Abstract
The cytochrome P450 enzymes MycCI and MycG are encoded within the mycinamicin biosynthetic gene cluster and are involved in the biosynthesis of mycinamicin II (a 16-membered macrolide antibiotic produced by Micromonospora griseorubida). Based on recent enzymatic studies, MycCI is characterized as the C-21 methyl hydroxylase of mycinamicin VIII, while MycG is designated multifunctional P450, which catalyzes hydroxylation and also epoxidation at C-14 and C-12/13 on the macrolactone ring of mycinamicin. Here, we confirm the functions of MycCI and MycG in M. griseorubida. Protomycinolide IV and mycinamicin VIII accumulated in the culture broth of the mycCI disruption mutant; moreover, the mycCI gene fragment complemented the production of mycinamicin I and mycinamicin II, which are produced as major mycinamicins by the wild strain M. griseorubida A11725. The mycG disruption mutant did not produce mycinamicin I and mycinamicin II; however, mycinamicin IV accumulated in the culture broth. The mycG gene was located immediately downstream of the self-resistance gene myrB. The mycG gene under the control of mycGp complemented the production of mycinamicin I and mycinamicin II. Furthermore, the amount of mycinamicin II produced by the strain complemented with the mycG gene under the control of myrBp was approximately 2-fold higher than that produced by the wild strain. In M. griseorubida, MycG recognized mycinamicin IV, mycinamicin V, and also mycinamicin III as the substrates. Moreover, it catalyzed hydroxylation and also epoxidation at C-14 and C-12/13 on these intermediates. However, C-14 on mycinamicin I was not hydroxylated.
INTRODUCTION
The cytochrome P450 enzymes (P450s) form a very large family of oxidative heme proteins, which are responsible for a diverse range of oxidative transformations across most life forms (12, 13). These reactions typically involve the modification of physiological and xenobiotic compounds and include the biosynthesis of various bioactive compounds (e.g., steroids, antibiotics, and signaling molecules). Approximately 40% of all known bacterial P450s are found in various species of the industrially important genus Streptomyces, which is the largest genus of the actinomycetes. Recent genome sequencing of the actinomycetes, particularly Streptomyces, has revealed an unexpectedly large number of genes encoding P450s (9, 16, 18, 24, 25). In secondary metabolic pathways, P450 genes are typically integrated within the biosynthetic cluster, where their products catalyze regiospecific and stereospecific oxidation of precursors. This results in structural diversity and also improved bioactivities of these molecules (21, 27). The P450s EryF (2) and EryK (30) are encoded within the erythromycin biosynthetic gene cluster and are involved in the biosynthesis of erythromycin A. Specifically, EryF catalyzes the hydroxylation of the macrolactone precursor 6-deoxyerthronolide B, while EryK catalyzes the formation of erythromycin D. As prototypic P450 hydroxylases involved in secondary metabolism, EryF and EryK exhibit strict substrate specificity. In contrast, PikC, which is involved in the methymycin/neomethymycin and pikromycin biosynthetic pathways of Streptomyces venezuelae, has broader substrate tolerance (33). This enzyme catalyzes the final hydroxylation step toward the 12-membered-ring macrolide YC-17 and also the 14-membered-ring macrolide narbomycin to produce methymycin/neomethymycin and pikromycin as major products. Recently, some P450 genes encoding multifunctional oxidation proteins have been identified in secondary metabolite biosynthetic clusters. For example, TamI, which is encoded within the tirandamycin biosynthetic gene cluster of Streptomyces sp. strain 307-9, has hydroxylation and also epoxidation functions (11). The enzyme is responsible for three hydroxylations and one epoxidation in the biosynthetic pathway from tirandamycin C to tirandamycin B. GfsF, which is involved in the biosynthetic pathway of Streptomyces graminofaciens, also has dual oxidation functions (20). This enzyme catalyzes stepwise epoxidation and hydroxylation during biosynthesis of the antibiotic FD-891.
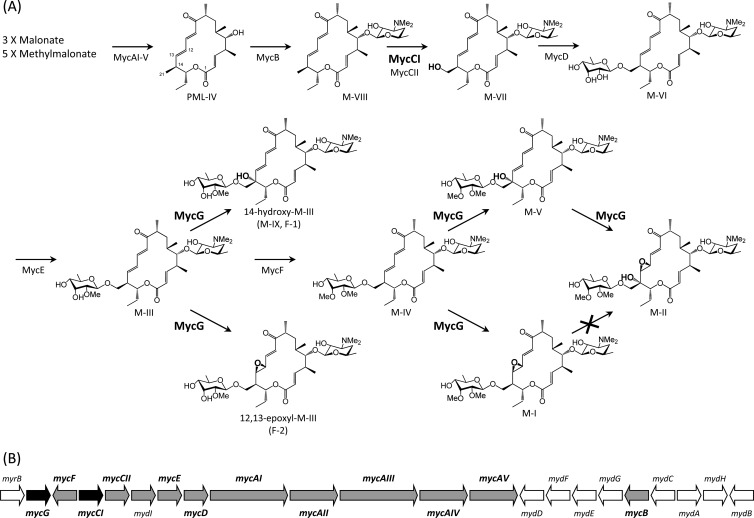
Mycinamicin, which is produced by Micromonospora griseorubida A11725, is a 16-membered macrolide antibiotic with strong antibacterial activity against Gram-positive bacteria (29). Mycinamicin consists of a macrolactone substituted with two different sugars, desosamine and mycinose. The nucleotide sequence of the complete mycinamicin biosynthetic gene cluster has been documented, and 22 open reading frames (ORFs) including two putative cytochrome P450 enzyme genes, mycCI and mycG, were identified in the cluster as shown in Fig. 1 (5). MycCI is closely related to ChmHI (32) and TylHI (8), both of which are responsible for methyl group hydroxylations of 16-membered ring macrolides. mycCI, which is located adjacent to the mycCII encoding ferredoxin, is the first gene of a putative polycistronic operon consisting of 10 genes (mycCI, mycCII, mydI, mycE, mycD, mycAI, mycAII, mycAIII, mycAIV, and mycAV) in the mycinamicin biosynthetic gene cluster. In a recent in vitro study, we characterized MycCI as the C-21 methyl hydroxylase of mycinamicin VIII (M-VIII), which is the earliest macrolide form in the postpolyketide synthase (PKS) tailoring pathway. Moreover, we revealed that the optimal activity of MycCI is dependent on the native ferredoxin MycCII (4). Using purified MycG proteins overexpressed in Escherichia coli cells, we also characterized the in vitro functions of MycG (4). MycG monooxygenase is the first P450 to possess multiple oxidation functions; the enzyme recognized mycinamicin IV (M-IV) as an initial substrate and thereafter catalyzed sequential hydroxylation and epoxidation steps with M-IV or mycinamicin V (M-V) as the substrates to generate mycinamicin II (M-II). However, mycinamicin I (M-I), which was formed by the epoxidation of M-IV, was not recognized as a substrate by MycG. Inouye et al. showed by genetic complementation analysis of an M-IV accumulation mutant of M. griseorubida that a P450 enzyme encoded by the mycG gene catalyzed hydroxylation and also epoxidation at C-14 and C-12/13 on the macrolactone ring of mycinamicin, and they estimated that M. griseorubida A11725 possessed the ability to convert low levels of M-I to M-II (17). In the present study, we isolated mycCI and mycG disruption mutants and their respective gene complementation strains in order to confirm the functions of mycCI and mycG in the mycinamicin biosynthetic pathway. Using the mycG disruption mutant, we also investigated whether M. griseorubida possessed the ability to convert M-I to M-II.
