Abstract
Cap-dependent mRNA translation requires the methylation of the mRNA guanosine cap by RNA guanine-7-methyltransferase (RNMT). mRNA cap methylation was recently described to be rate-limiting for a subset of mRNAs, and to be enhanced by expression of c-Myc and E2F1, although the biological significance of this finding was not investigated. Here it is reported that increased RNMT expression enhances cellular mRNA cap methyltransferase activity, promotes mammary epithelial cell transformation and co-operates with H-RasV12 or c-Myc to promote fibroblast cell transformation. Cyclin D1 is a prominent oncogene in epithelial tumours. A significant fraction of Cyclin D1 mRNA was found to be unmethylated on the mRNA cap and thus dormant in mammary epithelial cells. Cyclin D1 expression was increased by enhanced mRNA cap methylation. In summary, this report demonstrates that mRNA cap methylation is rate-limiting for expression of an oncogene and cell transformation.
Keywords: cyclin D1, cell transformation, mRNA cap methylation, mRNA translation, oncogene
Introduction
RNA polymerase II gene products undergo a series of modifications that are essential for efficient gene expression, including addition of the 5′ 7-methylguanosine cap (Shatkin, 1976; Shuman, 2002). In mammals, RNGTT (RNA guanylyltransferase and 5′-phosphatase) catalyses the capping reaction, which is the hydrolysis of the triphosphate of the first transcribed nucleotide and the addition of GMP to produce the mRNA guanosine cap, 5′ GpppX, where X is the first transcribed nucleotide (Furuichi and Shatkin, 2000). The cap protects mRNA from attack by exonucleases. A second enzyme, RNMT (RNA guanine-7 methyltransferase) catalyses the methylation of the guanosine cap at the 7 position to produce the 7-methylguanosine cap, 5′ m7GpppX. Methylation of the cap is required for efficient eIF4E binding and subsequent eIF4F complex formation, which is critical for mRNA binding to the 40S ribosomal subunit and initiation of translation (Furuichi and Shatkin, 2000; Gingras et al., 1999). The 7-methylguanosine cap also promotes splicing, nuclear export and polyA addition, although the extent to which this is observed appears to be gene-specific and species-specific. The majority of capping reactions occur co-transcriptionally, and both RNGTT and RNMT are recruited preferentially to the phosphorylated form of RNA polymerase II that is found at the initiation of transcription (Bentley, 2005).
Recent publications have described that a subset of mRNAs exist in the cell which are capped but not methylated, and that cap methylation of these mRNAs can be upregulated by c-Myc and E2F1 transcription factors (Cole and Cowling, 2009; Cowling and Cole, 2007). Most c-Myc transcriptional target genes and a subset of other genes were found to be upregulated by this mechanism and therefore, upregulation of mRNA cap methylation was proposed, but not proven, to be a significant effector of c-Myc in promoting cell proliferation and cell transformation (Cole and Cowling, 2008).
The S.cerevisiae and human mRNA cap methyltransferases, ADB1 and RNMT, are essential for cell viability (Chu and Shatkin, 2008; Mao et al., 1995; Shafer et al., 2005). However, it has not known whether endogenous RNMT is rate-limiting for mRNA translation and whether elevation of cap methyltransferase activity has an impact on cellular functions. In this report, RNMT is demonstrated to be rate-limiting for cellular cap methyltransferase activity, and enhancing this activity promotes Cyclin D1 expression and cell transformation.
