Abstract
Suppressors of cytokine signaling 1 and 3 (SOCS-1 and SOCS-3) are inhibitors of the Janus tyrosine kinase (JAK)/signal transducers and activators of transcription (STAT) pathway and function in a negative feedback loop during cytokine signaling. Abl transformation is associated with constitutive activation of JAK/STAT-dependent signaling. However, the mechanism by which Abl oncoproteins bypass SOCS inhibitory regulation remains poorly defined. Here, we demonstrate that coexpression of Bcr-Abl with SOCS-1 or SOCS-3 results in tyrosine phosphorylation of these SOCS proteins. Interestingly, SOCS-1 is highly tyrosine phosphorylated in one of five primary chronic myelogenous leukemia samples. Bcr-Abl-dependent tyrosine phosphorylation of SOCS-1 and SOCS-3 occurs mainly on Tyr 155 and Tyr 204 residues of SOCS-1 and on Tyr 221 residue of SOCS-3. We observed that phosphorylation of these SOCS proteins was associated with their binding to Bcr-Abl. Bcr-Abl-dependent phosphorylation of SOCS-1 and SOCS-3 diminished their inhibitory effects on the activation of JAK and STAT5 and thereby enhanced JAK/STAT5 signaling. Strikingly, disrupting the tyrosine phosphorylation of SOCS-1 or SOCS-3 impaired the expression of Bcl-XL protein and sensitized K562 leukemic cells to undergo apoptosis. Moreover, selective mutation of tyrosine phosphorylation sites of SOCS-1 or SOCS-3 significantly blocked Bcr-Abl-mediated tumorigenesis in nude mice and inhibited Bcr-Abl-mediated murine bone marrow transformation. Together, these results reveal a mechanism of how Bcr-Abl may overcome SOCS-1 and SOCS-3 inhibition to constitutively activate the JAK/STAT-dependent signaling, and suggest that Bcr-Abl may critically requires tyrosine phosphorylation of SOCS-1 and SOCS-3 to mediate tumorigenesis when these SOCS proteins are present in cells.
Introduction
Cytokine-mediated activation of Janus tyrosine kinases (JAKs) leads to phosphorylation of cytokine receptor, which assists in the recruitment of signal transducers and activators of transcription (STAT) protein, which is then phosphorylated, dimerized, and translocated to the nucleus to initiate transcription of specific target genes [1]. Previous studies have demonstrated that constitutive activation of JAK/STAT signaling is required for efficient transformation by the Abelson murine leukemia virus (A-MuLV), which expresses v-Abl [2]. There is considerable evidence that dysregulated JAK/STAT signaling plays a critical role in Bcr-Abl-induced malignant transformation [3,4]. JAKs and STAT5 were shown to be constitutively activated in Bcr-Abl-expressing cell lines and peripheral blood cells [5–7]. Although it was previously reported that Bcr-Abl can activate STAT5 independent of JAK [7], activation of JAK2 was detected in blood cells from patients with chronic myelogenous leukemia (CML) expressing Bcr-Abl [8]. Treatment of CML cell lines with JAK2 inhibitors or a kinase-inactive JAK2 mutant inhibited downstream effectors and blocked Bcr-Abl-mediated tumor formation [8–10]. Moreover, high STAT5 levels rendered CML cells resistant to imatinib and promoted tumor progression [11]. Recently, pimozide has been identified as STAT5 inhibitor that can control CML malignancy with imatinib [12]. In vivo experiments using mouse models have also portrayed STAT5 as an indispensible factor for induction and maintenance of Bcr-Abl-positive leukemia [13]. Together, these studies suggest that the JAK and STAT are important factors that contribute to Bcr-Abl-induced tumorigenesis.
An important mechanism for negative regulation of the JAK/STAT signaling pathway is mediated through members of the suppressor of cytokine signaling (SOCS) family [14]. Of the eight family members, SOCS-1 and SOCS-3 have been most extensively studied and are the most potent inhibitors of cytokine-induced signaling. SOCS-1 and SOCS-3 regulate JAK activity by at least two mechanisms. One mechanism involves direct interaction with JAKs by their kinase inhibitory region (KIR), which inhibits JAKs activity. The other mechanism involves interaction of SOCS box with the Elongin BC complex, which becomes part of an E3 ubiquitin ligase that targets JAKs to proteasomal degradation [15–17]. When overexpressed in cells, SOCS-1 and SOCS-3 can inhibit STAT activation induced by multiple cytokines stimulations.
Because activation of JAK/STAT signaling is required for transformation by several oncogenes, it has been proposed that the regulatory effects of SOCS-1 and SOCS-3 may need to be overcome to achieve cellular transformation. Indeed, SOCS-1 locus was methylated in different tumor types including hepatocellular carcinomas and multiple myeloma [18–20]. Several reports have found loss-of-function mutation of SOCS-1 gene in various malignancies [21–23]. Moreover, hypermethylation silencing of SOCS-3 facilitates cell growth in a variety of tumors, including human lung cancer and hepatocellular carcinoma [24–26]. SOCS-3 has been shown to function as an antisurvival agent in breast cancer [27]. Conversely, constitutive expression of SOCS-3 protects cells from growth inhibition in T-cell lymphoma treated with interferon α (IFN-α) [28]. Therefore, SOCS-3 is documented as an important regulator in tumor growth.
So far, no genetic mutations of SOCS-1 and SOCS-3 genes have been demonstrated in CML samples. The methylation status of SOCS-1 gene in CML samples has recently been addressed by several publications. One group demonstrated that the SOCS-1 gene was hypermethylated in 67% and 46% of the blastic and chronic phase CML samples, respectively, suggesting a relation between SOCS-1 gene hypermethylation and CML progression [29]. In contrast, a second group revealed no such correlation by showing unmethylated promoter region of SOCS-1 in all 56 CML patient samples [30]. A third group demonstrated that SOCS-1 was constitutively expressed in 49 (65%) of 75 patients with CML [31]. However, little information is available about methylation of SOCS-3 gene in patients with CML. The principal tyrosine phosphorylation residues of SOCS-3 have been identified [32], and the myeloproliferative disorder-associated JAK2(V617F) mutant can bypass the negative feedback of SOCS-3 through tyrosine phosphorylating SOCS-3 [33,34]. Together, these observations prompted us to explore the hypothesis that the functions of SOCS-1 and SOCS-3 may be altered in Bcr-Abl-positive cells.
In this study, we have found that Bcr-Abl signaling leads to tyrosine phosphorylation of SOCS-1 and SOCS-3 and thereby impairs the ability of SOCS-1 and SOCS-3 to inhibit the activation of the JAK/STAT signaling. Interestingly, SOCS-1 is highly tyrosine phosphorylated in one of five Bcr-Abl-positive CML samples. Disrupting the tyrosine phosphorylation of SOCS-1 and SOCS-3 promotes the apoptosis of K562 cells and blocks the tumor formation in nude mice. Together, these results reveal a requirement for tyrosine phosphorylation of SOCS-1 and SOCS-3 in Bcr-Abl-induced tumorigenesis in the presence of these SOCS proteins.
Materials and Methods
Antibodies
The following antibodies were used in this study: anti-phosphotyrosine clone 4G10 (Millipore, Billerica, MA); anti-JAK1, anti-phospho-JAK1, anti-His, anti-Bcr, and anti-Myc (Santa Cruz Biotechnology, Santa Cruz, CA); anti-JAK2 and anti-phospho-JAK2, anti-STAT5, and anti-phospho-STAT5 (Cell Signaling Technology, Boston, MA); anti-X-press (Invitrogen, Carlsbad, CA); anti-Flag (Sigma, St Louis, MO); anti-SOCS-1 polyclonal Ab (Abcam, Cambridge, MA), anti-SOCS-1 clone 4H1 (a gift from Dr. Doug Hilton, WEHI, Victoria, Australia). Anti-SOCS-3 antiserum was generated in the laboratory as described previously [35]. All other antibodies were obtained as previously described [36,37].
