Abstract
Three major types of opioid receptors, μ (MOR), δ (DOR), and κ (KOR), have been cloned and characterized. Each opioid receptor exhibits a distinct pharmacological profile as well as a distinct pattern of temporal and spatial expression in the brain, suggesting the critical role of transcription regulatory elements and their associated factors. Here, we report the identification of a minimum core promoter, in the 5′-flanking region of the mouse DOR gene, containing an E box and a GC box that are crucial for DOR promoter activity in NS20Y cells, a DOR-expressing mouse neuronal cell line. In vitro protein-DNA binding assays and in vivo transient transfection assays indicated that members of both the upstream stimulatory factor and Sp families of transcription factors bound to and trans-activated the DOR promoter via the E box and GC box, respectively. Furthermore, functional and physical interactions between these factors were critical for the basal as well as maximum promoter activity of the DOR gene. Thus, the distinct developmental emergence and brain regional distribution of the δ opioid receptor appear to be controlled, at least in part, by these two regulatory elements and their associated factors.
Opioids induce pharmacological as well as other physiological and cellular effects via opioid receptors (ORs)1 (1). Three major types of opioid receptors have been identified and cloned, namely the μ (MOR), δ (DOR), and κ (KOR), opioid receptors (2). ORs belong to the superfamily of G-protein-coupled receptors (3) modulating endocrine, immune, cardiovascular, and gastrointestinal functions. While all three ORs mediate opioid-induced analgesia, each receptor type displays a distinct pharmacological profile and a unique cell-type specific distribution pattern (4–5). Distinct molecular mechanisms probably coordinate the temporal and spatial expression of each receptor, but little is known of the regulatory elements and their associated transcription factors involved in the restricted expression of ORs.
In general, the localization of the ORs coincides with the pharmacological actions of the opioids (4). In the case of DOR, there is also a good correlation between the presence of DOR mRNA and δ-agonist binding sites (6). Although DOR is found in the peripheral nervous system and also in some immune cells, it is mainly confined to the central nervous system, in various densities at different regions of the brain (5). In addition, the expression of DOR is also tightly controlled during development. DOR appears later than MOR and KOR; in fact, DOR is not detectable prior to the postnatal stages of development, and even then its emergence lags behind than that of MOR and KOR (7). Levels of DOR mRNA or of δ-agonist binding sites can also be regulated by certain inducers in some cell lines. For example, DOR mRNA can be up-regulated by nerve growth factor (8), ethanol (9), or retinoic acid (10). In contrast, a reduction of DOR mRNA was also observed in the presence of prostaglandin E1, forskolin, or cyclic AMP analogue (11). Finally, DOR levels may be altered in certain disease states, such as inflammation (12) and lung cancer (13). All of these studies, together with the heterogeneity of the cell-type specific distribution, suggest that the spatial and temporal expression of DOR is under strict control, able to respond to specific physiological and pathological parameters. Therefore, to understand the molecular mechanism of the restricted expression pattern of DOR will be helpful to gain insights of its functions corresponding to different development status and physiological settings.
We previously reported isolation of mouse genomic clones of DOR, from which we determined two major transcription initiation sites, the exon-intron structure and 1.3-kb 5′-flanking sequence (14). The isolation and partial analysis of mouse genomic DNA of DOR has made it possible to study regulatory elements of the DOR gene. A 1.3-kb DNA fragment upstream from the translation start site (−1300 to +1 bp, with the translation start site designated as +1) of the DOR 5′-flanking region was sequenced and its sequence analyzed by sequence comparison with the Transcription Factors Database (15). This analysis indicated that the DOR gene has the features of a typical “housekeeping” gene. Thus, it lacks a classical TATA box, and there is no CCAAT box or a consensus initiator (16–18). However, the promoter region of the DOR gene is rich in G+C content and possesses several putative GC boxes. It has been suggested that the GC box is the binding site for members of the Sp1 transcription factor family (19–21). It is also known that Sp1 is important for both TATA and TATA-less promoters by interacting with TFIID (21) and involved in the transcription regulation of some cell- or tissue-specific genes (53–55). Therefore, to define the basal promoter of the DOR gene will be the foundation for elucidating the molecular mechanism of DOR expression.
Here, we report a functional analysis of the DOR promoter region in NS20Y, a mouse neuroblastoma cell line that constitutively expresses DOR. Using in vivo functional assays and in vitro protein-DNA binding assays, we have defined a minimal DOR promoter. We also demonstrate that the functional necessity of a putative Sp1 binding site as well as an E box for the transcription activation of DOR. We show that the E box and GC box, as well as the simultaneous binding and functional synergy between their associated factors, are crucial for the promoter activity of the DOR gene.
MATERIALS AND METHODS
Plasmid Construction
Luciferase fusion plasmids were constructed containing 1300 bp of upstream regulatory sequence (pD1300 construct; −1300 to +1 bp related to the translation start site as +1) or shorter upstream regulatory sequences of the mouse DOR gene. The pD1300 construct was created by ligating the 1300-bp fragment from the DOR promoter region with SacI and NcoI sites into a promoterless and enhancerless luciferase vector, pGL3-basic (Promega). The 5′-deletion (pD890, pD675, pD262, pD182, and pD150) and 3′-deletion (pD1300/150, pD1300/182, pD1300/262, and pD1300/675) constructs were generated by restriction digestion, blunt-ended by fill-in reaction, and religation. The 5′-deletion construct pD210 and the 3′-deletion construct pD1300/232 were prepared by recombinant polymerase chain reaction (PCR) with all of the upstream primers bearing the KpnI site and the downstream primers bearing the NcoI site. The PCR products were subcloned into KpnI and NcoI sites of the pGL3-basic vector, and the correct clones were confirmed by sequencing. The pD262/141 and pD141/262 constructs were prepared by PCR amplification of the DOR promoter fragment −262 to −141 with primers bearing a HindIII site. Then the PCR products were digested with HindIII and cloned into the HindIII site of the pGL3-basic vector. The correct clones of pD262/141 and pD141/262 were determined by sequencing. A HindIII linker mutation was introduced into the pD262 construct by PCR to generate a series of linker mutation constructs throughout the DOR promoter region (from −262 to −137). The mutated DOR promoter fragments (−262 to +1) of mutant constructs were sequenced and subcloned into pGL3-basic with NcoI and KpnI to generate linker scan mutant constructs pDm262 through pDm142. Each mutation construct in the linker scan analysis was designated by the position of the 5′-end nucleotide of its mutated sequence. Thus pDm262 represents the mutation construct containing the mutated sequence (AAGCTT) at positions −262 to −257; all other linker scan mutant constructs (pDm) contain the mutated sequence in the six nucleotides beginning with the indicated number. The double mutation construct, pD262Sp*/E*, was created using PCR to introduce a second HindIII linker into pDm226 and to replace the E box (CACGTG) with AAGCTT sequence. All of the mutation constructs were confirmed by DNA sequencing.
Cell Culture
Mouse neuroblastoma NS20Y cells were grown in DMEM medium with 10% heat-inactivated fetal calf serum in an atmosphere of 10% CO2 and 90% air at 37 °C. Schneider’s Drosophila line 2 (SL2) cells were grown at 22–24 °C in Schneider’s Drosophila medium (Life Technologies, Inc.) containing 10% heat-inactivated fetal calf serum.
Transient Transfection and Reporter Gene Activity Assay
NS20Y cells were transfected using the DOTAP (Roche Molecular Biochemicals) lipofection method as described previously (22). Briefly, cells at approximately 40% confluence were transfected with an equimolar amount of each test plasmid. Forty-eight hours after transfection, cells grown to confluence were washed and lysed with lysis buffer (Promega). To control for differences in transfection efficiency from dish to dish, a one-fifth molar ratio of pCH110 plasmid (Amersham Pharmacia Bio-tech) containing the β-galactosidase gene driven by the SV40 promoter was included in each transfection and used for normalization. Drosophila SL2 cells were transfected with CellFECTIN™ (Life Technologies, Inc.) as described in our previous report (23). Briefly, for each transfection, test plasmid and CellFECTIN were mixed and incubated at room temperature for 30 min, before adding to SL2 cells. Forty-eight hours after transfection, cells were washed and lysed. Normalization of the samples in the SL2 transient transfection followed the method described by Conn et. al. (24). The luciferase and β-galactosidase activities of each lysate were determined as described by the manufacturers (Promega and Tropix, respectively).
