Abstract
Deficits in the visual working memory (WM) system have been consistently reported in schizophrenia patients, but the relative contribution of initial perceptual encoding to these deficits remains unsettled. We assessed the role of visual perceptual encoding on performance on an object WM task. Schizophrenia patients (N=37) and nonpsychiatric control subjects (N=33) were tested on an object WM task involving three delay periods: 200 msec, 3 sec, and 10 sec. Schizophrenia patients performed significantly less accurately than controls on all three conditions. However, after controlling for the effect of perceptual encoding (accuracy on the 200 msec delay condition) on performance in the two memory load conditions, schizophrenia patients demonstrated intact WM in the 3 sec delay condition, and showed a weak trend for decreased accuracy on the 10 sec delay compared with controls. Analysis of individual differences in pattern of performance revealed that a distinct subgroup of poor encoder patients had a significantly greater reduction in accuracy at 3 sec than the other patient subgroups and controls. In contrast, among schizophrenia patients who performed poorly on the 10 sec delay, accuracy was equivalently reduced independent of encoding ability. WM deficits in controls were independent of encoding ability at both delay intervals. These results indicate that encoding ability titrates the magnitude of WM impairment in schizophrenia patients but not in controls, and that heterogeneity has to be taken into account to correctly estimate the effects of perceptual encoding on visual object WM deficits in schizophrenia.
INTRODUCTION
Working memory (WM) is a limited-capacity, short-term storage system for maintaining mental representations “online” for further processing in the service of response selection (Goldman-Rakic, 1992, Miyake and Shah, 1999) (Baddeley, 1986, Baddeley, 1992). An extensive research literature has confirmed that impaired WM is a cardinal feature of schizophrenia (Goldman-Rakic, 1991, Park and Holzman, 1992b, Keefe et al., 1995, Servan-Schreiber et al., 1996, Gold et al., 1997, Spindler et al., 1997, Coleman et al., 2002, Gold et al., 2010). Meta-analytic studies have found that WM deficits in schizophrenia are present in all modalities, across diverse methodologies, and are not accounted for by differences in IQ between patients and control subjects (Lee and Park, 2005, Forbes et al., 2009). Neuroimaging studies report abnormal neural activity during WM tasks in schizophrenia patients (Callicott et al., 2000, Barch et al., 2001, Barch et al., 2002, Kim et al., 2003, Manoach, 2003) and their unaffected first-degree relatives (Callicott et al., 2003). Further, neural circuits and neurotransmitter systems known to be abnormal in schizophrenia patients, particularly involving prefrontal dopamine, play key roles in WM processes; dysfunction in these systems is hypothesized to underlie WM deficits in schizophrenia (Goldman-Rakic, 1991, Barch, 2004, Seamans and Yang, 2004, Lisman et al., 2008). WM is critically involved in learning, problem solving, decision-making, anticipation, planning, and other cognitive functions that are frequently impaired in schizophrenia patients and is implicated in multiple neuropsychological deficits (Goldman-Rakic, 1994, Green, 1996, Silver et al., 2003).
Although most WM studies have focused on maintenance and executive processes (Callicott et al., 2000, Barch et al., 2001, Manoach, 2003), behavioral (Tek et al., 2002, Gold et al., 2003, Hartman et al., 2003, Lencz et al., 2003, Kim et al., 2006, Javitt et al., 2007), fMRI (Haenschel et al., 2007) and electroencephalographic (Haenschel et al., 2007, Dias et al., 2011) evidence also implicates abnormal encoding in WM impairments in schizophrenia patients. Much of the evidence for the role of impaired encoding in visual-spatial WM has come from delayed response (DR) and delayed match to sample (DMTS) tasks. The effects of encoding can be separated from the effects of maintenance and retrieval by comparing performance on 0-delay (i.e., testing memory immediately after the stimulus is removed) or non-memory (i.e., the stimulus remains present throughout the trial) conditions with longer delay conditions (i.e., ≥ 3 sec). Such designs do not always find evidence of impaired encoding in schizophrenia patients, possibly because ceiling effects may obscure underlying deficits in the patient group (Park and Holzman, 1992a, Javitt et al., 1997, Snitz et al., 1999). The fact that lengthening the delay interval beyond 1 second does not change the magnitude of performance differences between patients and controls implicates a role for encoding in impaired WM (Lee and Park, 2005).
In this study we assessed the role of visual perceptual encoding on performance on an object WM task. We measured encoding accuracy on a 200 msec delay interval in order to separate the effects of initial encoding from WM performance on 3 sec and 10 sec delay intervals. We hypothesized that perceptual encoding would be impaired in schizophrenia patients and would contribute to deficits in WM performance on the 3 sec and 10 sec delay intervals. We further hypothesized that worse performance would be observed in schizophrenia patients on the 3 sec and 10 sec delay intervals even in the absence of deficits in perceptual encoding. In addition, we examined whether inter-individual differences in performance across the 3 conditions could clarify discrete encoding and maintenance impairments.
