Abstract
A T cell-mediated immune response is initiated by the T cell receptor (TCR) interacting with peptide-bound MHC (pMHC) on an infected cell. The mechanism by which this interaction triggers intracellular phosphorylation of the TCR, which lacks a kinase domain, remains poorly understood. Here, we have introduced the TCR and associated signalling molecules into a nonimmune cell and reconstituted ligand-specific signalling when these cells are conjugated with antigen presenting cells. We show that signalling requires the differential segregation of a phosphatase and kinase in the plasma membrane. An artificial, chemically-controlled receptor system generates the same effect as TCR-pMHC, demonstrating that the binding energy of an extracellular protein-protein interaction can drive the spatial segregation of membrane proteins without a transmembrane conformational change. This general mechanism may extend to other receptors that rely on extrinsic kinases, including, as we demonstrate, chimaeric antigen receptors being developed for cancer immunotherapy.
In addition to intercellular communication mediated by soluble molecules, two cells can transmit signals through membrane-associated receptors and ligands. Adaptive immunity represents such a system, where the major histocompatibility complex (MHC) protein on the surface of antigen-presenting cells (APC) interacts with the T-cell receptor (TCR) on T lymphocytes. If the TCR binds peptide-bound MHC (pMHC) of the right complementarity, then the interaction results in tyrosine phosphorylation of the TCR (herein referred to as TCR “triggering”) and the initiation of signals that activate the T cell1. The TCR has no intrinsic kinase activity, unlike many other receptors2, and instead relies upon on a T-cell specific kinase called Lck3. Also distinct from other systems, the phosphorylatable tyrosine residues of the TCR (the ITAMs4) do not reside on the polypeptides that contact the pMHC (α, β) but instead are contained on tightly associated CD3 subunits (γ, δ, ε2, ζ2). The phosphylated ITAMs then bind a second kinase, ZAP70, which is subsequently activated and drives downstream signalling5.
Despite considerable work, the mechanism by which pMHC binding leads to TCR triggering remains poorly understood (reviewed in6). Some models propose that pMHC binding evokes a conformational change in the TCR that makes its cytoplasmic ITAM domains more accessible to Lck kinase7. Alternative triggering hypotheses include activation through the aggregation of TCR molecules6, and “kinetic segregation”8, where TCR phosphorylation is favoured by its partitioning into plasma membrane domains that contain Lck kinase but are depleted of CD45, an abundant transmembrane phosphatase. However, while TCR clustering9 and the segregation of CD45 away from the TCR have been observed10, it has not been established whether such events are necessary or sufficient for signal transduction across the plasma membrane. In addition, the physical basis of protein segregation within the plasma membrane is unclear.
Reconstitution of a biological phenomenon with defined components has proven to be a powerful means for dissecting molecular mechanisms. We have made use of this approach by introducing the genes encoding the TCR and other proteins required for regulating its phosphorylation into a non-immune cell and recapitulating TCR triggering when this cell forms a conjugate with an APC. Since each protein can be introduced separately and genetically engineered, this system has allowed us to test models of TCR triggering and the roles of individual proteins in a manner that is difficult to achieve with native T cells.
Reconstitution of regulated TCR triggering
We first sought to reconstitute Lck-mediated TCR phosphorylation in a non-immune cell and then determine which factors are needed to keep the TCR quiescent (Fig.1a). As the basis of our reconstitution, we expressed11 the complete set of protein chains of the 1G4 TCR12 in the plasma membrane of HEK cells (hereafter, “HEK-1G4”) (Supplementary Methods and Supplementary Fig.1). The expressed TCR complex did not show detectable phosphorylation (assayed by a phosphospecific antibody to the CD3ζ chain, an essential TCR subunit required for signalling13,14) unless Lck and ZAP70 were co-expressed (Fig.1b). Lck kinase activity, as detected by measuring levels of activating (Tyr394) and inhibitory (Tyr505) phosphorylation3, appeared to be unaffected by the presence of the TCR or ZAP70 (Fig.1b). However, ZAP70 activity, as measured by increased Tyr493 phosphorylation5, was only detectable in the presence of both Lck and the TCR (Fig.1b), which agrees with previous data that this kinase is inactive until it binds to phosphorylated CD3ζ ITAMs15 (Fig.1a). We confirmed the activity of ZAP70 by demonstrating phosphorylation of co-expressed LAT, its downstream substrate and critical adapter protein for T cell signalling (Supplementary Fig.2a).
