Abstract
Pax gene haploinsufficiency causes a variety of congenital defects. Renal-coloboma syndrome, resulting from mutations in Pax2, is characterized by kidney hypoplasia, optic nerve malformation, and hearing loss. Although this underscores the importance of Pax gene dosage in normal development, how differential levels of these transcriptional regulators affect cell differentiation and tissue morphogenesis is still poorly understood. We show that differential levels of zebrafish Pax2a and Pax8 modulate commitment and behavior in cells that eventually contribute to the otic vesicle and epibranchial placodes. Initially, a subset of epibranchial placode precursors lie lateral to otic precursors within a single Pax2a/8-positive domain; these cells subsequently move to segregate into distinct placodes. Using lineage-tracing and ablation analyses, we show that cells in the Pax2a/8+ domain become biased towards certain fates at the beginning of somitogenesis. Experiments involving either Pax2a overexpression or partial, combinatorial Pax2a and Pax8 loss of function reveal that high levels of Pax favor otic differentiation whereas low levels increase cell numbers in epibranchial ganglia. In addition, the Fgf and Wnt signaling pathways control Pax2a expression: Fgf is necessary to induce Pax2a, whereas Wnt instructs the high levels of Pax2a that favor otic differentiation. Our studies reveal the importance of Pax levels during sensory placode formation and provide a mechanism by which these levels are controlled.
Keywords: Pax2, Pax8, Epibranchial, Otic, Placode, Zebrafish
INTRODUCTION
The sensory organs of the vertebrate head develop from discrete regions of neurogenic epithelium known as cranial placodes. Placodes are transient ectodermal thickenings situated lateral to the forming central nervous system (CNS) and neural crest. Responding to extrinsic signals, cranial placode cells delaminate, migrate and condense to form diverse structures, including the otic vesicle and neurons of the cranial ganglia. The epibranchial (EB) placodes give rise to the EB ganglia, the facial, glossopharyngeal and vagal (also known as the geniculate, petrosal and nodose) ganglia that relay information from sensory organs to the CNS.
Cranial placodes are derived from the pan-placodal ectoderm (PPE) (Streit, 2004; Schlosser, 2005), which develops during late gastrulation as a horseshoe-shaped region adjacent to the neural plate and lateral to the neural crest (Brugmann et al., 2004; Litsiou et al., 2005; Ahrens and Schlosser, 2005). Once formed, the PPE is subdivided into distinct subdomains containing shared precursors for multiple placodes (Bailey et al., 2006; Schlosser, 2006). Previous studies suggested that otic and EB placode precursors might arise from a common subdomain of the PPE, the posterior placodal area (PPA) (Schlosser and Ahrens, 2004; Ohyama and Groves, 2004; Sun et al., 2007; Freter et al., 2008). However, one zebrafish study showed that glossopharyngeal and vagal (but not facial) placode precursors were induced from adjacent non-neural ectoderm by Fgf24 secreted from the PPA (Padanad and Riley, 2011). Thus, it is unclear whether the PPA contributes cells to the EB placodes.
The PPA is delineated by Paired-box2 (Pax2) and Pax8 expression at the onset of somitogenesis (Streit, 2002; Schlosser and Ahrens, 2004; Ohyama and Groves, 2004; Bouchard et al., 2004) with Pax8 and Pax2a acting redundantly in otic and EB placode development (Bouchard et al., 2004; Padanad and Riley, 2011). Humans with PAX2 haploinsufficiencies exhibit renal hypoplasia, vesicoureteric reflux and optic nerve coloboma (Alur et al., 2010; Schimmenti, 2011), whereas persistent expression of PAX2 causes a variety of cystic and dysplastic renal diseases (Cunliffe et al., 1998; Davis et al., 2011). This implies that levels of this transcriptional regulator must be strictly controlled (Eccles et al., 2002). However, whether different levels of Pax2a/8 play a role during sensory organ development is unknown.
Fgf and Wnt are both crucial during otic and EB placode induction. In zebrafish and chick, hindbrain- and mesoderm-derived Fgf signals act redundantly to induce EB placodes (Nechiporuk et al., 2006; Nikaido et al., 2007; Freter et al., 2008) and are required for initial otic placode induction. Attenuated Fgf signaling, coupled with increased hindbrain-derived Wnt8, subsequently leads to otic commitment and eventual inner ear patterning (Ohyama et al., 2006). Although Wnt and Fgf signaling requirements for defining otic and EB fate have been demonstrated, it is unclear how these signals control Pax2a/8 expression during placode resolution.
We show that in zebrafish, as in mouse and chick, the otic placode arises from a Pax2a+ PPA precursor domain that also contributes to the EB placodes. Through loss-of-function and overexpression analyses, we find that relative Pax2a/8 expression levels correlate with distinct placodes: high Pax2a/8 levels promote otic differentiation whereas cells with low Pax2a/8 levels acquire an EB bias. We show that Wnt pathway activation promotes high levels of Pax2a expression, biasing PPA cells towards otic commitment. We propose that Wnt directs PPA cell segregation, in part, by altering Pax2a/8 expression levels.
MATERIALS AND METHODS
Fish strains, maintenance, BIO and heat-shock treatments
Zebrafish husbandry was performed as described (Westerfield, 2000), and staging expressed in hours post-fertilization (hpf) (Kimmel et al., 1995). The following lines were used in this study: *AB, Tg(pax2a:GFP)e1 (Picker et al., 2002), TgBAC(phox2b:EGFP)w37 (Nechiporuk et al., 2006), pax2ab593/+ (Erickson et al., 2007), Tg(hsp70:ca-fgfr1)pd3 (Marques et al., 2008), Tg(hsp70:dnfgfr1-EGFP)pd1 (Lee et al., 2005), Tg(hsp70:tcfΔC-EGFP) (Martin and Kimelman, 2012) and Tg(hsp70:dkk1-GFP)w32 (Stoick-Cooper et al., 2006). Heterozygous pax2ab593/+ were used to generate homozygous offspring, identified by midbrain-hindbrain boundary loss. The GSK3-β inhibitor 6-bromoindirubin-3′-oxime (BIO; Sigma) was dissolved in DMSO at 10 mM and diluted to working concentrations in embryo medium. All heat-shock experiments were conducted at 10 hpf. Transgene expression (via EGFP reporter) was visible 45-60 minutes post heat-shock. Wild-type controls and heterozygous embryos obtained by outcrossing Tg(hsp70:ca-fgfr1)pd3 to *AB were heat-shocked between 36.9°C and 38°C for 45 minutes. Tg(hsp70:dnfgfr1-EGFP)pd1 were outcrossed to *AB and heat-shocked between 35°C and 37.2°C for 30 minutes. Tg(hsp70:Δtcf-EGFP)/+ fish were crossed to wild type and heat-shocked at 38°C or 39°C for 30 minutes. Tg(hsp70:dkk1-GFP)w32/+ fish were incrossed and embryos were heat-shocked at 39°C for 40 minutes. Following heat-shock, GFP+ embryos were identified under epifluorescence; GFP− embryos were used as controls.
Fate-mapping experiments
For photoconversion or fluorescein uncaging, Tg(pax2a:GFP)e1 embryos were injected with 100 pg of NLS-Kaede mRNA, 250 pg of patagRFP mRNA (Subach et al., 2010) or 1 nl of 1.25% caged fluorescein (Invitrogen) in 0.2 M KCl. At 11.5 hpf, embryos were mounted and labeling performed using an FV1000 confocal microscope under a 60×/NA=1.20 water objective (Olympus) using a small region of interest (ROI) in the Bleach function. Zoom was increased to 30× and selected cells excited with a 405 nm laser at 0.1% output. Only embryos with labeling entirely contained within the Pax2a domain were analyzed. Kaede-expressing embryos were live-imaged at 24 hpf to locate converted cells. In fluorescein uncaging experiments, embryos were fixed in 4% paraformaldehyde in PBS at 24 hpf and assayed for Pax2a expression.
Whole-mount in situ hybridization and immunostaining
Immunostaining and in situ hybridization were performed as described (Andermann et al., 2002). The following antibodies and riboprobes were used: anti-Pax2 (1:100, Covance), anti-GFP (1:1000, Invitrogen), anti-fluorescein (1:1000, Roche), anti-Elavl 3/4 (1:1000, Invitrogen; sold as anti-Hu C/D), anti-Dlx3b (1:100, Zirc), pax2a (Krauss et al., 1991), pax8 (Pfeffer et al., 1998), fgf3 (Kiefer et al., 1996) and fgf8 (Reifers et al., 1998). It is unknown whether the Pax2 antibody recognizes both Pax2a and Pax2b paralogs; however, immunostaining mirrored pax2a mRNA distribution. Whole-mount confocal images were obtained using an FV1000 (Olympus). Brightfield images were acquired with an AxioImager Z1 compound microscope (Zeiss). Assembly of z-stack images, heat-map analysis and mean fluorescence intensity measurements were performed using ImageJ (Abramoff et al., 2004). Comparisons of mean fluorescence intensities were carried out in parallel using the same dilution of anti-Pax2; confocal settings were consistent for each acquisition. Brightness and contrast were adjusted using Photoshop (Adobe).
Cellular ablations
Specific regions of the PPA in Tg(pax2a:GFP)e1 embryos were ablated at 11 hpf using previously described methods (Sagasti et al., 2009). Following ablations, the entire PPA was imaged. Embryos were allowed to develop to 24 hpf then were fixed and immunostained for Pax2a.
Pax2a misexpression
A bi-directional heat-shock-inducible construct was modified to contain the pax2a coding sequence (or egfp as control) with a dTomato reporter (Bajoghli et al., 2004). Each plasmid (20 pg) was injected into wild-type embryos, which were heat-shocked at 12 and 14 hpf at 38°C for 30 minutes.
