Abstract
Neuropeptide Y (NPY) is widely distributed in the human body and contributes to a vast number of physiological processes. Since its discovery, NPY has been implicated in metabolic regulation and, although interest in its role in central mechanisms related to food intake and obesity has somewhat diminished, the topic remains a strong focus of research concerning NPY signalling. In addition, a number of other uses for modulators of NPY receptors have been implied in a range of diseases, although the development of NPY receptor ligands has been slow, with no clinically approved receptor therapeutics currently available. Nevertheless, several interesting small molecule compounds, notably Y2 receptor antagonists, have been published recently, fueling optimism in the field. Herein we review the role of NPY in the pathophysiology of a number of diseases and highlight instances where NPY receptor signalling systems are attractive therapeutic targets.
Keywords: drug, neuropeptide, NPY, receptor, therapeutics
Neuropeptide Y and its Receptors
To date, neuropeptide Y (NPY) has been the subject of over 10,000 research papers identifying its numerous roles in the body that include, among other things, the control of feeding behaviour, cortical neural activity, heart activity and emotional regulation. Interestingly, NPY has also been implicated in several human diseases including obesity, alcoholism and depression, each of which might be considered to have behavioural or psychiatric components. NPY is a 36-amino acid peptide that acts as a neurotransmitter or neuromodulator depending on the context. It was originally identified and sequenced three decades ago (Tatemoto et al, 1982) and exhibits a 70% homology with peptide YY (PYY) and a 50% homology with pancreatic polypeptide (PP). All three of these 36-amino acid peptides are processed from 94 to 95 amino acid pro-hormones and have been grouped into the same so-called NPY family.
Neuropeptide Y and its receptors are expressed throughout the body. In the central nervous system, they are located in many brain regions and in the spinal cord. In the periphery, NPY is notably located in the sympathetic nervous system, where it acts with norepinephrine and adenosine triphosphate (ATP) to regulate cardiovascular and other functions. More recent findings suggest that NPY may even be involved in sweet and umami taste sensation (Herness & Zhao, 2009).
Heterogeneity among NPY receptors was first proposed based on studies of sympathetic neuroeffector junctions, in which differential NPY signalling pharmacology was observed (Wahlestedt et al, 1986). In this context, amidated carboxy-terminal NPY fragments selectively stimulate prejunctional NPY receptors called Y2-receptors, while post-junctional Y1-receptors are poorly activated by NPY fragments. Later work showed that the Y2-receptor also occurs post-junctionally in select systems such as in cardiac myocytes (McDermott et al, 1997; Wahlestedt et al, 1990). The diversity of the NPY receptor family is shown in Table 1. The Y1R, Y2R, Y4R and Y5R receptors have been grouped into the same family due to the fact that they all bind NPY, despite generally low sequence similarity. All NPY receptors are Gi protein-coupled receptors (Fig 1) and activation by NPY primarily results in decreased cyclic adenosine monophosphate (cAMP) production in the cell. Activation of the G protein complex by NPY can also lead to depressed Ca2+ channel and enhanced G protein coupled inwardly rectifying potassium channel (GIRK) currents (Acuna-Goycolea et al, 2005).
Table 1.
General overview of the NPY receptor family
| Human receptor | Peptide preference | cDNA cloned (year) | Comments | References |
|---|---|---|---|---|
| Y1R | PYY ≥ NPY [leu31,pro34]NPY ≫ NPY(13-36) | 1992 | Larhammar et al (1992); Herzog et al (1992) | |
| Y2R | PYY = NPY = NPY(13-36) ≫ [leu31,pro34]NPY | 1995 | Bard et al (1995); Gerald et al (1995) | |
| y3R | NPY ≫ PYY | Never | The putative y3R may actually be a multimerization of one or more of the other NPY receptors | Wahlestedt et al (1992); Movafagh et al (2006) |
| Y4R | PP > PYY > NPY | 1995 | Rose et al (1995) | |
| Y5R | NPY(2-36) ≥ NPY | 1996 | Gerald et al (1996); Weinberg et al (1996) | |
| y6R | N/A | 1996 | The human y6R is a non-functional pseudogene in the 5q31 region of the genome | Gregor et al (1996); Matsumoto et al (1996) |
The human Y1 receptor was cloned in 1992 (Herzog et al, 1992; Larhammar et al, 1992). The Y2 and Y4 receptors were first cloned in 1995 (Bard et al, 1995; Gerald et al, 1995; Rose et al, 1995), and the Y5 receptor in 1996 (Gerald et al, 1996; Weinberg et al, 1996). The neuropeptide y3 and y6 receptors have both been hypothesized in humans. The putative y3 receptor may be a multimerization of one or more NPY receptors (Movafagh et al, 2006; Wahlestedt et al, 1992). The y6 receptor is a non-functional pseudogene in humans, located in the 5q31 region of chromosome 5 (Gregor et al, 1996; Matsumoto et al, 1996).
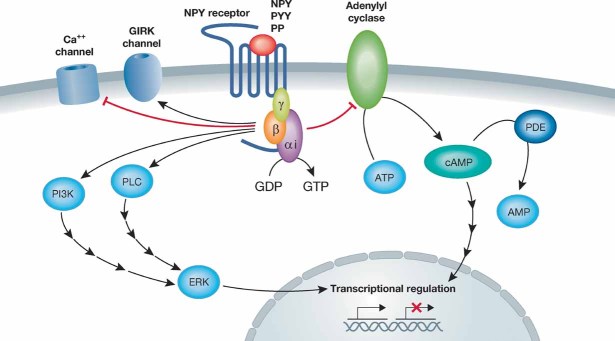
Figure 1. Typical intracellular signalling cascades for NPY receptors.
All NPY receptors couple to the Gi signalling cascade where the alpha subunit inactivates adenylyl cyclase. The betagamma subunit activates a number of different kinase cascades. Activation of the G protein complex can also lead to depressed Ca++ channel activity and enhanced G protein coupled inwardly rectifying potassium (GIRK) currents (Acuna-Goycolea et al, 2005). Initiation of these cellular signalling cascades has a multitude of effects such as the initiation of transcription or the stimulation or inhibition of hormone/neurotransmitter release, and is the basis for the physiological effects of NPY.
NPY in Obesity
A large number of studies on NPY have investigated its metabolic actions, especially related to food intake and obesity. A prevalent model for the study of obesity is the ob/ob mouse (Ingalls et al, 1950), which has been characterized as leptin deficient. Leptin is a regulator of NPY pathways and ob/ob mice have higher levels of NPY expression in the hypothalamus (Stephens et al, 1995). This, coupled to the fact that the central administration of NPY increases food intake, has led to much excitement about the idea that NPY systems regulate feeding behaviour.
Interestingly, however, NPY knockout mice are largely normal without any change in food intake or obesity (Erickson et al, 1996). On the other hand, Y1R knockout mice (Kushi et al, 1998) have higher body weights and more white adipose tissue, while both Y2R and Y5R knockout mice also have higher body weights, increased food intake and greater adipose deposition—though in the Y5R knockout mice this only occurs later in life (Marsh et al, 1998; Naveilhan et al, 1999). In contrast, Y4R knockout mice show reduced body weight and adipose tissue, which suggests an opposing action of Y4R in metabolism (Sainsbury et al, 2002).
Glossary
Angiogenesis
Development of blood vessels in the embryo or in an adult tissue.
Anxiolytic
A drug used for the treatment of symptoms of anxiety.
Inflammation
A poorly specific immune response of body tissues to infection, irritation or other injuries, characterized by pain, swelling, redness and heat.
Metastasis
The spreading of cancer from one part of the body to another.
Negative-feedback pathway
A network motif in which a node (hormone, protein, molecule, etc.) directly or indirectly negatively regulates itself in terms of production or activity.
Neurogenesis
The process by which neurons are generated from neural stem cells in the brain. Neurogenesis is most active in the young developing brain, but also occurs throughout life even into old age.
Nociceptive
The neural processes of encoding and processing noxious stimuli.
Pancreatic polypeptide (PP)
Pancreatic polypeptide was originally isolated from the pancreas and is believed to be the primary endogenous ligand for the Y4R (Kimmel et al, 1975). PP is released from the pancreas as a result of food intake and causes food absorption (Joehl & DeJoseph, 1986).
Peptide YY (PYY)
Peptide YY is mainly expressed in L-cells of the distal GI system (Tatemoto & Mutt, 1980). The peptide has affinity for all the NPY receptors but is 10-fold more potent at the Y1R, while both the full length and naturally occurring PYY(3-36) fragment have affinity for the Y2R. PYY causes nutrient and electrolyte absorption in the colon and distal GI tract, serving as a sensory hormone for the presence of excess nutritive matter (Vona-Davis & McFadden, 2007).
Pheochromocytoma
Adrenal gland tumour that causes overproduction of sympathetic nervous system hormones, such as epinephrine and norepinephrine.
Synaptic junctions (pre- and post-synaptic)
The place where a nerve impulse passes, in the form of an electric signal, from one neuron to another or to a muscle.
Umami
‘Savoriness’—one of the basic tastes (in addition to sweet, sour, bitter, salty).
