Abstract
Background
HIV infection and alcoholism each carries liability for disruption of brain structure and function integrity. Despite the considerable prevalence of HIV-alcoholism comorbidity, few studies have examined the potentially heightened burden of disease comorbidity.
Methods
Participants were 342 men and women: 110 alcoholics, 59 with HIV infection, 65 with HIV infection and alcoholism, and 108 healthy controls. This four-group design enabled examination of the independent and combined effects of HIV infection and alcoholism in addition to other factors (AIDS-defining events, hepatitis C infection, and age) on regional brain volumes derived from T1-weighted MR images.
Results
Brain volumes, expressed as Z-scores corrected for intracranial volume and age, were measured in 20 tissue and 5 ventricular and sulcal regions. The most profound and consistent volume deficits occurred with alcohol use disorders, notable in the cortical mantle, insular and anterior cingulate cortices, thalamus, corpus callosum, and frontal sulci. The HIV-only group had smaller thalamic and larger frontal sulcal volumes than controls. HIV disease-related factors associated with greater volume abnormalities included CD4 cell-count nadir, clinical staging, history of AIDS-defining events, infection age, and current age. Longer sobriety and less lifetime alcohol consumption were predictive of attenuated brain volume abnormalities in both alcohol groups.
Conclusions
Having a “triple hit”—HIV infection with alcoholism and AIDS—had an especially poor outcome on brain structures. That longer periods of sobriety and less lifetime alcohol consumption were predictive of attenuated brain volume abnormalities encourages the inclusion of alcohol recovery efforts in HIV/AIDS therapeutic settings.
Keywords: cortex, white matter, MRI, HIV, alcohol, AIDS
INTRODUCTION
Over 33 million people are living with HIV/AIDS infection, the fifth leading cause of death worldwide (1). With effective medication, HIV-infected individuals are living longer (2, 3), but with longevity, HIV infection has the potential of interacting with other factors that militate against maintaining health in older age. Notable factors (4-6) include age-related decline itself (7) and comorbidities common to the HIV-infected population, such as history of an AIDS- defining event and alcoholism (8). Levels of per capita alcohol consumption vary widely (9), with 70 million people worldwide estimated to have an alcohol use disorder (WHO, December 2001), and growing numbers of alcoholics test positive for HIV infection (10-16).
HIV infection and alcoholism each carries significant health liability. A Danish population-based cohort study (17) reported that HIV infection in highly active antiretroviral therapy (HAART)-treated persons with detectable viral loads and CD4 counts below 200, but no other disease comorbidity, reduced survival odds to 0.58 , dropping further for HIV-infected patients with other (non-drug/alcohol) disease comorbidity (0.30), and dramatically further (0.03) for those with drug or alcohol abuse comorbidity (17).
Alcoholism itself is associated with significant morbidity (18) and mortality (19). Alcohol dependent individuals, without obvious complications from nutritional deficiencies or hepatic disorders, demonstrate cortical shrinkage (20, 21). Supratentorial brain regions affected include cortical gray and white matter (22, 23), particularly prefrontal (24, 25) and parietal (26) areas , anterior hippocampus (27-29), amygdala (30), and thalamus (31-33). Whole-brain analyses have extended this profile to include the striatolimbic reward system (30). Given the considerable comorbidity of HIV infection and alcohol abuse (10-15), their combination poses a greater public health burden than either condition alone.
Through its role in facilitating HIV-risky behaviors, excessive alcohol use is increasingly recognized as a factor in the acquisition of HIV infection in the US (34) and worldwide (35). Continued alcohol exposure after HIV infection accelerates the course of HIV-disease progression in simian models (36, 37). This connection has been challenging to establish in humans because multiple relevant factors—alcohol dosage, premorbid susceptibility, comorbid drug use (e.g., 5, 38), cigarette smoking (39), and nutritional status—cannot be controlled in clinical settings (40).
Since the initial structural brain imaging study of HIV-infected adults showing basal ganglia volume deficits (41), MRI studies have sought patterns of sparing and involvement of regional brain volumes and factors that modify brain structural outcomes. In the pre- Antiretroviral Treatment (ART) era, white matter volume deficits and ventricular and sulcal enlargement were observed (42), although greater gray than white matter volume deficits accompanied advanced CDC symptom stage (43). A two-year longitudinal MRI study indicated widespread white matter volume loss and frontal, temporal, and parietal tissue loss in virally- suppressed HIV individuals relative to controls. HIV patients receiving antiretroviral therapy without complete viral suppression exhibited volume loss in gray and white matter (44). Low CD4 cell count (45) and high peripheral blood mononuclear cell load (46) have also been predictive of local cortical thinning, particularly in frontal and temporal regions. Lower nadir CD4 T-cell counts, higher (unexpectedly) current CD4 counts, detectable viral load, hepatitis C infection, and longer exposure to ART all predicted white matter and CSF volume abnormalities (6).
HIV-infected adults who had experienced an AIDS-defining event or had AIDS Dementia Complex (ADC) have thinner primary sensory, motor, and premotor cortices (45), smaller total gray matter and parietal cortical volumes, larger total ventricular size (47), and smaller corpora callosa measured with MRI (48, 49) and microstructural compromise measured with diffusion tensor imaging (50-54). Despite the benefit of antiretroviral therapies (cf., 55), there is evidence for potential neurotoxicity of long-term use of these potent pharmacotherapies on brain structure (56). For example, HIV-infected men with longer HIV infection duration and presumably longer time on HAART had smaller basal ganglia volumes (57). Regardless of history of AIDS or presence of HIV-related cognitive impairment or dementia, brain structure- function relations have been reported, where poorer performance on cognitive or motor tests correlated with smaller regional brain volumes (45, 48, 58-62) or compromised white matter integrity (50, 51, 63).
Our previous quantitative MRI studies identified a significant role of alcoholism in contributing to thinning of the corpus callosum and enlargement of ventricular and sulcal spaces in HIV-infected adults (48) that were exacerbated by presence of an AIDS-defining event. Herein, we used a four-group design to examine the effects of HIV infection alone, alcoholism alone, and their comorbidity on regional cortical, allocortical, subcortical, and CSF volumes. We anticipated that HIV-alcoholism comorbidity would be particularly harmful to frontal and parietal cortices, striatal and thalamic structures, and the corpus callosum. In addition, we examined the effect of other comorbidities (AIDS-defining events, hepatitis C infection, and age) on regional brain volumes. This approach enabled testing of the hypothesis that HIV infection may be emerging as a cortical disease (47, 57, 64) and may interact with alcoholism comorbidity (48), age (54), and disease progression (55).
METHODS
Participants
The participants were 342 men and women (age-range matched 25-69 years): 110 alcoholics (ALC), 59 with HIV infection (HIV), 65 with HIV infection and alcoholism (HIV+ALC), and 108 normal control subjects (NCS) unaffected by either disease. Of these 342 participants, 272 were drawn from our previous report on volume differences of the ventricular system and corpus callosum (48); the remaining 70 participants were newly recruited.
Recruitment
As previously described (48), patients were recruited by referral from local outpatient HIV/AIDS and alcohol and addiction treatment centers, presentations by project staff, and distribution of flyers at community events. Control participants were recruited by referral from patient participants, Internet posting, flyers, and word of mouth. Brief screening identified exclusionary factors—history of schizophrenia, bipolar disorder, neurological disease not related to alcohol use or HIV, or inability to undergo MRI. Those meeting initial criteria were invited for a detailed assessment at our laboratory or the AIDS Community Resource Center (ACRC), where trained medical research staff informed them about the full scope of the study and obtained written informed consent. Only HIV patients with CD4 count >100 mm3 and Karnofsky score (65) >70 (can care for self but unable to carry out normal activity) were considered for study enrollment.