Materials and Methods
Participants
The subject groups included 37 patients who met Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV) criteria for a diagnosis of schizophrenia or schizoaffective disorder (SZ) and 33 NC subjects (American Psychiatric Association, 1994). Demographic characteristics of the sample are presented in table 1. The groups did not differ in age, years of education, or familial socioeconomic status (SES) (Hollingshead and Redlich, 1958, Hollingshead, 1965). The schizophrenia group had a slightly larger proportion of males than the controls. NC participants had a higher mean estimated verbal IQ based on the vocabulary subtest of the Wechsler Adult Intelligence Scale-Revised (WAIS-R) (Wechsler, 1981). The patients were chronically ill outpatients (mean duration of illness = 16.7 years, SD = 8.8) and were moderately symptomatic as measured by the Brief Psychiatric Rating Scale (BPRS) (Overall and Gorham, 1962) (M = 44.7, SD = 15.1). The mean chlorpromazine-equivalent dose for the patients was 445 ± 249 mg/day (range = 198-990) (Davis, 1974, Woods, 2003). The NC group was restricted to individuals who did not meet DSM-IV criteria for any psychotic disorder (lifetime), bipolar disorder without psychotic features, or a schizophrenia-spectrum personality disorder, and who had no family history of psychosis, suicide, or psychiatric hospitalizations. Axis I disorders were assessed using the Structured Clinical Interview for DSM-IV, Patient Edition (Spitzer et al., 1994). Schizotypal, schizoid, and paranoid personality disorders were assessed in NC subjects using the Structured Interview for Schizotypal Symptoms (Version 1.5) (Kendler, 1989). An experienced clinician administered the interviews, and an independent group of senior diagnosticians reviewed the interview material and all available hospital records and assigned consensus diagnoses based on best estimate methods (Leckman et al., 1982). The interviews and diagnostic evaluations were performed blind to the experimental procedures and group membership. The following exclusion criteria applied to all participants: (a) lack of fluency in English; (b) history of serious head trauma or diagnosed organic brain disease; (c) history of substance abuse or dependence during the past 2 years or previous chronic dependence. All participants provided written informed consent and were paid for their participation.
Table 1. Demographic Characteristics of the Study Sample.
| Group | N | Age (yrs) |
Gender (% male) |
Estimated Verbal IQ* |
Years of Education |
SES |
|---|---|---|---|---|---|---|
| SZ | 37 | 39.8 (9.2) [26.8–52.0] |
60 | 99.2 (10.6) [85–120] |
13.9 (2.0) [12–18] |
2.7 (1.2) [1-5] |
| NC | 33 | 40.3 (12.4) [18.8-60.4] |
36 | 107.7 (9.6) [95-130] |
15.0 (2.9) [11-21] |
2.6 (1.1) [1-5] |
Mean (SD) [range]
Schizophrenia patients had significantly lower mean estimated verbal IQ (t=3.3, df=6, P=0.001) and tended to be disproportionately male compared with the NC group (χ2=3.6, df=1, P=0.06). Estimated verbal IQ data available on 28/33 controls.
Task and Stimuli
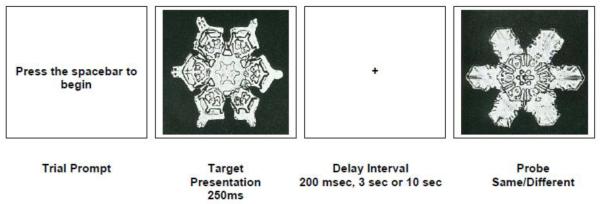
Participants were tested on an object recognition WM task that utilized complex visual stimuli chosen to minimize mneumonic strategies or verbal recoding of the stimuli. The stimuli were administered using a personal computer equipped with an 18-in monitor (45.7 cm) and a serial response box (Psychology Software Tools, Inc., Pittsburgh, PA). The task (Figure 1) was presented using a DMTS object WM paradigm. Subjects were presented with a target object, a grayscale image of a snowflake (Bentley and Humphreys, 1962) and were instructed to remember the object. The task employed three delay periods: a 200 msec (baseline) delay designed to tap initial perceptual encoding, and two WM conditions, a 3 sec, and a 10 sec delay period. The task was presented in three blocks of 40 trials each, organized by delay period. The order of presentation of the three blocks was semi-randomized.
Figure 1.
Snowflake Object Working Memory Task
To begin the experiment, subjects were presented with a brief prompt (“press the spacebar to begin”) followed by a target snowflake that was centrally illuminated on the computer screen for 250 msec and then replaced by a cross upon which subjects were instructed to fixate during the delay period. After the delay period, a second snowflake (probe) was illuminated on the computer screen, and subjects were instructed to indicate whether the probe snowflake was the same as the target snowflake by pressing a “Yes” or a “No” button on the response box. The probe remained on the screen until a response was made or 10 seconds elapsed. The target and probe snowflakes subtended about 13° of visual angle.
Statistical Analyses
The dependent measure for WM accuracy was the proportion correct score (# of correct responses/ # of trials). Planned comparisons between and within groups were carried out using Wilcoxon rank sum and signed-rank tests, respectively. Glass’ estimates of effect size (es) were calculated (Hedges, 1981). Repeated measures analysis of variance (ANOVA) was used to determine the effects of perceptual encoding ability on performance on the WM delay intervals.
Results
Summary statistics for accuracy scores of the two groups for the three delay intervals are presented in table 2. Schizophrenia patients were significantly less accurate than NC on all delay intervals: 200 msec (Z=2.97, P=0.003, es:1.27); 3 sec (Z=3.18, p=0.002, es:1.0); 10 sec (Z=3.77, P=0.0002, es:1.0). Both subject groups showed a significant decline in performance on the 3 and 10 sec delay intervals (table 2, figure 1 of supplementary materials) compared with the 200 msec perceptual encoding condition. Performance did not differ significantly between the 3 sec and 10 sec delay intervals in either group (SZ: S=54, P=0.2, es: 0.3; NC: S=105.5, P=0.1, es:0.17).
Table 2.