Although the experiments reported above in conjunction with additional studies using mouse models have yielded a wealth of information on the roles of these peptides and their receptors, we believe that there may be confounding factors contributing to the phenotypes seen. First, germline deletions of the peptide or receptors are likely to result in many compensatory changes to the underlying mouse physiology. Second, factors such as food seeking necessity, acute stress response or other general stressors associated with survival cannot be mimicked in the laboratory environment. Therefore, we and others hypothesize that other factors, most notably anxiety, play a role in the actions of NPY in metabolic diseases. For example, chronic stress combined with poor diet can lead to abdominal obesity via activation of NPY and resultant adipogenesis (Kuo et al, 2007, 2008). Kuo and colleagues have elegantly outlined a system for the effects of chronic stress on food intake as it relates to NPY (Kuo et al, 2008), which may become a prevalent model for the actions of NPY in other systems as well.
The discovery of Y2R and Y4R peptide agonists, as well as Y5R antagonists has increased interest in NPY family signalling in the context of obesity. As a result, receptor modulators such as obinepitide, MK-0557 or velneperit have entered into clinical trials (Small & Bloom, 2005). The first of these, obinepitide (TM30338; 7TM Pharma), which is a peptide agonist at both the Y2R and Y4R, is currently in phase I/II trials for the treatment of obesity. However, the other two, MK-0557 and velneperit, both Y5R antagonists, have failed to achieve clinically meaningful reductions in obesity (Erondu et al, 2006; Sato et al, 2009). Another small molecule, PYY(3-36), has been proposed to act as an endogenous Y2R agonist and possibly an antiobesity compound (Batterham et al, 2002), but this hypothesis has been challenged (Boggiano et al, 2005). Nevertheless, there is genetic evidence that a common Y2R gene variant is protective against obesity in men (Lavebratt et al, 2006) and Y2R agonists may have other shortcomings and side effects that lead to a lack of efficacy, most notably nausea, which occurred when either PYY(3-36) or obinepitide was given to humans (Feletou & Levens, 2005; Sato et al, 2009).
NPY in Anxiety, Depression and Epilepsy
The role of NPY in mood disorders has been studied extensively (Eaton et al, 2007; Thorsell et al, 2006; Wahlestedt et al, 1989). In patients with major depression, for example, several studies have found that central NPY levels, primarily measured in the cerebrospinal fluid, are markedly low and correlate inversely to anxiety (Heilig, 2004; Widdowson et al, 1992). Remarkably, only a few scattered efforts have focused on developing a NPY receptor modulator for psychiatric purposes.
The Y2 receptor is located presynaptically on neurons that contain NPY, and it negatively regulates NPY release (King et al, 1999). NPY is an anxiolytic-like substance, which has led us to speculate that a synthetic compound that blocks the Y2 receptor mediated negative-feedback pathway will likely also be anxiolytic (Thorsell et al, 2006). Antagonism of Y2R would thus be expected to increase NPY levels in the CNS and may prove useful in treating certain psychiatric diseases. Indeed, Y2R null mice demonstrate reduced anxiety-like behaviour compared with wild type controls (Redrobe et al, 2003). The administration of the Y2R antagonist N-[(1S)-4-[(Aminoiminomethyl)amino]-1-[[[2-(3,5-dioxo-1,2-diphenyl-1,2,4-triazolidin-4-yl)ethyl]amino]carbonyl]butyl]-1-[2-[4-(6,11-dihydro-6-oxo-5H-dibenz[b,e]azepin-11-yl)-1-piperazinyl]-2-oxoethyl]-cyclopentaneacetamide (BIIE0246), as well as Y1R agonists, induced antidepressant-like effects in mice (Rimondini et al, 2005). In contrast, an emerging role for NPY is as an anticonvulsant via activation of the Y2R, which was measured both by brain injection of NPY and by using NPY overexpressing viral vectors in rat hippocampi (Noe et al, 2009, 2010; Sperk et al, 2007). Therefore it is not known whether Y2R antagonists will have pro-convulsive side effects. On the other hand, Y2R brain penetrant agonists are almost sure to be anticonvulsant.
The Y1R has also been implicated in antianxiety behaviour in rats (Wahlestedt et al, 1993), as well as in the mediation of adult neuronal proliferation and hippocampal neurogenesis (Decressac et al, 2010; Hansel et al, 2001). Induction of neurogenesis in the hippocampus has been targeted as a potential treatment for depression, Alzheimer's disease and schizophrenia among other diseases (DeCarolis & Eisch, 2010). Presumably, NPY (or a Y1R agonist) could serve as an antidepressant (or cognitive enhancer) by induction of neurogenesis from neural stem cells. Though this remains to be tested, it is an emerging role for NPY that has some therapeutic potential.
NPY in Alcoholism
There is a large and compelling body of evidence implicating NPY and signaling in habitual alcohol use and abuse. First, data from human studies shows that NPY levels are reduced in the brain of alcoholic individuals compared to controls, though whether this is a cause or a consequence of alcohol abuse remains unclear (Mayfield et al, 2002). Second, in two corroborating studies of alcoholic families in the USA of European and African descent, a significant association of small nucleotide polymorphisms (SNPs) in the Y2R gene with alcohol, cocaine and co-morbid alcohol and cocaine dependence, as well as alcohol withdrawal symptoms, were observed (Lappalainen et al, 2002; Wetherill et al, 2008). This also suggests that the Y2R plays a role in addictive disorders other than alcoholism.
Several additional lines of evidence from animal models support the idea that the Y2R constitutes a viable target for the treatment of alcohol dependence. Although the Y1R, Y2R and Y5R have all been implicated in the modulation of alcohol intake, only Y2R messenger RNA (mRNA) has been found to be substantially reduced in alcohol-preferring rats (Caberlotto et al, 2001), suggesting that it modulates affinity for the drug. Further, Y2R knockout mice consume less ethanol than wild type mice (Thiele et al, 2004). Importantly, administration of the Y2R antagonist BIIE0246 to rats that had been exposed to ethanol vapour attenuated alcohol intake in a lever press ethanol administration paradigm; it did not affect rats without prior exposure (Rimondini et al, 2005; Thorsell et al, 2002). Nevertheless, additional work is still required to validate Y2R as a tractable clinical target and its antagonists as alternatives to current pharmacological treatments of alcohol dependence (such as the aldehyde dehydrogenase inhibitor disulfiram, the opioid receptor antagonist naltrexone and the functional glutamate receptor antagonist acamprosate).
NPY in Bone Physiology
It has become apparent in the last decade, mostly due to the efforts of Herzog and colleagues, that NPY plays a role in bone homeostasis (Lee & Herzog, 2009). Ob/ob (leptin deficient) mice are known to have greater bone density, and Ducy and colleagues have shown that ob/ob mice administered with NPY (i.c.v.) have decreased bone density compared to control mice (Ducy et al, 2000). This singular observation was made by histologically assessing vertebrae mass and volume, and has been followed up by others. However, it is not clear whether this effect is mediated through the Y2 or Y1 receptor. Y2R knockout mice have greater bone density, which is likely due to greater osteoclast (bone absorbing cell) activity mediated via the Y2R in the hypothalamus, as this was also seen in hypothalamus specific deletions of the Y2R (Baldock et al, 2002, 2006). Y1R knockout mice have greater bone mass and formation, and Y1 receptors have been observed on osteoblasts (bone forming cells) (Baldock et al, 2007). In Y2R knockout mice, the Y1R is also down-regulated, possibly due to an increase in NPY from a lack of Y2R mediated feedback (Lundberg et al, 2007). Thus, it remains to be determined whether it is the Y1R or the Y2R that is primarily involved in bone formation and which is most suited as a target for therapeutic intervention.
Current pharmacological treatment for osteoporosis (bone thinning) centres around a class of drugs called bisphosphonates, which includes products such as Fosamax (alendrate, Merck), Actonel (risedronate, Proctor & Gamble and Sanofi-Aventis) or Boniva (ibandronate, GlaxoSmithKlein and Roche Labs). Bisphosphonates incorporate into bone and prevent bone reabsorption by osteoclasts. However, because bisphosphonates reduce bone loss by interfering with osteoclast activity—rather than by promoting bone formation—they have relatively little effect in people with advanced osteoporosis who already have significant bone loss. NPY receptor modulators could supplement current pharmacotherapies. Y2R antagonists could reduce osteoclast activity via a hypothalamic mediated mechanism (unlike the metabolic inhibition that bisphosphonates confer) and could also lead to reduced Y1R function, which combined would lead to increased osteoblast function and increased bone density. Therefore, an increase in bone density would be expected, perhaps even leading to the reduction or loss of osteoporosis. It is also interesting to note that this bone building mechanism mediated by the NPY receptors might be related to sex steroids such as oestrogen (Heikkinen et al, 2004; Zengin et al, 2010), and so represents an attractive target for increasing bone volume in post-menopausal women without the need for hormone replacement therapy.
NPY in Pain
Neuropeptide Y has been strongly implicated in pathways related to nociceptive pain and inflammation. Naveilhan and colleagues tested the ability of capsaicin, which induces a burning pain sensation, to elicit a substance P response in wild type and Y1R knockout mice. Substance P is a sensory transmitter that is a key mediator of pain signalling. Y1R knockout mice did not present increased substance P levels, though the wild type mice did (Naveilhan et al, 2001). In the same study the authors showed that the Y1R knockout mice were more sensitive to a number of pain stimuli, including thermal and chemical. Other studies have broadened our understanding of the possible link between NPY and pain and it is now clear that both the Y1R and the Y2R are involved in mediating pain signalling in the brain (reviewed in Brumovsky et al, 2007). For instance, the injection of NPY elicits an analgesic response, which is thought to be a result of Y1R stimulation (Mantyh, 2002; Seybold et al, 2003); the Y2 receptor also plays a role (Moran et al, 2004).
