Abstract
Background/Objectives:
Numerous randomised controlled trials (RCTs) published in first tier medical journals have evaluated the health effects of diets high in protein. We conducted a rigorous systematic review of RCTs comparing higher- and lower-protein diets.
Methods:
We searched several electronic databases up to July 2011 for studies focusing on patient-important outcomes (for example, cardiovascular disease) and secondary outcomes such as risk factors for chronic disease (for example, adiposity).
Results:
We identified 111 articles reporting on 74 trials. Pooled effect sizes using standardised mean differences (SMDs) were small to moderate and favoured higher-protein diets for weight loss (SMD −0.36, 95% confidence interval (CI) −0.56 to −0.17), body mass index (−0.37, CI −0.56 to 0.19), waist circumference (−0.43, CI −0.69 to −0.16), blood pressure (systolic: −0.21, CI −0.32 to −0.09 and diastolic: −0.18, CI −0.29 to −0.06), high-density lipoproteins (HDL 0.25, CI 0.07 to 0.44), fasting insulin (−0.20, CI −0.39 to −0.01) and triglycerides (−0.51, CI −0.78 to −0.24). Sensitivity analysis of studies with lower risk of bias abolished the effect on HDL and fasting insulin, and reduced the effect on triglycerides. We observed nonsignificant effects on total cholesterol, low-density lipoproteins, C-reactive protein, HbA1c, fasting blood glucose, and surrogates for bone and kidney health. Adverse gastrointestinal events were more common with high-protein diets. Multivariable meta-regression analysis showed no significant dose response with higher protein intake.
Conclusions:
Higher-protein diets probably improve adiposity, blood pressure and triglyceride levels, but these effects are small and need to be weighed against the potential for harms.
Keywords: protein diet, GRADE, systematic review, weight loss, meta-analysis, randomised trial
Introduction
International dietary guidelines recommend healthy diets with higher energy intake from carbohydrates, a lower energy intake from fat and a still lower energy intake from protein.1, 2, 3 However, there has been an enduring interest in the ideal balance of these energy sources to achieve better health,4 including the ideal proportion of proteins in the diet. In fact, there is a wealth of literature that includes randomised controlled trials (RCTs) that have compared high-protein diets with low-protein diets and that were published in high-impact medical journals.5, 6 These studies have led to various and often contradictory conclusions about whether high-protein diets result in weight loss and other important health benefits and why.
Before developing health care recommendations, it is necessary to systematically review the evidence to determine the best estimates of effect and the quality of that evidence.7 The most rigorous study design to measure these effects will come from well-conducted RCTs, which can minimise the biases in observational studies in particular in studies that are at risk for residual confounding by lifestyle factors.7 A number of narrative reviews with their known biases have been published.8 These narrative reviews generally conclude that there is some evidence that high-protein diets may have beneficial effects on adiposity and other metabolic parameters, and hypothesise that the effects may be due to increased thermogenesis and satiety.9, 10, 11 One review, focusing on body composition and weight loss, although systematic in its methods and including randomised controlled studies, drew similar conclusions, but by combining randomised and nonrandomised studies the protection against bias and confounding by randomisation was lost.12 Other reviews have focused on single outcomes related to protein.13, 14
Therefore, we conducted a systematic review and meta-analysis of RCTs to assess the benefits and harms of higher-protein compared with lower-protein diets in the general population. Our specific objectives were (1) to systematically assess the effect of protein intake on health and other risk factors for chronic disease in RCTs; and (2) to perform a meta-regression analysis of these trials to test for a possible dose–response relationship between protein intake and health indicators.
Methods
Protocol, search strategy and selection criteria
Our methods generally follow the Cochrane Handbook for systematic reviews of intervention.15 We developed a systematic review protocol and searched Medline (1950 to July 2011), EMBASE (1980 to July 2011) and the Cochrane Central Register of Controlled Trials Register, CENTRAL (July 2011). The search strategies combined key subject terms and text words, and validated search filters15, 16 with no language restrictions (see Supplementary Information 1). We reviewed reference lists for additional studies. Two investigators independently screened all citations.
Studies were eligible if participants were adults (>18 years) and if at least 80% of participants did not have medically indicated dietary restrictions (for example, patients with diabetes or chronic renal disease), or their results were reported separately. Our aim was to include studies that would provide evidence applicable to a general population consuming a diet of commonly found foods and for which equipoise about the intervention existed at the time the study was conducted. This criterion meant that studies of people with hyperlipidemia, hypertension or metabolic syndrome were included. We included studies that were designed for weight loss or not, or provided food to participants or not, as long as the diet was based on foods that a general population could incorporate into a diet. For this reason, studies using special meal replacements or supplements were not eligible. We included RCTs that compared higher- versus lower-protein diets using a 5% difference in total energy intake. We considered protein intake based on the mean reported amount of protein consumption within each diet group closest to the time of follow-up. The choice of 5% was conservative when compared with the targeted difference in protein intake in most large randomised trials that is 10% or more.17, 18, 19 We included studies with co-interventions (for example, exercise programmes) only if they were applied similarly to both the higher and lower protein groups so that they would not mask or emphasise the effects of protein. For trials with a crossover design, we considered only the period before the crossover to avoid any carry-over effects or the last follow-up if the authors demonstrated no significant carry-over effects in their analyses. In studies that compared two or more higher-protein diets with one lower-protein diet, we used a split-half approach to include data of the lower-protein diet and compared the lower-protein group to each higher-protein diet group separately. For studies in which there were two higher-protein diets and two lower-protein diets, we included two comparisons between diets of similar caloric content. Given assumptions that initial effects of weight loss diets may be due to drastic water or muscle loss, the intervention period had to be 28 days or more.
