Abstract
Aims: Hydrogen sulfide (H2S), a novel gaseous mediator, has been recognized to protect neurons from overexcitation by enhancing the activity of the adenosine triphosphate-sensitive potassium (K-ATP) channel. However, no direct evidence supports that the K-ATP channel contributes to the neuroprotective effect of H2S in neurodegeneration. Herein, wild-type and Kir6.2 knockout (Kir6.2−/−) mice were used to establish the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson's disease (PD) so as to investigate the involvement of K-ATP channels in the neuroprotection of H2S. Results: Systemic administration of sodium hydrosulfide (NaHS) (an H2S donor, 5.6 mg/kg/day) for 7 days rescued MPTP-induced loss of dopaminergic (DA) neurons in substantia nigra compacta of both Kir6.2+/+ and Kir6.2−/− mice. Consistently, NaHS (100 μM) protected primary mesencephalic neurons against 1-methyl-4-phenylpyridinium (MPP+)-induced cytotoxicity in both genotypes. We further found that deficiency of mitochondrial uncoupling protein 2 (UCP2), which reduces reactive oxygen species (ROS) production and functions as upstream to the K-ATP channel in determining vulnerability of DA neurons, abolished the protective effects of H2S against either DA neuron degeneration in the PD mouse model or MPP+-induced injury in primary mesencephalic neurons. Rationally, UCP2 evokes mild uncoupling, which in turn diminishes ROS accumulation in DA neurons. Furthermore, H2S exerted neuroprotective effect via enhancing UCP2-mediated antioxidation and subsequently suppressing ROS-triggered endoplasmic reticulum stress as well as ultimately inhibiting caspase 12-induced neuronal apoptosis. Innovation and Conclusion: H2S protects DA neurons against degeneration in a UCP2 rather than Kir6.2/K-ATP channel-dependent mechanism, which will give us an insight into the potential of H2S in terms of opening up new therapeutic avenues for PD. Antioxid. Redox Signal. 17, 849–859.
Introduction
Parkinson's disease (PD) is characterized by the selective degeneration of dopaminergic (DA) neurons in the substantia nigra (SN) and aggregation of Lewy bodies in neurons. The incidence of the disorder increases with age, and more than 2% of the population aged over 65 are attacked by the disease. Therefore, PD is the second most prevalent neurodegenerative disease with only symptomatic treatment available (18). Clinically, the first-class drug to treat PD is L-DOPA, which solely ameliorates the symptoms but fails to retard DA neuron degeneration. It thus is crucial to develop effective therapeutic drugs and strategies that can reverse or alleviate the pathological process occurring in the DA neurons in PD patients.
Over the last decade, hydrogen sulfide (H2S) has been recognized as a novel gaseous mediator alongside nitric oxide and carbon monoxide (1). Endogenous H2S in mammalian body is produced by cystathionine β-synthase and cystathionine γ-lyase, which are two types of pyridoxal-5′-phosphate-dependent enzymes. Later on, Shibuya et al. found that 3-mercaptopyruvate sulfurtransferase, in combination with cysteine aminotransferase, also produces H2S with cysteine as a substrate in neurons (27). H2S is present at a relatively high level in brain of human, rat, and bovine (15). Furthermore, multiple lines of evidence suggest that H2S may function as a neuromodulator in the brain. In peripheral systems, H2S protects heart against ischemic injury, regulates vascular tone, controls the release of insulin, and modulates cell proliferation and apoptosis (2, 9, 29). Although the contribution is minor in comparison to the effect on increasing glutathione levels, H2S protects neurons from excessive excitation by enhancing the activity of adenosine triphosphate-sensitive potassium (K-ATP) channels (12, 13). However, no direct evidence supports that the K-ATP channel is responsible for the neuroprotective effect of H2S in vivo.
Innovation.
We here demonstrate that hydrogen sulfide (H2S) protects dopaminergic (DA) neurons against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced degeneration in an adenosine triphosphate-sensitive potassium channel–independent, but uncoupling protein 2 (UCP2)–dependent mechanism. Accordingly, we propose that H2S may enhance UCP2-induced mild uncoupling of mitochondrial oxidative phosphorylation. As such, reactive oxygen species generation is reduced, which in turn ameliorates oxidative products evoked endoplasmic reticulum stress and subsequent neuronal apoptosis. Ultimately, H2S alleviates the loss of substantia nigra compacta DA neurons and protects against MPTP-induced neurodegeneration. These findings will give us an insight into the potential of H2S in terms of opening up new therapeutic avenues for Parkinson's disease (PD). Promisingly, H2S-based neuroprotective therapies against PD may one day be developed.
K-ATP channels are unique channel proteins that directly couple the metabolic state of a cell to its electrical activity and distribute widely in brain. They are heteroctamers composed of pore-forming Kir6.x (6.1 or 6.2) subunits and sulfonylurea receptor (SUR1 or SUR2) regulatory subunits (25 26). Kir6.2 and SUR1 or SUR2B are expressed in neurons, whereas Kir6.1 and SUR1 or SUR2 are in astrocyte and microglia (31, 32, 36). H2S stimulates the K-ATP channel in the neurons, vascular smooth muscle cells, cardiomyocytes, and pancreatic β-cells, exerting its biological function. The direct evidence was from electrophysiological investigations since H2S increased K-ATP channel currents, which were blocked by antagonists like glibenclamide, in rat aorta and arteria mesenterica. H2S may bind to the cysteine residues (Cys6 and Cys26) of SUR1 or break the disulfide bonds between them. This will change the steric configuration and therefore open K-ATP channels (8, 19, 30). Nevertheless, the exact association of H2S and K-ATP channels is still in need of further exploration.
Although we reported that H2S may be a potential neuroprotectant for PD therapy (6), it remains largely unknown whether this neuroprotection of H2S is mediated by K-ATP channels. Therefore, we established a PD model by injection of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), a classic neurotoxin that destroys DA neurons, in both Kir6.2+/+ and Kir6.2−/− mice to investigate the involvement of K-ATP channels in the neuroprotection of H2S and to gain an insight into the potential strategy for PD therapy. Herein, we found unexpectedly that H2S protected DA neurons against MPTP-induced degeneration in a K-ATP channel-independent manner. However, mitochondrial mild uncoupling has been shown to predominantly reduce generation of reactive oxygen species (ROS), and decreased ROS will reduce the open probability of K-ATP channels. Therefore, uncoupling protein 2 (UCP2) may function as the upstream of the K-ATP channel in determining vulnerability of DA neurons (3). Our study showed that UCP2 mediated the protective effects of H2S against either DA neuron degeneration in the PD mouse model or MPP+-injured primary mesencephalic neurons. These findings indicate that the UCP2 rather than K-ATP channel is responsible for the neuroprotection of H2S.
