Abstract
Our goal is to develop countermeasures for pulmonary injury following unpredictable events such as radiological terrorism or nuclear accidents. We have previously demonstrated that captopril, an angiotensin converting enzyme (ACE) inhibitor, is more effective than losartan, an angiotensin type-1 receptor blocker, in mitigating radiation-pneumopathy in a relevant rodent model. In the current study we determined the dose modifying factors (DMFs) of captopril for mitigation of parameters of radiation pneumonitis. We used a whole animal model, irradiating 9–10-week-old female rats derived from a Wistar strain (WAG/RijCmcr) with a single dose of irradiation to the thorax of 11, 12, 13, 14 or 15 Gy. Our study develops methodology to measure DMFs for morbidity (survival) as well as physiological endpoints such as lung function, taking into account attrition due to lethal radiation-induced pneumonitis. Captopril delivered in drinking water (140–180 mg/m2/day, comparable with that given clinically) and started one week after irradiation has a DMF of 1.07–1.17 for morbidity up to 80 days (survival) and 1.21–1.35 for tachypnea at 42 days (at the peak of pneumonitis) after a single dose of ionizing radiation (X-rays). These encouraging results advance our goals, since DMF measurements are essential for drug labeling and comparison with other mitigators.
Keywords: radiation-pneumopathy, DMF, DRF, ACE inhibitors, breathing rate
INTRODUCTION
Development of countermeasures against radiation injuries after a radiological attack or nuclear accident has become a priority in several countries [1]. A first step is to identify safe and effective agents that can be administered after unintended exposures, to neutralize damage to radiosensitive organs and tissues. In countermeasure studies, agents delivered after irradiation but before the onset of injury are termed mitigators [2]. The relative efficacies of mitigators identified in preclinical studies need to be quantified by uniform assays to enable selection of the most promising candidate(s). The dose modifying factor (DMF) or dose reduction factor (DRF), the relative dose of irradiation required for a given effect in the drug-treated group as compared with a radiation-only group, has been described as the best measure of effectiveness. DMFs are essential to advance a drug for labeling as a radiation countermeasure [3, 4].
Our first goal has been to develop a suitable rat model to identify structural and functional derangements in the lung after a single, survivable radiation dose to the whole thorax [5–7]. This model is relevant to victims of radiological terrorism or accidents who receive high-dose upper-body exposure. Such victims have been described after nuclear explosions or accidents [8–10]. Our next goal was to use this model to identify agents that would mitigate the resulting injuries when therapy was started up to one week after irradiation following a mass casualty event; this delay was selected to provide a substantial window of time for mitigators to be delivered after biodosimetry to determine who needs them. Since suppressors of the renin-angiotensin system have been reported to attenuate radiation damage to the lungs of rats [6, 11, 12] and have been assessed for mitigation of clinical radiation injuries [13], we first tested these agents in our terrorism- or accident-relevant model. Assays were conducted between 35–80 days after radiation, which coincides with the first phase of lung injury: acute radiation pneumonitis. While an angiotensin converting enzyme (ACE) inhibitor, captopril, attenuated a wide range of radiation effects on lung structure and function, an angiotensin type-1 (AT1) receptor blocker, losartan, had more limited efficacy [6, 14]. In the current study we have quantified the beneficial effects of captopril by measuring DMFs for two endpoints (morbidity at 60 and 80 days and tachypnea at the peak of pneumonitis at 42 days). Because the steep dose response of the lungs to radiation demanded a need to account for attrition, we were required to develop methodology for determining DMFs at times when animals were lost to lethal pneumonitis.
MATERIALS AND METHODS
Animal model and irradiation
The Institutional Animal Care and Use Committee (IACUC) reviewed and approved all protocols in this study. Rats (WAG/RijCmcr) were housed in a moderate security barrier. Unanesthetized females were irradiated at 9–10 weeks of age at a weight of 120–140 g, with a single dose of irradiation of 11, 12, 13, 14 or 15 Gy limited to the thorax [5–7]. Irradiation was done with a 320-kVp orthovoltage source with a half-value layer of 1.4-mm Cu and a dose rate of 1.43 Gy/min. The radiation dose was delivered bilaterally to enhance uniformity. The head, kidneys and abdomen were shielded while the heart and a small portion of liver were exposed. Irradiated rats and age-matched controls (also female) were housed under identical conditions in a moderate-security barrier. Based upon directives from the IACUC of the Medical College of Wisconsin, rats were considered morbid and euthanized if they met specified veterinarian's criteria. These included at least three of the following: (i) greater than 10% loss in body weight; (ii) inactivity on two consecutive days, defined as no movement unless actively stimulated; (iii) lack of grooming that became worse after 24 hours; (iv) breathing rates of less than 50 or greater than 250 breaths per minute; and (v) hunched posture (similar to death pose) on two consecutive days.
