Abstract
Nonmutational resistance to linezolid is due to the presence of cfr, which encodes a methyltransferase responsible for methylation of A2503 in the 23S rRNA. The cfr gene was first described in animal isolates of staphylococci, and more recently, it has been identified in Staphylococcus aureus from human clinical infections, including in an outbreak of methicillin-resistant S. aureus. In enterococci, cfr has been described in an animal isolate of Enterococcus faecalis from China. Here, we report an isolate of linezolid-resistant E. faecalis (603-50427X) recovered from a patient in Thailand who received prolonged therapy with the antibiotic for the treatment of atypical mycobacterial disease. The isolate lacked mutations in the genes coding for 23S rRNA and L3 and L4 ribosomal proteins and belonged to the multilocus sequence type (MLST) 16 (ST16), which is commonly found in enterococcal isolates from animal sources. Resistance to linezolid was associated with the presence of cfr on an ∼97-kb transferable plasmid. The cfr gene environment exhibited DNA sequences similar to those of other cfr-carrying plasmids previously identified in staphylococci (nucleotide identity, 99 to 100%). The cfr-carrying plasmid was transferable by conjugation to a laboratory strain of E. faecalis (OG1RF) but not to Enterococcus faecium or S. aureus. The cfr gene was flanked by IS256-like sequences both upstream and downstream. This is the first characterization of the potential horizontal transferability of the cfr gene from a human linezolid-resistant isolate of E. faecalis.
INTRODUCTION
Linezolid was the first oxazolidinone introduced to clinical use (in 2000), and since then, it has been widely prescribed to treat infections caused by Gram-positive organisms and, in many instances, mycobacterial infections. Linezolid is currently approved by the Food and Drug Administration (FDA) for the treatment of complicated skin and skin structure infections and nosocomial pneumonia caused by susceptible organisms. Linezolid has an FDA indication for the treatment of vancomycin-resistant Enterococcus faecium (VRE) infections (including bacteremia) (61). Linezolid alters protein synthesis by binding to the 50S ribosomal subunit, with recent data suggesting that the oxazolidinone binds to the A site of the peptidyl-transferase center (PTC) of the bacterial ribosome, interfering with the positioning of aminoacyl-tRNA; as a result, protein synthesis is inhibited (71). Although the prevalence of linezolid resistance among Gram-positive organisms is still low (18, 56), the mechanisms of linezolid resistance have been extensively characterized. Indeed, the most common mechanism involves mutations in domain V of 23S rRNA (20, 67) with the G2576T (Escherichia coli numbering) substitution being the most frequently reported (18, 67). Additional mutations observed in resistant isolates include T2500A (44) C2192T (22), G2447T (34, 64), A2503G (34), T2504C (35), G2505A (50), G2766T (34), and C2461T (17). Additionally, mutations in the ribosomal proteins L3 and L4 have also been associated with linezolid resistance (17, 36–39). The above-mentioned mechanisms of linezolid resistance have been shown not to be transferable and are associated with previous exposure to linezolid.
In 2005, a Colombian patient developed nosocomial pneumonia with a linezolid-resistant methicillin-resistant Staphylococcus aureus (MRSA) isolate (3, 65). Characterization of the mechanism of resistance in the isolate indicated that cfr (for chloramphenicol-florfenicol resistance gene), encoding a methyltransferase that catalyzes the posttranscriptional methylation of nucleotide A2503 in the 23S rRNA, was responsible for the resistance phenotype (65). This gene had been previously described in a 17,108-bp transferable plasmid (pSCFS1) from an animal isolate of Staphylococcus sciuri (60) and had also been detected in several other staphylococci of animal origin in Europe (2, 26), which exhibited resistance to phenicols (florphenicol and chloramphenicol). Interestingly, Cfr methylation also affects susceptibility to other antimicrobial compounds, such as lincosamides, pleuromutilins, streptogramin A, and 16-member ring macrolides, which, like linezolid, all bind to the PTC on the 50S ribosomal subunit (63). This phenotype has been designated PhLOPSA (for phenicols, lincosamides, oxazolidinones, pleuromutilins, and streptogramin A antibiotics) (41). Linezolid resistance due to cfr in human clinical isolates of staphylococci has now been reported in several countries (5, 45–48), including a recent report of an outbreak of cfr-containing MRSA in a critical care unit in Spain (48). Dissemination of the cfr gene among staphylococci is of great concern, since evidence of transferability of the gene has been provided both in vitro and in vivo (45, 57). The initial report of cfr in a human isolate of MRSA suggested that a possible source of the gene may have been enterococci. Indeed, cfr has been detected in human clinical isolates of enterococci (7, 8), although a complete characterization of these isolates has not been published. In this work, we present the characterization of a human clinical isolate of linezolid-resistant Enterococcus faecalis carrying the cfr gene on a transferable plasmid.
(Parts of the results of the present study were presented at the 22nd European Congress of Clinical Microbiology and Infectious Diseases [ECCMID], London, England, 31 March to 3 April 2012.)
