Abstract
Increased oxidative stress is associated with various diseases and aging, while adaptation to heat stress is an important determinant of survival and contributes to longevity. However, the impact of oxidative stress on heat resistance remains largely unclear. Aim: In this study we investigated how oxidative stress impinges on heat stress responses. Results: We report that hydrogen-peroxide (H2O2) pretreatment inhibits both acquired thermotolerance and heat-induced Hsp70 expression in mammalian cells, as well as acquired thermotolerance in the nematode Caenorhabditis elegans, via RNA interference. Moreover, we demonstrate that elimination of RNA interference by silencing key enzymes in microRNA biogenesis, dcr-1 or pash-1, restores the diminished intrinsic thermotolerance of aged and H2O2-elimination compromised (catalase-2 and peroxiredoxin-2 deficient) worms. Innovation and Conclusion: These results uncover a novel post-transcriptional element in the regulation of heat stress adaptation under oxidative conditions that may have implications in disease susceptibility and aging. Antioxid. Redox Signal. 17, 890–901.
Introduction
Basic physiological processes such as metabolism, cellular signaling, and immunity are associated with the production of reactive oxygen species (ROS) (16). An accumulation of ROS, called oxidative stress, plays a critical role in various diseases and in aging (13, 16, 34). Although an excess of ROS generates diverse molecular and cellular damages and evokes a plethora of signaling events, how it is involved in the induction or aggravation of these pathological states is not entirely understood.
Increased resistance to heat stress protects against degenerative diseases in mammals (9, 32) and associates with longevity in Caenorhabditis elegans (10, 26). Intrinsic thermotolerance is maintained by multiple mechanisms. A preconditioning (i.e., heat) stress induces acquired thermotolerance, mediated by the heat shock response via heat shock factor (HSF1)-dependent induction of heat shock proteins (Hsp-s) (30, 47). Previous studies reported contrasting results of oxidative stress on HSF1 activation (2, 28) and Hsp70 levels (14, 22, 43). However, the effect of oxidative stress on thermotolerance remains largely unexplored.
RNA interference is a powerful post-transcriptional regulator of gene expression that operates via ∼22 nt microRNAs (miRNAs) (27). Genomic miRNA precursors are processed by highly specific RNases: the nuclear Drosha/PASH-1 produces hairpin pre-miRNAs, which are transported to the cytoplasm and cleaved to mature miRNAs by Dicer/DCR-1 (capital names indicate the respective nematode orthologs). Hence, Dicer/Drosha knockout is a reliable tool to investigate the general role of miRNAs (5, 41, 44). miRNAs bind to the mRNA 3′ untranslated region (3′UTR), repress translation, or promote mRNA degradation (27). miRNAs modulate diverse biological processes. Their connection with stress is exemplified by imparting robustness to gene expression networks in response to environmental change (24) and by the profound alterations of miRNA expression upon heat and oxidative stresses (25, 42, 49) [reviewed in (23)]. Heat and ischemic preconditioning-induced miRNAs induce Hsp70 and are cardioprotective during ischemia-reperfusion in mice (48, 49). Moreover, miRNAs modulate the life span and stress resistance of C. elegans involving DAF-16 and HSF1 (6, 11), underscoring a vital role of RNA interference in stress responses.
Innovation.
Oxidative stress is a serious cause of cell and tissue damage associated with many human diseases. Our observations beyond demonstrating a novel crosstalk between various types of stresses via RNA interference extend our understanding on how oxidative stress may debilitate physiological function. As RNA interference exhibits a significant functional conservation from nematodes to humans, we anticipate that the mechanism identified herein may be involved in human diseases and aging.
In this study we focused on the impact of oxidative stress on heat stress adaptation and found that hydrogen-peroxide (H2O2) pretreatment inhibited acquired thermotolerance in both COS-7 mammalian cells and in C. elegans. As an underlying mechanism, H2O2 inhibited the heat-induction of Hsp70 in cells, consistent with a recent study (1). Moreover, H2O2 prevented the heat-induction of an Hsp70 3′UTR reporter. H2O2-induced effects required Dicer, a key enzyme in miRNA biogenesis, in both cells and worms. We further found that RNAi against Dicer and Drosha orthologs restored the compromised thermotolerance of two worm strains deficient in H2O2 disposal. Finally, Dicer silencing delayed the decline of thermotolerance in aging worms and phenocopied the effect of the antioxidant N-acetyl-l-cysteine (NAC). Our results reveal RNA interference as a mediator of oxidative stress-induced inhibition of heat stress responses.
Results
H2O2 inhibits acquired thermotolerance and Hsp70 induction at the post-transcriptional level in COS-7 cells
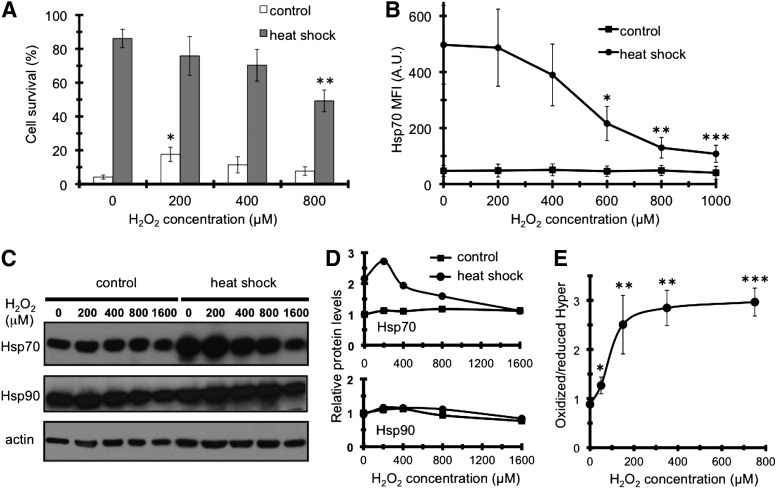
The effect of a transient H2O2 exposure on thermotolerance of COS-7 cells was determined by subjecting cells to a lethal heat stress 24 h after H2O2 and/or preconditioning heat treatments. Heat preconditioning elicited a large increase in survival (acquired thermotolerance, Fig. 1A). A prior H2O2 treatment slightly increased intrinsic thermotolerance. Importantly, it potently inhibited acquired thermotolerance in a concentration-dependent manner (Fig. 1A).
FIG. 1.
Hydrogen-peroxide (H2O2) impairs heat-preconditioned thermotolerance and Hsp70 heat-induction in COS-7 cells. (A) Effect of H2O2 on thermotolerance. Cells were treated by the indicated concentrations of H2O2 for 2 h, then kept at 37°C (control) or at 43°C for 30 min (heat shock). About 24 h later cells were subjected to a lethal heat stress (45°C, 60 min). Cell survival was analyzed 24 h later by Trypan blue exclusion. Values are means±standard deviations (SDs) of three experiments. (B, C) Effect of H2O2 on Hsp70 and Hsp90 protein levels. Cells were treated by H2O2 and heat shock as in panel A. Five hours later Hsp70 levels were analyzed by flow cytometry using a monoclonal antibody (B) or by Western blot using a polyclonal anti-Hsp70 and monoclonal anti-Hsp90 and anti-actin antibodies, respectively (C). Values are means±SDs of five experiments compared to their respective controls, and image is a representative of three experiments. (D) Densitometric analysis of relative Hsp70 and Hsp90 levels from (C). (E) H2O2 titration curve of cytosolic HyPer-C in COS-7 cells. The 490/420-nm fluorescence excitation ratio of HyPer was calculated after background fluorescence subtraction from two experiments. *p<0.05, **p<0.01, ***p<0.001.