Captopril therapy
Animals for each dose of irradiation were divided randomly into two groups (7–15 rats/group, see Table 1 for details) and one group was given the drug captopril (pharmaceutical-grade from Sigma, St. Louis, MO, USA). The drug was always started one week after irradiation and was continued until the end of the experiment (at 191 days). Rats are lost to pneumonitis from 35 days to 80 days after thoracic irradiation [15]. In the current study only one irradiated animal (14 Gy with captopril) out of 41 survivors was lost after 80 days (at 94 days). Captopril was dissolved in drinking water at 300 mg/l, which delivers 30–38 mg/kg/day or 140–180 mg/m2/day to a rat [6, 11, 12]; in comparison the approved range for use of captopril in humans is 12–250 mg/m2/day [16].
Table 1.
Animals remaining at 60 days after irradiation
| Irradiation alone |
Captopril |
|||
|---|---|---|---|---|
| Dose (Gy) | Number irradiated | Number remaining at 60 days | Number irradiated | Number remaining at 60 days |
| 11 | 7 | 7 | 7 | 7 |
| 12 | 7 | 7 | 8 | 8 |
| 13 | 7 | 1 | 8 | 6 |
| 14 | 7 | 3 | 8 | 7 |
| 15 | 14 | 0 | 15 | 6 |
The dose of captopril we administered to the rats was tested for biological potency. This was accomplished by measuring the increase in plasma renin activity (PRA) due to suppression of ACE activity by the drug in unirradiated rats. PRA was measured by radioimmunoassay (RIA) using a modification of the method of Sealey and Laragh [17]. Systolic blood pressure was measured in unirradiated rats using a tail-cuff (Visitec Systems, Apex, NC). Animals were conditioned to the apparatus and the reported blood pressure was the average of three readings taken on three consecutive days (13, 14 and 15) after the start of captopril [18].
Measurement of breathing rates
Breathing rates for each rat were measured every two weeks starting at four weeks after exposure as described previously [6, 7]. Rats were placed in a tight plexiglass jig which was put in a transparent, airtight box. A differential pressure transducer was connected to a data acquisition device (Dataq-DI 158U, Dataq Instruments Inc, Akron, OH, USA) to sense changes in pressure in the box. The frequency of pressure changes was recorded and analyzed. Recordings for a maximum of 10 min per animal after two training sessions were used. The training was needed to desensitize the rat to the jig and entire protocol in order to minimize stress. The mean breathing rate for each rat was then calculated from a minimum of four steady regions of the recording lasting greater than 15 s. If the measurement required more than 10 min of collection time, the rat was released and rested to prevent anxiety and drop in oxygen or increase in carbon dioxide inside the box. The breathing rate was expressed as breaths per minute. Data for breathing rates, blood pressure and PRA were calculated as mean ± standard error (SE).
Statistical methods for calculating DMF are described in the Results and Discussion section.
RESULTS AND DISCUSSION
DMF of captopril for survival
The DMF was measured by comparison of survival of rats given a single dose of 11, 12, 13, 14 or 15 Gy with or without captopril. The dose of drug tested did not affect blood pressure (119 ± 6 mm Hg in 10 animals on drug vs.123 ± 2 mm Hg in 10 age-matched rats). Captopril given for 10 days caused an increase in PRA to 31 ± 4 ng/ml/h in animals on drug vs. 16 ± 2 ng/ml/h in age-matched unirradiated rats. These results demonstrate the drug was biologically active.
A Cox proportional hazards model was fitted with log-transformed dose and the presence of drug as predictors (Fig. 1) for analysis of a full survival curve truncated at the end of pneumonitis (80 days). The presence of the drug affected the survival substantially with a hazard ratio (HR) = 0.30 (P = .0085). The DMF was estimated as DMF = 1.10 (SE 0.03).
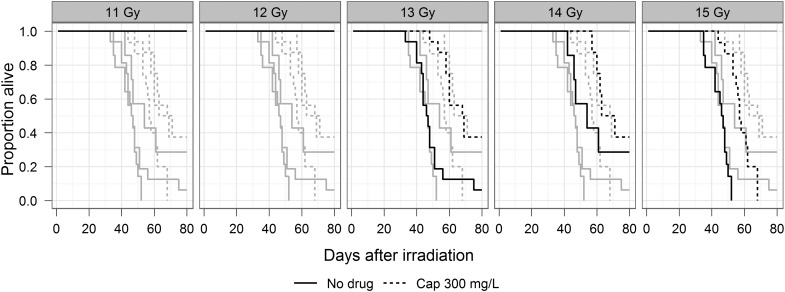
Fig. 1.
Kaplan–Meier plots of survival by radiation dose and treatment; survival truncated at 80 days.
The drug was started one week after irradiation. The numbers of rats in each group are shown in Table 1.
The complete dose response with five doses of irradiation is represented by gray lines while plots for each dose are individually highlighted in separate panels with black lines. As indicated in the figures, there was no morbidity with 11 and 12 Gy, with or without captopril.