MATERIALS AND METHODS
Bacterial isolates, species identification, and molecular typing.
A linezolid-resistant E. faecalis strain (603-50427X) was isolated in July 2010 from the skin of a 72-year-old diabetic woman in Bangkok, Thailand, who presented with multiple skin abscesses due to Mycobacterium abscessus and pulmonary tuberculosis. The patient received meropenem, amikacin, moxifloxacin, azithromycin, isoniazid, rifampin, ethambutol, metronidazole, and linezolid for at least 3 months prior to the isolation of the linezolid-resistant organism. The susceptibilities of the E. faecalis isolate are shown in Table 1. E. faecalis OG1RF (14, 51), rifampin- and fusidic acid-resistant derivatives of E. faecium GE1 (16), and S. aureus RN4220 (30) (RN4220-RF) were used as recipients for conjugation experiments (see below). Species identification of the enterococcal isolate and determination of the presence of vancomycin resistance genes were performed using a PCR assay as described previously (15). Typing of the organism was performed by multilocus sequence typing (MLST) using the established set of E. faecalis MLST primers (targeting aroE, gdh, gki, gyd, pstS, xpt, and yqiL) (1, http://efaecalis.mlst.net). DNA sequencing was performed in both strands using the dideoxynucleotide chain termination method (59) with fluorescent cycle sequencing with dye-labeled terminators (Applied Biosystems [Foster City, CA] BigDye Terminator V3.1 cycle sequencing kit) on an ABI Prism 3730xl DNA analyzer.
Table 1.
Antimicrobial susceptibilities of E. faecalis 603-50427X and OG1RF and transconjugants
| Antibiotic | MIC (μg/ml)a |
|||
|---|---|---|---|---|
| E. faecalis 603-50427X | OG1RF | Tc6 | Tc11 | |
| Linezolid | 32 | 1 | 8 | 8 |
| Linezolidb | 24 | 2 | 8 | 6 |
| Ampicillin | 2 | 4 | 1 | 1 |
| Vancomycin | 1 | 2 | 2 | 2 |
| Teicoplanin | ≤1 | ≤1 | ≤1 | ≤1 |
| Daptomycin | 1 | 2 | 1 | 1 |
| Ciprofloxacin | >4 | 2 | 1 | 1 |
| Levofloxacin | >4 | 2 | 1 | 1 |
| Chloramphenicol | 64 | 4 | 64 | 64 |
| Tigecycline | 0.06 | 0.12 | 0.12 | 0.12 |
| Quinupristin/dalfopristin | 4 | 16 | >16 | >16 |
| Tiamulin | 64 | >64 | >64 | >64 |
| Clindamycin | >128 | 32 | >128 | >128 |
| Fusidic acid | 8 | >128 | >128 | >128 |
| Rifampin | 0.25 | >128 | >128 | >128 |
Susceptibility testing and detection of linezolid resistance.
MICs were determined by a broth microdilution method following the Clinical and Laboratory Standards Institute (CLSI) guidelines (10, 11). Additional susceptibilities to linezolid were determined using Etest (BioMerieux, Marcy l'Étoile, France) on Mueller-Hinton agar (Oxoid Basingstoke, Hampshire, United Kingdom) following the recommendations of the manufacturer. The presence of mutations in genes encoding all copies of the 23S rRNA and ribosomal proteins L3 and L4 were investigated by PCR and sequencing. The 23S rRNA-encoding genes were PCR amplified used previously published primers (43), while the L3- and L4-encoding genes were amplified using specifically designed primers, as follows: ESP rplC-F, ATGACCAAAGGAATCTTAGGG; ESP rplC-R, CACAGCTGATTTGATWGTGATT; ESP rplD-F, GCCGAATGTAGCATTATTCAA; and ESP rplD-R, CAAGCACCTCCTCAATTTGAGT. The amplicons were sequenced on both strands, and the amino acid sequences were compared with those from wild-type linezolid-susceptible E. faecalis ATCC 29212. The resulting DNA sequences were compared with all E. faecalis isolates whose genomes have been sequenced (http://www.ncbi.nlm.nih.gov/genomes/geblast.cgi?taxid=1351). Detection of the presence of the cfr gene was performed by PCR using primers previously reported (27).
Bacterial mating and S1 nuclease assays.
Conjugative transfer of cfr was conducted by filter mating (66) using E. faecalis 603-50427X as the donor and E. faecalis OG1RF, E. faecium GE1, and S. aureus RN4220-RF as recipients. Selection of transconjugants was performed using brain heart infusion (BHI) agar plates supplemented with chloramphenicol (20 μg/ml) and fusidic acid (25 μg/ml). Purified single colonies of transconjugants were also plated on BHI agar containing chloramphenicol (20 μg/ml) and rifampin (100 μg/ml). Pulsed-field gel electrophoresis (PFGE) was performed in all transconjugants using the restriction enzyme SmaI (49) in order to confirm that they were derivatives of the recipient strain. Total DNA from E. faecalis transconjugants was subjected to S1 nuclease assays coupled with PFGE in order to determine the presence of transferred plasmids, as described previously (4). S1 nuclease-treated linearized plasmids were transferred to a nylon membrane and hybridized using a cfr probe (745 bp) (62).