Clinical evaluation
Structured Clinical Interview for DSM-IV (66) was used to identify patients who met criteria for alcohol dependence or abuse; exclude subjects who met lifetime criteria for schizophrenia or bipolar disorder or for nonalcohol substance dependence or abuse within the prior 3 months; identify volunteers meetingcriteria for depressive or anxiety disorder; and confirm that prospective controls did not meet DSM-IV criteria for any Axis I disorder. Quantity of lifetime alcohol consumption and date of last drink were obtained by interview (23, 67, 68). Participants were assigned to one of four groups: (1) HIV-infected patients who had never met criteria for Alcohol Dependence or Abuse or ever consumed an average of more than 6 drinks per day for men or 4 per day for women over any 30-day period (HIV); (2) HIV-infected patients who also met criteria for Alcohol Dependence or Abuse (HIV+ALC) within the past 3 years; (3) patients who met lifetime criteria for Alcohol Dependence within the past 3 years but were not HIV-infected (ALC); and (4) a control group (NCS) who were neither HIV-infected nor ever met criteria for alcohol abuse, dependence or other Axis I diagnoses. Participants were tested for HIV status and hepatitis C; additional blood tests characterized viral load and CD4 T-cell count in HIV-positive participants.
Group characteristics
Although the four groups were similar in handedness (69) and body mass index, the patient groups had fewer years of education, lower intelligence scores (National Adult Reading Test (70)), lower socioeconomic status (71), more depressive symptoms (72), and lower Global Assessment of Functioning scores (66) than controls and were more likely to have been cigarette smokers (Table 1). The two alcohol groups reported similar lifetime alcohol consumption levels, which were about 14 times higher than in the HIV or control groups. A similar proportion of alcoholics and HIV-only subjects (24.2% in each group) tested positive for hepatitis C virus, but the proportion was higher in the comorbid group (51.5%).
Table 1. Demographic characteristics of the four study groups (N=342): mean (SD) or frequency count.
| Control (C) | Alcohol (A) | HIV (H) | HIV+ALC (HA) | Group Differences χ2 or ANOVA (p≤05) |
|
|---|---|---|---|---|---|
| Sex: M/F | 52/56 | 74/36 | 40/19 | 47/18 | M>F in patient groups p=.0031 |
| Age (yrs) | 46.2 (11.8) |
48.1 (9.4) |
45.3 (8.6) |
45.3 (8.6) |
n.s. |
| Education (yrs) | 15.7 (2.3) |
14.0 (2.5) |
13.7 (2.7) |
12.8 1 (2.1) |
C>A=H>HA |
|
Handedness score
(RH=14-30; LH=50-70) |
22.9 (12.9) |
25.4 (14.5) |
25.8 (12.8) |
25.2 (12.3) |
n.s. |
| Body mass index | 26.1 (4.7) |
26.3 (4.3) |
26.0 (4.2) |
26.2 (4.0) |
n.s. |
|
Socioeconomic status
(lower score=higher status) |
26.7 (12.3) |
33.8 (14.8) |
38.2 (14.6) |
44.3 (11.3) |
C>A=H>HA |
| NART IQ | 112.3 (7.0) |
108.8 (8.6) |
106.0 (9.7) |
105.7 (7.9) |
C>A=H=HA |
|
Beck Depression
Inventory |
2.3 (2.8) |
9.4 (8.1) |
11.5 (9.1) |
12.3 (8.2) |
C<A=H=HA |
| n= | 45 | 51 | 54 | 56 | |
|
Global Assessment
of Functioning (GAF) |
81.9 V (8.5) |
61.4 (12.7) |
71.8 (10.9) |
60.4 (9.4) |
C>H>A=HA |
| n= | 47 | 59 | 59 | 64 | |
| Alcoholism onset age | — | 23.5 (8.8) |
— | 23.4 (8.2) |
n.s. |
| HIV onset age | — | — | 33.3 (7.7) |
34.9 (7.5) |
n.s. |
| CD4 cell count | — | — | 516.3 (279.7) |
470.2 (266.7) |
n.s. |
| CD4 cell count nadir | — | — | 203.7 (153.5) |
239.2 (196.4) |
n.s. |
| Viral load | — | — | 12738.9 (34928.2) |
12991.2 (24716.0) |
n.s. |
|
Hepatits C virus)
(positive/negative) |
— | 39/16 | 43/16 | 31/34 | p=.0053 A=H<HA |
|
Lifetime alcohol
consumption (kg) |
42.0 (59.0) |
973.9 (849.6) |
96.4 (193.0) |
957.7 (912.1) |
A=HA>C=H |
| Days since last drink | — | 131.5 (169.0) |
— | 222.2 JL (397.7) |
p=.0381 A<HA |
|
Smoker
(never/current or past) |
75/29 | 30/80 | 24/35 | 9/55 | C<H<C=HA p=.0001 |
| Self-Defined Ethnicity | |||||
| Caucasian | 61 | 71 | 33 | 42 | n.s. |
| African American | 37 | 31 | 20 | 17 | |
| Other | 10 | 8 | 6 | 6 |
CD4 cell count, CD4 nadir, current viral load, and estimated age at infection were statistically similar in the two HIV groups. Each HIV-infected participant was classified as ever having had an AIDS-defining event (CD4 count below 100 cells/μL or symptoms indicative of AIDS). Each also received a CDC classification of disease severity, where A=asymptomatic HIV infection; B=symptomatic conditions, not A or C; or C=AIDS-indicator conditions. A similar proportion of HIV and HIV+ALC participants were HIV-medication naive: 7/59 HIV and 9/65 HIV+ALC (p=.95). To quantify the potential effect of antiretroviral medication regimen reported at study entry, we calculated the CNS penetration effectiveness (CPE) index (56). No patient was clinically demented, and none showed evidence of opportunistic infections. Descriptive statistics of demographic data and clinical descriptors appear in Table 1.
MRI acquisition
MR data were collected on a GE 1.5T scanner. Brain volumes were derived from coronal T1-weighted SPoiled Gradient Recalled (SPGR) images (94, 2mm thick slices; TR=26ms, TE=5ms, flip angle=30°) and dual-echo Fast Spin-Echo (FSE) images (47, 4mm thick slices; TR=7150ms, TE=12/84ms, ETL=8). MRI quantification details appear in Supplemental Information.
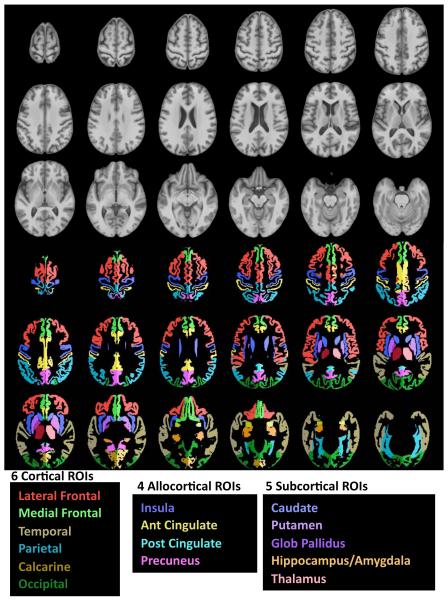
Regions of interest (ROIs)
We used 16 bilateral regions of cortex, allocortex, and subcortical structures (73) (Figure 1). Gray matter volume was computed for each cortical region, and tissue volume for each subcortical region. Also measured were CSF-filled volumes of the lateral ventricles, third ventricle, total cortical sulci, frontal sulci, and Sylvian fissures, based on ROIs drawn on the SRI24 SPGR template and reformatted into subject image space. The ventricles were identified by registration; for the cortical sulci and Sylvian fissure, the CSF segmentations from FAST (74) were multiplied against the ROIs.
Figure 1.
Top panel in gray scale: Axial slices from the SRI24 atlas of the superior (top left) to inferior (bottom right) brain regions. Bottom panel in color: 6 bilateral cortical and 4 allocortical gray matter regions and 5 subcortical tissue regions overlaid on the SRI24 atlas and color-coded by structure name.
Neuropsychological testing
Most of the HIV and HIV+ALC participants and about half the sample of controls and alcoholics completed the Digit Symbol subtest of the Wechsler Adult Intelligence Test-Revised (75). Standard administration involved transcribing symbols into a grid of empty boxes paired with a number (1-9). The standard raw score was the number of boxes correctly completed in 90 sec. Participants were also required to complete all 93 boxes; total grid completion time provided a measure of sustained attention (76).