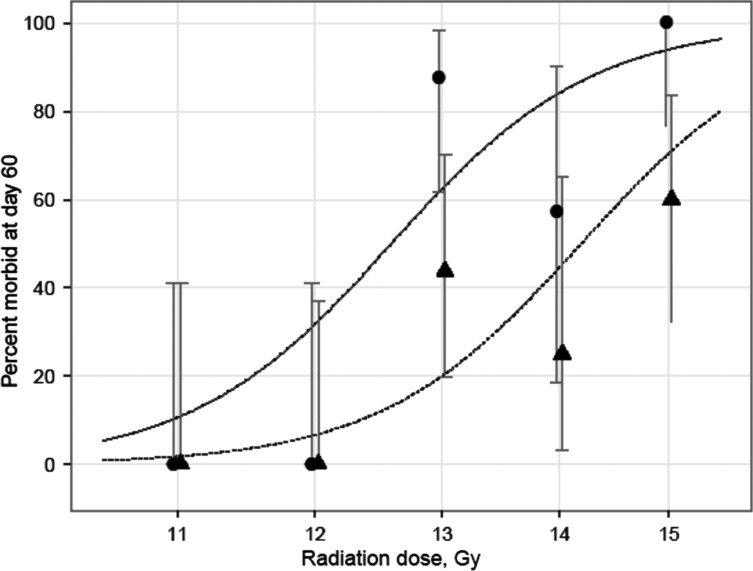
As an alternative, survival was considered as a binary endpoint and the DMF calculated by logistic regression. Calculation of DMF by logistic regression requires at least two dose groups in both control and drug-treated groups to have morbidity incidence that is neither 0 nor 100%. As a result a morbidity DMF could not be calculated for an interval prior to 50 days, when the first animals in the captopril group became morbid. No animals in any group required euthanasia for acute morbidity after 94 days, so that the DMF is unchanged after that time. Therefore, the morbidity DMF could be calculated for any interval between 50 and 94 days. At 60 days (Table 1 and Fig. 2) the morbidity DMF was 1.13 (SE 0.04); calculations of DMF for another interval, 80 days, give a similar value.
Fig. 2.
Observed and fitted probability of 60-day survival with exact binomial 95% confidence intervals versus radiation dose on log-scale. The X-coordinates of the observed values have been shifted slightly to avoid overlaps. The observed values are shown for captopril-treated (▲) and age-matched animals (•).
To our knowledge these are the first reports of a statistically significant DMF of captopril for mitigation of the effects of whole thoracic irradiation. At 13 Gy there was at least a 2-fold decrease in morbidity in rats given captopril (also see Table 1). The morbidity observed in Fig. 1 should be primarily due to pneumonitis since the lung is the most radiosensitive organ in the field, though we cannot eliminate the effect of radiation to the heart.
DMF of captopril for mitigating tachypnea at the peak of pneumonitis
Lung-specific injury was evaluated by measuring the increase in breathing rate caused by pneumonitis, since this is a simple non-invasive assay in rats. It can therefore be used to screen multiple agents and schedules. While breathing rates are non-invasive and can be measured serially over time, this parameter alone may not detect significant changes in lung physiology because of large breathing reserve. For tachypnea, the DMF was measured by comparison of irradiated rats given a single whole-thoracic dose of 11,12,13,14 or 15 Gy with or without captopril. Breathing rates increased after four weeks and peaked at six weeks (42 days) after irradiation, confirming the lung dysfunction in rats represented in Fig. 1.
We first examined the effect of breathing rates on residual survival, i.e. the survival after the measurement, using stratified Cox regression. The treatment groups form the strata. The effects of four-, six- and eight-week breathing rates were evaluated separately. Table 2 shows the results of this analysis. Both six- and eight-week breathing rates are predictive of the residual survival, with higher breathing rate associated with higher hazard of death, i.e. shorter survival.
Table 2.