The question of whether NPY is pro- or antinociceptive, or both, remains to be elucidated. It appears as though NPY serves as an analgesic peptide in the skin because subcutaneous injection of the peptide blocks thermal and mechanical pain: likely via the activation of the Y2R on Aδ and/or c sensory nerve fibres (Brumovsky et al, 2007; Tracey et al, 1995). Travelling further up the sensory nervous system, it is likely that NPY acts via both the Y1R and the Y2R to mediate pain signalling in the dorsal root ganglion. The proposed mechanism is via cross-excitation of other dorsal root ganglia (DRG), thus increasing the overall sensitivity of the system to pain inputs (Amir & Devor, 1996). Based on this, Brumovsky and colleagues have proposed that a peripherally active Y2R antagonist would be useful in reducing the pain sensitivity threshold in DRG, but this has not been directly tested (Brumovsky et al, 2007). Travelling from DRG to the spine, NPY and its receptors have been found to be expressed in spinal nerves (Brumovsky et al, 2005, 2006; Gibson et al, 1984); however, much less is known about what the NPY systems in the spine may have to do with pain sensation. It may be that NPY is partly responsible for the transmission of pain sensation from the periphery to the brain in the spine (Mantyh, 2002). Less still is known about the role of NPY signalling in the brain to mediate pain sensation; however, it is likely that NPY does play a role here: Jung and colleagues injected NPY into the brain stem and found an analgesic response after induction of neuropathic pain (Jung et al, 2009). Therefore, NPY appears to play a significant role in pain sensation along the entire pain pathway from the periphery to the brain stem, and perhaps beyond. We feel that the contribution of NPY is primarily as a modulator to pain threshold, but may also be directly responsible for transmitting pain sensation.
NPY in Cancer
Neuropeptide Y has been shown to be involved in a number of mechanisms involved in cancer progression, including cell proliferation, angiogenesis and metastasis (for a recent review see Ruscica et al, 2007). NPY has been shown to induce neural progenitor cell and smooth muscle cell proliferation, mediated by the Y1R (Erlinge et al, 1994; Hansel et al, 2001); and can induce the proliferation of neural crest tumours via the Y1R and Y2R (Kitlinska et al, 2005). Increased plasma levels of NPY can be a marker of neuroblastoma formation, though this is not useful as a diagnostic test due to high variability (Kitlinska, 2007). NPY also promotes angiogenesis (Zukowska-Grojec et al, 1998), and Y2R knockout mice have impaired angiogenic activity (Ekstrand et al, 2003).
Taken together with a number of other studies, there appears to be a clear link between NPY and cancer, and it is possible to state conclusively that NPY serves as a pro-oncogenic factor, especially for neuroblastomas and perhaps even breast and prostate cancers. Other cancer types have been mentioned, but with far less supporting evidence, which is not to say that NPY will not be implicated in other cancers. It is nevertheless too early to tell whether these findings will translate into potential cancer treatments, but this is an interesting area for further research as the generation of selective small molecule NPY receptor ligands continues. A Y1R or Y2R antagonist or even a non-selective antagonist of both receptors could be useful in preventing cancer progression.
NPY in Cardiovascular Regulation
The role of NPY in the cardiovascular system is one of the oldest and better characterized roles of NPY. Often in conjunction with norepenipherine, NPY is extensively involved in cardiovascular sympathetic regulation (McDermott & Bell, 2007). NPY is abundant in the heart and was identified as a cardiac peptide around the same time it was sequenced (Gu et al, 1983). It also acts in the sympathetic nervous system and potentiates norepinephrine-stimulated vasoconstriction (Wahlestedt et al, 1985). Plasma levels of NPY correlate with the activity of the sympathetic nervous system, as can be seen in different pathophysiological settings, such as in response to mild or heavy exercise (low sympathetic nervous system activation) and in heart disease or pheochromocytoma patients (with very high sympathetic nervous system output) (Adrian et al, 1983; Hulting et al, 1990). More recently, genome wide association studies have linked NPY to human coronary artery disease (CAD): SNPs in the NPY gene correlated to CAD in humans and even more so in early onset patients (Shah et al, 2009). In this same association study, the authors showed a very slight increase in NPY production in patients with the rs16147 A → G allele. They also showed that the antagonism of Y1Rs by N-[(1R)]-4-[(Aminoiminomethyl)amino-1-[[[(4-hydroxyphenyl)methyl]amino]carbonyl]butyl-a-phenylbenzeneacetamide (BIBP3226), a selective Y1R antagonist, reduced atherosclerosis. They thus conclude that the increase in NPY via Y1Rs is contributing to early onset CAD.
Y1R, Y2R and Y5R are expressed in various regions of the cardiovascular system (McDermott & Bell, 2007). The Y1R mediates a vasoconstrictor response, while the Y2R mediates slowing of the heart rate and Y2R knockout mice tend to have a higher heart rate (Matsuda et al, 2002; Smith-White et al, 2002). NPY activation of the Y5R has been implicated in heart function, primarily in promoting cardiac hypertrophy (thickening of the heart muscle) via MAP kinase signalling (Pellieux et al, 2000).
The potential for therapeutic NPY receptor modulators in cardiovascular disease is not clear. It can be hypothesized that a Y5R antagonist might be useful to prevent hypertrophy or a Y1R antagonist might be useful for inducing vasodilatation, or a Y2R agonist might be useful to treat mild bradycardia or an antagonist might be useful in mild tachycardia. Because of the modulatory role of NPY in sympathetic nervous system control of the heart, we feel that any NPY treatment would be useful in mild heart disease or prophylaxis against more severe acute heart problems such as cardiac arrest. The wide distribution of NPY receptors in the cardiovascular system should also be considered carefully in the planning and assessment of the trials of such drugs, as Y1R agonists might drive up blood pressure, while a Y2R antagonist may unintentionally increase heart rate.
NPY in the Gastrointestinal Tract
In the periphery, PYY, PP and NPY all regulate the gastrointestinal (GI) tract, but PYY appears to have the larger role. The NPY receptors are involved in numerous roles in the GI tract including adaptation to diet, GI motility, electrolyte balance, nutrient/water uptake, intestinal growth and gastric emptying (reviewed in Vona-Davis & McFadden, 2007). The most studied of these processes is the ‘illium break’ mechanism of PYY. Essentially, PYY is released in the small intestine in response to fat ingestion (Aponte et al, 1989; Lin et al, 1996). PYY then acts to inhibit electrolyte (liquid) entry into the small intestine, which effectively slows the passage of nutrient matter to allow absorption (Adrian et al, 1986; Gardiner et al, 2008). This effect of PYY in the gut is mediated via the Y1 and Y4 receptors (Cox & Tough, 2002). PYY also inhibits GI transit via a neuronal mechanism mediated by the Y2R (Cox, 2007). NPY also plays a role in gut health and may be involved in inflammatory bowel disease (IBD), as it was found that plasma and GI tract levels of NPY were significantly lower in patients with IBD. Patients with diarrhoeal IBD also had significantly lower levels of NPY than those with constipatory IBD, reinforcing the role of NPY as a proabsorptive peptide (Zhang et al, 2008).
Neuropeptide Y in the brain also plays a role in GI tract health. For example, intracerebroventricular injection of NPY in rats both reduces gastric erosion (Heilig & Murison, 1987) and reduces gastric acid secretion (Penner et al, 1993). Other studies have confirmed that the Y1R is an important component of these processes. The Y1R is likely also an important component of IBD progression as Y1R knockout mice appear to have a lower susceptibility to the disease (Hassani et al, 2005).
There is some potential to use NPY receptor modulators in GI diseases such as constipation/diarrhoea and IBD. It is likely that the administration of an NPY agonist, perhaps as a non-specific activator, would help in the treatment of IBD. This idea may be made even more attractive by the fact that such a therapeutic agent need not be taken up into the blood stream, and there have been some studies directed toward this idea. For example, in the case of diarrhoea, Litvak and colleagues used two stable peptide analogues of PYY, BIM-43073D and BIM-43004C and found that water absorption was increased in the guts of dogs treated with these analogues (Litvak et al, 1999). These findings support the idea that a Y1R selective agonist might promote gut health. On the other hand, the fact that Y1R knockout mice have a lower susceptibility to IBD also suggests that an antagonist would be effective and might be useful in the treatment of constipation and/or IBD. It seems likely that the Y2R and Y4R receptors will be important in resolving this discrepancy and, of course, the development of novel probes will help this discussion as well.
NPY in Circadian Regulation
In humans, intravenous injection of NPY into young men has been shown to enhance sleep quality and reduce the time it takes to get to sleep (Antonijevic et al, 2000). The levels of NPY in the bloodstream fluctuate over the course of a 24-h day, peaking every 6–8 h with the largest peak at approximately 16:00 (Lockinger et al, 2004). More recently, in an intricate set of experiments, NPY release was measured in the suprachiasmatic nucleus (SCN) of hamsters by microdialysis, which showed levels of the peptide rise and fall rhythmically throughout the day (Glass et al, 2010). Taken together each of these independent lines of evidence support a clear effect of NPY on circadian regulation, though no definitive study has been able to link them all together. It may be, as with the other roles of NPY, that the effects on circadian regulation modulate more fundamental systems such as melatonin, a major regulator of the sleep–wake cycle (Glass et al, 2010).