Results
NaHS protects DA neurons against MPTP-induced degeneration in a Kir6.2/K-ATP channel–independent manner
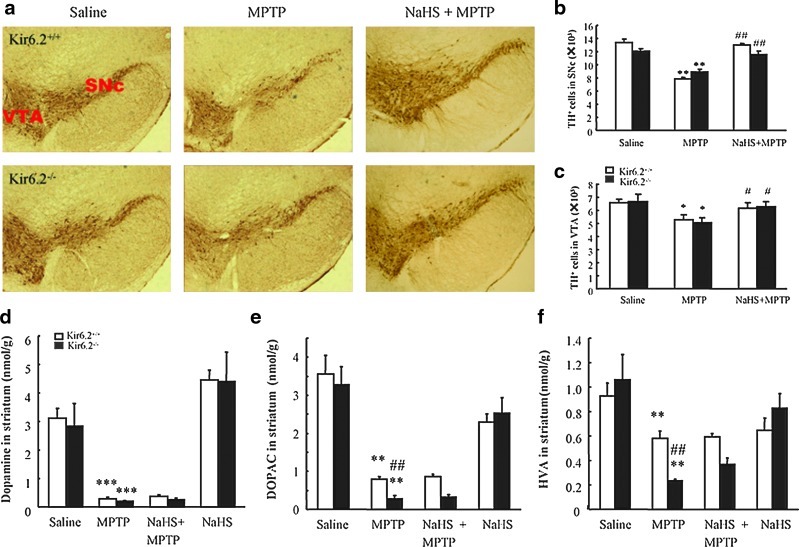
To demonstrate whether K-ATP channels contribute to the effect of H2S on DA neuron degeneration, we established an MPTP-induced subacute model of PD in wild type (WT) and Kir6.2−/− mice. As shown in Figure 1a, administration of MPTP (20 mg/kg, s.c.) for 5 days resulted in the dramatic degeneration of tyrosine-hydroxylase-positive (TH+) neurons in substantia nigra compacta (SNc) and ventral tegmental area (VTA). There was no significant difference in the number of TH+ neurons between WT and Kir6.2−/− mice in response to MPTP treatment. Treatment with sodium hydrosulfide (NaHS) at 5.6 mg/kg for 7 days remarkably rescued the loss of TH+ neurons induced by MPTP in both genotypic mice. Stereological statistics revealed that NaHS almost fully recovered the MPTP-reduced TH+ cell numbers in SNc and VTA of both Kir6.2+/+ and Kir6.2−/− mice to the basal level (Fig. 1b, c). These results indicate that H2S may exert a beneficial effect on DA neuron degeneration in a Kir6.2/K-ATP channel–independent manner.
FIG. 1.
NaHS protected DA neurons against MPTP-induced degeneration in midbrain of wild type and Kir6.2−/− mice. Immunohistochemical staining of THir neurons in SNc of mice with ×40 magnifications (a). Stereological counts of THir cells in mouse SNpc (b) and VTA (c). Data are presented as the mean±S.E.M., n=5 for each group. NaHS had no significant effect on MPTP-reduced levels of DA (d), DOPAC (e), and HVA (f) in the striatum of both genotypic mice. Data expressed as means±S.E.M. from eight mice per group. *p<0.05, **p<0.01, and ***p<0.001 versus saline group in corresponding genotype; #p<0.05 and ##p<0.01 versus corresponding MPTP-treated groups. MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; DA, dopaminergic; TH, tyrosine-hydroxylase; TH+, tyrosine-hydroxylase positive; THir, tyrosine-hydroxylase immunoreactive; VTA, ventral tegmental area; DOPAC, dihydroxyphenylacetic acid; HVA, homovanillic acid; NaHS, sodium hydrosulfide; SNc, substantia nigra compacta; SNpc, substantia nigra pars compacta; S.E.M., standard error of mean. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Depletion of nigrostriatal dopamine is an important pathological character for PD. To examine the effect of H2S on DA neurotransmitters in the MPTP-induced PD mouse model, we measured the levels of dopamine and its metabolic products by high-performance liquid chromatography coupled with electrochemical detection (HPLC-ECD). We found that MPTP treatment resulted in a dramatic reduction of dopamine (Fig. 1d), dihydroxyphenylacetic acid (DOPAC; Fig. 1e), and homovanillic acid (HVA; Fig. 1f) in striatum of both genotypes. However, NaHS (5.6 mg/kg), which alone had no significant effect on DA or its metabolic products, failed to rescue the decreased levels of neurotransmitters caused by MPTP. These data imply that H2S may ameliorate the structural degeneration of DA neuron before the rescue of dopamine metabolism and release.
NaHS protects primary mesencephalic neurons against MPP+-induced cytotoxicity in a Kir6.2/K-ATP channel–independent manner
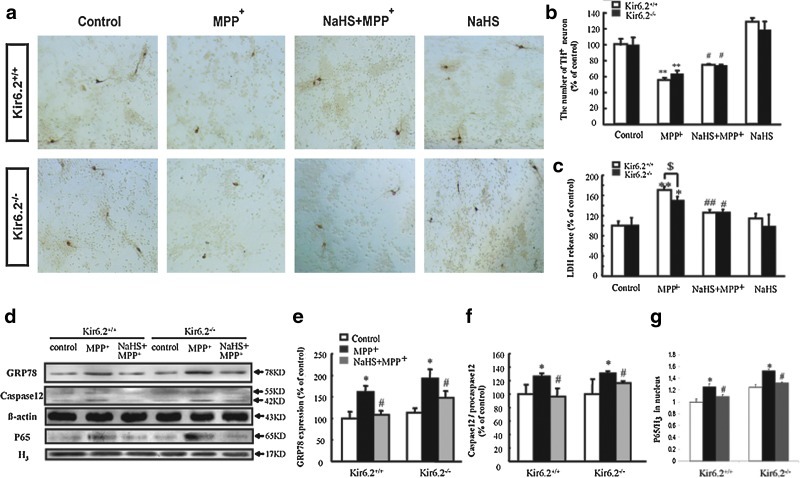
To investigate the potential mechanisms for the protective effect of H2S on PD, we isolated and cultured the primary mesencephalic neurons from WT and Kir6.2−/− mice. Immunocytochemistry showed that the number and morphology of TH+ neurons were equivalent under basal condition in both genotypes (Fig. 2a). Incubation of MPP+ (10 μM) for 48 h visibly decreased the mean length of neuritis and the number of TH+ neurons (Fig. 2a, b) and increased lactate dehydrogenase (LDH) release (Fig. 2c). Pretreatment with NaHS (100 μM, 15 min) significantly attenuated MPP+-reduced number of TH+ neurons and suppressed the MPP+-elevated LDH level to the same extent in both genotypes (Fig. 2a–c). These results further reveal that the protective effect of H2S on DA neuron degeneration in vitro is also independent of the Kir6.2/K-ATP channel.
FIG. 2.