Effect of breathing rate measured at 28, 42 and 56 days (four, six and eight weeks) on residual survival based on Cox regression stratified by treatment group
| Time (days) | Hazard ratio (HR) | 95% Confidence interval (CI) | P value |
|---|---|---|---|
| 28 | 1.002 | 0.977–1.027 | 0.883 |
| 42 | 1.008 | 1.001–1.015 | 0.020 |
| 56 | 1.019 | 1.006–1.032 | 0.005 |
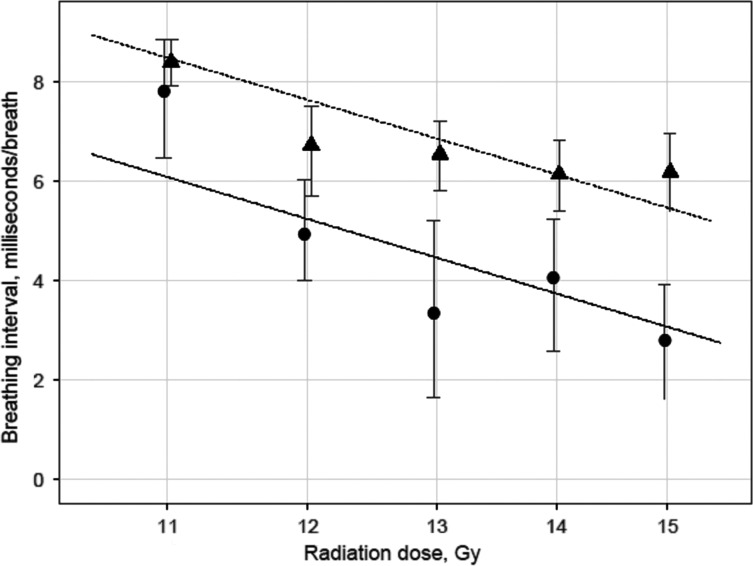
Breathing rate exhibited substantial variability, and thus Box–Cox analysis with a saturated model (each treatment–dose combination having an individual mean) was used to find the optimal power transformation. The estimated exponent of –0.55 implies that the inverse of breathing rate, the breathing interval (the number of seconds per breath) is most suited as a response variable. Since higher breathing rate and lower breathing interval are associated with more damage, the 42-day breathing interval was set to 0 for animals that died before this time.
Figure 3 shows the observed and fitted mean breathing interval at 42 days as a function of log-radiation dose. We estimated that the drug increased the breathing interval by 2.40 ms (P < 0.0001). The DMF for this outcome was estimated to be 1.28 (SE 0.07). We used breathing rates at 42 days to determine the DMF of captopril because it corresponds to the peak in breathing rates in irradiated non-treated rats and also because rats were being rapidly lost to morbidity at later times with the higher doses of irradiation.
Fig. 3.
Observed and fitted mean breathing interval at 42 days with 95% bootstrap confidence limits versus radiation dose on log scale. The X-coordinates of the observed values have been shifted slightly to avoid overlaps. The observed values are shown for captopril-treated (▲) and age-matched animals (•).
Incidental use [19, 20] and clinical trials of captopril [21] in irradiated lung cancer patients or patients receiving total body irradiation for bone marrow transplantation [13] demonstrated safety of this drug after radiation to the lungs. There is evidence that ACE inhibitors can mitigate radiation injuries to other organs [12, 22–24] enhancing the benefit of captopril as a radiomitigator. In addition, earlier studies indicated that multiple ACE inhibitors, including captopril [11, 25, 26], CL242817 [11, 25, 26] and CGS13945 [26] reduced radiation-induced pulmonary endothelial dysfunction. Those investigations employed partial volume irradiation to one lung with doses above 15 Gy. Because only one lung was injured the treatments were not lethal. Such models are less relevant to radiological terrorism and were designed for cancer therapies. Since these exposures did not affect survival, markers of endothelial dysfunction were used as surrogate endpoints to express the injury that resulted. The DRFs by the ACE inhibitors CGS13945 [26] and CL242817 for the endothelial marker plasminogen activator in these studies, were between 1.3 and 1.5 [25, 26] Functional endpoints such as survival or breathing rate were not evaluated [25, 26].
We have demonstrated histopathological changes in the lung (the gold standard for demonstrating radiation pneumonitis) between 28 and 56 days after single doses to the thorax in the same model described here. These changes corresponded with a rise in breathing rates [6, 7]. Captopril mitigated the changes observed after 56 days [6]. In addition to breathing rate and histology we have described at least eight other structural and radiation-induced functional derangements in the lungs at this time, including changes in the bronchoalveolar lavage, vascular reactivity, pulmonary vascular resistance, pulmonary arterial distensibility, pulmonary vascular density, etc. [5–7] These studies confirm that the morbidity and increase in breathing rate that we report here are due to radiation pneumonitis, although, as mentioned, the heart was also in the field. There was no deterioration in the heart after 10 Gy irradiation to the thorax only [27]. It is reported that doses around 15 Gy or above are required to injure the heart after partial body or localized exposures [27–33].
We used partial body irradiation, restricted to the thorax of rats in our study. There are two important reasons why we restricted irradiation to the thorax to measure the DMF for mitigation of lung injury by the ACE inhibitor captopril. (i) ACE inhibitors have been reported to mitigate radiation injuries to other organs such as kidney [34, 35], brain [36] and skin [37], thus mitigation of morbidity after total body irradiation (TBI) could reflect factors other than lung injury. (ii) Radiation doses sufficient to cause pneumonitis will first cause hematopoietic or gastrointestinal lethality in rats given TBI. Our model is relevant to victims who may survive partial body irradiation that shields the bone marrow and gut while exposing the whole volume of both lungs. Studies using TBI will evaluate the benefit of the drug in the context of multiple organ failure, a common effect of acute radiation injury. With a technique to measure DMF for lung function using breathing rates as an endpoint, we are now better prepared to test lung damage in more complicated models to assess other agents and schedules. Our model includes a single dose of thorax radiation at a relatively high dose rate, in the absence of any pharmacological anesthetic or inhalation agent, and is designed to simulate an unexpected radiological event.