Site-Directed Mutagenesis and Plasmid Construction
The mutants, SOCS-1(Y65F), SOCS-1(Y81F), SOCS-1(Y155F), SOCS-1(Y204F), SOCS-3(Y204F), SOCS-3(Y221F), and SOCS-3 (Y204, 221F), were generated by site-directed mutagenesis with the QuickChange XL system (Stratagene, La Jolla, CA). Six SOCS family members (SOCS 1, 2, 3, 5, 6, and 7) were subcloned into the pcDNA3.1 (-) vector, respectively. Wild-type (WT) SOCS-1, SOCS-3, and their mutants were subcloned into the pFLAG-CMV-5 vector and the retroviral vectors pMIG.-IRES-GFP and MSCV p210-IRES-GFP (a kind gift from Dr. Richard Van Etten, Tufts University, Boston, MA).
Virus Production and Generation of Stable K562 Cell Lines
Replication incompetent retroviruses were produced by transient cotransfection of 293T cells with pMIG bicistronic retroviral vector containing specific genes, pCL-Eco and pCL-VSV-G plasmids. K562 cell lines stably expressing specific genes were generated by infecting the cells with retroviruses encoding GFP alone or GFP and SOCS-1, SOCS-3, or their mutants as previously described [37].
Cell Extracts, Immunoprecipitation, and Western Blot
Preparation of cell extracts and immunoprecipitation were performed as previously described [5,37]. Briefly, cell extracts were immunoprecipitated overnight at 4°C with indicated antibodies. Samples were separated on SDS-polyacrylamide gel, transferred to a nitrocellulose membrane, and probed with antibodies as indicated.
Expression of GST Fusion Proteins and In Vitro Binding Experiment
GST fusion proteins were expressed in the bacteria BL21 (DE3) and purified as previously described [35,38]. Pull-down experiments were performed by incubating the beads with cell extracts treated with either mock or λ phosphatase. Bound materials were washed extensively and analyzed by Western blot.
Flow Cytometry and Apoptosis Assay
Cells were washed extensively in medium and cultured with etoposide (Sigma) for the indicated times. Then, cells were washed with phosphate-buffered saline buffer and stained with 1 µg/ml propidium iodode in phosphate-buffered saline. The samples were analyzed by fluorescence-activated cell sorter (BD Bioscience, San Jose, CA).
Nude Mouse Injection
Cells (1 x 107) were injected subcutaneously into female nude mouse (5–6 weeks old). Tumor growth was monitored and measured in volume (length x height x width) at the indicated time points during a 21-day period after inoculation. In addition, bioluminescent imaging was performed to detect tumors from GFP-expressing K562 cells. Mice were anesthetized using 2% isoflurane and imaged using a cooled CCD camera. Images were quantified as photons/s using the indigo software (Berthold Technologies, Germany). Bioluminescent imaging was performed at day 14 after inoculation.
Primary Murine Bone Marrow Transformation Assay
Bone marrow cells were freshly harvested from 5- to 6-week-old female Balb/c mice and then subjected to red cell lysis. Bcr-Abl-mediated bone marrow cell transformation was performed as previously described [39,40]. Infected cells were seeded in 96-well plates and cultured as previously described [36–38]. Ninety-six-well plates were then examined under a microscope to determine the transformed cell clones showing cytokine-independent growth, and transformation efficiency was scored by counting the number of wells containing the survivors 3 weeks after infection.
Results
SOCS-1 and SOCS-3 Are Tyrosine Phosphorylated in Bcr-Abl-Expressing Cells
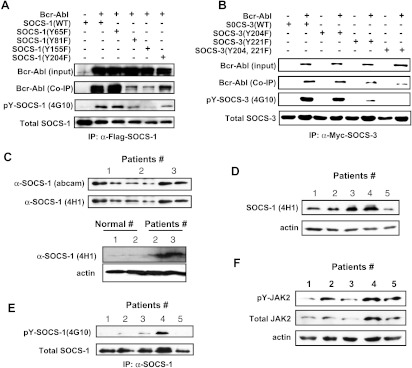
SOCS proteins constitute a class of negative regulators of JAK/STAT signaling pathway. However, little is known about how Bcr-Abl is able to overcome regulatory effects of SOCS proteins and impart constitutive activation of JAK/STAT pathway. Therefore, we determined whether Bcr-Abl could induce phosphorylation of SOCS proteins. We coexpressed Bcr-Abl with Xpress and His-tagged SOCS-1, 2, 3, 5, 6, and 7 in 293T cells. As shown in Figure 1A, SOCS-1 and SOCS-3 were clearly tyrosine phosphorylated in cells expressing Bcr-Abl. We also observed that Bcr-Abl was coimmunoprecipitated with SOCS-1 and SOCS-3. On the basis of these results, we focused on SOCS-1 and SOCS-3 in this study.
Figure 1.
SOCS-1 and SOCS-3 are tyrosine phosphorylated in Bcr-Abl expressing cells. (A) 293T cells were cotransfected with Bcr-Abl and SOCS-1, SOCS-2, SOCS-3, SOCS-5, SOCS-6, or SOCS-7 using Lipofectamine. After 48 hours of culture, cells were harvested and cell extracts were then prepared. The SOCS proteins were immunoprecipitated using the His-Tag, and immunoblot analysis was performed using X-press antibody, p-Tyr antibody (4G10), and Abl antibody (AB-2). (B and C) 293T cells were cotransfected with Bcr-Abl and either Flag-tagged SOCS-1 (B) or Flag-tagged SOCS-3 (C). SOCS-1 or SOCS-3 protein was then immunoprecipitated using Flag-tagged protein IP kit (Sigma). Shown are immunoblots probed with anti-SOCS-1 antibody (4H1), anti-SOCS-3 antiserum, and p-Tyr antibody (4G10).
To further confirm Bcr-Abl-dependent phosphorylation of SOCS-1 and SOCS-3, we repeated the cotransfection experiment using Flag-tagged SOCS-1 or SOCS-3 with Bcr-Abl. Indeed, SOCS-1 and SOCS-3 were found to be highly tyrosine phosphorylated in Bcr-Abl-expressing cells (Figure 1, B and C).
Identification of Bcr-Abl-Dependent Phosphorylation Sites of SOCS-1 and SOCS-3
We next sought to identify the tyrosine residues in SOCS-1 that could be phosphorylated by Bcr-Abl- All four tyrosine residues Y65, Y81, Y155, and Y204 were individually substituted with phenylalanine, and phosphorylation was analyzed in 293T cells cotransfected with Bcr-Abl and SOCS-1. The results showed that Bcr-Abl-dependent phosphorylation of SOCS-1 occurred mainly on Y155 and Y204, to a lesser extent, on Y81 residue (Figure 2A). Tyrosine residues at 81 and 155 are located in SH2 domain of SOCS-1, and tyrosine 204 is within the conserved SOCS box. Again, we observed that Bcr-Abl was brought down when SOCS-1 was immunoprecipitated (Figure 2A).
Figure 2.
Identification of Bcr-Abl-dependent phosphorylation sites of SOCS-1 and SOCS-3 and detection of SOCS-1 tyrosine phosphorylation in primary CML samples. (A) 293T cells were cotransfected with Bcr-Abl and either SOCS-1 or its mutants as described in Figure 1. Immunoblot analysis of whole cell lysates was performed to examine Bcr-Abl expression. Whole cell lysates were immunoprecipitated with anti-Flag-SOCS-1 antibody, and then precipitated proteins were examined for SOCS-1, Bcr-Abl, and tyrosine-phosphorylated SOCS-1 using antibodies as indicated. (B) Experiments were performed as described in A. 293T cells cotransfected with Bcr-Abl and Myc-tagged wild-type or mutant SOCS-3 were lysed and immunoprecipitated with anti-Myc antibody (9E10). Shown are immunoblots probed with indicated antibodies. (C) Lysates of peripheral blood white cells from three CML patients were examined using an mAb (4H1) directed against the N-terminal region of SOCS-1 and a rabbit polyclonal Ab directed against C-terminal SOCS box (upper panel). SOCS-1 protein levels were also examined in peripheral blood white cells from normal controls (lower panel). (D) Expression levels of SOCS-1 were examined in five CML samples (patients 1–3 are the same as in C) after normalizing to actin loading control. (E) Lysates derived from the five CML samples in D were immunoprecipitated with anti-SOCS-1 antibody. Precipitated proteins were probed with indicated antibodies by Western blot analysis. (F) Lysates as described in D were used to detect total JAK2 and pJAK2 levels. Shown is an immunoblot probed as indicated.