NaHS protected against MPP+-induced cytotoxicity in primary cultured mesencephalic neurons from wild-type and Kir6.2−/− mice. (a) Typical photograph of immunocytochemical staining showing THir neurons at ×100 magnifications. (b, c) Effects of NaHS on the number of TH+ neurons (b) and LDH release (c) in MPP+-treated DA neurons. (d) Typical western blotting revealed that NaHS alleviated ERS and apoptosis in primary mesencephalic neurons isolated from wild-type and Kir6.2−/− mice. Statistical analysis of GRP78 (e), caspase 12 (f), and p65/H3 (g) protein levels in neurons. Data are presented as the mean±S.E.M. of four individual experiments. **p<0.01 and *p<0.05 versus control group; ##p<0.01 and #p<0.05 versus corresponding MPP+-treated groups; $p<0.05 versus Kir6.2−/− group. ERS, endoplasmic reticulum stress; GRP, glucose-regulated protein; LDH, lactate dehydrogenase; MPP+, 1-methyl-4-phenylpyridinium. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
NaHS inhibits endoplasmic reticulum stress-triggered apoptosis in primary mesencephalic neurons isolated from WT and Kir6.2−/− mice
Glucose-regulated protein (GRP) 78, which induces expression in endoplasmic reticulum (ER) lumen and also acts as an apoptotic regulator during endoplasmic reticulum stress (ERS), has been thought to be a biomarker of ERS. As shown in Figure 2d, preincubation with NaHS (100 μM) for 15 min significantly suppressed the upregulation of GRP78 induced by MPP+ in both genotypes (Fig. 2e). Furthermore, the inductions of caspase 12 and p65 represent the activation level of ERS-triggered death pathway and inflammation, respectively. NaHS suppressed MPP+-upregulated expression of caspase 12 (Fig. 2f) and the translocation of p65 into nucleus (Fig. 2g) to a similar extent in both genotypic mesencephalic neurons. These data suggest that H2S may protect DA neurons against degeneration via inhibiting ERS-triggered apoptosis and inflammation.
NaHS attenuates MPTP-triggered glial cell activation and proliferation in the presence or absence of the Kir6.2/K-ATP channel
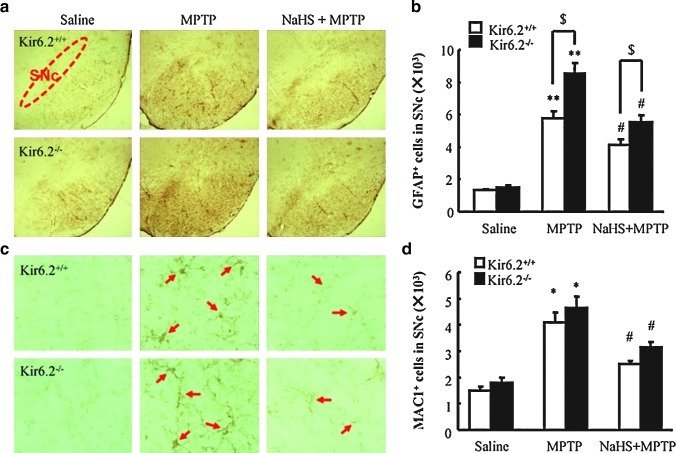
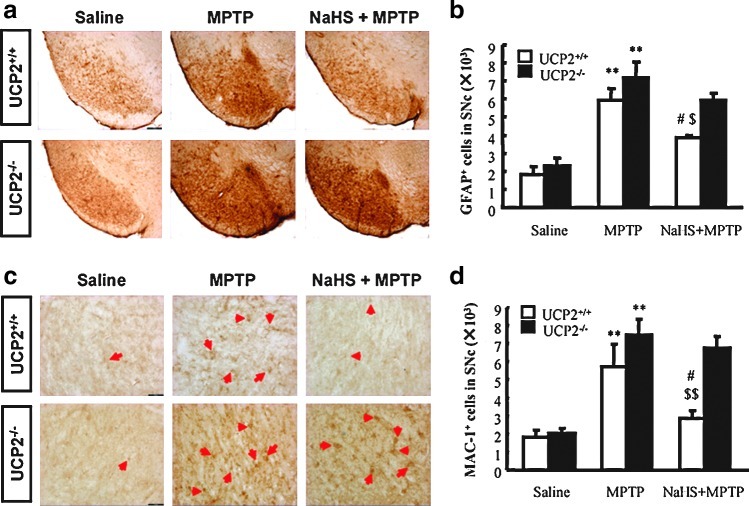
Glial proliferation and overactivation occur during the process of PD. Accompanied by the sustained secretion of inflammatory mediators, the persistent activation of microglia is regarded to be involved in the aggravation of DA neuron degeneration in PD. We therefore examined glial activation in the SNc of mice. As shown in Figure 3, the basal numbers of Glial fibrillary acidic protein (GFAP+, a marker for astrocytes) and Mac-1+ (also called CD11b/C18, a marker for microglia activation) cells were identical in SNc of WT and Kir6.2−/− mice. Administration of MPTP significantly increased the numbers of GFAP+ (426% and 587%, WT and Kir6.2−/−, respectively) and Mac-1+ cells (271% and 261%, WT and Kir6.2−/−, respectively) in SNc compared with those in saline-treated mice. NaHS treatment attenuated the proliferation of GFAP+ (306% and 378% of vehicle, WT and Kir6.2−/−, respectively) (Fig. 3a, b) and Mac-1+ cells (167% and 177% of vehicle, WT, and Kir6.2−/−, respectively) (Fig. 3c, d). These data suggest that low expression of Kir6.2 in astrocytes may be important to maintain the resting status of the glial cells and Kir6.2 knockout aggravated astrocyte activation. Moreover, inhibition of MPTP-induced glial excessive activation and subsequent neuroinflammation also contributes to the potential protective effect of H2S on PD.
FIG. 3.