Fig 1.
Mycinamicin biosynthetic pathway (A) and organization of mycinamicin biosynthetic gene cluster (B). The pathway from M-I to M-II is absent in M. griseorubida because there is no P450 enzyme which catalyzes hydroxylation at C-14 on M-I. Oxidative functionalities set up by cytochrome P450 enzymes are highlighted. Color codes in the myc gene cluster are as follows: black, cytochrome P450 genes; gray, mycinamicin biosynthetic genes shown in the biosynthetic pathway; and white, deoxysugar biosynthetic genes and the self-resistance gene. Me, methyl.
We previously demonstrated that the purified MycG protein recognized the mycinamicin biosynthetic intermediates M-IV and mycinamicin III (M-III); moreover, their hydroxylation compounds, assumed to be mycinamicin XV (M-XV) and mycinamicin IX (M-IX), respectively, were detected in the reaction mixtures as minor peaks (4). During analysis of the mycinamicins produced by mycE and mycF disruption mutants, some of the mycinamicin VI (M-VI) and M-III derivatives oxidized by MycG were detected as minor peaks in the culture broth (31). In the present study, we confirmed that M-III (the precursor of M-IV in the mycinamicin biosynthetic pathway) is converted into C-14-hydroxy-M-III (M-IX) and C-12/13-epoxy-M-III by the P450 enzyme encoded by the mycG gene in M. griseorubida.
MATERIALS AND METHODS
Strains, media, and culture conditions.
The strains used in this study are shown in Table 1. The culture conditions for M. griseorubida and E. coli were as described previously (31).
Table 1.
Strains and plasmids used in this study
| Strain or plasmid | Description or genotypea | Reference or Source |
|---|---|---|
| M. griseorubida | ||
| A11725 | Wild strain, mycinamicin producer | 29 |
| TPMA0016 | ΔmycF, amino acids (aa) 2 to 253 replaced with FRT-neo-oriT-FRT-attB cassette | 31 |
| TPMA0023 | ΔmycCI, aa 2 to 383 replaced with FRT-oriT-neo-FRT-attB cassette | This study |
| TPMA0025 | ΔmycG, aa 2 to 398 replaced with FRT-neo-oriT-FRT-attB cassette | This study |
| TPMA0027 | ΔmycCI, aa 2 to 383 replaced with FRT-neo-oriT-FRT-attB cassette | This study |
| TPMA0033 | pMG517 including mycCI and mycCII induced into mycCI deletion mutant TPMA0023 | This study |
| TPMA0034 | pMG518 including mycCI induced into mycCI deletion mutant TPMA0023 | This study |
| TPMA0040 | ΔmycF, aa 2 to 253 replaced with FRT-neo-oriT-FRT-attB cassette; ΔmycG, aa 2 to 283 replaced with FRT-aac(3)IV-oriT-FRT-attB cassette | This study |
| TPMA0046 | pMG519 including myrB and mycG induced into mycG deletion mutant TPMA0025 | This study |
| TPMA0047 | pMG520 including mycG induced into mycG deletion mutant TPMA0025 | This study |
| TPMA0062 | pMG521 including mycG controlled myrB promoter myrBp into mycG deletion mutant TPMA0025 | This study |
| E. coli | ||
| S17-1 | Conjugation donor | American Type Culture Collection (ATCC) |
| BW25113 | K-12 derivative, ΔaraBAD ΔrhaBAD | 14 |
| JM109 | General cloning host | TaKaRa, Japan |
| ATCC 25922 | Test strain for antibacterial assay | ATCC |
| M. luteus | ||
| ATCC 9341 | Test strain for antibacterial assay | ATCC |
| S. aureus | ||
| ATCC 25923 | Test strain for antibacterial assay | ATCC |
| Plasmids | ||
| pIJ790 | λ-Red (gam bet exo) cat araC rep101(Ts) | 14 |
| pIJ773 | Source of aac(3)IV-oriT for pMG510 | 14 |
| pIJ776 | Source of neo-oriT for pMG509 | 14 |
| pLITMUS38 | Cloning vector, bla | New England Biolabs |
| pUC19 | Cloning vector, bla | TaKaRa, Japan |
| pDrive | TA cloning vector, bla aph | Qiagen |
| pSET152 | aac(3)IV(Aprr) oriT attP int | 10 |
| pSAN-lac | bla tsr | 7 |
| pETmycG-NH | MycG protein-coding region inserted into pET21b | 4 |
| pMR01 | Cosmid clone containing some mycinamicin biosynthesis genes including mycE and mycF | 5 |
| pMG261 | Cosmid clone containing some mycinamicin biosynthesis and self-resistance genes including myrB, mycG, and mycF | 5 |
| pMG501 | 1.5-kb FRT-neo-oriT-FRT-attB cassette inserted into pLITMUS38 | 31 |
| pMG509 | 1.5-kb FRT-oriT-neo-FRT-attB cassette inserted into pLITMUS38 | This study |
| pMG510 | 1.4-kb FRT-aac(3)IV-oriT-FRT-attB cassette inserted into pLITMUS38 | This study |
| pMG511 | 8.8-kb BamHI fragment containing mycCI and mycCII inserted into pSAN-lac | This study |
| pMG512 | 9.2-kb EcoRI-HindIII fragment containing myrB, mycG, and mycF inserted into pSAN-lac | This study |
| pMG513 | mycCI replaced with FRT-neo-oriT-FRT-attB cassette on pMG511 | This study |
| pMG514 | mycCI replaced with FRT-oriT-neo-FRT-attB cassette on pMG511 | This study |
| pMG515 | mycG replaced with FRT-neo-oriT-FRT-attB cassette on pMG512 | This study |
| pMG516 | 5′-end region of mycG replaced with FRT-aac(3)IV-oriT-FRT-attB cassette on pMG512 | This study |
| pMG517 | 2.0-kb SphI-HindIII fragment including mycCI and mycCII inserted into pSET152 | This study |
| pMG518 | 1.8-kb SphI-KasI fragment including mycCI inserted into pSET152 | This study |
| pMG519 | 3.5-kb NotI-NruI fragment including myrB and mycG inserted into pSET152 | This study |
| pMG520 | 2.2-kb ApaLI-NruI fragment including mycG inserted into pSET152 | This study |
| pMG521 | mycG controlled myrB promoter myrBp inserted into pSET152 | This study |
FRT, FLP recombination target.