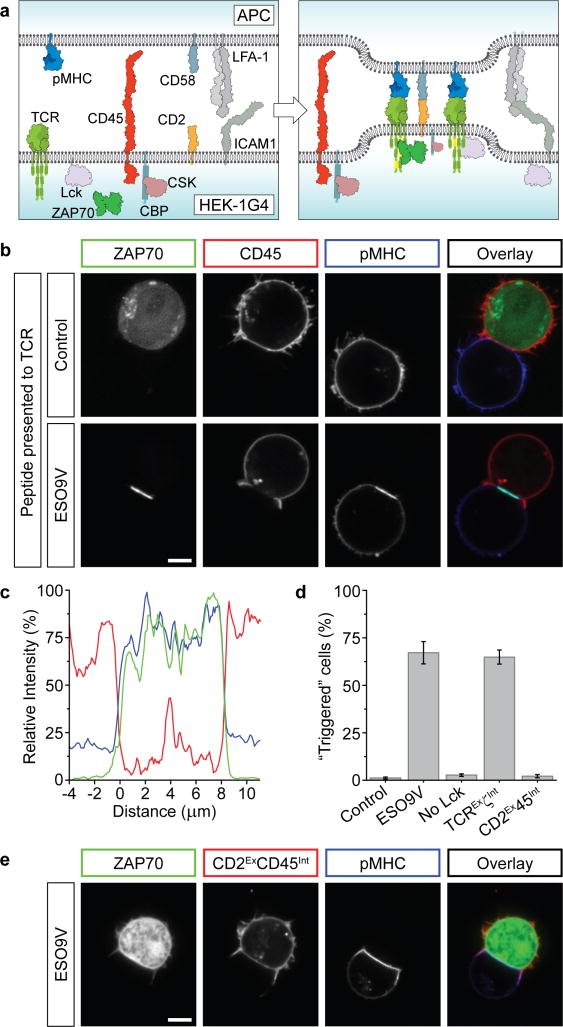
Figure 1. Regulatable TCR triggering in an engineered HEK cell line.
a, Schematic representation of molecules transfected into HEK cells. b, Western blot of phosphorylated proteins after transfection of HEK cells with selected molecules (green circles). c, Cells transfected with the TCR, Lck and ZAP70, were transfected with additional molecules (green circles), showing the synergistic action of modulatory proteins CSK/CBP and CD45 to restrain Lck. The decreased phosphorylation between lanes 5 and 7 is due to endogenous CSK recruitment. d, Confocal images of HEK-1G4 showing ZAP70-GFP recruitment to the plasma membrane in the presence of Lck. e, Quantitation of ZAP70 relocalisation with indicated molecules transfected in HEK-1G4 cells. Data presented as mean ± sem of 3 independent experiments (~300 cells per experiment). f, Addition of 100 μM pervanadate to HEK-1G4 cells (expressing components in lane 8 of c) caused the accumulation of membrane-localised ZAP70. Scale bars, 5 μm.
To establish a quiescent system that could be activated by pMHC, we next sought to restrain Lck’s kinase activity. CSK induces an “inactive” conformation of Lck by phosphorylating its C-terminus (Tyr505)16. However, co-expressing CSK and CSK-binding protein (CBP), which localises CSK to the plasma membrane (Fig. 1a), was insufficient to repress Lck phosphorylation of CD3ζ (Fig. 1c). CD45 is a tyrosine phosphatase that modulates T-cell signalling in a complex manner by dephosphorylating the inhibitory Tyr505 and activating Tyr394 of Lck17,18 and the ITAM tyrosines of the TCR (Fig. 1a). Co-expression of CD45 with Lck severely diminished Lck-induced ZAP70 activation but only modestly inhibited phosphorylation of the CD3ζ chain of the TCR (Fig. 1c). However, simultaneous expression of CSK/CBP and CD45 dramatically reduced CD3ζ phosphorylation (Fig. 1c). This synergy depended on CBP (Fig. 1c), suggesting that membrane recruitment of CSK is required for its potency. Thus, the two major activities known to repress TCR phosphorylation in T cells are sufficient, when combined, to keep the TCR in a quiescent state in this reconstituted cell system.
ZAP70 is normally cytosolic but binds to phosphorylated TCR ITAMs. The translocation of ZAP70-GFP from the cytosol to the plasma membrane therefore provides a microscopy-based assay of TCR triggering. Indeed, Lck expression in HEK-1G4 cells caused ZAP70-GFP to accumulate at the plasma membrane (Fig. 1d,e), while co-expression of CSK/CBP and CD45 delocalised ZAP70 to the cytoplasm (Fig. 1e). Furthermore, ZAP70-GFP translocated rapidly to the plasma membrane upon CD45 phosphatase inhibition by pervanadate (Fig. 1f, Supplementary Movie 1). Thus, this visual assay provides a dynamic readout of TCR triggering.
Reconstitution of a T cell-APC conjugate
Having identified a minimal set of components that could be expressed in HEK-1G4 cells to mimic the basal or “off” state of their T-cell counterpart, we next attempted to trigger signalling by having the 1G4 TCR interact with its ligand, the MHC class-I complex bound with a short peptide (ESO9V)19 and expressed in the Raji B-cell line as the antigen presenting cell (“APC”) (Fig. 2a; Supplementary Methods). Since HEK and Raji B-cells have no intrinsic affinity for each other (Supplementary Fig. 3a), we expressed the immune cell adhesion proteins CD2 and ICAM1 on the HEK-1G4 cells (Fig. 2a), which can interact with their respective counter-receptors CD58 and LFA-1 expressed endogenously on Raji cells (Supplementary Fig. 1). This strongly increased the conjugation between the two cell-types, with the LFA-1/ICAM1 interaction being the predominant driver (Supplementary Fig. 3a).