Sequencing of cfr and its genetic environment.
Total DNA was extracted by standard methodology using a DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA), following the manufacturer's recommendations, with a modification consisting of adding lysozyme (20 mg/ml) to the lysis buffer, followed by incubation for 30 min at 37°C. In order to sequence the cfr gene, primers Cfr-F (5′-TGTATGTTTTGACTTTCGGCACCGG-3′) and Cfr-R (5′-ATTATCTTCCACCCAGTAGTCC-3′), which are located 138 bp upstream and 133 bp downstream of cfr, respectively, in S. aureus CM-05 (accession number JN849634), were used for PCR amplifications. The PCR amplicon was purified using a QIAquick PCR purification kit (Qiagen, Valencia, CA), sequenced by fluorescent cycle sequencing with dye-labeled terminators, assembled using CodonCode Aligner (version 3.7.1; CodonCode Corp., Dedham, MA), and compared to reference sequences. The DNA sequence upstream and downstream of cfr in E. faecalis 603-50427X was obtained by inverse PCR methodology (53). Briefly, total DNA from the bacterial isolate was extracted and digested with EcoRI. The digested DNA was purified and religated using T4 ligase. The inverse primers 5′-TGCTCTGAATTTTGCTCTGCTAAGA-3′ (targeting nucleotides 203 to 227 of cfr) and 5′-GGAGAAGCAAACGAAGGGCAGG-3′ (nucleotides 907 to 928) were used for the PCR assay. The resulting amplicon was purified and cloned into the pGEM-T Easy Vector for sequencing with universal M13 primers.
Nucleotide sequence accession number.
The sequence of 1,274 bp upstream and and 1,170 bp downstream of the cfr gene has been deposited in GenBank under accession number JQ660368.
RESULTS AND DISCUSSION
Linezolid resistance in E. faecalis 603-50427X is associated with the presence of cfr on a plasmid.
E. faecalis 603-50427X exhibited resistance to linezolid with MICs of 32 μg/ml by broth microdilution and 24 μg/ml by Etest (Table 1). In order to determine the mechanism of resistance, we initially investigated the presence of mutations in the genes encoding domain V of the 23S rRNA (the most common mechanism found in clinical isolates) and in ribosomal proteins L3 and L4. Sequencing of the above-mentioned genes revealed no mutations when comparing the sequences with those from wild-type E. faecalis genomes available in the NCBI database (http://www.ncbi.nlm.nih.gov/genomes/geblast.cgi?taxid=1351). Instead, we were able to obtain a PCR product of the expected size (745 bp) when using primers previously used to amplify cfr from staphylococci. The amplicon was cloned and sequenced in its entirety on both strands, revealing a nucleotide sequence that was 100% identical to that of cfr genes from several human and animal staphylococcal isolates, including the original isolate of S. sciuri (25) and the first MRSA of human origin (65). The cfr gene from E. faecalis 603-50427X exhibited two nucleotide differences (G262→A and A367→G) from a cfr gene previously characterized from an animal isolate of E. faecalis (EF-01) (33), although the nucleotide changes caused an amino acid difference only in position 88 of the predicted enzyme (Glu→Lys). The putative Cfr amino acid sequence from E. faecalis 603-50427X had all the essential amino acids predicted to be required for biochemical activity, including the conserved CxxxCxxC motif characteristic of radical S-adenosyl-l-methionine (SAM) enzymes (24).
In several organisms, cfr has been described on plasmids, present as a monocistronic unit and associated with transposons, suggesting that the gene is likely to be mobilizable (40). Indeed, the cfr gene was initially described on a 17.1-kb plasmid from a multiresistant S. sciuri animal isolate and was designated pSCFS1, which also carries the rRNA methylase gene erm(33), the aminocyclitol phosphotransferase gene spc and the ABC transporter gene lsa(B), conferring resistance to macrolide-lincosamide-streptogramin B (MLSB) antibiotics, spectinomycin, and lincosamides, respectively (25). In order to determine the location of the cfr gene within the genome of E. faecalis 603-50427X, we performed S1 digestion, PFGE, and Southern hybridization with a cfr probe. This methodology has been used previously (4) to detect and estimate the sizes of enterococcal plasmids, since large plasmids are linearized on a PFGE gel. Figure 1 shows that cfr was indeed located on an E. faecalis plasmid of ca. 97 kb.
Fig 1.