Morpholino microinjections
The following antisense morpholino oligonucleotides (MO; Corvalis, OR, USA) were diluted to working concentrations in water and injected into TgBAC(phox2b:EGFP)w37 embryos: pax2a-MO (5′-ATATGGTGTCTCACCTATAGTGTGT-3′; gift from Joshua Waxman, Cincinnati Children's Hospital, OH, USA), pax8E5/I5-MO+pax8E9/I9-MO (Hans et al., 2004). Efficacy of pax2a-MO was assessed by RT-PCR using the following primers: pax2a-MO-F, 5′-GCAGAATACAAGCGGCAAAATC-3′; pax2a-MO-R, 5′-CGTAAACTCTCCACACTACCCTGAG-3′.
Transplantation experiments
Donor zygotes from Tg(hsp70:tcfΔC-EGFP)/+X*AB crosses were injected with 10 kD rhodamine-dextran (Invitrogen) in 0.2 M KCl. Embryos were dechorinated and allowed to develop to sphere and shield stage for donors and wild-type hosts, respectively. Twenty donor cells were transplanted into the presumptive placodal domain of host embryos (Kozlowski et al., 1997). Mosaic embryos were heat-shocked at 10 hpf at 39°C for 30 minutes, fixed at 24 hpf and immunostained for Pax2a and EGFP.
RESULTS
Pax2a-positive PPA precursors are spatially biased at the four-somite stage
Previous studies indicate that the EB and otic placodes arise from a common domain, the PPA, consisting of Pax2/8-positive precursors (Bouchard et al., 2004). However, a study in zebrafish showed that glossopharyngeal and vagal placode precursors arose from non-neural ectoderm lateral to the PPA (Padanad and Riley, 2011). We investigated whether the PPA contained both EB placode and otic precursors and whether these populations were segregated or intermingled. To address this, we utilized a Tg(pax2a:GFP)e1 line with GFP driven by a 5.3 kb portion of the pax2a promoter (Picker et al., 2002). By comparing expression of Tg(pax2a:GFP)e1 with Pax2a immunolabeling, we found that the transgene largely recapitulated endogenous early-stage Pax2a expression in the PPA. At 11 hpf, the transgene was expressed at low levels in a small population of cells lateral to the neural plate (data not shown). By 12 hpf, the GFP+ and Pax2a+ cells aggregated lateral to rhombomeres 5 and 6 (supplementary material Fig. S1A-A″ and Movie 1). Whereas at 24 hpf the transgene was expressed in all otic vesicle cells, it marked only a small subset of Pax2a+ cells in the facial and glossopharyngeal/vagal placodes (supplementary material Fig. S1B-B″). We concluded that the transgene contains necessary regulatory elements to mark PPA cells during early development, but is insufficient to drive GFP expression in most of the EB placodes.
We utilized early Tg(pax2a:GFP)e1 expression to mark specific cells within the PPA. Using a nuclear-localized Kaede protein (NLS-Kaede; 119 out of 139 specimens) or caged fluorescein-dextran (20 of 139), small groups of cells were labeled in discrete regions of the PPA (Fig. 1A,C). The initial location of the cells at 12 hpf was recorded and assigned to one of 12 domains (Fig. 1E). Importantly, we saw little variability in either cell number or dimensions of the GFP+ domain (107.8±7.2 cells; n=11; 1186 cells in total), indicating consistency of this domain between labeled embryos (supplementary material Fig. S1C,D). Additionally, few cells were added to this domain after 12 hpf (data not shown), which imparted little variation between labeled embryos. This is in contrast to a previous study suggesting that additional cells are recruited to this domain after 12 hpf (Bhat and Riley, 2011). This difference could be attributed to the more sensitive confocal detection technique used in our study, which allowed visualization of the most lateral, low-expressing Tg(pax2a:GFP)e1+ cells as early as 12 hpf. At 24 hpf, the fate of labeled cells was determined based on morphology and location of Kaede-labeled cells, and on Pax2a antibody labeling for fluorescein-positive cells (Fig. 1B,D).
Fig. 1.
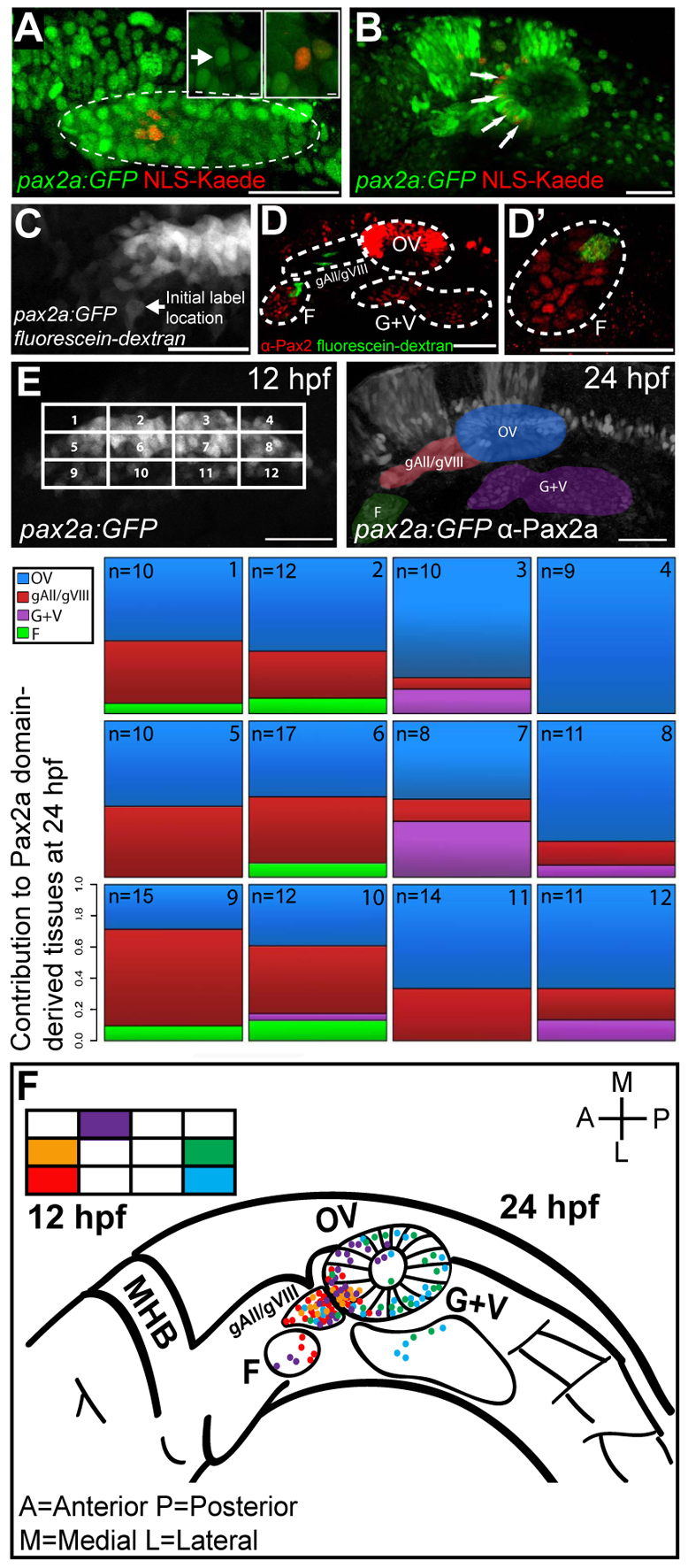
PPA fate-map. (A) NLS-Kaede photoconversion in the PPA (dashed outline) of a Tg(pax2a:GFP)e1 transgenic embryo at 12 hpf. Insets show single z-planes at high magnification before (left inset; arrow) and after (right inset) photoconversion. The Pax2a domain is delineated by GFP expression (dashed outline). (B) Confocal projection showing labeled cells (red; arrows) at 24 hpf. Red nuclei outside the otic vesicle are incidentally photoconverted epidermal cells. (C) Confocal projection of a caged fluorescein-dextran photoconversion (arrow) in the PPA of a Tg(pax2a:GFP)e1 transgenic embryo at 12 hpf. (D,D′) Confocal projection showing labeled cells (green) and Pax2a+ cells (red) at 24 hpf. (E) Fate map of the 12 hpf Pax2a domain, divided into 12 regions, each containing ~3×4 (12 total) cells except for corners where cell numbers vary. The location of labeled cells was assessed at 24 hpf and the proportion of labeling events recorded for each of the 12 regions. In instances in which multiple tissues were labeled at 24 hpf, proportions were calculated by dividing the number of endpoint label instances by the total number of labeled tissues resulting from labeling each region. (F) Fate map of all labeling events from five selected subregions (2, 5, 8, 9 and 12). F, facial placode; gAll/gVIII, anterior lateral line ganglion/acoustic ganglion; G+V, glossopharyngeal/vagal placodes; MHB, midbrain hindbrain boundary; OV, otic vesicle. Scale bars: 50 μm in main panels, 5 μm in insets.
The resulting fate map revealed a strong spatial bias within the PPA at 12 hpf. The subdomains of the PPA that contributed to the facial and glossopharyngeal/vagal placodes exhibited minimal overlap and corresponded to their respective anterior or posterior locations (Fig. 1E), suggesting that a subset of cells from the PPA contribute to the EB placodes (22 out of 139 labeling events).
In contrast to the spatial bias observed for the EB placode precursors, cells contributing to the otic vesicle were distributed throughout the Pax2a domain. However, anterolateral labeling events were more likely to label the acoustic (gVIII) and anterior lateral line (gAll) ganglia whereas posteromedial labeling marked the otic vesicle (Fig. 1F). We never observed labeled cells in the posterior lateral line ganglion. We found that otic vesicle patterning is based on the initial location of precursor cells in the PPA: cells from the anterior PPA preferentially segregated to the anterolateral otic vesicle (supplementary material Fig. S2A-C) whereas cells from the posterior and posteromedial regions were distributed throughout the posterior and posteromedial aspects (supplementary material Fig. S2C-E). The bias of anterior contributions to the anterolateral otic vesicle is consistent with previous observations indicating that the anterior neurogenic region is established prior to otic placode formation (Abello et al., 2010).