Figure 2. The exclusion of CD45 phosphatase is necessary and sufficient for TCR triggering.
a, Proteins expressed in HEK-1G4 for cell conjugation. b, HEK-1G4, expressing all components shown in a, were conjugated with APC (Raji cells) expressing cognate pMHC (ESO9V), or a control pMHC. Coloured boxes denote protein representation in the overlay image. c, A representative line profile of membrane fluorescence from CD45 (red), pMHC (blue) and ZAP70 (green) at the conjugate interface. d, Quantitation of triggering (defined as unambiguous recruitment of ZAP70 to conjugate region) for all conjugates described in text. Data is presented as the mean ± sem of independent experiments (n=4 or 5, 30-150 conjugates per experiment). e, Forcing CD45 into conjugate region by fusing to CD2 (CD2ExCD45Int) blocks TCR triggering (ZAP70 remains cytosolic). Quantitation is shown in d. Scale bars, 5 μm.
We found that the HEK-1G4 cells formed a cup-shaped contact around the APC expressing antigenic pMHC (Supplementary Movie 2), which was strikingly similar to T cell-APC conjugates20. pMHC was concentrated at the site of cell-cell interaction (Fig. 2b,c), and importantly, ZAP70 in the HEK-1G4 cells translocated to the plasma membrane in this region revealing that TCR triggering had occurred (Fig. 2b-d; confirmed by immunoblotting in Supplementary Fig. 2b). Triggering was rapid, and could be observed within one minute of cell contact (Supplementary Movie 3). Membrane translocation of ZAP70 did not occur when HEK-1G4 cells were conjugated with Raji cells expressing a control peptide-MHC complex (Fig. 2b,d) or in HEK-1G4 cells lacking transfected Lck (Fig. 2d, Supplementary Fig. 2b). We also found that the ITAMs of CD3ζ were sufficient for ligand-specific ZAP70 translocation by using a minimal TCR complex with the intracellular sequences of CD3γ,δ and ε truncated after the transmembrane domain (“TCRExζInt”, Fig. 2d). Although the APC used in these experiments expressed the cognate pMHC at high levels (~50 pMHC/μm2), ZAP70 recruitment was still observable at lower, more physiological levels of antigenic pMHC (~3 pMHC/μm2)21,22 (Supplementary Fig. 1 and 4). In summary, an APC expressing the appropriate pMHC is able to elicit a specific TCR triggering response from our reconstituted HEK-1G4 cell.
Disrupting the actin cytoskeleton severely inhibits T cell activation23, but its role in TCR triggering is uncertain. Depolymerisation of actin filaments in HEK-1G4 cells prior to mixing with APCs significantly decreased the number of conjugates (Supplementary Fig. 3b), but cells that did interact still showed ZAP70 recruitment (Supplementary Fig. 3c). This result suggests that the cytoskeleton facilitates the initial cell-cell interactions but is not essential for TCR triggering in this reconstituted cell system.
Mechanistic insight into how the TCR was triggered by pMHC came from the observation that CD45 phosphatase was excluded from the cell-cell interface where the TCR was bound to its cognate ligand (Fig. 2b,c) while Lck remained included (Supplementary Fig. 5a). Since complete repression of Lck phosphorylation of the TCR required both CD45 and CSK/CBP (Fig. 1c), the segregation of CD45 away from the TCR would be predicted to shift the steady state balance towards TCR phosphorylation. Previous studies have also found that the majority of CD45 is excluded from the interface of real T cell-APC conjugates24,25 and could be involved in triggering26,27. However, it has been difficult to ascertain whether CD45 exclusion is responsible for, or a consequence of TCR triggering. To show more definitively that CD45 exclusion has a causal role, we redirected phosphatase activity into the cell-cell interface by fusing the intracellular phosphatase domains of CD45 to the extracellular and transmembrane domains of CD2 (termed CD2ExCD45Int), since CD2 was localised within the conjugate interface (Supplementary Fig. 5b). The CD2ExCD45Int construct was clearly observed within the conjugate interface (Fig. 2e), and ZAP70 was no longer recruited to the conjugate region, indicating that TCR triggering was abolished (Fig. 2d,e). This result shows that CD45 exclusion is required for TCR triggering in our reconstituted cell system.
To show that CD45 exclusion and TCR triggering were not dependent on each other, we temporally separated the formation of the signalling zone from TCR triggering by engineering a TCR lacking its ITAMs but instead having an intracellular recruitment domain (FKBP) (TCRExFKBPInt, Supplementary Fig.6a). We expected this receptor to localise within the cell-cell interface through its extracellular interaction with pMHC, but unable to recruit ZAP70 in the absence of any signalling motifs. To initiate TCR phosphorylation and ZAP70 recruitment, an FKBP-binding domain (FRB) was fused to the cytoplasmic region of CD3ζ (FRB-CD3ζInt), which can be induced to dimerise with TCRExFKBPInt upon addition of the drug rapamycin (Supplementary Fig. 6a). CD45 and pMHC were excluded and concentrated from the conjugate interface, respectively in the absence of rapamycin. However, ZAP70 was recruited to the membrane in the zones of CD45 exclusion only when the separated components of the TCR (TCRExFKBPInt and FRB-CD3ζInt) were brought together with rapamycin (Supplementary Fig. 6b,c). Time lapse imaging showed no discernible delay between the translocation of FRB-CD3ζInt to the plasma membrane and the subsequent recruitment of ZAP70 (Supplementary Fig. 6d; Supplementary Movie 4).