NaHS attenuated MPTP-triggered glial cell activation and proliferation in the SN of wild-type and Kir6.2−/− mice. (a) Microphotographs of GFAP-ir cells in the SN with ×40 magnification. (b) Stereological counts of GFAP-ir cells in mouse SN. (c) Microphotographs of Mac1-ir cells in the SN with ×200 magnification. (d) Stereological counts of Mac-1-ir cells in mouse SN. n=5. Data are presented as the mean±S.E.M., **p<0.01 and *p<0.05 versus saline group; #p<0.05 versus corresponding MPTP-treated groups; $p<0.05 versus corresponding MPTP-treated Kir6.2−/− groups. SN, substantia nigra; GFAP, Glial fibrillary acidic protein. Arrows indicate Mac-1+ cells. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
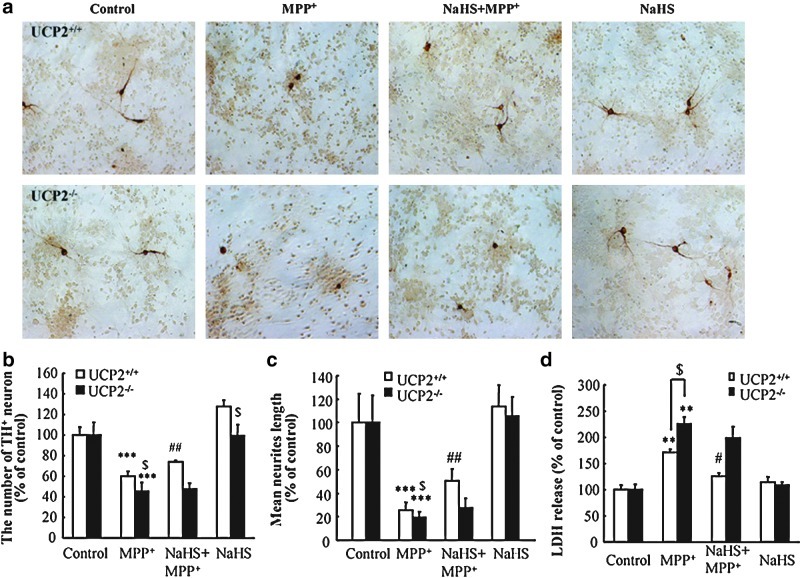
UCP2 deficiency abolishes the protective effects of NaHS against DA neuron degeneration in vivo and in vitro
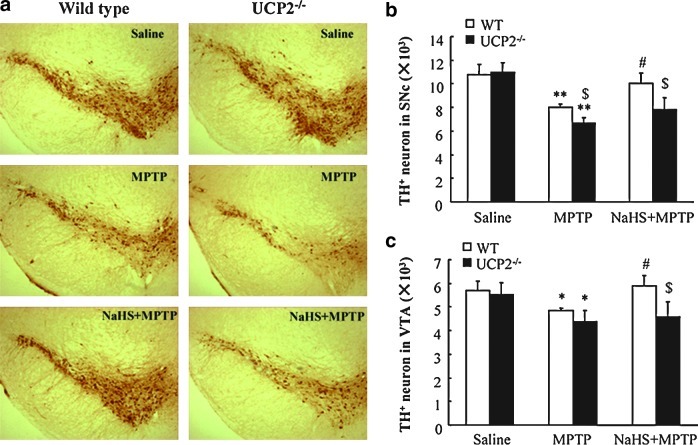
UCP2, located in the inner membrane of mitochondria, reduces ROS production in the respiratory chain and functions as the upstream to K-ATP channels in determine the vulnerability of DA neurons (3). To identify the potential target of H2S, we investigated the effect of H2S in MPTP-treated WT and Ucp2−/− mice. Immunostaining showed that MPTP induced obvious TH+ neuron loss in SNc of WT and Ucp2−/− mice. Notably, NaHS treatment could reverse SNc TH+ neuron damage in WT mice (Fig. 4a, b). However, UCP2 knockout abolished the protective effect of NaHS against MPTP-injured TH+ neurons. Similar results were also exhibited in the VTA region (Fig. 4c). In vitro study showed that MPP+ significantly reduced TH+ cell number and neurites length (Fig. 5a–c) as well as elevated LDH release (Fig. 5d) in the neurons isolated from WT and Ucp2−/− mice. In line with what we found in the in vivo study, NaHS treatment could only markedly attenuate MPP+-induced neuronal injuries in WT mice, but not in Ucp2−/− mice. These results suggest that UCP2 may be the action target of H2S and contribute to the neuroprotective effects of H2S against DA neuron degeneration.
FIG. 4.
UCP2 deficiency abolished the protective effect of NaHS on MPTP-injured TH+ neurons in SNc and VTA. (a) Microphotographs of THir neurons in SNpc of mice with ×40 magnification. Stereological counts of THir cells in SNpc (b) and VTA (c). Data are presented as the mean±S.E.M., n=5 for each group. *p<0.05 and **p<0.01 versus saline group in corresponding genotype; #p<0.05 versus corresponding MPTP-treated Ucp2+/+ groups; $p<0.05 versus corresponding Ucp2+/+ groups. UCP2, uncoupling protein 2. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
FIG. 5.
UCP2 knockout abolished the protective effect of NaHS on MPP+-induced neurotoxicity in primary cultured mesencephalic neurons. THir neurons were stained by immunocytochemistry with ×200 magnifications (a). Effects of NaHS on MPP+-induced neurotoxicity in DA neurons as assessed by the number of TH+ neurons (b), mean neurites length per cell (c), and LDH release (d). Four independent experiments were performed in duplicate. Data were presented as the mean±S.E.M. ***p<0.001 and **p<0.01 versus control group; ##p<0.01 and #p<0.05 versus MPTP-treated Ucp2+/+ group; $p<0.05 Ucp.2+/+ group versus Ucp2−/− group. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
UCP2 knockout revokes the prevention of NaHS on ERS-triggered neuronal apoptosis in the mesencephalon
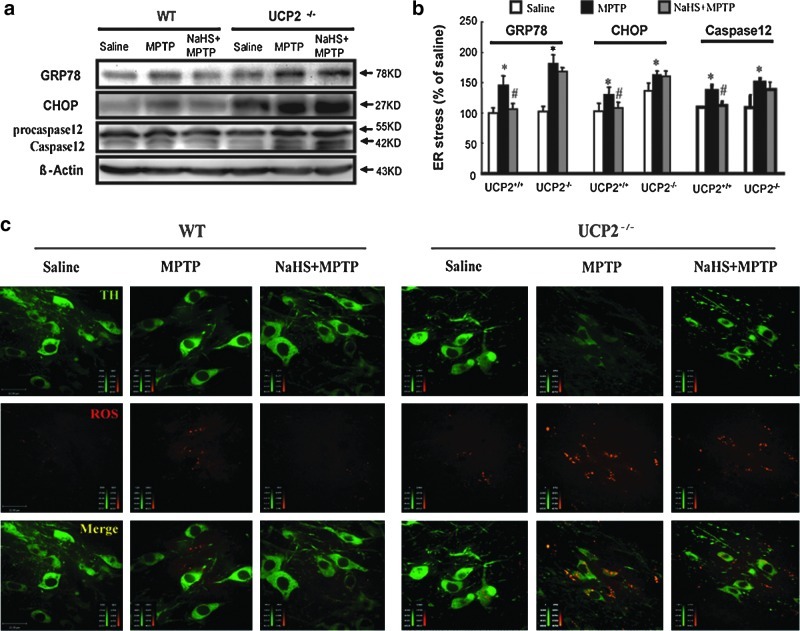
It has been demonstrated that ERS involves DA neuron degeneration in PD and that expression of C/EBP homologous protein (CHOP), which is an ERS-induced transcription factor, can be stimulated by MPTP in the PD mouse model. In this study, we found that treatment with NaHS for 7 days reversed the upregulated protein expression of GRP78, CHOP, and caspase 12 in the mesencephalon of WT mice treated with MPTP. However, these suppressive effects of NaHS on ERS were abolished by UCP2 knockout (Fig. 6a, b). These results suggest that H2S may suppress ERS-triggered apoptosis owing to the existence of UCP2.
FIG. 6.