Nuclear Extract Preparation
Nuclear extracts were prepared from NS20Y cells using the method described by Johnson et. al (25). Briefly, cells were grown to confluence, harvested, and washed with phosphate-buffered saline. All of the following steps were performed at 4 °C. The cells were resuspended in sucrose buffer (0.32 M sucrose, 3 mM CaCl2, 2 mM magnesium acetate, 0.1 mM EDTA, 10 mM Tris-HCl, pH 8.0, 1 mM dithiothreitol (DTT), 0.5 mM PMSF, and 0.5% Nonidet P-40). The lysate was microcentrifuged at 500 × g for 5 min to pellet the nuclei, which were washed with sucrose buffer without Nonidet P-40. The nuclei were resuspended in low salt buffer (20 mM Hepes, pH 7.9, 25% glycerol, 0.02 M KCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM DTT, and 0.5 mM PMSF), followed by addition of high salt buffer to extract the nuclei, with incubation for 20 min on a rotary platform. Diluent (2.5 vol. of 25 mM Hepes, pH 7.6, 25% glycerol, 0.1 mM EDTA, 0.5 mM DTT, and 0.5 mM PMSF) was added and the sample was microcentrifuged at 13,690 × g for 15 min. Aliquots of the supernatant (nuclear extract) were stored at −80 °C.
Electrophoretic Mobility Shift Assay (EMSA)
EMSA was performed with 32P-labeled double-stranded oligonucleotides that were incubated with nuclear extract in EMSA buffer (10 mM Tris, pH 7.5, 5% glycerol, 1 mM EDTA, pH 7.1, 50 mM NaCl, 1 mM DTT, 1 mM EDTA, and 0.1 mg/ml poly(dI-dC)). For oligonucleotide competition analysis, a 100-fold (or as indicated in figures) molar excess of competitor oligonucleotides was also added to the mixture. After incubation at 22 °C for 30 min, the mixture was analyzed on 5% nondenaturing polyacrylamide gels. For antibody supershift assays, 1 μl of monoclonal antibodies to Sp1, Sp2, Sp3, Sp4, USF1, USF2, or c-Myc (Santa Cruz Biotechnology, Inc.) was added to the mixture. The reaction was then incubated on ice for 1 h. Protein-DNA complexes and free DNA were fractionated on 5% poly-acrylamide gels in 1× Tris-glycine EDTA buffer (50 mM Tris, pH 8.3, 380 mM glycine, and 2 mM EDTA) at 4 °C and were visualized by autoradiography.
RESULTS
Functional Analysis of Promoter Activity of the Mouse DOR Gene
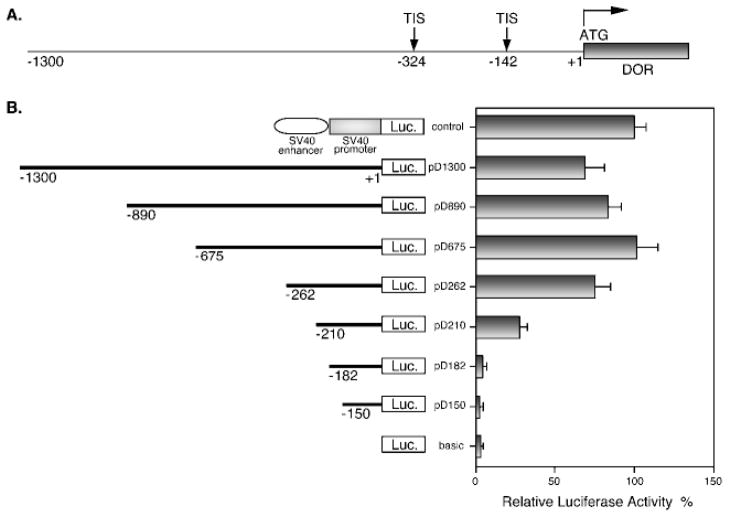
In order to identify the functional DOR promoter and elucidate the regulatory elements of the DOR gene, serial deletional analyses were performed with the 1.3-kb DNA fragment from upstream regulatory region of the DOR gene (Fig. 1A). A primary reporter construct and its serial deletion constructs were prepared from this 1.3-kb DNA fragment as described under “Materials and Methods.” This primary construct, designated as pD1300, and its serial 5′-deletion constructs, designated as pD890, pD675, pD262, pD210, pD182, and pD150, are illustrated in Fig. 1B. The pGL3-basic plasmid (designated as basic), containing no promoter and no enhancer, was included as a negative control, while the pGL3-control plasmid (designated as control) containing the SV40 promoter and SV40 enhancer was used as the positive control. The promoter activity of each construct was tested by transient transfection assays in NS20Y cells, a DOR-expressing neuronal cell line.
Fig. 1. Deletional analysis of the promoter of the δ-opioid receptor gene.
A, a schematic diagram representing the 5′-regulatory region of the mouse δ-opioid receptor gene from nucleotide −1300 to the translation start site (ATG), which is designated as +1. Arrows indicate two major transcription initiation sites (TIS). B, a series of DOR promoter/luciferase constructs were prepared and introduced into NS20Y cells as described under “Materials and Methods.” The line graph on the left is a schematic representation of the DOR promoter regions that were included in each construct. Each construct was named by the number of the 5′-end nucleotide of the inserted DOR promoter region. A positive control plasmid (control) containing the SV40 promoter and SV40 enhancer, as well as a negative control plasmid, the pGL3-basic vector (basic), were included in transient transfection assays. Luciferase activity of the transfectants was determined and normalized to β-galactosidase activity, then expressed for each construct as a percentage of the positive control plasmid (relative luciferase activity %). The histograms on the right represent the mean values of relative luciferase activity (%) from at least four independent transfection experiments with two different plasmid preparations. Error bars indicate the range of standard errors.
As shown in Fig. 1B, the luciferase reporter constructs with different 5′-ends from −1300 to −262 showed similar luciferase activities, with relatively minor variation. However, a dramatic decrease (more than 50%) in luciferase activity was seen with the 5′-end deletion construct pD210. Moreover, two deletion constructs with 5′-end deletion down to −182 and −150, respectively, displayed luciferase activity reduced to that of the pGL3-basic. Thus, these data indicated that the sequence from −262 to +1 is sufficient to express full promoter activity for the DOR gene.
A Minimum DOR Promoter Sequence Confers Promoter Activity in Both Orientations but of Different Magnitude
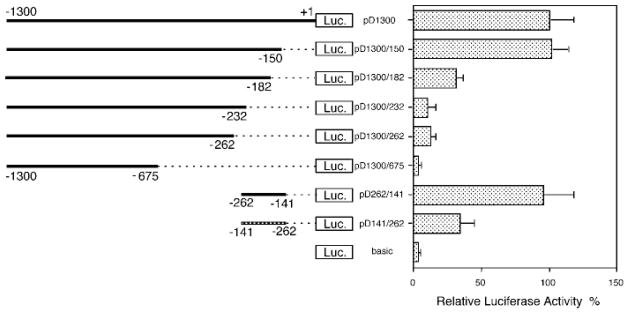
In addition to 5′-serial deletional analysis of the DOR promoter, a 3′-serial deletional analysis was also carried out to define the minimum core promoter of the DOR gene. As shown in Fig. 2, a construct containing a 3′-deletion of −150 to +1 of the DOR promoter region (designated as pD1300/150) did not affect activity of the DOR promoter. However, a deletion of up to −182 (pD1300/182) sharply reduced DOR promoter activity. Further 3′-end deletions of the DOR promoter region (constructs pD1300/232, pD1300/262, and pD1300/675) resulted in a still greater and ultimately total loss of DOR promoter activity (Fig. 2). Based on the combined data from the 3′- and 5′-serial deletional analyses (Figs. 1B and 2), the DOR promoter sequence from −262 to −150 provided more than 90% of the DOR promoter activity. Thus the sequence from −262 to −150 appeared to be essential for displaying the DOR promoter activity. Accordingly, a pair of DOR promoter constructs was created with the DOR promoter sequence from −262 to −141 in either orientation. The region of −262 to −141 includes a major transcription initiation site (Fig. 1A) and promoter activity of this region was examined. Surprisingly, both constructs were active in NS20Y cells (Fig. 2), although the promoter activity of the construct pD262/141 (in native orientation) was about 3-fold more active than that of the construct pD141/262 (with DOR promoter sequence in reverse orientation). Taken together, these results indicated that the promoter sequence between −262 and −141 is necessary and sufficient to provide the DOR promoter activity in both orientations but of different magnitude in NS20Y cells.
Fig. 2. Identification of the minimum DOR promoter.
A series of DOR promoter/luciferase constructs were prepared and introduced into NS20Y cells as described under “Materials and Methods.” The line graph on the left is a schematic representation of the DOR promoter regions that were included in each construct. Each construct was named by the numbers of both 5′- and 3′-end nucleotides of inserted DOR promoter DNA fragments. The pD262/141 construct represents the native orientation, while pD141/262 represents the reverse orientation of the DOR promoter sequence from −262 to −141, determined to be the minimum region expressing full promoter activity, as cloned into pGL3-basic. The pGL3-basic empty vector (basic) was included as the negative control. Luciferase activity was normalized to β-galactosidase activity and expressed as a percentage of the activity of the pD1300 construct. The histograms on the right represent the mean values of relative luciferase activity (%) of the pD1300 from four independent transfection experiments with two different plasmid preparations. Error bars indicate the range of standard errors.