Our a priori primary outcomes were direct patient-important outcomes, including death, cardiovascular diseases, cancer diagnosis, new onset diabetes, renal disease, starting dialysis, bone health/fractures, quality of life and adverse events. However, we expected little information for these primary outcomes and a priori determined to accept surrogate outcomes of the primary outcomes, including body mass index (BMI), weight, waist circumference, blood pressure, lipid levels (total cholesterol, high-density lipoproteins (HDL) and low-density lipoproteins (LDL)), C-reactive protein (CRP), serum HbA1c, fasting serum blood glucose, fasting serum insulin, triglycerides, serum creatinine and bone mineral density. One investigator extracted data from eligible studies and a second verified the information using a standardised, pilot tested form, and resolved disagreement by discussion. We contacted authors via email up to two times to request data. Event rates and continuous data were collected by available case analysis when provided by the author and at the last follow-up time of a study.15
Statistical analysis
We used random effect models to conduct meta-analyses of dichotomous outcomes, and mean differences (MD) or standardised mean differences (SMDs) of change scores for continuous outcomes using Review Manager 5.0. We interpreted an SMD <0.40 as a small effect size, 0.40–0.70 as moderate and >0.70 as large.15 Given these established ranges of effect sizes, effect sizes (and their confidence intervals (CIs)) ≤0.2 were interpreted as no difference if they were not statistically significant. To aid interpretation of SMDs, we translated them back to their original units by multiplying the pooled SMD with the pooled s.d. of the control group differences measured at 3 months.15
We conducted a priori subgroup analyses to determine whether or not the effect of higher-protein diets varied by caloric intake (for example, comparisons between diets that were hypocaloric, isocaloric or unlimited caloric intake). The subgroups consisted of studies comparing diets in which the higher-protein compared with the lower-protein diet yielded ≥100 kilocalories (kcal) more energy, ⩾100 kcal less or was within 100 kcal of the lower-protein diet. A difference of ∼0.45 kg (1 pound) in weight loss per month between higher- and lower-protein diets has been described in studies that we found during scoping exercises.11 We calculated that a 3500-kcal reduction in energy intake causes an ∼0.45 kg weight loss that, in turn, translates to a difference of ∼100 kcal in energy intake per day over 1 month. We performed post-hoc subgroup analyses by the percentage of protein intake in higher-protein diets to explore whether diets with a higher versus lower percentage protein intake resulted in different outcomes (that is, percentage protein intake >30% versus, 20–30%, versus <20%). We assessed heterogeneity of the results using the χ2 test and I2, and further explored the heterogeneity if I2 was >50%.15 We performed sensitivity analyses by excluding studies with 20% or greater loss of follow-up and dropouts, and created funnel plots for meta-analyses to assess publication bias. We planned to do a subgroup analysis by type of protein (animal versus plant sources), but too few studies reported the protein source preventing us from conducting these analyses.
We performed a multivariable meta-regression analysis to examine the dose–response relation between protein intake and the mean change in outcomes, weighted by the inverse of the variance and adjusted for important covariates. Two a priori models were constructed: (1) amount of protein intake as the main variable and duration of diet intervention, difference in total energy intake between the intervention and control diet, and baseline BMI (base model) as covariates and (2) the primary model with age, sex and difference in percentage of energy intake from carbohydrates between the intervention and control diet added to the model (full adjusted model). We used a random effects meta-regression model using Proc Mixed, SAS version 9.1, according to Morris.20
Secondary analysis
While we determined a priori to focus on change values because scoping of the literature indicated that many of the possibly eligible studies were small, many studies did not report change values. We, therefore, conducted a secondary analysis on the end of study values. Similar to the primary analysis, we used a random effects model in the meta-analyses and calculated SMDs by pooling results using data from the last follow-up reported in a study.
Quality of the body of evidence (confidence in the estimates of effect)
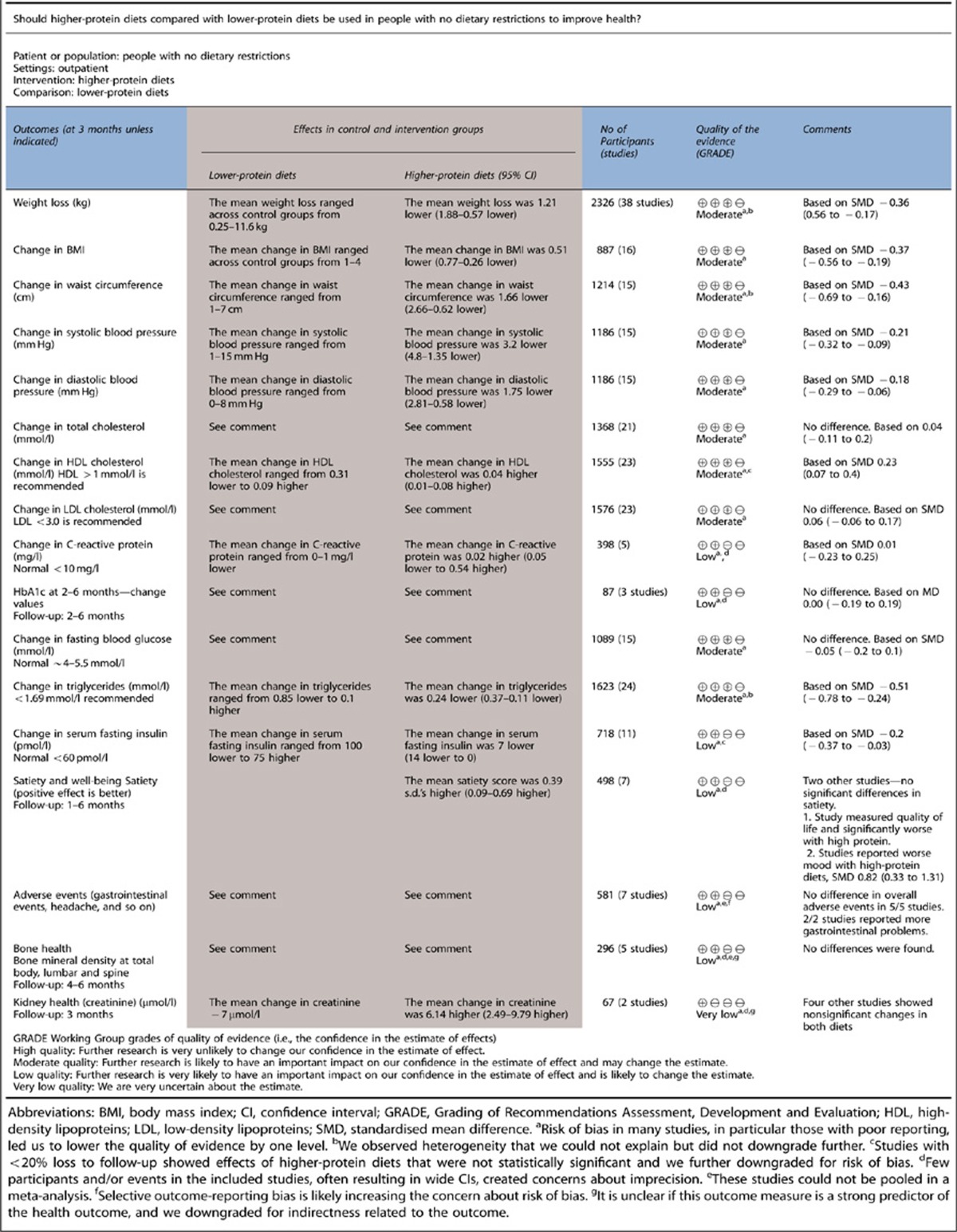
We assessed the quality of evidence (that is, the confidence in the estimates of effect) by outcome according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (www.gradeworkinggroup.org).7 The quality of evidence for each outcome is graded in four categories from high to very low (see Table 2 for detailed description of the categories).