SOCS-3 is known to be tyrosine-phosphorylated on Y204 and Y221 within the conserved SOCS box motif by several kinases [32]. In this study, we mutated these tyrosine residues to phenylalanine either individually or in combination and analyzed phosphorylation statuses of SOCS-3 in 293T cells. The level of phosphorylation of SOCS-3(Y221F) mutant was greatly reduced and that of SOCS-3 (Y204F) was slightly decreased (Figure 2B). The tyrosine phosphorylation of a mutant with replacement of both tyrosines 204 and 221 with phenylalanines (SOCS-3(Y204, 221F)) was undetectable (Figure 2B). Interestingly, we also found that Bcr-Abl was brought down when SOCS-3 was immunoprecipitated, and the amount of co-precipitated Bcr-Abl was decreased in correlation with the reduction of SOCS-3 phosphorylation (Figure 2B). The interaction between Bcr-Abl and SOCS proteins was further confirmed when anti-Flag was used to precipitate Bcr-Abl (Figure W1). Together, these results demonstrate that Bcr-Abl signaling leads to tyrosine phosphorylation of SOCS-1 and SOCS-3 and suggest that phosphorylation of these SOCS proteins is associated with their interaction with Bcr-Abl.
Tyrosine Phosphorylation of SOCS-1 Occurs in CML Patients
Of the eight family members, SOCS-1 is the most potent inhibitor of JAK/STAT signaling. Therefore, we next determined whether SOCS-1 is expressed and tyrosine phosphorylated in patients with Bcr-Abl-positive CML. To this end, we used two anti-SOCS-1 antibodies (see Materials and Methods) to detect SOCS-1 protein levels in these samples derived from chronic phases at diagnosis. Both antibodies detected a same band at ∼37 kDa (Figure 2C). As expected, the peripheral blood cells from normal controls exhibited an extremely low level of SOCS-1 protein (Figure 2C). Interestingly, after normalizing to actin loading control, we observed that levels of SOCS-1 protein were varied among five CML samples (Figure 2D). These data may support the previous idea that SOCS-1 gene is epigenetically regulated in some, but not all, patients with CML [29].
Next, we examined the SOCS-1 phosphorylation status of the cell lysates derived from the five patients with primary CML using immunoprecipitation experiments. We found that SOCS-1 derived from one of the CML samples was highly tyrosine phosphorylated. In addition, SOCS-1 in two samples was tyrosine phosphorylated to a small degree (Figure 2E). Interestingly, robust activation of JAK2 was detected in the CML sample containing highly tyrosine phosphorylated SOCS-1 (Figure 2F). The data may imply a correlation between SOCS-1 phosphorylation and the activation of JAK2 in CML. Moreover, JAK2 in the other three samples was also observed to be phosphorylated (Figure 2F). The results suggested that the inhibitory function of SOCS-1 may be altered in CML.
Bcr-Abl-Dependent Phosphorylation of SOCS-1 and SOCS-3 Alters Their Inhibitory Effects on JAK1 Activation and Disrupts Interaction between SOCS-1 and Elongin BC Complex
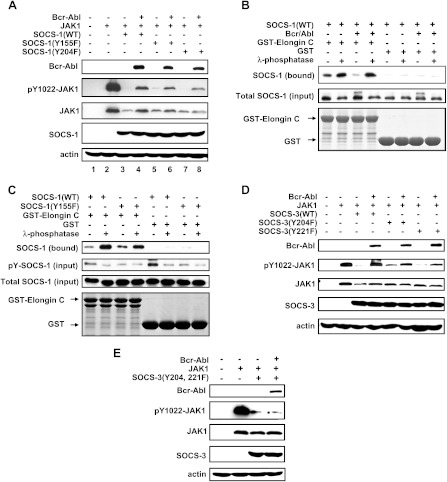
To determine whether Bcr-Abl-dependent tyrosine phosphorylation can alter SOCS-1 function, we investigated the effect of Bcr-Abl on SOCS-1-dependent JAK1 degradation in a transient transfection system using 293T cells. As expected, when SOCS-1 was cotransfected with JAK1, a marked decrease in JAK1 protein and phospho-JAK1 (pJAK1) was observed compared with cells expressing JAK1 alone (Figure 3A). This is consistent with previous studies demonstrating that SOCS-1 targets JAK to the proteasome for degradation [38]. In addition, mutant SOCS-1 carrying either Y155F or Y204F also significantly reduced JAK1 protein levels, demonstrating that this ability was not affected by the mutations. Importantly, when we coexpressed Bcr-Abl with JAK1 and SOCS-1, both JAK1 protein and pJAK1 levels were restored (Figure 3A, lane 4). The expression of Bcr-Abl had no significant effect on the levels of JAK1 protein and pJAK1 (Figure W2A). However, JAK1 and pJAK1 levels in the context of cells expressing SOCS-1(Y155F) or SOCS-1(Y204F) experienced a reduction with respect to those in cells expressing SOCS-1(WT) in the presence of Bcr-Abl (Figure 3A). These observations support the notion that Bcr-Abl signaling inhibits SOCS-1-dependent degradation of activated JAK1 through phosphorylation of SOCS-1.
Figure 3.
Bcr-Abl-dependent phosphorylation of SOCS-1 and SOCS-3 alters their inhibitory effects on JAK1 activation and disrupts interaction between SOCS-1 and Elongin BC complex. (A) JAK1 was cotransfected with empty vector, wild-type, or mutant SOCS-1 with or without Bcr-Abl in 293T cells as described in Figure 1. The levels of protein expression and tyrosine-phosphorylated JAK1(pY-JAK1) were examined by Western blot analysis. (B) The interaction between SOCS-1 and Elongin BC was examined by in vitro binding assays. Glutathione beads coupled to either GST alone or GST-Elongin C were incubated with extracts derived from 293T cells overexpressing Bcr-Abl and SOCS-1 and either treated with λ phosphatase or mock treated. SOCS-1 bound was detected by Western blot analysis. (C) GST pull-down experiments with glutathione beads were performed as described in B. Either GST beads or GST-Elongin C beads were incubated with extracts from K562 cells ectopically expressing SOCS-1(WT) or SOCS-1(Y155F). Cell lysates were either mock or λ phosphatase treated. The immunoblots were probed as indicated. (D and E) 293T cells were cotransfected with JAK1 and either empty vector, SOCS-3(WT), SOCS-3(Y204F), and SOCS-3(Y221F) (D) or SOCS-3(Y204, 221F) (E) with or without Bcr-Abl. Protein levels and pY-JAK1 were examined by Western blot analysis using indicated antibodies.
Because the interaction between SOCS-1 and the Elongin BC complex is thought to link JAK1 to degradation [38,41], we investigated whether Bcr-Abl-dependent phosphorylation of SOCS-1 had any effect on the interaction between SOCS-1 and Elongin C. The results from in vitro binding experiments showed that the amount of SOCS-1 that associated with Elongin C greatly decreased in the presence of Bcr-Abl, whereas the level of bound SOCS-1 dramatically increased when cell extracts were treated with λ phosphatase (Figure 3B). Furthermore, we introduced SOCS-1(WT) or SOCS-1(Y155F) into Bcr-Abl-expressing K562 cells. As expected, mutation of Y155F increased the amount of Elongin C bound SOCS-1 due to decreased tyrosine phosphorylation (Figure 3C). These data suggest that Bcr-Abl-dependent phosphorylation of SOCS-1 disrupts its interaction with Elongin C, and thereby the ability of SOCS-1 to target activated JAK1 to the proteasome is altered.