The mechanism of CD45 segregation
We next wished to explore what forces produce CD45 exclusion. Since a control peptide bound to pMHC did not elicit CD45 segregation (Fig. 2b), the adhesion pairs (CD2/CD58 and LFA-1/ICAM1) must be incapable of driving exclusion (Supplementary Fig. 5c). We next tested the TCR-pMHC interaction alone, and found that it was sufficient for conjugation (Supplementary Fig. 3a) and CD45 segregation (Fig. 3a). FRAP and FLIP experiments showed that the TCR remained mobile after pMHC binding and free to escape the conjugated region, showing that the segregated zone was not formed by an immobile aggregate and remained contiguous with the rest of the cell surface (Supplementary Fig. 7). Thus, the TCR-pMHC interaction is necessary and sufficient for CD45 exclusion, with no requirement for downstream TCR triggering/signalling.
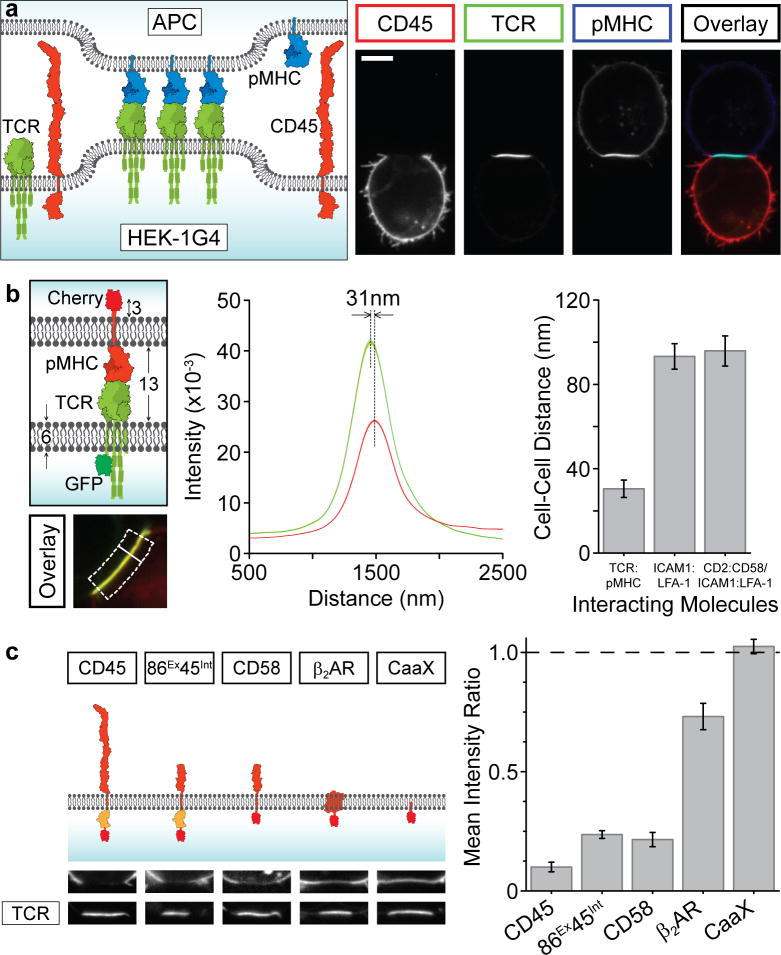
Figure 3. The TCR-pMHC interaction drives protein exclusion at conjugate regions.
a, A schematic and representative image dataset showing the TCR-pMHC interaction is sufficient to drive CD45 exclusion and its own clustering (scale bar, 5 μm). b, The intermembrane distance between the conjugates (see Supplementary Methods) was measured, shown schematically as the separation between the two fluorophores over a normal line (white line) averaged across the conjugate region (dotted box). This procedure was performed for the cognate TCR/pMHC interaction (n=20 cells), LFA-1/ICAM1 (n=23 cells) and CD2/CD58+LFA-1/ICAM1; n=20 cells) interactions in the presence of control pMHC, with mean ± sem shown. c, HEK cells were transfected with TCR-GFP and indicated molecule (fused to mCherry) and conjugated with APCs (CD45 phosphatase domains shown in orange). Representative images of conjugate region are shown, with quantitation of the ratio of fluorescence inside and outside of the interface. Data presented as mean ± sem (n=20) for each construct.
Next, we examined how the TCR-pMHC interaction affected the spacing of the two plasma membranes of the interacting cells. Using a subdiffraction-resolution method, we measured the separation between a GFP-tagged TCR in the HEK cells and mCherry-pMHC in the APC (Fig. 3b; see Supplemental Methods). The measured distance of ~31 nm (Fig. 3b) suggests a cell-cell separation of ~15 nm, which agrees well with the 13 nm cell-to-cell distance between T cells and conjugated APCs measured by EM28. In contrast, the membrane separation for Raji cells expressing control pMHC conjugated through adhesion molecules alone (ICAM-LFA1 or ICAM-LFA1/CD2-CD58) was much greater (Fig. 3b). These results show that the TCR-pMHC interaction brings the membranes much closer together than occurs with the adhesion molecules.