Vectors, DNA manipulation, and PCR.
The vectors and PCR primers used in this study are shown in Table 1 and Table S1 in the supplemental material, respectively. TaKaRa Ex Taq (TaKaRa, Japan), KOD FX (Toyobo, Japan), and PfuTurbo (Stratagene) DNA polymerases were used for amplification of DNA fragments by PCR. Plasmid and genomic DNA amplification, restriction enzyme digestion, fragment isolation, cloning, and DNA fragment amplification were performed according to standard procedures. Southern blot analysis was performed as described in our previous study (31).
Construction of the gene disruption cassettes FRT-oriT-neo-FRT-attB and FRT-aac(3)IV-oriT-FRT-attB.
The orientation of the resistance oriT fragment on pMG509, which contained gene disruption cassette FRT-oriT-neo-FRT-attB, was opposite that on pMG501. To construct pMG509, the 1.3-kb XbaI fragment including neo and oriT derived from pIJ776 was ligated with the 2.9-kb XbaI fragment derived from pMG501. The 1.3-kb XbaI fragment including aac(3)IV and oriT derived from pIJ773 was ligated with the 2.9-kb XbaI fragment derived from pMG501 to construct pMG510 containing gene disruption cassette FRT-aac(3)IV-oriT-FRT-attB. The resulting plasmids, pMG509 and pMG510, were digested with several restriction enzymes to confirm the direction of neo-oriT and aac(3)IV-oriT in these disruption cassettes.
Construction of plasmids.
The mycCI-disrupted plasmids, pMG513 and pMG514, were constructed using the λ-Red-mediated recombination system as described in our previous study (31). To construct pMG511, the 8.8-kb BamHI fragment containing mycCI and mycCII derived from pMR01, which was selected from the M. griseorubida A11725 genomic DNA library constructed with the cosmid vector pKC505 (26), was cloned into pSAN-lac. pKC505 possesses the apramycin resistance gene aac(3)IV as a marker. The disruption cassettes FRT-neo-oriT-FRT-attB and FRT-oriT-neo-FRT-attB were amplified by PCR with the primers DmycCIF and DmycCIR using the 1.5-kb EcoRV fragments from pMG501 and pMG509, respectively. E. coli BW25113/pIJ790 cells containing pMG511 were transformed with the amplified cassettes by electroporation. The resulting transformants were characterized by PCR with a set of oligonucleotides (RecognitionDmycCIF and RecognitionDmycCIR) priming outside the recombination region, and the resulting plasmids, pMG513 and pMG514, were used for disruption of the mycCI gene.
The mycG-disrupted plasmids, pMG515 and pMG516, were also constructed using the λ-Red-mediated recombination system. The 9.2-kb EcoRI-HindIII fragment containing myrB, mycG, and mycF derived from pMR261 was cloned into pSAN-lac to construct pMG512. To generate pMG515, the complete mycG region on pMG512 was replaced with the disruption cassette FRT-neo-oriT-FRT-attB, which was amplified with the primers DmycGF and DmycGR1 using the 1.5-kb EcoRV fragment from pMG501as the template. To generate pMG516, the 5′-end region of mycG on pMG512 was replaced with the disruption cassette FRT-aac(3)IV-oriT-FRT-attB, which was amplified with the primers DmycGF and DmycGR2 using the 1.4-kb EcoRV fragment from pMG510 as the template.
pMG517 and pMG518 were constructed to perform the genetic complementation studies for the mycCI disruption mutant. The 2.0-kb DNA fragment possessing the mycCI and mycCII clusters including the mycCI promoter region was amplified by PCR with the primers mycCIproF and mycCIIR. The 2.0-kb PCR product digested with SphI and HindIII was cloned into pUC19. After confirming the sequence of the cloned fragment, the SphI-HindIII fragment from the recombinant plasmid was blunted and inserted into the EcoRV site on pSET152 to generate pMG517. To construct pMG518 including the mycCI promoter and MycCI protein-coding region, the 1.8-kb SphI-KasI fragment from the cloned SphI-HindIII fragment was also blunted and inserted into the EcoRV site on pSET152.
For genetic complementation, studies with the mycG disruption mutant, pMG519, pMG520, and pMG521 were constructed. The 3.5-kb NotI-NruI and 2.2-kb ApaLI-NruI fragments from pMG512 were blunted and inserted into the EcoRV site on pSET152 to generate pMG519 containing the region from the myrB promoter to the mycG gene and pMG520 containing the region from the 3′end of myrB to mycG, respectively. The myrB gene promoter region, myrBp, was amplified by PCR with the primers myrBpF and myrBpR. The 0.7-kb PCR product digested with HindIII was cloned into pLITMUS38. After confirming the sequence of the cloned fragment, the 0.7-kb HindIII-NdeI fragment from the clone and the 1.2-kb NdeI-HindIII MycG protein-coding fragment, which was obtained from pETmycG-NH (4), were ligated with T4 DNA ligase, and then these ligated DNA fragments were digested with HindIII. The 1.9-kb HindIII fragment containing myrBp and mycG was blunted and inserted into the EcoRV site on pSET152 to generate pMG521.
Conjugation procedure.
The intergeneric conjugation from E. coli S17-1 to M. griseorubida was performed as described in our previous study (31). The genetic characteristics of the exconjugants, which were screened for resistance to the antibiotics used as recombination markers, were confirmed by PCR and Southern hybridization as described in our previous study (31).
Analysis of mycinamicins in the M. griseorubida culture.
To determine the mycinamicin production levels, M. griseorubida was cultured in 5 ml of MR0.1S broth with the appropriate antibiotics on a rotary shaker (150 rpm) at 27°C for 7 to 10 days. The broth was adjusted to a pH of 9 to 11 with 28% ammonia solution and extracted with an equal volume of ethyl acetate (EtOAc).