To examine whether the decrease in acquired thermotolerance is due to the inhibition of the heat shock response, we pretreated COS-7 cells with a series of H2O2 concentrations and monitored the heat induction of Hsp70 by flow cytometry (Fig. 1B). Cells, exposed to heat shock, exhibited an ∼10-fold induction of Hsp70, concordant with the induction of thermotolerance (cf. Fig. 1A). H2O2 treatment did not affect basal Hsp70 level, but inhibited Hsp70 heat induction in a concentration-dependent manner (Fig. 1B). Western blots using a polyclonal anti-Hsp70 antibody showed a similar inhibition of Hsp70, but not of the specific chaperone Hsp90 (Fig. 1C, D). These results exclude an H2O2-induced modification or degradation of Hsp70 as well as a general, stress-induced transcriptional or translational block. The efficacy of H2O2 was verified by cells expressing the H2O2-sensor Hyper-C (Fig. 1E) (12). Thus, H2O2 pretreatment compromises both acquired thermotolerance and Hsp70 heat-induction in COS-7 cells.
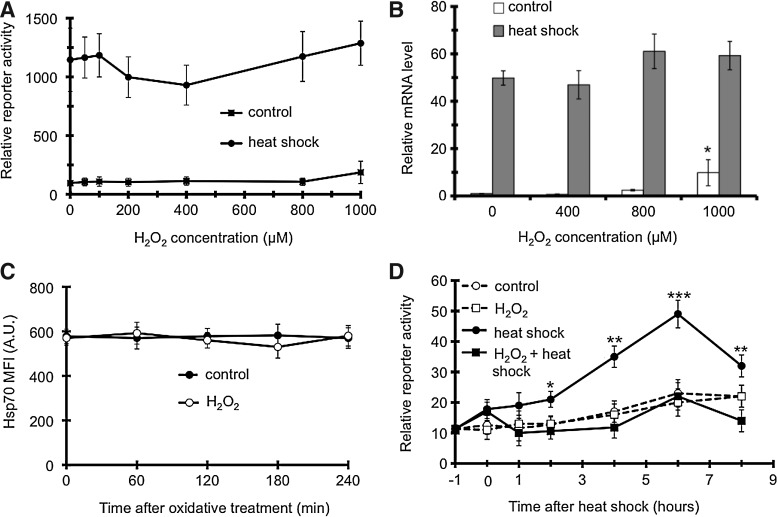
Next, we investigated the site of action of H2O2 along the heat shock regulon. Upon heat, misfolded proteins activate HSF1, which binds to heat shock promoter elements and induces hsp gene transcription (32). To assess the level of HSF1-dependent transactivation, we transfected COS-7 cells with a hsp70pr/luciferase vector and performed reporter gene assays after cells had either been oxidatively stressed and/or heat-shocked. Heat shock markedly induced reporter activity, while H2O2 treatment significantly affected neither basal nor heat-induced transactivation (Fig. 2A). Likewise, H2O2 treatment did not decrease hsp70 mRNA level (Fig. 2B). Thus, a transcriptional inhibition does not seem to underlie the H2O2-induced decrease in Hsp70 protein expression.
FIG. 2.
H2O2 inhibition of Hsp70 involves a potential post-transcriptional regulation. (A) H2O2 does not affect hsp70 promoter activation. Cells transfected with the hsp70.1pr/luc and control plasmids were treated as in Figure 1. Enzyme activities were measured 18 h later, and their ratios were expressed. (B) H2O2 does not diminish hsp70 (HSPA1A) mRNA expression. Cells were treated as in Figure 1. mRNA levels were determined 1-h after treatments by quantitative reverse transcriptase–polymerase chain reaction and expressed relative to β-actin. (C) H2O2 does not affect Hsp70 protein turnover. Cells were heat shocked as above, after 2 h at 37°C cells were incubated in the absence (control), or presence of 800 μM of H2O2 for 2 h, and then harvested at the indicated timepoints, and analyzed by flow cytometry. (D) H2O2 inhibits the heat-induced luciferase reporter translation mediated by the Hsp70 3′ untranslated region (3′UTR). Cells transfected with a pGL3/luc/hsp70.1 3′-UTR and control plasmids were treated by 650 μM H2O2 for 2 h, and then kept at 37°C or heat shocked. At the indicated timepoints enzyme activities were determined, and expressed as a ratio. Values are means±SDs of three experiments. n.s., non-significant, *p<0.05, **p<0.01, ***p<0.001.
To assess, if H2O2 could down-regulate Hsp70 post-translationally, we changed the order of stresses (i.e., employed H2O2 after heat shock) and followed the Hsp70 protein level by flow cytometry (Fig. 2C). H2O2 did not change the heat shock-induced sustained elevation of Hsp70, which excluded the possibility of an accelerated Hsp70 turnover.
The 3′UTR is intimately connected with the post-transcriptional regulation of mRNAs. To investigate the molecular events at the Hsp70 3′UTR, we took use of a reporter harboring the mouse hsp70.1 3′UTR fused to Firefly luciferase (18). Monitoring luciferase activity provided an estimate of the impact of the hsp70 3′UTR on the translation of luciferase mRNA following H2O2 and/or heat shock treatments. 3′UTR reporter activity displayed a time-dependent increase after heat-shock peaking at 6 h (Fig. 2C). This finding is consistent with early reports in Drosophila and mammalian cells on the role of the 3′UTR in the regulation of Hsp70 protein synthesis during heat shock (33, 38). Neither H2O2 nor the combination of H2O2 and heat shock increased luciferase activity above the baseline demonstrating that H2O2 entirely prevented the heat-induced activation by the hsp70 3′UTR (Fig. 2C).
RNA interference mediates H2O2-induced inhibition of Hsp70 induction and acquired thermotolerance in COS-7 cells
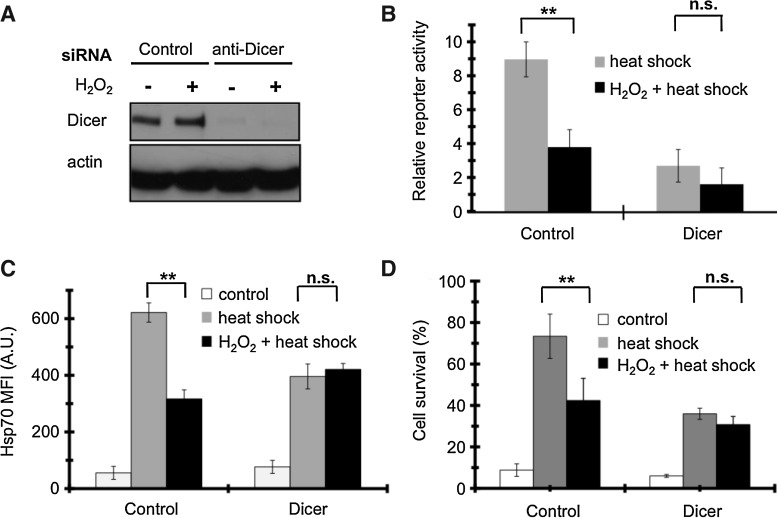
RNA interference is a powerful modulator of stress responses (23). To address whether RNA interference may mediate the events involving the Hsp70 3′UTR, we blocked miRNA maturation by anti-Dicer siRNA transfection. Only the siRNA, but not H2O2 led to a knock-down of Dicer (Fig. 3A). Intriguingly, anti-Dicer siRNA led to a large decrease in heat-induced 3′UTR reporter activity, suggesting that Dicer was necessary for the 3′UTR-mediated translational activation of the luciferase mRNA upon heat shock. This inhibition was comparable to that induced by H2O2, and a combination of anti-Dicer siRNA and H2O2 was not additive (Fig. 3B). Thus, H2O2 prevents the heat-induced Hsp70 3′UTR activation primarily via RNA interference.