Mean Accuracy Scores (and Standard Deviations) for the Three Delay Intervals
| Group/Delay | N | 200 ms | 3 sec | 10 sec |
|---|---|---|---|---|
| Schizophrenia | 37 | 0.86 (0.10)* | 0.76 (0.13)**, † | 0.73 (0.11)***, †† |
| Controls | 33 | 0.93 (0.05) | 0.86 (0.10)‡ | 0.85 (0.11)‡‡ |
Between group differences:
P<0.003 (Z=2.97, es-1.27);
P<0.002 (Z=3.18, es=1.0);
P<0.0002 (Z=3.77, es=1.0).
Schizophrenia patient within group differences:
P<.0.0001(S=248.0, es=0.83);
P<0.0001(S=285.5, es=1.17).
NC within group differences:
p<0.002(S=140.0, es=0.68);
P<0.0001(S=182.5, es=0.77).
In order to separate the effects of perceptual encoding from the effects of maintenance and retrieval in the two delay conditions, we calculated change-from-perceptual encoding scores for both intervals: (3 sec accuracy - 200 msec accuracy; 10 sec accuracy - 200 msec accuracy). When perceptual encoding was accounted for in this fashion, patients did not differ significantly in accuracy from NC in the 3 sec delay condition (Z=-1.26, P=0.21, es:0.39), a three-fold reduction in effect size suggesting that perceptual encoding had accounted for their poorer average accuracy on the 3 sec delay. Patients tended to be less accurate than NC in the 10 sec delay condition (Z=−1.90, P=0.06, es:0.47), indicating that the longer interval was more taxing on WM maintenance and retrieval capacities of patients. Accuracy did not change significantly within either group between the 3 and 10 sec delay intervals when baseline performance was taken into account (P’s >0.1; SZ es:0.3; NC es:0.04). These results are consistent with the finding that accuracy in the 3 sec (r=0.54, n= 70, P<0.0001) and 10 sec delay conditions (r=0.54, n= 70, P<0.0001) were correlated with accuracy in the 200 msec condition (r=0.54, n= 70, P<0.0001).
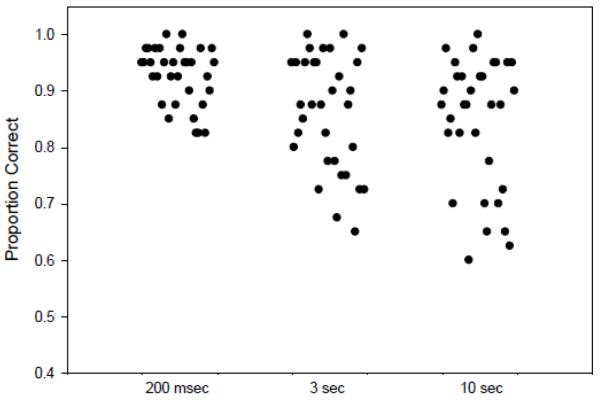
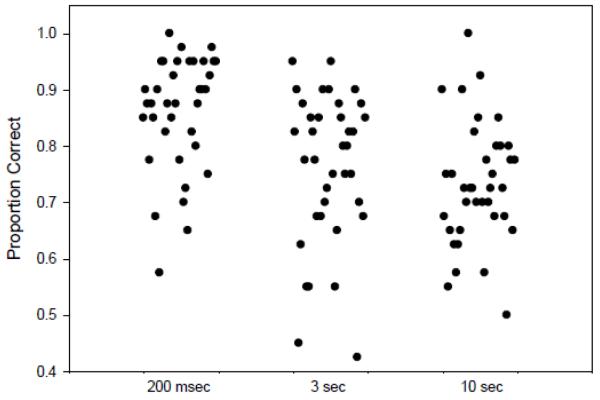
We set a cut-off for good versus poor performance after inspecting the distributions of accuracy scores (figures 2a, 2b). The maximum difference between the groups occurred at 90% accuracy in all conditions, with NC having more subjects (76%) with ≥90% accuracy at 200 msec than did patients (49%) [X2 =5.4, df=1, P=0.02]. Accuracy scores on the 200 msec delay condition of ≥ 90% were used to distinguish good encoders from poor encoders (<90%).
Figure 2.
a. Distribution of Accuracy Scores in NC Subjects for the Delay Intervals
b. Distribution of Accuracy Scores in Schizophrenia Patients for the Three Delay Intervals
Inspection of the individual data points revealed three distinct subgroups: 1) poor perceptual encoders with poor accuracy at 3 sec (16/34 or 47% of patients; 7/32 or 22% of NC), 2) good encoders with poor accuracy at 3 sec (15/34 or 44% of patients; 11/32 or 34% of NC), and 3) good encoders with high accuracy at 3 sec (3/34 or 9% of SZ; 14/32 or 44% of NC). The accuracy score means and standard deviations and samples sizes for the 3 and 10 sec delay conditions are reported for each group in Table 3 (see also figures 2 and 3 of supplementary materials). Among patients with impaired accuracy at 3 sec, poor encoders (n=16) performed worse than good encoders (n=15) (Z=2.421, P=0.02). Among both patients and controls, good encoders with good accuracy had equivalent mean values (NC: mean =0.95, SD=0.03, n=14; SZ: mean=0.92, SD=0.03, n=3).