Intermediate feeding into the M. griseorubida culture plate and sample preparation for high-performance liquid chromatography (HPLC) analysis were performed using a protocol modified from our previous study (3). A seed culture was grown in 5 ml of MR0.1S broth for 5 days, and 150 μl of this seed culture was then spread on agar plates containing 15 ml of MR0.1S. Next, 1 ml of mycinamicin biosynthetic intermediate (500 μg) in 30% dimethyl sulfoxide-water was overlaid on the agar plate seed culture. After 8 days, the agar was homogenized and extracted with a 2-fold volume of EtOAc containing 1% triethylamine at 50°C; the extract was then concentrated in vacuo.
An equal volume of 0.1% trifluoroacetic acid (TFA) was added to the organic layer or the EtOAc solution to dissolve the crude extracts. The water layer containing mycinamicins was adjusted to a pH of 9 to 11 with 28% ammonia solution and extracted with an equal volume of EtOAc. The organic layer was concentrated in vacuo, and each residue was dissolved in methanol for HPLC analyses with a diode array detector model L-2450 (Hitachi, Japan) and liquid chromatography-mass spectrometry (LC-MS) analyses with a liquid chromatograph mass spectrometer model LCMS2010 (Shimadzu, Japan).
Fermentation, isolation, and identification of compounds F-1 and F-2.
M. griseorubida TPMA0016 was cultured in 300-ml Erlenmeyer flasks containing 50 ml of MR0.1S broth. The flasks were incubated on a rotary shaker (120 rpm) at 27°C for 3 to 5 days. Next, 3-ml aliquots of the culture were transferred into each of 10 500-ml Sakaguchi flasks, containing 300 ml of FMM medium; these flasks were incubated on a rotary shaker (120 rpm) at 27°C for 11 days. The broth filtrate was adjusted to a pH of 9 to 11 with 28% ammonia solution and extracted with EtOAc. Next, the mycelium was extracted with EtOAc containing 1% triethylamine, and the extract was concentrated in vacuo. The combined crude extracts were dissolved with EtOAc, after which an equal volume of 0.1% TFA was added. The water layer containing mycinamicins was adjusted to a pH of 9 to 11 with 28% ammonia solution and extracted with an equal volume of EtOAc. After concentrating the organic layer in vacuo, the extracts were applied to a silica gel column (22 cm by 3 cm, silica gel 60; Merck) and eluted with a chloroform–methanol–28% ammonia solution (100:10:1). The fractions containing antibacterial metabolites were applied to a preparative HPLC system with a column Shim-pack PREP-ODS (inside diameter of 250 mm by 20 mm; Shimadzu, Japan) using the mobile phases acetonitrile (MeCN)–0.06% TFA (35:65) (flow rate, 10 ml/min) or MeCN–0.06% TFA (25:75) (flow rate, 20 ml/min) for collecting F-1 or F-2, respectively. Each fraction, which contained F-1 or F-2, was adjusted to a pH of 9 to 11 with 28% ammonia solution, and the fractions were then extracted with an equal volume of EtOAc. After concentrating the organic solution to dryness in vacuo, F-1 (12.6 mg) and F-2 (10.9 mg) were obtained. The purified F-1 and F-2 were characterized by 1H nuclear magnetic resonance (1H NMR; 600 MHz) and 13C NMR (150 MHz) spectroscopy (JEOL JNM-ECA600). The 1H NMR and 13C NMR spectra of the products were assigned by a combination of 1H-1H correlation spectroscopy (COSY), distortionless enhancement by polarization transfer (DEPT) spectroscopy, and 1H-13C heteronuclear correlation spectroscopy (HETCOSY) and compared with those of other mycinamicins (15, 19).
Antibacterial activity.
Antibacterial activities were determined by microbroth dilution method using Mueller-Hinton medium (Becton, Dickinson and Company) (6). After the addition of test compounds, Gram-positive bacteria Staphylococcus aureus ATCC 25923 and Micrococcus luteus ATCC 9341 and the Gram-negative bacterium E. coli ATCC 25922 were grown at 37°C for 20 to 24 h. MICs were defined as the lowest concentration in which an 80% inhibition of growth was observed.
RESULTS AND DISCUSSION
Functions of the mycCI and mycG genes in M. griseorubida.
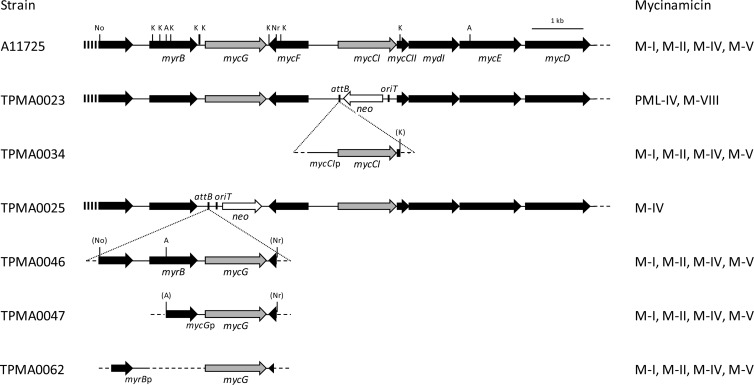
In this study, isolation of gene disruption mutants was performed with disruption cassettes containing a bacteriophage ϕC31 attB attachment site from Streptomyces lividans (28) as described in our previous study (31). The plasmid pMG514, in which the protein-coding region of mycCI on pMG511 was replaced with the disruption cassette FRT-oriT-neo-FRT-attB, was introduced into strain A11725 by intergeneric conjugation. The neomycin-resistant (Neor) and thiostrepton-sensitive (Thios) transconjugant TPMA0023 was subjected to PCR to confirm that the chromosomal copy of A11725 mycCI was deleted by double crossover (data not shown). Moreover, the deletion of the mycCI gene in strain TPMA0023 was confirmed by Southern blot analysis (data not shown). The mycCI disruption mutant TPMA0023 did not produce M-I and M-II, which were major products of the wild strain A11725, when cultured in MR0.1S broth; however, protomycinolide IV (PML-IV) and M-VIII were detected in the EtOAc extract from the culture broth (Fig. 2; see also Fig. S1 in the supplemental material). For genetic complementation of the mycCI disruption mutant TPMA0023, pMG518 including mycCI was transferred to TPMA0023 by intergeneric conjugation. The transconjugant TPMA0034, isolated from the conjugation plate containing apramycin and nalidixic acid, produced M-I and M-II as major products (Fig. 2; see also Fig. S1 in the supplemental material). The amount of M-II produced by TPMA0034 was approximately 66% of that produced by the wild strain A11725. Insertion of pMG518 into the portable attB site on the chromosome of TPMA0034 was confirmed by Southern blot analysis (data not shown); moreover, PCR was performed with several primer pairs for TPMA0034 to confirm that the mycCI gene was inserted into the chromosome by site-specific recombination between the portable attB site on the chromosome and the attP site on pMG518 (data not shown). In this study, we isolated another mycCI disruption mutant, TPMA0027, in which the direction of the neo gene was the same as that of the mycinose biosynthesis gene cluster. However, the total amounts of PML-IV and M-VIII in the culture broth of TPMA0027 were lower than those in the culture broth of TPMA0023, in which the direction of the neo gene in the disruption cassette was opposite that of the mycinose biosynthesis genes in the mycinamicin biosynthetic gene cluster (data not shown); therefore, it was concluded that the direction of the neo gene transcription exerted a negative effect for mycinamicin productivity. Indeed, such a relationship between the direction of a replaced gene and the productivity of mycinamicin biosynthetic intermediates was previously observed in mycE disruption mutants (31).