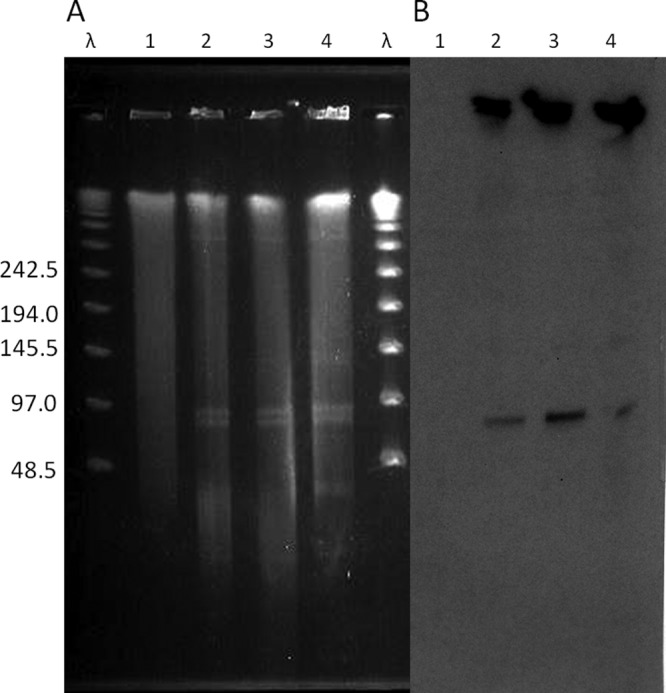
Localization and transfer of the cfr gene determined by S1-PFGE and Southern blot hybridization. S1 digestion of total DNA of E. faecalis strains was followed by PFGE (A) and Southern blot analysis with a cfr probe (B). λ denotes the lambda ladder (molecular sizes in kilobases are shown on the left). Lane 1, E. faecalis OG1RF; lanes 2 and 3, E. faecalis transconjugants Tc6 and Tc11, respectively; lane 4, E. faecalis 603-50427X. The figure is a composite of gels and hybridizations from different experiments.
Conjugative transfer of linezolid resistance to E. faecalis OG1RF via a cfr-carrying plasmid.
The transferability of the cfr gene was evaluated by performing filter-mating experiments, as described previously (66). As the recipients of the mating experiments, we used E. faecalis OG1RF (14, 51), E. faecium GE1 (16), and S. aureus RN4220-RF (30). We were able to successfully transfer cfr to E. faecalis OG1RF (efficiency, 2.6 × 10−5 transconjugants per donor), but not to E. faecium or S. aureus, suggesting that the cfr-carrying plasmid was not able to replicate in a heterologous host. Two purified single colonies from the E. faecalis OG1RF transconjugants, designated Tc6 and Tc11, were characterized by PCR (targeting cfr), PFGE, S1 nuclease digestion, and hybridization in order to compare DNA banding and plasmid profiles between the donor and the corresponding transconjugants. Figure 1 confirms that the cfr-carrying plasmid was transferred to the recipient E. faecalis strain via conjugation. Of note, several other plasmids were also transferred during the mating experiments, indicating that several mobilizable plasmids are present in the clinical strain E. faecalis 603-50427X (Fig. 1). The lack of transfer to an S. aureus recipient was not surprising, since it has been shown that some enterococcal plasmids may be unstable in staphylococcal backgrounds. Indeed, it has been shown that enterococcal plasmids containing the vanA gene cluster involved in vancomycin resistance are unstable in S. aureus (54). Moreover, the efficient transfer of DNA between bacteria of different species may be limited by one or more restriction/modification systems (23). In fact, at least three types of restriction systems that drastically reduce the frequency of horizontal gene transfer have been described in S. aureus (12). The lack of plasmid transfer to E. faecium was also not surprising, since most E. faecalis plasmids may not have the ability to replicate in E. faecium hosts. As an example, pheromone-responsive plasmids that are commonly found in E. faecalis isolates are rarely described in E. faecium (14).
The transfer of the cfr plasmid was associated with a 3- to 4-fold increase in the linezolid MIC (2 μg/ml to 6 μg/ml and 8 μg/ml in the transconjugants Tc11 and Tc6, respectively) (Table 1). Moreover, increases in the MICs of chloramphenicol, clindamycin, and quinopristin/dalfopristin (part of PhLOPSA) were also observed in the transconjugant strains, supporting the notion that cfr was an important contributor to the resistance phenotype in the parental strain (Table 1). The increase of linezolid MICs observed in the transconjugants did not reach the level observed in E. faecalis 603-50427X, suggesting that other factors may influence susceptibility to linezolid in the original clinical strain. This phenomenon has also been observed in staphylococci when cfr has been expressed on plasmids in heterologous hosts (65).
Sequence type and genetic environment of the cfr gene in E. faecalis 603-50427X.