Identification of the GC Box and Its Associated Factors of the DOR Promoter
In order to localize the important cis elements for regulation of the DOR promoter, a series of linker scan mutations throughout the minimum DOR promoter region (−262 to −141) of the pD262 construct were performed. This construct was used because, as discussed earlier, this was the largest 5′-end deletion retaining full promoter activity. The promoter activities of these linker-mutated constructs were examined, with several mutant constructs displaying impaired promoter activities (Table I).
Table I. Linker scan analysis defines two crucial elements of the DOR promoter.
The DOR promoter region (from −262 to −137) was mutated with HindIII linker (AAGCTT) at 6-bp intervals by using the PCR-based oligonucleotide-directed mutagenesis with the pD262 as DNA template. The positions of mutated sequence of each mutant construct are described under “Materials and Methods.” The linker scan mutant constructs and pD262 were transfected into NS20Y cells, and luciferase activity of each construct was determined. Luciferase activity was normalized to β-galactosidase activity and expressed as a percentage of the activity of the pD262 construct. The numbers on the left represent the mean values of relative luciferase activity (%) of the pD262 from three independent transfection experiments with two different plasmid preparations. The numbers on the right represent the standard errors. Three constructs (pDm226, pDm190 and pDm184), displaying 35% or lower promoter activities than that of pD262, are indicated with asterisks and reveal the locations of two crucial elements for the DOR promoter activity.
| Constructs | Relative activity | Constructs | Relative activity | Constructs | Relative activity |
|---|---|---|---|---|---|
| % | % | % | |||
| pD262 | 100 ± 13 | pDm220 | 58 ± 7 | pDm178 | 77 ± 9 |
| pDm262 | 98 ± 12 | pDm214 | 67 ± 8 | pDm172 | 85 ± 8 |
| pDm256 | 103 ± 18 | pDm208 | 76 ± 10 | pDm166 | 97 ± 15 |
| pDm250 | 114 ± 16 | pDm202 | 94 ± 12 | pDm160 | 90 ± 18 |
| pDm244 | 90 ± 12 | pDm196 | 128 ± 20 | pDm154 | 105 ± 15 |
| pDm238 | 120 ± 15 | pDm190* | 14 ± 4 | pDm148 | 92 ± 13 |
| pDm232 | 79 ± 12 | pDm184* | 7 ± 3 | pDm142 | 91 ± 17 |
| pDm226* | 35 ± 5 |
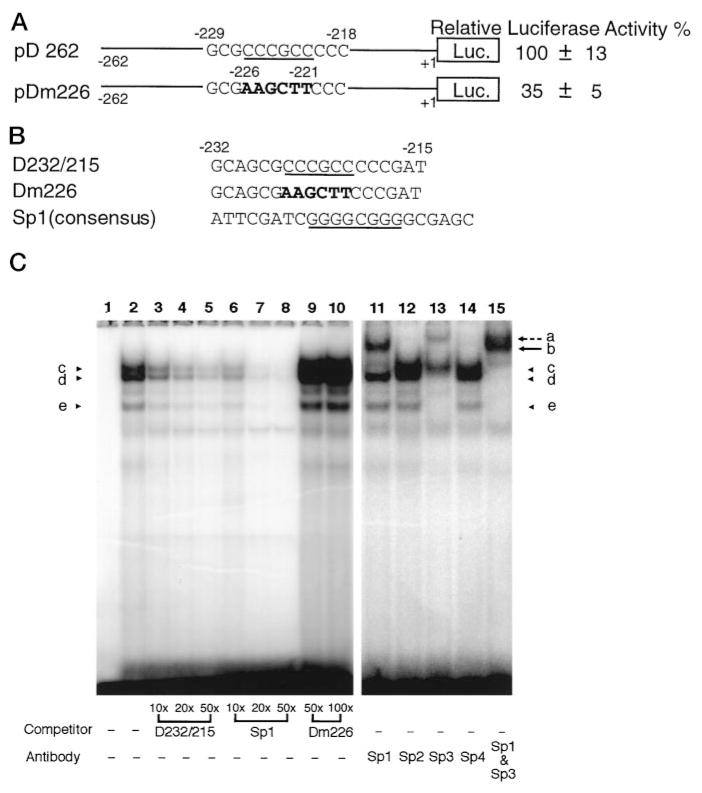
By comparing the sequences of the mutated region from the mutant constructs exhibiting reduced promoter activities to the consensus sequences of known transcription factors, two putative binding sites of known transcription factors were identified from three mutant constructs that showed more than 60% decrease in promoter activities (Table I, pDm226, pDm190, and pDm184). One of these binding sites was a GC box (shown in Fig. 3A, underlined), which is the putative binding site for transcription factors of the Sp1 family (19–21). Mutation of this GC box (pDm226) resulted in about 65% decrease in promoter activity of that of the wild type DOR promoter in NS20Y cells (Fig. 3A).
Fig. 3. Identification of the GC box and its associated factors of the DOR promoter.
A, the effect of the GC box mutation. The DOR promoter construct, pD262, contains the wild-type promoter sequence from −262 to +1. The GC box consensus in pD262 is underscored, and the mutated sequence in pDm226 is shown in boldface. The activities of promoter constructs were examined by transient transfection assays using NS20Y cells. Luciferase activity was normalized to β-galactosidase activity and expressed as a percentage of the activity of the pD262 construct. The data on the right represent the mean values of relative lu-ciferase activity (%) of pD262 from four independent transfection experiments. B, three oligonucleotides were used in EM-SAs to identify the Sp binding site in the DOR promoter region as well as its binding factors. D232/215 was a portion of the DOR promoter region from −232 to −215 containing the GC box consensus (underlined). Dm226 contains the same sequence as D232/215 except 6 mutated bp shown in boldface. Sp1 consensus oligo-nucleotides were a commercial product from Santa Cruz Biotechnology, Inc. C, EMSAs were performed by using D232/215 as the probe in the absence (lane 1) or presence (lanes 2–15) of nuclear extracts from NS20Y cells. Lanes 3–10, various amount of different unlabeled competitors were included as indicated. Lanes 11–15, 1 μl of different anti-Sp Abs were added to each reaction as indicated. Protein-DNA complexes of the D232/215 probe are marked by arrowheads c, d, and e. The anti-Sp1 Ab supershifted band is indicated by arrow b. The anti-Sp3 Ab supershifted band is indicated by broken arrow a.
In order to identify the transcription factors bound to this GC box of the DOR promoter, EMSAs were performed using nuclear extracts prepared from NS20Y cells. The oligonucleotide D232/215, representing the DOR promoter sequence from −232 to −215, which includes the putative binding site for the Sp1 family (Fig. 3B, D232/215, underlined), was used as the probe. Three major bands representing protein-DNA complexes were observed in the presence of the nuclear extracts (Fig. 3C, lane 2, arrowheads c–e). These three complexes were all sequence-specific, because formation of all of them could be prevented by the presence of unlabeled D232/215 in a dose-dependent manner (Fig. 3C, lanes 3–5). Competition with the Sp1 consensus oligonucleotide (Fig. 3B) also blocked the formation of the protein-DNA complexes c–e in a dose-dependent manner (Fig. 3C, lanes 6–8). However, formation of these three bands was not affected by using the Dm226 oligonucleotide, which contained the same sequence as D232/215 except the GC box was replaced by the linker sequence AAGCTT (Fig. 3B, Dm226, boldface), as the competitor (Fig. 3C, lanes 9 and 10). These results suggest that Sp1 or Sp1-like factors bind to the DOR GC box.
At least four transcription factors, Sp1, Sp2, Sp3, and Sp4, are known to belong to the Sp family (19–21). In order to identify those members binding to the DOR GC box, immuno-supershift assays were carried out with Sp1, Sp2, Sp3, or Sp4 antibodies (Abs). As shown in Fig. 3C, the protein-DNA complex band c was retarded by anti-Sp1 Ab to the position of band b (Fig. 3C, lane 11, arrow b). The anti-Sp3 Ab could recognize both bands d and e, shifting both bands to a higher position, band a (Fig. 3C, lane 13, broken arrow a). Furthermore, all three bands, c, d, and e, could be converted to bands a and b in the presence of both anti-Sp1 and anti-Sp3 Abs (Fig. 3C, lane 15). It was of interest that the amount of band b, the Sp1-D232/215 complex, recognized by anti-Sp1 Ab was more abundant than that of band a, the Sp3-D232/215 complex detected by anti-Sp3 Ab (Fig. 3C, lanes 11, 13, and 15, bands a and b). However, none of the bands was recognized by either anti-Sp2 or anti-Sp4 Ab (Fig. 3C, lanes 12 and 14). Taken together, these results demonstrate that the GC box of the DOR promoter is the binding site for the Sp1 and Sp3 transcription factors. Mutation of this site resulted in eliminating the binding of both Sp1 and Sp3 to the DOR promoter and diminished the DOR promoter activity.