Fig 2.
Physical maps of the region, including mycCI, mycG, and the flanking genes of the wild strain Micromonospora griseorubida A11725, mycCI disruption mutant TPMA0023, and mycG disruption mutant TPMA0025 and the DNA fragments introduced into these disruption mutants for the gene complementation study. The major mycinamicins in the culture broth of the wild strain, disruption mutants, and respective complementation strains (TPMA0034, mycCI; TPMA0046, TPMA0047, and TPMA0062, mycG) were detected with high-performance liquid chromatography (HPLC) (see Fig. S2 and S4 in the supplemental material). The relevant restriction sites are indicated as follows: A, ApaLI; K, KasI; No, NotI; and Nr, NruI. Mycinamicins in the ethyl acetate (EtOAc) extracts from the culture broth were analyzed with HPLC. Trace amounts of M-IV and M-V were detected in strains A11725, TPMA0034, TPMA0046, TPMA0047, and TPMA0062.
To isolate the mycG disruption mutant, pMG515, on which the complete mycG region was replaced with the disruption cassette FRT-oriT-neo-FRT-attB, was introduced into strain A11725 by intergeneric conjugation. The resulting transconjugant TPMA0025 (Neor, Thios) was subjected to Southern blot analysis to confirm that the mycG gene was deleted from the chromosomal DNA from A11725 (data not shown). Moreover, deletion of the mycG gene by double crossover between the pMG515 and A11725 chromosomes was confirmed by PCR (data not shown). When the mycG disruption mutant TPMA0025 was cultured in MR0.1S broth, M-I, M-II, and M-V were not detected in the EtOAc extract; however, M-IV did accumulate in the culture broth (Fig. 2; see also Fig. S2 in the supplemental material). The mycG gene was located immediately downstream of myrB, encoding 23S rRNA methyltransferase for self-resistance, and there were at least two promoters, mycGp and myrBp, which would be able to control the expression of mycG. A 2.7-kb fragment including the MycG protein-coding region and mycGp previously restored M-I and M-II production of the M-IV accumulation mutant, which was obtained with N-methyl-N′-nitro-N-nitrosoguanidine treatment (17). However, it was assumed that the promoter myrBp, which is located immediately upstream of myrB, could also control the expression of mycG. For the genetic complementation of the mycG disruption mutant TPMA0025, three kinds of plasmids—pMG519, pMG520, and pMG521—were constructed. pMG519 possessed the myrBp and mycGp regions upstream of mycG, and therefore, the expression of mycG on this plasmid could be controlled by myrBp and/or mycGp. In pMG520, a 2.2-kb DNA fragment, which included the region from the 3′ end of myrB to mycG, was inserted; the expression of mycG on this plasmid was controlled by mycGp. The mycG complementation plasmids pMG519 and pMG520 were introduced into the mycG disruption mutant TPMA0025. The resulting transconjugants, TPMA0046 and TPMA0047, produced M-II (5.2 μg/ml and 4.1 μg/ml, respectively). The amount of M-II produced by TPMA0046 and TPMA0047 was almost the same as that produced by the wild strain A11725 (4.4 μg/ml) (Fig. 2; see also Fig. S2). Moreover, M-I and M-V production by these complementation strains was restored (Fig. 2; see also Fig. S2). Insertion of the complementation plasmids into the portable attB site on the chromosome of the respective gene complementation strains was confirmed by Southern blot analysis (data not shown); the size of the complementation DNA fragments inserted into the chromosomes of these complementation strains was confirmed by PCR (data not shown). On the mycG complementation plasmid pMG521, the open reading frame of mycG was directly attached to the amplified myrBp region. The nucleotide sequence of the 1.9-kb HindIII fragment containing myrBp and mycG on pMG521 was identified to be the same as that of the wild strain A11725 except for the connection site NdeI between the myrBp and mycG regions. The transconjugant TPMA0062 produced M-II (9.3 μg/ml); the amount was approximately 2-fold higher than that produced by the wild strain A11725. Moreover, M-I and M-V productivities by TPMA0062 were higher than that of A11725. The insertion of this complementation plasmid into the portable attB site and the size of the complementation DNA fragment inserted into the chromosome were confirmed by Southern blot analysis and PCR, respectively (data not shown). We hypothesize that the transcription of mycG is driven by these two promoters, myrBp and mycGp. It is interesting to speculate which promoter is the main regulator of mycG expression during mycinamicin biosynthesis, and in our future studies, we should investigate which promoter mainly controls the transcription of mycG by performing experiments such as Northern blotting, reverse transcriptase (RT)-PCR, and S1 nuclease mapping.
Multifunctional P450 enzyme MycG in mycinamicin producer.