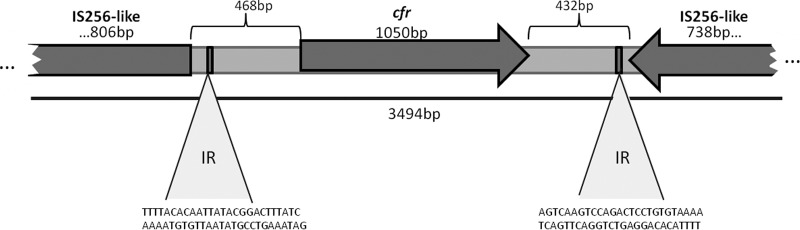
MLST typing of E. faecalis 603-50427X indicated that it belonged to sequence type 16 (ST16). Unlike the majority of clinical isolates of E. faecalis belonging to clonal complex 2 (CC2) and CC9 (31, 58), isolates belonging to ST16 have been associated with both animal and human origins (9, 19, 52, 55). This finding is of particular interest, since the cfr gene has been characterized mainly in isolates from animal origin, and it suggests that E. faecalis 603-50427X may have been transmitted by direct contact with an animal source. In order to determine the genetic environment surrounding the cfr gene on the plasmid, we obtained the sequences 1,274 bp upstream and 1,170 bp downstream of the cfr gene (JQ660368). BLASTn analysis of these sequences showed a high degree of nucleotide identity (99%) to IS256-like sequences present in plasmid pSS-01, which was originally described in a Staphylococcus cohnii strain recovered from a porcine nasal swab in Shandong Province, China (69) (Fig. 2). Our findings indicate that cfr in E. faecalis 603-50427X is flanked by the same mobile transposable elements, a phenomenon that has been described in other cfr-carrying organisms (5, 26, 64, 68). IS256 belongs to the mutator transposase family and is widespread in the genomes of several multiresistant enterococci and staphylococci (6). IS256 consists of an open reading frame encoding a transposase protein flanked by noncoding regions harboring imperfect inverted repeats (IRs) (21). As shown in Fig. 2, we found two imperfect IRs flanking cfr in E. faecalis 603-50427X, suggesting that the gene may also be transferable via IS256-mediated transposition. Of note, this insertion sequence has been associated with aminoglycoside resistance (29, 42) and with the ica operon in biofilm producers of Staphylococcus epidermidis (28, 29). Since a high prevalence of transposons with IS256 sequences in enterococcal isolates has been reported from Japan and Thailand (70, 32) and many cfr-carrying strains have been detected in the last 2 years in China (13, 33, 68, 69, 72), it is tempting to speculate that cfr has an important potential for dissemination from animals to humans in Southeast Asia.
Fig 2.
Genetic environment of cfr in E. faecalis 603-50427X. The cfr gene is flanked in both sites by IS256-like sequences. The 26-bp IR sequences are shown.
In summary, we report the first characterization of a transferable cfr-carrying plasmid of human origin in an E. faecalis isolate (ST16), conferring resistance to linezolid. The genetic environment suggests high potential for dissemination of this gene, which confers multidrug resistance.
ACKNOWLEDGMENTS
Cesar A. Arias is supported by grants R00 AI R00 AI72961 and R01 AI093749 from the National Institute of Allergy and Infectious Diseases (NIAID). Lorena Diaz was partially supported by a graduate scholarship from The Instituto Colombiano para el Desarrollo de la Ciencia y Tecnología, Francisco José de Caldas, COLCIENCIAS; the American Society of Microbiology Latin American Fellowship for Epidemiology; and the Universidad El Bosque Graduate Fellowship. Pattarachai Kiratisin is supported by the Siriraj Research Development Fund.
Footnotes
Published ahead of print 9 April 2012
REFERENCES
- 1. Aanensen DM, Spratt BG. 2005. The multilocus sequence typing network: mlst.net. Nucleic Acids Res. 33:W728–W733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Argudín MA, et al. 2011. Virulence and resistance determinants of German Staphylococcus aureus ST398 isolates from nonhuman sources. Appl. Environ. Microbiol. 77:3052–3060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arias CA, et al. 2008. Clinical and microbiological aspects of linezolid resistance mediated by the cfr gene encoding a 23S rRNA methyltransferase. J. Clin. Microbiol. 46:892–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arias CA, Panesso D, Singh KV, Rice LB, Murray BE. 2009. Cotransfer of antibiotic resistance genes and a hylEfm-containing virulence plasmid in Enterococcus faecium. Antimicrob. Agents Chemother. 53:4240–4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bonilla H, et al. 2010. Multicity outbreak of linezolid-resistant Staphylococcus epidermidis associated with clonal spread of a cfr-containing strain. Clin. Infect. Dis. 51:796–800 [DOI] [PubMed] [Google Scholar]
- 6. Byrne ME, Rouch DA, Skurray RA. 1989. Nucleotide sequence analysis of IS256 from the Staphylococcus aureus gentamicin-tobramycin-kanamycin-resistance transposon Tn4001. Gene 81:361–367 [DOI] [PubMed] [Google Scholar]
- 7. Cercenado E, et al. 2010. Emerging linezolid resistance: dissemination of the cfr gene among Staphylococcus aureus, Staphylococcus epidermidis, Enterococcus faecium and Enterococcus faecalis and inability of the Etest method for detection, abstr C2-1490. Abstr. 50th Intersci. Conf. Antimicrob. Agents Chemother., Boston, MA [Google Scholar]