Results
RNMT increases mRNA cap methyltransferase activity
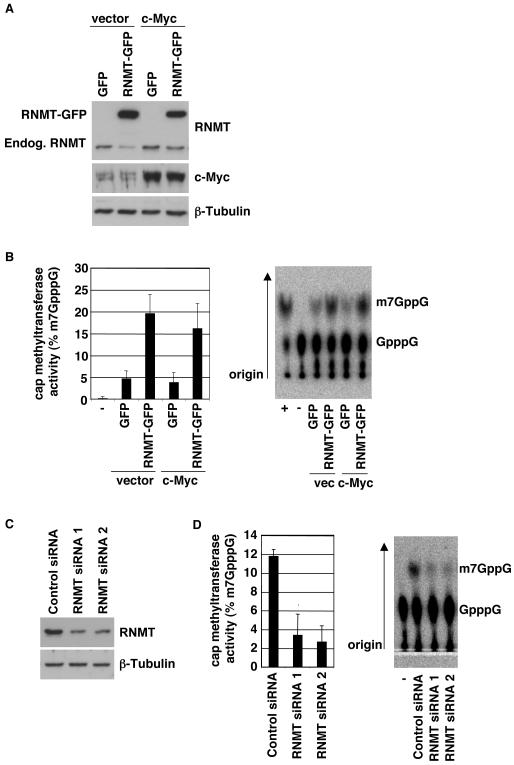
In order to increase mRNA cap methyltransferase activity, TERT-immortalised human mammary epithelial cells (IMEC) were transduced by retro-viral infection with the human RNA guanine-7 methyltransferase gene, RNMT, fused to Green Fluorescent Protein (GFP) (DiRenzo et al., 2002; Wen and Shatkin, 2000). Following retroviral infection, pools of cells were drug-selected and RNMT was detected by Western Blot (Figure 1a). The mRNA cap methyltransferase activity of the IMEC line extracts was measured. Cell extracts were incubated with guanosine-capped, in vitro transcribed RNA and the methyl donor, S-adenosyl methionine. The resultant cap methylation of the in vitro transcribed RNA was detected by thin layer chromatography (Pillutla et al., 1998), (Figure 1b). Expression of RNMT-GFP resulted in a four-fold increase in cap methyltransferase activity compared to the GFP control. Since GFP is a large protein which may potentially alter the activity and action of proteins to which it is fused, the same experiment was carried out using RNMT fused to the ten amino acid HA tag. HA-RNMT expression in IMECs was also found to increase mRNA cap methyltransferase activity (supplementary Figure 1a and b).
Figure 1. Increased RNMT (RNA guanine-7-methyltransferase) expression increases cellular mRNA cap methyltransferase activity.
a) Whole cell extracts from the IMEC (Tert-immortalised human mammary epithelial cell) lines indicated were analysed by Western Blot for expression of RNMT, RNMT-GFP, c-MYC and β-Tubulin. The migration of endogenous RNMT and RNMT-GFP is indicated. Cells were lysed in Triton lysis buffer (10 mM Tris, pH 7.05, 50 mM NaCl, 30 mM Na4 pyrophosphate, 50 mM NaF, 5 μM ZnCl2, 10% glycerol, 0.5% Triton X-100, 10μM Leupeptin, 1μM Pepstatin and 0.1mg/ml Aprotinin). Polyclonal anti-RNMT antibodies were raised against full length recombinant GST-RNMT in sheep and affinity-purified and other antibodies were purchased from Santa Cruz Biotechnologies (Santa Cruz, CA, USA). b) The same extracts were analysed for their cap methyltransferase activity. Assay was adapted from (Pillutla et al., 1998). A 55 base strand of in vitro transcribed RNA was capped using Vaccina Virus capping enzyme and 32P alpha-GTP according to manufacturers instructions (Epicentre Biotechnologies, Madison, WI, USA). 10-40ng G32P-RNA was incubated with 2μg cell extract in 10μl 50mM Tris pH 8, 6mM KCl, 1.25mM MgCl2, 100nM S-adenosyl methionine at 37°C for 10mins. RNA was purified, precipitated and resuspended in 4μl 50mM NaAcetate and 0.25units P1 nuclease for 30mins at 37°C. Cap (GpppG) and methyl-cap (m7GpppG) were resolved in 0.4M Ammonium Sulphate thin layer chromatography using PEI cellulose plates. Standards were visualised by UV light to establish correct migration. Labelled spots were visualised and quantified by autoradiography, and percentage conversion of GpppG to m7GppG was calculated. Average results for three independent experiments are shown and error bars indicate the standard deviation. A representative assay is also shown including a negative control (-), in which no extract was added and a positive control (+), in which recombinant RNMT was added. c) IMEC cells were transfected with two independent siRNAs directed against RNMT or Cyclophilin B (negative control), according to manufacturer’s instructions (Dharmacon Inc, Lafayette, CO, USA). After 24hrs, cell extracts were prepared and analysed for their cap methyltransferase activity, as above, except 4μg of cell extract was used.