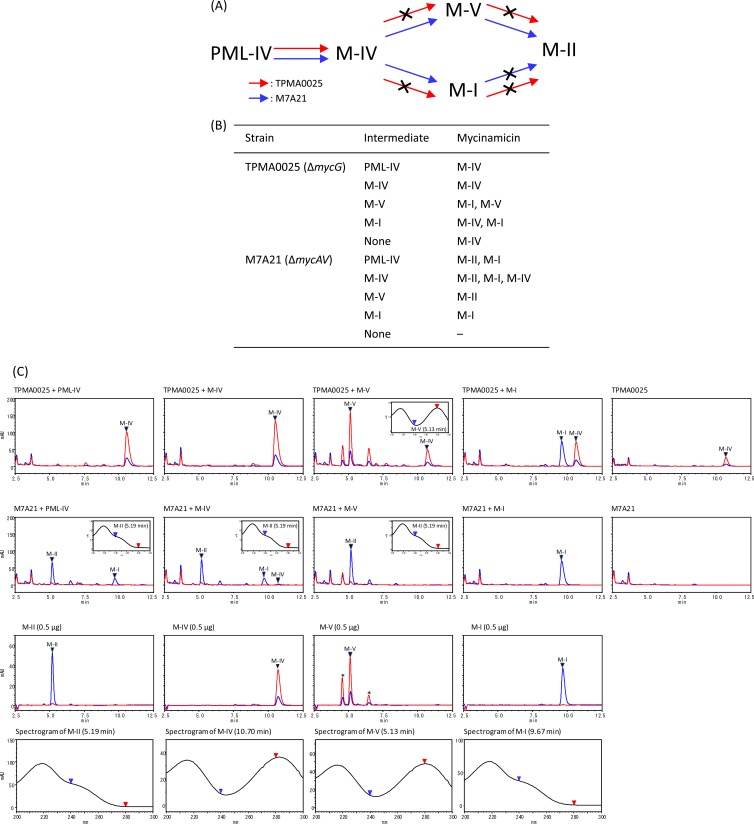
The mycG disruption mutant TPMA0025 did not produce M-I, M-II, and M-V, and the mycinamicin biosynthetic intermediate M-IV accumulated in the culture broth. In our previous enzymatic study, the cytochrome P450 protein MycG catalyzed hydroxylation and also epoxidation at C-14 and C-12/13 on the macrolactone ring of mycinamicin. In particular, M-IV was an initial substrate of MycG; moreover, MycG converted M-IV into M-V and M-I and M-V into M-II. However, M-I was not recognized as a substrate by MycG and was not converted into M-II (3). On the other hand, it was proposed by Inouye et al. that M. griseorubida A11725 possessed the ability of low-level conversion from M-I to M-II (17). Therefore, to confirm M-I hydroxylation reaction in M. griseorubida, a bioconversion study for mycinamicin biosynthetic intermediates was performed by using the mycG disruption mutant TPMA0025 and the PKS gene mycAV disruption mutant M7A21 (3) (Fig. 3). The intermediates PML-IV, M-IV, M-V, and M-I were added individually into plate cultures of TPMA0025 and M7A21, and the culture plates were incubated at 27°C for 8 days. PML-IV was converted into M-IV by TPMA0025 as would be expected according to the biosynthetic pathway shown in Fig. 1A. In contrast, M-IV, M-V, and M-I were not converted into any other intermediates because of the loss of MycG activity in TPMA0025. On the other hand, M7A21, which retained its MycG activity, converted M-IV into M-II and M-I and M-V into M-II; however, it did not convert M-I into M-II (Fig. 1A). These results indicated that M-I was not converted into M-II by M. griseorubida but was a shunt metabolite in mycinamicin biosynthesis.
Fig 3.
(A) Mycinamicin biosynthetic pathway of the mycG disruption mutant TPMA0025 and the mycAV disruption mutant M7A21 based on a bioconversion study of mycinamicin biosynthetic intermediates (B). The crossed arrows in panel A represent the undetected pathways in the mutants. (B) Bioconversion of mycinamicin biosynthetic intermediates by TPMA0025 and M7A21. Mycinamicin intermediates (500 μg) were added to culture plates of TPMA0025 and M7A21, and the culture plates were incubated at 27°C for 8 days. Mycinamicins in the ethyl acetate (EtOAc) extracts from the culture plates fed mycinamicin biosynthetic intermediates were analyzed with HPLC culture plate fed M-IV (C). Trace amounts of M-IV were detected in M7A21 culture plate fed M-IV. (C) HPLC chromatograms of the EtOAc extract obtained from culture plates fed with mycinamicin intermediates (PML-IV, M-IV, M-V, and M-I) of TPMA0025 and M7A21 and UV spectrograms of M-II and M-V detected in the EtOAc extract. HPLC chromatograms of the mycinamicin intermediates are shown with UV spectrograms. The purity of M-V was approximately 70%; other compounds included in the M-V powder are shown with asterisks. HPLC conditions: column, ODS-80Tm (Tosoh); mobile phase, MeCN, 0.06% TFA (35:65); flow rate, 0.8 ml/min; UV wavelength, 200 to 300 nm. Blue line, at 240 nm; red line, at 280 nm.
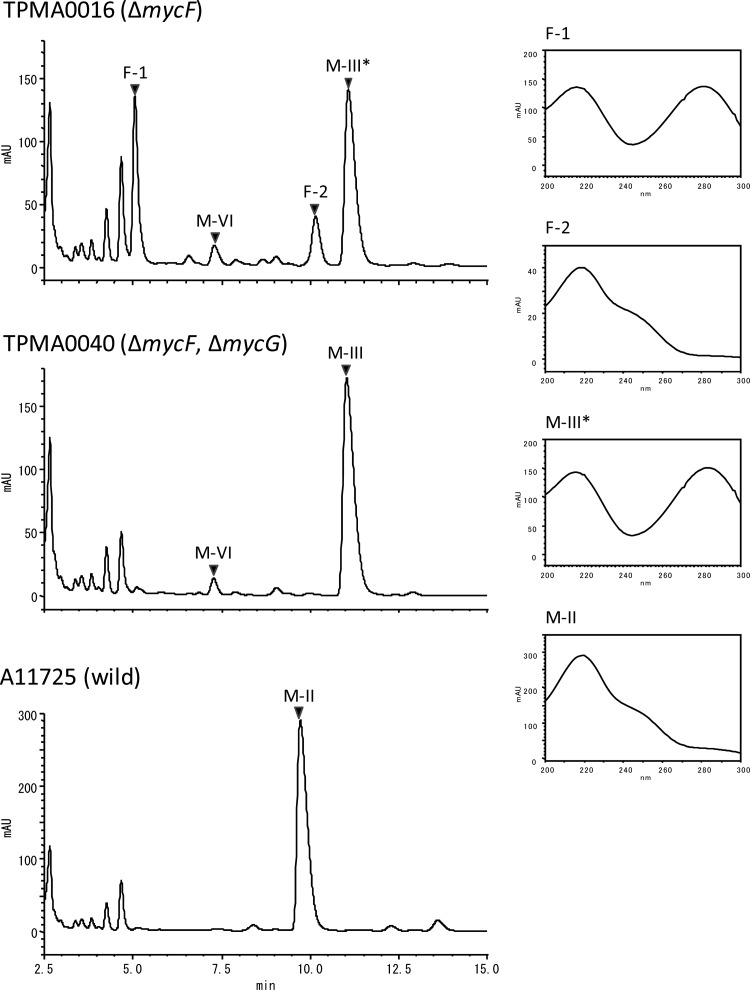
In our previous enzymatic study, MycG recognized M-IV as an initial substrate. Moreover, mycinose at C-21 of the mycinamicin macrolactone ring was required for efficient catalysis by MycG (4). Mycinose in M-IV was generated from 6-deoxyallose via javose by two O-methyltransferases, MycE and MycF (22). The MycF protein catalyzes methylation at the C3″-OH group of javose in M-III. In a recent study, we observed that M-III (C3″-demethyl M-IV) accumulated in the culture broth of the mycF disruption mutants TPMA0004 and TPMA0016; furthermore, two unknown metabolites, F-1 and F-2, which were assumed to be hydroxylated and epoxidized M-IIIs, respectively, were detected in the EtOAc extracts of the mutants (31). We postulated that the hydroxylation and epoxidation of M-III were catalyzed by the P450 protein MycG in M. griseorubida. Thus, we isolated the mycF and mycG double-disruption mutant and identified the structure of the metabolites F-1 and F-2. To generate the mycG disruption plasmid pMG516, the 5′-end region of mycG on pMG512 was replaced with the disruption cassette FRT-aac(3)IV-oriT-FRT-attB. pMG516 was introduced into the mycF disruption mutant TPMA0016, and partial deletion of mycG on the chromosome in the resulting transconjugant TPMA0040 (Aprr Neor) was confirmed by PCR (data not shown). The mycF and mycG double-disruption mutant TPMA0040 accumulated M-III and M-VI in the culture broth; however, F-1 and F-2, which were produced by TPMA0016, were not detected in the EtOAc extract from the culture broth of TPMA0040 (Fig. 4).