Proteins with extended extracellular domains (such as CD45) might be prevented from entering regions of close membrane apposition, as suggested by the kinetic segregation model8. We tested this hypothesis by fusing the intracellular phosphatase domains of CD45 to an extracellular domain (from CD86) of comparable size to the TCR (termed CD86ExCD45Int). CD86ExCD45Int was also excluded from the cell-cell interface, although its exclusion (Fig. 3c) and TCR triggering (Supplementary Fig. 8a,b) were somewhat lower than seen with the large CD45 construct (Fig. 3c). Because of this unanticipated exclusion of a protein with a small extracellular domain, we next tested mCherry fusion proteins of a series of membrane proteins with different properties: CD58, a transmembrane protein with an equivalently-sized extracellular domain to CD86, β2-adrenergic receptor (β2AR), a seven transmembrane protein with small extracellular loops, and CaaX, a short targeting sequence that confers prenylation and links the fluorophore to the inner leaflet of the bilayer (Fig. 3c). CD58 was excluded to a similar level as CD86ExCD45Int, showing that the segregation of the latter construct was not due to the intracellular phosphatase domains of CD45 (Fig. 3c). β2AR, a protein with a large lateral footprint, was also partially excluded (Fig. 3c), possibly through a crowding effect arising from the high density of pMHC-TCR in the conjugate region. However, the CaaX prenylated mCherry was evenly distributed throughout the cell membrane, showing no exclusion by TCR-pMHC (Fig. 3c).
The above experiments cannot explain why CD2 (Supplementary Fig. 5b) and CD2ExCD45Int (Fig. 2e), whose extracellular domains are similar in size to CD86, were not excluded from the cell-cell contact zone (Fig. 3c). We speculated that binding of CD2 to its ligand (CD58) endogenously expressed on the APC provided a counteracting force to constrain CD2 within the conjugate region. If this were true, then expressing CD28, the binding partner for CD86, on the APC should diminish the TCR-pMHC mediated segregation of CD86ExCD45Int. Indeed, when CD86ExCD45Int-expressing HEK-1G4 cells were conjugated with APCs co-expressing CD28, CD86ExCD45Int now localised at the cell-cell interface and TCR triggering was greatly diminished (Supplementary Fig. 8b,c).
In summary, the TCR-pMHC interaction alone is capable of excluding plasma membrane proteins with extracellular extensions and that exclusion can be overcome by the energy provided by binding to a protein partner on the APC.
Triggering of artificial receptors
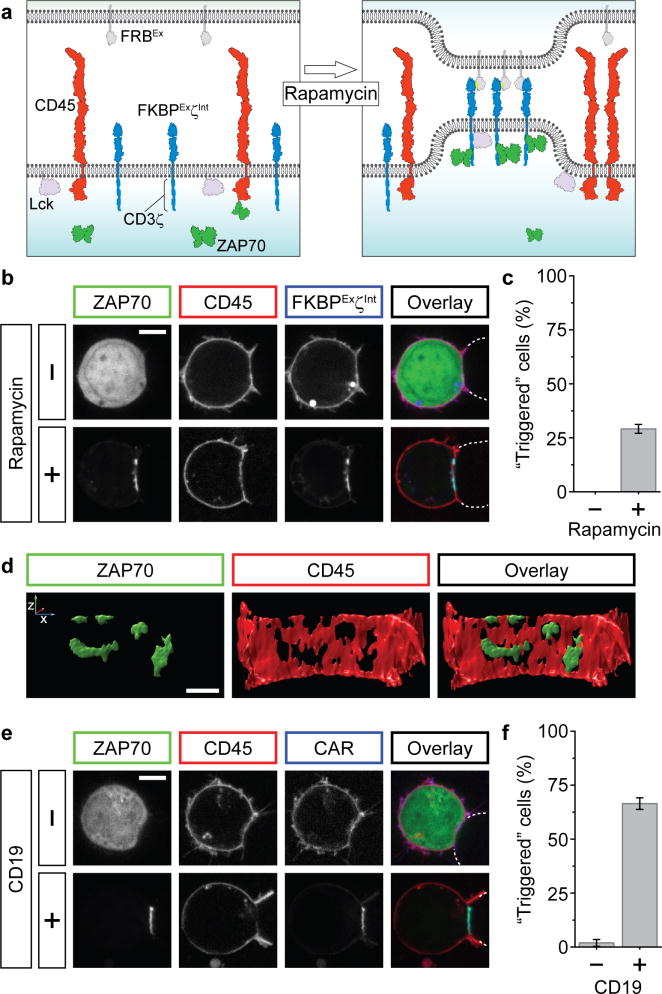
The preceding experiments suggested that TCR triggering results from CD45 segregation, which is driven by the binding interaction between the TCR and pMHC. If this is true, then interactions between extracellular domains of membrane proteins with the proper spacing and affinity might elicit comparable effects to TCR-pMHC, as has been shown in T cells28. To explore this idea, we engineered a chemically-controlled, cell surface receptor system consisting of a transmembrane protein with extracellular FKBP and the intracellular CD3ζ domains expressed in the HEK cell (FKBPExζInt; mimicking the TCR) and a transmembrane protein with an extracellular FRB expressed in the APC (mimicking the pMHC) (Fig. 4a). FKBPExζInt and FRBEx will only interact in the presence of rapamycin, forming a complex that spans a similar distance to TCR-pMHC. In the absence of rapamycin, conjugates formed (through the LFA1-ICAM1 interaction) but ZAP70 was not recruited to the membrane (Fig. 4b,c). However, with rapamycin, FKBPExζInt receptor clusters were observed at the cell-cell interface, even with low levels of FRBEx ligand that are equivalent to physiological densities of antigen pMHC (~5 molecules/μm2; Supplementary Fig. 1). Furthermore, CD45 was excluded from and ZAP70 was recruited to these receptor clusters, indicating that “triggering” had occurred (Fig. 4b-d).