We next investigated the effects of tyrosine-phosphorylated SOCS-3 on regulating the activation of JAK1. We found that, although JAK1 protein levels were only slightly decreased by coexpressing SOCS-3, a dramatic reduction of pJAK1 was observed in the presence of SOCS-3 (Figure 3D). Interestingly, the results from the experiment coexpressing Bcr-Abl with SOCS-3 and JAK1 showed a restoration of the levels of pJAK1 compared with that in cells expressing JAK1 (Figure 3D). When cells were cotransfected with JAK1 and SOCS-3 (Y204F), SOCS-3(Y221F), or SOCS-3(Y204, 221F), a dramatic decrease in pJAK1 was also observed although the JAK1 protein levels were not significantly changed (Figure 3, D and E). Importantly, even if Bcr-Abl was present, phosphorylation of JAK1 was still maintained at low levels in cells expressing these SOCS-3 mutants (Figure 3, D and E). Together, these results suggest that Bcr-Abl-dependent tyrosine phosphorylation of SOCS-1 and SOCS-3 abolishes their abilities to inhibit the activation of JAK1.
Bcr-Abl-Dependent Phosphorylation of SOCS-1 and SOCS-3 Impairs Their Ability to Negatively Regulate JAK2 Activation
It has been shown that JAK2 is constitutively tyrosine phosphorylated in a number of Bcr-Abl-expressing cells [8]. Because SOCS proteins negatively regulate JAK2 activity, we reasoned that the ability of SOCS proteins to regulate activated JAK2 has been impaired in these cells. To address this possibility, SOCS1 or SOCS-3 was coexpressed with JAK2 and either with or without Bcr-Abl in 293T cells. When overexpressed in 293T cells, JAK2 became activated independently of Bcr-Abl oncoprotein (Figure W2B). Our data showed that the protein levels of JAK2 were not significantly affected by the expression of SOCS-1, SOCS-3, or their mutants, regardless of the presence of Bcr-Abl (Figure 4). In contrast, phosphorylation of JAK2 was dramatically inhibited by these SOCS proteins (Figure 4). Interestingly, when Bcr-Abl was coexpressed with JAK2 and either SOCS-1 or SOCS-3, a marked increase in phospho-JAK2 (pJAK2) levels was observed compared with cells expressing JAK2 and SOCS-1 or SOCS-3 but without Bcr-Abl (Figure 4, A and B, lanes 4). However, this effect was abrogated when tyrosine phosphorylation sites-mutated SOCS-1 or SOCS-3 was expressed in cells (Figure 4). Strikingly, pJAK2 levels in cells expressing Bcr-Abl and SOCS-1(Y204F), SOCS-3(Y221F), or SOCS-3(Y204, 221F) were reduced to levels similar to those observed in the absence of Bcr-Abl (Figure 4, A and B, lanes 8, and C, lane 6). Together, these data suggest that, after being tyrosine phosphorylated in Bcr-Abl-expressing cells, the ability of SOCS-1 and SOCS-3 to negatively regulate JAK2 activation is impaired.
Figure 4.
Bcr-Abl-dependent phosphorylation of SOCS-1 and SOCS-3 impairs their ability to negatively regulate JAK2 activation. (A) 293T cells were cotransfected with JAK2 and empty vector, wild-type, or mutant SOCS-1 in the presence or absence of Bcr-Abl as described in Figure 1. Cell lysates were examined for levels of JAK2, phosphor-JAK2, Bcr-Abl, and SOCS-1 using immunoblot analysis assay. (B and C) Experiments were carried out as described in A. JAK2 was coexpressed with either empty vector, SOCS-3(WT), SOCS-3(Y204F), and SOCS-3(Y221F) (B) or SOCS-3(Y204, 221F) (C) in the presence or absence of Bcr-Abl. Shown are immunoblots probed as indicated.
Activation of JAK/STAT Signaling in Bcr-Abl Positive K562 Leukemic Cells Is Attenuated When Tyrosine Phosphorylation of SOCS-1 or SOCS-3 Is Disrupted
Activated JAK/STAT signaling is thought to play a critical role in Bcr-Abl-mediated tumorigenicity [3,4]. Indeed, we observed that JAK2 and STAT5 were phosphorylated in K562 leukemic cells (Figure 5). To explore whether tyrosine phosphorylation status of SOCS-1 and SOCS-3 determines their ability to negatively regulate JAK/STAT activation in leukemic cells, we generated K562 cell lines stably expressing GFP alone, SOCS-1(WT), SOCS-3(WT), or their mutants using bicistronic retroviruses (Figures W3 and W4). Importantly, our experiments demonstrated that tyrosine phosphorylation of SOCS-1(WT) or SOCS-3(WT) proteins is Bcr-Abl kinase dependent in K562 cells (Figure 5A). The cell lines infected with the retroviruses encoding SOCS or their mutants expressed comparable levels of these proteins (Figure 5, B and C). Interestingly, we observed that, in K562 cells expressing SOCS-1(WT) or SOCS-3(WT), endogenous JAK2 and STAT5 were constitutively activated and SOCS-1 and SOCS-3 were tyrosine phosphorylated (Figure 5, B and C). However, the levels of pJAK2 and pSTAT5 were significantly decreased in cells expressing SOCS-1(Y155F) or SOCS-1(Y204F) compared with the control cells. Surprisingly, SOCS-1(Y204F) displayed more profound effects on the activation of JAK2 and STAT5 than SOCS-1 (Y155F) did, although SOCS-1(Y204F) was phosphorylated to a greater degree than SOCS-1(Y155F) (Figure 5B). The data suggest that Bcr-Abl-dependent tyrosine phosphorylation of SOCS-1 at Y204 within SOCS box is critical for altering SOCS-1 function.
Figure 5.
Activation of JAK/STAT signaling in K562 cells is attenuated when tyrosine phosphorylation of SOCS-1 or SOCS-3 is disrupted. (A) Shown are immunoblots of K562 cells ectopically expressing SOCS-1(WT) or SOCS-3(WT). Cells were treated with or without imatinib and probed with indicated antibodies. (B) K562 cells ectopically expressing Myc-tagged wild-type or mutant SOCS-1 were lysed and examined for endogenous Bcr-Abl, JAK2, phospho-JAK2 (pJAK2), STAT5, and phospho-STAT5 (pSTAT5). The same lysates were immunoprecipitated with anti-Myc antibody (9E10), and precipitated proteins were then examined for SOCS-1 total protein and pY-SOCS-1 levels. (C) Immunoblot of lysates from K562 cells ectopically expressing Flag-tagged wild-type or mutant SOCS-3 was probed as indicated. The lysates were also immunoprecipitated with anti-Flag antibody and then examined for SOCS-3 and pY-SOCS-3 levels by Western blot analysis.
Similarly, the levels of pJAK2 and pSTAT5 were dramatically reduced in K562 cells expressing SOCS-3(Y221F) or SOCS-3(Y204, 221F) without affecting the total protein levels of JAK2 and STAT5 (Figure 5C). K562 cells expressing SOCS-3(Y204F) exhibited a slightly decreased level of pJAK2 but unchanged level of pSTAT5 compared with control cells. Together, these experiments demonstrated that Bcr-Abl-dependent tyrosine phosphorylation of SOCS-1 and SOCS-3 coincided with the activation of JAK2 and STAT5 in K562 leukemic cells.