Our fate-mapping experiments indicated that most PPA cells did not undergo extensive rearrangements after 12 hpf; however, a subset can contribute to more distal locations (e.g. anterior PPA cells contributed to the otic vesicle; Fig. 1F). To visualize this, we labeled small clusters of PPA cells at 12 hpf and analyzed their behavior using live imaging (supplementary material Movies 2, 3). These analyses confirmed that although PPA cells generally retained their anteroposterior (A-P) position, cells occasionally exhibited extensive movement (supplementary material Movie 2). Cells not incorporated into the otic vesicle were also found migrating towards EB placodes (supplementary material Movie 3).
Our fate-mapping data support the assertion that not all cells of the EB placodes arise from the PPA (Padanad and Riley, 2011). However, a subset of the PPA contributes to the EB placodes; at 12 hpf, these cells exhibited a spatial bias for EB placode precursors. In addition, the bulk of the otic vesicle arises from the posteriomedial regions of the PPA, with anterior regions contributing to the neurogenic anterolateral portion of the otic vesicle and the gAll/gVIII.
Cells of the Pax2a domain are required for normal development of the epibranchial and otic placodes
Our fate-mapping data demonstrated spatial restriction within the PPA by 12 hpf. To confirm this, we unilaterally ablated different regions of the PPA in 12 hpf transgenic Tg(pax2a:GFP)e1 embryos. Embryos were then immunostained for Pax2a at 24 hpf to visualize EB placodes and otic vesicles on the ablated and unablated (contralateral) sides. Ablating the anterior PPA yielded dramatic reductions in the facial placode versus the contralateral side (Fig. 2A,C,D) without affecting the otic vesicle or glossopharyngeal/vagal placodes. Conversely, ablating the posterior PPA resulted in a near-complete loss of the otic vesicle and a marked reduction in the glossopharyngeal/vagal placodes whereas the facial placode was unaffected (Fig. 2B,E,F). Thus, consistent with our fate-map, cells exhibit co-linearity between their initial positions in the PPA and their destinations within resolved placodes.
Fig. 2.
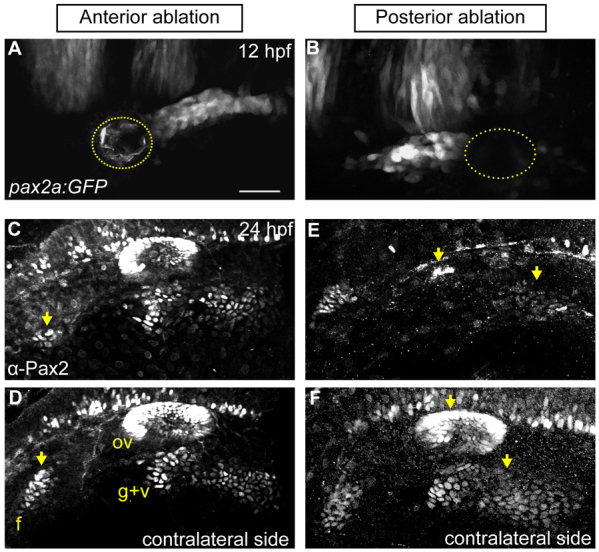
PPA cells are required for normal epibranchial and otic placode development. (A,B) Confocal projection of a Tg(pax2a:GFP)e1 transgenic zebrafish embryo; anterior quarter (A) or posterior third (B) of the PPA was ablated at 12 hpf. Ablated region indicated by dotted line. (C-F) Embryos shown in A and B were Pax2a immunolabeled at 24 hpf, showing expression in otic vesicle and EB placodes. (C,D) Ablated and contralateral sides of the embryo in A. Facial placode on the ablated side exhibits an 81% reduction versus contralateral control; otic and posterior EB placodes are unaffected. (E,F) Ablated and contralateral sides of the embryo in B. Ablated side shows 95% loss of the otic vesicle and 72% reduction in the glossopharyngeal/vagal placodes versus control. Facial placode is unaffected. Arrows indicate corresponding placodes on ablated and control sides. Scale bar: 50 μm
Differential levels of Pax2a expression in the PPA
As the PPA is defined by the expression of Pax2a, we investigated next whether different levels of Pax2a affect differentiation of the otic and EB placodes. At 11 hpf, PPA cells exhibited low and uniform levels of Pax2a (Fig. 3A,A′). However, by 12 hpf, Pax2a+ progenitors had assumed different expression levels with a significant increase in the number of high-expressing cells in the posteromedial region (Fig. 3B,B′,D). These differential levels of Pax2a persisted in PPA-derived structures; cells in the EB placodes expressed Pax2a at low levels, whereas the otic vesicle expressed Pax2a at high levels in the medial-most aspect and at lower levels in the lateral-most aspect (Fig. 3C,C′). We also observed differential levels of pax2a mRNA that mirrored differential protein levels in PPA cells at 12 hpf and in the otic vesicle and EB placodes at 24 hpf, indicating that differences in Pax2a protein originate at the transcriptional level (supplementary material Fig. S3A-C).
Fig. 3.
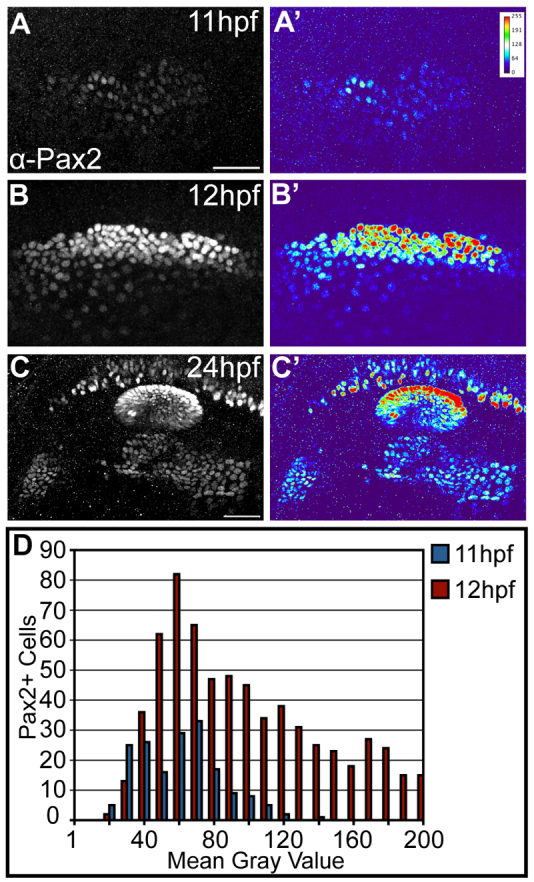
Differential levels of Pax2a expression in the zebrafish PPA. (A,A′) Low, uniform Pax2a expression at 11 hpf. Heat map in A′ shows fluorescence intensity. (B,B′) At 12 hpf, Pax2a expression levels occur in a wider range. (C,C′) Fully segregated placodal precursors with structures exhibiting differential Pax2a expression at 24 hpf. The otic placode exhibits high Pax2a expression; the EB placodes display low levels (C′). (D) Pax2a fluorescence mean gray value (MGV) distribution at 11 and 12 hpf (n≥175 cells, seven embryos per time point). Note significant increase in the number of cells with high Pax2a expression at 12 hpf (χ2-test; P<<0.001). High Pax2a levels defined as MGV>100. Scale bars: 50 μm.
Another Pax gene, pax8, has been described as functionally redundant to pax2a during otic and EB placode formation (Padanad and Riley, 2011). Unfortunately, levels of Pax8 protein cannot be examined owing to the lack of a zebrafish-compatible antibody. pax8 transcripts are seen in zebrafish as early as 8 hpf in presumptive PPA (Pfeffer et al., 1998). We found that pax8 expression was discernable in the PPA at 11 and 12 hpf as well as in EB placodes and discrete foci of the otic vesicle at 24 hpf (supplementary material Fig. S3D-F). Thus, like pax2a, levels of pax8 transcripts were also differentially regulated in PPA cells. These observations suggest that heterogeneous levels of Pax2a and Pax8 expression might reflect early cell biases towards specific placodal destinations.
High levels of Pax2a instruct an otic placode bias
Because Pax2a expression levels correlate with PPA cell identity, we investigated whether overexpression or reduction of Pax2a would alter precursor commitment or differentiation. We conducted overexpression experiments using a bi-directional heat-shock-inducible promoter driving Pax2a (or EGFP control) in one direction and the dTomato reporter in the other. DNA was injected into wild-type embryos followed by heat-shock at 12 and 14 hpf, resulting in mosaic expression. The construct induced Pax2a expression comparable to high endogenous levels; 96% of cells that were positive for dTomato also expressed high levels of Pax2a at 15 hpf (supplementary material Fig. S4). Induced Pax2a was quickly turned over and was undetectable in most dTomato+ cells by 24 hpf (Fig. 4B); at this stage, we assessed relative numbers of dTomato+ cells in the EB placodes and otic vesicle (Fig. 4A,B). Following induction at 12 hpf and 14 hpf, 57% and 65% of dTomato+ cells contributed to the ear versus 44% and 45% in control embryos, respectively (Fig. 4C). There was also a significant decrease in facial placode cell contribution in Pax2a overexpression embryos (11% and 10% versus 20% and 17% in controls). We also observed a significant progressive decrease in cells populating the glossopharyngeal/vagal placodes in both induction conditions (controls: 36% and 38%; Pax2a overexpression: 32% and 25%; Fig. 4C). To examine this bias, we used live imaging to follow Pax2a-overexpressing cells in Tg(pax2a:GFP)e1 embryos. We observed that Pax2a+ overexpressing cells from the lateral non-neural ectoderm moved medially to join the forming otic vesicle; dTomato+ cells that did not integrate died off more frequently versus GFP+ controls (supplementary material Movies 4, 5). These data indicate that high levels of Pax2a alter cell behavior resulting in preferential segregation to the otic placode versus the EB placodes.