Figure 4. Artificial receptor systems can cause CD45 exclusion and triggering.
a, Schematic of the chemically-inducible receptor system. FRB fused to a transmembrane segment (FRBEx) replaces pMHC on the APC and FKBPExζInt (fusion of FKBP, CD86 and intracellular CD3ζsignalling motifs) replaces the TCR. Rapamycin induces FKBPExζInt-FRBEx interaction. Additional molecules have been omitted for clarity. b. Rapamycin addition causes ZAP70 accumulation, denoting receptor triggering (gamma correction applied to lower images). FRBEx-expressing cells shown by dotted lines (scale bar 5 μm). c, Quantitation of rapamycin-induced triggering (mean ± sem over four experiments). d, A deconvolved 3D-rendering of the reconstituted cell interface shown in b (scale bar 2 μm). e, Reconstituted HEK cells with the TCR replaced by a chimaeric antigen receptor (CAR) specific for CD19 (see text) were conjugated with either CD19- (Jurkat) or CD19+ (Raji) cells, marked by dotted lines (scale bar 5 μm). f, Quantitation of CAR-mediated triggering (mean ± sem over three experiments).
Our artificial receptor is structurally analogous to chimaeric antigen receptors (CARs), which have an extracellular single-chain antibody fragment fused to a transmembrane sequence and cytoplasmic CD3ζ ITAMs. When the CD19-specific CAR29, which has shown promise in recent clinical trials as a gene therapy treatment of leukaemia29, was expressed in our reconstituted HEK cells and these cells were conjugated with Raji B cells (which are CD19+), we observed CAR clustering, ZAP70 recruitment and CD45 exclusion (Fig. 4e,f). This triggering was ligand-specific, since conjugation with CD19- cells did not produce these effects (Fig. 4e,f). Interestingly, the interface of many CD19-CAR HEK cell and CD19+-Raji B cell conjugates showed a highly convoluted membrane surface (Supplementary Movie 5), which was not seen for cells interacting via either TCR/pMHC or FKBPExζInt–FRBEx. This membrane effect could be a product of high affinity antibody binding and might warrant further investigation for its relevance to CAR potency or potential side effects.
Conclusion
Our reconstitution experiments reveal a physical mechanism for TCR triggering that differs from dimerization or conformational change models proposed for many cell surface receptors. We find that the binding energy of the TCR-pMHC interaction generates an exclusion force for membranes proteins with large and/or unligated extracellular domains, even in the absence of downstream signalling. By linking an inhibitory phosphatase activity to a transmembrane protein (CD45) that is subject to the exclusion force and an activating kinase (Lck) to the inner leaflet of the membrane that is not, the TCR-pMHC interaction can shift the kinase-phosphatase balance and thus trigger the TCR, as first suggested by the kinetic segregation model8. Since the FKBP-rapamycin-FRB or antibody-antigen modules can replace the extracellular TCR-pMHC interaction, conformational changes in the TCR are unlikely to be critical for the fundamental mechanism of triggering. Coreceptors and actin also do not appear to be essential for transducing TCR-pMHC binding across the plasma membrane, although they may very likely be necessary for achieving the high sensitivity and full selectivity of T cell activation. This could be tested in the future by pushing our reconstituted system to respond to very low antigen densities using more sensitive readouts of triggering.
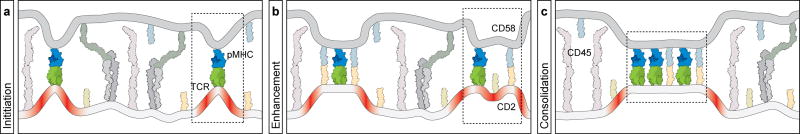
The precise mechanism by which the TCR-pMHC interaction results in protein exclusion remains unanswered, although our data provide certain clues. Exclusion according to the kinetic segregation model is based on the greater size of the extracellular domain of CD45 compared to the TCR-pMHC complex. However, while size influences the extent of exclusion, our data reveals that membrane proteins with small extracellular domains are excluded, suggesting that additional forces must be acting on the system. We speculate upon the driving force in the following model, which incorporates previous findings30-34. Within the initial adhesion mediated by LFA-1/ICAM1 between the T cell and APC, transient fluctuations bring the two membranes in closer apposition, allowing TCR and pMHC molecules to interact (Fig.5a). The binding energy of this interaction must be sufficient to overcome unfavourable membrane bending and compression of the large proteins that constitute the glycocalyx, such as CD45, which occur when the two membranes regions are brought close together (Fig.5b). In a critical next step, separate regions of close membrane apposition passively consolidate into larger, contiguous regions, resulting in a net decrease in membrane bending as has been previously suggested35. Proteins that do not provide any binding energy will be excluded over time, since as they diffuse from the region, interacting molecules will be further clustered as the area of close apposition is minimised (Fig.5c). These same principles may explain how TCR microdomains initiate when T cells interact with pMHC on a supported lipid bilayer36-38 (note the small exclusion zones in our experiments at low ligand densities (Supplementary Fig.4 and Fig.4d)).