Table 3. Mean (SD) Accuracy for the 3 and 10 sec Delay Intervals in Individuals with Poor Accuracy1 at 3 seconds and in Individuals with Poor Accuracy1 at 10 seconds: Subgroups Stratified on the Basis of Good2 versus Poor1 Initial Encoding.
| Subgroups (%) | Accuracy: 3 Second Delay | Accuracy: 10 Second Delay | |
|---|---|---|---|
| NC3,4 | Poor Encoders | 0.79 (0.08), n=7 | 0.70 (0.08), n=5+ |
| Good Encoders | 0.79 (0.07), n=11 | 0.79 (0.09), n=13+ | |
| SZ3,4 | Poor Encoders | 0.68 (0.13), n=16 | 0.70 (0.09), n=18* |
| Good Encoders | 0.79 (0.09), n=15 | 0.72 (0.07), n=15* |
P=0.08
P=0.02
Poor accuracy at 3 seconds or 10 seconds denotes the proportion of correct responses below 0.90 (<90% correct).
Good accuracy at 3 seconds or 10 seconds denotes the proportion of correct responses on or above 0.90 (≥90% correct).
All poor encoders had poor accuracy on both the 3 and 10 second delay conditions.
The average accuracy values for good encoders on the 3 and 10 second delay conditions were equivalent in the two groups (3 seconds - NC: 0.95 (0.03), n=14; SZ: 0.92 (0.03), n=3; 10 seconds - NC: 0.94 (0.03), n=12; SZ: 0.94 (0.05), n=3)
Among NC with impaired accuracy at 3 sec, however, accuracy was identical independent of encoding ability (Z=0.05, P=0.96). Importantly, accuracy was equivalently compromised in patients and NC with good initial encoding but impaired performance at 3 sec (Z=−0.31, P=0.75). Thus, the group difference in performance on the 3 sec delay interval seems to be due to a distinct subgroup (n=16, 47%) of patients with impaired perceptual encoding.
A similar analysis was done after forming the same subgroups for the 10 second delay condition. These results are also reported in Table 3. Among patients who performed poorly at 10 sec, accuracy was equivalently reduced independent of encoding ability (Z=0.67, P=0.50), suggesting that factors related to the increased duration of the retention interval played a greater role than perceptual encoding ability in poor WM performance (Table 3). However, among NC who performed poorly at 10 sec, good encoders (n = 13) tended to be less impaired than poor encoders (n = 5) (Z=−1.75, P=0.08), suggesting a facilitative effect of good encoding for meeting the increased retention demands. The only significant difference between patients and NC on the 10 sec delay occurred in subjects with good encoding but impaired 10 sec accuracy. Schizophrenia patients who were good encoders (n = 15) were more impaired than NC (n = 13) who were good encoders (Z=2.11, P=0.04), providing further support for the notion that factors other than encoding ability contribute to poor patient performance at longer delay intervals. Patients and controls with poor perceptual encoding and poor accuracy at 10 sec did not differ in accuracy (Z=−0.07, P=0.94). Patients and controls with normal initial encoding and good accuracy in the 10 sec condition also did not differ in accuracy (Z=0.0, p=1.0).
Discussion
This study examined the role of perceptual encoding on performance during an object WM task involving three delay intervals: a perceptual encoding condition (200ms) and two WM delay conditions (3 sec and 10 sec). Schizophrenia patients were significantly less accurate than controls on all three conditions. However, when the effects of perceptual encoding were separated from the effects of memory and retrieval, patients did not differ significantly from NC in the 3 sec condition. This result suggests that the worse mean performance of patients on the 3 sec delay interval was largely determined by poor initial encoding. In contrast, among patients who performed poorly at 10 sec, accuracy was equivalently reduced independent of encoding ability.
Impaired Perceptual Encoding or Impaired WM?
The distribution of accuracy scores in both groups (table 2) was not consistent with the uniform ceiling effects reported in previous studies that used 0-delay conditions. Indeed, substantial subgroups of patients (47%) and NC (22%) had difficulty with perceptual encoding. However, impaired initial perceptual encoding accounted for poor performance in the 3 sec delay only in patients. Poor encoding patients performed significantly worse in the 3 sec delay condition than good encoders with impaired accuracy on the 3 sec delay. These individual differences in perceptual encoding ability in patients are consistent with the well-documented heterogeneity of performance on cognitive measures in SZ patients (Heinrichs, 2004). In particular, previous studies have shown selective deficits in verbal, but not nonverbal, WM in subgroups of patients who performed normally on screening tests of attention and perception (Wexler et al., 1998, Bruder et al., 2004, Bruder et al., 2011). In contrast, patients who performed abnormally on the screening tests of attention and perception showed more generalized WM and cognitive deficits. Importantly, between-group comparisons of patients and NC that do not take into account heterogeneity in perceptual encoding ability result in two related inaccuracies: underestimating the magnitude of the encoding deficit in the subgroup with impaired encoding and overestimating it in the group as a whole (Buchsbaum and Rieder, 1979, Coleman et al., 2010).
The present study confirms prior findings implicating impaired initial perceptual encoding as the basis for what has been interpreted as WM deficits as well as substantial within-group variability in perceptual encoding in schizophrenia patients. Indeed, one DMTS object WM study showed that patients needed an approximately fivefold increase in stimulus duration relative to control subjects in order to effectively encode stimuli into WM [M(SD) = 2163(457) versus 421(473) msec, respectively].(Hartman et al., 2003) The relatively short stimulus duration (250 msec) used in the present study may have been too short for the subgroups of patients and NC who were poor encoders. An alternative design would extend stimulus exposure times to determine whether longer stimulus durations normalize performance in these groups. Nevertheless, our results and those of others (Hartman et al., 2003, Neufeld, 2007, Badcock et al., 2008) implicate speed of visual perceptual encoding in the encoding deficit we observed in a subgroup of schizophrenia patients. Our results are also consistent with results from other cognitive tasks showing that increased latency of stimulus encoding is a key cognitive deficit of schizophrenia (Neufeld, 2007). Notably, the 250 msec stimulus duration was adequate for those subgroups of patients (53%) and NC (79%) who were able to encode at high levels of accuracy.