FIG. 3.
RNA interference mediates H2O2-induced inhibition of Hsp70 induction and acquired thermotolerance in COS-7 cells. (A) Effect of H2O2 treatment and anti-Dicer siRNA on Dicer protein level. Two days after transfecton by anti-Dicer or control siRNA, respectively, cells were treated with 800 μM H2O2 for 2 h. Protein levels were analyzed by Western blot. Image is a representative of three experiments. (B) Effect of Dicer siRNA and H2O2 on the Hsp70 3′UTR activation. Cells undergoing a 2-day co-transfection with a control/Dicer siRNA and the 3′UTR reporter plasmids were treated by 650 μM H2O2 for 2 h, and then heat shocked. About 6 h later enzyme activities were determined and expressed as a ratio. (C) Effect of Dicer siRNA and H2O2 on Hsp70 protein expression. Cells transfected with a control/Dicer siRNA were treated by 800 μM H2O2 for 2 h, and then kept at 37°C or heat shocked. Five hours later Hsp70 levels were analyzed by flow cytometry. (D) Effect of Dicer siRNA and H2O2 on heat preconditioned thermotolerance. Cells transfected with a control/Dicer siRNA were treated by 800 μM H2O2 for 2 h, and then kept at 37°C or heat shocked. Lethal heat stress and survival assay was performed as in Figure 1A. Values are means±SDs of three experiments. n.s., non-significant, *p<0.05, **p<0.01, ***p<0.001.
To investigate how the inhibition of the 3′UTR by RNA interference is reflected in Hsp70 translation, we determined Hsp70 protein levels in anti-Dicer siRNA-transfected cells undergoing H2O2 and heat shock treatments. Dicer silencing inhibited Hsp70 heat-induction to approximately two-thirds of the control siRNA transfected value, comparable to the effect of Dicer silencing (Fig. 3C). Remarkably, H2O2 could not further reduce Hsp70 expression in Dicer-silenced cells, suggesting that the effect of H2O2 required an intact RNA interference.
These results suggested that RNA interference might play a role in the H2O2-induced inhibition of acquired thermotolerance. Indeed, we found that Dicer siRNA reduced acquired thermotolerance in heat-preconditioned cells, which was similar to the effect of H2O2 (Fig. 3D). Moreover, H2O2 did not further diminish thermotolerance in Dicer-silenced cells, in agreement with our observations on Hsp70 induction (cf. Fig. 3C). Hence, we conclude that RNA interference mediates the H2O2-induced inhibition of heat stress adaptation in COS-7 cells.
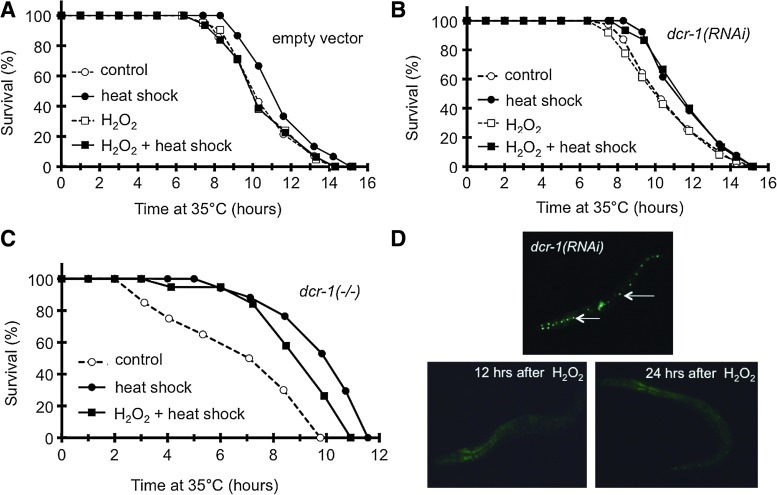
H2O2 inhibits aquired thermotolerance through DCR-1 in C. elegans
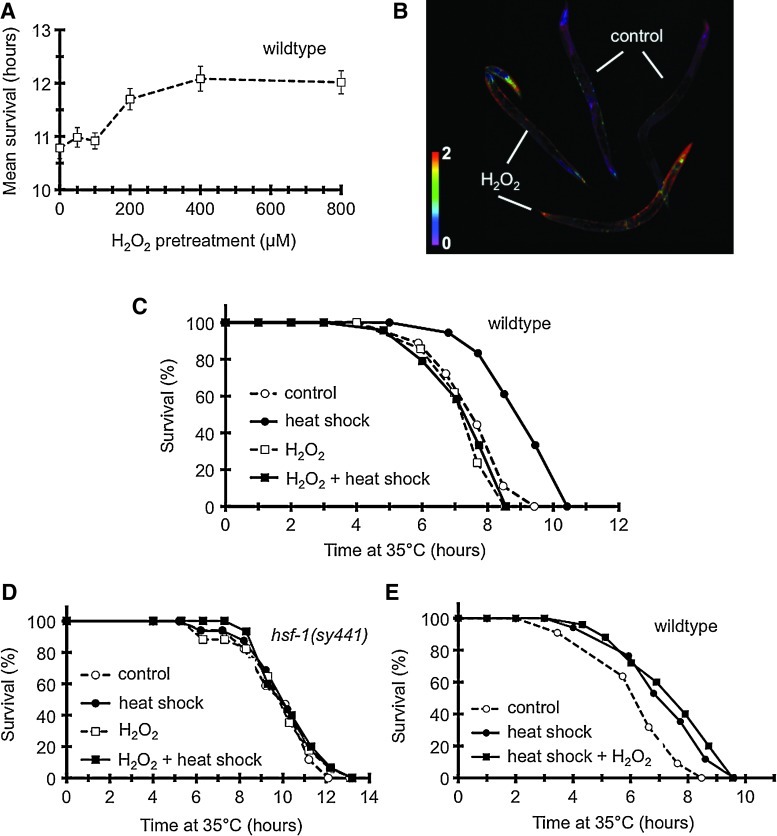
To address if the effect of H2O2 on heat stress adaptation was conserved during evolution, we used C. elegans, a powerful model system exhibiting an organismal complexity. In search of an H2O2-exposure that did not cause significant damage in nematodes, we found that a treatment by 100 μM for 1 h was below the threshold to induce oxidative tolerance to a lethal H2O2 challenge (Fig. 4A). This concentration induced a rapid signal elevation in the pharynx and intestine of worms ubiquitously expressing HyPer (Fig. 4B) (3). We used the 100-μM pretreatment to investigate its effect on nematodal thermotolerance. A preconditioning heat shock at 30°C for 2 h resulted in a 20%–40% increase in thermotolerance (Fig. 4C). A prior H2O2 treatment did not affect intrinsic thermotolerance of worms; however, it entirely abolished acquisition of thermotolerance by the preconditioning heat shock.
FIG. 4.