Figure 5. A model for steps in TCR-mediated segregation based on membrane bending and energy minimisation.
The schematic uses equivalent molecule representations as previous figures (only extracellular domains are shown for simplicity), and boxed regions highlight features of each panel. a, After initial adhesion driven by large receptors such as LFA-1, transient fluctuations in the intermembrane distance permit encounters between TCR and pMHC whose binding interaction overcomes energetically unfavourable membrane bending (red regions). b, Additional molecules (such as CD2/CD58) provide additional binding energy that stabilise regions of local membrane bending and may enhance the local exclusion of larger molecules by TCR-pMHC interactions. c, Consolidation of discrete contact regions serves to minimise unfavourable membrane bending and leads to the exclusion of smaller proteins that do not provide any countering ligand-binding free energy. See text for details.
Overall, our data suggests how the binding energy associated with specific molecular recognition events that take place between two interacting cells can be transduced into intracellular biochemical reactions that change cell behaviour. This model provides a plausible mechanism to explain how chimaeric antigen receptors trigger T cells to kill cancerous cells29 and may apply to other cell types that signal using membrane-bound receptors and ligands.
Methods Summary
Multiple proteins were expressed in HEK cells using a combination of transient and stable expression by lentiviral transduction; proteins were expressed at close to physiological levels and in the correct localisation as described in the Supplementary Methods. To create cell conjugates, the two cell types were centrifuged, resuspended at high density, and placed on glass-bottomed dishes for live-cell imaging at 37°C using spinning disc confocal microscopy. Image analysis, including the intermembrane distance algorithm, was performed using ImageJ and Matlab (Supplementary Methods). Full methods and supplementary material accompany this paper.
Supplementary Material
Acknowledgments
We thank Anton van der Merwe and Vincenzo Cerundolo (University of Oxford) for the 1G4 TCR sequence, Art Weiss (UCSF) for cell lines and helpful advice, Carl June (University of Pennsylvania) for the CD19 CAR construct, Nico Stuurman (UCSF) and Kurt Thorn (Nikon Imaging Facility, UCSF) for microscopy help and members of the Vale lab for discussions. RDV is an HHMI investigator and JRJ is a fellow of the Jane Coffin Childs Memorial Fund.
References
- 1.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palacios EH, Weiss A. Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene. 2004;23:7990–8000. doi: 10.1038/sj.onc.1208074. [DOI] [PubMed] [Google Scholar]
- 4.Love PE, Hayes SM. ITAM-mediated signaling by the T-cell antigen receptor. Cold Spring Harb Perspect Biol. 2010;2:a002485. doi: 10.1101/cshperspect.a002485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Au-Yeung BB, et al. The structure, regulation, and function of ZAP-70. Immunol Rev. 2009;228:41–57. doi: 10.1111/j.1600-065X.2008.00753.x. [DOI] [PubMed] [Google Scholar]
- 6.van der Merwe PA, Dushek O. Mechanisms for T cell receptor triggering. Nat Rev Immunol. 2011;11:47–55. doi: 10.1038/nri2887. [DOI] [PubMed] [Google Scholar]
- 7.Xu C, et al. Regulation of T cell receptor activation by dynamic membrane binding of the CD3epsilon cytoplasmic tyrosine-based motif. Cell. 2008;135:702–713. doi: 10.1016/j.cell.2008.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis SJ, van der Merwe PA. The kinetic-segregation model: TCR triggering and beyond. Nat Immunol. 2006;7:803–809. doi: 10.1038/ni1369. [DOI] [PubMed] [Google Scholar]
- 9.Lillemeier BF, et al. TCR and Lat are expressed on separate protein islands on T cell membranes and concatenate during activation. Nat Immunol. 2010;11:90–96. doi: 10.1038/ni.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varma R, Campi G, Yokosuka T, Saito T, Dustin ML. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25:117–127. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szymczak AL, et al. Correction of multi-gene deficiency in vivo using a single ‘self-cleaving’ 2A peptide-based retroviral vector. Nat Biotechnol. 2004;22:589–594. doi: 10.1038/nbt957. [DOI] [PubMed] [Google Scholar]
- 12.Aleksic M, et al. Dependence of T cell antigen recognition on T cell receptor-peptide MHC confinement time. Immunity. 2010;32:163–174. doi: 10.1016/j.immuni.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holst J, et al. Scalable signaling mediated by T cell antigen receptor-CD3 ITAMs ensures effective negative selection and prevents autoimmunity. Nat Immunol. 2008;9:658–666. doi: 10.1038/ni.1611. [DOI] [PubMed] [Google Scholar]
- 14.Irving BA, Weiss A. The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell. 1991;64:891–901. doi: 10.1016/0092-8674(91)90314-o. [DOI] [PubMed] [Google Scholar]