Slowed consolidation of WM has been reported in schizophrenia patients (Knight et al., 1985, Fuller et al., 2005, Vogel et al., 2006). We cannot rule out the possibility that a combination of deficits in speed of processing during initial encoding and slowed WM consolidation contributed to our findings. However, unless initial encoding takes place, WM consolidation cannot occur. Further, if slowed WM consolidation accounted for the findings, patients would have been expected to perform better on the 3 sec delay than on the 200 msec delay, but both groups did worse on the 3 sec delay. A backward masking effect from the probe also seems unlikely since our task involved a 250 msec exposure to perceive the stimulus before the probe was presented 200 msec later.
Perceptual Encoding and Early Visual Processing
Our finding implicating a perceptual encoding deficit is consistent with an extensive literature showing dysfunction in schizophrenia patients during the earliest stages of visual information processing (McGhie and Chapman, 1961, Miller et al., 1979, Saccuzzo and Braff, 1981, Green and Walker, 1986). Haenschel and colleagues carried out an object WM study using parallel event-related potentials (ERPs) and functional magnetic resonance imaging (fMRI) in early-onset schizophrenia. They linked impaired generation of event-related components (e.g., P1, P370) reflecting early stage visual processes to decreased blood oxygenation levels in extrastriate visual areas during encoding of abstract shapes (Haenschel et al., 2007). Specifically, patients showed reduced amplitude of various ERP components and decreased brain activation in highly overlapping areas of the visual cortex during both encoding and retrieval.
The visual cortex is organized into two major visual streams - object (what) information and spatial (where) information, which are processed in the ventral and dorsal visual streams, respectively (Merigan and Maunsell, 1993, Callaway, 1998). Ventral stream processing of object information has been linked to the lateral occipital cortex (LOC) by high-density electrical mapping (Doniger et al., 2000, Sehatpour et al., 2010) and fMRI (Malach et al., 1995, Green et al., 2009, Sehatpour et al., 2010) studies. Visual input from the ventral and dorsal visual streams, corresponding to the parvocellular and magnocellular visual systems, respectively, converges within the LOC. ERP studies of visual processing suggest that the initial stages of ventral stream processing are generally intact in schizophrenia patients whereas impaired magnocellular/dorsal stream functioning may be responsible for secondary downstream impairment within ventral stream object processing regions in the LOC (Doniger et al., 2002, Sehatpour et al., 2010, Dias et al., 2011, Martinez et al., 2011). Thus, the visual perceptual encoding deficit implicated in this study and others may reflect magnocellular dysfunction contributing to a secondary processing impairment within the ventral stream pathway and object processing regions in the LOC.
Relation between Initial Encoding and Length of Delay Interval
In the longer delay condition patients tended to perform less accurately than NC, even after encoding ability was taken into account. Notably, initial encoding ability cannot fully account for patient performance in this condition, suggesting that factors related to “on-line” maintenance of a durable representation in WM played a greater role in impaired performance than did initial encoding. Our findings implicating impaired initial perceptual encoding in WM memory deficits at shorter delay intervals and deficits in maintaining an accurate representation within WM at longer intervals, are consistent with other work showing both abnormal encoding and WM maintenance deficits in schizophrenia (Tek et al., 2002, Glahn et al., 2003, Lencz et al., 2003). Taken together, these findings are consistent with recent evidence showing that early sensory and later cognitive ERP components contribute independently to impaired visual WM performance (Dias et al., 2011).
In summary, we showed that a distinct subgroup of schizophrenia patients with poor visual perceptual encoding accounted for worse object WM performance in patients than controls on a 3 sec delay condition. The visual encoding deficit implicates early visual processing, likely reflecting processes related to the speed of visual perceptual encoding in WM impairments in schizophrenia. Impaired accuracy in patients on the 10 sec delay was unrelated to initial perceptual encoding, thereby implicating WM maintenance deficits. Our findings indicate that object WM deficits in some patients, at least at shorter delay intervals, are largely a function of poor initial perceptual encoding. Our results also suggest a disproportionately greater role for processes related to maintenance and retrieval in object WM deficits observed at longer delay intervals. Although behavioral results cannot be conclusively linked to specific neurophysiological processes, our results are consistent with other data implicating dysfunction of both bottom-up sensory processing brain regions in the visual cortex as well as in top-down higher cortical storage areas involved in the active maintenance of information during longer delay intervals (Tek et al., 2002, Lencz et al., 2003, Dias et al., 2011).
Supplementary Material
Acknowledgements
This work was supported by the National Institute of Mental Health (R01 MH071523), the Sidney R. Baer, Jr. Foundation, the Essel Foundation, and the National Association for Research on Schizophrenia and Depression. The authors are grateful to Anne Gibbs for subject recruitment and to study participants for dedicating their time and effort.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Michael Coleman and Deborah Levy conceived of the study, designed it, and wrote the manuscript. Olga Krastoshevsky carried out the study and wrote the manuscript. Xiawei Tu and Nancy R. Mendell analyzed the data and contributed to the interpretation.
Conflicts of Interest
The authors report no conflicts of interest.