Results
Trial flow
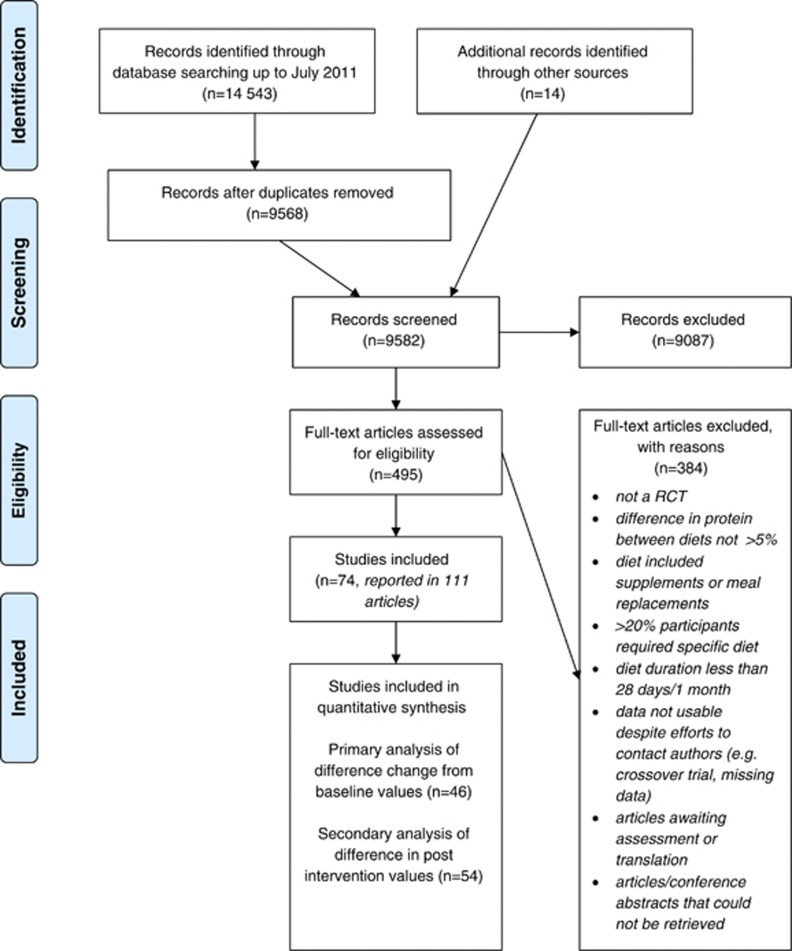
The search identified 9658 unique citations for screening. We identified 111 articles describing 74 eligible studies (Figure 1). We contacted authors of 19 articles and received responses from 15. The table of included studies and references to all articles are provided in Supplementary Information 2, and the table of excluded studies and reasons for exclusion are provided in Supplementary Information 3.
Figure 1.
PRISMA Flow Diagram of results of search.
Study characteristics
Participants were either healthy, overweight, obese, hypertensive or had elevated lipid levels. The median age was 45 years, median BMI was 33 (range: 22–43) and studies included more women than men (64% versus 36%). The median protein content of the higher-protein diets was 27% of the total energy intake (range: 16–45%) and 18% (range: 5–23%) in the lower-protein diets. The MD across studies of the total energy intake from protein was 10%. Only 11 studies (15%) reported the amount of protein as vegetable or meat protein. Most studies were performed in North America. Few studies (34%) measured adverse events. The majority of studies (80%) measured outcomes at <6 months (Table 1 and Supplementary Information 2 for more details).
Table 1. Key characteristics of included randomised controlled trialsa.
| Participant characteristics | Median (range) unless indicated otherwise |
|---|---|
| Number of participants | 54 (5–405) |
| Age | 45 years |
| Male/female (mean across studies) | 36%/64% |
| BMI | 33 (22–43) |
| Diet characteristics | |
| Total daily energy intake (approximate kcal) | |
| Lower-protein diets | 1500 (1100–2800) |
| Higher-protein diets | 1500 (900–2800) |
| Protein (% total daily energy intake) | |
| Lower-protein diets | 18% (5–23%) |
| Higher-protein diets | 27% (16–45%) |
| Carbohydrate (% total daily energy intake) | |
| Lower-protein diets | 55% (27–78%) |
| Higher-protein diets | 38% (5–62%) |
| Fat (% total daily energy intake) | |
| Lower-protein diets | 26% (11–53%) |
| Higher-protein diets | 32% (13–68%) |
| Study characteristics | Number of studies (%) |
| Crossover trial | 9 (14%) |
| Loss to follow-up and dropouts ≥20% | 27 (36%) |
| Caloric content (difference between diets) | |
| Higher-protein diets with >100 calories more than lower-protein diets | 13 (18%) |
| Higher-protein diets within 100 calories of lower-protein diets | 43 (58%) |
| Higher-protein diets with >100 calories fewer than lower-protein diets | 6 (8%) |
| Reporting adverse events (for example, bone and kidney health, other adverse events) | 25 (34%) |
| Greatest duration of diet (see inclusion criteria): | |
| 1 to <3 months | 32 (43%) |
| 3 months to <6 months | 30 (41%) |
| 6 months or greater | 12 (16%) |
| Reporting type of protein (animal versus vegetable) | 11 (15%) |
Abbreviation: BMI, body mass index.