Sp1 Transcription Factor trans-Activates the DOR Promoter in Vivo
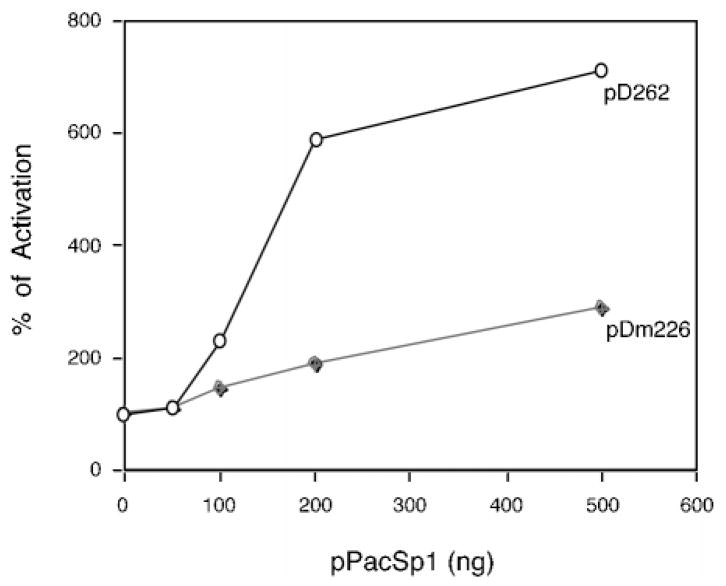
It has been reported that the Sp1 transcription factor plays a critical role in the basal transcription of several genes with TATA-less promoters (16, 21). In order to demonstrate that the Sp1 factor activates the DOR promoter via direct interaction with the DOR GC box in vivo, we performed co-transfection assays in Drosophila SL2 cells, which do not express endogenous Sp1 protein (26). As shown in Fig. 4, co-transfection assays using the Sp1 expression vector and pD262 DOR promoter construct in Drosophila SL2 cells demonstrated that Sp1 can specifically activate the promoter of the pD262 construct in a dose-dependent manner. Mutation of the DOR GC box (construct pDm226) diminished this trans-activation of the DOR promoter by Sp1 (Fig. 4). Thus, we concluded that Sp1 not only binds to the GC box of the DOR promoter in vitro but also in this manner trans-activates the DOR promoter in vivo. However, mutation of the GC box did not completely eliminate DOR promoter activity in SL2 cells, which might be due to the binding of overexpressed Sp1 protein to unidentified low affinity sites within the DOR promoter region.
Fig. 4. Sp1 trans-activates the DOR promoter via Sp1 binding site.
Drosophila SL2 cells were co-transfected with the indicated amounts of pPacSp1 plasmid and either pD262 (open circles; the wild type DOR promoter construct) or pDm226 (closed diamonds; the mutant construct of the DOR promoter with mutated Sp1 site). Activation was expressed as a percentage of the activity of each construct in the presence of the indicated amount of pPacSp1 divided by the activity of the construct without effector (arbitrarily defined as 100%).
Identification of the E Box and Its Associated Factors of the DOR Promoter
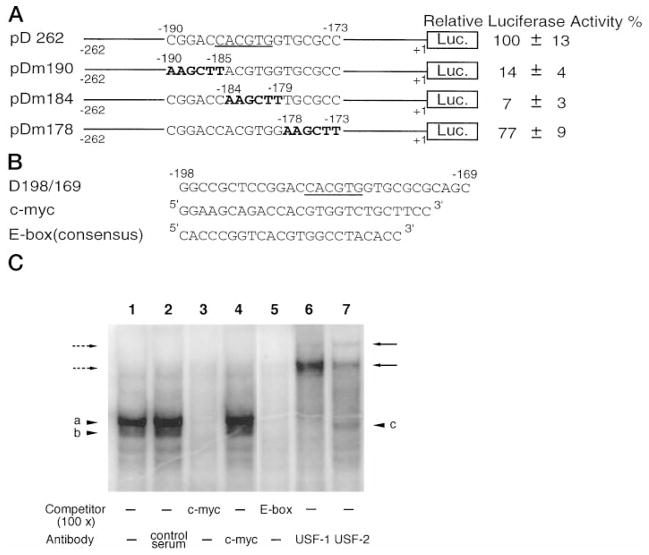
As noted earlier, two putative binding sites of known transcription factors were identified in the DOR promoter region by linker scanning and sequence comparison. In addition to the GC box, a second binding site was an E box (Fig. 5A, underlined), which is a putative binding site for the transcription factors of the basic helix-loop-helix leucine zipper (bHLH/LZ) family (27). In NS20Y cells, mutations of the E box in the DOR promoter region (pDm190, pDm184) abolished more than 80% of the promoter activity exhibited by the wild type construct pD262 (Fig. 5A). In contrast, a promoter construct (pDm178) retained most of the promoter activity while its E box was intact (Fig. 5A). Several members of the bHLH/LZ family are able to bind to the sequence (-CACGTG-) of the E box (26). Therefore, by using EMSA, we tested several members of this family as candidates for binding to the promoter E box of DOR gene. These included c-Myc (28–29), which plays a role in cell proliferation and differentiation that could be relevant to the limited expression pattern of DOR, as well as upstream stimulatory factor (USF), USF1 and USF2, because of their ubiquitous expression.
Fig. 5. Identification of the E box and its associated factors of the DOR promoter.
A, the effect of the E box mutation. The E box consensus in pD262 is underscored, and the mutated sequences in mutant constructs pDm190, pDm184, and pDm178 are shown in boldface. The activities of promoter constructs were examined by transient transfection assays in NS20Y cells. Luciferase activity was standardized to β-galactosidase activity and expressed as a percentage of the activity of the pD262 construct. The data on the right represent the mean values of relative luciferase activity (%) of pD262 from four independent transfection experiments. B, three oligonucleotides were used in EMSAs to identify the binding factors of the E box in the DOR promoter region. The oligonucleotide D198/169 contained the sequence from −198 to −169 of the DOR promoter. Both c-Myc and E box consensus oligonucleotides used as unlabeled competitors were commercial products from Santa Cruz Biotech-nology, Inc. C, EMSA was performed by using D198/169 as the probe with NS20Y nuclear extracts (lanes 1–7). Lane 1, control reaction; lane 2, 1 μl of control serum; lane 3, 100-fold excess of c-Myc competitor; lane 4, 1 μl of anti-c-Myc Ab; lane 5, 100-fold excess of E box competitor; lane 6, 1 μl of anti-USF1 Ab; lane 7, 1 μl of anti-USF2 Ab. The USF-D198/169 complexes are marked by arrowheads a and b. The putative USF1 homodimer is indicated by arrowhead c. The USF-D198/169 complexes recognized by anti-USF1 Ab are marked by broken arrows on the left. The USF-D198/169 complexes recognized by anti-USF2 Ab are marked by arrows on the right.
As illustrated in Fig. 5B, an oligonucleotide (D198/169) containing the E box and its flanking sequence of the DOR promoter was used as the probe; and two consensus oligonucleotides, an E box and a c-Myc binding sequence, were used as the unlabeled competitors. Results show in Fig. 5C that nuclear proteins of NS20Y cells formed two major protein-DNA complexes with D198/169 (lane 1, arrowheads a and b). Formation of these two major protein-DNA complexes, bands a and b, could be blocked by either the c-Myc (Fig. 5C, lane 3) or the E box consensus oligonucleotide (Fig. 5C, lane 5). The anti-USF1 Ab shifted both bands a and b to higher positions (Fig. 5C, lane 6, marked by broken arrows on the left), while the anti-USF2 Ab also retarded portions of these bands (Fig. 5C, lane 7, marked by arrows on the right). However, the anti-c-Myc Ab did not recognize any of the protein-DNA complexes formed (Fig. 5C, lane 4). Thus, it appears that USF1 and USF2, but not c-Myc, were the nuclear factors bound to the DOR E box in vitro. Moreover, it was obvious that both of the protein-DNA complexes represented by bands a and b contain USF1, because the anti-USF1 Ab could shift both bands to higher positions (Fig. 5C, lane 6). In contrast, only a portion of the protein-DNA complexes was recognized by anti-USF2 Ab (Fig. 5C, lane 7). Thus, we conclude that in NS20Y cells, USF1 homodimers (Fig. 5C, lane 7, band c) and USF1/USF2 heterodimers, but not USF2 homodimers, were present and bound to the DOR E box.