Fig. 4.
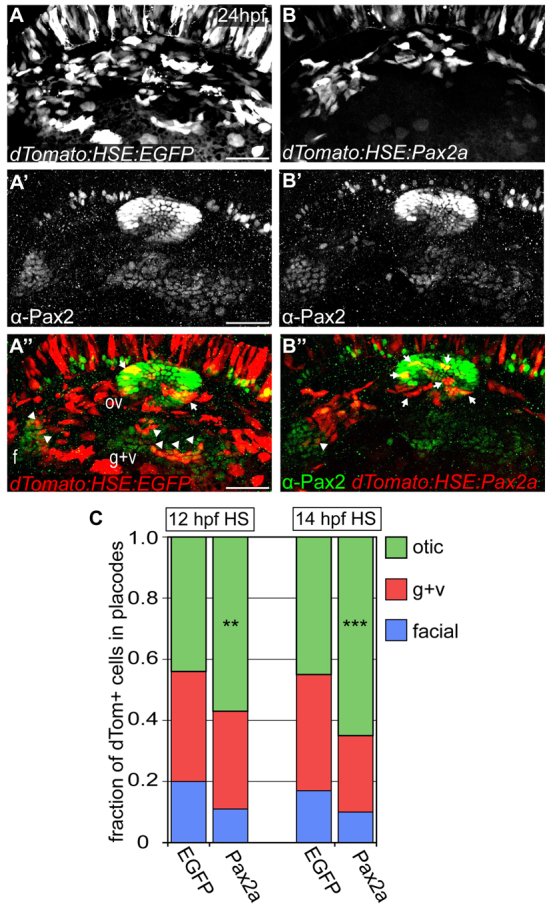
High levels of Pax2a bias otic contribution. (A-B″) Zebrafish embryos expressing bi-directional heat-shock-inducible plasmid (heat-shocked at 12 and 14 hpf) driving egfp (A-A″) or pax2a (B-B″) in one direction and dTomato (dTom) in the other. Arrows indicate Pax2a-misexpressing cells sequestered to otic placode. Arrowheads indicate cells segregated to EB placodes. (C) Relative contribution of dTomato+ cells to the otic vesicle and EB placodes at 24 hpf in EGFP (controls) and Pax2a (overexpression) embryos. Pax2a-overexpressing cells are prone to otic contribution, segregating less frequently to EB placodes versus controls (n≥192 cells from 8-12 embryos per condition; χ2-test; **P<0.016, ***P<0.001). f, facial placode; g+v, glossopharyngeal/vagal placodes; HS, heat-shock; ov, otic vesicle. Scale bars: 50 μm.
Partial knockdown of pax transcripts increases cell numbers in the epibranchial ganglia
We posited that if raising levels of Pax2a biases precursor cells towards otic commitment, reducing Pax2a levels might affect commitment or differentiation of PPA cells to EB placodes. Previous studies indicate that both Pax2a and Pax8 are required during otic and EB placode development (Padanad and Riley, 2011). However, whether differential levels of these factors bias cell commitment is unknown. Injection of 1 ng of pax8 morpholino (pax8-MO) into zygotes from a heterozygous pax2a+/− incross resulted in severe reductions or absence of EB ganglia (supplementary material Fig. S5A,B) in ~25% of progeny, supporting gene redundancy during EB development. We subsequently examined the effect of partial combinatorial reductions in Pax2a and Pax8 on the otic vesicle and EB ganglia.
The pax8-MO used in our studies was characterized previously (Hans et al., 2004). Doses of pax2a splice-blocking MO that yield partial knockdown were estimated based on Pax2a expression levels in the PPA at 12 hpf using RT-PCR (supplementary material Fig. S6 and Fig. S5I). Injections of partial knockdown concentrations of pax2a-MO (3.0 ng) into wild-type embryos markedly reduced levels of Pax2a in the PPA versus controls at 12 hpf. Transgenic zygotes were injected with either pax2a-MO or pax8-MO alone, or in combination at increasing concentrations. The numbers of cells in the forming cranial ganglia were quantified at 50 hpf using TgBAC(phox2b:EGFP)w37 expression (Nechiporuk et al., 2006). Suboptimal concentrations of individual pax morpholinos alone yielded no change in cranial ganglion cell numbers versus controls (supplementary material Fig. S5). However, when partial knockdown concentrations were co-injected, we saw a significant increase in the numbers of Phox2b+ neurons in the facial, glossopharyngeal and anteriormost small vagal ganglia. As dosages increased, ganglion sizes decreased (Fig. 5A-C), indicating loss of precursor differentiation (Padanad and Riley, 2011). We also observed a concomitant reduction (albeit not statistically significant) in the size of the otic vesicle (Fig. 5D). Therefore, relative levels of Pax2a/8 expression instruct cell commitment during subsequent placode segregation, with low levels favoring EB placode differentiation and higher levels favoring otic differentiation.
Fig. 5.
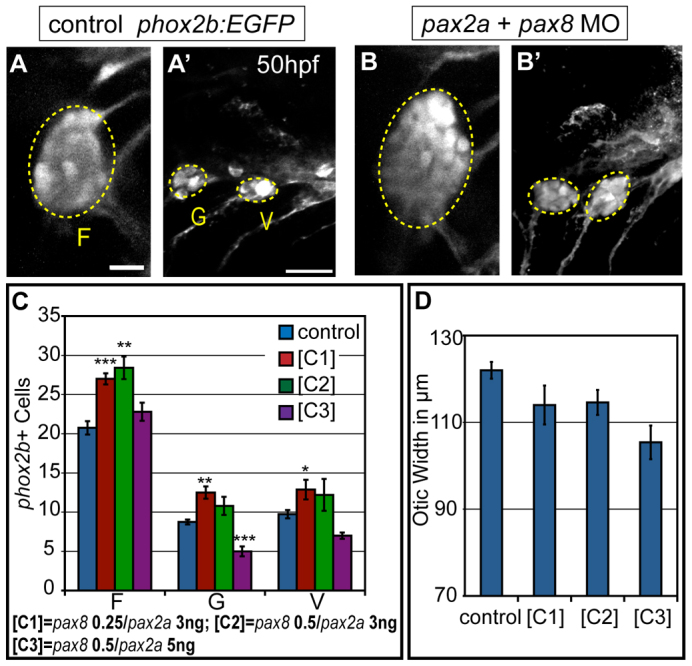
Partial knockdown of pax2a and pax8 transcripts increases cell numbers in EB ganglia. Zebrafish zygotes carrying the TgBAC(phox2b:EGFP)w37 transgene were injected with different amounts of pax2a+pax8 MOs; EB ganglion cell numbers were assayed at 50 hpf. (A,A′) Confocal projection in uninjected control. Facial (F), glossopharyngeal (G) and vagal (V) ganglia (dashed ovals) contain 20, nine and eight EGFP-positive cells, respectively. (B,B′) Confocal projection of an embryo injected with 3+0.25 ng of pax2a+pax8 MOs. Note increased size of G and small V ganglia (F, 29; G, 13; V, 13 cells). (C) Quantification of cells in F, G and small V ganglia in controls and embryos that received increasing doses of pax2a+pax8 MOs. Note significant size increase in all ganglia at [C1]. ***P<0.001, **P<0.005, *P<0.05. (D) Quantification of otic width (longest A-P segment in μm) of controls and embryos receiving increasing doses of pax2a+pax8 MOs (Student's t-test, P=0.056). n≥33 cells, eight embryos per condition. Error bars represent s.e.m. Scale bars: 10 μm in A; 25 μm in A′.
Fgf regulates the number of Pax2a-positive progenitors but does not control levels of Pax2a
Previous studies demonstrated that Fgf signaling was necessary for Pax2 expression to initiate otic and EB development (Ladher et al., 2010); however, Fgf must be attenuated during later stages for continued otic development (Freter et al., 2008). We investigated whether similar Fgf signaling requirements exist in zebrafish and whether Fgf levels bias PPA cell behavior. We examined Pax2a expression in transgenic embryos carrying Tg(hsp70:ca-fgfr1), a heat-shock-inducible form of constitutively active Fgfr1 (Marques et al., 2008). The transgene was differentially induced using a range of heat-shock temperatures (36.9-38°C). Transgenic embryos were heat-shocked at 10 hpf and Pax2a expression analyzed at 24 hpf (supplementary material Fig. S7A,B). At the highest heat-shock induction temperature, Pax2a+ cell numbers increased more than threefold in the facial placode, whereas Pax2a+ cell numbers in the otic vesicle and the glossopharyngeal/vagal placodes decreased significantly (supplementary material Fig. S7D). Examination of intermediate stages (12-18 hpf) and time-lapse imaging of Tg(pax2a:GFP)e1 revealed that Pax2a+ cells were continuously generated from non-neural ectoderm lateral to the PPA between 14 and 18 hpf in induced Tg(hsp70:ca-fgfr1) embryos, but not in wild-type controls (supplementary material Movies 1, 6). Increased facial placode size is probably explained by this dramatic increase in Pax2a+ precursors and not higher proliferation levels (supplementary material Fig. S7F).
Consistent with the live imaging and previous studies (Hans et al., 2007; Padanad et al., 2012), the overall number of otic cells (as measured by Dlx3b expression) increased at 18 hpf following Tg(hsp70:ca-fgfr1) induction, although these cells appeared to be more dispersed than in controls (supplementary material Fig. S8A,C). This increase in cell number was also accompanied by a significant increase in apoptosis after 18 hpf (supplementary material Fig. S7G), which accounts for smaller otic vesicles in Tg(hsp70:ca-fgfr1) embryos at 24 hpf. However, we cannot rule out the possibility that the observed increase in cell death was due to persistently high levels of transgene expression. In summary, our data indicate that high levels of Fgf activity favor the formation of facial and otic, but not glossopharyngeal/vagal, placodes.