The window of one week that we used before starting the drug would allow biodosimetry and other evaluation of risk to the lungs in a mass casualty event, as well as avoiding acute gastrointestinal toxicities that may be aggravated by oral intake [38].
Other agents have been described to mitigate radiation pneumonitis using models relevant to accidental exposures. Since reactive oxygen species are generated by irradiation, superoxide dismutases (SODs) such as the Eukarion class of drugs were tested in rats [39, 40]. Eukarion 207 (delivered by subcutaneous pumps) reduced tachypnea in female Sprague Dawley rats irradiated with 10 Gy to the lungs only. Survival was not assessed in these studies as all irradiated rats survived. The soy isoflavone genistein (given in the food) had similar effects [39]. In a different model (12 Gy to the lung with or without TBI (4 Gy)) one of four schedules of genistein mitigated tachypnea during pneumonitis but did not improve survival. Another SOD mimetic, Mn porphyrin (MnTE-2-Py5+), again delivered by subcutaneous pumps was given to Fischer-344 rats after irradiation of one lung with 28 Gy [41]. This partial lung irradiation was not lethal. Histopathology of the irradiated lung after 10 weeks was improved if the drug was started 2, 6 and 12 h after irradiation but not at 24–72 h. Statins (lovastatin) improved survival of female C57BL/6 mice when given immediately or eight weeks after 15 Gy to the thorax [42]. The breathing rates however were not altered by the drug. To our knowledge, the DMFs for survival or tachypnea induced by radiation have not been measured for any drug, making this one of the first such reports.
In summary our studies demonstrate that acute radiation pneumopathy can be mitigated in female rats by captopril, an agent that suppresses the renin-angiotensin system. The drug can be started one week after irradiation. The results need to be confirmed with male animals, younger or older rats and in other species. The present study establishes basic techniques for assessing DMFs.
This study describes a DMF of 1.1 and 1.28 by captopril for improvement of morbidity and tachypnea, respectively. Tachypnea is a more sensitive endpoint of radiation lung injury than survival because it can be measured as a continuous variable. A DMF of 1.1 means that on average a 10% higher dose of radiation can be tolerated when captopril is given in this schedule. However, because the sigmoidal dose–response curve for survival from lung injury is so steep, there may be minimal effect at the highest and lowest tolerated doses and more benefit at doses in between. No radio-mitigator has been approved for use by the US Food and Drug Administration (FDA), so it is not known what effect is sufficient for a countermeasure to be approved. Mitigation regimens have achieved DMFs of 1.23 in kidney [18], 1.13–1.18 in the gastrointestinal tract [43, 44], 1.1–1.5 in skin [45] and 1.1–1.5 for marrow [46, 47]. To our knowledge this is the first mitigator (i.e. a drug started after irradiation) in which a statistically significant DMF for radiation pneumonitis has been determined for single dose, whole-thorax radiation.
Results described here achieve an important step forward towards our goal, to identify and develop radio-mitigators for delayed radiation injuries. The next steps will be to extend testing to large animal models and to understand the mechanism(s) of mitigation by captopril in order to address the FDA ‘Animal Rule’ [25, 26]. For a drug to be considered for marketing approval in the USA, it must satisfy the ‘Animal Rule’ [48, 49] when human studies are not ethical, and clearly irradiation of normal humans to test mitigators is not ethical. Exposure of large volumes of the lungs to irradiation would fall into this category. Our study clearly addresses two of the four important considerations defined by the FDA that need to be met to develop captopril as a mitigator of radiation pneumonitis after accidental exposure. The first is that the animal study endpoints must correlate to increased survivability or prevention of significant morbidity in humans. The second is that the pharmacokinetic and pharmacodynamic data for the proposed product are available and can be used to select an effective dose in humans. The third is that a therapeutic or beneficial effect of the drug must be demonstrated in more than one species, which we have only partially addressed. The fourth requirement is a detailed understanding regarding the mechanism of toxicity of radiation in humans and the mechanism by which the drug reduces or prevents adverse effects. While the mechanism of radiation pneumonitis is unclear we have observed lung vasculopathies following single dose radiation exposure in rats. ACE inhibitors have multiple actions that affect the vasculature and could be responsible for radiation mitigation such as inhibiting angiotensin II formation, increasing bradykinin [51] and angiotensin-(1–7) levels [52], and blocking matrix metalloproteases-2&9 that affect vascular remodeling [53, 54]. Further research is necessary to investigate the mechanism through which captopril mitigates radiation injury to the lung.
ACKNOWLEDGEMENTS
This work was funded by RC1 AI81294 and NIH/NIAID agreements U19 AI67734. The statistical analysis was supported by grant 1UL1RR031973 from the CTSI program of the NIH. We also acknowledge the contribution of the Irradiation Core facility for conducting irradiation and dosimetry, and thank Jayashree Narayanan for her valuable help in preparing the manuscript.