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Fourth edition American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Badcock JC, Badcock DR, Read C, Jablensky A. Examining encoding imprecision in spatial working memory in schizophrenia. Schizophr. Res. 2008;100:144–152. doi: 10.1016/j.schres.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working Memory. Oxford University Press, Inc; Oxford: 1986. [Google Scholar]
- Baddeley A. Working memory. Science. 1992;255:556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Barch DM. Pharmacological manipulation of human working memory. Psychopharmacology. 2004;174:126–135. doi: 10.1007/s00213-003-1732-3. [DOI] [PubMed] [Google Scholar]
- Barch DM, Carter CS, Braver TS, Sabb FW, MacDonald A, 3rd, Noll DC, Cohen JD. Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Arch Gen Psychiatry. 2001;58:280–288. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- Barch DM, Csernansky JG, Conturo T, Snyder AZ. Working and long-term memory deficits in schizophrenia: is there a common prefrontal mechanism? J. Abnorm. Psychol. 2002;111:478–494. doi: 10.1037//0021-843x.111.3.478. [DOI] [PubMed] [Google Scholar]
- Bentley WA, Humphreys WJ. Snow Crystals. Dover Publications, Inc.; New York: 1962. [Google Scholar]
- Bruder GE, Alschuler DM, Kroppmann CJ, Fekri S, Gil RB, Jarskog LF, Harkavy-Friedman JM, Goetz R, Kayser J, Wexler BE. Heterogeneity of auditory verbal working memory in schizophrenia. J. Abnorm. Psychol. 2011;120:88–97. doi: 10.1037/a0021661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder GE, Wexler BE, Sage MM, Gil RB, Gorman JM. Verbal memory in schizophrenia: Additional evidence of subtypes having different cognitive deficits. Schizophr. Res. 2004;68:137–147. doi: 10.1016/S0920-9964(03)00156-7. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Rieder RO. Biological heterogeneity and psychiatric research. Platelet MAO activity as a case study. Arch. Gen. Psychiatry. 1979;36:1163–1169. doi: 10.1001/archpsyc.1979.01780110017001. [DOI] [PubMed] [Google Scholar]
- Callaway EM. Local circuits In primary visual cortex of the Macaque monkey. Ann. Rev. Neurosci. 1998;21:47–74. doi: 10.1146/annurev.neuro.21.1.47. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, Goldberg TE, Weinberger DR. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb. Cortex. 2000;10:1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Egan MF, Mattay VS, Bertolino A, Bone AD, Verchinksi B, Weinberger DR. Abnormal fMRI response of the dorsolateral prefrontal cortex in cognitively intact siblings of patients with schizophrenia. Am. J. Psychiatry. 2003;160:709–719. doi: 10.1176/appi.ajp.160.4.709. [DOI] [PubMed] [Google Scholar]
- Coleman MJ, Matthysse S, Barnard J, Lo Y, Levy DL, Rubin DB, Holzman PS. Spatial and object working memory in schizophrenia patients: A Bayesian item-response theory analysis. J. Abnorm. Psychol. 2002;111:425–435. doi: 10.1037//0021-843x.111.3.425. [DOI] [PubMed] [Google Scholar]
- Coleman MJ, Titone D, Krastoshevsky O, Krause V, Huang Z, Mendell NR, Eichenbaum HE, Levy DL. Reinforcement ambiguity and novelty do not account for transitive inference deficits in schizophrenia. Schizophr. Bull. 2010;36:1187–1200. doi: 10.1093/schbul/sbp039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM. Dose equivalents of antipsychotic drugs. J. Psychiatr. Res. 1974;11:65–69. doi: 10.1016/0022-3956(74)90071-5. [DOI] [PubMed] [Google Scholar]
- Dias EC, Butler PD, Hoptman MJ, Javitt DC. Early sensory contributions to contextual encoding deficits in schizophrenia. Arch. Gen. Psychiatry. 2011;68:654–664. doi: 10.1001/archgenpsychiatry.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doniger GM, Foxe JJ, Murray MM, Higgins BA, Javitt DC. Impaired visual object recognition and dorsal/ventral stream interaction in schizophrenia. Arch. Gen. Psychiatry. 2002;59:1011–1020. doi: 10.1001/archpsyc.59.11.1011. [DOI] [PubMed] [Google Scholar]
- Doniger GM, Foxe JJ, Murray MM, Higgins BA, Snodgrass JG, Schroeder CE, Javitt DC. Activation timecourse of ventral visual stream object-recognition areas: High density electrical mapping of perceptual closure processes. J. Cogn Neurosci. 2000;12:615–321. doi: 10.1162/089892900562372. [DOI] [PubMed] [Google Scholar]
- Forbes NF, Carrick LA, McIntosh AM, Lawrie SM. Working memory in schizophrenia: A meta-analysis. Psychol. Med. 2009;39:889–905. doi: 10.1017/S0033291708004558. [DOI] [PubMed] [Google Scholar]
- Fuller RL, Luck SJ, McMahon RP, Gold JM. Working memory consolidation is abnormally slow in schizophrenia. J. Abnorm. Psychol. 2005;114:279–290. doi: 10.1037/0021-843X.114.2.279. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Therman S, Manninen M, Huttunen M, Kaprio J, Lönnqvist J, Cannon TD. Spatial working memory as an endophenotype for schizophrenia. Biol. Psychiatry. 2003;53:624–626. doi: 10.1016/s0006-3223(02)01641-4. [DOI] [PubMed] [Google Scholar]
- Gold JM, Carpenter C, Randolph C, Goldberg TE, Weinberger DR. Auditory working memory and Wisconsin Card Sorting Test performance in schizophrenia. Arch. Gen. Psychiatry. 1997;54:159–165. doi: 10.1001/archpsyc.1997.01830140071013. [DOI] [PubMed] [Google Scholar]