Statistical analysis
Group differences were tested with one-factor and group-by-brain region repeated- measures analysis of variance (ANOVA); only effects involving group differences were of interest and are reported. Pair-wise comparisons were made with hypothesis-driven Scheffé tests (p≤.05), with the prediction that patient group tissue volumes would be smaller and CSF volumes larger than those of the control group. Relations between variables were tested with Pearson product-moment correlations (r) and, where appropriate, Spearman Rank Order (Rho) correlations. Correlations were conducted within each patient group and tested one-tailed, with the general hypothesis that smaller tissue volumes and larger CSF volumes would be associated with older age, greater disease burden, and poorer test performance. Significant correlates were then used in multiple regression analysis to identify correlates contributing uniquely to the variance of the brain volume of interest.
Analyses were based on regional brain volumes expressed as standardized Z-scores adjusted for variation in supratentorial intracranial volume and age measured in the control group (23, 77, 78) (See Supplemental Information for description of Z-scores).
RESULTS
Cortical Volumes
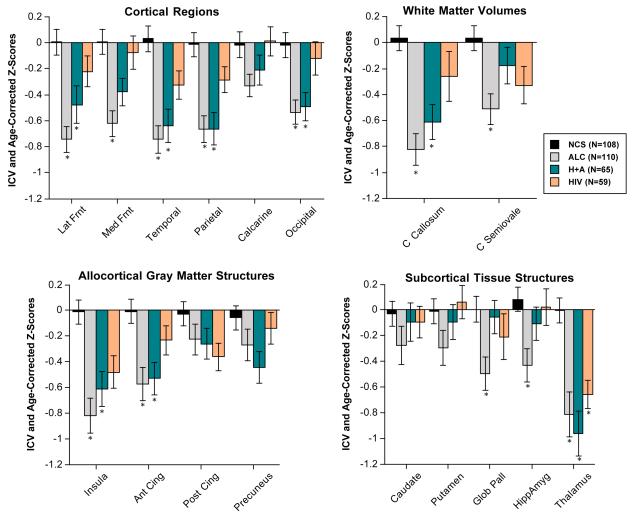
A repeated-measures ANOVA (four groups by six cortical-region gray matter volumes) yielded a significant group effect (F(3,338)=17.302, p=.0001) but no group-by-region interaction (F(15, 1690)=1.251, n.s.). Followup tests with Scheffé criterion (p≤.05) indicated volume deficits were present in the two alcoholic groups; all regions were affected except the calcarine cortex in ALC and all but the medial frontal and calcarine cortices in HIV+ALC (Figure 2). Despite showing a 0.3 to 1.0 SD deficit in five of the six regions, none of the cortical volumes of the HIV- only group met Scheffé criterion for statistically significant difference from those of the control group.
Figure 2.
Mean±S.E.M of each regional brain tissue volume, expressed as ICV- and age- corrected Z-scores, for each of the four study groups. Low scores are in the direction of tissue deficits. * denotes significant differences from controls (Scheffé posthoc tests p≤.05).
Allocortical, Subcortical, and White Matter Volumes
An ANOVA comparing the four allocortical gray matter regional volumes (insula, precuneus, and anterior and posterior cingulate cortex) of the four groups revealed a significant group effect (F(3,338)=10.076, p=.0001) and interaction (F(9,1014)=2.633, p=.0052). Scheffé tests indicated significant volume deficits in the anterior cingulate and insular regions of both alcoholic groups and a trend for an insular deficit in the HIV-only group.
When comparing the five subcortical structures measured as tissue volumes (caudate nucleus, putamen, globus pallidus, hippocampus/amygdala, and thalamus) across the four subject groups, the group effect (F(3,338)=7.3729, p=.0001) and interaction (F(12,1352)=3.2914, p=.001) were significant. Here, the ALC group had volume deficits of the globus pallidus, hippocampus/amygdala, and thalamus, whereas the two HIV-infected groups had volume deficits in the thalamus only (Figure 2).
The ANOVA for the two white matter measures (Figure 2) indicated a group effect (F(3,338)=8.681, p=.0001) and interaction (F(3,338)=3.600, p=.0138). Compared with controls, the ALC and HIV+ALC groups had volume deficits of the corpus callosum, but only the ALC group had significantly smaller centrum semiovale volumes than controls.
Sulcal and Ventricular Volumes
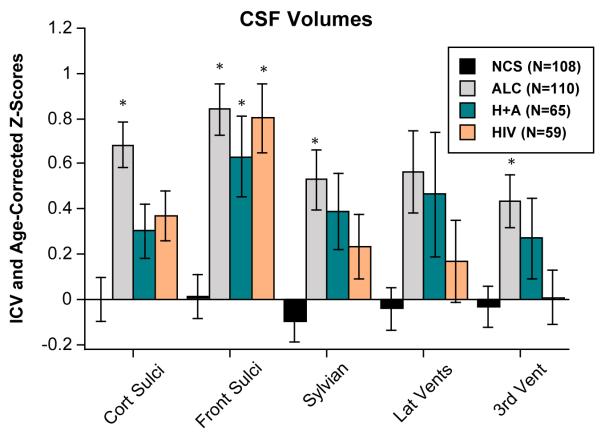
In contrast with tissue measures, CSF measures revealed volume abnormalities in all three patient groups (Figure 3). Specifically, an ANOVA comparing the frontal and Sylvian CSF volumes across the four groups yielded a significant group effect (F(3,338)=12.684, p=.0001). Although the volume enlargement of the Sylvian fissure was significant only in the ALC group, the ALC, HIV, and HIV+ALC groups had larger volumes than controls. For the ventricles, only the ALC group showed significant enlargement, which was restricted to the third ventricle.
Figure 3.
Mean±S.E.M of each regional ventricular and sulcal volume, expressed as ICV- and age-corrected Z-scores, for each of the four study groups. High scores are in the direction of CSF-space expansion. * denotes significant differences from controls (Scheffé posthoc tests p≤.05).
Relations between Disease Variables and Regional Brain Volume Abnormalities
HIV-infection Variables
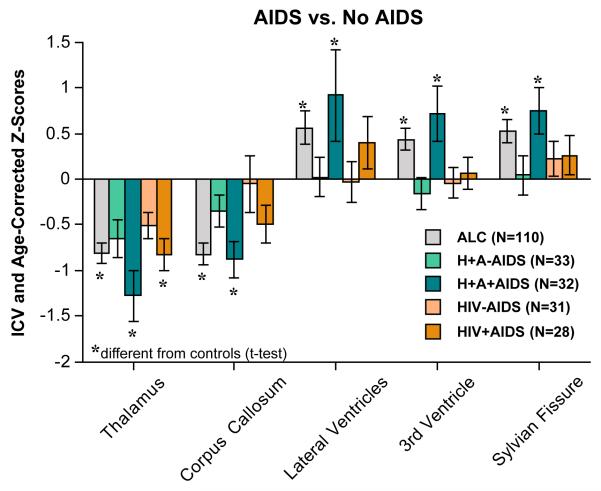
These analyses examined differences in regional brain volumes related to HIV-infection variables in the two HIV groups (HIV and HIV+ALC). ANOVAs using presence vs. absence of a lifetime AIDS-defining event and the two HIV groups revealed that, irrespective of diagnostic group, smaller thalamic (p=.0244) and callosal (p=.0351) volumes and larger CSF volumes [lateral (p=.0475) and third (p=.0246) ventricles ] occurred in the HIV groups with a history of an AIDS-defining event. Followup t-tests showed that HIV+ALC+AIDS had smaller callosal volumes (p=.0446) and larger Sylvian (p=.0355) and third ventricular (p=.0141) volumes than HIV+ALC without AIDS (Figure 4). Further analysis using the CDC 3-category classification identified a disproportionately large volume deficit in parietal cortex of the HIV- only group in the C (−.82 SD) relative to the A (−.14 SD) and B (−.13 SD) category groups (p=.0344).