- 8. Cercenado E, Marin M, Gama B, Iglesias C, Bouza E. 2011. In vitro activity of torezolid (TR-700) against linezolid-resistant gram-positive clinical isolates possessing the cfr methyltransferase gene and/or ribosomal gene mutations. C2-945. Abstr. 51st Intersci. Conf. Antimicrob. Agents Chemother [Google Scholar]
- 9. Chowdhury SA, et al. 2009. A trilocus sequence typing scheme for hospital epidemiology and subspecies differentiation of an important nosocomial pathogen, Enterococcus faecalis. J. Clin. Microbiol. 47:2713–2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clinical and Laboratory Standards Institute 2009. M07–A8 Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 8th ed Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 11. Clinical and Laboratory Standards Institute 2011. MS100-S21 Performance standards for antimicrobial susceptibility testing: 21st informational supplement. CLSI, Wayne, PA [Google Scholar]
- 12. Corvaglia AR, et al. 2010. A type III-like restriction endonuclease functions as a major barrier to horizontal gene transfer in clinical Staphylococcus aureus strains. Proc. Natl. Acad. Sci. U. S. A. 107:11954–11958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dai L, et al. 2010. First report of the multidrug resistance gene cfr and the phenicol resistance gene fexA in a Bacillus strain from swine feces. Antimicrob. Agents Chemother. 54:3953–3955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dunny GM, Brown BL, Clewell DB. 1978. Induced cell aggregation and mating in Streptococcus faecalis: evidence for a bacterial sex pheromone. Proc. Natl. Acad. Sci. U. S. A. 75:3479–3483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dutka-Malen S, Evers S, Courvalin P. 1995. Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J. Clin. Microbiol. 33:1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eliopoulos GM, Wennersten C, Moellering RC., Jr 1982. Resistance to beta-lactam antibiotics in Streptococcus faecium. Antimicrob. Agents Chemother. 22:295–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Endimiani A, et al. 2011. Emergence of linezolid-resistant Staphylococcus aureus after prolonged treatment of cystic fibrosis patients in Cleveland, Ohio. Antimicrob. Agents Chemother. 55:1684–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Farrell DJ, Mendes RE, Ross JE, Sader HS, Jones RN. 2011. LEADER Program results for 2009: an activity and spectrum analysis of linezolid using 6,414 clinical isolates from 56 medical centers in the United States. Antimicrob. Agents Chemother. 55:3684–3690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Freitas AR, Novais C, Ruiz-Garbajosa P, Coque TM, Peixe L. 2009. Clonal expansion within clonal complex 2 and spread of vancomycin-resistant plasmids among different genetic lineages of Enterococcus faecalis from Portugal. J. Antimicrob. Chemother. 63:1104–11011 [DOI] [PubMed] [Google Scholar]
- 20. Gonzales RD, et al. 2001. Infections due to vancomycin-resistant Enterococcus faecium resistant to linezolid. Lancet 357:1179. [DOI] [PubMed] [Google Scholar]
- 21. Hennig S, Ziebuhr W. 2010. Characterization of the transposase encoded by IS256, the prototype of a major family of bacterial insertion sequence elements. J. Bacteriol. 192:4153–4163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Howe RA, et al. 2003. Activity of AZD2563, a novel oxazolidinone, against Staphylococcus aureus strains with reduced susceptibility to vancomycin or linezolid. Antimicrob. Agents Chemother. 47:3651–3652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jeltsch 2003. Maintenance of species identity and controlling speciation of bacteria: a new function for restriction/modification systems? Gene 317:13–16 [DOI] [PubMed] [Google Scholar]
- 24. Kaminska KH, et al. 2010. Insights into the structure, function and evolution of the radical-SAM 23S rRNA methyltransferase Cfr that confers antibiotic resistance in bacteria. Nucleic Acids Res. 38:1652–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kehrenberg C, Ojo KK, Schwarz S. 2004. Nucleotide sequence and organization of the multiresistance plasmid pSCFS1 from Staphylococcus sciuri. J. Antimicrob. Chemother. 54:936–939 [DOI] [PubMed] [Google Scholar]
- 26. Kehrenberg C, Schwarz S. 2006. Distribution of florfenicol resistance genes fexA and cfr among chloramphenicol-resistant Staphylococcus isolates. Antimicrob. Agents Chemother. 50:1156–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kehrenberg C, Schwarz S, Jacobsen L, Hansen LH, Vester B. 2005. A new mechanism for chloramphenicol, florfenicol and clindamycin resistance: methylation of 23S ribosomal RNA at A2503. Mol. Microbiol. 57:1064–1073 [DOI] [PubMed] [Google Scholar]
- 28. Koskela A, Nilsdotter-Augustinsson A, Persson L, Soderquist B. 2009. Prevalence of the ica operon and insertion sequence IS256 among Staphylococcus epidermidis prosthetic joint infection isolates. Eur. J. Clin. Microbiol. Infect. Dis. 28:655–660 [DOI] [PubMed] [Google Scholar]