We also conditionally inhibited Fgf signaling using Tg(hsp70:dnfgfr1-EGFP): a heat-shock-inducible dominant-negative form of Fgfr1 (Lee et al., 2005). The transgene was induced at 10 hpf using a range of heat-shock conditions (35-37.2°C) and Pax2a expression was analyzed at 24 hpf (supplementary material Fig. S7A,C). Here, the facial placode was severely reduced or completely absent, and the glossopharyngeal/vagal placodes failed to form. At higher induction temperatures, the otic vesicle was severely reduced (supplementary material Fig. S7E), confirming a requirement for Fgf during placode formation.
We then investigated whether modulating Fgf alters otic vesicle and EB placode size via Pax2a. Tg(hsp70:dnfgfr1-EGFP) and Tg(hsp70:ca-fgfr1) embryos were heat-shocked at 10 hpf at 36.3°C and 37.°C, respectively, and levels of Pax2a expression were analyzed at 12 hpf (supplementary material Fig. S7N). We found that the distribution of Pax2a intensity levels were similar between experimental conditions and control. We conclude that Fgf signaling is sufficient to induce Pax2a+ precursors from non-neural ectoderm and that persistent Fgf favors formation of the facial and otic placode, but not glossopharyngeal/vagal placodes. However, Fgf does not establish heterogeneous Pax2a levels in PPA progenitors.
Overactivation of Wnt biases PPA cells to an otic commitment
Studies in chick, Xenopus and mouse demonstrated that canonical Wnt activation is required to restrict a subset of posteromedial PPE cells to an otic fate (Freter et al., 2008; Park and Saint-Jeannet, 2008; Ohyama et al., 2006). Based on this and our observation that high-expressing Pax2a cells segregate to the otic placode, we hypothesized that Wnt might influence otic differentiation by inducing high levels of Pax2a within the PPA. To test this, embryos were treated from 11 hpf with BIO, which inhibits GSK3-β-mediated degradation of β-catenin, thereby overactivating canonical Wnt signaling (Sato et al., 2003). At 12 hpf, we observed dramatic Pax2a level increases in treated embryos (Fig. 6A-C). These increases were dose dependent, with average fluorescence intensities of 107.1 and 151.6 for 2.5 and 5.0 μM BIO conditions, respectively (88.4 for control). The proportion of PPA cells expressing high levels of Pax2a increased in the presence of 5.0 μM BIO (P<0.001, χ2-test; Fig. 6G). Pax2a expression analyses at 24 hpf revealed dramatic increases in otic vesicle Pax2a+ cell numbers (2.5-fold increase for 2.5 μM BIO and 1.5-fold increase for 5.0 μM BIO), with a concomitant 80% reduction in the facial placode at 2.5 μM BIO (Fig. 6D-F,I). At 5.0 μM BIO, the otic vesicle was enlarged and the EB placodes completely absent. The increase in otic cells correlated with higher Pax2a expression levels in the otic vesicle (Fig. 6H). Loss of EB placodes was not due to developmental delay, as EB ganglia were either severely reduced or completely absent at 50 hpf in TgBAC(phox2b:EGFP) embryos treated with BIO between 11 and 24 hpf (supplementary material Fig. S9). Importantly, BIO-induced effects were specific to the canonical Wnt pathway, as these phenotypes were lost when the Wnt pathway was blocked downstream of GSK3-β via Tg(hsp70:tcfΔC-EGFP) activation (Martin and Kimelman, 2012) (supplementary material Fig. S10).
Fig. 6.
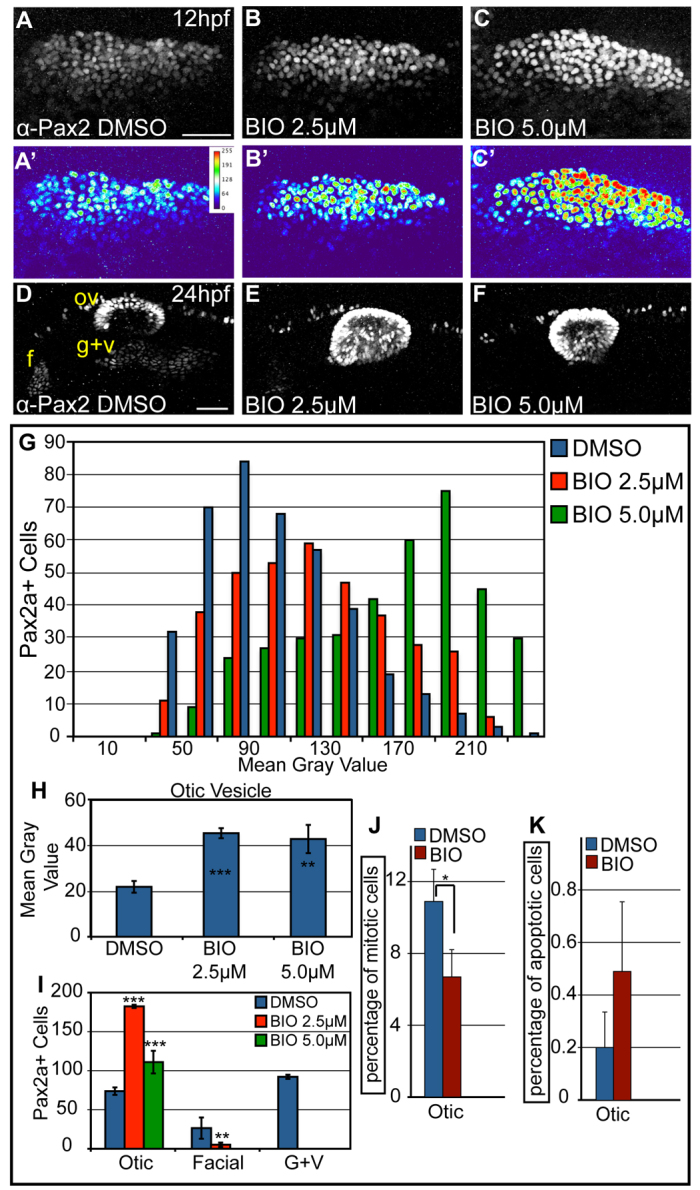
Overactivation of Wnt signaling increases levels of Pax2a expression and biases cells to an otic commitment. (A-C) Confocal projections of DMSO- and BIO-treated zebrafish embryos between 11 and 12 hpf and analyzed for Pax2a expression in the PPA. (A′-C′) Heat maps show increased Pax2a levels after BIO treatment. (D-F) Confocal projection of embryos treated with DMSO or BIO between 11 and 24 hpf and immunolabeled with Pax2a. In BIO-treated embryos, the otic vesicle is larger, whereas EB placodes are significantly reduced (80-100% decrease) (G) Analysis of PPA Pax2a+ cells in control and BIO-treated embryos (2.5 and 5.0 μM) at 12 hpf (n≥354 cells, five embryos per condition) shows dose-dependent increases in expression from low (MGV<120) to high (MGV>120) (χ2, P<<0.001). (H) Comparison of MGVs for Pax2a fluorescence in the otic vesicle showed a significant increase in Pax2a expression levels following BIO treatment (Student's t-test; **P<0.007; ***P<0.001). (I) Pax2a+ cell number in the otic vesicle showed a 2.47-fold increase in 2.5 μM BIO and a 1.5-fold increase in 5.0 μM BIO versus controls (Student's t-test; **P<0.01; ***P<0.001). (J) Percentage of Pax2a+ mitotic cells in the otic vesicle dropped at 18 hpf following 10-hour-long BIO treatment (*P<0.05; n=12 embryos per condition). (K) The percentage of Pax2a+ cells that were also Caspase-3+ at 18 hpf in embryos treated with BIO between 10-18 hpf (n=12 embryos per condition) was unchanged. f, facial placode; g+v, glossopharyngeal/vagal placodes; MGV, mean gray value; ov, otic vesicle. Scale bars: 50 μm.
Next, we endeavored to determine the cellular bases of the BIO-induced phenotype. Cell proliferation was not responsible for increased Dlx3b+ otic progenitors (Fig. 6J; supplementary material Fig. S8B,E), whereas the decrease in EB placodal progenitors was not due to increased cell death (Fig. 6K). Live imaging of Tg(pax2a:GFP)e1 embryos treated with BIO between 11 and 24 hpf revealed that anterior PPA cells failed to move rostrally, remaining in the presumptive otic region (supplementary material Movie 7). This demonstrates that Wnt activation alters PPA cell behavior towards otic differentiation, possibly through high levels of Pax2a expression.
Inhibition of Wnt signaling reduces Pax2a expression levels and favors formation of the facial placode
We determined next whether inhibiting the canonical Wnt pathway affects Pax2a levels and PPA progenitor cell behavior. We used Tg(hsp70:tcfΔC-EGFP), a heat-shock-inducible dominant-negative form of the Wnt effector Tcf3 fused to EGFP (Martin and Kimelman, 2012). We heat-shocked transgenic embryos for 30 minutes at 38°C or 39°C at 10 hpf, using EGFP-negative siblings as controls. At 12 hpf, with Wnt signaling loss, we observed dose-dependent decreases in PPA Pax2a expression levels (χ2-test, P<0.001; Fig. 7A-C,G). The otic vesicle was dramatically reduced by 24 hpf, with significant concomitant facial placode expansion (Fig. 7D-F). We measured Pax2a protein levels in the otic placode and observed decreases of 63% at 38°C and 75% at 39°C (Student's t-test: P<0.0033, P<0.0017, respectively; Fig. 7H). We also observed fewer Pax2a-expressing cells in the otic placode in both conditions: 75% and 91% reductions (P<<0.001) with concurrent 1.4- and 2.5-fold increases (P<0.05, P<0.005, respectively) in facial placodes (Fig. 7I). The glossopharyngeal/vagal placode was reduced significantly under both conditions (71% reduction; Fig. 7I). These effects were recapitulated by the conditional induction of the Wnt antagonist Dkk1 using the Tg(hsp70:dkk1-GFP) line (supplementary material Fig. S11). Wnt signaling loss did not alter levels of cell proliferation or cell death in the otic/EB precursors (Fig. 7J,K). Interestingly, we observed a more modest reduction in Dlx3b+ otic cells (25%) versus Pax2a+ cells (61%) at 18 hpf, confirming that a subset of otic vesicle cells either do not express Pax2a or express Pax2a at low levels (supplementary material Fig. S8D,E). These results indicate that the absence of Wnt signaling promotes low Pax2a levels in the PPA and favors facial placodal development; conversely, high levels of Wnt signaling induce high Pax2a levels and promote otic development.