Disrupting the Tyrosine Phosphorylation of SOCS-1 or SOCS-3 Sensitizes K562 Cells to Undergo Apoptosis
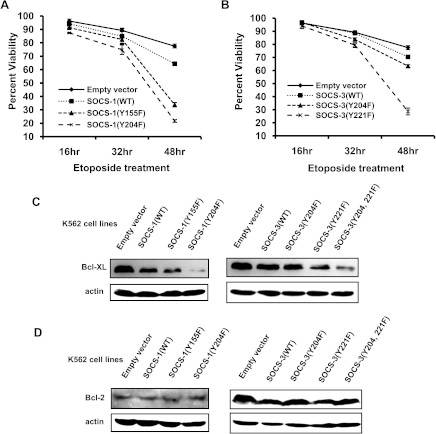
Because activation of JAK2 and STAT5 was inhibited by disrupting the tyrosine phosphorylation of SOCS-1 or SOCS-3 and given that activation of JAK2/STAT5 signaling contributes to increased cell survival, we hypothesized that decreasing the levels of tyrosine-phosphorylated SOCS-1 or SOCS-3 might sensitize K562 cells to undergo apoptosis in response to drug treatment. As shown in Figure 6A, 77.5% of K562 cells expressing GFP control and 64.4% of cells expressing SOCS-1 (WT) remained viable after treatment with etoposide for 48 hours under our culture condition. However, only 33.8% of K562 cells expressing SOCS-1(Y155F) and 21.7% of cells expressing SOCS-1 (Y204F) were viable under the same culture conditions (Figure 6A). As expected, 70.4% of cells expressing SOCS-3(WT) remained viable after treatment with etoposide for 48 hours, which was comparable to that of control cells (Figure 6B). Strikingly, only 28.7% of K562 cells expressing SOCS-3(Y221F) were viable, whereas 63.4% of K562 cells expressing SOCS-3(Y204F) were viable under the same conditions (Figure 6B). Together, these data indicate that disrupting the tyrosine phosphorylation of SOCS-1 or SOCS-3 sensitizes K562 cells to undergo apoptosis.
Figure 6.
Disrupting the tyrosine phosphorylation of SOCS-1 or SOCS-3 promotes K562 cells to undergo apoptosis. (A) K562 cells ectopically expressing empty vector, SOCS-1, or its mutants were treated with 50 µM etoposide at indicated times. Apoptosis and cell survival were analyzed by the propidium iodide staining and measured by flow cytometry. Plotted are the results from three independent experiments. Error bars, SD. (B) K562 cells expressing empty vector, SOCS-3(WT), SOCS-3(Y204F), or SOCS-3(Y221F) were exposed to etoposide and cell survival was examined as described in A. Three independent experiments were performed. Error bars, SD. (C and D) K562 cell lines described above were analyzed by Western blot analysis to detect Bcl-XL protein levels (C) and Bcl-2 protein levels (D).
Previous studies have suggested that inefficient apoptotic signaling in Bcr-Abl transformed cells may be attributed to the STAT5-dependent expression of antiapoptotic Bcl-XL protein [42]. Therefore, we reasoned that increased apoptosis of K562 cells expressing SOCS mutants presented above was likely due to impaired expression of Bcl-XL. To test this possibility, we examined the levels of Bcl-XL and Bcl-2 in K562 cell lines stably expressing GFP control, SOCS-1(WT), SOCS-3 (WT), or their mutants. Indeed, we observed that the level of Bcl-XL significantly decreased in K562 cells expressing SOCS-1(Y155F), SOCS-1(Y204F), SOCS-3(Y221F), or SOCS-3(Y204, 221F) compared with those in cells expressing wild-type SOCS proteins or GFP alone (Figure 6C). In contrast, no significant changes in protein expression of Bcl-2 were seen in cells expressing these SOCS mutants (Figure 6D).
Selective Mutation of Tyrosine Phosphorylation Sites of SOCS-1 or SOCS-3 Completely Blocks Tumor Formation Caused by K562 Cells in Mouse Model
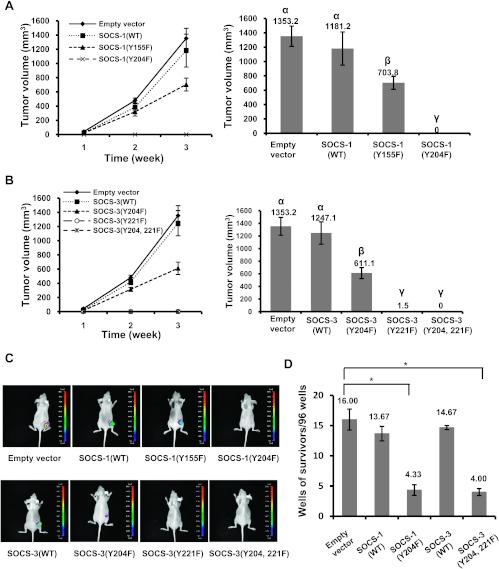
An important extension of our hypothesis was to establish whether tyrosine phosphorylation of SOCS-1 or SOCS-3 is required for Bcr-Abl-induced tumorigensis. To this end, we injected nude mice subcutaneously with K562 cells stably expressing SOCS-1(WT), SOCS-1(Y155F), SOCS-1(Y204F), or GFP alone. Tumor growth was examined every week after inoculation. Tumors were detected about 7 days after inoculation in most of the nude mice challenged with K562 cells expressing SOCS-1(WT), SOCS-1(Y155F), or GFP control. Importantly, tumors formed by cells expressing GFP or SOCS-1(WT) grew clearly faster than tumors formed by cells expressing SOCS-1(Y155F). However, during the 3 weeks after inoculation, tumors were invisible in all mice receiving K562 cells expressing SOCS-1(Y204F), suggesting that phosphorylation of tyrosine 204 residue within SOCS-1 box is required for tumor formation caused by K562 cells (Figures 7, A and C, and W5A).
Figure 7.
Decreasing tyrosine phosphorylation levels of SOCS-1 or SOCS-3 efficaciously inhibits tumor formation in mouse model and transformation of primary murine bone marrow cells. (A) Nude mice were subcutaneously injected with K562 cells stably expressing empty vector, SOCS-1(WT), SOCS-1(Y155F), or SOCS-1(Y204F). The mice were monitored for visible signs of tumor growth, and tumor volumes were measured at indicated time points. The groups labeled by letter a are different from the ones labeled by letter b at the significant level of P ≤ .01, and the groups of a and b are different from the ones labeled by letter c at the significant level of P ≤ .001. Plotted are the results from three independent experiments. Error bars, SD; n = 16. (B) Nude mice were inoculated with K562 cells stably expressing empty vector, SOCS-3(WT), SOCS-3(Y204F), SOCS-3(Y221F), or SOCS-3(Y204, 221F). Tumor growth was analyzed as described in A. Letters a, b, and c indicate the mean values that are different at the significant level of P ≤ .001. Error bars, SD; n = 20. (C) Nude mice were subcutaneously injected with K562 cells as described in A and B. Over a 14-day period after inoculation, tumors formed by K562 cells stably expressing empty vector (GFP), wild-type, or mutant SOCS-1 (upper panel) and wild-type or mutant SOCS-3 (lower panel) were measured by bioluminescent imaging. Shown are representative images from several experiments with similar results. (D) Bone marrow cells from Balb/cmice were infected with retroviruses expressing Bcr-Abl and GFP, SOCS-1(WT), SOCS-1(Y204F), SOCS-3 (WT), or SOCS-3(Y204, 221F) and plated on 96-well plates. Transformation efficiency was scored as described in Materials and Methods. Plotted are the results from three independent experiments. Error bars, SEM; n = 3. *P < .01.
To test the involvement of SOCS-3 phosphorylation in tumor formation, nude mice were inoculated subcutaneously with K562 cells expressing SOCS-3(WT), its mutants, or GFP control. We found that tumor growth was inhibited by Y204F mutation and was completely blocked by Y221F mutation or Y204/221F double mutation of SOCS-3 (Figures 7, B and C, and W5B). These experiments were repeated at least three times to ensure specificity of the results and consistency of data.
To further examine the involvement of tyrosine phosphorylation of SOCS-1 and SOCS-3 in Bcr-Abl-mediated cellular transformation, we generated bicistronic retroviruses encoding Bcr-Abl and GFP, SOCS-1(WT), SOCS-3(WT), SOCS-1(Y204F), or SOCS-3(Y204, 221F) because these mutants had profound effect on the tumor growth (Figure W6). Primary murine bone marrow cells were infected with equal titer of the viruses and the capacity of these viruses to transform bone marrow cells was measured by counting the number of Bcr-Abl-transformed cell clones. As shown in Figure 7D, cells infected with viruses carrying Bcr-Abl-IRES-GFP, Bcr-Abl-IRES-SOCS-1 (WT), or Bcr-Abl-IRES-SOCS-3(WT) displayed Bcr-Abl transformation with average results of 16.00, 13.67, and 14.67 wells, showing growth of cell clones per 96-well plate, respectively. Importantly, under the same conditions, expression of SOCS-1(Y204F) or SOCS-3 (Y204, 221F) significantly decreased Bcr-Abl transformation efficiency to 4.33 and 4.00 wells per 96-well plate, respectively (Figure 7D). Taken together, these experiments provide strong evidence that Bcr-Abl-mediated tumorigenesis critically requires robust tyrosine phosphorylation of SOCS-1 and SOCS-3 when these SOCS proteins are present in the cells.