UCP2 mediated the suppressive effect of NaHS on ROS-triggered ERS in the midbrain. Western blotting analysis of GRP78, CHOP, and caspase 12 protein levels in the midbrain of mice (a, b). UCP2 knockout abolished the inhibitory effect of NaHS on MPTP-induced ROS accumulation in SNc TH+ neurons (c). Photos were taken at ×600 magnification. Green color represents TH neuron and red color represents ROS. Scale bar=22 μm. Data are presented as the mean±S.E.M., n=4 for each group. *p<0.05 versus saline group; #p<0.05 versus corresponding MPTP-treated groups. ROS, reactive oxygen species; CHOP, C/EBP homologous protein. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
UCP2 deletion abolishes the inhibitory effect of NaHS on ROS accumulation in TH+ neurons in SN
Given that intracellular ROS accumulation evokes ERS, we continued to examine ROS production in TH+ neurons in the presence or absence of UCP2 to explore the potential mechanism whereby UCP2 deficiency abolished the effect of H2S on DA neuron degeneration. It was found that MPTP triggered ROS accumulation not only in TH+ neurons but also in astrocytes surrounding TH+ neurons in our previous study (data not shown). As shown in Figure 6c, ROS levels were much higher in SNc TH+ neurons of Ucp2−/− mice treated with and without MPTP compared with those in WT mice, respectively. Moreover, H2S treatment significantly scavenged the accumulation of ROS in WT TH+ neurons, but failed to diminish ROS level in TH+ neurons of Ucp2−/− mice. These findings indicate that existence of UCP2 suppresses intracellular ROS production and mediates the antioxidative role of H2S, whereas UCP2 deletion increases mitochondrial ROS production and further abolishes the protective effects of H2S.
NaHS fails to alleviate MPTP-triggered glial cell activation and proliferation in the absence of UCP2
As shown in Figure 7, there was no significant difference of the morphology and basal amounts of GFAP+ as well as Mac-1+ cells in SNc between WT and Ucp2−/− mice. Administration of MPTP resulted in a significant activation and proliferation of GFAP+ and Mac-1+ cells in SNc compared with those in saline-treated mice. NaHS treatment attenuated the MPTP-enhanced proliferation of GFAP+ (Fig. 7a, b) and Mac-1+ cells (Fig. 7c, d) in WT mice. However, NaHS failed to alleviate MPTP-induced glial cell proliferation in the SNc of Ucp2−/− mice. These data suggest that UCP2-mediated antioxidation involves the inhibitory effect of NaHS on glial cell activation and the subsequent neuroinflammation.
FIG. 7.
UCP2 deletion abolished the inhibitory effect of NaHS on MPTP-triggered glial cell activation and proliferation. (a) Microphotographs of GFAP-ir cells in the SN with ×40 magnification. (b) Stereological counts of GFAP-ir cells in mouse SN. (c) Microphotographs of Mac1-ir cells in the SN with ×200 magnification. (d) Stereological counts of Mac-1-ir cells in mouse SN. n=4. Data are presented as the mean±S.E.M. **p<0.01 versus saline group; #p<0.05 versus corresponding MPTP-treated groups; $p<0.05 and $$p<0.01 versus corresponding Ucp2−/− groups. Arrows indicate Mac-1+ cells. Scale bar=100 μm. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Discussion
We demonstrated in the present study that the Kir6.2/K-ATP channel, which is abundantly expressed in SN DA neurons, was not responsible for the neuroprotective effects of H2S in the MPTP mouse model of PD. Deficiency of UCP2, which functions as the upstream of K-ATP channels (3), abolished the protective effect of H2S on DA neuronal degeneration. Our findings indicate that H2S may target UCP2 to attenuate ROS-triggered ERS and subsequent neuronal apoptosis, suggesting the potential of H2S in prospective therapy for PD.
Among numerous ion channels, the K-ATP channel is a unique one to integrate energy cues with membrane excitability-dependent processes (17, 21). It is inhibited by ATP at the physiologic concentration range, but stimulated when ATP falls during metabolic depletion (28). K-ATP channels contributed to multiple pathological and physiological functions of H2S (30). However, they were not found to mediate the anti-oxidant effects of H2S in the central neurons system (33). The present study was, therefore, designed to investigate the contribution of K-ATP channels in the therapeutic effects of H2S in the MPTP-induced subacute model of PD with Kir6.2−/− mice. Although Birgit Liss et al. reported that Kir6.2 knockout recovered DA neuron loss in SNc in the chronic PD model (16), we found that deficiency of Kir6.2 had no effect on DA neuron degeneration in both SNc and VTA in the subacute MPTP-induced PD model. Notably, our results showed that H2S reversed the loss of DA neurons induced by MPTP in both WT and Kir6.2−/− mice. In vitro study further revealed that H2S protected mesencephalic TH+ neurons against MPP+-induced cytotoxicity in both genotypes. These findings indicate that the beneficial effect of H2S on PD model mice is independent of the Kir6.2/K-ATP channel. In agreement of previous findings (16), we found that dopamine production and survival of DA neurons seemed out of step. H2S failed to rescue MPTP-induced depletion of dopamine and its metabolic products in striatum of both genotypic mice at current experiment condition, suggesting that although NaHS rescued DA neuron loss in SNc, the TH in these survived neurons may not able to produce DA synchronously. This is consistent with the previous finding that structural recovery of DA neuron degeneration is before the amelioration of neuronal function in vivo (37).
So far, the exact mechanisms for DA neuron degeneration in the progress of PD have not been fully understood. It involves multiple pathological impairments such as mitochondrial oxidative stress, inflammation, dysfunction of ubiqutin-protease system, and deposition of abnormal protein. Among these, ER is the major signal-transducing organelle that continuously responds to environmental cues (22, 24) and provides a unique oxidizing compartment for the folding of membrane and secretory proteins (11). Recent studies reveal that ER stress also occurs in the neurodegeneration of PD and MPTP evokes the accumulation of unfolding or misfolding protein and up-regulate expression of GRP78, which in turn initiates the unfolding protein response (4, 23, 38). Recently, Krishnan et al. reported that suppression of endogenous H2S production decreased the phosphorylation of protein kinase-like endoplasmic reticulum kinase, thus reducing its activation in response to ER stress (14). In the present study, we found consistently that H2S attenuated MPTP-induced ER stress and the subsequent apoptosis. Moreover, absence of the Kir6.2/K-ATP channel could not impair the suppressive effect of H2S on ER stress in MPP+-injured DA neurons. These data suggest that the K-ATP channel is not responsible for the neuroprotective effect of H2S in the MPTP-induced PD model.
UCP2, localized in the inner mitochondrial membrane, uncouples respiration from ATP synthesis by providing an alternative route for the protons to enter into the mitochondrial matrix and also functions as a transporter of fatty acids into the mitochondrial matrix (20). The primary physiological function of mild uncoupling is to suppress oxygen radical production accompanied by restricting mitochondrial permeability transition pore opening and Ca2+ accumulation (20). It also plays an important role in regulating the energy balance in many tissues such as pancreas, liver, and brain (10). It has been reported that UCP2 modulated intracellular energy metabolism and thus was involved in aging, diabetes, and neurodegeneration (5, 34). It has been proposed that UCP2 may be upstream to the K-ATP channel in determining vulnerability of DA neurons (3). We therefore identified whether UCP2 was a potential target for the action of H2S by establishing an MPTP PD model in Ucp2−/− mice. Our findings showed that UCP2 knockout could abolish the protective effects of H2S against DA neuron damage in both in vivo and in vitro studies, indicating that UCP2 may be responsible for the neuroprotective action of H2S.