Dietary amounts are actual dietary intake by participants, not targeted intake in study design.
Quality of the body of evidence
Less than 10% of the studies reported adequate randomisation and/or allocation concealment. Almost 40% of the studies had greater than 20% loss to follow-up or dropout rates. We did not detect publication bias in the meta-analyses. The summary of findings table describes the quality of the body of evidence for each outcome (Table 2).
Table 2. Summary of findings table.
Health outcomes
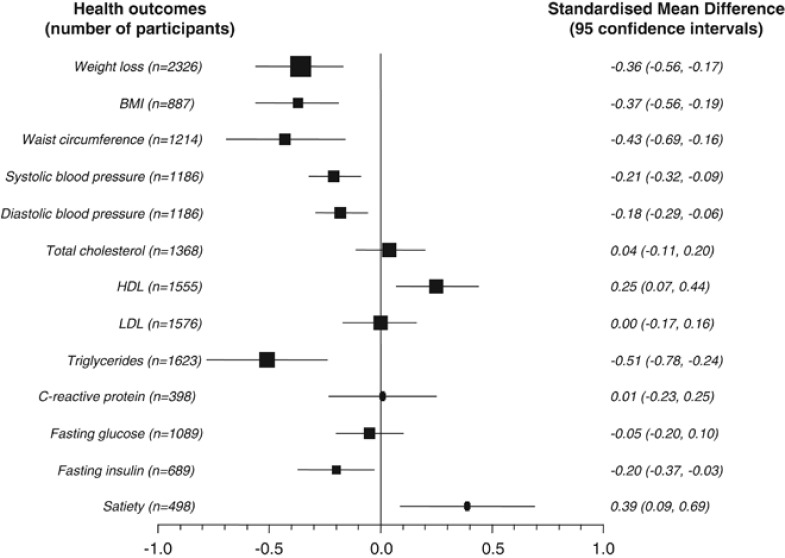
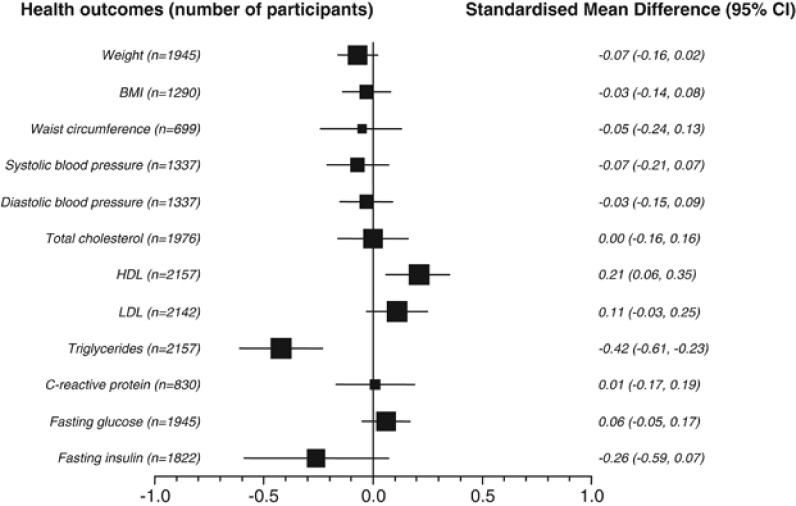
Figure 2 summarises the results of the meta-analyses for all measured outcomes as pooled effect sizes or SMDs. The results of the meta-analyses for each outcome are provided in Supplementary Information 4. No included study measured the incidence of death, cancer, diabetes, heart attack, stroke, dialysis and other renal disease, or fractures.
Figure 2.
Results of meta-analysis of outcomes using SMDs (difference between change values from baseline).
Weight loss and adiposity
A total of 38 studies provided data for the meta-analysis for weight change (2326 participants), 16 for BMI (887 participants) and 15 for waist circumference (1214 participants). The pooled standardised effect sizes for changes in weight loss (−0.36, CI −0.56 to −0.17, P=0.0002), BMI (−0.37, CI −0.56 to −0.19, P<0.0001) and waist circumference (−0.43, CI −0.69 to −0.16, P=0.00001) were statistically significant and represented small to moderate effects. However, there was inconsistency (heterogeneity) across trials for weight loss (I2=77%, P<0.00001) and waist circumference (I2=75%, P<0.0001). Exploration of whether effects on adiposity varied depending on energy content showed no significant effects (interaction terms: weight loss P=0.31; BMI P=0.21; waist circumference P=0.53). In the meta-regression, other covariates did not influence waist circumference. However, a higher BMI at the start of a study (in both models), and a more similar energy intake between diets (in the base model only) was associated with greater weight loss in people following the higher-protein diet. In contrast, the magnitude of the reduction in BMI was not associated with greater differences in protein content (in the full model only). In the sensitivity analyses excluding studies with more than 20% loss to follow-up and dropouts, effect sizes were similar for weight loss and BMI, and smaller for waist circumference (−0.2, CI −0.34 to −0.07, P=0.003).
Translated to an effect at 3 months, the meta-analysis shows a greater weight loss of 1.21 kg (CI −1.88 to −0.57), a greater decrease in BMI (−0.51 kg/m2, CI −0.77 to −0.26) and a greater loss in waist circumference (−1.66 cm, CI −2.66 to −0.62) in higher compared with lower-protein diets (moderate-quality evidence for all outcomes).
Blood pressure
Meta-analysis of 15 studies (1186 participants) showed a small statistically significant decrease in systolic blood pressure (SMD −0.21, CI −0.32 to −0.09, P=0.0004) and diastolic blood pressure (SMD −0.18, CI −0.29 to 0.06, P=0.003). The effect's estimates were similar in the sensitivity analyses using only studies with <20% loss to follow-up/dropout. There was no interaction between diet type with different caloric content for systolic (P=0.17) or diastolic blood pressure (P=0.42), nor any association of covariates in the meta-regression analyses. Of note, however, was a significant change with higher-protein diets at 20–30% energy intake, but not with 30% or more. Translated to an effect at 3 months, the meta-analysis showed a greater mean decrease of 3.2 mm Hg (CI −4.80 to −1.35) in systolic blood pressure and 1.75 mm Hg (CI −2.81 to −0.58) in diastolic blood pressure with higher- compared with lower-protein diets (moderate-quality evidence).