Fig 4.
HPLC chromatograms (220 nm) of the EtOAc extracts obtained from the culture broths of the mycF disruption mutant TPMA0016, mycF and mycG double-disruption mutant TPMA0040, and the wild strain A11725 and UV spectrograms of M-II, M-III, F-1 (14-hydroxy-M-III, M-IX), and F-2 (12,13-epoxyl-M-III) on these chromatograms. HPLC conditions: column, ODS-80Tm (Tosoh, Japan); mobile phase, MeCN, 0.06% TFA (30:70); flow rate, 0.8 ml/min; UV wavelength, 200 to 300 nm.
F-1 and F-2 were isolated and purified from 3.0 liters of TPMA0016 culture broth to obtain purified F-1 (12.6 mg) and F-2 (10.9 mg). The purified F-1 and F-2 were characterized by 1H NMR (600 MHz) and 13C NMR (150 MHz) spectroscopy. From their NMR chemical shifts (see Tables S2 and S3 in the supplemental material), molecular weight (m/z 698), and UV absorption spectra (Fig. 4), the structures of F-1 and F-2 were determined as C-14-hydroxy-M-III and C-12/13-epoxy-M-III, respectively. The 13C NMR data for F-1 were almost the same as those for C-14-hydroxy-M-III, which was named mycinamicin IX (19); the 1H NMR data for F-1 supported the structure. Moreover, the UV spectrum suggested the presence of an α,β-unsaturated lactone (216 nm) and an α,β,γ,δ-unsaturated ketone (281 nm). In contrast, the UV spectrum of F-2 suggested the presence of an α,β-unsaturated lactone (218 nm) and a γ,δ-epoxy-α,β-unsaturated ketone (240 nm). The chemical shifts of C-12 and C-13 were identical at 59.0 ppm, which was almost the same as those of M-I (59.6 ppm and 59.0 ppm, respectively), indicating an epoxide ring. Moreover, the chemical shift of C″3 was 69.5 ppm, which was almost the same as that of M-III (69.8 ppm), and the lack of the 2″-OCH3 was also observed in the 1H NMR and 13C NMR spectra. Therefore, F-2 was identified as C-12/13-epoxy-M-III, which was previously reported by Mierzwa et al. (23).
Antibacterial activities of C-14-hydroxy-M-III and C-12/13-epoxy-M-III were compared with those of M-III and M-IV (see Table S4 in the supplemental material). All tested compounds inhibited the growth of Gram-positive bacteria. MICs of C-12/13-epoxy-M-III were 0.20 μg/ml and 0.05 μg/ml against S. aureus ATCC 25923 and M. luteus ATCC 9341, respectively, and antibacterial activity of C-12/13-epoxy-M-III was as same as those of M-III and M-IV. However, the activity of C-14-hydroxy-M-III was lower than those of other tested compounds.
From the bioconversion study, we recognized that MycG converted M-IV into M-V and M-I and M-V into M-II in M. griseorubida but did not recognized M-I as a substrate. Moreover, the results of mycF and mycG double disruption indicated that M-III was also recognized as a substrate by MycG and that the MycG P450 protein catalyzes hydroxylation and also epoxidation at C-14 and C-12/13 on M-III in M. griseorubida. The results in this study support our previous enzymatic study using purified MycG proteins overexpressed in E. coli cells (4). MycG was previously shown to catalyze hydroxylation at the 6β-position of testosterone (1). Thus, MycG has a wide substrate specificity and multifunctional oxidation activity and plays an important role in the synthesis of mycinamicins by M. griseorubida.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Akira Arisawa (Mercian Co., Japan) for donating pSAN-lac and Keith F. Chater (John Innes Centre, United Kingdom) for donating E. coli BW25113 and plasmids pIJ790, pIJ773, and pIJ776. We thank Shingo Fujisaki (Toho University) for help with LC-MS analysis and Wei Li (Toho University) for help with NMR spectroscopic analyses.