- Gold JM, Hahn B, Zhang WW, Robinson BM, Kappenman ES, Beck VM, Luck SJ. Reduced capacity but spared precision and maintenance of working memory representations in schizophrenia. Arch Gen Psychiatry. 2010;67:570–577. doi: 10.1001/archgenpsychiatry.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Wilk CM, McMahon RP, Buchanan RW, Luck SJ. Working memory for visual features and conjunctions in schizophrenia. J. Abnorm. Psychol. 2003;112:61–71. [PubMed] [Google Scholar]
- Goldman-Rakic PS. Prefrontal cortical dysfunction in schizophrenia: The relevance of working memory. In: Carroll BJ, Barrett JE, editors. Psychopathology and the Brain. Raven Press, Ltd.; New York: 1991. pp. 1–23. [Google Scholar]
- Goldman-Rakic PS. Working memory and the mind. Sci. Am. 1992;267:110–117. doi: 10.1038/scientificamerican0992-110. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J. Neuropsychiatry Clin. Neurosci. 1994;6:348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- Green M, Walker E. Symptom correlates of vulnerability to backward masking in schizophrenia. Am. J. Psychiatry. 1986;143:181–186. doi: 10.1176/ajp.143.2.181. [DOI] [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am. J. Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Green MF, Lee J, Cohen MS, Engel SA, Korb AS, Nuechterlein KH, Wynn JK, Glahn DC. Functional neuroanatomy of visual masking deficits in schizophrenia. Arch. Gen. Psychiatry. 2009;66:1295–1303. doi: 10.1001/archgenpsychiatry.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenschel C, Bittner RA, Haertling F, Rotarska-Jagiela A, Maurer K, Singer W, Linden DE. Contribution of impaired early-stage visual processing to working memory dysfunction in adolescents with schizophrenia: A study with event-related potentials and functional magnetic resonance imaging. Arch. Gen. Psychiatry. 2007;64:1229–1240. doi: 10.1001/archpsyc.64.11.1229. [DOI] [PubMed] [Google Scholar]
- Hartman M, Steketee M,C, Silva S, Lanning K, McCann H. Working memory and schizophrenia: Evidence for slowed encoding. Schizophr. Res. 2003;59:99–113. doi: 10.1016/s0920-9964(01)00366-8. [DOI] [PubMed] [Google Scholar]
- Hedges LV. Distribution theory for Glass’s estimator of effect size and related estimators. J. Ed. Statistics. 1981;6:107–128. [Google Scholar]
- Heinrichs RW. Meta-analysis and the science of schizophrenia: Variant evidence or evidence of variants? Neurosci. Biobehav. Rev. 2004;28:379–394. doi: 10.1016/j.neubiorev.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Two Factor Index of Social Position. Yale Station; New Haven, CT: 1965. Privately Printed. [Google Scholar]
- Hollingshead AB, Redlich FC. Social Class and Mental Illness. John Wiley & Sons, Inc.; New York: 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Rabinowicz E, Silipo G, Dias EC. Encoding vs. retention: Differential effects of cue manipulation on working memory performance in schizophrenia. Schizophr. Res. 2007;91:159–168. doi: 10.1016/j.schres.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Strous RD, Grochowski S, Ritter W, Cowan N. Impaired precision, but normal retention, of auditory sensory (“echoic”) memory information in schizophrenia. J. Abnorm. Psychol. 1997;106:315–324. doi: 10.1037//0021-843x.106.2.315. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Roitman SE, Harvey PD, Blum CS, DuPre RL, Prieto DM, Davidson M, Davis KL. A pen-and-paper human analogue of a monkey prefrontal cortex activation task: Spatial working memory in patients with schizophrenia. Schizophr. Res. 1995;17:25–33. doi: 10.1016/0920-9964(95)00027-j. [DOI] [PubMed] [Google Scholar]
- Kendler KS. Structured Interview for Schizotypal Symptoms (SISS, version 1.5) Department of Psychiatry, Medical College of Virginia; Richmond, Virginia: 1989. [Google Scholar]
- Kim J-J, Kwon JS, Park HJ, Youn T, Kang DH, Kim MS, Lee DS, Lee MC. Functional disconnection between the prefrontal and parietal cortices during working memory processing in schizophrenia: A [15O]H2O PET study. Am J Psychiatry. 2003;160:919–923. doi: 10.1176/appi.ajp.160.5.919. [DOI] [PubMed] [Google Scholar]
- Kim J, Park S, Shin YW, Lee KJ, Kwon JS. Self-initiated encoding facilitates object working memory in schizophrenia: Implications for the etiology of working memory deficit. Schizophr. Res. 2006;82:65–74. doi: 10.1016/j.schres.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Knight RA, Elliott DS, Freedman EG. Short-term visual memory in schizophrenics. J. Abnorm. Psychol. 1985;94:427–442. doi: 10.1037//0021-843x.94.4.427. [DOI] [PubMed] [Google Scholar]
- Leckman J, Sholomskas D, Thompson W, Belanger A, Weissman M. Best estimate of lifetime psychiatric diagnosis. Arch. Gen. Psychiatry. 1982;39:879–883. doi: 10.1001/archpsyc.1982.04290080001001. [DOI] [PubMed] [Google Scholar]