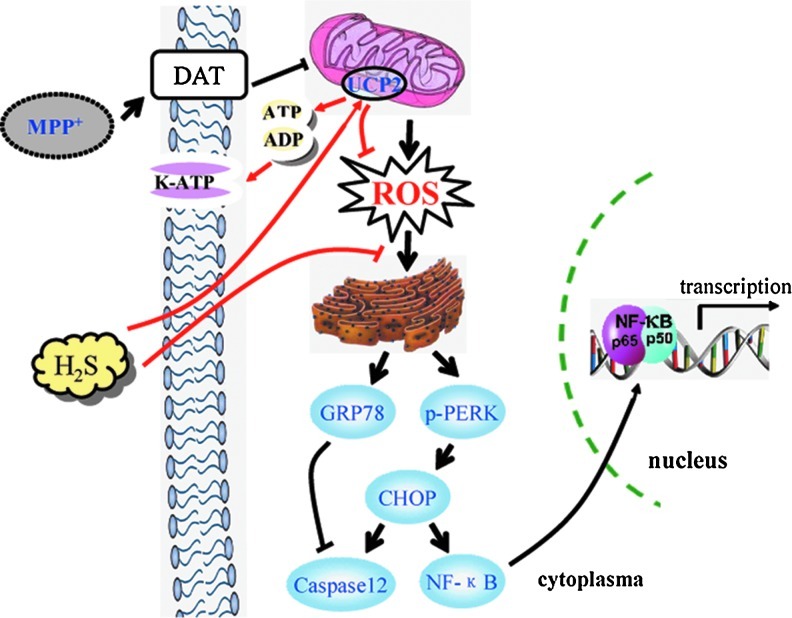
MPTP is converted to MPP+ by monoamine oxidase-B expressed in glial cells and then transported into DA neurons by dopamine transporter. MPP+ selectively inhibits mitochondrial complex I, triggering ROS production and ERS. As a result, upregulated GRP78 and CHOP activate gene transcription, leading to glial cell activation-induced neuroinflammation or caspase 12-mediated neuronal apoptosis. H2S may act on UCP2 and facilitate it to inhibit MPTP-induced ROS accumulation and subsequently triggered ERS, whereby preventing DA neurons from degeneration (Fig. 8). UCP2 deficiency impairs the inhibitory action on mitochondrial ROS production and further leads to the accumulation of higher level oxygen radicals. Consequently, this deficit may largely weaken or abolish the antioxidative effect of H2S on both neurons and glial cells. These findings suggest that UCP2 may act as a potential target of H2S on protecting DA neurons via antioxidation.
FIG. 8.
Schematic model for the mechanism of H2S in protecting against MPP+-induced neuronal injury. MPP+ is selectively transported into DA neurons by DAT and subsequently inhibits the activity of mitochondrial complex I. Accumulated ROS evokes the upregulation of GRP78 and further triggers ERS. Consequently, p65 nuclear translocation-induced gene transcription or caspase 12-mediated apoptosis occurs in DA neurons. H2S-stimulated mitochondria uncoupling reduces the ratio of ATP/ADP, which in turn opens K-ATP channels. On the other hand, H2S enhances UCP2 function and attenuates ROS production, suppressing ERS and apoptosis induced by MPP+. DAT, dopamine transporter; K-ATP, adenosine triphosphate-sensitive potassium; H2S, hydrogen sulfide. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Although our study revealed the neuroprotection of H2S was independent of Kir6.2/K-ATP channels in the PD mouse model, we does not exclude the possibility that K-ATP channels contribute to the cytoprotective actions of H2S in other pathophysiological processes. Since UCP2 uncouples oxidative phosphorylation and reduces the synthesis of ATP (5, 7), it acts as a potential modulator of the K-ATP channel via regulating the ratio of ATP and ADP. H2S may therefore activate UCP2, which in turn decreases ATP level and subsequently opens K-ATP channels. In this case, UCP2 may be upstream molecule of K-ATP channels and is responsible for the neuroprotective effect of H2S on PD. Therefore, although it is not essential for the neuroprotection of H2S in the PD model, the K-ATP channel can still be activated by H2S and mediates multiple functions in other pathophysiological processes.
Materials and Methods
The study protocol was approved by the Institutional Animal Care and Use Committee of Nanjing Medical University.
Animals and reagents
Kir6.2 knockout C57BL/6J mice were donated by Professor Miki (Chiba University, Japan) (25), of which 12–16–weeks-old, 26–32-g male Kir6.2+/+ and Kir6.2−/− mice were used. UCP2 knockout C57BL/6J mice were donated by Professor Chen-Yu Zhang (Nanjing University, P.R. China) with the same age and weight. The expressions of Kir6.2 and UCP2 were detected by RT-PCR (Supplementary Fig. S1; Supplementary Data are available online at www.liebertonline.com/ars)
NaHS and MPTP were purchased from Sigma-Aldrich, while probenecid was from Jinan Times Pharmacology Co. Ltd. MPTP was dissolved in saline and preserved at 0°C, while probenecid was dissolved in dimethyl sulfoxide (DMSO) just 30 min before injection.
Animal model and drug administration
Each type of mice (Kir6.2+/+, Kir6.2−/−, Ucp2+/+, and Ucp2−/−) was divided into four groups: vehicle, MPTP, MPTP+NaHS (5.6 mg kg−1•day−1, intraperitoneal injection [i.p]), and NaHS alone groups. MPTP (20 mg kg−1, subcutaneous [s.c]) was injected 1 h before injection of probenecid (250 mg kg−1, i.p.) for 5 days in MPTP group. Mice in NaHS treatment group received NaHS (5.6 mg kg−1, i.p.) injection for 7 days and MPTP administration 30 min after NaHS injection from day 3 to day 7. In NaHS alone group, NaHS (5.6 mg kg−1, ip) was given for 7 days. The same volume of saline was injected in the 7 days in the vehicle group. Animals were sacrificed 3.5 days after the last injection of MPTP.
Immunohistochemical studies and quantitative evaluation
Immunostaining method was described in the previous publication (6). Images were observed and photos were taken under a confocal microscope (Axiovert LSM510; Carl Zeiss Co.). The immunostaining signals were quantitatively analyzed using the Optical Fractionator method with Microbrightfield Stereo-Investigator software (Stereo Investigator software; Microbrightfield). The total number of TH-immunoreactive (IR) neurons, GFAP-IR astrocytes, and Mac-1-IR microglia in entire extent of substantia nigra pars compacta were counted from four samples per group.
High-performance liquid chromatography
Striatum tissues were collected for measurement of DA, DOPAC, and HVA with HPLC with ECD. HPLC/ECD consisted of BAS LC-4C, a reversed-phase C18 column (Ultrasphere ODS 4.6×250 mm, 5 μm), chromograph interface DA-5, and solvent delivery system. The mobile phase consisted of 0.1 M citrate, 0.075 M Na2HPO4, 0.1 M EDTA, 1.0 mM 1-heptanesulfonic acid, and 10% methanol, pH 3.9.