Lipid levels
The 21 studies (1368 participants) that reported total cholesterol showed no difference and similar nonsignificant changes in studies with <20% follow-up. Subgroup analysis showed no significant differences by caloric content of diet (interaction term P=0.31). About 23 studies (∼1550 participants) were included in the analyses for HDL and LDL cholesterol. HDL cholesterol was significantly increased with higher-protein diets (SMD 0.25, CI 0.07–0.44, P=0.007). However, we observed heterogeneity (I2=65%, P<0.00001), and the change in HDL was no longer significant in the sensitivity analysis restricted to studies with <20% loss to follow-up/dropout. Subgroup analysis showed no significant difference with diets of different caloric content (P=0.38) or protein. In addition, the meta-regression (full model) across studies showed an inverse association between greater differences in protein content of the diets and improvements in HDL, and the lower carbohydrate content of the higher-protein diet was associated with a higher HDL. There was no difference in LDL cholesterol in the meta-analysis (SMD 0.00, CI −0.17 to 0.16, P=0.97), with similar results for studies with <20% loss to follow-up, and nonsignificant differences by caloric content (P=0.68). However, it was positively associated with duration of the diet in the meta-regression. At 3 months, the effect sizes translate to no difference in total cholesterol and LDL cholesterol with higher-protein diets. However, we observed a significantly greater increase in HDL cholesterol of 0.04 mmol/l (CI 0.01–0.08) with higher- compared with lower-protein diets (moderate-quality evidence for all outcomes).
C-reactive protein
The pooled effect size of five studies (398 participants) showed no significant difference in CRP (0.01, CI −0.23 to 0.25, P=0.95). At 3 months, this effect would translate into a nonsignificant increase of 0.02 mg/l (CI −0.05 to 0.54) (low-quality evidence). Subgroup analysis showed nonsignificant differences with caloric content between higher- and lower-protein diets (P=0.61 for the interaction term), and there were no associations with covariates in the meta-regression.
HbA1c
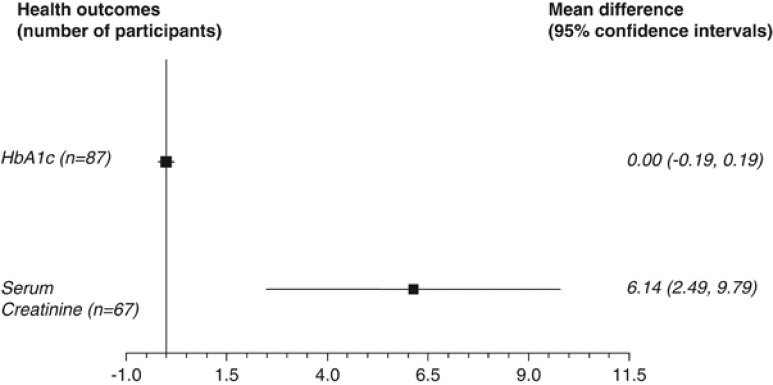
Three studies (87 participants) measured the change in HbA1c at 2–6 months but the data could be pooled. Another study reported nonsignificant differences in reduction in HbA1c.21 In the studies that could be meta-analysed, as shown in Figure 3, we observed no difference in HbA1c levels between higher- or lower-protein diets (MD 0.00, CI −0.19 to 0.19, P=0.96) (low-quality evidence).
Figure 3.
Results of meta-analysis of outcomes using MDs (difference between change values from baseline).
Fasting blood glucose
Pooled analysis of 15 studies (1089 participants) showed no difference in fasting blood glucose (SMD −0.05, CI −0.20 to 0.10, P=0.49) (moderate-quality evidence). When comparing the results across studies with diets of different caloric content, we detected no difference (P=0.47 for the interaction term). Meta-regression also showed no association of the effect with covariates.
Triglycerides
The 24 studies (1623 participants) included in the meta-analysis demonstrated a moderate statistically significant decrease in triglycerides (SMD −0.51, CI −0.78 to −0.24, P=0.0002) but heterogeneity was high (I2=85%, P<0.00001), and there was no interaction with higher-protein diets of different caloric content (P=0.60) or associations with covariates in the meta-regression. A smaller effect was seen in studies with <20% follow-up (SMD −0.34, CI −0.46 to −0.21). Based on the SMD from the meta-analysis, there was a greater reduction in triglycerides (−0.24 mmol/l, CI −0.37 to −0.11) at 3 months with higher- compared with lower-protein diets (moderate-quality evidence).
Fasting blood insulin
Pooled analysis of 10 studies (718 participants) showed a small significant effect (SMD −0.20, CI −0.37 to −0.03, P=0.02). However, in the meta-analysis of studies with <20% follow-up the effect was nonsignificant. There was no significant interaction with energy intake. Meta-regression of the eight studies, however, showed no association of differences in the covariates with fasting blood insulin. The overall pooled SMD translates into a 7 pmol/l decrease (CI −14 to 0) after 3 months of consuming a higher-protein diet with restricted calories (low-quality evidence).
Bone health
No study measured the incidence of fractures. Changes in bone mineral density were measured in five studies at 2–6 months.22, 23, 24, 25 There was no difference in total body bone mean difference (MD 0.00, CI −0.02 to 0.03, P=0.82) in three pooled studies, a statistically nonsignificant difference reported in two studies,22, 23 and a statistically nonsignificant increase at the lumbar spine (MD 0.60, CI −0.34 to 1.54, P=0.21) in two pooled studies.24, 25 However, we observed heterogeneity (I2=67%, P=0.05). Change in bone mineral density at the hip was measured in one study enrolling 130 participants25 and the MD was nonsignificant (MD 0.61, CI −0.13 to 1.34, P=0.11). The quality of the evidence for these outcomes was low.