- Lee J, Park S. Working memory impairments in schizophrenia: A meta-analysis. J. Abnorm. Psychol. 2005;114:599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- Lencz T, Bilder RM, Turkel E, Goldman RS, Robinson D, Kane JM, Lieberman JA. Impairments in perceptual competency and maintenance on a visual delayed match-to-sample test in first-episode schizophrenia. Arch. Gen. Psychiatry. 2003;60:238–243. doi: 10.1001/archpsyc.60.3.238. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malach R, Reppas JB, Benson RR, Kwong KK, Jiang H, Kennedy WA, Ledden PJ, Brady TJ, Rosen BR, Tootell RB. Object-related activity revealed by functional magnetic resonance imaging in human occipital cortex. Proc. Natl. Acad. Sci. U. S A. 1995;92:8135–8139. doi: 10.1073/pnas.92.18.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS. Prefrontal cortex dysfunction during working memory performance in schizophrenia: Reconciling discrepant findings. Schizophr. Res. 2003;60:285–298. doi: 10.1016/s0920-9964(02)00294-3. [DOI] [PubMed] [Google Scholar]
- Martinez A, Hillyard SA, Bickel S, Dias EC, Butler PD, Javitt DC. Consequences of magnocellular dysfunction on processing attended information in schizophrenia. Cereb. Cortex. 2011 doi: 10.1093/cercor/bhr195. in press, PMID: 21840846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhie A, Chapman J. Disorders of attention and perception in early schizophrenia. Br. J. Med. Psychol. 1961;34:103–117. doi: 10.1111/j.2044-8341.1961.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Merigan WH, Maunsell JHR. How parallel are the primate visual pathways? Ann. Rev. Neurosci. 1993;16:369–402. doi: 10.1146/annurev.ne.16.030193.002101. [DOI] [PubMed] [Google Scholar]
- Miller S, Saccuzzo D, Braff D. Information processing deficits in remitted schizophrenics. J. Abnorm. Psychol. 1979;88:446–449. [PubMed] [Google Scholar]
- Miyake A,P, Shah P. Models of Working Memory: Mechanisms of Active Maintenance and Executive Control. Cambridge University Press; New York: 1999. [Google Scholar]
- Neufeld RW. On the centrality and significance of stimulus-encoding deficit in schizophrenia. Schizophr. Bull. 2007;33:982–993. doi: 10.1093/schbul/sbm056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. Brief Psychiatric Rating Scale. Psychol. Rep. 1962;10:799–812. [Google Scholar]
- Park S, Holzman PS. Association of working memory deficit and eye tracking dysfunction in schizophrenia. Schizophr. Res. 1992;11:55–61. doi: 10.1016/0920-9964(93)90038-k. [DOI] [PubMed] [Google Scholar]
- Park S, Holzman PS. Schizophrenics show spatial working memory deficits. Arch. Gen. Psychiatry. 1992;49:975–982. doi: 10.1001/archpsyc.1992.01820120063009. [DOI] [PubMed] [Google Scholar]
- Saccuzzo DP, Braff DL. Early information processing deficit in schizophrenia: New findings using schizophrenic subgroups and manic control subjects. Arch. Gen. Psychiatry. 1981;38:175–179. doi: 10.1001/archpsyc.1981.01780270061008. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog. Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Sehatpour P, Dias EC, Butler PD, Revheim N, Guilfoyle DN, Foxe JJ, Javitt DC. Impaired visual object processing across an occipital-frontal-hippocampal brain network in schizophrenia: An integrated neuroimaging study. Arch. Gen. Psychiatry. 2010;67:772–782. doi: 10.1001/archgenpsychiatry.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servan-Schreiber D, Cohen JD, Steingard S. Schizophrenic deficits in the processing of context: A test of a theoretical model. Arch. Gen. Psychiatry. 1996;53:1105–1112. doi: 10.1001/archpsyc.1996.01830120037008. [DOI] [PubMed] [Google Scholar]
- Silver H, Feldman P, Bilker W, Gur RC. Working memory deficit as a core neuropsychological dysfunction in schizophrenia. Am. J. Psychiatry. 2003;160:1809–1816. doi: 10.1176/appi.ajp.160.10.1809. [DOI] [PubMed] [Google Scholar]
- Snitz BE, Curtis CE, Zald DH, Katsanis J, Iacono WG. Neuropsychological and oculomotor correlates of spatial working memory performance in schizophrenia patients and controls. Schizophr. Res. 1999;38:37–50. doi: 10.1016/s0920-9964(98)00178-9. [DOI] [PubMed] [Google Scholar]
- Spindler KA, Sullivan EV, Menon V, Lim KO, Pfefferbaum A. Deficits in multiple systems of working memory in schizophrenia. Schizophr. Res. 1997;27:1–10. doi: 10.1016/S0920-9964(97)00074-1. [DOI] [PubMed] [Google Scholar]
- Spitzer R, Williams J, Gibbon M, First M. Structured Clinical Interview for DSM-IV. American Psychiatric Association; Washington, D.C.: 1994. [Google Scholar]
- Tek C, Gold J, Blaxton T, Wilk C, McMahon RP, Buchanan RW. Visual perceptual and working memory impairments in schizophrenia. Arch. Gen. Psychiatry. 2002;59:146–153. doi: 10.1001/archpsyc.59.2.146. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Woodman GF, Luck SJ. The time course of consolidation in visual working memory. J. Exp. Psychol. Hum. Percept. 2006;32:1436–1451. doi: 10.1037/0096-1523.32.6.1436. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale - Revised. Psychological Corporation; New York: 1981. [Google Scholar]
- Wexler BE, Stevens AA, Bowers AA, Sernyak MJ, Goldman-Rakic PS. Word and tone working memory deficits in schizophrenia. Arch. Gen. Psychiatry. 1998;55:1093–1096. doi: 10.1001/archpsyc.55.12.1093. [DOI] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J. Clin. Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.