REFERENCES
- 1.Coleman CN, Stone HB, Moulder JE, et al. Medicine. Modulation of radiation injury. Science. 2004;304:693–4. doi: 10.1126/science.1095956. [DOI] [PubMed] [Google Scholar]
- 2.Stone HB, Moulder JE, Coleman CN, et al. Models for evaluating agents intended for the prophylaxis, mitigation and treatment of radiation injuries. Report of an NCI Workshop, December 3-4, 2003. Radiat Res. 2004;162:711–28. doi: 10.1667/rr3276. [DOI] [PubMed] [Google Scholar]
- 3.Williams JP, Brown SL, Georges GE, et al. Animal models for medical countermeasures to radiation exposure. Radiat Res. 2010;173:557–8. doi: 10.1667/RR1880.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.FDA. New drug and biological drug products: evidence needed to demonstrate effectiveness of new drugs when human efficacy studies are not ethical or feasible. Final rule. Fed Reg. 2002;67:37988–98. [PubMed] [Google Scholar]
- 5.Ghosh SN, Wu Q, Mader M, et al. Vascular injury after whole thoracic x-ray irradiation in the rat. Int J Radiat Oncol Biol Phys. 2009;74:192–9. doi: 10.1016/j.ijrobp.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghosh SN, Zhang R, Fish BL, et al. Renin-Angiotensin system suppression mitigates experimental radiation pneumonitis. Int J Radiat Oncol Biol Phys. 2009;75:1528–36. doi: 10.1016/j.ijrobp.2009.07.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang R, Ghosh SN, Zhu D, et al. Structural and functional alterations in the rat lung following whole thoracic irradiation with moderate doses: injury and recovery. Int J Radiat Biol. 2008;84:487–97. doi: 10.1080/09553000802078396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fliedner TM, Dorr H, Meineke V. Multi-organ involvement as a pathogenetic principle of the radiation syndromes: a study involving 110 case histories documented in SEARCH and classified as the bases of haematopoietic indicators of effect. BJR Suppl. 2005;27:1–8. [Google Scholar]
- 9.UNSCEAR. In: Sources and Effects of Ionizing Radiation. Vienna: United Nations Scientific Committee on the Effects of Atomic Radiation; 2000. Exposures and effect of the Chernobyl accident (Annex J) pp. 453–566. [Google Scholar]
- 10.Shank B. Toxicity due to total body irradiation. In: Shrieve D, Loeffler J, editors. Human Radiation Injury. Philadelphia: Lippincott Williams & Wilkins; 2010. pp. 133–9. [Google Scholar]
- 11.Molteni A, Moulder JE, Cohen EF, et al. Control of radiation-induced pneumopathy and lung fibrosis by angiotensin-converting enzyme inhibitors and an angiotensin II type 1 receptor blocker. Int J Radiat Biol. 2000;76:523–32. doi: 10.1080/095530000138538. [DOI] [PubMed] [Google Scholar]
- 12.Molteni A, Wolfe LF, Ward WF, et al. Effect of an angiotensin II receptor blocker and two angiotensin converting enzyme inhibitors on transforming growth factor-beta (TGF-beta) and alpha-actomyosin (alpha SMA), important mediators of radiation-induced pneumopathy and lung fibrosis. Curr Pharm Des. 2007;13:1307–16. doi: 10.2174/138161207780618777. [DOI] [PubMed] [Google Scholar]
- 13.Cohen EP, Bedi M, Irving AA, et al. Mitigation of Late Renal and Pulmonary Injury after Hematopoietic Stem Cell Transplantation. Int J Radiat Oncol Biol Phys. 2011 doi: 10.1016/j.ijrobp.2011.05.081. (Epub ahead of print 19 November 2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jenkins P, Watts J. An improved model for predicting radiation pneumonitis incorporating clinical and dosimetric variables. Int J Radiat Oncol Biol Phys. 2011;80:1023–29. doi: 10.1016/j.ijrobp.2010.03.058. [DOI] [PubMed] [Google Scholar]
- 15.Kma L, Gao F, Fish B, et al. Angiotensin Converting Enzyme Inhibitors Mitigate Collagen Synthesis Induced by a Single Dose of Radiation to the Whole Thorax. J Radiat Res. 2012;53:10–17. doi: 10.1269/jrr.11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.PDR. Montvale: PDR network LLC. Physician's Desk Reference, 65th Edition. 2011. pp. 2394–7.