Interestingly, the role that NPY plays in feeding behaviour might be linked to circadian regulation (Edelsbrunner et al, 2009). Food intake is higher in rats that have been continuously deprived of sleep for anywhere from 2 to 4 (the maximum tested) days. In the same rats, there is a significant up-regulation of hypothalamic NPY mRNA levels that precedes this feeding behaviour (Martins et al, 2010). Therefore, it has been hypothesized that the role of NPY in feeding behaviour may have more to do with circadian regulation than previously thought. This is an interesting line of thinking, though due to the lack of information it would be difficult to speculate whether this role could be a therapeutic target. There is, however, a lot of room for the development of this idea, and it may be one of the most interesting new areas for research that will benefit from new small molecule probes.
Current NPY Receptor Ligands
Antagonists
The availability of useful NPY receptor ligands for either in vitro or in vivo work is still limited, despite a clear need (Table 2) (Grundemar & Bloom, 1997). Most of the selective NPY receptor ligands are peptides, which limit in vivo or clinical applications (Table 3). There are, however, several non-peptide receptor antagonists of the Y1R, including BIBP3226, which is potent and selective, except for a mild affinity for the Neuropeptide FF receptor (Mollereau et al, 2001). N-[(1R)-1-[[[[4-[[(Aminocarbonyl)amino]methyl]phenyl]methyl]amino]carbonyl]-4-[(aminoiminomethyl)amino]butyl]-a-phenyl-benzeneacetamide (BIBO3304) is another Y1R antagonist with a better selectivity profile and higher affinity (Dumont et al, 2000).
Table 2.
The hypothesized utility of indicated NPY receptor modulators listed for indicated diseases
| Disease | Y1 receptor | Y2 receptor | Y4 receptor | Y5 receptor | ||||
|---|---|---|---|---|---|---|---|---|
| Agonist | Antagonist | Agonist | Antagonist | Agonist | Antagonist | Agonist | Antagonist | |
| Obesity | − | + | + | +(1) | +(2) | ? | − | +(3) |
| Anxiety and depression | ++ | − | + | ++ | ? | + | + | − |
| Epilepsy | − | ? | ++ | − | ? | ? | ? | ? |
| Alcoholism | ++ | − | − | ++ | ? | + | + | − |
| Bone metabolism | − | + | − | + | − | + | ? | ? |
| Pain | + | + | + | +(4) | ? | ? | ? | ? |
| Cancer | − | + | − | + | −/? | −/? | − | + |
| Cardiovascular disease | −/? | + | −/+(5) | −/? | − | − | + | − |
| Intestinal disease (6) | + | + | + | + | −/? | + | ? | ? |
| Circadian disorders | + | +/? | + | +/? | ? | ? | ? | ? |
| Alzheimer's disease | +/? | ? | +/? | ? | ? | ? | ? | ? |
++, Strong supporting evidence for utility; +, that there may be some utility; ?, little evidence either way for utility or opposing views; −, evidence for lack of utility or that the molecule would be counterproductive.
Note: (1) This may be useful for peripheral antagonism in adipose tissue. (2) A dual Y2 and Y4 agonist from 7TM pharma is currently in clinical trials for obesity. (3) Clinical trials of two Y5R antagonists have yielded insufficient clinical efficacy. (4) Proposed as a peripheral antagonist in (Brumovsky et al, 2007). (5) Depending on the site of action. (6) Depending on the indication, NPY ligands may be useful for IBD, malabsorption and constipation among others.
Table 3.
Representative NPY receptor ligands
| Agonists | Antagonists | |
|---|---|---|
| Y1R | NPY ≥ PYY ≫ PP | BIBP3226 (1) |
| Leu31,Pro34-NPY | BIBO3304 | |
| [Pro30,Nle31,Bpa32,Leu34]NPY(28-36) | 1229U91 (GR231118) (2) | |
| J-104870 | ||
| J-115814 | ||
| BW1911U90 | ||
| BMS193885 | ||
| Y2R | NPY ≥ PYY ≫PP | BIIE0246 |
| PYY(3-36) | SF-11 | |
| NPY(13-36) | JNJ-5207787 | |
| Obinepitide (TM30338) | JNJ-31020028 | |
| Soluble pyridyl analogue #36 from (Lunniss et al,2009) | ||
| Y4R | PP > PYY > NPY | UR-AK49 ? (3) |
| 1229U91 (GR231118) (2) | ||
| Obinepitide (TM30338) | ||
| Y5R | NPY ≥ PYY ≥ PP | MK-0557 |
| [Ala31,Aib32]NPY | S-2367 (Velneperit) | |
| L152,804 | ||
| 2-36[K4,RYYSA(19-23)]PP |
Ligands marked in bold are small molecules, non-peptide ligands. Note: (1) BIBP3226 also antagonizes the neuropeptide FF receptor (Ki = ∼100 nM).
(2) 1229U91 (GR231118) is a non-specific peptide based ligand that is both a Y1R antagonist and a Y4R partial agonist. (3) UR-AK49 was found to be a small molecule antagonist for the Y4R, however the very low potency of this compound (IC50 = 68 µM) blunts its usefulness. UR-AK49 may serve as a lead compound for optimization.
BIIE0246, another non-peptide molecule, is the most widely used Y2R antagonist (Doods et al, 1999). However, BIIE0246 is a large molecule (MW ≍900) and binds to mu and kappa opioid and α1A adrenergic receptors with submicromolar affinities, as well as to several other receptors with low micromolar affinities. Moreover, this drug cannot cross the blood brain barrier (Brothers et al, 2010), limiting its use. There is a need to develop other Y2R antagonists and we (Brothers et al, 2010) and others, including Johnson & Johnson (Shoblock et al, 2010), GlaxoSmithKline (Lunniss et al, 2009) and Novartis (under patents WO2009050201, WO2009050200 and WO2009050197), have been working to find selective Y2R antagonists. The Johnson & Johnson and GSK compounds currently offer better potency than our compounds. On the other hand, our compounds have fewer patent-related restrictions and we have a greater diversity of chemical scaffolds for additional development efforts, which are currently underway. Our most potent Y2R antagonist, SF-11, is now being sold by Tocris and we are certain that as a result of the greater availability of compounds and the parallel development efforts, a useful Y2R antagonist for human use will soon appear.
While no potent and selective non-peptide Y4R antagonists have been developed so far, there are many Y5 receptor antagonists. Some of these have entered into clinical trials for the treatment of obesity. Notably, two Y5R antagonists are well tolerated in patients, MK-0557 (Merck & Co., Inc.) and S-2367 (velneperit, Shionogi & Co, Ltd). Both antagonists were tested in phase II trials for obesity, but were withdrawn given their lack of clinically meaningful effects, though each had some effect on weight loss (Sato et al, 2009). It is unlikely that these compounds will ever enter the clinic as they stand. However, they may be very useful to use for study of the Y5 receptor contributions to obesity and also other diseases.
Agonists
Non-peptide small molecule NPY receptor agonists are severely lacking. Despite many efforts, none have been reported to date for any of the NPY receptors. Peptide agonists, however, are common, including the recent description of a Y1R peptide agonist smaller than NPY, [Pro30, Nle31, Bpa32, Leu34]NPY(28-36) (Zwanziger et al, 2009). Several selective peptide agonists have been identified for Y2R such as NPY(13-36) and PYY(3-36). 1229U91 (Roche) was originally identified as an antagonist of Y1R (Hegde et al, 1995) but it is also a potent Y4R agonist peptide (Schober et al, 1998). There are other clinically interesting peptide-based NPY receptor agonists, such as obinepitide (TM30338; 7TM Pharma), mentioned at the beginning of this review.
The utility of NPY receptor ligands as therapeutics remains largely unproven, especially in light of the failure of the Y5R antagonist, MK-0557 in obesity clinical trials (Erondu et al, 2006). It may well be that more than a single NPY receptor may need to be targeted in order to observe a meaningful effect. The outcome of obinepitide trials will be telling on this point.
Conclusion
There is a severe and pressing need to develop useful NPY related small molecule ligands to conclusively assess NPY's role in pathologies, such as those outlined here, as well as to underpin its molecular partners in these settings and to clarify the clinical therapeutic potential of NPY signalling pathways. The potential therapeutic usefulness of NPY receptor ligands is still not clear, nor is the potential side effect profile of hypothetical NPY receptor small molecules. While investigators in the NPY field are to be applauded for having performed their research in the absence of such tools, the growing repertoire of NPY, which includes roles in feeding, alcoholism, anxiety, bone formation and pain, among other conditions (as well as potentially undiscovered roles), means that new tools are needed to further explore these activities.
Pending issues
Neuropeptide Y receptor drug discovery: until recently there has been a lack of available small molecule NPY receptor therapeutics. Therefore there is little useful data on human efficacy and side effect profiles of NPY related compounds. A general feeling, in part based on the lack of severe phenotype in NPY receptor null mice, is that NPY receptor antagonists may not actually show problematic mechanism based toxicity. Therefore, the issue with NPY receptor antagonists, by shutting down endogenous NPY tone, is whether they would provide clinically useful efficacy profiles. The development of additional conditional NPY receptor knockout mice in tandem with drug discovery efforts may help this discussion.