In order to confirm that cellular mRNA cap methyltransferase activity is dependent on RNMT, RNMT expression was inhibited by transfection of two independent siRNAs (Figure 1c). Inhibition of endogenous RNMT expression resulted in a corresponding reduction in mRNA cap methyltransferase activity (Figure 1d). This confirms that RNMT is the major mRNA cap methyltransferase in IMECs.
c-Myc has been reported to enhance mRNA cap methylation of specific mRNAs (Cole and Cowling, 2009; Cowling and Cole, 2007), and therefore the effect of elevated c-Myc expression on RNMT expression and cellular cap methyltransferase activity was investigated. The panel of IMEC lines was transduced with a c-Myc expression construct or vector control by retroviral infection and pools of cells were drug-selected. Elevated c-Myc expression did not result in changes in RNMT expression or total cellular cap methyltransferase activity (Figure 1a and b, supplementary Figure 1a and b). Therefore c-Myc-induced mRNA cap methylation is not a result of increased RNMT expression or total cellular RNMT activity.
RNMT transforms human mammary epithelial cells
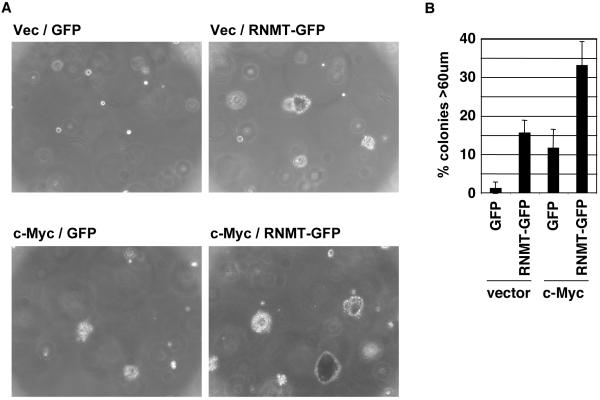
The impact of elevated RNMT expression on cell proliferation and cell transformation was investigated. Elevated RNMT-GFP and HA-RNMT expression did not result in increased cell proliferation of plated cells (not shown), but did result in enhanced cell transformation (Figure 2 a and b and supplementary Figure 1c). Cell transformation was assessed by measuring the ability of the IMEC lines to proliferate in an anchorage-independent manner in the soft agar transformation assay. IMEC lines have been shown previously to be unable to proliferate in suspension unless they have elevated expression of an oncogene such as c-Myc (Cowling et al., 2007). After 7 days incubation in suspension, the size of the resultant colonies was measured (Figure 2b), and after 14 days, micrographs of the IMEC lines were taken (Figure 2a). In accordance with previous publications, vector control IMECs did not proliferate in an anchorage-independent manner, whereas elevated c-Myc expression resulted in 12% of plated cells proliferating into significant colonies (larger than 60μm in diameter). Expression of RNMT-GFP also resulted in 16% of plated cells growing into significant colonies, and combined exogenous expression of c-Myc and RNMT resulted in 33% of plated cells growing into significant colonies. The transformation assays were also performed using IMEC cells expressing RNMT-HA, with similar results to those obtained using RNMT-GFP (Supplementary Figure 1c).