- 17.Sealey JE, Laragh JH. How to do a plasma renin assay. Cardiovascular Medicine. 1977;2:1079–1092. [Google Scholar]
- 18.Moulder JE, Cohen EP, Fish BL. Captopril and losartan for mitigation of renal injury caused by single-dose total-body irradiation. Radiat Res. 2011;175:29–36. doi: 10.1667/RR2400.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang LW, Fu XL, Clough R, et al. Can angiotensin-converting enzyme inhibitors protect against symptomatic radiation pneumonitis? Radiat Res. 2000;153:405–10. doi: 10.1667/0033-7587(2000)153[0405:caceip]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 20.Bezjak A, Soyfer V, Yi Q, et al. Radiation pneumonitis in lung cancer patients - The neglected patient-related variables. Int J Radiat Oncol Biol Phys. 2005;63:S229–S229. [Google Scholar]
- 21.Radiation Therapy Oncology Group. A Phase II Randomized Trial with Captopril in Patients Who Have Received Radiation Therapy +/- Chemptherapy for Stage II-IIIB Non-Small Cell Lung Cancer, Stage I Central Non-Small Cell Lung Cancer, or Limited-Stage Small-Cell Lung Cancer. Philadelphia: Radiation Therapy Oncology Group; 2009. [Google Scholar]
- 22.Cohen EP, Joines MM, Moulder JE. Prevention and treatment of radiation injuries-The role of the renin-angiotensin system. In: Rubin P, Constine LS, Mark LB, Okunieff P, editors. Late Effects of Cancer Treatment on Normal Tissues. Heidelberg: Springer-Verlag; 2008. pp. 69–76. [Google Scholar]
- 23.Jenrow KA, Brown SL, Liu J, et al. Ramipril mitigates radiation-induced impairment of neurogenesis in the rat dentate gyrus. Radiat Oncol. 2010;5:6–8. doi: 10.1186/1748-717X-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen EP, Fish BL, Moulder JE. Mitigation of radiation injuries via suppression of the renin-angiotensin system: emphasis on radiation nephropathy. Curr Drug Targets. 2010;11:1423–9. doi: 10.2174/1389450111009011423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ward WF, Lin PJ, Wong PS, et al. Radiation pneumonitis in rats and its modification by the angiotensin-converting enzyme inhibitor captopril evaluated by high-resolution computed tomography. Radiat Res. 1993;135:81–7. [PubMed] [Google Scholar]
- 26.Ward WF, Molteni A, Kim YT, et al. Structure-function analysis of angiotensin-converting enzyme inhibitors as modifiers of radiation-induced pulmonary endothelial dysfunction in rats. Br J Radiol. 1989;62:348–54. doi: 10.1259/0007-1285-62-736-348. [DOI] [PubMed] [Google Scholar]
- 27.Baker JE, Fish BL, Su J, et al. 10 Gy total body irradiation increases risk of coronary sclerosis degeneration of heart structure function in a rat model. Int J Radiat Biol. 2009;85:1089–100. doi: 10.3109/09553000903264473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cilliers GD, Harper IS, Lochner A. Radiation-induced changes in the ultrastructure and mechanical function of the rat heart. Radiother Oncol. 1989;16:311–26. doi: 10.1016/0167-8140(89)90044-3. [DOI] [PubMed] [Google Scholar]
- 29.Fajardo LF, Stewart JR. Experimental radiation-induced heart disease. I. Light microscopic studies. Am J Pathol. 1970;59:299–316. [PMC free article] [PubMed] [Google Scholar]
- 30.Lauk S, Kiszel Z, Buschmann J, et al. Radiation-induced heart disease in rats. Int J Radiat Oncol Biol Phys. 1985;11:801–8. doi: 10.1016/0360-3016(85)90314-1. [DOI] [PubMed] [Google Scholar]
- 31.Yeung TK, Hopewell JW. Effects of single doses of radiation on cardiac function in the rat. Radiother Oncol. 1985;3:339–45. doi: 10.1016/s0167-8140(85)80047-5. [DOI] [PubMed] [Google Scholar]
- 32.Yeung TK, Lauk S, Simmonds RH, et al. Morphological and functional changes in the rat heart after X irradiation: strain differences. Radiat Res. 1989;119:489–99. [PubMed] [Google Scholar]
- 33.Baker JE, Moulder JE, Hopewell JW. Radiation as a risk factor for cardiovascular disease. Antioxid Redox Signal. 2011;15:1945–56. doi: 10.1089/ars.2010.3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moulder JE. Pharmacological intervention to prevent or ameliorate chronic radiation injuries. Semin Radiat Oncol. 2003;13:73–84. doi: 10.1053/srao.2003.50007. [DOI] [PubMed] [Google Scholar]
- 35.Moulder JE. Post-irradiation approaches to treatment of radiation injuries in the context of radiological terrorism and radiation accidents: a review. Int J Radiat Biol. 2004;80:3–10. doi: 10.1080/09553000310001642920. [DOI] [PubMed] [Google Scholar]