In contrast, there can be little doubt that a NPY receptor agonist would be efficacious in humans. However, agonist related side effect profiles are more difficult to predict and cannot be predicated on knockout models. There is also a confounding species distinction when using agonists in animal models, an important consideration in discussions of potential psychiatric effects. Most drug discovery efforts with NPY have focused on central nervous system applications, but it remains possible that compounds that do not pass the blood brain barrier have greater immediate clinical utility. Though, by nature, there is also the greatest potential for adaptation to such peripheral and modulatory NPY receptor drugs.
It is an interesting time for NPY research as small molecule receptor ligands will likely become increasingly available. Because NPY receptor knockout mice are largely normal, it is possible to presume that NPY's physiological roles are generally rather subtle and modulatory in nature. If so, receptor antagonists are perhaps not likely to show great efficacy, but the safe use of these in humans would arguably be less of a concern. Agonist compounds, on the other hand, would likely be efficacious due to the widespread presence of NPY receptors but could therefore have severe pleiotropic effects. In our opinion, the identification of small molecule compounds that display high degrees of NPY receptor subtype selectivity must be a focus for the research community over the next several years.
Acknowledgments
Our current work on NPY is funded by the National Institute on Alcohol Abuse and Alcoholism (1U01AA018665). We thank Dr. Lars Grundemar for critical reading of this manuscript.
The authors declare that they have no conflict of interest.
References
- Acuna-Goycolea C, Tamamaki N, Yanagawa Y, Obata K, van den Pol AN. Mechanisms of neuropeptide Y, peptide YY, and pancreatic polypeptide inhibition of identified green fluorescent protein-expressing GABA neurons in the hypothalamic neuroendocrine arcuate nucleus. J Neurosci. 2005;25:7406–7419. doi: 10.1523/JNEUROSCI.1008-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian TE, Allen JM, Terenghi G, Bacarese-Hamilton AJ, Brown MJ, Polak JM, Bloom SR. Neuropeptide Y in phaeochromocytomas and ganglioneuroblastomas. Lancet. 1983;2:540–542. doi: 10.1016/s0140-6736(83)90570-6. [DOI] [PubMed] [Google Scholar]
- Adrian TE, Savage AP, Bacarese-Hamilton AJ, Wolfe K, Besterman HS, Bloom SR. Peptide YY abnormalities in gastrointestinal diseases. Gastroenterology. 1986;90:379–384. doi: 10.1016/0016-5085(86)90936-4. [DOI] [PubMed] [Google Scholar]
- Amir R, Devor M. Chemically mediated cross-excitation in rat dorsal root ganglia. J Neurosci. 1996;16:4733–4741. doi: 10.1523/JNEUROSCI.16-15-04733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonijevic IA, Murck H, Bohlhalter S, Frieboes RM, Holsboer F, Steiger A. Neuropeptide Y promotes sleep and inhibits ACTH and cortisol release in young men. Neuropharmacology. 2000;39:1474–1481. doi: 10.1016/s0028-3908(00)00057-5. [DOI] [PubMed] [Google Scholar]
- Aponte GW, Park K, Hess R, Garcia R, Taylor IL. Meal-induced peptide tyrosine tyrosine inhibition of pancreatic secretion in the rat. FASEB J. 1989;3:1949–1955. doi: 10.1096/fasebj.3.8.2721855. [DOI] [PubMed] [Google Scholar]
- Baldock PA, Sainsbury A, Couzens M, Enriquez RF, Thomas GP, Gardiner EM, Herzog H. Hypothalamic Y2 receptors regulate bone formation. J Clin Invest. 2002;109:915–921. doi: 10.1172/JCI14588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldock PA, Allison S, McDonald MM, Sainsbury A, Enriquez RF, Little DG, Eisman JA, Gardiner EM, Herzog H. Hypothalamic regulation of cortical bone mass: opposing activity of Y2 receptor and leptin pathways. J Bone Miner Res. 2006;21:1600–1607. doi: 10.1359/jbmr.060705. [DOI] [PubMed] [Google Scholar]
- Baldock PA, Allison SJ, Lundberg P, Lee NJ, Slack K, Lin EJ, Enriquez RF, McDonald MM, Zhang L, During MJ, et al. Novel role of Y1 receptors in the coordinated regulation of bone and energy homeostasis. J Biol Chem. 2007;282:19092–19102. doi: 10.1074/jbc.M700644200. [DOI] [PubMed] [Google Scholar]
- Bard JA, Walker MW, Branchek TA, Weinshank RL. Cloning and functional expression of a human Y4 subtype receptor for pancreatic polypeptide, neuropeptide Y, and peptide YY. J Biol Chem. 1995;270:26762–26765. doi: 10.1074/jbc.270.45.26762. [DOI] [PubMed] [Google Scholar]
- Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- Boggiano MM, Chandler PC, Oswald KD, Rodgers RJ, Blundell JE, Ishii Y, Beattie AH, Holch P, Allison DB, Schindler M, et al. PYY 3-36 as an anti-obesity drug target. Obes Rev. 2005;6:307–322. doi: 10.1111/j.1467-789X.2005.00218.x. [DOI] [PubMed] [Google Scholar]
- Brothers SP, Saldanha SA, Spicer TP, Cameron M, Mercer BA, Chase P, McDonald P, Wahlestedt C, Hodder PS. Selective and brain penetrant neuropeptide y y2 receptor antagonists discovered by whole-cell high-throughput screening. Mol Pharmacol. 2010;77:46–57. doi: 10.1124/mol.109.058677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumovsky P, Stanic D, Shuster S, Herzog H, Villar M, Hokfelt T. Neuropeptide Y2 receptor protein is present in peptidergic and nonpeptidergic primary sensory neurons of the mouse. J Comp Neurol. 2005;489:328–348. doi: 10.1002/cne.20639. [DOI] [PubMed] [Google Scholar]
- Brumovsky P, Hofstetter C, Olson L, Ohning G, Villar M, Hokfelt T. The neuropeptide tyrosine Y1R is expressed in interneurons and projection neurons in the dorsal horn and area X of the rat spinal cord. Neuroscience. 2006;138:1361–1376. doi: 10.1016/j.neuroscience.2005.11.069. [DOI] [PubMed] [Google Scholar]
- Brumovsky P, Shi TS, Landry M, Villar MJ, Hokfelt T. Neuropeptide tyrosine and pain. Trends Pharmacol Sci. 2007;28:93–102. doi: 10.1016/j.tips.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Caberlotto L, Thorsell A, Rimondini R, Sommer W, Hyytia P, Heilig M. Differential expression of NPY and its receptors in alcohol-preferring AA and alcohol-avoiding ANA rats. Alcohol: Clin Exp Res. 2001;25:1564–1569. [PubMed] [Google Scholar]
- Cox HM. Neuropeptide Y receptors; antisecretory control of intestinal epithelial function. Auton Neurosci. 2007;133:76–85. doi: 10.1016/j.autneu.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Cox HM, Tough IR. Neuropeptide Y, Y1, Y2 and Y4 receptors mediate Y agonist responses in isolated human colon mucosa. Br J Pharmacol. 2002;135:1505–1512. doi: 10.1038/sj.bjp.0704604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarolis NA, Eisch AJ. Hippocampal neurogenesis as a target for the treatment of mental illness: a critical evaluation. Neuropharmacology. 2010;58:884–893. doi: 10.1016/j.neuropharm.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decressac M, Wright B, David B, Tyers P, Jaber M, Barker RA, Gaillard A. Exogenous neuropeptide Y promotes in vivo hippocampal neurogenesis. Hippocampus. 2010 doi: 10.1002/hipo.20765. DOI: 10.1002/hipo.20765. [DOI] [PubMed] [Google Scholar]
- Doods H, Gaida W, Wieland HA, Dollinger H, Schnorrenberg G, Esser F, Engel W, Eberlein W, Rudolf K. BII E0246: a selective and high affinity neuropeptide Y Y(2) receptor antagonist. Eur J Pharmacol. 1999;384:R3–R5. doi: 10.1016/s0014-2999(99)00650-0. [DOI] [PubMed] [Google Scholar]
- Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- Dumont Y, Cadieux A, Doods H, Fournier A, Quirion R. Potent and selective tools to investigate neuropeptide Y receptors in the central and peripheral nervous systems: BIB03304 (Y1) and CGP71683A (Y5) Can J Physiol Pharmacol. 2000;78:116–125. [PubMed] [Google Scholar]
- Eaton K, Sallee FR, Sah R. Relevance of neuropeptide Y (NPY) in psychiatry. Curr Top Med Chem. 2007;7:1645–1659. doi: 10.2174/156802607782341037. [DOI] [PubMed] [Google Scholar]