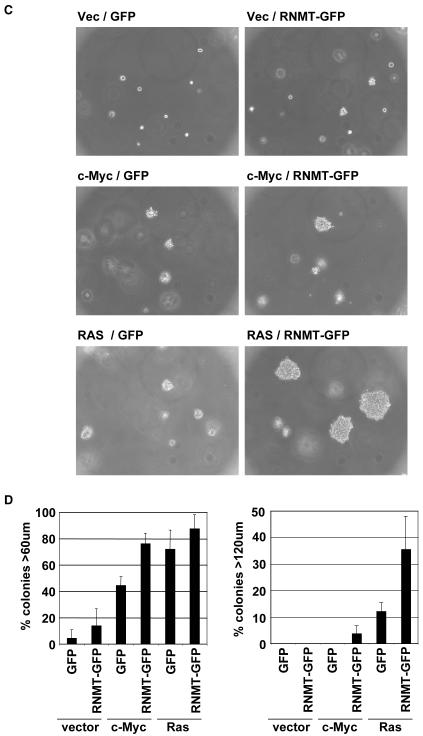
Figure 2. RNMT promotes cell transformation.
a,b) IMEC cells and c,d) Rat1A fibroblasts expressing the proteins indicated were used in the soft agar transformation assay, performed according to (Cowling et al., 2006) with the following modifications. 2.5 × 104 IMECs/ml were plated in 0.3% noble agar/IMEC medium supplemented with 5% FBS (Invitrogen). Cells were fed with 250μl IMEC medium/5% FBS every 2nd day, starting with the day of plating. Rat1A cells were plated in DMEM/10% FCS and fed every 4th day, starting with the day of plating. a, c) Micrographs of colonies at 14 and 7 days, respectively. b, d) After 7 days the size of colonies was measured for 100 plated cells using a graticle. Chart depicts the percentage of cells developing into colonies of the size indicated at 7 days. Average results for three independent experiments are shown and error bars indicate the standard deviation.
RNMT enhances Ras and c-Myc induced cell transformation
In order to expand on these findings, a similar set of transformation assays were performed in Rat1A cells which are also unable to proliferate in an anchorage-independent manner unless they have elevated expression of an oncogene. In this set of experiments, RNMT was exogenously expressed in combination with c-Myc, H-RasV12, an activated Ras mutant or vector control. After 7 days growth in suspension the size of resultant colonies was measured (Figure 2d), and light micrographs were taken (Figure 2c). As expected, exogenous expression of c-Myc and H-RasV12 resulted in significant anchorage-independent colony growth (45% and 72% plated cells growing into colonies larger than 60μm in diameter, respectively). Exogenous expression of RNMT did result in a small increase in anchorage-independent colony growth, although this was not statistically significant. Expression of RNMT, however, did significantly increase the number of c-Myc-induced colonies larger than 60μm in diameter from 45% to 76% of plated cells, and increased the number of H-Ras V12 induced colonies larger than 120uM in diameter from 12% to 36% of plated cells.