A prior H2O2 treatment inhibits aquired thermotolerance in an HSF1-dependent manner in Caenorhabditis elegans. (A) Effect of preconditioning H2O2 treatments on oxidative tolerance. Oxidative stress was applied in the liquid nematode growth medium (NGM) for 1 h at 20°C, 12–14 h before a lethal oxidative challenge. Data are means±SDs of two separate experiments. (B) Intensity-normalized ratio image demonstrating a rapid rise of oxidized/reduced HyPer ratio in jrIs[Prpl-17::HyPer] worms in response to a 1-min challenge by 100 μM H2O2 in liquid NGM. Representative image from five independent experiments. (C) Effect of H2O2 (100 μM for 1 h) on intrinsic and acquired thermotolerance induced by a preconditioning heat shock (heat shock, 30° for 2 h). Lethal heat stress was employed 12 h later. Only heat shock induces a significant difference in survival (p<0.0001). (D) No change in thermotolerance by a preconditioning heat shock and/or an H2O2 treatment in hsf-1(sy441) mutant worms (p>0.1). (E) H2O2 employed after the preconditioning heat shock does not abrogate acquired thermotolerance (p>0.1 compared to heat shock). (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
To address whether H2O2 would affect an HSF1-dependent process, we employed the hsf-1(sy441) point mutant strain harboring a truncated transactivation domain that prevented the heat-induction of HSF1-target genes (15). In line with recently published data of McColl and colleagues (29), HSF1 was required for aquired, but not for intrinsic thermotolerance (Fig. 4D). H2O2 treatment was not additive to the hsf-1(sy441) background; it affected neither basal nor heat-preconditioned survival. Moreover, in wild-type worms, H2O2, if applied after the preconditioning heat shock, was unable to inhibit acquired thermotolerance (Fig. 4E), suggesting that H2O2 needs to precede heat preconditioning. Thus, H2O2 specifically inhibits the acquisition of HSF1-dependent thermotolerance in C. elegans.
If, similarly to mammalian cells, H2O2 inhibited the heat shock response via RNA interference in C. elegans, then worms deficient in miRNA synthesis would escape from the H2O2-dependent inhibition of thermotolerance. Investigating this hypothesis we found that silencing the Dicer ortholog by dcr-1(RNAi) restored the acquired thermotolerance of H2O2-treated worms to levels comparable to heat shock alone (Fig. 5A, B). dcr-1(RNAi) per se did not affect thermotolerance (Fig. 5B). We obtained similar results using loss-of-function dcr-1 mutant nematodes (Fig. 5C). The efficiency of dcr-1 silencing and the lack of a general disruption of RNA interference by H2O2, respectively, were demonstrated by an RNA interference reporter strain (20) (Fig. 5D). We made attempts to investigate an analogous involvement of Hsp70 regulation. Unfortunately, a number of antibodies were unable to detect nematode Hsp70. Quantitative polymerase chain reaction (PCR) measurements revealed a tendency of H2O2 preconditioning to augment heat-induced hsp-70 mRNA expression. However, dcr-1(RNAi) significantly altered neither heat-induced mRNA level nor the H2O2-induced elevation (Supplementary Fig. S1; Supplementary Data are available online at www.liebertonline.com/ars). Despite the unclear involvement of Hsp70, RNA interference is required for H2O2 to inhibit acquired thermotolerance in worms.
FIG. 5.
DCR-1 mediates the H2O2-induced inhibition of thermotolerance in C. elegans. Effect of H2O2 on intrinsic and acquired thermotolerance in worms fed by empty (EV, A) or dcr-1(RNAi) (B) vectors, respectively. Treatments were as in Figure 4. Note that the activatory effect of preconditioning heat shock was less pronounced on RNAi plates. In EV-fed worms (A) only heat shock, while in dcr-1(RNAi)-fed worms (B) both heat shock as well as H2O2+heat shock induced a significantly higher survival compared with controls (p<0.001). (C) Both heat shock and H2O2+heat shock induces a significant increase in thermotolerance in dcr-1(ok247);unc-32(e189) nematodes (p<0.0001 vs. control). Survival curves are representatives of three experiments yielding similar results. (D) H2O2 does not compromise RNA interference. Epifluorescence image demonstrating increased expression of pajm::GFP (harboring an anti-GFP hairpin siRNA in addition to the GFP sequence) in the GR1401 RNA interference reporter strain fed by dcr-1(RNAi). Arrows point to specific dots localized to epithelial seam cells. In contrast, H2O2 (100 μM for 1 h) treatment followed by a 12- or 24-h recovery did not inhibit GFP silencing. Please note the autoflurescence of oxidatively stressed worms. Representative image from three independent experiments. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
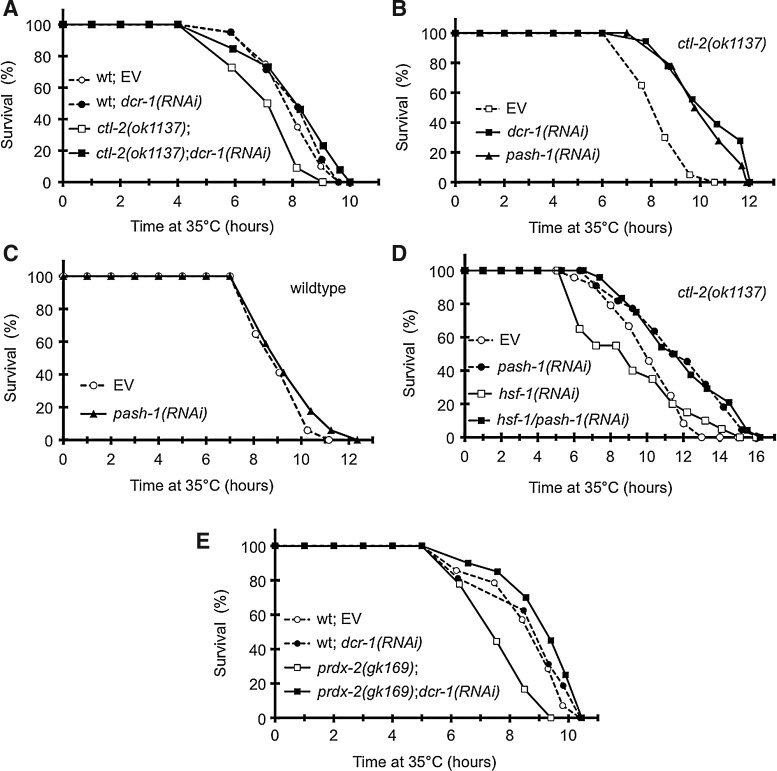
Inhibition of RNA interference restores thermotolerance in endogenous models of oxidative stress
Next we asked how thermotolerance might be affected by chronic genetic disturbances in antioxidant defense. Antioxidant enzymes provide protection against oxidative stress by removing ROS. Catalase-2 is a peroxisomal enzyme involved in H2O2 elimination accounting for ∼80% of total catalase activity in the worm (39). ctl-2 loss of function elevates endogenous H2O2 levels (3), decreases oxidative tolerance and shortens lifespan (39). We observed that ctl-2(ok1137) animals exhibited impaired intrinsic thermotolerance compared to wild type, which was completely restored by dcr-1(RNAi) (Fig. 6A). Silencing the Drosha ortholog PASH-1, the other key enzyme in miRNA biogenesis, phenocopied the effect of dcr-1(RNAi) in the ctl-2(ok1137) strain (Fig. 6B) without affecting wild-type thermotolerance (Fig. 6C). Neither the survival decrease in ctl-2(ok1137) nor the amelioration by pash-1(RNAi) was prevented by hsf-1(RNAi) (Fig. 6D). Hence, ctl-2 loss of function modulates intrinsic thermotolerance, not involving the HSF1-Hsp axis.
FIG. 6.
Loss of RNA interference rescues thermotolerance in nematodes with genetic defects of H2O2 disposal. (A) Effect of dcr-1(RNAi) on thermotolerance of N2 and ctl-2(ok1137) worms. ctl-2(ok1137) worms exhibited significantly shorter survival (p<0.001), while other survivals were not significantly different (p>0.2), compared to N2 control. (B) pash-1(RNAi) phenocopies dcr-1(RNAi) by inducing a significant increase in thermotolerance of ctl-2(ok1137) (p<0.0001) compared to that of the EV control. (C) pash-1(RNAi) does not change thermotolerance of wild-type worms (p>0.1) compared to that of the EV control. (D) pash-1(RNAi) extends thermotolerance independently of hsf-1 in ctl-2(ok1137) worms [p<0.01 vs. pash-1/hsf-1(RNAi)]. (E) Effect of dcr-1(RNAi) on thermotolerance of N2 and prdx-2(gk169) worms. prdx-2(gk169) worms fed by EV exhibited significantly shorter (p<0.0001), while those fed by dcr-1(RNAi) exhibited slightly longer survival (p<0.05) compared to N2 control. Survival curves are representatives of three independent experiments giving similar results.