- Edelsbrunner ME, Painsipp E, Herzog H, Holzer P. Evidence from knockout mice for distinct implications of neuropeptide-Y Y2 and Y4 receptors in the circadian control of locomotion, exploration, water and food intake. Neuropeptides. 2009;43:491–497. doi: 10.1016/j.npep.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrand AJ, Cao R, Bjorndahl M, Nystrom S, Jonsson-Rylander AC, Hassani H, Hallberg B, Nordlander M, Cao Y. Deletion of neuropeptide Y (NPY) 2 receptor in mice results in blockage of NPY-induced angiogenesis and delayed wound healing. Proc Natl Acad Sci USA. 2003;100:6033–6038. doi: 10.1073/pnas.1135965100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JC, Clegg KE, Palmiter RD. Sensitivity to leptin and susceptibility to seizures of mice lacking neuropeptide Y. Nature. 1996;381:415–421. doi: 10.1038/381415a0. [DOI] [PubMed] [Google Scholar]
- Erlinge D, Brunkwall J, Edvinsson L. Neuropeptide Y stimulates proliferation of human vascular smooth muscle cells: cooperation with noradrenaline and ATP. Regul Pept. 1994;50:259–265. doi: 10.1016/0167-0115(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Erondu N, Gantz I, Musser B, Suryawanshi S, Mallick M, Addy C, Cote J, Bray G, Fujioka K, Bays H, et al. Neuropeptide Y5 receptor antagonism does not induce clinically meaningful weight loss in overweight and obese adults. Cell Metab. 2006;4:275–282. doi: 10.1016/j.cmet.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Feletou M, Levens NR. Neuropeptide Y2 receptors as drug targets for the central regulation of body weight. Curr Opin Investig Drugs. 2005;6:1002–1011. [PubMed] [Google Scholar]
- Gardiner JV, Jayasena CN, Bloom SR. Gut hormones: a weight off your mind. J Neuroendocrinol. 2008;20:834–841. doi: 10.1111/j.1365-2826.2008.01729.x. [DOI] [PubMed] [Google Scholar]
- Gerald C, Walker MW, Vaysse PJ, He C, Branchek TA, Weinshank RL. Expression cloning and pharmacological characterization of a human hippocampal neuropeptide Y/peptide YY Y2 receptor subtype. J Biol Chem. 1995;270:26758–26761. doi: 10.1074/jbc.270.45.26758. [DOI] [PubMed] [Google Scholar]
- Gerald C, Walker MW, Criscione L, Gustafson EL, Batzl-Hartmann C, Smith KE, Vaysse P, Durkin MM, Laz TM, Linemeyer DL, et al. A receptor subtype involved in neuropeptide-Y-induced food intake. Nature. 1996;382:168–171. doi: 10.1038/382168a0. [DOI] [PubMed] [Google Scholar]
- Gibson SJ, Polak JM, Anand P, Blank MA, Morrison JF, Kelly JS, Bloom SR. The distribution and origin of VIP in the spinal cord of six mammalian species. Peptides. 1984;5:201–207. doi: 10.1016/0196-9781(84)90207-9. [DOI] [PubMed] [Google Scholar]
- Glass JD, Guinn J, Kaur G, Francl JM. On the intrinsic regulation of neuropeptide Y release in the mammalian suprachiasmatic nucleus circadian clock. Eur J Neurosci. 2010;31:1117–1126. doi: 10.1111/j.1460-9568.2010.07139.x. [DOI] [PubMed] [Google Scholar]
- Gregor P, Feng Y, DeCarr LB, Cornfield LJ, McCaleb ML. Molecular characterization of a second mouse pancreatic polypeptide receptor and its inactivated human homologue. J Biol Chem. 1996;271:27776–27781. doi: 10.1074/jbc.271.44.27776. [DOI] [PubMed] [Google Scholar]
- Grundemar L, Bloom SR. Neuropeptide Y and Drug Development. San Diego: Academic Press; 1997. [Google Scholar]
- Gu J, Polak JM, Adrian TE, Allen JM, Tatemoto K, Bloom SR. Neuropeptide tyrosine (NPY)—a major cardiac neuropeptide. Lancet. 1983;1:1008–1010. doi: 10.1016/s0140-6736(83)92642-9. [DOI] [PubMed] [Google Scholar]
- Hansel DE, Eipper BA, Ronnett GV. Neuropeptide Y functions as a neuroproliferative factor. Nature. 2001;410:940–944. doi: 10.1038/35073601. [DOI] [PubMed] [Google Scholar]
- Hassani H, Lucas G, Rozell B, Ernfors P. Attenuation of acute experimental colitis by preventing NPY Y1 receptor signaling. Am J Physiol Gastrointest Liver Physiol. 2005;288:G550–G556. doi: 10.1152/ajpgi.00182.2004. [DOI] [PubMed] [Google Scholar]
- Hegde SS, Bonhaus DW, Stanley W, Eglen RM, Moy TM, Loeb M, Shetty SG, DeSouza A, Krstenansky J. Pharmacological evaluation of 1229U91, a novel high-affinity and selective neuropeptide Y-Y1 receptor antagonist. J Pharmacol Exp Ther. 1995;275:1261–1266. [PubMed] [Google Scholar]
- Heikkinen AM, Niskanen LK, Salmi JA, Koulu M, Pesonen U, Uusitupa MI, Komulainen MH, Tuppurainen MT, Kroger H, Jurvelin J, et al. Leucine7 to proline7 polymorphism in prepro-NPY gene and femoral neck bone mineral density in postmenopausal women. Bone. 2004;35:589–594. doi: 10.1016/j.bone.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Heilig M. The NPY system in stress, anxiety and depression. Neuropeptides. 2004;38:213–224. doi: 10.1016/j.npep.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Heilig M, Murison R. Intracerebroventricular neuropeptide Y protects against stress-induced gastric erosion in the rat. Eur J Pharmacol. 1987;137:127–129. doi: 10.1016/0014-2999(87)90191-9. [DOI] [PubMed] [Google Scholar]
- Herness S, Zhao FL. The neuropeptides CCK and NPY and the changing view of cell-to-cell communication in the taste bud. Physiol Behav. 2009;97:581–591. doi: 10.1016/j.physbeh.2009.02.043. [DOI] [PubMed] [Google Scholar]
- Herzog H, Hort YJ, Ball HJ, Hayes G, Shine J, Selbie LA. Cloned human neuropeptide Y receptor couples to two different second messenger systems. Proc Natl Acad Sci USA. 1992;89:5794–5798. doi: 10.1073/pnas.89.13.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulting J, Sollevi A, Ullman B, Franco-Cereceda A, Lundberg JM. Plasma neuropeptide Y on admission to a coronary care unit: raised levels in patients with left heart failure. Cardiovasc Res. 1990;24:102–108. doi: 10.1093/cvr/24.2.102. [DOI] [PubMed] [Google Scholar]
- Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. J Hered. 1950;41:317–318. doi: 10.1093/oxfordjournals.jhered.a106073. [DOI] [PubMed] [Google Scholar]
- Joehl RJ, DeJoseph MR. Pancreatic polypeptide inhibits amylase release by rat pancreatic acini. J Surg Res. 1986;40:310–314. doi: 10.1016/0022-4804(86)90192-7. [DOI] [PubMed] [Google Scholar]
- Jung SJ, Chang JW, Won R, Cha MH, Nam TS, Lee HJ, Lee BH. Modulation of neuropathic pain by galanin and neuropeptide Y at the level of the medulla in rats. Int J Neurosci. 2009;119:1941–1955. doi: 10.1080/00207450903263661. [DOI] [PubMed] [Google Scholar]
- Kimmel JR, Hayden LJ, Pollock HG. Isolation and characterization of a new pancreatic polypeptide hormone. J Biol Chem. 1975;250:9369–9376. [PubMed] [Google Scholar]
- King PJ, Widdowson PS, Doods HN, Williams G. Regulation of neuropeptide Y release by neuropeptide Y receptor ligands and calcium channel antagonists in hypothalamic slices. J Neurochem. 1999;73:641–646. doi: 10.1046/j.1471-4159.1999.0730641.x. [DOI] [PubMed] [Google Scholar]
- Kitlinska J. Neuropeptide Y (NPY) in neuroblastoma: effect on growth and vascularization. Peptides. 2007;28:405–412. doi: 10.1016/j.peptides.2006.08.038. [DOI] [PubMed] [Google Scholar]
- Kitlinska J, Abe K, Kuo L, Pons J, Yu M, Li L, Tilan J, Everhart L, Lee EW, Zukowska Z, et al. Differential effects of neuropeptide Y on the growth and vascularization of neural crest-derived tumors. Cancer Res. 2005;65:1719–1728. doi: 10.1158/0008-5472.CAN-04-2192. [DOI] [PubMed] [Google Scholar]
- Kuo LE, Kitlinska JB, Tilan JU, Li L, Baker SB, Johnson MD, Lee EW, Burnett MS, Fricke ST, Kvetnansky R, et al. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat Med. 2007;13:803–811. doi: 10.1038/nm1611. [DOI] [PubMed] [Google Scholar]