Discussion
SOCS proteins are identified as negative regulators of JAK/STAT signaling and play important roles in many immunologic and pathologic processes [43,44]. A previous study has shown that v-Abl can bypass SOCS-1 inhibition and reduce its ability to inhibit JAK1 activation through phosphorylation of SOCS-1 [38]. It has been shown that SOCS-3 is tyrosine phosphorylated in cells stimulated with cytokines such as IL-2, IL-3, and growth factors [32]. Interestingly, the myeloproliferative disorder-associated JAK2(V617F) mutant can escape negative regulation of SOCS-3 through tyrosine phosphorylation of this SOCS protein [33]. Although JAK/STAT signaling plays an important role in Bcr-Abl-induced tumorigenicity, the precise mechanism by which Bcr-Abl overcomes regulatory effects of SOCS proteins and imparts constitutive activation of JAK/STAT signaling is still unknown. Here, our experiments provide the first evidence that SOCS-1 and SOCS-3 are both tyrosine phosphorylated in a Bcr-Abl-dependent manner. We have further identified the Bcr-Abl-dependent tyrosine phosphorylation sites of SOCS-1 and SOCS-3. These observations imply that Bcr-Abl may alter function of SOCS-1 and SOCS-3 through robust tyrosine phosphorylation of these SOCS proteins to constitutively activate JAK/STAT signaling. However, although our results indicate that Bcr-Abl is associated with SOCS-1 and SOCS-3 in cells, it is still unclear whether the binding between Bcr-Abl and SOCS is direct and whether Bcr-Abl directly phosphorylates SOCS proteins. Conversely, it is also unclear whether this phosphorylation is important in physiological (non-CML) setting. These issues remain to be further addressed.
Our data show that Bcr-Abl-dependent phosphorylation of SOCS-1 and SOCS-3 diminishes their inhibitory effects on JAK1 and JAK2 activation. Importantly, the results reveal that Bcr-Abl-dependent tyrosine phosphorylation of SOCS proteins impairs their activity to negatively regulate STAT5 activation in K562 leukemic cells. In addition, we demonstrate that disrupting the tyrosine phosphorylation of SOCS-1 or SOCS-3 sensitizes K562 cells to undergo apoptosis. Consistent with this altered apoptosis profile, a decreased level of Bcl-XL was detected in K562 cells expressing the phosphorylation site-mutated SOCS proteins. Because expression of Bcl-XL is transcriptionally activated by STAT5 [42], it is most likely that ectopically expressed SOCS mutants inactivate STAT5 and thereby suppress STAT5-dependent expression of Bcl-XL, which may contribute to the enhanced apoptosis of the cells. Interestingly, we further found that selective targeting of tyrosine phosphorylation sites of SOCS-1 or SOCS-3 completely blocks tumor formation caused by K562 cells in nude mouse model and significantly inhibits Bcr-Abl-mediated murine bone marrow transformation. These experiments provide strong evidence that Bcr-Abl-mediated tumorigenesis critically requires inability of SOCS-1 and SOCS-3 through robust tyrosine phosphorylation of these SOCS proteins when they are present in the cells.
It was interesting to determine whether tyrosine phosphorylation of SOCS-1 and SOCS-3 also occurs in other Abl-transformed cell lines besides K562 cell. To test this possibility, we examined the SOCS-1 and SOCS-3 phosphorylation status in a v-Abl-transformed cell line described previously [37]. Interestingly, we detected significant amount of tyrosine phosphorylated SOCS-3 but very low level of SOCS-1 tyrosine phosphorylation in the v-Abl-transformed cells ectopically expressing these SOCS proteins (Figure W7). These data are consistent with a previous study suggesting that v-Abl signaling leads to SOCS-1 phosphorylation mainly on nontyrosine residues [38]. In addition, we found previously that expression of Pim kinases downstream of v-Abl signaling resulted in an increased amount of phosphorylated SOCS-1 and thereby promoted v-Abl-mediated cellular transformation [37]. Based on these data, it is likely that Pim kinases are involved in v-Abl-mediated SOCS-1 phosphorylation [37,38]. Together, these experiments demonstrated that Abl oncogenes may alter SOCS function through the phosphorylation of these SOCS proteins on tyrosine or nontyrosine residues.
Both SOCS-1 and SOCS-3 contain a highly conserved C-terminal region termed SOCS box. The SOCS boxes of SOCS-1 and SOCS-3 have been thought to participate in the formation of an E3 ubiquitin ligase complex that is assumed to degrade the activated signaling complex [45]. Interestingly, although Bcr-Abl-dependent tyrosine phosphorylation of SOCS-1 occurs on Tyr 81, Tyr 155, and Tyr 204 residues, Y204F mutation seems to have the strongest impact on activation of JAK2 and STAT5. Our results indicate that Tyr 204 within SOCS-1 box and Tyr 221 within SOCS-3 box are key residues for altering SOCS function through phosphorylation. These data suggest that SOCS boxes of these SOCS proteins are critical for SOCS activity to negatively regulate JAK and STAT5 activation downstream of Bcr-Abl signaling. Previous studies revealed that v-Abl signaling could lead to phosphorylation of SOCS-1 on nontyrosine residues [38]. The present report is the first one to assess the tyrosine phosphorylation status of SOCS-1 and SOCS-3 in Bcr-Abl-expressing cells. The question of whether Bcr-Abl signaling, like v-Abl, can lead to SOCS phosphorylation on nontyrosine residues remains to be further determined.
Although methylation of SOCS-1 gene has been observed in patients with CML [29], there is increasing evidence that SOCS-1 is constitutively expressed in CML samples [31]. More recently, SOCS-1 expression was further confirmed in more than 50% of patients with CML [46]. The constitutive expression of SOCS-3 was also previously found in most CML cell lines that are resistant to treatment with IFN-α [47]. Furthermore, most of the blast cells from patients in CML blast crisis showed constitutive expression of SOCS-3 [47]. SOCS-1 and SOCS-3 are known potent inhibitors of JAK/STAT signaling. However, the mechanism by which Bcr-Abl bypasses SOCS regulation to constitutively activate JAK/STAT pathway in CML cells has not been explored. In this study, tyrosine-phosphorylated SOCS-1 was detected in three of five primary CML samples, which express Bcr-Abl. We understand that our CML sample size is limited, and our sample set did not enable us to dissect protein expression and phosphorylation of many signal transduction molecules at various levels to identify sites of potential pathway activation after altering the SOCS function in CML cells. Another large-scale study could increase the statistical power of our results obtained from CML samples. Also, we did not investigate the SOCS-3 expression in CML patients in this study, which remains an ongoing task.
In summary, we demonstrate that Bcr-Abl-dependent tyrosine phosphorylation of SOCS-1 and SOCS-3 alters inhibitory function of these SOCS proteins. On the basis of these findings, our model suggests that SOCS needs to be bypassed for transformation to occur and may reveal a mechanism by which Abl oncogenes overcome SOCS-1 and SOCS-3 inhibition. Thus, SOCS may be therapeutically useful for treatment of Abl-induced malignancies known to involve constitutive activation of JAK/STAT signaling.
Supplementary Material
Abbreviations
- SOCS
suppressor of cytokine signaling
- JAK
Janus tyrosine kinase
- STAT
signal transducers and activators of transcription
- CML
chronic myelogenous leukemia
Footnotes
This work was supported by National Basic Research Program (973) of China (2009CB918902), Natural Science Foundation of China (81171943, 30971476), intramural grant of the Chinese Academy of Sciences (2010-Biols-CAS-0204), and Hundreds of Talents Program of Chinese Academy of Sciences 2009-2013.