RNMT promotes cyclin D1 translation
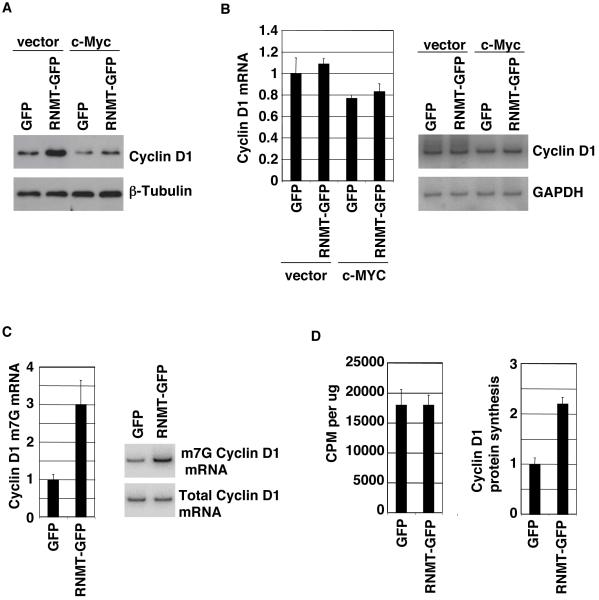
The implication of the finding that RNMT promotes cell transformation is that a gene or genes which are rate-limiting for cell transformation have increased mRNA cap methylation and mRNA translation in response to RNMT expression. Mammary tumours are associated with increased expression of several oncogenes, including c-Myc, Ras and Cyclin D1, which were potential candidates for regulation at the level of cap methylation in the RNMT-expressing mammary epithelial cell (IMEC) line. c-Myc and Ras protein levels were unresponsive to elevated RNMT expression (Figure 1a and not shown), however, Cyclin D1 protein was induced in response to RNMT-GFP (Figure 3a), and HA-RNMT expression (Supplementary Figure 1a). (Cyclin D1 is a Myc-repressed gene (Philipp et al., 1994), and a reduction in Cyclin D1 protein levels was observed in the c-Myc-expressing cell lines (Figure 3a and b)). Total Cyclin D1 mRNA levels were not induced in response to RNMT (Figure 3b), but the level of Cyclin D1 m7G (methyl capped) mRNA was upregulated 3-fold (Figure 3c). mRNA cap methylation was determined by anti-7-methylguanosine antibody immunoprecipitation of m7G mRNA followed by Cyclin D1-specific RT-PCR (Cole and Cowling, 2009). The substrate for the anti-methylguanosine immunoprecipitation was highly purified cellular RNA which is free from binding proteins and therefore RNA interaction with the antibody is dependent on the guanosine cap being methylated, rather than a binding protein masking the methylguanosine epitope. Similar results were also gained by expression of HA-RNMT (Supplementary figure 1d and e). The rate of Cyclin D1 protein synthesis was measured in the IMEC lines by incubation with radiolabelled amino acids. These experiments were performed in the presence of the proteasome inhibitor, MG132, using conditions that had previously been established to inhibit Cyclin D1 degradation (not shown). Amino-acid incorporation into total acid-precipitable material did not alter in response to elevated RNMT expression, indicating that total protein synthesis was unaffected (Figure 3d). However, when Cyclin D1 was immunoprecipitated from the same extracts and label incorporation was measured, Cyclin D1 protein synthesis was found to be upregulated in response to RNMT expression, which correlated with the observed increase in Cyclin D1 m7G mRNA levels and protein expression (Figure 3d).
Figure 3. RNMT promotes Cyclin D1 mRNA cap methylation and translation.
All experiments were performed in the IMEC lines indicated. a) Expression of Cyclin D1 and β-Tubulin was analysed by Western Blot. b) Cyclin D1 mRNA levels were determined by RT-PCR performed using Superscript One Step RT-PCR System (Invitrogen, Carlsbad, CA, USA). Reactions were labelled using 32P labelled-primers, resolved by gel electrophoresis and quantified by phosphoimager. A representative gel is shown. Reactions were determined to be in the linear range by performing a titration of input mRNA. Reactions were normalised to rRNA levels since the cellular rRNA content was unchanged in response to expression of RNMT. c) Cyclin D1 m7G mRNA levels were determined by anti-m7G antibody immunoprecipitation followed by RT-PCR, in the cell lines indicated, performed according to (Cole and Cowling, 2009). A representative gel is shown. d) The IMEC lines were labelled with 35S cysteine and methionine and incorporation per μg protein extract and into Cyclin D1 was determined. 10cm plates of IMECs were incubated in 4ml regular growth medium minus cysteine and methionine containing 10μM MG132 for 30mins at 37°C. 220μCi Express Protein Labelling Mix containing 35S cysteine and methionine (Perkin Elmer) was added for 15mins. Cells were lysed, normalised for protein content and 35S-cysteine and methionine incorporation into TCA-precipitated protein was detected using a scintillation counter. Immunoprecipitations performed on half of each extract using 2.5μl polyclonal anti-Cyclin D1 or control polyclonal antibodies were resolved by gel electrophoresis. Label incorporation into Cyclin D1 was detected by phosphoimager and expressed as values relative to input. For all charts, average results for three independent experiments are shown and error bars indicate the standard deviation.