To test whether the observed phenomena might be attributed to the general impairment of H2O2 elimination, we examined the lack of peroxiredoxin-2, involved in H2O2 reduction in the cytosol. prdx-2(gk169) worms, similarly to the ctl-2(ok1137) strain, are susceptible to H2O2 injury, and display a shortened lifespan (37). We found that prdx-2 knockout also markedly decreased C. elegans thermotolerance (Fig. 6E). Importantly, dcr-1(RNAi) prevented thermotolerance inhibition in prdx-2(gk169) worms. Together these data suggest that genetic defects in H2O2 elimination compromise heat stress adaptation via RNA interference.
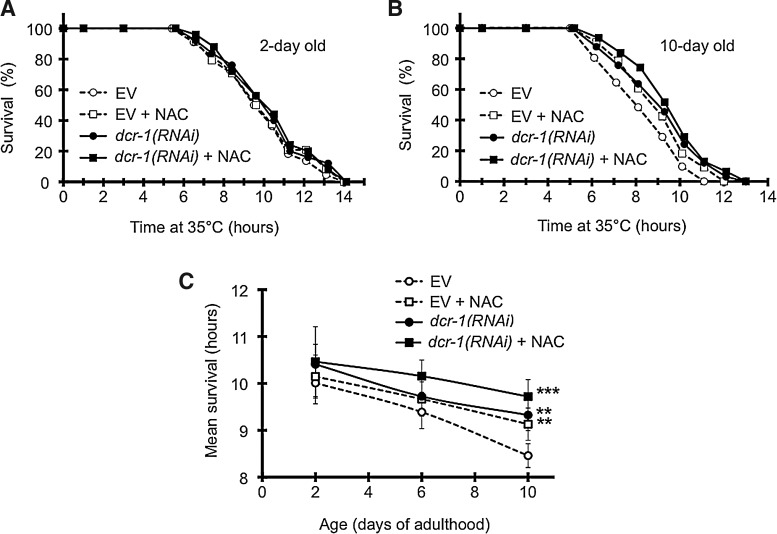
Inhibition of RNA interference delays age-dependent decline of thermotolerance
Aging is characterized by a collapse of proteostasis and an impairment of the heat shock response in C. elegans (4). Consistent with this, we observed a decline in C. elegans thermotolerance during aging (Fig. 7A–C). Oxidative stress and H2O2 increases during aging and ROS are considered a major cause of aging (3, 34). To address if oxidative stress affected thermotolerance during aging, we treated worms with the small molecular antioxidant, NAC. Intriguingly, NAC was able to reduce the decline of thermotolerance during aging resulting in a milder slope and a significant difference at the old worms at day 10 of age (Fig. 7A–C).
FIG. 7.
Loss of RNA interference and the antioxidant N-acetyl-l-cysteine ameliorate age-associated decline of thermotolerance in C. elegans. Thermotolerance of 2-day (A) and 10-day (B) old nematodes treated by dcr-1(RNAi) and/or 5 mM N-acetyl-l-cysteine (NAC; from day 1 of adulthood). There was no significant difference in survival between treatments at day 2 (p>0.1). The 10-day old control (EV) exhibited a significantly shorter survival (p<0.001 vs. 2-day EV). All dcr-1(RNAi) and/or 5 mM NAC induced a significant increase in survival (p<0.001 vs. 10-day old EV), which approached (p=0.028 10-day EV+NAC vs. 2-day EV), and became non-significant (p>0.05 10-day dcr-1(RNAi) and dcr-1(RNAi)+NAC vs. 2-day EV) compared with the survival of the 2-day old EV control. Survivals of 10-day dcr-1(RNAi) and dcr-1(RNAi)+NAC strains were not significantly different (p>0.4). (C) Mean thermotolerance of nematodes treated by dcr-1(RNAi) and/or 5 mM NAC as a function of age. Panels are representatives of two independent experiments yielding similar results. **p<0.01, ***p<0.001.
Then we asked, whether RNA interference was involved in the age-associated decline of thermotolerance of worms. dcr-1(RNAi) did not significantly influence the thermotolerance of young animals at day 1 (Fig. 5), but efficiently suppressed the age-induced decline of thermotolerance similarly to NAC treatment (Fig. 7A–C). Moreover, the combination of dcr-1(RNAi) with NAC was not significantly different from the effect of dcr-1(RNAi) at any time points tested. These findings indicate that RNA interference is involved in the oxidative stress-induced age-dependent decline of heat stress adaptation in C. elegans.
Discussion
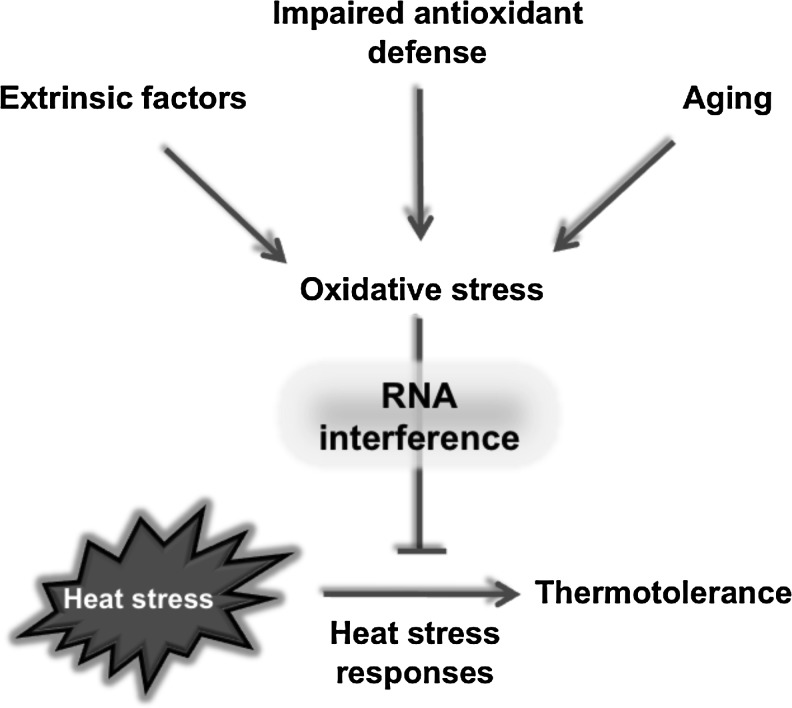
In this study, we have presented evidence that oxidative stress inhibits the adaptive responses to heat stress in both mammalian cells and C. elegans. Silencing Dicer and Drosha orthologs, key enzymes specific to miRNA maturation reveals a conserved role for RNA interference. In mammalian cells H2O2 abolishes a positive action of RNA interference on acquired thermotolerance. Inhibition of RNA interference does not alter thermotolerance in young nematodes, suggesting that H2O2 may induce miRNA(s) that inhibit the acquisition of thermotolerance. Intrinsic thermotolerance decrease of prdx-2 and ctl-2 knockouts and aged worms might require accumulation of miRNA(s) inhibiting HSF1-independent processes. Despite species-specific and context-dependent mechanisms, our results provide support to the modulation of stress responses by RNA interference (Fig. 8) (23).
FIG. 8.
Model for the role of RNA interference in the modulation of heat stress responses by oxidative stress. Oxidative stress may be induced by various sources, such as increased production/extrinsic factor (H2O2), decreased elimination (impaired defense, endogenous mutants), or a combination of the two (aging). RNA interference mediates an inhibitory action of oxidative stress and reduces heat resistance.