- 15.Deindl S, et al. Structural basis for the inhibition of tyrosine kinase activity of ZAP-70. Cell. 2007;129:735–746. doi: 10.1016/j.cell.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 16.Bergman M, et al. The human p50csk tyrosine kinase phosphorylates p56lck at Tyr-505 and down regulates its catalytic activity. EMBO J. 1992;11:2919–2924. doi: 10.1002/j.1460-2075.1992.tb05361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hermiston ML, Xu Z, Weiss A. CD45: a critical regulator of signaling thresholds in immune cells. Annu Rev Immunol. 2003;21:107–137. doi: 10.1146/annurev.immunol.21.120601.140946. [DOI] [PubMed] [Google Scholar]
- 18.Saunders AE, Johnson P. Modulation of immune cell signalling by the leukocyte common tyrosine phosphatase, CD45. Cell Signal. 2010;22:339–348. doi: 10.1016/j.cellsig.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Chen JL, et al. Structural and kinetic basis for heightened immunogenicity of T cell vaccines. J Exp Med. 2005;201:1243–1255. doi: 10.1084/jem.20042323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 21.Altan-Bonnet G, Germain RN. Modeling T cell antigen discrimination based on feedback control of digital ERK responses. PLoS Biol. 2005;3:e356. doi: 10.1371/journal.pbio.0030356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manz BN, Jackson BL, Petit RS, Dustin ML, Groves J. T-cell triggering thresholds are modulated by the number of antigen within individual T-cell receptor clusters. Proc Natl Acad Sci U S A. 2011;108:9089–9094. doi: 10.1073/pnas.1018771108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valitutti S, Dessing M, Aktories K, Gallati H, Lanzavecchia A. Sustained signaling leading to T cell activation results from prolonged T cell receptor occupancy. Role of T cell actin cytoskeleton. J Exp Med. 1995;181:577–584. doi: 10.1084/jem.181.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson KG, Bromley SK, Dustin ML, Thomas ML. A supramolecular basis for CD45 tyrosine phosphatase regulation in sustained T cell activation. Proc Natl Acad Sci U S A. 2000;97:10138–10143. doi: 10.1073/pnas.97.18.10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leupin O, Zaru R, Laroche T, Muller S, Valitutti S. Exclusion of CD45 from the T-cell receptor signaling area in antigen-stimulated T lymphocytes. Curr Biol. 2000;10:277–280. doi: 10.1016/s0960-9822(00)00362-6. [DOI] [PubMed] [Google Scholar]
- 26.Irles C, et al. CD45 ectodomain controls interaction with GEMs and Lck activity for optimal TCR signaling. Nat Immunol. 2003;4:189–197. doi: 10.1038/ni877. [DOI] [PubMed] [Google Scholar]
- 27.He X, Woodford-Thomas TA, Johnson KG, Shah DD, Thomas ML. Targeting of CD45 protein tyrosine phosphatase activity to lipid microdomains on the T cell surface inhibits TCR signaling. Eur J Immunol. 2002;32:2578–2587. doi: 10.1002/1521-4141(200209)32:9<2578::AID-IMMU2578>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 28.Choudhuri K, Wiseman D, Brown MH, Gould K, van der Merwe PA. T-cell receptor triggering is critically dependent on the dimensions of its peptide-MHC ligand. Nature. 2005;436:578–582. doi: 10.1038/nature03843. [DOI] [PubMed] [Google Scholar]
- 29.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaw AS, Dustin ML. Making the T cell receptor go the distance: a topological view of T cell activation. Immunity. 1997;6:361–369. doi: 10.1016/s1074-7613(00)80279-4. [DOI] [PubMed] [Google Scholar]
- 31.Qi SY, Groves JT, Chakraborty AK. Synaptic pattern formation during cellular recognition. Proc Natl Acad Sci U S A. 2001;98:6548–6553. doi: 10.1073/pnas.111536798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weikl TR, Lipowsky R. Pattern formation during T-cell adhesion. Biophys J. 2004;87:3665–3678. doi: 10.1529/biophysj.104.045609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coombs D, Dembo M, Wofsy C, Goldstein B. Equilibrium thermodynamics of cell-cell adhesion mediated by multiple ligand-receptor pairs. Biophys J. 2004;86:1408–1423. doi: 10.1016/S0006-3495(04)74211-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alakoskela JM, et al. Mechanisms for size-dependent protein segregation at immune synapses assessed with molecular rulers. Biophys J. 2011;100:2865–2874. doi: 10.1016/j.bpj.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burroughs NJ, Wulfing C. Differential segregation in a cell-cell contact interface: the dynamics of the immunological synapse. Biophys J. 2002;83:1784–1796. doi: 10.1016/S0006-3495(02)73944-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yokosuka T, et al. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat Immunol. 2005;6:1253–1262. doi: 10.1038/ni1272. [DOI] [PubMed] [Google Scholar]
- 37.Campi G, Varma R, Dustin ML. Actin and agonist MHC-peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J Exp Med. 2005;202:1031–1036. doi: 10.1084/jem.20051182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bunnell SC, et al. T cell receptor ligation induces the formation of dynamically regulated signaling assemblies. J Cell Biol. 2002;158:1263–1275. doi: 10.1083/jcb.200203043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.