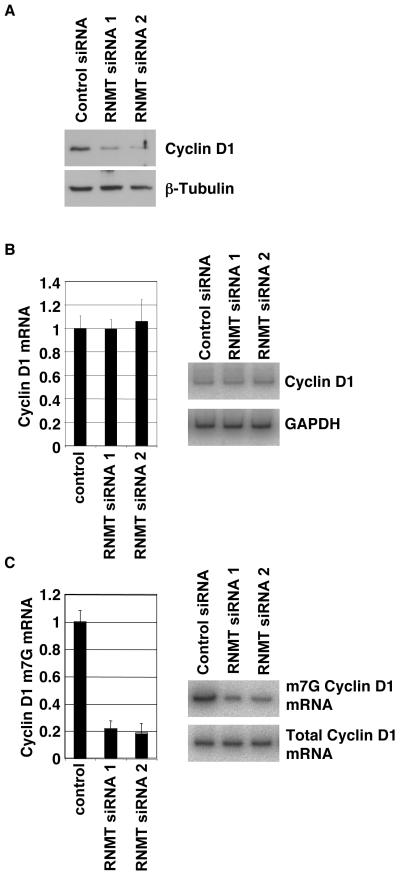
The experiments described above demonstrate that elevated RNMT expression results in increased Cyclin D1 mRNA cap methylation and protein expression. To further validate the relationship between RNMT and Cyclin D1, expression of endogenous RNMT was inhibited by transfection of two independent siRNAs (Figure 1c). A reduction in RNMT expression correlated with reduced Cyclin D1 mRNA cap methylation (Figure 4c) and reduced expression of Cyclin D1 protein (Figure 4a). However, reduced RNMT expression had no effect on Cyclin D1 mRNA levels (Figure 4b). Therefore, endogenous RNMT is necessary for Cyclin D1 mRNA cap methylation and protein expression.
Figure 4. RNMT is necessary for Cyclin D1 mRNA cap methylation and translation.
IMEC cells were transfected with two independent siRNAs directed against RNMT or Cyclophilin B (negative control), as in figure 1. After 24hrs, cells were lysed and protein and RNA was extracted. a) Whole cell extracts were analysed by Western Blot for expression of RNMT, Cyclin D1 and β-Tubulin. RNA was analysed for relative expression level of b) total Cyclin D1 mRNA and c) Cyclin D1 m7G mRNA. Representative gels are shown. For all charts, average results for three independent experiments are shown and error bars indicate the standard deviation.
Discussion
In this report, RNMT was demonstrated to be rate-limiting for mRNA cap methyltransferase activity and cell transformation. Of note, elevated expression of RNMT in TERT-immortalised human mammary epithelial cells was sufficient to transform an equivalent number of cells as elevated expression of c-Myc (Figure 2). RNMT and mRNA cap methyltransferase activity have not been previously reported to be transforming or oncogenic, and future work will be focussed on investigating the expression and activity of RNMT in tumours. In support of a role for RNMT in oncogenesis, elevated RNMT mRNA expression level was noted in adrenal, bladder, liver and lung carcinomas relative to normal tissue in ONCOMINE (www.oncomine.org), a web-based microarray database and data-mining platform (Rhodes et al., 2004).