Figure 4.
Mean±S.E.M of each regional brain volume, expressed as ICV- and age- corrected Z-scores, for each of the patient study groups divided by presence or absence of an AIDS-defining event. Low scores for tissue volumes and high scores for CSF volumes are in the direction of abnormality. * denotes significant differences from controls (t-tests p≤.05).
Although current CD4 cell count did not correlate with any regional volumes in either HIV group, lower CD4 nadir showed modest correlations with smaller volumes of the putamen (r=.32, p=.0324) and thalamus (r=.31, p=.0384) in the HIV-only group. Followup multiple regressions, considering the potential additional influence of age at scan and age at HIV infection on these brain volumes, found that only CD4 nadir endured as a significant unique predictor of putamen volumes (p=.0179).
HIV-only subjects showed a relation between higher viral load and larger Sylvian fissure volumes (Rho=.32, p=.0151). Higher CPE scores were modestly related to smaller total white matter volumes of the HIV (Rho=−.28, p=.0318) and HIV+ALC (Rho=−.25, p=.0475) groups.
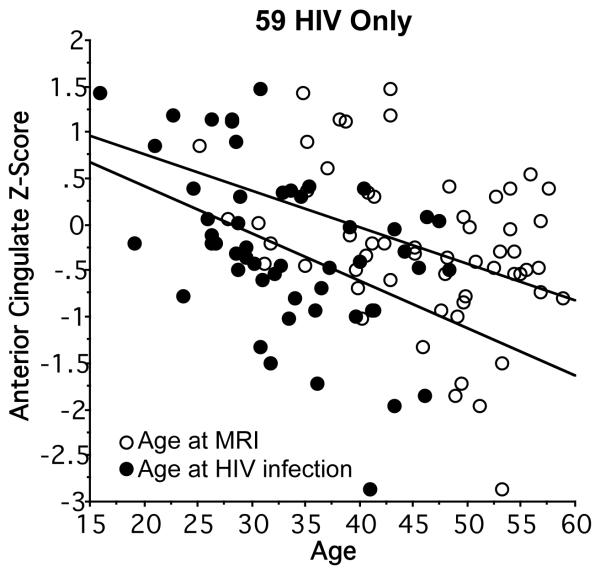
We next used bivariate and then multiple regression to test whether any regional brain volumes were related to age at MRI or age at HIV infection. Within the HIV-only group, both older current age (r=−.40, p=.0017) and estimated age at infection (r=−.45, p=.001) correlated with smaller age-adjusted volumes of the anterior cingulate cortex (Figure 5). Together, age and age at HIV infection accounted for 22% of the anterior cingulate volume variance. In multiple regression analysis, age at HIV onset was a significant unique predictor (p=.0354) over age (p=.3516); neither age variable uniquely predicted age-adjusted regional volumes in the HIV+ALC group.
Figure 5.
Linear regressions showing older age at MRI and older age at HIV-infection onset correlating with anterior cingulate ICV- and age-corrected Z-scores in the HIV-only group.
Alcoholism and Other Disease Variables
Age and estimated lifetime alcohol consumption each correlated with a number of regional volumes in the ALC group. Because these variables can be confounded, we conducted a series of multiple regression analyses, which entered age and lifetime alcohol consumption as predictors of regional brain volumes. The results in the ALC-only group indicated that together, age (despite use of age-adjusted Z- scores) and alcohol consumption accounted for a significant portion of the variance in five brain regions, with the unique contribution of each variable differing by region: age at MRI accounted over and above total alcohol consumption for volumes of the medial frontal cortex (age p=.0073; alcohol p=.1208), thalamus (age p=.0014; alcohol p=.0651), and cortical sulci (age p=.0154; alcohol p=.7354); by contrast, total alcohol consumption accounted over and above age for volumes of the anterior cingulum (age p=.5596; alcohol p=.0139) and insula (age p=.5130; alcohol p=.0026). Unlike these findings in the ALC-only group, the HIV+ALC group did not show simple correlations between age or amount of lifetime alcohol consumption and regional brain volumes, and when used together, age and lifetime alcohol consumption were not significant predictors of brain volumes in any region.
Seropositivity for hepatitis C virus was highly prevalent in all three disease groups (Table 1), but its presence was related to only a few regional volume abnormalities in the HIV group and to none in the HIV+ALC or ALC groups. The HIV subgroup with hepatitis C virus had smaller anterior cingulate volumes (−.69 SD; p=.0101) and larger frontal sulcal volumes (Z-score=1.37 SD; p=.0206) than the HIV subgroup without hepatitis C (anterior cingulate volume Z-score= −.06 SD; frontal sulcal volume Z-score= .59 SD).
Shorter periods of sobriety correlated with larger volumes of the third ventricle in the ALC (Rho=−.24, p=.0118) and HIV+ALC (Rho=−.28, p=.0242) groups and Sylvian fissures in the HIV+ALC group (Rho=−.31, p=.0147). Regional brain volume abnormalities were not related to history of smoking in any diagnostic group.
Relations between Neuropsychological Test Performance and Brain Volume Abnormalities
One-way ANOVAs and followup Scheffé tests identified group effects (p=.0001) for both measures of the Digit Symbol Substitution test (Table 2). For the 90 sec. output, the ALC and HIV+ALC groups performed more poorly than the HIV or control groups. The pattern of group differences was similar for grid completion. Within-group correlations used a directional family-wise Bonferroni correction for multiple comparisons (20 brain regional volumes) and required p=.005 (α=.05, one-tailed) for statistical significance. Within the HIV+ALC group, lower output in 90 sec. and slower time to grid completion correlated with smaller volumes of occipital cortex and hippocampus/amygdala and larger volumes of frontal sulci and third ventricle (Table 3). Additional correlations were present between longer grid completion time and smaller insula and precuneus volumes. No correlation was significant in the HIV, ALC, or control groups.
Table 2. Digit Symbol Test Scores of Each Group.
| Group ANOVA | |||||
|---|---|---|---|---|---|
| Control (C) | ALC (A) | HIV (H) | HIV+ALC (HA) |
Sheffé (p≤.05) | |
|
Digit Symbol (output)
90 sec. raw score |
|||||
| mean= | 54.8 | 47 | 50.1 | 41.7 | 0.0001 |
| SE= | 2.10 | 1.64 | 1.80 | 9.74 | C=H>A=HA |
| n= | 38 | 41 | 40 | 54 | |
| Grid completion time (sec). | |||||
| mean= | 152.1 | 172.7 | 164.6 | 194.9 | 0.0001 |
| SE= | 6.25 | 5.44 | 5.68 | 6.74 | C=H<HA * |
| n= | 38 | 41 | 40 | 54 |
The alcoholic group did not differ significantly from the remaining three groups.
Table 3. Digit Symbol Score Correlations with Brain Volumes: HIV+ALC Group.
| HIV+ALC | Digit Symbol | |||
|---|---|---|---|---|
| 90 sec. | Time to Complete | |||
| Brain Region | r | p | r | p |
| Lateral Frontal | .04 | 0.75 | -.09 | 0.51 |
| Medial Frontal | .16 | 0.24 | -.13 | 0.36 |
| Temporal | .20 | 0.14 | -.30 | 0.0296 |
| Parietal | .26 | 0.059 | -.37 | 0.006 |
| Calcarine | .32 | 0.019 | -.32 | 0.0172 |
| Occipital | .41 | 0.0019 | -.40 | 0.0027 |
| Caudate/Putamen | .10 | 0.46 | .05 | 0.74 |
| Globus Pallidus | .11 | 0.41 | .22 | 0.12 |
| Insula | .34 | 0.012 | -.39 | 0.0035 |
| Anterior Cingulum | .17 | 0.21 | -.22 | 0.11 |
| Posterior Cingulum | .26 | 0.0595 | -.30 | 0.0299 |
| Precuneus | .30 | 0.0258 | -.39 | 0.0038 |
| Hipp/Amyg | .38 | 0.0042 | -.39 | 0.0032 |
| Thalamus | .25 | 0.065 | -.25 | 0.064 |
| Corpus Callosum | .04 | 0.80 | -.13 | 0.36 |
| Centrum Semiovale | .07 | 0.61 | -.17 | 0.21 |
| Frontal Sulci | -.38 | 0.005 | .48 | 0.0002 |
| Sylvian Fissure | -.20 | 0.14 | .30 | 0.0295 |
| Lateral Ventricles | -.20 | 0.14 | .21 | 0.12 |
| Third Ventricle | -.37 | 0.0055 | .35 | 0.0087 |
Family-wise Bonferroni correction for 20 comparisons in bold: p<.005
DISCUSSION
The most profound and consistent volume deficits were identified in the two groups with alcohol use disorders. Regions showing volume abnormalities in both groups were the lateral frontal, temporal, parietal, and occipital cortices, insular and anterior cingulate cortices, thalamus, corpus callosum, and frontal sulci. By contrast, significant volume abnormalities selective to the alcoholism-only group included the medial frontal cortex, but those in the HIV- only group were limited to the thalamus and frontal sulci. Thus, a history of alcoholism greatly expanded the scope of deficit present in HIV-infected individuals.
