, Cole SW, Irwin MR. Mindfulness meditation training effects on CD4+ T lymphocytes in HIV-1 infected adults: a small randomized controlled trial. Brain Behav Immun. 2009;23(2):184–8. doi: 10.1016/j.bbi.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hofmann SG, Sawyer AT, Witt AA, Oh D. The Effect of Mindfulness-Based Therapy on Anxiety and Depression: A Meta-Analytic Review. Journal of Consulting and Clinical Psychology. 2010;78(2):169–83. doi: 10.1037/a0018555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Obermeyer CM. HIV in the Middle East. BMJ. 2006;333(7573):851. doi: 10.1136/bmj.38994.400370.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kabat-Zinn J. Full Catastrophe Living: Using the Wisdom of Your Body and Mind to Face Stress, Pain, and Illness. New York: Dell Publishing; 1990. [Google Scholar]
- 34.Kabat-Zinn J. Mindfulness-Based Interventions in Context: Past, Present, and Future. Clinical Psychology: Science & Practice. 2003;10(2):144. [Google Scholar]
- 35.Kabat-Zinn J. Wherever you go, there you are: Mindfulness meditation in everyday life. New York: Hyperion Books; 1994. [Google Scholar]
- 36.Balter M. How does HIV overcome the body’s T cell bodyguards? Science. 1997;278(5342):1399–400. doi: 10.1126/science.278.5342.1399. [DOI] [PubMed] [Google Scholar]
- 37.Travis JW. Wellness workbook for health professionals. Mill Valley, CA: Wellness Resource Center; 1977. [Google Scholar]
- 38.Kabat-Zinn J, Lipworth L, Burney R. The clinical use of mindfulness meditation for the self-regulation of chronic pain. Journal of behavioral medicine. 1985;8(2):163–90. doi: 10.1007/BF00845519. [DOI] [PubMed] [Google Scholar]
- 39.Kabat-Zinn J, Massion AO, Kristeller J, Peterson LG, Fletcher KE, Pbert L, Lenderking WR, Santorelli SF. Effectiveness of a meditation-based stress reduction program in the treatment of anxiety disorders. Am J Psychiatry. 1992;149:936–43. doi: 10.1176/ajp.149.7.936. [DOI] [PubMed] [Google Scholar]
- 40.Williams KA, Kolar MM, Reger BE, Pearson JC. Evaluation of a Wellness-Based Mindfulness Stress Reduction intervention: a controlled trial. American Journal of Health Promotion. 2001;15(6):422. doi: 10.4278/0890-1171-15.6.422. [DOI] [PubMed] [Google Scholar]
- 41.Derogatis LR. Symptom Checklist-90-Revised (SCL-90-R) Minneapolis, MN: NCS Assessments; 1975. [Google Scholar]
- 42.Bech P, Allerup P, Maier W, Albus M, Lavori P, Ayuso JL. The Hamilton scales and the Hopkins Symptom Checklist (SCL-90). A cross-national validity study in patients with panic disorders. The British Journal of Psychiatry. 1992;160(2):206. doi: 10.1192/bjp.160.2.206. [DOI] [PubMed] [Google Scholar]
- 43.Schmitz N, Hartkamp N, Kiuse J, Franke GH, Reister G, Tress W. The Symptom Check-List-90-R (SCL-90-R): a German validation study. Quality of Life Research. 2000;9(2):185. doi: 10.1023/a:1008931926181. [DOI] [PubMed] [Google Scholar]
- 44.Derogatis LR, Savitz KL. The SCL-90-R and Brief Symptom Inventory (BSI) in primary care. Handbook of psychological assessment in primary care settings. 2000;236:297–334. [Google Scholar]
- 45.Morone NE, Greco CM, Weiner DK. Mindfulness meditation for the treatment of chronic low back pain in older adults: A randomized controlled pilot study. Pain. 2008;134:310–319. doi: 10.1016/j.pain.2007.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morris SB, DeShon RP. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychological Methods. 2002;7(1):105–25. doi: 10.1037/1082-989x.7.1.105. [DOI] [PubMed] [Google Scholar]
- 47.McCune JM. The dynamics of CD4+ T-cell depletion in HIV disease. Nature. 2001;410(6831):974–979. doi: 10.1038/35073648. [DOI] [PubMed] [Google Scholar]
- 48.Cole SW, Korin YD, Fahey JL, Zack JA. Norepinephrine accelerates HIV replication via protein kinase A-dependent effects on cytokine production. The Journal of Immunology. 1998;161(2):610. [PubMed] [Google Scholar]
- 49.Sloan EK, Capitanio JP, Tarara RP, Mendoza SP, Mason WA, Cole SW. Social stress enhances sympathetic innervation of primate lymph nodes: mechanisms and implications for viral pathogenesis. The Journal of Neuroscience. 2007;27(33):8857. doi: 10.1523/JNEUROSCI.1247-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Antoni MH, Carrico AW, Durán RE, Spitzer S, Penedo F, Ironson G, Fletcher MA, Klimas N, Schneiderman N. Randomized clinical trial of cognitive behavioral stress management on human immunodeficiency virus viral load in gay men treated with highly active antiretroviral therapy. Psychosom Med. 2006;68:143–51. doi: 10.1097/01.psy.0000195749.60049.63. [DOI] [PubMed] [Google Scholar]
- 51.Petrie KJ, Fontanilla I, Thomas MG, Booth RJ, Pennebaker JW. Effect of written emotional expression on immune function in patients with human immunodeficiency virus infection: a randomized trial. Psychosomatic Medicine. 2004;66(2):272. doi: 10.1097/01.psy.0000116782.49850.d3. [DOI] [PubMed] [Google Scholar]