Abstract
Background
Since the plant hormone gibberellin (GA) was discovered as a fungal toxin that caused abnormal elongation of rice shoots, the physiological function of GA has mainly been investigated in relation to the regulation of plant height. However, an indispensable role for GA in root growth has been elucidated by using severely GA-depleted plants, either with a gene mutation in GA biosynthesis or which have been treated by an inhibitor of GA biosynthesis. The molecular sequence of GA signalling has also been studied to understand GA functions in root growth.
Scope
This review addresses research progress on the physiological functions of GA in root growth. Concentration-dependent stimulation of elongation growth by GA is important for the regulation of plant height and root length. Thus the endogenous level of GA and/or the GA sensitivity of shoots and roots plays a role in determining the shoot-to-root ratio of the plant body. Since the shoot-to-root ratio is an important parameter for agricultural production, control of GA production and GA sensitivity may provide a strategy for improving agricultural productivity. The sequence of GA signal transduction has recently been unveiled, and some component molecules are suggested as candidate in planta regulatory sites and as points for the artificial manipulation of GA-mediated growth control.
Conclusions
This paper reviews: (1) the breakthrough dose–response experiments that show that root growth is regulated by GA in a lower concentration range than is required for shoot growth; (2) research on the regulation of GA biosynthesis pathways that are known predominantly to control shoot growth; and (3) recent research on GA signalling pathways, including GA receptors, which have been suggested to participate in GA-mediated growth regulation. This provides useful information to suggest a possible strategy for the selective control of shoot and root growth, and to explain how GA plays a role in rosette and liana plants with tall or short, and slender or thick axial organs.
Keywords: Ancymidol, GA receptors, GA sensitivity, gibberellin, GID1, Lactuca sativa, low concentration of GA, Pisum sativum, root elongation, root thickening, rosette form, shoot-to-root ratio
INTRODUCTION
Plant growth is regulated by multiple environmental factors and nutritional supply; however, most of the regulatory events are mediated by plant hormones. Among these plant hormones, gibberellin (GA) has been known to control plant height ever since its discovery in studies of an abnormally elongating disease of rice seedlings caused by a fungal infection by Gibberella fujikuroi (Takahashi et al., 1955, 1957, 1991).
Exogenously applied GA strongly promotes the elongation growth of shoots in many plants, as shown in Fig. 1, whereas it does not show such clear effects on roots, even in GA-sensitive pea plants (Fig. 2). Therefore, the question arises of whether GA is a plant hormone that regulates shoot growth but does not affect root growth. The answer to this question is a resounding ‘no’. A GA requirement for root growth has been elucidated by GA depletion experiments in GA-deficient mutants and/or treatment with growth retardants. Some growth retardants were discovered to suppress shoot elongation by inhibiting endogenous production of GA (Rademacher, 1991). By using precise concentrations of such chemical inhibitors of GA biosynthesis in combination with GA-deficient mutants, GA has been shown to control root growth at a considerably lower concentration than is necessary for controlling shoot growth (Tanimoto, 1994). It is now recognized that GA plays an indispensable role in both shoots and roots in many plants. The next question is how shoot and root growth is quantitatively regulated by GA in one and the same plant body.
Fig. 1.

Typical effect of gibberellin in the enhancement of stem growth. Two cabbage plants (on the right) were treated with GA3 for 5 months. Stem growth was strongly enhanced as compared with the control plant (to the left) to which no GA was applied.
Fig. 2.