HIV disease-related factors associated with greater volume abnormalities included CD4 cell-count nadir, clinical staging, and history of an AIDS-defining event. Brain regional abnormalities particularly affected by an AIDS-defining event were the ventricular system, which we had previously observed in a subset of these subjects (79). Newly identified is the vulnerability of the thalamus to HIV infection and CD4 nadir. Having a “triple hit”—HIV infection with alcoholism with AIDS—had an especially poor outcome on brain structures, including the corpus callosum, Sylvian fissure, and third ventricle. Possibly contributing to the plight of the comorbid group are their disadvantaged socioeconomic status and extra HCV burden, factors that were excessive relative to the single-disease groups.
Within the HIV-only group, clinical categorization by CDC staging revealed that those with the most advanced classification (C) had the largest volume deficits in parietal cortex (cf., 47, 80). This association lends converging validity to this staging process with regard to its neural substrates and also supports emerging concepts of HIV infection as invading the cortex (45, 54, 61, 81). Two factors have the potential of promoting brain structural deterioration in HIV-infection even without concomitant alcohol use disorders. Firstly, pharmacological agents used in ART can have neurotoxic effects (82). In the current cohort, smaller white matter volumes of the centrum semiovale were related to higher CPE regardless of alcoholism history (cf., 6, 44). Secondly, age may be relevant: smaller volumes of the anterior cingulate cortex were related to older age at HIV infection in the HIV-only group. These findings are consistent with the neuroinflammatory response of brain tissue (for review, 83), especially white matter (84), to a subset of antiviral agents and the greater vulnerability of the aging brain to such insults (85), exacerbated by alcohol (83). The absence of volume deficits in basal ganglia structures was surprising, given that striatal dysmorphology is commonly reported in HIV- infected groups (41,57). Whether the lack of current striatal volume deficits could be attributable to successful viral suppression or other factors promoting health, lower CD4 cell- count nadir, which occurred prior to MRI, was predictive of smaller putamen and also thalamic volumes. This relation suggests the possibility that these subcortical structures are selectively vulnerable to immunological compromise associated with severe decline in systemic CD4 cell population.
In the ALC-only group, significant volume deficits involved all four cortical lobes, insula, anterior cingulate cortex, globus pallidus, hippocampus, thalamus, and corpus callosum. Abnormally large CSF volumes in the alcoholics were present in the cortical sulci, notably the frontal and Sylvian regions and third ventricle. In contrast with the HIV-only group, the HIV+ALC group showed widespread abnormalities similar to those observed in the ALC-only group that included lateral frontal, temporal, parietal, occipital, insular, anterior cingulate cortical regions, thalamus, corpus callosum, and frontal sulci. Further evidence for an alcoholism effect was the presence of modest correlations between greater third ventricular volumes in ALC and HIV+ALC with shorter periods of sobriety and between smaller thalamic volumes in the ALC group with greater lifetime alcohol consumption. That the comorbid group did not show even greater volume abnormalities than the ALC group could be attributable to the longer sobriety of the comorbid group, allowing time for volume recovery.
The functional meaning of volume abnormalities was challenged with the Digit Symbol Substitution test, which assesses several components of cognitive and motor processes commonly impaired with HIV infection (e.g., 62, 86, 87) or alcoholism (e.g., 76, 88, 89, 90). Processes include directed and sustained attention, hand-eye coordination involving working memory for transcribed symbols, and psychomotor speed (cf., 76). Although deficits in symbol output and time to grid completion were present in the two alcoholism groups, relations between poorer performance scores and greater abnormalities in regional brain volumes were present only in the comorbid group. The brain regions involved in these correlations represented visual memory circuitry nodes (occipital cortex, hippocampus/amygdala, and third ventricle) and nodes of attentional control (frontal sulci, insula, and precuneus), areas likely to participate in digit-symbol transcription under continuous performance conditions.
The research strategy taken herein prescribed direct examination of alcoholism rather than “controlling for” or excluding it as a nuisance variable in identifying factors contributing to the neural consequences of HIV infection (cf., 91). The high prevalence of alcohol use disorders in the HIV-infected community (16), its negative effects on response to medication and compliance (8), and its properties in promoting HIV transmission (92, 93) provide the imperative to gain knowledge about the independent and combined effects of alcoholism and HIV infection. Recent evidence indicates that early detection of HIV infection when coupled with urgent treatment can reduce progression of the virus or even eradicate the infection (47). Individuals with alcohol use disorders who become infected with HIV are at heightened risk for failing to detect their condition for prolonged periods, thereby missing a critical window for optimal treatment efficacy (8). That longer periods of sobriety and lower lifetime alcohol consumption levels were predictive of attenuated brain volume abnormalities further encourages inclusion of alcohol recovery efforts in HIV/AIDS therapeutic settings (94).
Supplementary Material
Acknowledgment
The authors owe great thanks to Crystal Caldwell for exceptional efforts in recruitment and screening.
Support
AA017347, AA005965, AA012388, AA010723, AA017168, EB008381
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions A.P. and T.R. analyzed the MRI data and participated in manuscript writing and figure preparation. M.J.R. and S.A.S. provided clinical and neuropsychological data for analysis and editorial comments on the manuscript. C.A.K. and S.D. provided patient referrals, HIV infection information, and editorial comments on the manuscript. E.V.S. performed statistical analysis, figure preparation, and manuscript writing. E.V.S., the corresponding author, had full access to all data in the study and takes final responsibility for the decision to submit the paper for publication.