Kidney health
Serum creatinine levels from 1 to 12 months were measured in six studies. Data from two studies at 3 months could be pooled26, 27 and showed a significant increase between diets (MD 6.14 μmol/l, CI 2.49–9.79, P=0.001) (very low-quality evidence). The other four studies reported nonsignificant changes in both diet groups.5, 28, 29
Quality of life and well-being
Quality of life was measured in one study and showed a nonsignificant decrease.6 Mood was pooled in two studies 31, 32 and showed large effects, which meant worse mood with higher-protein diets (SMD 0.82, CI 0.33–1.31, P=0.001). But pleasure was not significantly different between diets.33 Satiety (feeling full after meals) and satisfaction with the diet were measured in nine studies after 1–6 months, of which seven could be pooled. There was a small increase in satiety with higher-protein diets (SMD 0.39, CI 0.09–0.69, P=0.03). The quality of evidence for these outcomes is low.
Adverse events
A total of 22 studies measured and reported adverse events. Five studies analysed composites of adverse events and found no difference between the diets. Gastrointestinal effects were specifically reported in four studies but could not be pooled because relevant data were not available. Appel18 reported a significant increase in bloating or fullness with higher-protein diets (risk ratio 2.11, CI 0.98–4.53, P=0.05), a nonsignificant increase in dry mouth (risk ratio 2.00, CI 0.70–5.72, P=0.20), and poor appetite (risk ratio 2.83, CI 1.15–7.01, P=0.02). However, Due34 and Leidy33 found no significant differences between diets in appetite or desire to eat. Yancy5 reported significantly more gastrointestinal problems with higher-protein diets: constipation (68% versus 35% P<0.001), halitosis (38% versus 8% P<0.001) and diarrhoea (23% versus 7% P=0.02). Fatigue did not differ between groups.18 General weakness (25% versus 8% P=0.01), headache (60% versus 40% P=0.03), muscle cramps (35% versus 7% P=0.001) and rash (13% versus 0% P=0.006) were all significantly increased with higher-protein diets.5 The quality of evidence for these outcomes is low.
Dose response and overall effects of covariates
In the multivariable analysis (see Supplementary Information 5), the base and full models that adjusted for duration of diet intervention, differences in energy intakes, as well as carbohydrate intakes between intervention and control group, baseline BMI and age, and percentage of males in the studies showed no significant dose response with higher protein intake on outcomes. The beneficial effect of higher protein intake on BMI and HDL cholesterol was significantly greater when the difference in protein content between intervention and control diets was smaller. These effects were found when variation in carbohydrate intake (and consequently fat content), age and sex were taken into account in the fully adjusted model.
Secondary analysis of end of study values
The results of the secondary analysis of end of study scores for all health outcomes are displayed in Figure 4 and Supplementary Information 6. Generally the results are consistent with those adjusted for the baseline values (our primary analysis). The small to moderate positive effects on HDL and triglycerides were consistent with the effects found in the primary analysis. However, in this analysis, higher-protein diets had smaller or no effect on adiposity and blood pressure.
Figure 4.
Results of secondary analysis for each outcome as SMDs (difference in the end of study values).
Discussion
In this systematic review of 74 RCTs, the first of on this topic, we compared higher- with lower-protein diets. While no study assessed effects on direct patient-important outcomes, moderate-quality evidence suggests that higher-protein diets probably lead to greater weight loss, and loss in BMI and waist circumference than lower-protein diets. After 3 months, the observed effects translate into a 1.21-kg greater weight loss, a greater reduction in BMI by 0.51 units and a 1.66-cm greater reduction in waist circumference. However, we cannot rule out that the effect of higher-protein diets on weight loss could have been modified by BMI at the start of the diet (that is, the greater the BMI the greater the weight loss seen with high-protein diets). Across studies, we did not observe that greater differences in protein intake were associated with better adiposity outcomes. In fact, we observed that the beneficial effect of higher protein intake on BMI and HDL cholesterol in the intervention diets appear larger with smaller increases in protein content.
We found moderate-quality evidence that higher-protein diets had nonsignificant effects or no effect on surrogate cardiovascular disease outcomes: blood pressure, total cholesterol, LDL cholesterol and CRP. However, we observed a small positive effect on HDL cholesterol. We also observed moderate reductions in triglycerides with higher-protein diets although this effect was smaller when studies at lower risk of bias were analysed separately (that is, studies with <20% loss to follow-up). These results do corroborate results of studies suggesting that higher-protein diets may result in better lipid profiles although these effects are likely to be small.35 The results of the meta-regression also suggest that the lower carbohydrate content of the high-protein diet contributes to this positive change, although greater differences in protein intake were not positively associated with better HDL profiles.
Low to moderate-quality evidence suggests effects of higher-protein diets on outcomes related to diabetes: HbA1c and fasting blood glucose. We did find small reductions in fasting blood insulin with higher-protein diets but this effect was no longer significant in studies with <20% loss to follow-up. We found very limited and low-quality evidence (due to a high likelihood of selective outcome-reporting bias) for more adverse gastrointestinal events with higher- compared with lower-protein diets.
Limitations and strengths
The limitations of this systematic review relate to the available research evidence. We conducted this research to identify RCTs that measured patient-important outcomes, such as incidences of stroke, heart attacks and diabetes. However, no study focused on these outcomes and, therefore, the evidence we describe here is based on pre-defined surrogate outcomes, such as adiposity measures, lipid and other blood parameters. Although some of these may be valid surrogates for future disease (for example, blood pressure, HDL and LDL cholesterol, and waist circumference) and some are of direct importance to patients (for example, weight loss), the value of other outcomes is unclear (for example, fasting blood insulin). We made every attempt to reduce the risk of ‘double counting' participants in the analyses by carefully evaluating studies that were reported in multiple articles (often without reference to the original study).