USF Binding Contributes to the Promoter Activity of the DOR Gene
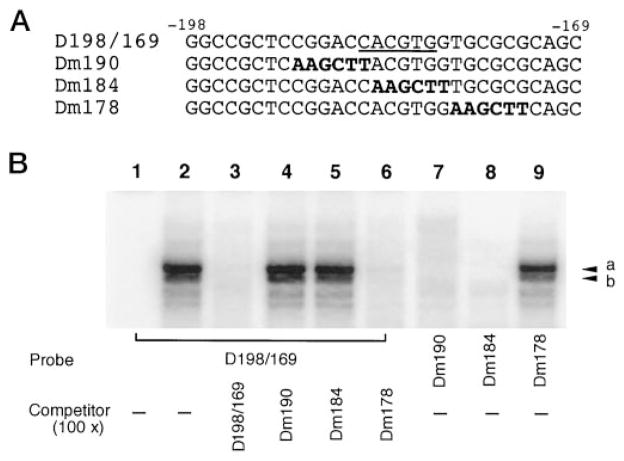
The data in Fig. 5A suggest that a functional E box (−185 to −180) resides in the core promoter of the DOR gene. Mutations of this site (pDm184 and pDm190) abolished the promoter activity. In order to provide physical evidence for the impaired USF-binding ability of the mutated DOR E box and correlate the in vitro binding activity to the reduced promoter activities of pDm184 and pDm190 in vivo, an EMSA was performed. In addition to the oligonucleotide D198/169 described above (Fig. 5B), three oligonucleotides, Dm190, Dm184, and Dm178, were synthesized, corresponding to the DOR promoter sequence −198 to −169 in mutant constructs, pDm190, pDm184, and pDm178, respectively. Both D198/169 and Dm178 contained the intact E box, while Dm190 and Dm184 contained the mutated E box (Fig. 6A).
Fig. 6. USF-binding is critical for DOR promoter activity.
A, the oligonucleotide D198/169 contained the DOR promoter sequence from −198 to −169 including the consensus E box (underscored). Three different oligonucleotides, Dm190, Dm184, and Dm178, all contained the same sequence as D198/169 except for a 6-bp region mutated as indicated in boldface. B, EMSAs were performed in the absence (lane 1) or presence (lanes 2–9) of NS20Y nuclear extracts. Lanes 1–6, D198/169 was used as the probe in the absence (lanes 1 and 2) or presence of different unlabeled competitors as indicated (lanes 3–6). Lane 7, Dm190 was the probe. Lane 8, Dm184 was the probe. Lane 9, Dm178 was the probe. Major protein-DNA complexes are marked by arrowheads a and b.
As shown in Fig. 6B, the probe D198/169 formed two distinct protein-DNA complex bands with NS20Y nuclear proteins (lane 2, arrowheads a and b). Formation of these bands could be blocked by the probe itself and by Dm178 (Fig. 6B, lanes 3 and 6), but not by either Dm190 or Dm184 (Fig. 6B, lanes 4 and 5). Moreover, when using Dm190 and Dm184 as the probe, there was no specific complex formed (Fig. 6B, lanes 7 and 8). However, when Dm178 was used as the probe, it displayed USF binding ability similar to that of the D198/169 (Fig. 6B, lane 9). The intact E box binding ability of oligonucleotide Dm178 was consistent with the strong promoter activity of the construct pDm178 (Fig. 5A). In contrast, abolished E box binding abilities of Dm190 and Dm184 were in agreement with diminished promoter activities of pDm190 and pDm184, respectively (Fig. 5A). Thus, USF binding to the DOR E box reflects its trans-activation effect on the promoter activity of the DOR gene.
USF2 trans-Activates the DOR Promoter in Vivo
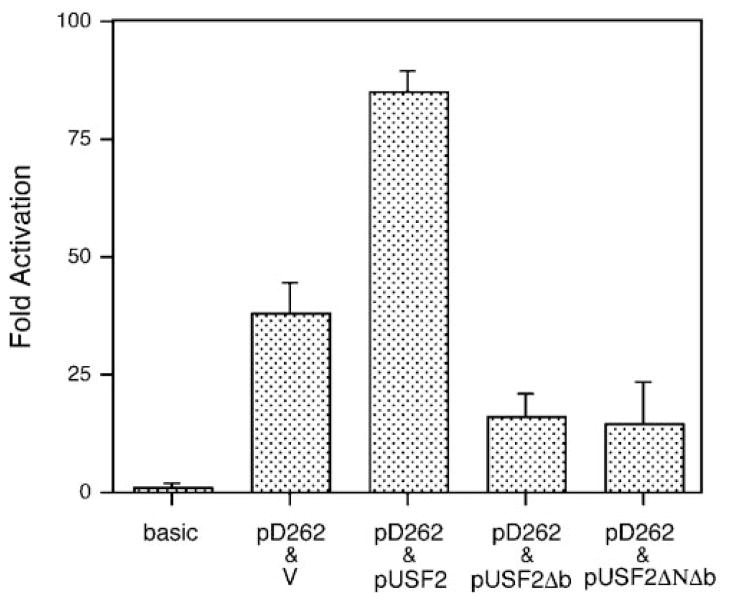
The data in Fig. 5C indicate that USF2 could only be detected in the form of heterodimers. Since the heterodimer of USF1/2 is most likely acting as a transcription activator in vivo (30), this suggests that USF2 may be critical for the DOR expression. In order to evaluate the functional role of USF2 in DOR promoter activity in vivo, co-transfection assays were carried out in NS20Y cells with the promoter construct pD262 and the USF2 expression vector.
As shown in Fig. 7, the promoter activity of pD262 was elevated more than 2-fold when it was co-transfected with wild type USF2. As noted earlier, USF belongs to the bHLH/LZ family. USF1 and USF2 are capable of binding to DNA in the form of either homodimers or heterodimers with each other, through their leucine zipper dimerization domain (31). The basic region (b) of these proteins provides their DNA binding affinity and specificity, with deletion of this motif abolishing DNA binding. To evaluate the role of USF2 in DOR promoter activity, further experiments were carried out with USF2Δb, in which only the basic region was deleted, and USF2ΔNΔb, in which both the basic region and the N-terminal trans-activation domain were deleted. Since both of these USF2 derivatives retain the helix-loop-helix and leucine zipper region, however, they should be able to dimerize with either USF1 or USF2. Thus, they would be predicted to act as dominant negative forms of USF2, able to form dimers but unable to bind to the E box (32). As shown in Fig. 7, the DOR promoter construct, pD262, displayed more than a 50% loss in promoter activity when it was co-transfected with either pUSF2Δb or pUSF2ΔNΔb. Together with the significant trans-activation of USF2, these data provide direct evidence that USF2 does act on the DOR E box and trans-activate the DOR promoter in vivo.
Fig. 7. USF trans-activates the DOR promoter in vivo.
NS20Y cells were transfected with a total of 3 μg of DNA, including 2 μg of the reporter construct (pD262 or pGL3-basic as indicated) and 1 μg of a plasmid expressing USF2 (pUSF2) or a dominant negative form of USF2 (pUSF2Δb, pUSF2ΔNΔb) or empty expression vector (v). Activation was expressed as luciferase activities relative to the luciferase activity of pGL3-basic, arbitrarily defined as 1. Results are means of three different experiments. Error bars indicate the range of standard errors.
Functional Interaction of the DOR GC Box and E Box
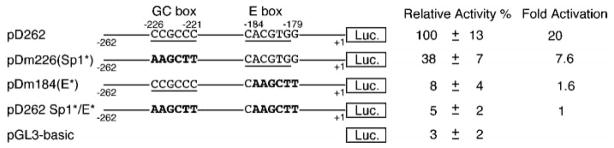
Our data presented earlier demonstrate that both E box and GC box are important for the DOR promoter activity and are located 40–90 bp upstream of one major transcription initiation site (position −142, Fig. 1A). In addition, their associated factors, USF and Sp1, have been reported to interact with TAFII55, a transcription factor involved in the basal transcription machinery (33). This suggests that both USF and Sp1 might contribute to the basal transcription of DOR and functionally interact with each other via a common transcription factor partner. To investigate the possibility of functional interaction between them, a double-mutation construct, pD262Sp1*/E*, was created as described under “Materials and Methods.” This double-mutation construct, featuring alterations in both the GC box and E box, was tested for promoter activity in NS20Y cells, along with pD262 (wild-type), pDm184 (E*) (E box mutated), pDm226 (Sp1*) (GC box mutated), and the empty reporter gene vector (pGL3-basic).