- Kuo LE, Czarnecka M, Kitlinska JB, Tilan JU, Kvetnansky R, Zukowska Z. Chronic stress, combined with a high-fat/high-sugar diet, shifts sympathetic signaling toward neuropeptide Y and leads to obesity and the metabolic syndrome. Ann N Y Acad Sci. 2008;1148:232–237. doi: 10.1196/annals.1410.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushi A, Sasai H, Koizumi H, Takeda N, Yokoyama M, Nakamura M. Obesity and mild hyperinsulinemia found in neuropeptide Y-Y1 receptor-deficient mice. Proc Natl Acad Sci USA. 1998;95:15659–15664. doi: 10.1073/pnas.95.26.15659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappalainen J, Kranzler HR, Malison R, Price LH, Van Dyck C, Rosenheck RA, Cramer J, Southwick S, Charney D, Krystal J, et al. A functional neuropeptide Y Leu7Pro polymorphism associated with alcohol dependence in a large population sample from the United States. Arch Gen Psychiatry. 2002;59:825–831. doi: 10.1001/archpsyc.59.9.825. [DOI] [PubMed] [Google Scholar]
- Larhammar D, Blomqvist AG, Yee F, Jazin E, Yoo H, Wahlested C. Cloning and functional expression of a human neuropeptide Y/peptide YY receptor of the Y1 type. J Biol Chem. 1992;267:10935–10938. [PubMed] [Google Scholar]
- Lavebratt C, Alpman A, Persson B, Arner P, Hoffstedt J. Common neuropeptide Y2 receptor gene variant is protective against obesity among Swedish men. Int J Obes (London) 2006;30:453–459. doi: 10.1038/sj.ijo.0803188. [DOI] [PubMed] [Google Scholar]
- Lee NJ, Herzog H. NPY regulation of bone remodelling. Neuropeptides. 2009;43:457–463. doi: 10.1016/j.npep.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Lin HC, Zhao XT, Wang L, Wong H. Fat-induced ileal brake in the dog depends on peptide YY. Gastroenterology. 1996;110:1491–1495. doi: 10.1053/gast.1996.v110.pm8613054. [DOI] [PubMed] [Google Scholar]
- Litvak DA, Iseki H, Evers BM, Greeley GH, Jr, Hellmich MR, Iwase K, Balasubramaniam A, Townsend CM., Jr Characterization of two novel proabsorptive peptide YY analogs, BIM-43073D and BIM-43004C. Dig Dis Sci. 1999;44:643–648. doi: 10.1023/a:1026686214004. [DOI] [PubMed] [Google Scholar]
- Lockinger A, Koberle D, Konig PS, Saria A, Herold M, Cornelissen G, Halberg F. Neuropeptide chronomics in clinically healthy young adults: circaoctohoran and circadian patterns. Peptides. 2004;25:533–542. doi: 10.1016/j.peptides.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Lundberg P, Allison SJ, Lee NJ, Baldock PA, Brouard N, Rost S, Enriquez RF, Sainsbury A, Lamghari M, Simmons P, et al. Greater bone formation of Y2 knockout mice is associated with increased osteoprogenitor numbers and altered Y1 receptor expression. J Biol Chem. 2007;282:19082–19091. doi: 10.1074/jbc.M609629200. [DOI] [PubMed] [Google Scholar]
- Lunniss GE, Barnes AA, Barton N, Biagetti M, Bianchi F, Blowers SM, Caberlotto L, Emmons A, Holmes IP, Montanari D, et al. The identification and optimisation of novel and selective diamide neuropeptide Y Y2 receptor antagonists. Bioorg Med Chem Lett. 2009;19:4022–4025. doi: 10.1016/j.bmcl.2009.06.035. [DOI] [PubMed] [Google Scholar]
- Mantyh PW. Neurobiology of substance P and the NK1 receptor. J Clin Psychiatry. 2002;63:6–10. [PubMed] [Google Scholar]
- Marsh DJ, Hollopeter G, Kafer KE, Palmiter RD. Role of the Y5 neuropeptide Y receptor in feeding and obesity. Nat Med. 1998;4:718–721. doi: 10.1038/nm0698-718. [DOI] [PubMed] [Google Scholar]
- Martins PJ, Marques MS, Tufik S, D'Almeida V. Orexin activation precedes increased NPY expression, hyperphagia, and metabolic changes in response to sleep deprivation. Am J Physiol Endocrinol Metab. 2010;298:E726–E734. doi: 10.1152/ajpendo.00660.2009. [DOI] [PubMed] [Google Scholar]
- Matsuda H, Brumovsky PR, Kopp J, Pedrazzini T, Hokfelt T. Distribution of neuropeptide Y Y1 receptors in rodent peripheral tissues. J Comp Neurol. 2002;449:390–404. doi: 10.1002/cne.10303. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Nomura T, Momose K, Ikeda Y, Kondou Y, Akiho H, Togami J, Kimura Y, Okada M, Yamaguchi T. Inactivation of a novel neuropeptide Y/peptide YY receptor gene in primate species. J Biol Chem. 1996;271:27217–27220. doi: 10.1074/jbc.271.44.27217. [DOI] [PubMed] [Google Scholar]
- Mayfield RD, Lewohl JM, Dodd PR, Herlihy A, Liu J, Harris RA. Patterns of gene expression are altered in the frontal and motor cortices of human alcoholics. J Neurochem. 2002;81:802–813. doi: 10.1046/j.1471-4159.2002.00860.x. [DOI] [PubMed] [Google Scholar]
- McDermott BJ, Bell D. NPY and cardiac diseases. Curr Top Med Chem. 2007;7:1692–1703. doi: 10.2174/156802607782340939. [DOI] [PubMed] [Google Scholar]
- McDermott BJ, Millar BC, Dolan FM, Bell D, Balasubramaniam A. Evidence for Y1 and Y2 subtypes of neuropeptide Y receptors linked to opposing postjunctional effects observed in rat cardiac myocytes. Eur J Pharmacol. 1997;336:257–265. doi: 10.1016/s0014-2999(97)01258-2. [DOI] [PubMed] [Google Scholar]
- Mollereau C, Gouarderes C, Dumont Y, Kotani M, Detheux M, Doods H, Parmentier M, Quirion R, Zajac JM. Agonist and antagonist activities on human NPFF(2) receptors of the NPY ligands GR231118 and BIBP3226. Br J Pharmacol. 2001;133:1–4. doi: 10.1038/sj.bjp.0704049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran TD, Colmers WF, Smith PA. Opioid-like actions of neuropeptide Y in rat substantia gelatinosa: Y1 suppression of inhibition and Y2 suppression of excitation. J Neurophysiol. 2004;92:3266–3275. doi: 10.1152/jn.00096.2004. [DOI] [PubMed] [Google Scholar]
- Movafagh S, Hobson JP, Spiegel S, Kleinman HK, Zukowska Z. Neuropeptide Y induces migration, proliferation, and tube formation of endothelial cells bimodally via Y1, Y2, and Y5 receptors. FASEB J. 2006;20:1924–1926. doi: 10.1096/fj.05-4770fje. [DOI] [PubMed] [Google Scholar]
- Naveilhan P, Hassani H, Canals JM, Ekstrand AJ, Larefalk A, Chhajlani V, Arenas E, Gedda K, Svensson L, Thoren P, et al. Normal feeding behavior, body weight and leptin response require the neuropeptide Y Y2 receptor. Nat Med. 1999;5:1188–1193. doi: 10.1038/13514. [DOI] [PubMed] [Google Scholar]
- Naveilhan P, Hassani H, Lucas G, Blakeman KH, Hao JX, Xu XJ, Wiesenfeld-Hallin Z, Thoren P, Ernfors P. Reduced antinociception and plasma extravasation in mice lacking a neuropeptide Y receptor. Nature. 2001;409:513–517. doi: 10.1038/35054063. [DOI] [PubMed] [Google Scholar]
- Noe F, Frasca A, Balducci C, Carli M, Sperk G, Ferraguti F, Pitkanen A, Bland R, Fitzsimons H, During M, et al. Neuropeptide Y overexpression using recombinant adeno-associated viral vectors. Neurotherapeutics. 2009;6:300–306. doi: 10.1016/j.nurt.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noe F, Vaghi V, Balducci C, Fitzsimons H, Bland R, Zardoni D, Sperk G, Carli M, During MJ, Vezzani A. Anticonvulsant effects and behavioural outcomes of rAAV serotype 1 vector-mediated neuropeptide Y overexpression in rat hippocampus. Gene Ther. 2010;17:643–652. doi: 10.1038/gt.2010.23. [DOI] [PubMed] [Google Scholar]
- Pellieux C, Sauthier T, Domenighetti A, Marsh DJ, Palmiter RD, Brunner HR, Pedrazzini T. Neuropeptide Y (NPY) potentiates phenylephrine-induced mitogen-activated protein kinase activation in primary cardiomyocytes via NPY Y5 receptors. Proc Natl Acad Sci USA. 2000;97:1595–1600. doi: 10.1073/pnas.030533197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner SB, Smyth DD, Glavin GB. Effects of neuropeptide Y and [Leu31,Pro34] neuropeptide Y on experimental gastric lesion formation and gastric secretion in the rat. J Pharmacol Exp Ther. 1993;266:339–343. [PubMed] [Google Scholar]
- Redrobe JP, Dumont Y, Herzog H, Quirion R. Neuropeptide Y (NPY) Y2 receptors mediate behaviour in two animal models of anxiety: evidence from Y2 receptor knockout mice. Behav Brain Res. 2003;141:251–255. doi: 10.1016/s0166-4328(02)00374-1. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Thorsell A, Heilig M. Suppression of ethanol self-administration by the neuropeptide Y (NPY) Y2 receptor antagonist BII E0246: evidence for sensitization in rats with a history of dependence. Neurosci Lett. 2005;375:129–133. doi: 10.1016/j.neulet.2004.10.084. [DOI] [PubMed] [Google Scholar]
- Rose PM, Fernandes P, Lynch JS, Frazier ST, Fisher SM, Kodukula K, Kienzle B, Seethala R. Cloning and functional expression of a cDNA encoding a human type 2 neuropeptide Y receptor. J Biol Chem. 1995;270:22661–22664. doi: 10.1074/jbc.270.39.22661. [DOI] [PubMed] [Google Scholar]