Our findings on the post-transcriptional inhibition of Hsp70 expression offer a potential molecular mechanism underlying the H2O2-induced compromise of acquired thermotolerance. Early reports demonstrating a heat-induced stabilization of hsp70 mRNA by its 3′UTR (33, 38) and the decrease in heat-induced hsp70 mRNA by H2O2 in glioma cells (1) suggested that H2O2 may prevent mRNA stabilization. However, our results showing no impact of H2O2 on hsp70 mRNA and inhibition of 3′UTR reporter, respectively, are consistent with a compromised translation by H2O2. Interestingly, inflammatory cytokines inhibit colonic Hsp70 translation by recruiting its mRNA to stress granules (17, 18). Although it may be one plausible mechanism, our results using Dicer knockdown suggest the involvement of miRNA(s). Possible scenarios include an H2O2-induced decrease of activatory miRNA(s), or displacement/domination of heat-induced activatory miRNA(s) by H2O2-induced inhibitory/neutral miRNA(s) from the hsp70 mRNA. Both mechanisms are generally employed by RNA interference (23, 27). Moreover, recent articles provide evidence on miRNAs either inhibiting (miR-378*, miR-711, miR-146a, miR-146b-5b) (35, 46), or ischemic preconditioning-induced miRNAs (miR-1, miR-21, miR-24 or others) (48, 49) activating Hsp70 expression. Identification of the exact mechanism(s) and miRNA(s), as well as an analogous Hsp70 regulation in nematodes requires further studies. Nevertheless, our study raises the idea that pathophysiological oxidative conditions (inflammation, wound healing, aging) might employ RNA interference to post-transcriptionally regulate Hsp70 in various tissues (7, 18, 36).
Our use of mutants deficient in H2O2 elimination demonstrates that a chronic disturbance in ROS metabolism impairs intrinsic thermotolerance, independently of HSF1 (Fig. 6). This defect can entirely be reversed by blocking miRNA maturation, suggesting a profound post-transcriptional remodeling of heat stress adaptation by RNA interference in response to oxidative stress. McColl et al. elegantly showed that increased intrinsic thermotolerance in daf-2 mutant worms is mediated by a daf-16-dependent translational response (29). A common motif in the two studies is that RNA interference or translation do not limit survival in young worms; however, they differentially condition heat resistance in both long-lived insulin-like signaling mutants and short-lived oxidative defense-deficient mutants and aged worms, respectively [(29) and our study]. It is tempting to speculate that the daf-16-regulated response of insulin signaling mutants might involve miRNAs. The clarification of a possible interaction of the translational response and RNA interference in the regulation of stress resistance remains the task of future studies.
Aging in the worm is characterized by an increased accumulation of ROS as well as a collapse of protein homeostasis (4). Our results on the age-induced decline of intrinsic thermotolerance support these observations, and use of the antioxidant NAC demonstrates a progressive causal role for ROS in decline of stress resistance during aging (Fig. 7). Importantly, both the comparable pattern of NAC and dcr-1(RNAi) protection and the lack of significant synergism imply a substantially overlapping mode of action. Moreover, increased protection by dcr-1(RNAi) suggests that RNA interference adversely affects heat resistance with aging. Single miRNAs do not seem to play an essential role in C. elegans development and growth, but both RNA interference and single miRNAs are indispensable to ensure proper development during environmental stress (20, 24, 31). Likewise, there is an extensive change in miRNA expression during C. elegans aging (11, 19) and several individual miRNAs similarly modulate longevity and stress resistance in C. elegans (11). Inhibition of the entire RNA interference in adulthood provides strong evidence to the general dysregulation of miRNAs in aging and in oxidative stress with a negative impact on stress resistance (Figs. 6 and 7). It remains to be seen whether RNA interference would pose a trade-off between fine-tuning developmental programs and growth during stress in exchange for a self-maintenance later in life. Our results imply that beyond well-characterized stress-responsive HSF1 and DAF-16 pathways, RNA interference may offer a novel target to alleviate decline of stress responses during aging.
Materials and Methods
Materials
Reagents for cell culture were from Invitrogen. Solutions for flow cytometry were from BD Biosciences. Electrophoresis and blotting reagents were from Bio-Rad. N-acetyl-l-cysteine and H2O2 were from Sigma. All other reagents were from either from Sigma or Fluka.
Cell culture and survival
COS-7 cells were obtained from the ATCC. Cells were cultured as described (40). Cell survival was analyzed by Trypan Blue exclusion 24 h after challenge.
Determination of protein levels
Flow cytometry using a fluorescein-isothiocyanate-conjugated monoclonal anti-Hsp70 antibody (StressGen), cell lysis, and Western blotting using a polyclonal anti-Hsp70 antibody (21), or antibodies against Hsp90 (Stressgen), Dicer (CST), actin (Sigma) was carried out as previously described (40).
Transfection and reporter gene assays
Cells were transfected at a density of 40% using Lipofectamine (Invitrogen). Control/anti-Dicer siRNA (Quiagen) was introduced at 100 nM. Further treatments were applied at 48 h post-transfection. For the hsp70-promoter reporter gene assay, cells were transfected with 0.35 μg hsp70.1pr/Firefly luciferase plasmid (Rick Morimoto, Northwestern University) and cytomegalovirus/β-galactosidase plasmids, while for the 3′UTR reporter assay 0.35 μg pGL3 basic or pGL3/hsp70.1 3′-UTR plasmid (Eugene Chang, University of Chicago) and thymidine kinase/Renilla luciferase plasmids were employed, respectively. Treatments were performed 24 h post-transfection. About 18 h post-treatment reporter activities were measured using commercial assay kits (Promega) and expressed.
C. elegans strains and RNA interference
Strains were obtained from the CGC, if not otherwise specified. The following strains were used in this study: wild type (N2), jrIs[Prpl-17::HyPer], PS3551 hsf-1(sy441), BB1 dcr-1(ok247);unc-32(e189) III, VC289 prdx-2(gk169) II, VC574 ctl-2(ok1137) II, and the GFP RNAi-reporter GR1401 (Gary Ruvkun, Harvard University). Strains were backcrossed to the wild type at east three times to clear potential background mutations, and were maintained as described (8). RNAi was performed as described by feeding worms with HT115(DE3) bacteria transformed with empty vector, dcr-1(RNAi) Gary Ruvkun (Harvard University) or pash-1(RNAi) (Source BioScience) vectors, repectively (45). Experiments were carried out in the second generation. Experiments were performed in the second generation with synchronized young 1-day-old adults, except for age-related thermotolerance.
Thermotolerance assay
Thermotolerance was performed on nematode growth medium plates at 35°C till complete extinction of the population using 25 animals per condition in at least two independent trials. Viability was determined hourly by assaying for movement in response to gentle prodding.
mRNA expression analysis
mRNA was prepared using the GeneJET RNA Purification Kit (Fermentas). mRNA was reverse transcribed using the RevertAid™ cDNA Synthesis Kit (Fermentas). Quantitative PCR was performed in an ABI 7300 System by Taqman Gene Expression Assays: HSPA1A: Hs_00359147_s1; β-actin: Hs_99999903_m1 (Applied Biosystems). Relative amounts of hsp70 mRNA were determined using the Comparative Cycle Treshold Method for quantitation and normalized to actin mRNA levels. Please see Supplementary Materials and Methods for the analysis of hsp70 mRNA expression in nematodes.