Many recent publications have correlated an increase in total protein synthesis with cell transformation (Pandolfi, 2004; White, 2008). However, the rate of total protein synthesis was not altered in response to RNMT expression, and therefore the pro-transforming effect of RNMT in mammary epithelial cells is likely to be mediated by a restricted number of biologically significant mRNAs. Cyclin D1, an oncogene which is over expressed in 40-90% of cases of invasive breast cancer (Roy and Thompson, 2006), was found to be induced in response to RNMT expression, correlating with increased mRNA cap methylation and translation (Figure 3). Therefore, a significant proportion of Cyclin D1 mRNA is not methylated on the 5′ guanosine cap and thus is dormant in epithelial cells, and can be rapidly activated and translated by upregulation of mRNA cap methylation. Cyclin D1 is the first example of oncogene expression being regulated at the level of mRNA cap methylation.
In this report, RNMT and c-Myc co-operate to promote mammary epithelial cell transformation (fig. 2). Cyclin D1 is transcriptionally repressed by c-Myc, and the minor elevation in Cyclin D1 levels in response to RNMT in the c-Myc/IMECs is unlikely to make a significant contribution to cell transformation. Other examples of biologically significant mRNAs have been reported to be upregulated by mRNA cap methylation, and these are potential candidates for mediating Myc-RNMT co-transformation. These include most c-Myc transcriptional target genes, which were demonstrated to be targets for Myc-dependent mRNA cap methylation (Cole and Cowling, 2009). Amongst these are genes that have been implicated in or demonstrated to promote cell transformation or tumoroigenesis, including eIF4E (Wendel et al., 2004; Wendel et al., 2007), Nol5a (Goy et al., 2006; Vallat et al., 2007), and HSP60 (Tsai et al., 2008).
Supplementary Material
Acknowledgements
I would like to thank Mike Cole for the generous gift of the anti-m7G antibodies, Aaron Shatkin for providing the RNMT-GFP construct, Julian Downward for providing the H-RasV12 construct and James DiRenzo for providing IMEC cells. I would also like to thank all of the above and the Cowling lab for advice. This work was funded by a MRC Career Development Award and a Tenovus Scotland Project Grant.
Footnotes
The author declares no conflict of interest.
References
- Bentley DL. Rules of engagement: co-transcriptional recruitment of pre-mRNA processing factors. Curr Opin Cell Biol. 2005;17:251–6. doi: 10.1016/j.ceb.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Chu C, Shatkin AJ. Apoptosis and autophagy induction in mammalian cells by small interfering RNA knockdown of mRNA capping enzymes. Mol Cell Biol. 2008;28:5829–36. doi: 10.1128/MCB.00021-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MD, Cowling VH. Transcription-independent functions of MYC: regulation of translation and DNA replication. Nat Rev Mol Cell Biol. 2008;9:810–5. doi: 10.1038/nrm2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MD, Cowling VH. Specific regulation of mRNA cap methylation by the c-Myc and E2F1 transcription factors. Oncogene. 2009 doi: 10.1038/onc.2008.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowling VH, Chandriani S, Whitfield ML, Cole MD. A conserved Myc protein domain, MBIV, regulates DNA binding, apoptosis, transformation, and G2 arrest. Mol Cell Biol. 2006;26:4226–39. doi: 10.1128/MCB.01959-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowling VH, Cole MD. The Myc transactivation domain promotes global phosphorylation of the RNA polymerase II carboxy-terminal domain independently of direct DNA binding. Mol Cell Biol. 2007;27:2059–73. doi: 10.1128/MCB.01828-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowling VH, D’Cruz CM, Chodosh LA, Cole MD. c-Myc transforms human mammary epithelial cells through repression of the Wnt inhibitors DKK1 and SFRP1. Mol Cell Biol. 2007;27:5135–46. doi: 10.1128/MCB.02282-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiRenzo J, Signoretti S, Nakamura N, Rivera-Gonzalez R, Sellers W, Loda M, et al. Growth factor requirements and basal phenotype of an immortalized mammary epithelial cell line. Cancer Res. 2002;62:89–98. [PubMed] [Google Scholar]