This article refers to supplementary materials, which are designated by Figures W1 to W7 and are available online at www.neoplasia.com.
References
- 1.Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol. 2007;178:2623–2629. doi: 10.4049/jimmunol.178.5.2623. [DOI] [PubMed] [Google Scholar]
- 2.Danial NN, Rothman P. JAK-STAT signaling activated by Abl oncogenes. Oncogene. 2000;19:2523–2531. doi: 10.1038/sj.onc.1203484. [DOI] [PubMed] [Google Scholar]
- 3.Van Etten RA. Oncogenic signaling: new insights and controversies from chronic myeloid leukemia. J Exp Med. 2007;204:461–465. doi: 10.1084/jem.20062335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helgason GV, Karvela M, Holyoake TL. Kill one bird with two stones: potential efficacy of BCR-ABL and autophagy inhibition in CML. Blood. 2011;118:2035–2043. doi: 10.1182/blood-2011-01-330621. [DOI] [PubMed] [Google Scholar]
- 5.Chai SK, Nichols GL, Rothman P. Constitutive activation of JAKs and STATs in BCR-Abl-expressing cell lines and peripheral blood cells derived from leukemic patients. J Immunol. 1997;159:4720–4728. [PubMed] [Google Scholar]
- 6.Shuai K, Halpern J, ten Hoeve J, Rao X, Sawyers CL. Constitutive activation of STAT5 by the BCR-ABL oncogene in chronic myelogenous leukemia. Oncogene. 1996;13:247–254. [PubMed] [Google Scholar]
- 7.Ilaria RL, Jr, Van Etten RA. P210 and P190(BCR/ABL) induce the tyrosine phosphorylation and DNA binding activity of multiple specific STAT family members. J Biol Chem. 1996;271:31704–31710. doi: 10.1074/jbc.271.49.31704. [DOI] [PubMed] [Google Scholar]
- 8.Xie S, Wang Y, Liu J, Sun T, Wilson MB, Smithgall TE, Arlinghaus RB. Involvement of Jak2 tyrosine phosphorylation in Bcr-Abl transformation. Oncogene. 2001;20:6188–6195. doi: 10.1038/sj.onc.1204834. [DOI] [PubMed] [Google Scholar]
- 9.Samanta AK, Lin H, Sun T, Kantarjian H, Arlinghaus RB. Janus kinase 2: a critical target in chronic myelogenous leukemia. Cancer Res. 2006;66:6468–6472. doi: 10.1158/0008-5472.CAN-06-0025. [DOI] [PubMed] [Google Scholar]
- 10.Samanta A, Perazzona B, Chakraborty S, Sun X, Modi H, Bhatia R, Priebe W, Arlinghaus R. Janus kinase 2 regulates Bcr-Abl signaling in chronic myeloid leukemia. Leukemia. 2011;25:463–472. doi: 10.1038/leu.2010.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warsch W, Kollmann K, Eckelhart E, Fajmann S, Cerny-Reiterer S, Holbl A, Gleixner KV, Dworzak M, Mayerhofer M, Hoermann G, et al. High STAT5 levels mediate imatinib resistance and indicate disease progression in chronic myeloid leukemia. Blood. 2011;117:3409–3420. doi: 10.1182/blood-2009-10-248211. [DOI] [PubMed] [Google Scholar]
- 12.Nelson EA, Walker SR, Weisberg E, Bar-Natan M, Barrett R, Gashin LB, Terrell S, Klitgaard JL, Santo L, Addorio MR, et al. The STAT5 inhibitor pimozide decreases survival of chronic myelogenous leukemia cells resistant to kinase inhibitors. Blood. 2011;117:3421–3429. doi: 10.1182/blood-2009-11-255232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoelbl A, Schuster C, Kovacic B, Zhu B, Wickre M, Hoelzl MA, Fajmann S, Grebien F, Warsch W, Stengl G, et al. Stat5 is indispensable for the maintenance of Bcr/Abl-positive leukaemia. EMBO Mol Med. 2010;2:98–110. doi: 10.1002/emmm.201000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wormald S, Hilton D. Inhibitors of cytokine signal transduction. J Biol Chem. 2003;279:821–824. doi: 10.1074/jbc.R300030200. [DOI] [PubMed] [Google Scholar]
- 15.Zhang JG, Farley A, Nicholson SE, Willson TA, Zugaro LM, Simpson RJ, Moritz RL, Cary D, Richardson R, Hausmann G, et al. The conserved SOCS box motif in suppressors of cytokine signaling binds to elongins B and C and may couple bound proteins to proteasomal degradation. Proc Natl Acad Sci USA. 1999;96:2071–2076. doi: 10.1073/pnas.96.5.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ungureanu D, Saharinen P, Junttila I, Hilton DJ, Silvennoinen O. Regulation of Jak2 through the ubiquitin-proteasome pathway involves phosphorylation of Jak2 on Y1007 and interaction with SOCS-1. Mol Cell Biol. 2002;22:3316–3326. doi: 10.1128/MCB.22.10.3316-3326.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waiboci LW, Ahmed CM, Mujtaba MG, Flowers LO, Martin JP, Haider MI, Johnson HM. Both the suppressor of cytokine signaling 1 (SOCS-1) kinase inhibitory region and SOCS-1 mimetic bind to JAK2 autophosphorylation site: implications for the development of a SOCS-1 antagonist. J Immunol. 2007;178:5058–5068. doi: 10.4049/jimmunol.178.8.5058. [DOI] [PubMed] [Google Scholar]
- 18.Galm O, Yoshikawa H, Esteller M, Osieka R, Herman JG. SOCS-1, a negative regulator of cytokine signaling, is frequently silenced by methylation in multiple myeloma. Blood. 2003;101:2784–2788. doi: 10.1182/blood-2002-06-1735. [DOI] [PubMed] [Google Scholar]
- 19.Yoshikawa H, Matsubara K, Qian GS, Jackson P, Groopman JD, Manning JE, Harris CC, Herman JG. SOCS-1, a negative regulator of the JAK/STAT pathway, is silenced by methylation in human hepatocellular carcinoma and shows growth-suppression activity. Nat Genet. 2001;28:29–35. doi: 10.1038/ng0501-29. [DOI] [PubMed] [Google Scholar]
- 20.Saudemont A, Hamrouni A, Marchetti P, Liu J, Jouy N, Hetuin D, Colucci F, Quesnel B. Dormant tumor cells develop cross-resistance to apoptosis induced by CTLs or imatinib mesylate via methylation of suppressor of cytokine signaling 1. Cancer Res. 2007;67:4491–4498. doi: 10.1158/0008-5472.CAN-06-1627. [DOI] [PubMed] [Google Scholar]
- 21.Melzner I, Bucur AJ, Bruderlein S, Dorsch K, Hasel C, Barth TF, Leithauser F, Moller P. Biallelic mutation of SOCS-1 impairs JAK2 degradation and sustains phospho-JAK2 action in the MedB-1 mediastinal lymphoma line. Blood. 2005;105:2535–2542. doi: 10.1182/blood-2004-09-3701. [DOI] [PubMed] [Google Scholar]
- 22.Weniger MA, Melzner I, Menz CK, Wegener S, Bucur AJ, Dorsch K, Mattfeldt T, Barth TFE, Möller P. Mutations of the tumor suppressor gene SOCS-1 in classical Hodgkin lymphoma are frequent and associated with nuclear phospho-STAT5 accumulation. Oncogene. 2006;25:2679–2684. doi: 10.1038/sj.onc.1209151. [DOI] [PubMed] [Google Scholar]
- 23.Mottok A, Renne C, Willenbrock K, Hansmann ML, Brauninger A. Somatic hypermutation of SOCS1 in lymphocyte-predominant Hodgkin lymphoma is accompanied by high JAK2 expression and activation of STAT6. Blood. 2007;110:3387–3390. doi: 10.1182/blood-2007-03-082511. [DOI] [PubMed] [Google Scholar]