The strengths of this review include the systematic approach to a topic that has been characterised by unsystematic methods to find, report, synthesise and interpret studies. In fact, our study is the most comprehensive of this topic that has seen many high-profile publications in key medical journals. In addition, this review is the first to use meta-analyses of RCTs to determine the effects of higher- versus lower-protein diets on health outcomes. We used standard systematic review methodology to perform this review and assess the risk of bias in studies and grade the quality of the body of evidence. Furthermore, in this review we synthesised a vast number of studies that included diets with an actually higher protein content rather than studies in which the interventions were labelled as high protein. Thus, we took a mechanistic approach to determine the health effects of actually consuming a diet higher in protein rather than evaluating the health effects in a population that intended to consume a diet higher in protein. In this way, we attempted to overcome the confounding effects of adherence, providing information about whether or not higher-protein diets should be recommended. Pooling the data of many RCTs provided sufficient power to draw conclusions about many of the outcomes. However, pooling the data also meant that studies that differed in other ways were analysed together (for example, hypocaloric and ad libitum diets). For this reason, we performed subgroup analyses and multivariate meta-regressions to explore heterogeneity and the potential for effect modification. We found no consistent subgroup differences or effect modifiers, including a lack of impact by the amount of protein intake (for example, the higher the protein intake the better the outcome), by energy or carbohydrate content, by BMI at the start of the diet, by age, by sex or by duration of the study. While our primary analyses focused on a comparison of change values, that is, the difference between the changes from baseline to the end of study values, our secondary analyses of post-intervention values generally confirmed these results although the changes were generally smaller and less likely to be statistically significant.
Implications
In this systematic review, we observed effects on weight loss and BMI, which contradict the findings by Krieger et al.12 who observed no effect of higher protein intake on body mass but on reducing fat-free mass. That review, however, included single arms from observational studies, as well as from RCTs, which even after adjustment for covariates may not have accounted for other known or unknown confounding factors leading to potentially misleading results. However, the associations we observed were not accompanied by a clear effect on all other outcomes. Additionally, our findings suggest that protein diets emphasising modest instead of large increases in protein content are more likely to have favourable effects on risk factors (for example, BMI and HDL), and thus a moderate amount of protein intake may be of greater benefit. More research is needed about the effects of high-protein diets on long-term patient-important outcomes such as cardiovascular outcomes, adiposity outcomes and the effects on quality of life after dietary changes. Further work is also needed to determine if the type of protein (for example, animal versus vegetable) could result in different effects. Greater rigour with respect to allocation concealment and sequence generation, blinding of assessors and avoiding loss to follow-up would decrease the risks to bias in these lifestyle intervention studies.
In practice, higher-protein diets may have little effect on intermediate cardiovascular outcomes, such as blood pressure and glucose levels. High-protein diets probably result in small improvements in weight loss and other measures of adiposity and selected lipid parameters. However, the importance of a slightly greater weight loss, loss in waist size or drop in triglycerides would need to be balanced against any potential harms that could occur.
Acknowledgments
This review was funded by Barilla, SpA, Parma, Italy. The funding organisation was not involved in the analysis of the study and the final decision to submit for publication; but one author was an employee of the sponsor and she was involved in the review and interpretation of the data in the manuscript. However, the final decision about interpretation rested with the first and corresponding author.
NS, EAA and HJS designed the review, conducted the literature search, screened, analysed and interpreted the data, and wrote the manuscript. RM screened and abstracted the data. AM and DH-A were involved in the analysis and draft of the manuscript. MB helped with the critical revision of the manuscript. HJS had overall supervision of the review and conceived the review.
NS and HJS received grant support from Barilla, SpA to conduct the review. MB was an employee of Barilla, SpA. All other authors responded 'no‘ to all questions on the disclosure form of the International Committee of Medical Journal Editors.
Footnotes
Supplementary Information accompanies the paper on European Journal of Clinical Nutrition website (http://www.nature.com/ejcn)
Supplementary Material
References
- United States Department of Agriculture ChooseMyPlate.gov. . http://www.choosemyplate.gov ( accessed 9 April 2012).
- Health Canada Canada's Food Guide. . http://www.hc-sc.gc.ca/fn-an/food-guide-aliment/index-eng.php ( accessed 9 April 2012).
- UK Department of Health in association with the Welsh Government, the Scottish Government and the Food Standards Agency in Northern Ireland Eatwellplate. . http://www.nhs.uk/Livewell/Goodfood/Pages/eatwell-plate.aspx ( accessed 9 April 2012).
- Astrup A, Meinert Larsen T, Harper A. Atkins and other low-carbohydrate diets: hoax or an effective tool for weight loss. Lancet. 2004;364:897–899. doi: 10.1016/S0140-6736(04)16986-9. [DOI] [PubMed] [Google Scholar]
- Yancy WS, Olsen MK, Guyton JR, Bakst RP, Westman EC, Yancy William S, et al. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: a randomised, controlled trial. Ann Intern Med. 2004;140:769–777. doi: 10.7326/0003-4819-140-10-200405180-00006. [DOI] [PubMed] [Google Scholar]
- Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859–873. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Vist G, Kunz R, Falck-Ytter Y, Alonso-Coello P, for the GRADE Working Group et al. Rating quality of evidence and strength of recommendations GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antman EM, Lau J, Kupelnick B, Mosteller F, Chalmers TC. A comparison of results of meta-analyses of randomised control trials and recommendations of clinical experts. Treatments for myocardial infarction. JAMA. 1992;268:240–248. [PubMed] [Google Scholar]