Fig. 7.
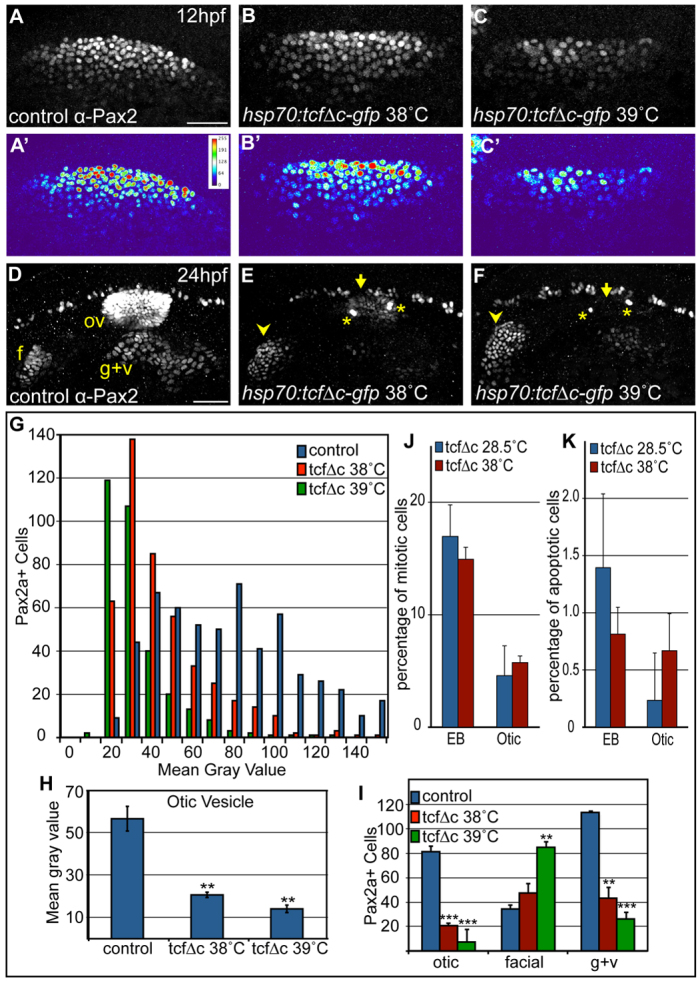
Inhibition of Wnt signaling reduces Pax2a levels resulting in a cell segregation shift from otic to facial placode. (A-C′) Confocal projections from control and Tg(hsp70:tcfΔC-EGFP) zebrafish embryos heat-shocked at 10 hpf and analyzed for Pax2a expression at 12 hpf. Note decreased Pax2a levels in heat map images as heat-shock stringency increases (A′-C′). (D-F) Control and Tg(hsp70:tcfΔC-EGFP)+ embryos heat-shocked at 10 hpf and analyzed for Pax2a expression at 24hpf (arrowheads, facial placode; arrows, otic vesicle; asterisks, putative otic sensory patches). (G) PPA Pax2a expression MGVs at 12 hpf. Note significant shift from high to low Pax2a levels with increased heat-shock stringency (χ2-test; P<<0.001). (H) Average MGV of Pax2a expression in the otic vesicle was significantly reduced in Tg(hsp70:tcfΔC-EGFP)+ embryos (heat-shocked at 39°C) versus controls (n≥317 cells from five embryos per condition; unpaired t-test; **P<0.003). (I) Quantification of Pax2a+ cells in control and Wnt-inhibited embryos (heat-shocked at 39°C) reveals significant otic vesicle and glossopharyngeal/vagal placode cell losses, with concurrent increases in facial placode (***P<0.001; **P<0.01). (J) Percentage of mitotic cells (by pH3 immunolabeling) at 18 hpf following heat-shock of Tg(hsp70:tcfΔC-EGFP) embryos at 10 hpf was unchanged versus uninduced controls (n=12 embryos per condition). (K) There is no significant change in Caspase-3+ cell percentage at 18 hpf following heat-shock of Tg(hsp70:tcfΔC-EGFP) embryos at 10 hpf (n=12 embryos per condition). Error bars represent s.e.m. f, facial placode; g+v, glossopharyngeal/vagal placodes; MGV, mean gray value; ov, otic vesicle. Scale bars: 50 μm.
Wnt activation is cell-autonomously required for otic development
Wnt regulation of Pax2a in the PPA may be direct or indirect. Direct activation of Wnt signaling leads to otic commitment in chick and mouse (Freter et al., 2008; Ohyama et al., 2006). However, a previous zebrafish study suggested that canonical Wnt signaling does not directly affect otic development; rather, Wnt maintains hindbrain expression of fgf3 and fgf8, both of which are required for otic induction (Phillips et al., 2004). However, in our hands, modulating Wnt activity did not disrupt fgf3/8 expression in the hindbrain (supplementary material Fig. S12). To explore further whether intracellular Wnt activity drives high levels of Pax2a expression and subsequent otic commitment, we employed the Tg(hsp70:tcfΔC-EGFP) line to perform mosaic analyses. A small number of Tg(hsp70:tcfΔC-EGFP)+ (or wild-type) donor cells were transplanted into wild-type hosts. Mosaic embryos were heat-shocked at 10 hpf and Pax2a expression examined at 24 hpf (Fig. 8). We reasoned that if intracellular activation of the Wnt pathway is required for otic development, then PPA cells deficient for Wnt activity (TcfΔC-EGFP+ cells) should be excluded from the otic vesicle. Indeed, the vast majority of Tg(hsp70:tcfΔC-EGFP)+ donor cells (92%) contributed to the EB placodes (Fig. 8B-D) versus 42% in controls (χ2-test: P<<0.001; Fig. 8A,D). Interestingly, although very few Tg(hsp70:tcfΔC-EGFP)+ donor cells were found in the otic vesicle, a substantial number of donor cells contributed to the presumptive gAll/gVIII (Fig. 8C,D). These data reveal a cell-autonomous requirement for Wnt signaling in PPA cells for otic development.
Fig. 8.
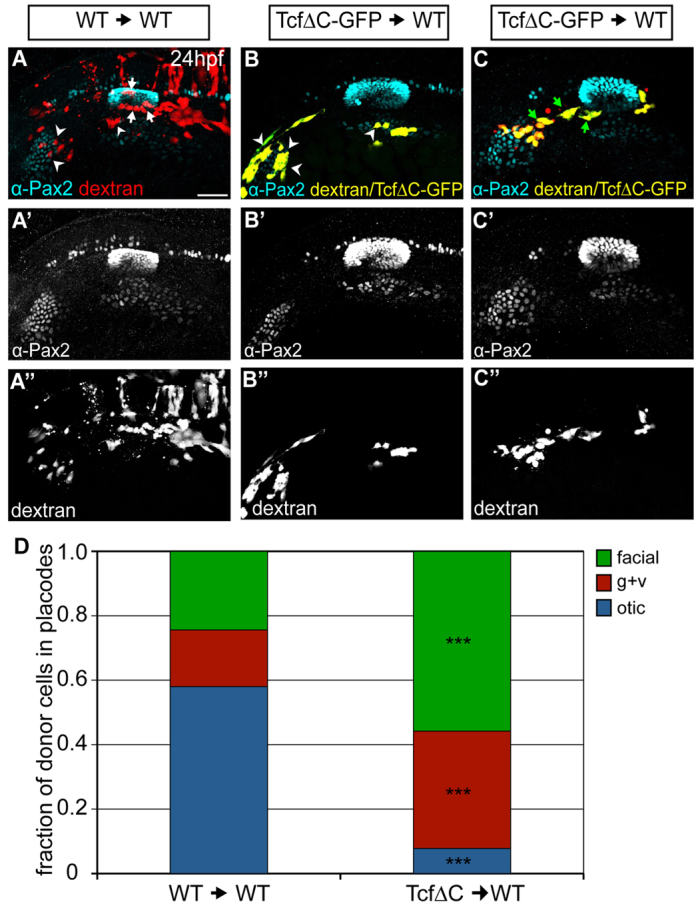
Wnt activation is required cell-autonomously for otic commitment. (A-C″) Wild-type zebrafish hosts containing rhodamine-dextran-positive cells from wild-type (red, A) or Tg(hsp70:tcfΔC-EGFP) (yellow, B,C) donors were heat-shocked at 10 hpf and immunolabeled for Pax2a (cyan) at 24 hpf. Cells derived from Tg(hsp70:tcfΔC-EGFP) donors appear yellow owing to colocalization of EGFP and rhodamine-dextran. Arrowheads indicate donor cells in EB placodes; white arrows mark donor cells in the otic vesicle, green arrows mark donor cells in presumptive gAll/gVIII. (D) Relative contributions of donor cells to otic vesicle and EB placodes at 24 hpf in wild-type and Tg(hsp70:tcfΔC-EGFP) transplants. Tg(hsp70:tcfΔC-EGFP)+ cells are biased towards facial and glossopharyngeal/vagal placodes, rarely segregating to the otic vesicle [total of 77 and 131 donor cells counted from 11 Tg(hsp70:tcfΔC-EGFP) and ten wild-type transplants, respectively; χ2-test; ***P<<0.001]. Scale bar: 50 μm.