- 24.He B, You L, Uematsu K, Zang K, Xu Z, Lee AY, Costello JF, McCormick F, Jablons DM. SOCS-3 is frequently silenced by hypermethylation and suppresses cell growth in human lung cancer. Proc Natl Acad Sci USA. 2003;100:14133–14138. doi: 10.1073/pnas.2232790100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niwa Y, Kanda H, Shikauchi Y, Saiura A, Matsubara K, Kitagawa T, Yamamoto J, Kubo T, Yoshikawa H. Methylation silencing of SOCS-3 promotes cell growth and migration by enhancing JAK/STAT and FAK signalings in human hepatocellular carcinoma. Oncogene. 2005;24:6406–6417. doi: 10.1038/sj.onc.1208788. [DOI] [PubMed] [Google Scholar]
- 26.Weber A, Hengge UR, Bardenheuer W, Tischoff I, Sommerer F, Markwarth A, Dietz A, Wittekind C, Tannapfel A. SOCS-3 is frequently methylated in head and neck squamous cell carcinoma and its precursor lesions and causes growth inhibition. Oncogene. 2005;24:6699–6708. doi: 10.1038/sj.onc.1208818. [DOI] [PubMed] [Google Scholar]
- 27.Barclay JL, Anderson ST, Waters MJ, Curlewis JD. SOCS3 as a tumor suppressor in breast cancer cells, and its regulation by PRL. Int J Cancer. 2009;124:1756–1766. doi: 10.1002/ijc.24172. [DOI] [PubMed] [Google Scholar]
- 28.Brender C, Lovato P, Sommer VH, Woetmann A, Mathiesen AM, Geisler C, Wasik M, Odum N. Constitutive SOCS-3 expression protects T-cell lymphoma against growth inhibition by IFNα. Leukemia. 2005;19:209–213. doi: 10.1038/sj.leu.2403610. [DOI] [PubMed] [Google Scholar]
- 29.Liu TC, Lin SF, Chang JG, Yang MY, Hung SY, Chang CS. Epigenetic alteration of the SOCS1 gene in chronic myeloid leukaemia. Br J Haematol. 2003;123:654–661. doi: 10.1046/j.1365-2141.2003.04660.x. [DOI] [PubMed] [Google Scholar]
- 30.Hatirnaz O, Ure U, Ar C, Akyerli C, Soysal T, Ferhanoğlu B, Özçelik T, Ozbek U. The SOCS-1 gene methylation in chronic myeloid leukemia patients. Am J Hematol. 2007;82:729–730. doi: 10.1002/ajh.20886. [DOI] [PubMed] [Google Scholar]
- 31.Roman-Gomez J, Jimenez-Velasco A, Castillejo JA, Cervantes F, Barrios M, Colomer D, Heiniger A, Torres A. The suppressor of cytokine signaling-1 is constitutively expressed in chronic myeloid leukemia and correlates with poor cytogenetic response to interferon-α. Haematologica. 2004;89:42–48. [PubMed] [Google Scholar]
- 32.Cacalano NA, Sanden D, Johnston JA. Tyrosine-phosphorylated SOCS-3 inhibits STAT activation but binds to p120 RasGAP and activates Ras. Nat Cell Biol. 2001;3:460–465. doi: 10.1038/35074525. [DOI] [PubMed] [Google Scholar]
- 33.Hookham MB, Elliott J, Suessmuth Y, Staerk J, Ward AC, Vainchenker W, Percy MJ, McMullin MF, Constantinescu SN, Johnston JA. The myeloproliferative disorder-associated JAK2 V617F mutant escapes negative regulation by suppressor of cytokine signaling 3. Blood. 2007;109:4924–4929. doi: 10.1182/blood-2006-08-039735. [DOI] [PubMed] [Google Scholar]
- 34.Elliott J, Suessmuth Y, Scott LM, Nahlik K, McMullin MF, Constantinescu SN, Green AR, Johnston JA. SOCS3 tyrosine phosphorylation as a potential bio-marker for myeloproliferative neoplasms associated with mutant JAK2 kinases. Haematologica. 2009;94:576–580. doi: 10.3324/haematol.2008.002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen XP, Losman JA, Cowan S, Donahue E, Fay S, Vuong BQ, Nawijn MC, Capece D, Cohan VL, Rothman P. Pim serine/threonine kinases regulate the stability of Socs-1 protein. Proc Natl Acad Sci USA. 2002;99:2175–2180. doi: 10.1073/pnas.042035699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo G, Qiu X, Wang S, Chen Y, Rothman PB, Wang Z, Wang G, Chen JL. Oncogenic E17K mutation in the pleckstrin homology domain of AKT1 promotes v-Abl-mediated pre-B-cell transformation and survival of Pim-deficient cells. Oncogene. 2010;29:3845–3853. doi: 10.1038/onc.2010.149. [DOI] [PubMed] [Google Scholar]
- 37.Chen JL, Limnander A, Rothman PB. Pim-1 and Pim-2 kinases are required for efficient pre-B-cell transformation by v-Abl oncogene. Blood. 2008;111:1677–1685. doi: 10.1182/blood-2007-04-083808. [DOI] [PubMed] [Google Scholar]
- 38.Limnander A, Danial NN, Rothman PB. v-Abl signaling disrupts SOCS-1 function in transformed pre-B cells. Mol Cell. 2004;15:329–341. doi: 10.1016/j.molcel.2004.06.041. [DOI] [PubMed] [Google Scholar]
- 39.MacPartlin M, Smith AM, Druker BJ, Honigberg LA, Deininger MW. Bruton's tyrosine kinase is not essential for Bcr-Abl-mediated transformation of lymphoid or myeloid cells. Leukemia. 2008;22:1354–1360. doi: 10.1038/leu.2008.126. [DOI] [PubMed] [Google Scholar]
- 40.Ko J, Patel N, Ikawa T, Kawamoto H, Frank O, Rivera RR, Van Etten RA, Murre C. Suppression of E-protein activity interferes with the development of BCR-Abl-mediated myeloproliferative disease. Proc Natl Acad Sci USA. 2008;105:12967–12972. doi: 10.1073/pnas.0805073105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kamizono S, Hanada T, Yasukawa H. The SOCS box of SOCS-1 accelerates ubiquitin-dependent proteolysis of TEL-JAK2. J Biol Chem. 2001;276:12530–12538. doi: 10.1074/jbc.M010074200. [DOI] [PubMed] [Google Scholar]
- 42.Gesbert F, Griffin JD. Bcr/Abl activates transcription of the Bcl-X gene through STAT5. Blood. 2000;96:2269–2276. [PubMed] [Google Scholar]
- 43.Yoshimura A, Nishinakamura H, Matsumura Y, Hanada T. Negative regulation of cytokine signaling and immune responses by SOCS proteins. Arthritis Res Ther. 2005;7:100–110. doi: 10.1186/ar1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007;7:454–465. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- 45.Boyle K, Zhang JG, Nicholson SE, Trounson E, Babon JJ, McManus EJ, Nicola NA, Robb L. Deletion of the SOCS box of suppressor of cytokine signaling 3 (SOCS3) in embryonic stem cells reveals SOCS box-dependent regulation of JAK but not STAT phosphorylation. Cell Signal. 2009;21:394–404. doi: 10.1016/j.cellsig.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghaith F, Abdou S, El-Bendary A, Shahin D, Eid M, Megeed WA, El-Sheikh I, Farrag W, Yousuf S. Prognostic relevance of 9q34 deletion and the suppressor of cytokine signalling-1 in CML patients. Int J Lab Hematol. 2010;32:103–112. doi: 10.1111/j.1751-553X.2009.01143.x. [DOI] [PubMed] [Google Scholar]
- 47.Sakai I, Takeuchi K, Yamauchi H, Narumi H, Fujita S. Constitutive expression of SOCS3 confers resistance to IFN-α in chronic myelogenous leukemia cells. Blood. 2002;100:2926–2931. doi: 10.1182/blood-2002-01-0073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.