The results shown in Fig. 8 demonstrated that simultaneous mutation of the GC box and E box (pD262Sp1*/E*) almost completely abolished the DOR promoter activity of pD262, reducing its luciferase activity to the same level as that of the empty reporter gene vector (pGL3-basic). However, comparison of the luciferase activities of the constructs pD262, pDm226 (Sp1*), pDm184 (E*), and pD262Sp1*/E* suggests a cooperative relationship between the GC box and E box to the promoter activity. First, the promoter activity of pD262Sp1*/E* (construct with both the E box and GC box mutated) is arbitrarily set as 1-fold (Fig. 8, fold activation). Then, a 20-fold activation is observed with pD262 (construct with both the E box and GC box intact). When this is compared with the 7.6-fold activation of pDm226 (intact E box and mutated GC box) and 1.6-fold activation of pDm184 (intact GC box and mutated E box), it is clear that the effect is substantially more than additive. Thus, it suggests a functional synergy between the GC box and E box for the DOR promoter activity. Therefore, both the GC box and E box are required for the maximum promoter activity of the DOR gene, although the E box obviously played the major role and contributed more than the GC box did to the promoter activity.
Fig. 8. Functional interaction of the GC box and E box of the DOR gene.
NS20Y cells were transfected with various DOR promoter/luciferase constructs containing either wild type DOR promoter (pD262), or DOR promoter with mutated GC box (pDm226), or DOR promoter with mutated E box (pDm184), or DOR promoter with both mutated GC box and mutated E box (pD262Sp1*/E*). Luciferase activities were normalized to β-galactosidase activity and expressed relative to wild type promoter activity, arbitrarily defined as 100%. Activation was expressed as luciferase activities relative to the luciferase activity of pD262Sp1*/E*, arbitrarily defined as 1. The underlined sequences represent either the GC box or E box, as indicated. Mutant sequences are shown in boldface.
Physical Interaction of USF and Sp1/Sp3 on the DOR Promoter
In addition to functional synergy, the proximity of the DOR GC box and E box also implies the possible physical interaction between their associated factors. This was investigated by EMSA competition assay with NS20Y nuclear extracts. As shown in Fig. 9A, all three probes featured the sequence from the same promoter region of the DOR gene (−232 to −169) containing both the GC box and E box. In one of these probes, designated as D232/169, both the GC box and E box were intact. In the second probe, designated as D232/169E*, the GC box was intact while the E box was mutated. The third probe, designated as D232/169Sp1*, contained an intact E box and mutated GC box.
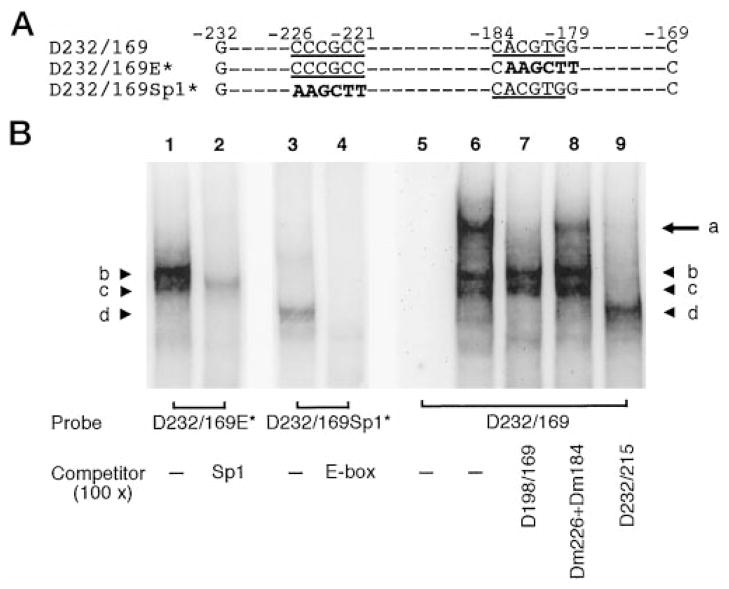
Fig. 9. Physical interaction of the GC box and E box of the DOR promoter.
A, three oligonucleotides were used as probes in EMSA shown in panel B. D232/169 contained the DOR promoter sequence from −232 to −169, with both the GC box and E box (underlined). D232/169E* contained the same sequence as D232/169 except the E box was mutated (in bold). D232/169Sp1* containing the same sequence as D232/169 except the GC box was mutated (in bold). B, EMSA was performed with NS20Y nuclear extracts and indicated probes. Lanes 1, 3, and 6, no competitor; lane 5, probe only; lane 2, in the presence of Sp1 consensus oligonucleotide (as shown in Fig. 3B) as the competitor; lane 4, in the presence of E box consensus (as shown in Fig. 5B) as the competitor; lane 7, in the presence of D198/169 (as shown in Fig. 5B) as the competitor; lane 8, in the presence of the Dm226 and Dm184 (as shown in Fig. 3B and 6A) as the competitors; lane 9, in the presence of the D232/215 (as shown in Fig. 3B) as the competitor.
As shown in Fig. 9B, nuclear factors and the probe D232/169E* formed bands with the characteristics of Sp-DNA complexes (Fig. 9B, lanes 1 and 2, bands b and c), since formation of these bands was blocked by the presence of Sp1 consensus oligonucleotide. Likewise, the bands formed using the probe D232/169Sp1* did not appear in the presence of E box consensus oligonucleotide (Fig. 9B, lanes 3 and 4, band d). Thus, mutation of the E box abolishing USF binding and mutation of the GC box blocking Sp1/Sp3 binding to the DOR promoter region were consistent with the data shown earlier. Interestingly, the probe D232/169, which contained both an intact GC box and E box, formed an additional band of larger size (Fig. 9B, lane 6, band a) than the bands corresponding to the Sp-D232/169 complexes and USF-D232/169 complexes (Fig. 9B, lane 6, bands b–d). Formation of this band could be abolished by oligonucleotides containing either the DOR E box, D198/169, or the DOR GC box, D232/215 (Fig. 9B, lanes 7 and 9) but not by the mutated DOR promoter sequences, Dm184 and Dm226 (Fig. 9B, lane 8). Taken together, these results demonstrate that binding of either USF or Sp1/Sp3 to the DOR promoter sequence did not affect binding of the other factor. However, formation of USF-Sp-D232/169 complexes indicates that simultaneous binding of USF and Sp factors to the DOR promoter sequence was highly favored since almost no binding of USF alone could be seen when both the GC box and E box were intact (Fig. 9B, lane 6, band d). This suggests that a direct or additional factor-mediated physical interaction between USF and Sp factors occurred when both the E box and GC box were present in the DOR promoter region. The mechanism of physical interaction between USF, Sp, and/or additional factors on the DOR promoter needs to be further investigated.
It was also observed that much lesser amounts of USF-D232/169 complexes were formed in the presence of USF-Sp physical interaction (Fig. 9B, lanes 6 and 8, band d) than in the absence of this interaction (Fig. 9B, lane 9, band d). In contrast, the amount of Sp-D232/169 complexes was largely unaffected by the presence or absence of the USF-Sp physical interaction (Fig. 9B, lanes 6–8, bands b and c). It suggests the presence of excess amount of Sp-D232/169 complexes and limited amount of USF-D232/169 complexes in the EMSA competition assays. Thus, binding of USF to the DOR promoter sequence is more critical than that of Sp1/Sp3 in NS20Y cells. This conclusion is also in agreement with the results of functional assays noted earlier, that the E box contributes more than the GC box does to the promoter activity of the DOR gene.
DISCUSSION
The goal of this study was to identify the cis-elements and trans-acting factors critical to transcriptional regulation of the DOR gene. Based on the results of 5′- and 3′-end serial deletion analyses, we have localized the core promoter controlling this expression to a minimal region situated between −262 and −141 bp upstream of the DOR gene’s translation initiation site (Fig. 2). Surprisingly, this minimum promoter sequence conferred promoter activity in both orientations, although with different magnitudes. The promoter with orientation-independent promoter activity is uncommon in transcription regulation. The mechanism behind this unusual observation remains to be determined.
Based on our results, two cis-elements were identified to be crucial for the DOR promoter activity. The first cis-element is a GC box. Sp1 and Sp3 were shown bound to this element (Fig. 3C). Sp1 often acts as an activator of Sp1 binding sites (34), and also plays an essential role in transcription by tethering preinitiation complexes to the promoter through interaction with TFIID at both TATA and TATA-less promoters (21, 35–36). We demonstrated that Sp1 is an activator of DOR expression by showing that Sp1 is the major binding factor on the DOR GC box in NS20Y cells (Fig. 3C, lanes 11, 13, and 15), and that it activates the DOR promoter in Drosophila SL2 cells (Fig. 4). Sp3, in contrast, was defined originally as an inhibitor of Sp1-mediated activation, based on its competition with Sp1 for the Sp1-binding site (19, 37–38). Recently, however, Sp3 has been shown to function as a dual-function regulatory factor, also possessing stimulatory activity in some circumstances (39–40). In any case, the relative levels of Sp1 and Sp3 proteins are likely to be important determinants of transcription activity in cells that express both factors (41–42). While both Sp1 and Sp3 were present and bound to the DOR promoter GC box in NS20Y cells, the amount of Sp1-containing complexes was much greater than that of the Sp3-containing complexes in vitro (Fig. 3C, lane 11, band a and lane 13, band b). Thus, further studies will be needed to determine whether Sp3 can activate DOR expression, or whether it simply acts as an inhibitor to modulate Sp1-mediated expression of the DOR in vivo.