Analysis of H2O2 levels and fluorescence microscopy
Fluorescence measurements in COS-7 cells transfected by HyPer-C (Miklós Geiszt, Semmelweis University) were performed as described (12). HyPer titration was achieved by sequential addition of increasing concentrations of H2O2. Mean fluorescence intensities over individual cells were calculated from 3-min recordings. H2O2 in worms was monitored using the jrIs[Prpl-17::HyPer] strain, ubiquitously expressing the H2O2-biosensor HyPer, was used. Worms were immobilized and imaged as described (3).
Statistical analysis
Data were analyzed using SPSS software 15.0 (SPSS, Inc.). Survival curves were compared by the log-rank test. If not stated otherwise, all experiments were repeated at least three times. Variables were expressed as mean±standard deviation. Statistical significance was indicated as follows: *p<0.05, **p<0.01, ***p<0.001.
Supplementary Material
Abbreviations Used
- 3′UTR
3′ untranslated region
- H2O2
hydrogen-peroxide
- HSF1
heat shock transcription factor 1
- Hsp
heat shock protein
- miRNA
microRNA
- NAC
N-acetyl-l-cysteine
- NGM
nematode growth medium
- PCR
polymerase chain reaction
- ROS
reactive oxygen species
- MFI
mean fluorescence intensity
Acknowledgments
We thank Eugene Chang, Miklós Geiszt, Gary Ruvkun, and Rick Morimoto for reagents; Tibor Vellai for help in setting up our worm lab; Melinda Zana and Balázs Enyedi (cellular HyPer measurements), Zsolt Rónai (qPCR), Bea Gilányi, and Ákos Putics for technical help; Eszter Daubner and members of the Sőti Group for discussions; the anonymous reviewers for their helpful advice; and the Caenorhabditis Genetics Center for nematode strains. This work was supported by grants from the EU (FP6-518230, FP7-200970, TÁMOP-4.2.2/B-10/1-2010-0013), a grant of the Hungarian Science Foundation/Norway Grants (NNF-78794), the Hungarian Science Foundation (OTKA-K69105 and OTKA-K83314). During the completion of this study, C.S. was a Bolyai Research Scholar of the Hungarian Academy of Sciences.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Adachi M. Liu Y. Fujii K. Calderwood SK. Nakai A. Imai K. Shinomura Y. Oxidative stress impairs the heat stress response and delays unfolded protein recovery. PLoS One. 2009;4:e7719. doi: 10.1371/journal.pone.0007719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn SG. Thiele DJ. Redox regulation of mammalian heat shock factor 1 is essential for Hsp gene activation and protection from stress. Genes Dev. 2003;17:516–528. doi: 10.1101/gad.1044503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Back P. De Vos WH. Depuydt GG. Matthijssens F. Vanfleteren JR. Braeckman BP. Exploring real-time in vivo redox biology of developing and aging Caenorhabditis elegans. http://dx.doi.org/10.1016/j.freeradbiomed.2011.11.037. Free Rad Biol Med. 2012;2011;52:850–859. doi: 10.1016/j.freeradbiomed.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Zvi A. Miller EA. Morimoto RI. Collapse of proteostasis represents an early molecular event in Caenorhabditis elegans aging. Proc Natl Acad Sci U S A. 2009;106:14914–14919. doi: 10.1073/pnas.0902882106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernstein E. Kim SY. Carmell MA. Murchison EP. Alcorn H. Li MZ. Mills AA. Elledge SJ. Anderson KV. Hannon GJ. Dicer is essential for mouse development. Nat Genet. 2003;35:215–217. doi: 10.1038/ng1253. [DOI] [PubMed] [Google Scholar]
- 6.Boehm M. Slack F. A developmental timing microRNA and its target regulate life span in C. elegans. Science. 2005;310:1954–1957. doi: 10.1126/science.1115596. [DOI] [PubMed] [Google Scholar]
- 7.Bonelli MA. Alfieri RR. Petronini PG. Brigotti M. Campanini C. Borghetti AF. Attenuated expression of 70-kDa heat shock protein in WI-38 human fibroblasts during aging in vitro. Exp Cell Res. 1999;252:20–32. doi: 10.1006/excr.1999.4614. [DOI] [PubMed] [Google Scholar]
- 8.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Currie RW. Karmazyn M. Kloc M. Mailer K. Heat-shock response is associated with enhanced postischemic ventricular recovery. Circ Res. 1988;63:543–549. doi: 10.1161/01.res.63.3.543. [DOI] [PubMed] [Google Scholar]
- 10.Cypser JR. Johnson TE. Multiple stressors in Caenorhabditis elegans induce stress hormesis and extended longevity. J Gerontol A Biol Sci Med Sci. 2002;57:B109–B114. doi: 10.1093/gerona/57.3.b109. [DOI] [PubMed] [Google Scholar]
- 11.de Lencastre A. Pincus Z. Zhou K. Kato M. Lee SS. Slack FJ. MicroRNAs both promote and antagonize longevity in C. elegans. Curr Biol. 2010;20:2159–2168. doi: 10.1016/j.cub.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Enyedi B. Varnai P. Geiszt M. Redox state of the endoplasmic reticulum is controlled by Ero1L-alpha and intraluminal calcium. Antioxid Redox Signal. 2010;13:721–729. doi: 10.1089/ars.2009.2880. [DOI] [PubMed] [Google Scholar]
- 13.Finkel T. Radical medicine: treating ageing to cure disease. Nat Rev Mol Cell Biol. 2005;6:971–976. doi: 10.1038/nrm1763. [DOI] [PubMed] [Google Scholar]
- 14.Gosslau A. Ruoff P. Mohsenzadeh S. Hobohm U. Rensing L. Heat shock and oxidative stress-induced exposure of hydrophobic protein domains as common signal in the induction of hsp68. J Biol Chem. 2001;276:1814–1821. doi: 10.1074/jbc.M008280200. [DOI] [PubMed] [Google Scholar]
- 15.Hajdu-Cronin YM. Chen WJ. Sternberg PW. The L-type cyclin CYL-1 and the heat-shock-factor HSF-1 are required for heat-shock-induced protein expression in Caenorhabditis elegans. Genetics. 2004;168:1937–1949. doi: 10.1534/genetics.104.028423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halliwell B. Gutteridge JMC. xxxvi. Oxford; New York: Oxford University Press; 2007. Free Radicals in Biology and Medicine; p. 851. [8] p. of plates p. [Google Scholar]
- 17.Hu S. Claud EC. Musch MW. Chang EB. Stress granule formation mediates the inhibition of colonic Hsp70 translation by interferon-gamma and tumor necrosis factor-alpha. Am J Physiol Gastrointest Liver Physiol. 2010;298:G481–G492. doi: 10.1152/ajpgi.00234.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu S. Zhu X. Triggs JR. Tao Y. Wang Y. Lichtenstein L. Bissonnette M. Musch MW. Chang EB. Inflammation-induced, 3'UTR-dependent translational inhibition of Hsp70 mRNA impairs intestinal homeostasis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1003–G1011. doi: 10.1152/ajpgi.00027.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ibanez-Ventoso C. Yang M. Guo S. Robins H. Padgett RW. Driscoll M. Modulated microRNA expression during adult lifespan in Caenorhabditis elegans. Aging Cell. 2006;5:235–246. doi: 10.1111/j.1474-9726.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- 20.Kim JK. Gabel HW. Kamath RS. Tewari M. Pasquinelli A. Rual JF. Kennedy S. Dybbs M. Bertin N. Kaplan JM. Vidal M. Ruvkun G. Functional genomic analysis of RNA interference in C. elegans. Science. 2005;308:1164–1167. doi: 10.1126/science.1109267. [DOI] [PubMed] [Google Scholar]