Footnotes
Published ahead of print 30 April 2012
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1. Agematu H, et al. 2006. Hydroxylation of testosterone by bacterial cytochromes P450 using the Escherichia coli expression system. Biosci. Biotechnol. Biochem. 70:307–311 [DOI] [PubMed] [Google Scholar]
- 2. Andersen JF, Hutchinson CR. 1992. Characterization of Saccharopolyspora erythraea cytochrome P-450 genes and enzymes, including 6-deoxyerythronolide B hydroxylase. J. Bacteriol. 174:725–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anzai Y, Ishii Y, Yoda Y, Kinoshita K, Kato F. 2004. The targeted inactivation of polyketide synthase mycAV in the mycinamicin producer, Micromonospora griseorubida, and a complementation study. FEMS Microbiol. Lett. 238:315–320 [DOI] [PubMed] [Google Scholar]
- 4. Anzai Y, et al. 2008. Functional analysis of MycCI and MycG, cytochrome P450 enzymes involved in biosynthesis of mycinamicin macrolide antibiotics. Chem. Biol. 15:950–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anzai Y, et al. 2003. Organization of the biosynthetic gene cluster for the polyketide macrolide mycinamicin in Micromonospora griseorubida. FEMS Microbiol. Lett. 218:135–141 [DOI] [PubMed] [Google Scholar]
- 6. Anzai Y, et al. 2010. Isolation and characterization of 23-O-mycinosyl-20-dihydro-rosamicin: a new rosamicin analogue derived from engineered Micromonospora rosaria. J. Antibiot. 63:325–328 [DOI] [PubMed] [Google Scholar]
- 7. Arisawa A, Tsunekawa H, Okamura K, Okamoto R. 1995. Nucleotide sequence analysis of the carbomycin biosynthetic genes including the 3-O-acyltransferase gene from Streptomyces thermotolerans. Biosci. Biotech. Biochem. 59:582–588 [DOI] [PubMed] [Google Scholar]
- 8. Baltz RH, Seno ET. 1981. Properties of Streptomyces fradiae mutants blocked in biosynthesis of the macrolide antibiotic tylosin. Antimicrob. Agents Chemother. 20:214–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bentley SD, et al. 2002. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417:141–147 [DOI] [PubMed] [Google Scholar]
- 10. Bierman M, et al. 1992. Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene 116:43–49 [DOI] [PubMed] [Google Scholar]
- 11. Carlson JC, et al. 2011. Tirandamycin biosynthesis is mediated by co-dependent oxidative enzymes. Nat. Chem. 3:628–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Coon MJ. 2005. Cytochrome P450: nature's most versatile biological catalyst. Annu. Rev. Pharmacol. Toxicol. 45:1–25 [DOI] [PubMed] [Google Scholar]
- 13. Guengerich FP. 2001. Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicity. Chem. Res. Toxicol. 14:611–650 [DOI] [PubMed] [Google Scholar]
- 14. Gust B, Challis GL, Fowler K, Kieser T, Chater KF. 2003. PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc. Natl. Acad. Sci. U. S. A. 100:1541–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hayashi M, et al. 1981. Mycinamicins, new macrolide antibiotics. III Isolation and structures of mycinamicin aglycones, mycinolide IV and V. J. Antibiot. 34:346–349 [DOI] [PubMed] [Google Scholar]
- 16. Ikeda H, et al. 2003. Complete genome sequence and comparative analysis of the industrial microorganism Streptomyces avermitilis. Nat. Biotechnol. 21:526–531 [DOI] [PubMed] [Google Scholar]
- 17. Inouye M, Takada Y, Muto N, Beppu T, Horinouchi S. 1994. Characterization and expression of a P-450-like mycinamicin biosynthesis gene using a novel Micromonospora-Escherichia coli shuttle cosmid vector. Mol. Gen. Genet. 245:456–464 [DOI] [PubMed] [Google Scholar]
- 18. Ishikawa J, et al. 2004. The complete genomic sequence of Nocardia farcinica IFM 10152. Proc. Natl. Acad. Sci. U. S. A. 101:14925–14930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kinoshita K, Takenaka S, Suzuki H, Morohoshi T, Hayashi M. 1992. Mycinamicins, new macrolide antibiotics. XIII. Isolation and structures of novel fermentation products from Micromonospora griseorubida (FERM BP-705). J. Antibiot. 45:1–9 [DOI] [PubMed] [Google Scholar]
- 20. Kudo F, Motegi A, Mizoue K, Eguchi T. 2010. Cloning and characterization of the biosynthetic gene cluster of 16-membered macrolide antibiotic FD-891: involvement of a dual functional cytochrome P450 monooxygenase catalyzing epoxidation and hydroxylation. Chembiochem 11:1574–1582 [DOI] [PubMed] [Google Scholar]
- 21. Lamb DC, et al. 2003. Cytochrome P450 complement (CYPome) of the avermectin-producer Streptomyces avermitilis and comparison to that of Streptomyces coelicolor A3(2). Biochem. Biophys. Res. Commun. 307:610–619 [DOI] [PubMed] [Google Scholar]
- 22. Li S, Anzai Y, Kinoshita K, Kato F, Sherman DH. 2009. Functional analysis of MycE and MycF, two O-methyltransferases involved in the biosynthesis of mycinamicin macrolide antibiotics. Chembiochem 10:1297–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mierzwa R, Truumees I, Patel M, Marquez J, Gullo V. 1985. High-performance preparative isolation and purification of several mycinamicins. J. Liq. Chromatogr. 8:1697–1709 [Google Scholar]
- 24. Ohnishi Y, et al. 2008. Genome sequence of the streptomycin-producing microorganism Streptomyces griseus IFO 13350. J. Bacteriol. 190:4050–4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oliynyk M, et al. 2007. Complete genome sequence of the erythromycin-producing bacterium Saccharopolyspora erythraea NRRL23338. Nat. Biotechnol. 25:447–453 [DOI] [PubMed] [Google Scholar]
- 26. Richardson MA, Kuhstoss S, Solenberg P, Schaus NA, Rao RN. 1987. A new shuttle cosmid vector, pKC505, for streptomycetes: its use in the cloning of three different spiramycin-resistance genes from a Streptomyces ambofaciens library. Gene 61:231–241 [DOI] [PubMed] [Google Scholar]
- 27. Rix U, Fischer C, Remsing LL, Rohr J. 2002. Modification of post-PKS tailoring steps through combinatorial biosynthesis. Nat. Prod. Rep. 19:542–580 [DOI] [PubMed] [Google Scholar]
- 28. Rodriguez E, et al. 2003. Rapid engineering of polyketide overproduction by gene transfer to industrially optimized strains. J. Ind. Microbiol. Biotechnol. 30:480–488 [DOI] [PubMed] [Google Scholar]
- 29. Satoi S, Muto N, Hayashi M, Fujii T, Otani M. 1980. Mycinamicins, new macrolide antibiotics. I. Taxonomy, production, isolation, characterization and properties. J. Antibiot. 33:364–376 [DOI] [PubMed] [Google Scholar]
- 30. Stassi D, Donadio S, Staver MJ, Katz L. 1993. Identification of a Saccharopolyspora erythraea gene required for the final hydroxylation step in erythromycin biosynthesis. J. Bacteriol. 175:182–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tsukada S, et al. 2010. Gene targeting for O-methyltransferase genes, mycE and mycF, on the chromosome of Micromonospora griseorubida producing mycinamicin with a disruption cassette containing the bacteriophage ϕC31 attB attachment site. FEMS Microbiol. Lett. 304:148–156 [DOI] [PubMed] [Google Scholar]
- 32. Ward SL, et al. 2004. Chalcomycin biosynthesis gene cluster from Streptomyces bikiniensis: novel features of an unusual ketolide produced through expression of the chm polyketide synthase in Streptomyces fradiae. Antimicrob. Agents Chemother. 48:4703–4712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xue Y, Wilson D, Zhao L, Liu Sherman H-WDH. 1998. Hydroxylation of macrolactones YC-17 and narbomycin is mediated by the pikC-encoded cytochrome P450 in Streptomyces venezuelae. Chem. Biol. 5:661–667 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.