The E box is another crucial cis-element for the DOR promoter activity (Fig. 5). Two ubiquitous transcription factors, USF1 and USF2, were identified bound to this E box (Fig. 5C). USF2 was also demonstrated to trans-activate the DOR promoter in vivo (Fig. 7) and found binding to the DOR E box only in the form of heterodimer (Fig. 5C). As with Sp1 and Sp3, different ratios of these homo- and heterodimers of USF are found in different cell types (30, 43). In the case of NS20Y cells, we found that heterodimers of USF1/2 comprised about 80% of the USF binding activity to the DOR E box, with the remaining 20% of binding activity contributed by USF1 homodimers. We observed no binding of USF2 homodimers to the DOR promoter E box (Fig. 5C). The functional roles of USF1/2 heterodimers and USF1 homodimers in NS20Y cells are not clear. However, USF1/2 heterodimer is most likely acting as a transcription activator in vivo (30), while USF1 homodimer may play a dual-function role for the DOR promoter (44). Thus the ratio between homo- and heterodimers of USF1 and USF2 may also determine the magnitude of the DOR promoter activity. In any case, our results suggest that both USF1 and USF2 are playing a critical role in the regulation of DOR expression.
Substantial evidence indicates that USF interacts with other transcription factors, such as TFIID, AP1, ETS-1, and Stat-1 (33, 45–47). In this report, we demonstrate a physical interaction between USF and Sp1/Sp3 (Fig. 9). In NS20Y cells, several observations suggest that the formation of USF-D232/169 complexes is the rate-limiting step in the formation of USF-Sp-D232/169 complexes. First, only trace amounts of USF-D232/169 complexes were detected (Fig. 9, lanes 6 and 8, band d) under conditions in which USF-Sp-D232/169 complexes were observed (Fig. 9, lanes 6 and 8, band a), while excess amounts of the Sp1/Sp3-D232/169 complexes were present (Fig. 9, lanes 6 and 8, bands b and c). Second, there was always substantial Sp1/Sp3 binding to D232/169 (Fig. 9, lanes 1, 6, 7, and 8, bands b and c). Finally, the complex of USF-D232/169 increased dramatically when formation of USF-Sp-D232/169 complex was disrupted by the Sp1 site-containing oligonucleotide, D232/215 (Fig. 9, lanes 6 and 9, band d). Thus, almost all of the USF bound to D232/169, forming higher molecular weight complexes with Sp1/Sp3, while excess amounts of the Sp1/Sp3-D232/169 complexes were left. It indicates that the USF binding determines the extent of simultaneous binding of USF and Sp factors to the DOR promoter. In addition to these EMSA studies indicating that USF binding to the E box plays a decisive role in DOR promoter activation, a similar conclusion is consistent with the results of our mutation experiments. Thus, mutation of the DOR E box abolished promoter activity much more effectively than mutation of the DOR GC box. Furthermore, since the DOR promoter is a TATA-less promoter, the formation of the preinitiation complex may rely on both the GC box and E box in the DOR promoter region, especially the close location of them to the transcription initiation site. The physical interaction between the DOR E box and GC box reported here, as well as their interactions with TAFII55 (33), make it possible that both the E box and GC box are involved in the basal transcription of the TATA-less DOR promoter.
As mentioned earlier, the expression of the DOR is restricted (5). The 5′-promoter region of the DOR gene displays high expression activity in DOR-expressing neuronal cell lines, including NS20Y (Fig. 1B) and NMB (data not shown). Thus luciferase was expressed with a magnitude of 7-fold greater or more in these cells than in cells not expressing DOR, including hepatocytes, neuro2A, and Chinese hamster ovary cells (data not shown). However, the preferential expression of the DOR promoter constructs for DOR-expressing cell lines disappeared when the minimum promoter region (−262 to −141) was impaired by deletions. Thus, the DOR minimum promoter region appears to contain an element or elements controlling the cell-type specificity of these DOR promoter constructs. A similar phenomenon is also found in the regulation of the MOR gene (22) and human complement component C4 gene (52), in that the minimum promoter provides the basal promoter activity as well as preferential cell-type expression. Dissection of the minimum promoter region at a finer level may be helpful in further defining the mechanisms underlying cell-type specific expression of DOR.
Recently, cross talk has been reported between cell- or tissue-specific transcription factors and ubiquitous USF or Sp1 (46–48, 53–55). A combinatorial activity of an associated cell-specific activator and ubiquitous USF or Sp1 is able to direct the transcription in a cell-type specific manner (49–51, 55). Moreover, there is evidence indicating the existence of a family of proteins able to bind the Sp1 consensus-binding motif, and it has been suggested that a complex interaction of several factors at this site may be involved in tissue specific gene transcription, e.g. that the Sp1/Sp3 ratios in some cells and Sp1 activity determined by signal transduction (41–42, 56–57) might modulate the promoter activity in a cell-type specific manner. Therefore, either the GC box or the E box, or indeed an association between the relative abundance and state of activity of the trans-acting factors binding these elements, or their transcription factor partners with cell-type specificity, may provide the basis for selective cell-specific transcriptional regulation of the DOR gene.
Acknowledgments
We thank Dr. H. C. Towle (University of Min-nesota, Minneapolis, MN) for the generous gift of USF2, USF2Δb, and USF2 ΔNΔb expression plasmids. We thank Dr. R. Tjian (University of California, Berkeley, CA) for the kind gift of pPacSp1 plasmid. We thank Dr. Y. Sarne and Dr. Y. Wollman (Tel Aviv University, Tel Aviv, Israel) for the kind gift of NMB cells. We thank Dr. A. P. Smith for helping with the preparation of the manuscript.
Footnotes
This work was supported by National Institutes of Health Grants DA-00546, DA-01583, DA-11806, and KO5-DA-70554 and by the A. & F. Stark Fund of the Minnesota Medical Foundation.
The abbreviations used are: OR, opioid receptor; DOR, δ-opioid receptor; MOR, μ-opioid receptor; KOR, κ-opioid receptor; PCR, polym-erase chain reaction; EMSA, electrophoresis mobility shift assay; Ab, antibody; bHLH/LZ, basic-helix-loop-helix leucine zipper; USF, upstream stimulatory factor; bp, base pair(s); kb, kilobase pair(s); PMSF, phenylmethylsulfonyl fluoride; DTT, dithiothreitol; DMEM, Dulbecco’s modified Eagle’s medium.