Conflicts of interest All authors report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.UNAIDS . Report on the Global HIV/AIDS Epidemic: Executive Summary. UNAIDS; Geneva, Switzerland: 2008. [Google Scholar]
- 2.Goodkin K, Wilkie FL, Concha M, Hinkin CH, Symes S, Baldewicz TT, et al. Aging and neuro-AIDS conditions and the changing spectrum of HIV-1-associated morbidity and mortality. J Clin Epidemiol. 2001;54(Suppl 1):S35–43. doi: 10.1016/s0895-4356(01)00445-0. [DOI] [PubMed] [Google Scholar]
- 3.Hooshyar D, Hanson DL, Wolfe M, Selik RM, Buskin SE, McNaghten AD. Trends in perimortal conditions and mortality rates among HIV-infected patients. Aids. 2007;21:2093–2100. doi: 10.1097/QAD.0b013e3282e9a664. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez R, Cherner M. Co-factors in HIV neurobehavioural disturbances: substance abuse, hepatitis C and aging. Int Rev Psychiatry. 2008;20:49–60. doi: 10.1080/09540260701872028. [DOI] [PubMed] [Google Scholar]
- 5.Martin-Thormeyer EM, Paul RH. Drug abuse and hepatitis C infection as comorbid features of HIV associated neurocognitive disorder: neurocognitive and neuroimaging features. Neuropsychol Rev. 2009;19:215–231. doi: 10.1007/s11065-009-9101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jernigan TL, Archibald SL, Fennema-Notestine C, Taylor MJ, Theilmann RJ, Julaton MD, et al. Clinical factors related to brain structure in HIV: the CHARTER study. J Neurovirol. 2011;17:248–257. doi: 10.1007/s13365-011-0032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hardy DJ, Vance DE. The neuropsychology of HIV/AIDS in older adults. Neuropsychol Rev. 2009;19:263–272. doi: 10.1007/s11065-009-9087-0. [DOI] [PubMed] [Google Scholar]
- 8.Bryant KJ, Nelson S, Braithwaite RS, Roach D. Integrating HIV/AIDS and alcohol research. Alcohol Research & Health. 2010;33:167–178. [PMC free article] [PubMed] [Google Scholar]
- 9.Room R, Babor T, Rehm J. Alcohol and public health. Lancet. 2005;365:519–530. doi: 10.1016/S0140-6736(05)17870-2. [DOI] [PubMed] [Google Scholar]
- 10.Bonacini M. Alcohol use among patients with HIV infection. Ann Hepatol. 2011;10:502–507. [PubMed] [Google Scholar]
- 11.Miguez MJ, Shor-Posner G, Morales G, Rodriguez A, Burbano X. HIV treatment in drug abusers: impact of alcohol use. Addict Biol. 2003;8:33–37. doi: 10.1080/1355621031000069855. [DOI] [PubMed] [Google Scholar]
- 12.Cook RL, Sereika SM, Hunt SC, Woodward WC, Erlen JA, Conigliaro J. Problem drinking and medication adherence among persons with HIV infection. J Gen Intern Med. 2001;16:83–88. doi: 10.1111/j.1525-1497.2001.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samet JH, Phillips SJ, Horton NJ, Traphagen ET, Freedberg KA. Detecting alcohol problems in HIV-infected patients: use of the CAGE questionnaire. AIDS Res Hum Retroviruses. 2004;20:151–155. doi: 10.1089/088922204773004860. [DOI] [PubMed] [Google Scholar]
- 14.Conigliaro J, Justice AC, Gordon AJ, Bryant K. Role of alcohol in determining human immunodeficiency virus (HIV)-relevant outcomes: A conceptual model to guide the implementation of evidence-based interventions into practice. Med Care. 2006;44:S1–6. doi: 10.1097/01.mlr.0000223659.36369.cf. [DOI] [PubMed] [Google Scholar]
- 15.Samet JH, Walley AY, Bridden C. Illicit drugs, alcohol, and addiction in human immunodeficiency virus. Panminerva Med. 2007;49:67–77. [PubMed] [Google Scholar]
- 16.Justice A, Sullivan L, Fiellin D. HIV/AIDS, comorbidity, and alcohol: Can we make a difference? Alcohol Research & Health. 2010;33:258–266. [PMC free article] [PubMed] [Google Scholar]
- 17.Obel N, Omland LH, Kronborg G, Larsen CS, Pedersen C, Pedersen G, et al. Impact of non-HIV and HIV risk factors on survival in HIV-infected patients on HAART: a population-based nationwide cohort study. PLoS ONE. 2011;6:e22698. doi: 10.1371/journal.pone.0022698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rehm J, Room R, Graham K, Monteiro M, Gmel G, Sempos CT. The relationship of average volume of alcohol consumption and patterns of drinking to burden of disease: an overview. Addiction. 2003;98:1209–1228. doi: 10.1046/j.1360-0443.2003.00467.x. [DOI] [PubMed] [Google Scholar]
- 19.Katcher BS, Reiter RB, Aragon TJ. Estimating alcohol-related premature mortality in San Francisco: use of population-attributable fractions from the global burden of disease study. BMC Public Health. 2010;10:682. doi: 10.1186/1471-2458-10-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang GJ, Volkow ND, Roque CT, Cestaro VL, Hitzemann RJ, Cantos EL, et al. Functional importance of ventricular enlargement and cortical atrophy in healthy subjects and alcoholics as assessed with PET, MR imaging, and neuropsychologic testing. Radiology. 1993;186:59–65. doi: 10.1148/radiology.186.1.8416587. [DOI] [PubMed] [Google Scholar]
- 21.Hommer DW, Momenan R, Kaiser E, Rawlings RR. Evidence for a gender-related effect of alcoholism on brain volumes. Am J Psychiatry. 2001;158:198–204. doi: 10.1176/appi.ajp.158.2.198. [DOI] [PubMed] [Google Scholar]
- 22.Jernigan TL, Butters N, DiTraglia G, Schafer K, Smith T, Irwin M, et al. Reduced cerebral grey matter observed in alcoholics using magnetic resonance imaging. Alcoholism: Clinical and Experimental Research. 1991;15:418–427. doi: 10.1111/j.1530-0277.1991.tb00540.x. [DOI] [PubMed] [Google Scholar]
- 23.Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, Lane B, et al. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: A quantitative MRI study. Alcoholism: Clinical and Experimental Research. 1992;16:1078–1089. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- 24.Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcoholism: Clinical and Experimental Research. 1997;21:521–529. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- 25.Cardenas VA, Studholme C, Gazdzinski S, Durazzo TC, Meyerhoff DJ. Deformation-based morphometry of brain changes in alcohol dependence and abstinence. Neuroimage. 2007;34:879–887. doi: 10.1016/j.neuroimage.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fein G, Di Sclafani V, Cardenas VA, Goldmann H, Tolou-Shams M, Meyerhoff DJ. Cortical gray matter loss in treatment-naive alcohol dependent individuals. Alcoholism: Clinical and Experimental Research. 2002;26:558–564. [PMC free article] [PubMed] [Google Scholar]
- 27.Agartz I, Momenan R, Rawlings RR, Kerich MJ, Hommer DW. Hippocampal volume in patients with alcohol dependence. Arch Gen Psychiatry. 1999;56:356–363. doi: 10.1001/archpsyc.56.4.356. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Anterior hippocampal volume deficits in nonamnesic, aging chronic alcoholics. Alcoholism: Clinical and Experimental Research. 1995;19:110–122. doi: 10.1111/j.1530-0277.1995.tb01478.x. [DOI] [PubMed] [Google Scholar]
- 29.Beresford TP, Arciniegas DB, Alfers J, Clapp L, Martin B, Du Y, et al. Hippocampus volume loss due to chronic heavy drinking. Alcohol Clin Exp Res. 2006;30:1866–1870. doi: 10.1111/j.1530-0277.2006.00223.x. [DOI] [PubMed] [Google Scholar]
- 30.Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, et al. Decreased Volume of the Brain Reward System in Alcoholism. Biol Psychiatry. 2008;64:192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcoholism: Clinical and Experimental Research. 2005;29:1590–1600. doi: 10.1097/01.alc.0000179368.87886.76. [DOI] [PubMed] [Google Scholar]
- 32.Sullivan EV. Compromised pontocerebellar and cerebellothalamocortical systems: speculations on their contributions to cognitive and motor impairment in nonamnesic alcoholism. Alcoholism: Clinical and Experimental Research. 2003;27:1409–1419. doi: 10.1097/01.ALC.0000085586.91726.46. [DOI] [PubMed] [Google Scholar]
- 33.Chanraud S, Martelli C, Delain F, Kostogianni N, Douaud G, Aubin HJ, et al. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology. 2007;32:429–438. doi: 10.1038/sj.npp.1301219. [DOI] [PubMed] [Google Scholar]