In situ detection of ROS
Dihydroethidium (Molecular Probes) was used to investigate the local in situ production of ROS. An intravenous injection of 200 μl dihydroedithium (DHE; stock solution, 100 mg/ml in DMSO, diluted 1:100 with sterile saline before injection) was administered through the caudal vein. Three hours after DHE injection, mice were overdosed with dimethyl ether and transcardially perfused with 4% paraformaldehyde (0.1% glutaraldehyde and 15% picric acid in phosphate buffer). After postfixing overnight in paraformaldehyde without glutaraldehyde, sections at 30 μm were cut through the SN using a Leica freezing microtome. To observe ROS in SNc DA cells, sections were incubated overnight with TH antibody (1:3000). After washing for three times, sections were then incubated with the fluorescent secondary antibody, donkey anti-mouse IgG 488 Alexafluore (1:200; Molecular Probes), to visualize TH-IR cells.
Identification and quantification of TH+ neurons and neuronal processes
Mesencephalic neuron primary culture was performed according to the previous publication (35). After incubation with MPP+ (10 μM) for 48 h with or without pretreatment of NaHS (100 μM), cells were rinsed carefully with 0.1 M phosphate buffer solution (PBS) (pH 7.2) and fixed with 4% paraformaldehyde, followed by blocking with PBST (0.03% Triton) containing 10% bovine serum albumin. Cells were then incubated at 4°C overnight with the primary antibody (the mouse monoclonal anti-TH at 1:3000; Sigma). After washing for three times, cells were exposed to 1:800 dilutions of HRP-conjugated goat anti-mouse IgG (Chemicon) for 1 h at room temperature. Immunostaining was visualized with 3,3′-diaminobenzidine. The number of THir neurons was counted in 10 randomly selected fields (1.13 mm2/field) at ×100 magnification on a Nikon Optical TE2000-S inverted microscope. The values were normalized to that obtained from control culture. The average number of THir cells in control cultures ranged from 20 to 30 per field, while THir cells made up about 5% of all cells in primary culture. Cell processes were determined at ×200 magnification. Photomicrographs were taken with a Nikon Optical TE2000-S inverted microscope. Each THir cell process was traced from soma to the end of the process, realized by the measurement function of Image Pro Plus 5.1.
Assay of LDH release
After incubation with MPP+ for 48 h, LDH release was measured in the culture medium with an LDH diagnostic kit (Jiancheng Bioengineering) in accordance with the manufacturer's instructions. LDH activity was calculated by data measured from absorbance at 490 nm. Data were expressed as percentages of LDH release gained from control treatment.
Western blotting analysis
The method was described in previous publication (6). Different primary antibodies (mouse anti-Actin, CHOP, and H3 at 1:1000 [Cell Signaling]; rabbit anti-GRP78 and Caspase 12 at 1:1000 and rabbit anti-p65 at 1:600 [Cell Signaling]) and the secondary antibody (1:1000) were used in the present study.
Statistical analysis
All data were presented as mean±standard error of mean. Statistical significance was assessed with one-way analysis of variance followed by the post hoc Student-Newman-Keuls test between control and samples treated with various factors. Differences with p-values less than 0.05 were considered statistically significant.
Supplementary Material
Abbreviations Used
- CHOP
C/EBP homologous protein
- DA
dopaminergic
- DHE
dihydroedithium
- DMSO
dimethyl sulfoxide
- DOPAC
dihydroxyphenylacetic acid
- ECD
electrochemical detection
- ERS
endoplasmic reticulum stress
- GFAP
glial fibrillary acidic protein
- GRP
glucose-regulated protein
- HPLC
high-performance liquid chromatography
- H2S
hydrogen sulfide
- HVA
homovanillic acid
- IR
immunoreactive
- K-ATP
adenosine triphosphate-sensitive potassium
- LDH
lactate dehydrogenase
- MPP+
1-methyl-4-phenylpyridinium
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NaHS
sodium hydrosulfide
- PBS
phosphate buffer solution
- PD
Parkinson's disease
- ROS
reactive oxygen species
- S.E.M.
standard error of mean
- SN
substantia nigra
- SNc
substantia nigra compacta
- TH
tyrosine-hydroxylase
- UCP2
uncoupling protein 2
- VTA
ventral tegmental area
- WT
wild type
Acknowledgments
We appreciate Prof. Miki (Chiba University, Japan) for donating Kir6.2−/− mice and appreciate Prof. Chen-Yu Zhang (Nanjing University, P.R. China) for donating Ucp2−/− mice. The work reported herein was supported by the grants from the National Key Program of Basic Research of China (No. 2011CB504103), the National Natural Science Foundation of China (No. 81030060 and No. 30873055), the National Science & Technology Major Project (No. 2012ZX09304-001), and Jiangsu Province's Key Discipline of Medicine (XK201117).
Author Disclosure Statement
The authors declare no conflicts of interest.
References
- 1.Abe K. Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bannenberg GL. Vieira HL. Therapeutic applications of the gaseous mediators carbon monoxide and hydrogen sulfide. Expert Opin Ther Pat. 2009;19:663–682. doi: 10.1517/13543770902858824. [DOI] [PubMed] [Google Scholar]
- 3.Deutch AY. Winder DG. A channel to neurodegeneration. Nat Med. 2006;12:17–18. doi: 10.1038/nm0106-17. [DOI] [PubMed] [Google Scholar]
- 4.Holtz WA. O'Malley KL. Parkinsonian mimetics induce aspects of unfolded protein response in death of dopaminergic neurons. J Biol Chem. 2003;278:19367–19377. doi: 10.1074/jbc.M211821200. [DOI] [PubMed] [Google Scholar]
- 5.Hong Y. Fink BD. Dillon JS. Sivitz WI. Effects of adenoviral overexpression of uncoupling protein-2 and -3 on mitochondrial respiration in insulinoma cells. Endocrinology. 2001;142:249–256. doi: 10.1210/endo.142.1.7889. [DOI] [PubMed] [Google Scholar]
- 6.Hu LF. Lu M. Tiong CX. Dawe GS. Hu G. Bian JS. Neuroprotective effects of hydrogen sulfide on Parkinson's disease rat models. Aging Cell. 2010;9:135–146. doi: 10.1111/j.1474-9726.2009.00543.x. [DOI] [PubMed] [Google Scholar]
- 7.Hurtaud C. Gelly C. Chen Z. Levi-Meyrueis C. Bouillaud F. Glutamine stimulates translation of uncoupling protein 2mRNA. Cell Mol Life Sci. 2007;64:1853–1860. doi: 10.1007/s00018-007-7039-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang B. Tang G. Cao K. Wu L. Wang R. Molecular mechanism for H(2)S-induced activation of K(ATP) channels. Antioxid Redox Signal. 2010;12:1167–1178. doi: 10.1089/ars.2009.2894. [DOI] [PubMed] [Google Scholar]