References
- 1.Herz A, editor. Handbook of Experimental Pharmacology. Vol. 104. Springer Verlag; New York: 1993. [Google Scholar]
- 2.Kieffer BL. Cell Mol Neurobiol. 1995;15:615–635. doi: 10.1007/BF02071128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Standifer KM, Pasternak GW. Cell Signalling. 1997;9:237–248. doi: 10.1016/s0898-6568(96)00174-x. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein A, Naidu A. Mol Pharmacol. 1989;36:265–272. [PubMed] [Google Scholar]
- 5.Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Trends Neurosci. 1988;11:308–314. doi: 10.1016/0166-2236(88)90093-8. [DOI] [PubMed] [Google Scholar]
- 6.Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, Watson SJ. J Comp Neurol. 1994;350:412–438. doi: 10.1002/cne.903500307. [DOI] [PubMed] [Google Scholar]
- 7.Zhu Y, Hsu M, Pintar JE. J Neurosci. 1998;18:2538–2549. doi: 10.1523/JNEUROSCI.18-07-02538.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abood ME, Tao Q. J Pharmacol Exp Ther. 1995;274:1566–1573. [PubMed] [Google Scholar]
- 9.Jenab S, Inturrisi CE. Brain Res Mol Brain Res. 1997;47:44–48. doi: 10.1016/s0169-328x(97)00061-2. [DOI] [PubMed] [Google Scholar]
- 10.Beczkowska IW, Buck J, Inturrisi CE. Brain Res Bull. 1996;39:193–199. doi: 10.1016/0361-9230(95)02104-3. [DOI] [PubMed] [Google Scholar]
- 11.Gylys KH, Tran N, Magendzo K, Zaki P, Evans CJ. Neuroreport. 1997;8:2369–2372. doi: 10.1097/00001756-199707070-00053. [DOI] [PubMed] [Google Scholar]
- 12.Sharp BM, Shahabi N, McKean D, Li MD, McAllen K. J Neuroimmunol. 1997;78:198–202. doi: 10.1016/s0165-5728(97)00101-x. [DOI] [PubMed] [Google Scholar]
- 13.Schreiber G, Campa MJ, Prabhakar S, O’Briant K, Bepler G, Patz EF., Jr Anticancer Res. 1998;18:1787–1792. [PubMed] [Google Scholar]
- 14.Augustin LB, Feisheim RF, Min BH, Fuchs SM, Fuchs JA, Loh HH. Biochem Biophys Res Commun. 1995;207:111–119. doi: 10.1006/bbrc.1995.1160. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh D. Nucleic Acids Res. 1993;21:3117–3118. doi: 10.1093/nar/21.13.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gill G, Tjian R. Curr Opin Genet Dev. 1992;2:236–242. doi: 10.1016/s0959-437x(05)80279-5. [DOI] [PubMed] [Google Scholar]
- 17.Smale ST, Baltimore D. Cell. 1989;57:103–113. doi: 10.1016/0092-8674(89)90176-1. [DOI] [PubMed] [Google Scholar]
- 18.Javahery R, Khachk A, Lo K, Zenzie-Gregory B, Smale ST. Mol Cell Biol. 1994;14:116–127. doi: 10.1128/mcb.14.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kingsley C, Winoto A. Mol Cell Biol. 1992;12:4251–4261. doi: 10.1128/mcb.12.10.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hagen G, Muller S, Beato M, Suske G. Nucleic Acids Res. 1992;20:5519–5525. doi: 10.1093/nar/20.21.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pugh BF, Tjian R. Cell. 1990;61:1187–1197. doi: 10.1016/0092-8674(90)90683-6. [DOI] [PubMed] [Google Scholar]
- 22.Ko JL, Minnerath SR, Loh HH. Biochem Biophys Res Commun. 1997;234:351–357. doi: 10.1006/bbrc.1997.6640. [DOI] [PubMed] [Google Scholar]
- 23.Ko JL, Liu HC, Minnerath SR, Loh HH. J Biol Chem. 1998;273:27678–27685. doi: 10.1074/jbc.273.42.27678. [DOI] [PubMed] [Google Scholar]
- 24.Conn KJ, Rich CB, Jensen DE, Fontanilla MR, Bashir MM, Rosenbloom J, Foster JA. J Biol Chem. 1996;271:28853–28860. doi: 10.1074/jbc.271.46.28853. [DOI] [PubMed] [Google Scholar]
- 25.Johnson DR, Levant S, Bale AE. BioTechniques. 1995;19:192–105. [PubMed] [Google Scholar]
- 26.Courey AJ, Tjian R. Cell. 1988;55:887–898. doi: 10.1016/0092-8674(88)90144-4. [DOI] [PubMed] [Google Scholar]
- 27.Marco KB, Bossone SA, Patel AJ. Annu Rev Biochem. 1992;61:809–860. doi: 10.1146/annurev.bi.61.070192.004113. [DOI] [PubMed] [Google Scholar]
- 28.Blackwood EM, Eisenman RN. Science. 1991;251:1211–1217. doi: 10.1126/science.2006410. [DOI] [PubMed] [Google Scholar]
- 29.Kerhoff E, Bister K, Klempnauer KH. Proc Natl Acad Sci U S A. 1991;88:4323–4327. doi: 10.1073/pnas.88.10.4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sirito M, Lin Q, Maity T, Sawadogo M. Nucleic Acids Res. 1994;22:427–433. doi: 10.1093/nar/22.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bresnick EH, Felsenfeld G. J Biol Chem. 1994;269:21110–21116. [PubMed] [Google Scholar]
- 32.Kaytor EN, Shih H, Towle HC. J Biol Chem. 1997;272:7525–7531. doi: 10.1074/jbc.272.11.7525. [DOI] [PubMed] [Google Scholar]
- 33.Chiang CM, Roeder RG. Science. 1995;267:531–536. doi: 10.1126/science.7824954. [DOI] [PubMed] [Google Scholar]
- 34.Lania L, Majello B, De Luca P. Int J Biochem Cell Biol. 1997;29:1313–1323. doi: 10.1016/s1357-2725(97)00094-0. [DOI] [PubMed] [Google Scholar]
- 35.Gill G, Pascal E, Tseng ZH, Tjian R. Proc Natl Acad Sci U S A. 1994;81:192–196. doi: 10.1073/pnas.91.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanese N, Saluja D, Vassallo MF, Chen JL, Admon A. Proc Natl Acad Sci U S A. 1996;93:13611–13616. doi: 10.1073/pnas.93.24.13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hagen G, Muller S, Beato M, Suske G. EMBO J. 1994;13:3843–3851. doi: 10.1002/j.1460-2075.1994.tb06695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Majello B, De Luca P, Suske G, Lania L. Oncogene. 1995;10:1841–1848. [PubMed] [Google Scholar]
- 39.Dennig J, Beato M, Suske G. EMBO J. 1996;15:5659–5667. [PMC free article] [PubMed] [Google Scholar]
- 40.Majello B, De Luca P, Lania L. J Biol Chem. 1997;272:4021–4026. doi: 10.1074/jbc.272.7.4021. [DOI] [PubMed] [Google Scholar]
- 41.Kubo T, Kohno K, Ohga T, Taniguchi K, Kawanami K, Wada M, Kuwano M. Cancer Res. 1995;55:3860–3864. [PubMed] [Google Scholar]
- 42.Apt D, Watts RM, Suske G, Bernard HU. Virology. 1996;224:281–291. doi: 10.1006/viro.1996.0530. [DOI] [PubMed] [Google Scholar]
- 43.Viollet B, Lefrancois-Martinez AM, Henrion A, Kahn A, Raymondjean M, Martinez A. J Biol Chem. 1996;271:1405–1415. doi: 10.1074/jbc.271.3.1405. [DOI] [PubMed] [Google Scholar]
- 44.Ghosh AK, Datta PK, Jacob ST. Oncogene. 1997;14:589–594. doi: 10.1038/sj.onc.1200866. [DOI] [PubMed] [Google Scholar]
- 45.Blanar MA, Rutter WJ. Science. 1992;256:1014–1018. doi: 10.1126/science.1589769. [DOI] [PubMed] [Google Scholar]
- 46.Siewekw MH, Tekotte H, Jarosch U, Graf T. EMBO J. 1998;17:1728–1739. doi: 10.1093/emboj/17.6.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muhlethaler-Mottet A, Berardino WD, Otten LA, Mach B. Immunity. 1998;8:157–166. doi: 10.1016/s1074-7613(00)80468-9. [DOI] [PubMed] [Google Scholar]
- 48.Lanigan TM, Russo AF. J Biol Biochem. 1997;272:18316–18324. doi: 10.1074/jbc.272.29.18316. [DOI] [PubMed] [Google Scholar]
- 49.Ricco A, Pedone PV, Lund LR, Olesen T, Olsen HS, Andreasen PA. Mol Cell Biol. 1992;12:1846–1855. doi: 10.1128/mcb.12.4.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bresnick EH, Felsenfeld G. J Biol Chem. 1993;268:18824–18834. [PubMed] [Google Scholar]
- 51.Navankasattusas S, Sawadogo M, van Bilsen M, Dang CV, Chien KR. Mol Cell Biol. 1994;14:7331–7339. doi: 10.1128/mcb.14.11.7331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vaishnaw AK, Mitchell TJ, Rose SJ, Walport MJ, Morley BJ. J Immunol. 1998;160:4353–4360. [PubMed] [Google Scholar]
- 53.Korner M, Rattner A, Mauxion F, Sen R, Citri Y. Neuron. 1989;3:563–572. doi: 10.1016/0896-6273(89)90266-3. [DOI] [PubMed] [Google Scholar]
- 54.Pecorino LT, Darrow AL, Strickland S. Mol Cell Biol. 1991;11:3139–3147. doi: 10.1128/mcb.11.6.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Solecki D, Schwarz S, Wimmer E, Lipp M, Bernhardt G. J Biol Chem. 1997;272:5579–5586. doi: 10.1074/jbc.272.9.5579. [DOI] [PubMed] [Google Scholar]
- 56.Borellini F, Aquino A, Josephs SF, Glazer RI. Mol Cell Biol. 1990;10:5541–5547. doi: 10.1128/mcb.10.10.5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rohlff C, Ahmad S, Borellini F, Lei J, Glazer RI. J Biol Chem. 1997;272:21137–21141. doi: 10.1074/jbc.272.34.21137. [DOI] [PubMed] [Google Scholar]