- 29. Kozitskaya S, et al. 2004. The bacterial insertion sequence element IS256 occurs preferentially in nosocomial Staphylococcus epidermidis isolates: association with biofilm formation and resistance to aminoglycosides. Infect. Immun. 72:1210–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kreiswirth BN, et al. 1983. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 305:709–712 [DOI] [PubMed] [Google Scholar]
- 31. Leavis HL, Bonten MJ, Willems RJ. 2006. Identification of high-risk enterococcal clonal complexes: global dispersion and antibiotic resistance. Curr. Opin. Microbiol. 9:454–460 [DOI] [PubMed] [Google Scholar]
- 32. Leelaporn A, Yodkamol K, Waywa D, Pattanachaiwit S. 2008. A novel structure of Tn4001-truncated element, type V, in clinical enterococcal isolates and multiplex PCR for detecting aminoglycoside resistance genes. Int. J. Antimicrob. Agents 31:250–254 [DOI] [PubMed] [Google Scholar]
- 33. Liu Y, et al. 2012. First report of the multidrug resistance gene cfr in Enterococcus faecalis of animal origin. Antimicrob. Agents Chemother. 56:1650–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Livermore DM, Mushtaq S, Warner M, Woodford N. 2009. Activity of oxazolidinone TR-700 against linezolid-susceptible and -resistant staphylococci and enterococci. J. Antimicrob. Chemother. 63:713–715 [DOI] [PubMed] [Google Scholar]
- 35. Livermore DM, Warner M, Mushtaq S, North S, Woodford N. 2007. In vitro activity of the oxazolidinone RWJ-416457 against linezolid-resistant and -susceptible staphylococci and enterococci. Antimicrob. Agents Chemother. 51:1112–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Locke JB, Hilgers M, Shaw KJ. 2009. Mutations in ribosomal protein L3 are associated with oxazolidinone resistance in staphylococci of clinical origin. Antimicrob. Agents Chemother. 53:5275–5278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Locke JB, et al. 2010. Structure-activity relationships of diverse oxazolidinones for linezolid-resistant Staphylococcus aureus strains possessing the cfr methyltransferase gene or ribosomal mutations. Antimicrob. Agents Chemother. 54:5337–5343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Locke JB, Hilgers M, Shaw KJ. 2009. Novel ribosomal mutations in Staphylococcus aureus strains identified through selection with the oxazolidinones linezolid and torezolid (TR-700). Antimicrob. Agents Chemother. 53:5265–5274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Locke JB, Morales G, Hilgers M, Rahawi GCKS, Picazo J, Shaw KJ, Stein JL. 2010. Elevated linezolid resistance in clinical cfr-positive Staphylococcus aureus isolates is associated with co-occurring mutations in ribosomal protein L3. Antimicrob. Agents Chemother. 54:5352–5355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Locke JB, Rahawi S, Lamarre J, Mankin AS, Shaw KJ. 2012. Genetic environment and stability of cfr in methicillin-resistant Staphylococcus aureus CM05. Antimicrob. Agents Chemother. 56:332–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Long KS, Poehlsgaard J, Kehrenberg C, Schwarz S, Vester B. 2006. The Cfr rRNA methyltransferase confers resistance to phenicols, lincosamides, oxazolidinones, pleuromutilins, and streptogramin A antibiotics. Antimicrob. Agents Chemother. 50:2500–2505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lyon BR, Gillespie MT, Skurray RA. 1987. Detection and characterization of IS256, an insertion sequence in Staphylococcus aureus. J. Gen. Microbiol. 133:3031–3038 [DOI] [PubMed] [Google Scholar]
- 43. Marshall SH, Donskey CJ, Hutton-Thomas R, Salata RA, Rice LB. 2002. Gene dosage and linezolid resistance in Enterococcus faecium and Enterococcus faecalis. Antimicrob. Agents Chemother. 46:3334–3336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Meka VG, et al. 2004. Linezolid resistance in sequential Staphylococcus aureus isolates associated with a T2500A mutation in the 23S rRNA gene and loss of a single copy of rRNA. J. Infect. Dis. 190:311–317 [DOI] [PubMed] [Google Scholar]
- 45. Mendes RE, et al. 2010. First report of Staphylococcal clinical isolates in Mexico with linezolid resistance caused by cfr: evidence of in vivo cfr mobilization. J. Clin. Microbiol. 48:3041–3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mendes RE, et al. 2008. First report of cfr-mediated resistance to linezolid in human staphylococcal clinical isolates recovered in the United States. Antimicrob. Agents Chemother. 52:2244–2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mendes RE, et al. 2010. Assessment of linezolid resistance mechanisms among Staphylococcus epidermidis causing bacteraemia in Rome, Italy. J. Antimicrob. Chemother. 65:2329–2335 [DOI] [PubMed] [Google Scholar]
- 48. Morales G, et al. 2010. Resistance to linezolid is mediated by the cfr gene in the first report of an outbreak of linezolid-resistant Staphylococcus aureus. Clin. Infect. Dis. 50:821–825 [DOI] [PubMed] [Google Scholar]
- 49. Murray BE, Singh KV, Heath JD, Sharma BR, Weinstock GM. 1990. Comparison of genomic DNAs of different enterococcal isolates using restriction endonucleases with infrequent recognition sites. J. Clin. Microbiol. 28:2059–2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.North SE, Ellington MJ, Johnson AP, Livermore DM, Woodford N.Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother., abstr C1-1417.2005. [Google Scholar]