- Ruscica M, Dozio E, Motta M, Magni P. Relevance of the neuropeptide Y system in the biology of cancer progression. Curr Top Med Chem. 2007;7:1682–1691. doi: 10.2174/156802607782341019. [DOI] [PubMed] [Google Scholar]
- Sainsbury A, Schwarzer C, Couzens M, Jenkins A, Oakes SR, Ormandy CJ, Herzog H. Y4 receptor knockout rescues fertility in ob/ob mice. Genes Dev. 2002;16:1077–1088. doi: 10.1101/gad.979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Ogino Y, Mashiko S, Ando M. Modulation of neuropeptide Y receptors for the treatment of obesity. Expert Opin Ther Pat. 2009;19:1401–1415. doi: 10.1517/13543770903251722. [DOI] [PubMed] [Google Scholar]
- Schober DA, Van Abbema AM, Smiley DL, Bruns RF, Gehlert DR. The neuropeptide Y Y1 antagonist, 1229U91, a potent agonist for the human pancreatic polypeptide-preferring (NPY Y4) receptor. Peptides. 1998;19:537–542. doi: 10.1016/s0196-9781(97)00455-5. [DOI] [PubMed] [Google Scholar]
- Seybold VS, McCarson KE, Mermelstein PG, Groth RD, Abrahams LG. Calcitonin gene-related peptide regulates expression of neurokinin1 receptors by rat spinal neurons. J Neurosci. 2003;23:1816–1824. doi: 10.1523/JNEUROSCI.23-05-01816.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SH, Freedman NJ, Zhang L, Crosslin DR, Stone DH, Haynes C, Johnson J, Nelson S, Wang L, Connelly JJ, et al. Neuropeptide Y gene polymorphisms confer risk of early-onset atherosclerosis. PLoS Genet. 2009;5:e1000318. doi: 10.1371/journal.pgen.1000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoblock JR, Welty N, Nepomuceno D, Lord B, Aluisio L, Fraser I, Motley ST, Sutton SW, Morton K, Galici R, et al. In vitro and in vivo characterization of JNJ-31020028 (N-(4-{4-[2-(diethylamino)-2-oxo-1-phenylethyl]piperazin-1-yl}-3-fluorophe nyl)-2-pyridin-3-ylbenzamide), a selective brain penetrant small molecule antagonist of the neuropeptide Y Y(2) receptor. Psychopharmacology (Berlin) 2010;208:265–277. doi: 10.1007/s00213-009-1726-x. [DOI] [PubMed] [Google Scholar]
- Small CJ, Bloom SR. The therapeutic potential of gut hormone peptide YY3-36 in the treatment of obesity. Expert Opin Investig Drugs. 2005;14:647–653. doi: 10.1517/13543784.14.5.647. [DOI] [PubMed] [Google Scholar]
- Smith-White MA, Herzog H, Potter EK. Role of neuropeptide Y Y(2) receptors in modulation of cardiac parasympathetic neurotransmission. Regul Pept. 2002;103:105–111. doi: 10.1016/s0167-0115(01)00368-8. [DOI] [PubMed] [Google Scholar]
- Sperk G, Hamilton T, Colmers WF. Neuropeptide Y in the dentate gyrus. Prog Brain Res. 2007;163:285–297. doi: 10.1016/S0079-6123(07)63017-9. [DOI] [PubMed] [Google Scholar]
- Stephens TW, Basinski M, Bristow PK, Bue-Valleskey JM, Burgett SG, Craft L, Hale J, Hoffmann J, Hsiung HM, Kriauciunas A, et al. The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature. 1995;377:530–532. doi: 10.1038/377530a0. [DOI] [PubMed] [Google Scholar]
- Tatemoto K, Mutt V. Isolation of two novel candidate hormones using a chemical method for finding naturally occurring polypeptides. Nature. 1980;285:417–418. doi: 10.1038/285417a0. [DOI] [PubMed] [Google Scholar]
- Tatemoto K, Carlquist M, Mutt V. Neuropeptide Y—a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature. 1982;296:659–660. doi: 10.1038/296659a0. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Naveilhan P, Ernfors P. Assessment of ethanol consumption and water drinking by NPY Y(2) receptor knockout mice. Peptides. 2004;25:975–983. doi: 10.1016/j.peptides.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Rimondini R, Heilig M. Blockade of central neuropeptide Y (NPY) Y2 receptors reduces ethanol self-administration in rats. Neurosci Lett. 2002;332:1–4. doi: 10.1016/s0304-3940(02)00904-7. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Slawecki CJ, El Khoury A, Mathe AA, Ehlers CL. The effects of social isolation on neuropeptide Y levels, exploratory and anxiety-related behaviors in rats. Pharmacol Biochem Behav. 2006;83:28–34. doi: 10.1016/j.pbb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Tracey DJ, Romm MA, Yao NN. Peripheral hyperalgesia in experimental neuropathy: exacerbation by neuropeptide Y. Brain Res. 1995;669:245–254. doi: 10.1016/0006-8993(94)01265-j. [DOI] [PubMed] [Google Scholar]
- Vona-Davis LC, McFadden DW. NPY family of hormones: clinical relevance and potential use in gastrointestinal disease. Curr Top Med Chem. 2007;7:1710–1720. doi: 10.2174/156802607782340966. [DOI] [PubMed] [Google Scholar]
- Wahlestedt C, Edvinsson L, Ekblad E, Hakanson R. Neuropeptide Y potentiates noradrenaline-evoked vasoconstriction: mode of action. J Pharmacol Exp Ther. 1985;234:735–741. [PubMed] [Google Scholar]
- Wahlestedt C, Yanaihara N, Haakanson R. Evidence for different pre- and post-junctional receptors for neuropeptide Y and related peptides. Regul Pept. 1986;13:307–318. doi: 10.1016/0167-0115(86)90048-0. [DOI] [PubMed] [Google Scholar]
- Wahlestedt C, Ekman R, Widerlov E. Neuropeptide Y (NPY) and the central nervous system: distribution effects and possible relationship to neurological and psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 1989;13:31–54. doi: 10.1016/0278-5846(89)90003-1. [DOI] [PubMed] [Google Scholar]
- Wahlestedt C, Grundemar L, Hakanson R, Heilig M, Shen GH, Zukowska-Grojec Z, Reis DJ. Neuropeptide Y receptor subtypes, Y1 and Y2. Ann N Y Acad Sci. 1990;611:7–26. doi: 10.1111/j.1749-6632.1990.tb48918.x. [DOI] [PubMed] [Google Scholar]
- Wahlestedt C, Regunathan S, Reis DJ. Identification of cultured cells selectively expressing Y1-, Y2-, or Y3-type receptors for neuropeptide Y/peptide YY. Life Sci. 1992;50:PL7–PL12. doi: 10.1016/0024-3205(92)90342-m. [DOI] [PubMed] [Google Scholar]
- Wahlestedt C, Pich EM, Koob GF, Yee F, Heilig M. Modulation of anxiety and neuropeptide Y-Y1 receptors by antisense oligodeoxynucleotides. Science. 1993;259:528–531. doi: 10.1126/science.8380941. [DOI] [PubMed] [Google Scholar]
- Weinberg DH, Sirinathsinghji DJ, Tan CP, Shiao LL, Morin N, Rigby MR, Heavens RH, Rapoport DR, Bayne ML, Cascieri MA, et al. Cloning and expression of a novel neuropeptide Y receptor. J Biol Chem. 1996;271:16435–16438. doi: 10.1074/jbc.271.28.16435. [DOI] [PubMed] [Google Scholar]
- Wetherill L, Schuckit MA, Hesselbrock V, Xuei X, Liang T, Dick DM, Kramer J, Nurnberger ,JI, Jr, Tischfield JA, Porjesz B, et al. Neuropeptide Y receptor genes are associated with alcohol dependence, alcohol withdrawal phenotypes, and cocaine dependence. Alcohol Clin Exp Res. 2008;32:2031–2040. doi: 10.1111/j.1530-0277.2008.00790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdowson PS, Ordway GA, Halaris AE. Reduced neuropeptide Y concentrations in suicide brain. J Neurochem. 1992;59:73–80. doi: 10.1111/j.1471-4159.1992.tb08877.x. [DOI] [PubMed] [Google Scholar]
- Zengin A, Zhang L, Herzog H, Baldock PA, Sainsbury A. Neuropeptide Y and sex hormone interactions in humoral and neuronal regulation of bone and fat. Trends Endocrinol Metab. 2010;21:411–418. doi: 10.1016/j.tem.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Zhang H, Yan Y, Shi R, Lin Z, Wang M, Lin L. Correlation of gut hormones with irritable bowel syndrome. Digestion. 2008;78:72–76. doi: 10.1159/000165352. [DOI] [PubMed] [Google Scholar]
- Zukowska-Grojec Z, Karwatowska-Prokopczuk E, Rose W, Rone J, Movafagh S, Ji H, Yeh Y, Chen WT, Kleinman HK, Grouzmann E, et al. Neuropeptide Y: a novel angiogenic factor from the sympathetic nerves and endothelium. Circ Res. 1998;83:187–195. doi: 10.1161/01.res.83.2.187. [DOI] [PubMed] [Google Scholar]
- Zwanziger D, Bohme I, Lindner D, Beck-Sickinger AG. First selective agonist of the neuropeptide Y1-receptor with reduced size. J Pept Sci. 2009;15:856–866. doi: 10.1002/psc.1188. [DOI] [PubMed] [Google Scholar]