- 21.Kurucz I. Tombor B. Prechl J. Erdo F. Hegedus E. Nagy Z. Vitai M. Koranyi L. Laszlo L. Ultrastructural localization of Hsp-72 examined with a new polyclonal antibody raised against the truncated variable domain of the heat shock protein. Cell Stress Chaperones. 1999;4:139–152. doi: 10.1379/1466-1268(1999)004<0139:ulohew>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JS. Seo JS. Differential expression of two stress-inducible hsp70 genes by various stressors. Exp Mol Med. 2002;34:131–136. doi: 10.1038/emm.2002.19. [DOI] [PubMed] [Google Scholar]
- 23.Leung AK. Sharp PA. MicroRNA functions in stress responses. Mol Cell. 2010;40:205–215. doi: 10.1016/j.molcel.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X. Cassidy JJ. Reinke CA. Fischboeck S. Carthew RW. A microRNA imparts robustness against environmental fluctuation during development. Cell. 2009;137:273–282. doi: 10.1016/j.cell.2009.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin Y. Liu X. Cheng Y. Yang J. Huo Y. Zhang C. Involvement of MicroRNAs in hydrogen peroxide-mediated gene regulation and cellular injury response in vascular smooth muscle cells. J Biol Chem. 2009;284:7903–7913. doi: 10.1074/jbc.M806920200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lithgow GJ. White TM. Melov S. Johnson TE. Thermotolerance and extended life-span conferred by single-gene mutations and induced by thermal stress. Proc Natl Acad Sci U S A. 1995;92:7540–7544. doi: 10.1073/pnas.92.16.7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Q. Paroo Z. Biochemical principles of small RNA pathways. Ann Rev Biochem. 2010;79:295–319. doi: 10.1146/annurev.biochem.052208.151733. [DOI] [PubMed] [Google Scholar]
- 28.Manalo DJ. Lin Z. Liu AY. Redox-dependent regulation of the conformation and function of human heat shock factor 1. Biochemistry. 2002;41:2580–2588. doi: 10.1021/bi0159682. [DOI] [PubMed] [Google Scholar]
- 29.McColl G. Rogers AN. Alavez S. Hubbard AE. Melov S. Link CD. Bush AI. Kapahi P. Lithgow GJ. Insulin-like signaling determines survival during stress via posttranscriptional mechanisms in C. elegans. Cell Metab. 12:260–272. doi: 10.1016/j.cmet.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McMillan DR. Xiao X. Shao L. Graves K. Benjamin IJ. Targeted disruption of heat shock transcription factor 1 abolishes thermotolerance and protection against heat-inducible apoptosis. J Biol Chem. 1998;273:7523–7528. doi: 10.1074/jbc.273.13.7523. [DOI] [PubMed] [Google Scholar]
- 31.Miska EA. Alvarez-Saavedra E. Abbott AL. Lau NC. Hellman AB. McGonagle SM. Bartel DP. Ambros VR. Horvitz HR. Most Caenorhabditis elegans microRNAs Are Individually Not Essential for Development or Viability. PLoS Genet. 2007;3:e215. doi: 10.1371/journal.pgen.0030215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morimoto RI. Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev. 2008;22:1427–1438. doi: 10.1101/gad.1657108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moseley PL. Wallen ES. McCafferty JD. Flanagan S. Kern JA. Heat stress regulates the human 70-kDa heat-shock gene through the 3'-untranslated region. Am J Physiol. 1993;264:L533–L537. doi: 10.1152/ajplung.1993.264.6.L533. [DOI] [PubMed] [Google Scholar]
- 34.Muller FL. Lustgarten MS. Jang Y. Richardson A. Van Remmen H. Trends in oxidative aging theories. Free Radic Biol Med. 2007;43:477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 35.Namba T. Tanaka K. Hoshino T. Azuma A. Mizushima T. Suppression of expression of heat shock protein 70 by gefitinib and its contribution to pulmonary fibrosis. PLoS ONE. 2011;6:e27296. doi: 10.1371/journal.pone.0027296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oberringer M. Baum HP. Jung V. Welter C. Frank J. Kuhlmann M. Mutschler W. Hanselmann RG. Differential expression of heat shock protein 70 in well healing and chronic human wound tissue. Biochem Biophys Res Commun. 1995;214:1009–1014. doi: 10.1006/bbrc.1995.2386. [DOI] [PubMed] [Google Scholar]
- 37.Olahova M. Taylor SR. Khazaipoul S. Wang J. Morgan BA. Matsumoto K. Blackwell TK. Veal EA. A redox-sensitive peroxiredoxin that is important for longevity has tissue- and stress-specific roles in stress resistance. Proc Natl Acad Sci U S A. 2008;105:19839–19844. doi: 10.1073/pnas.0805507105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petersen RB. Lindquist S. Regulation of HSP70 synthesis by messenger RNA degradation. Cell Regul. 1989;1:135–149. doi: 10.1091/mbc.1.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petriv OI. Rachubinski RA. Lack of peroxisomal catalase causes a progeric phenotype in Caenorhabditis elegans. J Biol Chem. 2004;279:19996–20001. doi: 10.1074/jbc.M400207200. [DOI] [PubMed] [Google Scholar]
- 40.Putics A. Vegh EM. Csermely P. Soti C. Resveratrol induces the heat-shock response and protects human cells from severe heat stress. Antioxid Redox Signal. 2008;10:65–75. doi: 10.1089/ars.2007.1866. [DOI] [PubMed] [Google Scholar]
- 41.Rehwinkel J. Natalin P. Stark A. Brennecke J. Cohen SM. Izaurralde E. Genome-wide analysis of mRNAs regulated by Drosha and Argonaute proteins in Drosophila melanogaster. Mol Cell Biol. 2006;26:2965–2975. doi: 10.1128/MCB.26.8.2965-2975.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simone NL. Soule BP. Ly D. Saleh AD. Savage JE. Degraff W. Cook J. Harris CC. Gius D. Mitchell JB. Ionizing radiation-induced oxidative stress alters miRNA expression. PLoS One. 2009;4:e6377. doi: 10.1371/journal.pone.0006377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steels EL. Watson K. Parsons PG. Relationships between thermotolerance, oxidative stress responses and induction of stress proteins in human tumour cell lines. Biochem Pharmacol. 1992;44:2123–2129. doi: 10.1016/0006-2952(92)90338-j. [DOI] [PubMed] [Google Scholar]
- 44.Tang F. Kaneda M. O'Carroll D. Hajkova P. Barton SC. Sun YA. Lee C. Tarakhovsky A. Lao K. Surani MA. Maternal microRNAs are essential for mouse zygotic development. Genes Dev. 2007;21:644–648. doi: 10.1101/gad.418707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Timmons L. Court DL. Fire A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene. 2001;263:103–112. doi: 10.1016/s0378-1119(00)00579-5. [DOI] [PubMed] [Google Scholar]
- 46.Tranter M. Helsley RN. Paulding WR. McGuinness M. Brokamp C. Haar L. Liu Y. Ren X. Jones WK. Coordinated post-transcriptional regulation of Hsp70.3 gene expression by microRNA and alternative polyadenylation. J Biol Chem. 2011;286:29828–29837. doi: 10.1074/jbc.M111.221796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walker GA. Lithgow GJ. Lifespan extension in C. elegans by a molecular chaperone dependent upon insulin-like signals. Aging Cell. 2003;2:131–139. doi: 10.1046/j.1474-9728.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- 48.Yin C. Salloum FN. Kukreja RC. A novel role of microRNA in late preconditioning: upregulation of endothelial nitric oxide synthase and heat shock protein 70. Circ Res. 2009;104:572–575. doi: 10.1161/CIRCRESAHA.108.193250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yin C. Wang X. Kukreja RC. Endogenous microRNAs induced by heat-shock reduce myocardial infarction following ischemia-reperfusion in mice. FEBS Lett. 2008;582:4137–4142. doi: 10.1016/j.febslet.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.