- 51. Oliver DR, Brown BL, Clewell DB. 1977. Analysis of plasmid deoxyribonucleic acid in a cariogenic strain of Streptococcus faecalis: an approach to identifying genetic determinants on cryptic plasmids. J. Bacteriol. 130:759–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Olsen RH, Schønheyder HC, Christensen H, Bisgaard M. 2012. Enterococcus faecalis of human and poultry origin share virulence genes supporting the zoonotic potential of E. faecalis. Zoonoses Public Health 59:256–263 [DOI] [PubMed] [Google Scholar]
- 53. Pavlopoulos A. 2011. Identification of DNA sequences that flank a known region by inverse PCR. Methods Mol. Biol. 772:267–275 [DOI] [PubMed] [Google Scholar]
- 54. Périchon B, Courvalin P. 2009. VanA-type vancomycin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 53:4580–4587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Quiñones D, Kobayashi N, Nagashima S. 2009. Molecular epidemiologic analysis of Enterococcus faecalis isolates in Cuba by multilocus sequence typing. Microb. Drug Resist. 15:287–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ross JE, Farrell DJ, Mendes RE, Sader HS, Jones RN. 2011. Eight-year (2002–2009) summary of the linezolid (Zyvox Annu. Appraisal of Potency and Spectrum; ZAAPS) program in European countries. J. Chemother. 23:71–76 [DOI] [PubMed] [Google Scholar]
- 57. Ruiz de Gopegui E, Juan C, Zamorano L, Pérez JL, Oliver A. 2012. Transferable multidrug resistance plasmid carrying cfr associated with tet(L), ant(4′)-Ia and dfrK genes from a clinical methicillin-resistant Staphylococcus aureus ST125 strain. Antimicrob. Agents Chemother. 56:2139–2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ruiz-Garbajosa P, et al. 2006. Multilocus sequence typing scheme for Enterococcus faecalis reveals hospital-adapted genetic complexes in a background of high rates of recombination. J. Clin. Microbiol. 44:2220–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sanger F, Nicklen S, Coulson AR. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. U. S. A. 74:5463–5467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schwarz S, Werckenthin C, Kehrenberg C. 2000. Identification of a plasmid-borne chloramphenicol-florfenicol resistance gene in Staphylococcus sciuri. Antimicrob. Agents Chemother. 44:2530–2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Senior K. 2000. FDA approves first drug in new class of antibiotics. Lancet 29:1523. [DOI] [PubMed] [Google Scholar]
- 62. Singh KV, Coque TM, Weinstock GM, Murray BE. 1998. In vivo testing of an Enterococcus faecalis efaA mutant and use of efaA homologs for species identification. FEMS Immunol. Med. Microbiol. 21:323–331 [DOI] [PubMed] [Google Scholar]
- 63. Smith LK, Mankin AS. 2008. Transcriptional and translational control of the mlr operon, which confers resistance to seven classes of protein synthesis inhibitors. Antimicrob. Agents Chemother. 52:1703–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Swaney SM, et al. Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr C-104.1998. [Google Scholar]
- 65. Toh SM, et al. 2007. Acquisition of a natural resistance gene renders a clinical strain of methicillin-resistant Staphylococcus aureus resistant to the synthetic antibiotic linezolid. Mol. Microbiol. 64:1506–1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tomita H, Pierson C, Lim SK, Clewell DB, Ike Y. 2002. Possible connection between a widely disseminated conjugative gentamicin resistance (pMG1-like) plasmid and the emergence of vancomycin resistance in Enterococcus faecium. J. Clin. Microbiol. 40:3326–3333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tsiodras S, et al. 2001. Linezolid resistance in a clinical isolate of Staphylococcus aureus. Lancet 38:207–208 [DOI] [PubMed] [Google Scholar]
- 68. Wang Y, et al. 2011. Detection of the staphylococcal multiresistance gene cfr in Proteus vulgaris of food animal origin. J. Antimicrob. Chemother. 66:2521–2526 [DOI] [PubMed] [Google Scholar]
- 69. Wang Y, et al. 2012. Distribution of the multidrug resistance gene cfr in Staphylococcus spp. isolates from swine farms in China. Antimicrob. Agents Chemother. 56:1485–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Watanabe S, et al. 2009. Genetic diversity of enterococci harboring the high-level gentamicin resistance gene aac(6′)-Ie-aph(2′)-Ia or aph(2′)-Ie in a Japanese hospital. Microb. Drug Resist. 15:185–194 [DOI] [PubMed] [Google Scholar]
- 71. Wilson DN, et al. 2008. The oxazolidinone antibiotics perturb the ribosomal peptidyl-transferase center and effect tRNA positioning. Proc. Natl. Acad. Sci. U. S. A. 105:13339–13344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zhang WJ, et al. 2011. The new genetic environment of cfr on plasmid pBS-02 in a Bacillus strain. J. Antimicrob. Chemother. 66:1174–1175 [DOI] [PubMed] [Google Scholar]