- 9.Kapoor A. Thiemermann C. Hydrogen sulfide, neurogenic inflammation, and cardioprotection: a tale of rotten eggs and vanilloid receptors. Crit Care Med. 2010;38:728–730. doi: 10.1097/CCM.0b013e3181cab0ee. [DOI] [PubMed] [Google Scholar]
- 10.Kashemsant N. Chan CB. Impact of uncoupling protein-2 overexpression on proinsulin processing. J Mol Endocrinol. 2006;37:517–526. doi: 10.1677/jme.1.02091. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- 12.Kimura Y. Dargusch R. Schubert D. Kimura H. Hydrogen sulfide protects HT22 neuronal cells from oxidative stress. Antioxid Redox Signal. 2006;8:661–670. doi: 10.1089/ars.2006.8.661. [DOI] [PubMed] [Google Scholar]
- 13.Kimura Y. Goto Y. Kimura H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid Redox Signal. 2010;12:1–13. doi: 10.1089/ars.2008.2282. [DOI] [PubMed] [Google Scholar]
- 14.Krishnan N. Fu C. Pappin DJ. Tonks NK. H2S-Induced sulfhydration of the phosphatase PTP1B and its role in the endoplasmic reticulum stress response. Sci Signal. 2011;4:ra86. doi: 10.1126/scisignal.2002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SW. Hu YS. Hu LF. Lu Q. Dawe GS. Moore PK. Wong PT. Bian JS. Hydrogen sulphide regulates calcium homeostasis in microglial cells. Glia. 2006;54:116–124. doi: 10.1002/glia.20362. [DOI] [PubMed] [Google Scholar]
- 16.Liss B. Haeckel O. Wildmann J. Miki T. Seino S. Roeper J. K-ATP channels promote the differential degeneration of dopaminergic midbrain neurons. Nat Neurosci. 2005;8:1742–1751. doi: 10.1038/nn1570. [DOI] [PubMed] [Google Scholar]
- 17.Miki T. Liss B. Minami K. Shiuchi T. Saraya A. Kashima Y. Horiuchi M. Ashcroft F. Minokoshi Y. Roeper J. Seino S. ATP-sensitive K+ channels in the hypothalamus are essential for the maintenance of glucose homeostasis. Nat Neurosci. 2001;4:507–512. doi: 10.1038/87455. [DOI] [PubMed] [Google Scholar]
- 18.Moore DJ. West AB. Dawson VL. Dawson TM. Molecular pathophysiology of Parkinson's disease. Annu Rev Neurosci. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- 19.Mustafa AK. Gadalla MM. Sen N. Kim S. Mu W. Gazi SK. Barrow RK. Yang G. Wang R. Snyder SH. H2S signals through protein S-sulfhydration. Sci Signal. 2009;2:ra72. doi: 10.1126/scisignal.2000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagy TR. Blaylock ML. Garvey WT. Role of UCP2 and UCP3 in nutrition and obesity. Nutrition. 2004;20:139–144. doi: 10.1016/j.nut.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 21.Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440:470–476. doi: 10.1038/nature04711. [DOI] [PubMed] [Google Scholar]
- 22.Ron D. Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 23.Ryu EJ. Harding HP. Angelastro JM. Vitolo OV. Ron D. Greene LA. Endoplasmic reticulum stress and the unfolded protein response in cellular models of Parkinson's disease. J Neurosci. 2002;22:10690–10698. doi: 10.1523/JNEUROSCI.22-24-10690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schroder M. Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 25.Seino S. Miki T. Gene targeting approach to clarification of ion channel function: studies of Kir6.x null mice. J Physiol. 2004;554:295–300. doi: 10.1113/jphysiol.2003.047175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seino S. Miki T. Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog Biophys Mol Biol. 2003;81:133–176. doi: 10.1016/s0079-6107(02)00053-6. [DOI] [PubMed] [Google Scholar]
- 27.Shibuya N. Tanaka M. Yoshida M. Ogasawara Y. Togawa T. Ishii K. Kimura H. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal. 2009;11:703–714. doi: 10.1089/ars.2008.2253. [DOI] [PubMed] [Google Scholar]
- 28.Sun XL. Hu G. ATP-sensitive potassium channels: a promising target for protecting neurovascular unit function in stroke. Clin Exp Pharmacol Physiol. 2010;37:243–252. doi: 10.1111/j.1440-1681.2009.05190.x. [DOI] [PubMed] [Google Scholar]
- 29.Szabo C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 30.Tang G. Wu L. Wang R. Interaction of hydrogen sulfide with ion channels. Clin Exp Pharmacol Physiol. 2010;37:753–763. doi: 10.1111/j.1440-1681.2010.05351.x. [DOI] [PubMed] [Google Scholar]
- 31.Thomzig A. Pruss H. Veh RW. The Kir6.1-protein, a pore-forming subunit of ATP-sensitive potassium channels, is prominently expressed by giant cholinergic interneurons in the striatum of the rat brain. Brain Res. 2003;986:132–138. doi: 10.1016/s0006-8993(03)03222-0. [DOI] [PubMed] [Google Scholar]
- 32.Thomzig A. Wenzel M. Karschin C. Eaton MJ. Skatchkov SN. Karschin A. Veh RW. Kir6.1 is the principal pore-forming subunit of astrocyte but not neuronal plasma membrane K-ATP channels. Mol Cell Neurosci. 2001;18:671–690. doi: 10.1006/mcne.2001.1048. [DOI] [PubMed] [Google Scholar]
- 33.Tyagi N. Moshal KS. Sen U. Vacek TP. Kumar M. Hughes WM., Jr. Kundu S. Tyagi SC. H2S protects against methionine-induced oxidative stress in brain endothelial cells. Antioxid Redox Signal. 2009;11:25–33. doi: 10.1089/ars.2008.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vogler S. Goedde R. Miterski B. Gold R. Kroner A. Koczan D. Zettl UK. Rieckmann P. Epplen JT. Ibrahim SM. Association of a common polymorphism in the promoter of UCP2 with susceptibility to multiple sclerosis. J Mol Med (Berl) 2005;83:806–811. doi: 10.1007/s00109-005-0661-5. [DOI] [PubMed] [Google Scholar]
- 35.Xie J. Duan L. Qian X. Huang X. Ding J. Hu G. K(ATP) channel openers protect mesencephalic neurons against MPP+-induced cytotoxicity via inhibition of ROS production. J Neurosci Res. 2010;88:428–437. doi: 10.1002/jnr.22213. [DOI] [PubMed] [Google Scholar]
- 36.Zhou F. Yao HH. Wu JY. Ding JH. Sun T. Hu G. Opening of microglial K(ATP) channels inhibits rotenone-induced neuroinflammation. J Cell Mol Med. 2008;12:1559–1570. doi: 10.1111/j.1582-4934.2007.00144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou QY. Palmiter RD. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell. 1995;83:1197–1209. doi: 10.1016/0092-8674(95)90145-0. [DOI] [PubMed] [Google Scholar]
- 38.Zhu JH. Horbinski C. Guo F. Watkins S. Uchiyama Y. Chu CT. Regulation of autophagy by extracellular signal-regulated protein kinases during 1-methyl-4-phenylpyridinium-induced cell death. Am J Pathol. 2007;170:75–86. doi: 10.2353/ajpath.2007.060524. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.