- 34.Howe CJ, Cole SR, Ostrow DG, Mehta SH, Kirk GD. A prospective study of alcohol consumption and HIV acquisition among injection drug users. AIDS. 2011;25:221–228. doi: 10.1097/QAD.0b013e328340fee2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fritz K, Morojele N, Kalichman S. Alcohol: the forgotten drug in HIV/AIDS. Lancet. 2010;376:398–400. doi: 10.1016/S0140-6736(10)60884-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bagby GJ, Zhang P, Purcell JE, Didier PJ, Nelson S. Chronic binge ethanol consumption accelerates progression of simian immunodeficiency virus disease. Alcohol Clin Exp Res. 2006;30:1781–1790. doi: 10.1111/j.1530-0277.2006.00211.x. [DOI] [PubMed] [Google Scholar]
- 37.Nelson S, Bagby GJ. Alcohol and HIV Infection. Trans Am Clin Climatol Assoc. 2010;122:244–253. [PMC free article] [PubMed] [Google Scholar]
- 38.Jernigan TL, Gamst AC, Archibald SL, Fennema-Notestine C, Mindt MR, Marcotte TL, et al. Effects of methamphetamine dependence and HIV infection on cerebral morphology. Am J Psychiatry. 2005;162:1461–1472. doi: 10.1176/appi.ajp.162.8.1461. [DOI] [PubMed] [Google Scholar]
- 39.Durazzo TC, Rothlind JC, Cardenas VA, Studholme C, Weiner MW, Meyerhoff DJ. Chronic cigarette smoking and heavy drinking in human immunodeficiency virus: consequences for neurocognition and brain morphology. Alcohol. 2007;41:489–501. doi: 10.1016/j.alcohol.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hahn JA, Samet JH. Alcohol and HIV Disease Progression: Weighing the Evidence. Curr HIV/AIDS Rep. 2010;7:226–233. doi: 10.1007/s11904-010-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aylward EH, Henderer JD, McArthur JC, Brettschneider PD, Harris GJ, Barta PE, et al. Reduced basal ganglia volume in HIV-1-associated dementia: results from quantitative neuroimaging. Neurology. 1993;43:2099–2104. doi: 10.1212/wnl.43.10.2099. [DOI] [PubMed] [Google Scholar]
- 42.Stout J, Ellis R, Jernigan T, Archibald S, Abramson I, Wolfson T, et al. Progressive cerebral volume loss in human immunodeficiency virus infection: a longitudinal volumetric magnetic resonance imaging study. Arch Neurol. 1998;55:161–168. doi: 10.1001/archneur.55.2.161. [DOI] [PubMed] [Google Scholar]
- 43.Di Sclafani V, Mackay RD, Meyerhoff DJ, Norman D, Weiner MW, Fein G. Brain atrophy in HIV infection is more strongly associated with CDC clinical stage than with cognitive impairment. Journal of International Neuropsychological Society. 1997;3:276–287. [PMC free article] [PubMed] [Google Scholar]
- 44.Cardenas V, Meyerhoff D, Studholme C, Kornak J, Rothlind J, Lampiris H, et al. Evidence for ongoing brain injury in human immunodeficiency virus-positive patients treated with antiretroviral therapy. J Neurovirol. 2009:1–10. doi: 10.1080/13550280902973960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thompson PM, Dutton RA, Hayashi KM, Toga AW, Lopez OL, Aizenstein HJ, et al. Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T lymphocyte decline. Proc Natl Acad Sci U S A. 2005;102:15647–15652. doi: 10.1073/pnas.0502548102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kallianpur KJ, Kirk GR, Sailasuta N, Valcour V, Shiramizu B, Nakamoto BK, et al. Regional Cortical Thinning Associated with Detectable Levels of HIV DNA. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohen RA, Harezlak J, Schifitto G, Hana G, Clark U, Gongvatana A, et al. Effects of nadir CD4 count and duration of human immunodeficiency virus infection on brain volumes in the highly active antiretroviral therapy era. J Neurovirol. 2010;16:25–32. doi: 10.3109/13550280903552420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pfefferbaum A, Rosenbloom MJ, Rohlfing T, Adalsteinsson E, Kemper CA, Deresinski S, et al. Contribution of alcoholism to brain dysmorphology in HIV infection: Effects on the ventricles and corpus callosum. Neuroimage. 2006;33:239–251. doi: 10.1016/j.neuroimage.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 49.Thompson PM, Dutton RA, Hayashi KM, Lu A, Lee SE, Lee JY, et al. 3D mapping of ventricular and corpus callosum abnormalities in HIV/AIDS. Neuroimage. 2006;31:12–23. doi: 10.1016/j.neuroimage.2005.11.043. [DOI] [PubMed] [Google Scholar]
- 50.Pfefferbaum A, Rosenbloom MJ, Rohlfing T, Kemper CA, Deresinski S, Sullivan EV. Frontostriatal fiber bundle compromise in HIV infection without dementia. AIDS. 2009;23:1977–1985. doi: 10.1097/QAD.0b013e32832e77fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pfefferbaum A, Rosenbloom MJ, Adalsteinsson E, Sullivan EV. Diffusion tensor imaging with quantitative fiber tracking in HIV infection and alcoholism comorbidity: Synergistic white matter damage. Brain. 2007;130:48–64. doi: 10.1093/brain/awl242. [DOI] [PubMed] [Google Scholar]
- 52.Hoare J, Fouche JP, Spottiswoode B, Joska JA, Schoeman R, Stein DJ, et al. White matter correlates of apathy in HIV-positive subjects: A Diffusion Tensor Imaging study. J Neuropsychiatry Clin Neurosci. 2010;22:313–320. doi: 10.1176/jnp.2010.22.3.313. [DOI] [PubMed] [Google Scholar]
- 53.Gongvatana A, Schweinsburg BC, Taylor MJ, Theilmann RJ, Letendre SL, Alhassoon OM, et al. White matter tract injury and cognitive impairment in human immunodeficiency virus-infected individuals. J Neurovirol. 2009;15:187–195. doi: 10.1080/13550280902769756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Towgood KJ, Pitkanen M, Kulasegaram R, Fradera A, Kumar A, Soni S, et al. Mapping the brain in younger and older asymptomatic HIV-1 men: Frontal volume changes in the absence of other cortical or diffusion tensor abnormalities. Cortex. 2011 doi: 10.1016/j.cortex.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 55.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC, et al. Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65:65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Becker JT, Sanders J, Madsen SK, Ragin A, Kingsley L, Maruca V, et al. Subcortical brain atrophy persists even in HAART-regulated HIV disease. Brain Imaging Behav. 2011;5:77–85. doi: 10.1007/s11682-011-9113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Castelo JM, Courtney MG, Melrose RJ, Stern CE. Putamen hypertrophy in nondemented patients with human immunodeficiency virus infection and cognitive compromise. Arch Neurol. 2007;64:1275–1280. doi: 10.1001/archneur.64.9.1275. [DOI] [PubMed] [Google Scholar]
- 59.Paul RH, Ernst T, Brickman AM, Yiannoutsos CT, Tate DF, Cohen RA, et al. Relative sensitivity of magnetic resonance spectroscopy and quantitative magnetic resonance imaging to cognitive function among nondemented individuals infected with HIV. J Int Neuropsychol Soc. 2008;14:725–733. doi: 10.1017/S1355617708080910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Becker JT, Maruca V, Kingsley LA, Sanders JM, Alger JR, Barker PB, et al. Factors affecting brain structure in men with HIV disease in the post-HAART era. Neuroradiology. 2011 doi: 10.1007/s00234-011-0854-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kuper M, Rabe K, Esser S, Gizewski ER, Husstedt IW, Maschke M, et al. Structural gray and white matter changes in patients with HIV. J Neurol. 2011;258:1066–1075. doi: 10.1007/s00415-010-5883-y. [DOI] [PubMed] [Google Scholar]
- 62.Sullivan EV, Rosenbloom MJ, Rohlfing T, Kemper CA, Deresinski S, Pfefferbaum A. Pontocerebellar contribution to ataxia and psychomotor slowing in HIV infection without dementia. Brain Imaging and Behavior. 2011;5:12–24. doi: 10.1007/s11682-010-9107-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tate DF, Conley J, Paul RH, Coop K, Zhang S, Zhou W, et al. Quantitative diffusion tensor imaging tractography metrics are associated with cognitive performance among HIV-infected patients. Brain Imaging Behav. 2010;4:68–79. doi: 10.1007/s11682-009-9086-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cohen RA, Gongvatana A. The persistence of HIV-associated neurocognitive dysfunction and the effects of comorbidities. Neurology. 2011;75:2052–2053. doi: 10.1212/WNL.0b013e318200d833. [DOI] [PubMed] [Google Scholar]