DISCUSSION
Our study, in context with previous work, suggests a new model for the patterning of the PPA into distinct placodes. During early somitogenesis, Fgf signals from the neural tube and head mesoderm promote formation of the multipotent Pax2a/8-positive PPA domain that gives rise to the otic placode and contributes cells to EB placodes. Shortly after the PPA is specified, Wnt signaling from the neural tube promotes otic commitment in the posteromedial PPA, potentially by driving high levels of Pax2a/8 expression. We speculate that, in parallel with other factors, these differential levels of Pax2a/8 modulate cellular behaviors that contribute to segregation and subsequent morphogenesis of otic or EB progenitors. Signals from the PPA, including Fgf24 for glossopharyngeal/vagal placodes (Padanad and Riley, 2011) and an unknown signal for the facial placode, induce additional EB placode cells from non-neural ectoderm.
PPA cell segregation is biased during early somitogenesis
Our fate-mapping and ablation experiments show that only a subset of PPA cells contribute to EB placodes; however, PPA formation is required for proper EB placode induction. Fate mapping indicated that EB placode precursors within the PPA are axially restricted, corresponding to the future anteroposterior position of their respective placodes. However, only 16% of labeling events marked EB placodes, indicating a limited contribution of the PPA to the EB placodes. Live-imaging experiments revealed that PPA contribution to EB placodes was limited (supplementary material Movie 3). By contrast, labeling large regions of non-neural ectoderm immediately adjacent to the PPA resulted in labeling within both EB placodes and intervening ectoderm (data not shown), supporting the notion that additional EB placode contributors are derived through induction from the non-neural ectoderm. Recent work showed that PPA-derived Fgf24 signals to the lateral ectoderm were required to induce glossopharyngeal/vagal placodal precursors (Padanad and Riley, 2011). This is reiterated by our ablation experiments, as loss of the posterior PPA yielded a marked reduction in the glossopharyngeal/vagal placodes. Interestingly, ablation of the anterior PPA led to near-complete loss of the facial placode, indicating that other PPA-derived signals are required for facial placode formation. Our present and previous work suggest that this signal is an Fgf, as inhibition of Fgf receptor activity between 11.5 and 16 hpf completely blocked formation of the facial placode without affecting the otic placode (Nechiporuk et al., 2006).
Unlike EB placode precursors, otic vesicle precursors are found throughout the Pax2a domain. However, labeling posteromedial regions resulted in contributions throughout the otic vesicle, whereas labeling of anterolateral regions yielded a majority of labeled cells within the anterolateral side of the otic vesicle, a neurogenic region that gives rise to the acoustic ganglion (Bricaud and Collazo, 2006). Consistent with our fate mapping, posterior PPA ablation yields near-complete loss of the otic vesicle, indicating that most otic precursors lie in the posterior region of this domain. By contrast, previous work in chick indicated extensive cell mixing of otic and EB precursors; however, the labeling was performed at earlier embryonic stages (3 somites and earlier vs 5-6 somites in the present study) and much larger regions of ectoderm were labeled using DiI or DiO dyes (Streit, 2002). A more recent study in frog indicated that progressively less cell mixing occurred between the otic and EB precursors when they were labeled at later developmental stages (Pieper et al., 2011). The anterolateral-posteromedial bias suggests that positional information (perhaps a morphogen gradient) confers otic fates. Reinforcing this idea, it has been shown that Fgf3 determines anterior otic characteristics whereas Hedgehog (Hh) defines lateral/posterior identity (Hammond et al., 2010; Hammond and Whitfield, 2011). Other factors subsequently assign neurogenic fate to anterior PPA cells. This is consistent with chick studies showing that Fgf8 expression is sufficient to induce PPA expression of Sox3, a key factor in specifying the neurogenic region of the otic placode (Abello et al., 2010).
Pax2a levels regulate PPA cell behavior
Through loss- and gain-of-function experiments, we determined that changes in Pax2a and Pax8 levels influence otic and EB precursor segregation. Importantly, the PPA can form in the absence of Pax2a/8 activity, as previous studies showed that pax2a transcript was expressed in the PPA of Pax2a mutants injected with pax8 morpholino (Hans et al., 2004; Mackereth et al., 2005). Thus, differential levels of Pax2a/8 probably affect cellular behavior/differentiation after the PPA is specified. Modulation of Pax2a/8 levels lead to modest (but statistically significant) changes in otic and EB segregation. We attribute these to several factors. First, during Pax2a overexpression experiments, only a subset of transgenic cells were able to intercalate within the otic vesicle, whereas the remainder of high-expressing Pax2a cells underwent cell death. Second, the partial depletion experiments led to a 20-30% increase in EB ganglia, consistent with the limited contribution of the PPA to the EB domain. It is notable that the otic vesicle was composed of both high (medial) and low (lateral) Pax2a-expressing cells, suggesting that other factor(s), in addition to Pax2a/8, contribute to segregation of otic and EB precursors in the PPA. In summary, although Pax2a/8 levels clearly influence commitment/differentiation of cells within the otic or EB domain, further experiments are needed to determine how Pax2a/8 levels modulate cellular behaviors of the PPA.
Roles of Fgf and Wnt pathways in regulating Pax2a expression
Our previous study demonstrated that Fgf signaling is necessary to induce Pax2a in PPA cells (Nechiporuk et al., 2006). Corroborating this idea, we observed that Fgf could impart placodal identity to non-neural ectoderm by inducing Pax2a expression. Wnt can subsequently act upon these cells to induce high Pax2a (and possibly Pax8) expression levels, thereby influencing cellular behavior. Surprisingly, not all EB placodes exhibited the same responses to modulating Fgf and Wnt signaling pathways. We observed that the number of Pax2a+ cells contributing to the facial placode related directly to levels of Fgf signaling, whereas increased Wnt activity resulted in facial placode loss. However, a measurable reduction of the glossopharyngeal/vagal placodes was seen under both of these conditions. The reduction following decreased Wnt activity might be secondary to otic domain reductions (fewer Pax2a+ and Dlx3a+ otic cells); this would lead to a drop in the Fgf24 signaling necessary for posterior EB placode formation. Reinforcing this idea, we observed that absence or low levels of intracellular Wnt did not prevent cells from contributing to glossopharyngeal/vagal placodes in mosaic embryos, as Tg(hsp70:tcfΔC-EGFP)+ donor cells readily contributed to the these placodes.
Our mosaic experiments showed that, in contrast to previous work (Phillips et al., 2004), Wnt signaling is directly activated in a subpopulation of the PPA to drive high levels of Pax2a expression, biasing these cells to otic vesicle formation. Wnt is also a demonstrated dorsalizing signal during otic placode morphogenesis (Ohyama et al., 2006). Indeed, when Wnt signaling was overactivated using the BIO inhibitor, otic vesicle cells became supernumerary, and all expressed high levels of Pax2a. Furthermore, mediolateral extension of the otic vesicle was expanded versus activated Fgf and wild types.
Conclusions
Our studies show that relative levels of Pax2a/8 can influence placode segregation and tissue morphogenesis. The actions of Pax2a during these processes are regulated by extrinsic factors, Wnt and Fgf, in order to achieve the temporal and spatial resolution necessary for proper sensory structure formation. Differential levels of crucial transcription factors could play vital roles in other biological processes, such as cell commitment decisions, organ patterning and tumorigenesis.
Supplementary Material
Acknowledgements
We thank Drs Brigande, Christian and McGraw for critical reading of the manuscript; and Dr Waxman (Cincinnati Children's Hospital) for pax2a morpholino.