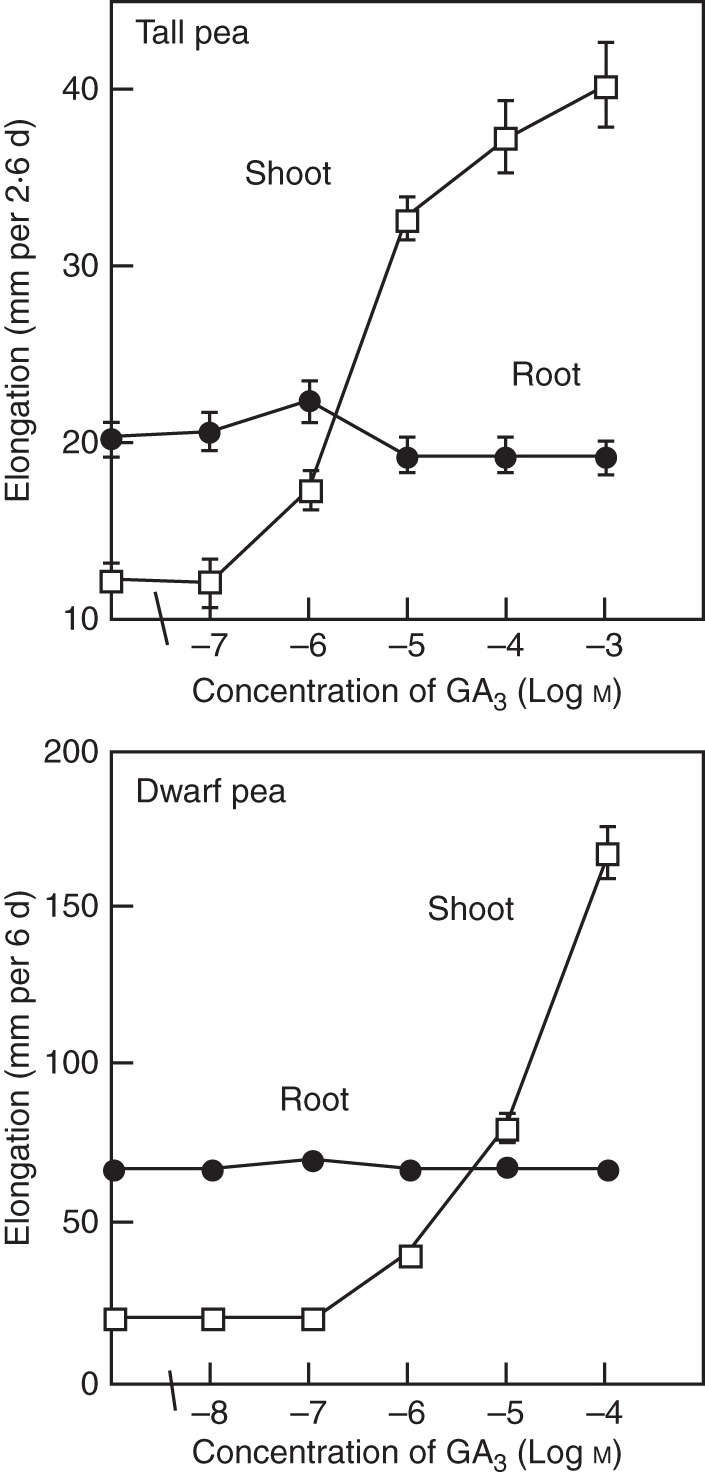
Effect of GA3 concentration on the elongation growth of pea seedlings. Shoot elongation was strongly enhanced by GA3 supplied by hydroponics, whereas root elongation was not affected. Tall pea (‘Alaska’) and dwarf pea (‘Little Marvel’) varieties were used. (Adapted from Tanimoto, 1988, 2002.)
Since the shoot/root ratio is important for agricultural production, practical methods to change the shoot/root ratio have long been awaited, as demonstrated by ‘the green revolution’ where partial suppression of shoot growth resulted in more cereal production (Sasaki et al., 2002; Hedden, 2003). Moreover, there are diverse plant shapes with different shoot/root ratios in nature. Extreme examples are liana (climbing) plants, such as pea and kudzu (Pueraria lobata), which contrast with rosette plants such as radish and dandelion (Taraxacum officinale), the latter having short stems but well-developed roots. It is an interesting physiological question as to how stem and root growth can be separately controlled in these plants.
Although the molecular mechanism of GA-mediated elongation growth itself is still under investigation, molecular events of GA signal transduction, including GA receptor molecules, have been revealed. Additionally, the GA biosynthesis pathway and its regulation have been investigated to show the local regulation of active GA production in a plant body, suggesting the separate regulation of GA production in roots and shoots.
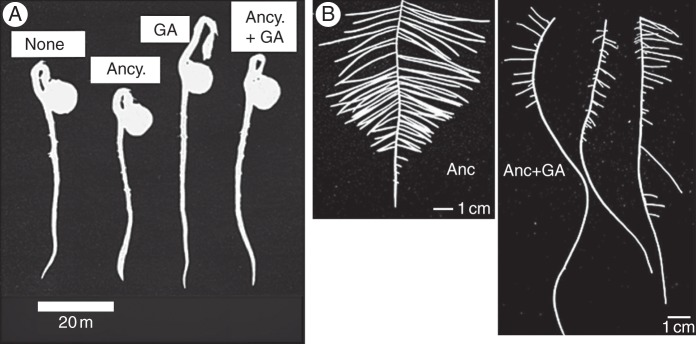
Inhibitors of GA biosynthesis have been shown to cause swelling of onion bulb cells and to change their microtubule and microfibril orientation (Mita and Shibaoka, 1984; Shibaoka, 1994). In GA depletion experiments, either by inhibition of GA biosynthesis or using GA-deficient mutants, remarkable thickening of the root was observed in pea and lettuce, while slender roots were induced by GA treatment (Tanimoto, 1987, 1988, 1994).