- 65.Karnofsky DA. The clinical evaluation of chemotherapeutic agents in cancer. In: MacLeod CM, editor. Evaluation of Chemotherapeutic Agents. Columbia University Press; New York: 1949. pp. 191–205. [Google Scholar]
- 66.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) Version 2.0. Biometrics Research Department, New York State Psychiatric Institute; New York, NY: 1998. [Google Scholar]
- 67.Skinner HA. Development and Validation of a Lifetime Alcohol Consumption Assessment Procedure. Addiction Research Foundation; Toronto, Canada: 1982. [Google Scholar]
- 68.Skinner HA, Sheu WJ. Reliability of alcohol use indices: The lifetime drinking history and the MAST. J Stud Alcohol. 1982;43:1157–1170. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- 69.Crovitz HF, Zener KA. Group test for assessing hand and eye dominance. Am J Psychol. 1962;75:271–276. [PubMed] [Google Scholar]
- 70.Nelson HE. The National Adult Reading Test (NART) Nelson Publishing Company; Windsor, Canada: 1982. [Google Scholar]
- 71.Hollingshead AB, Redlich FC. Social Class and Mental Illness. John Wiley and Sons; New York: 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- 73.Pfefferbaum A, Chanraud S, Pitel A-L, Müller-Oehring EM, Shankaranarayanan A, Alsop D, et al. Cerebral blood flow in posterior cortical nodes of the default mode network decreases with task engagement but remains higher than in most brain regions. Cereb Cortex. 2011;21:233–244. doi: 10.1093/cercor/bhq090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smith S. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wechsler D. Wechsler Adult Intelligence Scale - Revised. The Psychological Corporation; San Antonio, TX: 1981. [Google Scholar]
- 76.Sassoon SA, Fama R, Rosenbloom MJ, O’Reilly A, Pfefferbaum A, Sullivan EV. Component cognitive and motor processes of the Digit Symbol Test: Differential deficits in alcoholism, HIV infection and their comorbidity. Alcoholism: Clinical and Experimental Research. 2007;31:1315–1324. doi: 10.1111/j.1530-0277.2007.00426.x. [DOI] [PubMed] [Google Scholar]
- 77.Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- 78.Mathalon DH, Sullivan EV, Rawles JM, Pfefferbaum A. Correction for head size in brain-imaging measurements. Psychiatry Research: Neuroimaging. 1993;50:121–139. doi: 10.1016/0925-4927(93)90016-b. [DOI] [PubMed] [Google Scholar]
- 79.Pfefferbaum A, Adalsteinsson E, Sullivan EV. Dysmorphology and microstructural degradation of the corpus callosum: Interaction of age and alcoholism. Neurobiol Aging. 2006;27:994–1009. doi: 10.1016/j.neurobiolaging.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 80.Pfefferbaum A, Adalsteinsson E, Sullivan EV. Cortical NAA deficits in HIV infection without dementia: influence of alcoholism comorbidity. Neuropsychopharmacology. 2005;30:1392–1399. doi: 10.1038/sj.npp.1300723. [DOI] [PubMed] [Google Scholar]
- 81.Cohen RA, Harezlak J, Gongvatana A, Buchthal S, Schifitto G, Clark U, et al. Cerebral metabolite abnormalities in human immunodeficiency virus are associated with cortical and subcortical volumes. J Neurovirol. 2011;16:435–444. doi: 10.3109/13550284.2010.520817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, et al. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol. 2002;8:136–142. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- 83.Persidsky Y, Ho W, Ramirez SH, Potula R, Abood ME, Unterwald E, et al. HIV-1 infection and alcohol abuse: neurocognitive impairment, mechanisms of neurodegeneration and therapeutic interventions. Brain Behav Immun. 2011;25(Suppl 1):S61–70. doi: 10.1016/j.bbi.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xing HQ, Hayakawa H, Izumo K, Kubota R, Gelpi E, Budka H, et al. In vivo expression of proinflammatory cytokines in HIV encephalitis: an analysis of 11 autopsy cases. Neuropathology. 2009;29:433–442. doi: 10.1111/j.1440-1789.2008.00996.x. [DOI] [PubMed] [Google Scholar]
- 85.Ances BM, Vaida F, Yeh MJ, Liang CL, Buxton RB, Letendre S, et al. HIV infection and aging independently affect brain function as measured by functional magnetic resonance imaging. J Infect Dis. 2010;201:336–340. doi: 10.1086/649899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cohen RA, de la Monte S, Gongvatana A, Ombao H, Gonzalez B, Devlin KN, et al. Plasma cytokine concentrations associated with HIV/hepatitis C coinfection are related to attention, executive and psychomotor functioning. J Neuroimmunol. 2011;233:204–210. doi: 10.1016/j.jneuroim.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Smurzynski M, Wu K, Letendre S, Robertson K, Bosch RJ, Clifford DB, et al. Effects of central nervous system antiretroviral penetration on cognitive functioning in the ALLRT cohort. Aids. 2011;25:357–365. doi: 10.1097/QAD.0b013e32834171f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rosenbloom MJ, Sassoon SA, Fama R, Sullivan EV, Pfefferbaum A. Frontal Callosal Fiber Integrity Selectively Predicts Coordinated Psychomotor Performance in Chronic Alcoholism. Brain Imaging Behav. 2008;2:74–83. doi: 10.1007/s11682-007-9017-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ratti MT, Bo P, Giardini A, Soragna D. Chronic alcoholism and the frontal lobe: which executive functions are impaired? Acta Neurol Scand. 2002;105:276–281. doi: 10.1034/j.1600-0404.2002.0o315.x. [DOI] [PubMed] [Google Scholar]
- 90.Beatty WW, Hames KA, Blanco CR, Nixon SJ, Tivis LJ. Visuospatial perception, construction and memory in alcoholism. J Stud Alcohol. 1996;57:136–143. doi: 10.15288/jsa.1996.57.136. [DOI] [PubMed] [Google Scholar]
- 91.Basso MR, Bornstein RA. Neurobehavioural consequences of substance abuse and HIV infection. J Psychopharmacol. 2000;14:228–237. doi: 10.1177/026988110001400306. [DOI] [PubMed] [Google Scholar]
- 92.Pandrea I, Happel KI, Amedee AM, Bagby GJ, Nelson S. Alcohol’s role in HIV transmission and disease progression. Alcohol Research & Health. 2010;33:203–218. [PMC free article] [PubMed] [Google Scholar]
- 93.Marcondes MC, Watry D, Zandonatti M, Flynn C, Taffe MA, Fox H. Chronic alcohol consumption generates a vulnerable immune environment during early SIV infection in rhesus macaques. Alcohol Clin Exp Res. 2008;32:1583–1592. doi: 10.1111/j.1530-0277.2008.00730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Samet JH, Walley AY. Interventions targetting HIV-infected risky drinkers: Drops in the bottle. Alcohol Research & Health. 2010;33:267–279. [PMC free article] [PubMed] [Google Scholar]
- 95.Likar B, Viergever MA, Pernus F. Retrospective correction of MR intensity inhomogeneity by information minimization. EEE Transactions on Medical Imaging. 2001;20:1398–1410. doi: 10.1109/42.974934. [DOI] [PubMed] [Google Scholar]
- 96.Rohlfing T, Maurer CR. Nonrigid image registration in shared-memory multiprocessor environments with application to brains, breasts, and bees. IEEE Trans Inf Technol Biomed. 2003;7:16–25. doi: 10.1109/titb.2003.808506. [DOI] [PubMed] [Google Scholar]
- 97.Rohlfing T, Maurer CR. Multi-classifier framework for atlas-based image segmentation. Pattern Recognition Letters. 2005;26:2070–2079. [Google Scholar]
- 98.Rohlfing T, Zahr NM, Sullivan EV, Pfefferbaum A. The SRI24 multi-channel atlas of normal adult human brain structure. Hum Brain Mapp. 2010;31:798–819. doi: 10.1002/hbm.20906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation maximization algorithm. IEEE Transactions Medical Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.