- Brehm BJ, D'Alessio DA. Benefits of high-protein weight loss diets: enough evidence for practice. Curr Opin Endocrinol Diabetes Obes. 2008;15:416–421. doi: 10.1097/MED.0b013e328308dc13. [DOI] [PubMed] [Google Scholar]
- Halton TL, Hu FB. The effects of high protein diets on thermogenesis, satiety and weight loss: a critical review. J Am Coll Nutr. 2004;23:373–385. doi: 10.1080/07315724.2004.10719381. [DOI] [PubMed] [Google Scholar]
- Clifton PM, Keogh J. Metabolic effects of high-protein diets. Curr Atheroscler Rep. 2007;9:472–478. doi: 10.1007/s11883-007-0063-y. [DOI] [PubMed] [Google Scholar]
- Krieger JW, Sitren HS, Daniels MJ, Langkamp-Henken B. Effects of variation in protein and carbohydrate intake on body mass and composition during energy restriction: a meta-regression. Am J Clin Nutr. 2006;83:260–274. doi: 10.1093/ajcn/83.2.260. [DOI] [PubMed] [Google Scholar]
- Darling AL, Millward DJ, Torgerson DJ, Hewitt CE, Lanham-New SA. Dietary protein and bone health: a systematic review and meta-analysis. Am J Clin Nutr. 2009;90:1674–1692. doi: 10.3945/ajcn.2009.27799. [DOI] [PubMed] [Google Scholar]
- torf-van der Kuil W, Engberink MF, Brink EJ, van Baak MA, Bakker SJ, Navis G, et al. Dietary protein and blood pressure: a systematic review. PloS One. 2010;5:e12102. doi: 10.1371/journal.pone.0012102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S.(eds)Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 (updated September 2008) The Cochrane Collaboration; 2008 : http://www.cochrane-handbook.org ( (accessed 25 February 2010). [Google Scholar]
- Wong SS, Wilczynski NL, Haynes RB. Developing optimal search strategies for detecting clinically sound treatment studies in EMBASE. J Med Libr Assoc. 2006;94:41–47. [PMC free article] [PubMed] [Google Scholar]
- Larsen TM, Dalskov SM, van Baak M, Jebb SA, Papadaki A, Pfeiffer AF, Diet, Obesity, and Genes (Diogenes) Project et al. Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med. 2010;363:2102–2113. doi: 10.1056/NEJMoa1007137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ER, et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomised trial. JAMA. 2005;294:2455–2464. doi: 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- Brinkworth GD, Noakes M, Buckley JD, Keogh JB, Clifton PM. Long-term effects of a very-low-carbohydrate weight loss diet compared with an isocaloric low-fat diet after 12 months. Am J Clin Nutr. 2009;90:23–32. doi: 10.3945/ajcn.2008.27326. [DOI] [PubMed] [Google Scholar]
- Morris CN. Parametric empirical Bayes inference: theory and applications. J Am Stat Assoc. 1983;78:47–55. [Google Scholar]
- Jenkins DJA, Wong JMW, Kendall CWC, Esfahani A, Ng VWY, Leong TCK, et al. The effect of a plant-based low-carbohydrate (‘eco-atkins') diet on body weight and blood lipid concentrations in hyperlipidemic subjects. Arch Intern Med. 2009;169:1046–1054. doi: 10.1001/archinternmed.2009.115. [DOI] [PubMed] [Google Scholar]
- Campbell WW, Tang M. Protein intake, weight loss, and bone mineral density in postmenopausal women. J Gerontol A Biol Sci Med Sci. 2010;65:1115–1122. doi: 10.1093/gerona/glq083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josse AR, Atkinson SA, Tarnopolsky MA, Phillips SM. Diets higher in dairy foods and dietary protein support bone health during diet- and exercise-induced weight loss in overweight and obese premenopausal women. J Clin Endocrinol Metab. 2012;97:251–260. doi: 10.1210/jc.2011-2165. [DOI] [PubMed] [Google Scholar]
- Skov AR, Haulrik N, Toubro S, Molgaard C, Astrup A. Effect of protein intake on bone mineralization during weight loss: a 6-month trial. Obes Res. 2002;10:432–438. doi: 10.1038/oby.2002.60. [DOI] [PubMed] [Google Scholar]
- Thorpe MP, Jacobson EH, Layman DK, He X, Kris-Etherton PM, Evans EM, et al. A diet high in protein, dairy, and calcium attenuates bone loss over twelve months of weight loss and maintenance relative to a conventional high-carbohydrate diet in adults. J Nutr. 2008;138:1096–1100. doi: 10.1093/jn/138.6.1096. [DOI] [PubMed] [Google Scholar]
- Greene PJ, Devecis J, Willett WC. Effects of low-fat vs ultra-low-carbohydrate weight-loss diets: A 12-week pilot feeding study. J Parent Enter Nutr. 2004;28:S13–S14. [Google Scholar]
- Leidy HJ, Carnell NS, Mattes RD, Campbell WW. Higher protein intake preserves lean mass and satiety with weight loss in pre-obese and obese women. Obesity. 2007;15:421–429. doi: 10.1038/oby.2007.531. [DOI] [PubMed] [Google Scholar]
- Brinkworth GD, Buckley JD, Noakes M, Clifton PM. Renal function following long-term weight loss in individuals with abdominal obesity on a very-low-carbohydrate diet vs high-carbohydrate diet. J Am Diet Assoc. 2010;110:633–638. doi: 10.1016/j.jada.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Ferrara LA, Innelli P, Palmieri V, Limauro S, De Luca G. Effects of different dietary protein intakes on body composition and vascular reactivity. Eur J Clin Nutr. 2006;60:643–649. doi: 10.1038/sj.ejcn.1602363. [DOI] [PubMed] [Google Scholar]
- Jenkins DJ, Kendall CW, Vidgen E, Augustin LS, Parker T, Faulkner D, et al. Effect of high vegetable protein diets on urinary calcium loss in middle-aged men and women. Eur J Clin Nutr. 2003;57:376–382. doi: 10.1038/sj.ejcn.1601530. [DOI] [PubMed] [Google Scholar]
- Brinkworth GD, Buckley JD, Noakes M, Clifton PM, Wilson CJ. Long-term effects of a very low-carbohydrate diet and a low-fat diet on mood and cognitive function. Arch Intern Med. 2009;169:1873–1880. doi: 10.1001/archinternmed.2009.329. [DOI] [PubMed] [Google Scholar]
- Schweiger U, Laessle R, Kittl S, Dickhaut B, Schweiger M, Pirke KM, et al. Macronutrient intake, plasma large neutral amino acids and mood during weight-reducing diets. J Neural Transm. 1986;67:77–86. doi: 10.1007/BF01243361. [DOI] [PubMed] [Google Scholar]
- Leidy HJ, Mattes RD, Campbell WW, Leidy Heather J, Mattes Richard D, Campbell Wayne W. Effects of acute and chronic protein intake on metabolism, appetite, and ghrelin during weight loss. Obesity. 2007;15:1215–1225. doi: 10.1038/oby.2007.143. [DOI] [PubMed] [Google Scholar]
- Due A, Toubro S, Skov AR, Astrup A. Effect of normal-fat diets, either medium or high in protein, on body weight in overweight subjects: a randomised 1-year trial. Int J Obes Relat Metab Disord. 2004;28:1283–1290. doi: 10.1038/sj.ijo.0802767. [DOI] [PubMed] [Google Scholar]
- Clifton PM, Bastiaans K, Keogh JB. High protein diets decrease total and abdominal fat and improve CVD risk profile in overweight and obese men and women with elevated triacylglycerol. Nutr Metab Cardiovasc Dis. 2009;19:548–554. doi: 10.1016/j.numecd.2008.10.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.