Footnotes
Funding
This work was funded by National Institutes of Health grants [HD055303 to A.V.N., 2T32GM071338-06 to M.N.M. and GM079203 to D.K.]. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.076075/-/DC1
References
- Abello G., Khatri S., Radosevic M., Scotting P., Giraldez F., Alsina B. (2010). Independent regulation of Sox3 and Lmx1b by FGF and BMP signaling influences the neurogenic and non-neurogenic domains in the chick otic placode. Dev. Biol. 339, 166-178 [DOI] [PubMed] [Google Scholar]
- Abramoff M. D., Magalhaes P. J., Ram S. J. (2004). Image processesing with ImageJ. Biophotonics Int. 11, 36-42 [Google Scholar]
- Ahrens K., Schlosser G. (2005). Tissues and signals involved in the induction of placodal Six1 expression in Xenopus laevis. Dev. Biol. 288, 40-59 [DOI] [PubMed] [Google Scholar]
- Alur R. P., Vijayasarathy C., Brown J. D., Mehtani M., Onojafe I. F., Sergeev Y. V., Boobalan E., Jones M., Tang K., Liu H., et al. (2010). Papillorenal syndrome-causing missense mutations in PAX2/Pax2 result in hypomorphic alleles in mouse and human. PLoS Genet. 6, e1000870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andermann P., Ungos J., Raible D. W. (2002). Neurogenin1 defines zebrafish cranial sensory ganglia precursors. Dev. Biol. 251, 45-58 [DOI] [PubMed] [Google Scholar]
- Bailey A. P., Bhattacharyya S., Bronner-Fraser M., Streit A. (2006). Lens specification is the ground state of all sensory placodes, from which FGF promotes olfactory identity. Dev. Cell 11, 505-517 [DOI] [PubMed] [Google Scholar]
- Bajoghli B., Aghaallaei N., Heimbucher T., Czerny T. (2004). An artificial promoter construct for heat-inducible misexpression during fish embryogenesis. Dev. Biol. 271, 416-430 [DOI] [PubMed] [Google Scholar]
- Bhat N., Riley B. B. (2011). Integrin-α5 coordinates assembly of posterior cranial placodes in zebrafish and enhances Fgf-dependent regulation of otic/epibranchial cells. PLoS ONE 6, e27778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard M., Souabni A., Busslinger M. (2004). Tissue-specific expression of cre recombinase from the Pax8 locus. Genesis 38, 105-109 [DOI] [PubMed] [Google Scholar]
- Bricaud O., Collazo A. (2006). The transcription factor six1 inhibits neuronal and promotes hair cell fate in the developing zebrafish (Danio rerio) inner ear. J. Neurosci. 26, 10438-10451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugmann S. A., Pandur P. D., Kenyon K. L., Pignoni F., Moody S. A. (2004). Six1 promotes a placodal fate within the lateral neurogenic ectoderm by functioning as both a transcriptional activator and repressor. Development 131, 5871-5881 [DOI] [PubMed] [Google Scholar]
- Cunliffe H., McNoe L., Ward T., Devriendt K., Brunner H., Eccles M. (1998). The prevalence of PAX2 mutations in patients with isolated colobomas or colobomas associated with urogenital anomalies. J. Med. Genet. 35, 806-812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. L., Matsumura L., Weeks D. A., Troxell M. L. (2011). PAX2 Expression in Wilms tumors and other childhood neoplasms. Am. J. Surg. Pathol. 35, 1186-1194 [DOI] [PubMed] [Google Scholar]
- Eccles M. R., He S., Legge M., Kumar R., Fox J., Zhou C., French M., Tsai R. W. S. (2002). PAX genes in development and disease: the role of PAX2 in urogenital tract developments. Int. J. Dev. Biol. 46, 535-544 [PubMed] [Google Scholar]
- Erickson T., Scholpp S., Brand M., Moens C. B., Waskiewicz A. J. (2007). Pbx proteins cooperate with Engrailed to pattern the midbrain-hindbrain and diencephalic-mesencephalic boundaries. Dev. Biol. 301, 504-517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter S., Muta Y., Mak S.-S., Rinkwitz S., Ladher R. K. (2008). Progressive restriction of otic fate: the role of FGF and Wnt in resolving inner ear potential. Development 135, 3415-3424 [DOI] [PubMed] [Google Scholar]
- Hammond K. L., Whitfield T. T. (2011). Fgf and Hh signalling act on a symmetrical pre-pattern to specify anterior and posterior identity in the zebrafish otic placode and vesicle. Development 18, 3977-3987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond K. L., van Eeden F. J. M., Whitfield T. T. (2010). Repression of Hedgehog signalling is required for the acquisition of dorsolateral cell fates in the zebrafish otic vesicle. Development 137, 1361-1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hans S., Liu D., Westerfield M. (2004). Pax8 and Pax2a function synergistically in otic specification, downstream of the Foxi1 and Dlx3b transcription factors. Development 131, 5091-5102 [DOI] [PubMed] [Google Scholar]
- Hans S., Christison J., Liu D., Westerfield M. (2007). Fgf-dependent otic induction requires competence provided by Foxi1 and Dlx3b. BMC Dev. Biol. 7, 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer P., Strahle U., Dickson C. (1996). The zebrafish Fgf-3 gene: cDNA sequence, transcript structure and genomic organization. Gene 168, 211-215 [DOI] [PubMed] [Google Scholar]
- Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B., Schilling T. F. (1995). Stages of embryonic development of the zebrafish. Am. J. Anat. 203, 253-310 [DOI] [PubMed] [Google Scholar]
- Kozlowski D., Murakami T., Ho R., Weinberg E. (1997). Regional cell movement and tissue patterning in the zebrafish embryo revealed by fate mapping with caged fluorescein. Biochem. Cell Biol. 75, 551-562 [PubMed] [Google Scholar]
- Krauss S., Johansen T., Korzh V., Fjose A. (1991). Expression of the zebrafish paired box gene pax [zf-b] during early neurogenesis. Development 113, 1193-1206 [DOI] [PubMed] [Google Scholar]
- Ladher R. K., O'Neill P., Begbie J. (2010). From shared lineage to distinct functions: the development of the inner ear and epibranchial placodes. Development 137, 1777-1785 [DOI] [PubMed] [Google Scholar]
- Lee Y., Grill S., Sanchez A., Murphy-Ryan M., Poss K. D. (2005). Fgf signaling instructs position-dependent growth rate during zebrafish fin regeneration. Development 132, 5173-5183 [DOI] [PubMed] [Google Scholar]
- Litsiou A., Hanson S., Streit A. (2005). A balance of FGF, BMP and WNT signalling positions the future placode territory in the head. Development 132, 4051-4062 [DOI] [PubMed] [Google Scholar]
- Mackereth M. D., Kwak S. J., Fritz A., Riley B. B. (2005). Zebrafish pax8 is required for otic placode induction and plays a redundant role with Pax2 genes in the maintenance of the otic placode. Development 132, 371-382 [DOI] [PubMed] [Google Scholar]
- Marques S. R., Lee Y., Poss K. D., Yelon D. (2008). Reiterative roles for FGF signaling in the establishment of size and proportion of the zebrafish heart. Dev. Biol. 321, 397-406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B. L., Kimelman D. (2012). Canonical Wnt signaling dynamically controls multiple stem cell fate decisions during vertebrate body formation. Dev. Cell 22, 223-232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nechiporuk A., Linbo T., Poss K. D., Raible D. W. (2006). Specification of epibranchial placodes in zebrafish. Development 134, 611-623 [DOI] [PubMed] [Google Scholar]
- Nikaido M., Doi K., Shimizu T., Hibi M., Kikuchi Y., Yamasu K. (2007). Initial specification of the epibranchial placode in zebrafish embryos depends on the fibroblast growth factor signal. Dev. Dyn. 236, 564-571 [DOI] [PubMed] [Google Scholar]
- Ohyama T., Groves A. K. (2004). Generation of Pax2-Cre mice by modification of a Pax2 bacterial artificial chromosome. Genesis 38, 195-199 [DOI] [PubMed] [Google Scholar]
- Ohyama T., Mohamed O. A., Taketo M. M., Dufort D., Groves A. K. (2006). Wnt signals mediate a fate decision between otic placode and epidermis. Development 133, 865-875 [DOI] [PubMed] [Google Scholar]
- Padanad M. S., Riley B. B. (2011). Pax2/8 proteins coordinate sequential induction of otic and epibranchial placodes through differential regulation of foxi1, sox3 and fgf24. Dev. Biol. 351, 90-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padanad M. S., Bhat N., Guo B., Riley B. B. (2012). Conditions that influence the response to Fgf during otic placode induction. Dev. Biol. 364, 1-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park B. Y., Saint-Jeannet J. P. (2008). Hindbrain-derived Wnt and Fgf signals cooperate to specify the otic placode in Xenopus. Dev. Biol. 324, 108-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer P. L., Gerster T., Lun K., Brand M., Busslinger M. (1998). Characterization of three novel members of the zebrafish Pax2/5/8 family: dependency of Pax5 and Pax8 expression on the Pax2.1 (noi) function. Development 125, 3063-3074 [DOI] [PubMed] [Google Scholar]
- Phillips B. T., Storch E. M., Lekven A. C., Riley B. B. (2004). A direct role for Fgf but not Wnt in otic placode induction. Development 131, 923-931 [DOI] [PubMed] [Google Scholar]
- Picker A., Scholpp S., Böhli H., Takeda H., Brand M. (2002). A novel positive transcriptional feedback loop in midbrain-hindbrain boundary development is revealed through analysis of the zebrafish pax2.1 promoter in transgenic lines. Development 129, 3227-3239 [DOI] [PubMed] [Google Scholar]
- Pieper M., Eagleson G. W., Wosniok W., Schlosser G. (2011). Origin and segregation of cranial placodes in Xenopus laevis. Dev. Biol. 360, 257-275 [DOI] [PubMed] [Google Scholar]
- Reifers F., Bohli H., Walsh E. C., Crossley P. H., Stainier D., Brand M. (1998). Fgf8 is mutated in zebrafish acerebellar (ace) mutants and is required for maintenance of midbrain-hindbrain boundary development and somitogenesis. Development 125, 2381-2395 [DOI] [PubMed] [Google Scholar]
- Sagasti A., O'Brien G. S., Rieger S., Martin S. M., Cavanaugh A. M., Portera-Cailliau C. (2009). Two-photon axotomy and time-lapse confocal imaging in live zebrafish embryos. J. Vis. Exp. 24, 1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N., Meijer L., Skaltsounis L., Greengard P., Brivanlou A. H. (2003). Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat. Med. 10, 55-63 [DOI] [PubMed] [Google Scholar]
- Schimmenti L. A. (2011). Renal coloboma syndrome. Eur. J. Hum. Genet. 102, 1-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosser G. (2005). Evolutionary origins of vertebrate placodes: insights from developmental studies and from comparisons with other deuterostomes. J. Exp. Zool. B Mol. Dev. Evol. 304, 347-399 [DOI] [PubMed] [Google Scholar]
- Schlosser G. (2006). Induction and specification of cranial placodes. Dev. Biol. 294, 303-351 [DOI] [PubMed] [Google Scholar]
- Schlosser G., Ahrens K. (2004). Molecular anatomy of placode development in Xenopus laevis. Dev. Biol. 271, 439-466 [DOI] [PubMed] [Google Scholar]
- Stoick-Cooper C. L., Weidinger G., Riehle K. J., Hubbert C., Major M. B., Fausto N., Moon R. T. (2006). Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development 134, 479-489 [DOI] [PubMed] [Google Scholar]
- Streit A. (2002). Extensive cell movements accompany formation of the otic placode. Dev. Biol. 249, 237-254 [DOI] [PubMed] [Google Scholar]
- Streit A. (2004). Early development of the cranial sensory nervous system: from a common field to individual placodes. Dev. Biol. 276, 1-15 [DOI] [PubMed] [Google Scholar]
- Subach F. V., Patterson G. H., Renz M., Lippincott-Schwartz J., Verkhusha V. V. (2010). Bright monomeric photoactivatable red fluorescent protein for two-color super-resolution sptPALM of live cells. J. Am. Chem. Soc. 132, 6481-6491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S. K., Dee C. T., Tripathi V. B., Rengifo A., Hirst C. S., Scotting P. J. (2007). Epibranchial and otic placodes are induced by a common Fgf signal, but their subsequent development is independent. Dev. Biol. 303, 675-686 [DOI] [PubMed] [Google Scholar]
- Westerfield M. (2000). The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio), 4th edn Eugene, OR: University of Oregon Press; [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