- Furuichi Y, Shatkin AJ. Viral and cellular mRNA capping: past and prospects. Adv Virus Res. 2000;55:135–84. doi: 10.1016/S0065-3527(00)55003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras AC, Raught B, Sonenberg N. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu Rev Biochem. 1999;68:913–63. doi: 10.1146/annurev.biochem.68.1.913. [DOI] [PubMed] [Google Scholar]
- Goy A, Stewart J, Barkoh BA, Remache YK, Katz R, Sneige N, et al. The feasibility of gene expression profiling generated in fine-needle aspiration specimens from patients with follicular lymphoma and diffuse large B-cell lymphoma. Cancer. 2006;108:10–20. doi: 10.1002/cncr.21500. [DOI] [PubMed] [Google Scholar]
- Mao X, Schwer B, Shuman S. Yeast mRNA cap methyltransferase is a 50-kilodalton protein encoded by an essential gene. Mol Cell Biol. 1995;15:4167–74. doi: 10.1128/mcb.15.8.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandolfi PP. Aberrant mRNA translation in cancer pathogenesis: an old concept revisited comes finally of age. Oncogene. 2004;23:3134–7. doi: 10.1038/sj.onc.1207618. [DOI] [PubMed] [Google Scholar]
- Philipp A, Schneider A, Vasrik I, Finke K, Xiong Y, Beach D, et al. Repression of cyclin D1: a novel function of MYC. Mol Cell Biol. 1994;14:4032–43. doi: 10.1128/mcb.14.6.4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillutla RC, Yue Z, Maldonado E, Shatkin AJ. Recombinant human mRNA cap methyltransferase binds capping enzyme/RNA polymerase IIo complexes. J Biol Chem. 1998;273:21443–6. doi: 10.1074/jbc.273.34.21443. [DOI] [PubMed] [Google Scholar]
- Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, Ghosh D, et al. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6:1–6. doi: 10.1016/s1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy PG, Thompson AM. Cyclin D1 and breast cancer. Breast. 2006;15:718–27. doi: 10.1016/j.breast.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Shafer B, Chu C, Shatkin AJ. Human mRNA cap methyltransferase: alternative nuclear localization signal motifs ensure nuclear localization required for viability. Mol Cell Biol. 2005;25:2644–9. doi: 10.1128/MCB.25.7.2644-2649.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatkin AJ. Capping of eucaryotic mRNAs. Cell. 1976;9:645–53. doi: 10.1016/0092-8674(76)90128-8. [DOI] [PubMed] [Google Scholar]
- Shuman S. What messenger RNA capping tells us about eukaryotic evolution. Nat Rev Mol Cell Biol. 2002;3:619–25. doi: 10.1038/nrm880. [DOI] [PubMed] [Google Scholar]
- Tsai YP, Teng SC, Wu KJ. Direct regulation of HSP60 expression by c-MYC induces transformation. FEBS Lett. 2008;582:4083–8. doi: 10.1016/j.febslet.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Vallat LD, Park Y, Li C, Gribben JG. Temporal genetic program following B-cell receptor cross-linking: altered balance between proliferation and death in healthy and malignant B cells. Blood. 2007;109:3989–97. doi: 10.1182/blood-2006-09-045377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y, Shatkin AJ. Cap methyltransferase selective binding and methylation of GpppG-RNA are stimulated by importin-alpha. Genes Dev. 2000;14:2944–9. doi: 10.1101/gad.848200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendel HG, De Stanchina E, Fridman JS, Malina A, Ray S, Kogan S, et al. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428:332–7. doi: 10.1038/nature02369. [DOI] [PubMed] [Google Scholar]
- Wendel HG, Silva RL, Malina A, Mills JR, Zhu H, Ueda T, et al. Dissecting eIF4E action in tumorigenesis. Genes Dev. 2007;21:3232–7. doi: 10.1101/gad.1604407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RJ. RNA polymerases I and III, non-coding RNAs and cancer. Trends Genet. 2008;24:622–9. doi: 10.1016/j.tig.2008.10.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.