- 36.Ryu S, Kolozsvary A, Jenrow KA, et al. Mitigation of radiation-induced optic neuropathy in rats by ACE inhibitor ramipril: importance of ramipril dose and treatment time. J Neurooncol. 2007;82:119–24. doi: 10.1007/s11060-006-9256-4. [DOI] [PubMed] [Google Scholar]
- 37.Kohl RR, Kolozsvary A, Brown SL, et al. Differential radiation effect in tumor and normal tissue after treatment with ramipril, an angiotensin-converting enzyme inhibitor. Radiat Res. 2007;168:440–5. doi: 10.1667/RR0707.1. [DOI] [PubMed] [Google Scholar]
- 38.Otterson MF, Leming SC, Callison J, et al. Safety of enalapril after abdominal irradiation. J Invest Med. 2012 (in press) [Google Scholar]
- 39.Mahmood J, Jelveh S, Calveley V, et al. Mitigation of radiation-induced lung injury by genistein and EUK-207. Int J Radiat Biol. 2011;87:889–901. doi: 10.3109/09553002.2011.583315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calveley VL, Jelveh S, Langan A, et al. Genistein can mitigate the effect of radiation on rat lung tissue. Radiat Res. 2010;173:602–11. doi: 10.1667/RR1896.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gauter-Fleckenstein B, Fleckenstein K, Owzar K, et al. Early and late administration of MnTE-2-PyP5+ in mitigation and treatment of radiation-induced lung damage. Free Radic Biol Med. 2010;48:1034–43. doi: 10.1016/j.freeradbiomed.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams JP, Hernady E, Johnston CJ, et al. Effect of administration of lovastatin on the development of late pulmonary effects after whole-lung irradiation in a murine model. Radiat Res. 2004;161:560–7. doi: 10.1667/rr3168. [DOI] [PubMed] [Google Scholar]
- 43.Kim H, Bernard ME, Flickinger J, et al. The autophagy-inducing drug carbamazepine is a radiation protector and mitigator. Int J Radiat Biol. 2011;87:1052–60. doi: 10.3109/09553002.2011.587860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang L, Sun W, Wang J, et al. Mitigation effect of an FGF-2 peptide on acute gastrointestinal syndrome after high-dose ionizing radiation. Int J Radiat Oncol Biol Phys. 2010;77:261–8. doi: 10.1016/j.ijrobp.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hopewell JW, Robbins ME, van den Aardweg GJ, et al. The modulation of radiation-induced damage to pig skin by essential fatty acids. Br J Cancer. 1993;68:1–7. doi: 10.1038/bjc.1993.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.MacVittie TJ, Farese AM, Jackson W. Defining the full therapeutic potential of recombinant growth factors in the post radiation-accident environment: the effect of supportive care plus administration of G-CSF. Health Phys. 2005;89:546–55. doi: 10.1097/01.hp.0000173143.69659.5b. [DOI] [PubMed] [Google Scholar]
- 47.Satyamitra M, Uma Devi P, Murase H, et al. In vivo postirradiation protection by a vitamin E analog, alpha-TMG. Radiat Res. 2003;160:655–61. doi: 10.1667/rr3077. [DOI] [PubMed] [Google Scholar]
- 48.Snoy PJ. Establishing efficacy of human products using animals: the US food, drug administration's ‘animal rule’. Vet Pathol. 2010;47:774–8. doi: 10.1177/0300985810372506. [DOI] [PubMed] [Google Scholar]
- 49.US Department of Health and Human Services, Food and Drug Administration, Draft guidance for industry—essential elements to address efficacy under the animal rule. Fed Regist. 2009;74:3610–11. [Google Scholar]
- 50.Goodfriend TL, Elliott ME, Catt KJ. Angiotensin receptors and their antagonists. N Engl J Med. 1996;334:1649–54. doi: 10.1056/NEJM199606203342507. [DOI] [PubMed] [Google Scholar]
- 51.Yang HY, Erdos EG, Levin Y. A dipeptidyl carboxypeptidase that converts angiotensin I and inactivates bradykinin. Biochim Biophys Acta. 1970;214:374–6. doi: 10.1016/0005-2795(70)90017-6. [DOI] [PubMed] [Google Scholar]
- 52.Chappell MC, Pirro NT, Sykes A, et al. Metabolism of angiotensin-(1-7) by angiotensin-converting enzyme. Hypertension. 1998;31:362–7. doi: 10.1161/01.hyp.31.1.362. [DOI] [PubMed] [Google Scholar]
- 53.Sorbi D, Fadly M, Hicks R, et al. Captopril inhibits the 72 kDa and 92 kDa matrix metalloproteinases. Kidney Int. 1993;44:1266–72. doi: 10.1038/ki.1993.378. [DOI] [PubMed] [Google Scholar]
- 54.Prontera C, Mariani B, Rossi C, et al. Inhibition of gelatinase A (MMP-2) by batimastat and captopril reduces tumor growth and lung metastases in mice bearing Lewis lung carcinoma. Int J Cancer. 1999;81:761–6. doi: 10.1002/(sici)1097-0215(19990531)81:5<761::aid-ijc16>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]