This short review describes in detail the progress of research on GA-mediated regulation of root growth, and presents a recent hypothesis for ‘GA sensitivity’, as well as discussing possible methods to change the shoot/root ratio by regulating the GA concentration and GA sensitivity. Brief details of the materials and methods used in some of the experiments referred to in this review are given in the Appendix.
REGULATION OF ROOT ELONGATION AND THICKNESS BY GA DEPLETION AND GA SUPPLEMENTATION
Gibberellin and root elongation
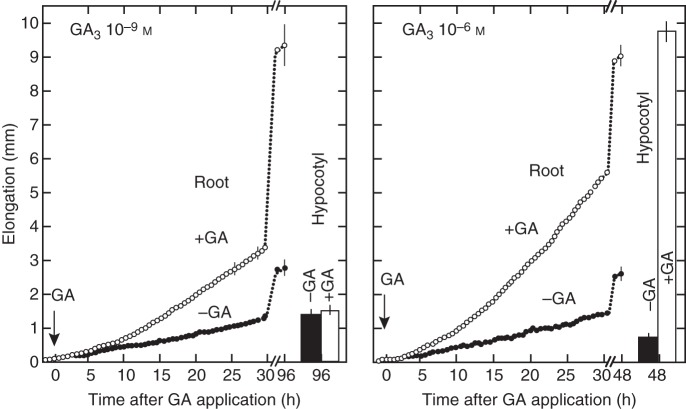
An inhibitor of GA biosynthesis, ancymidol (Coolbaugh and Hamilton, 1976; Coolbaugh et al., 1978), strongly suppresses hypocotyl elongation of lettuce seedlings at a relatively low concentration at which root growth is not correspondingly suppressed (Fig. 3; Tanimoto, 1987). Gibberellin shows clear stimulation of root elongation under growth-suppressed conditions induced by this inhibitor of GA biosynthesis in lettuce (Tanimoto, 1987), a tall variety of pea (Tanimoto, 1988) and in dwarf pea (Fig. 4; Tanimoto, 1994). When the endogenous GA level is decreased by the GA biosynthesis inhibitor or by mutation of a GA biosynthesis gene, root growth is stunted and abnormal thickening is induced. These phenomena are completely reversed by the exogenous application of GA3 at a rather low nanomolar concentration (Fig. 4), which is insufficient to promote shoot elongation. The phenomenon has been further confirmed by time course experiments using a rhizometer, in which lettuce seedlings are dipped in hydroponics containing growth regulators for 2 min at 30 min intervals during a 4 d experiment (Fig. 5; Tanimoto, 2002). It was shown that GA3 enhances root elongation but not that of the hypocotyl at 1 nm, whereas hypocotyl elongation is stimulated at 1 µm GA3.
Fig. 3.
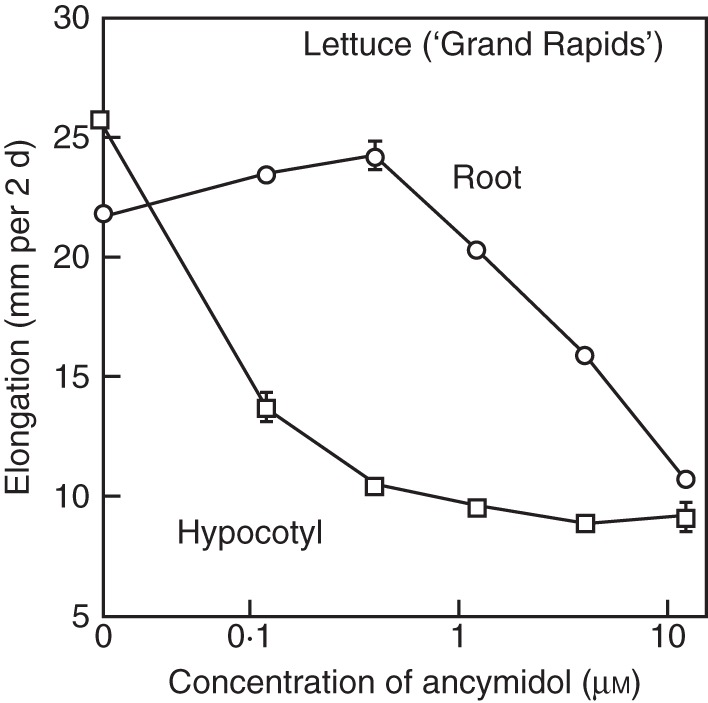
Concentration-dependent inhibition by ancymidol of hypocotyl and root elongation in Lactuca sativa. (Adapted from Tanimoto, 1987.)
Fig. 4.
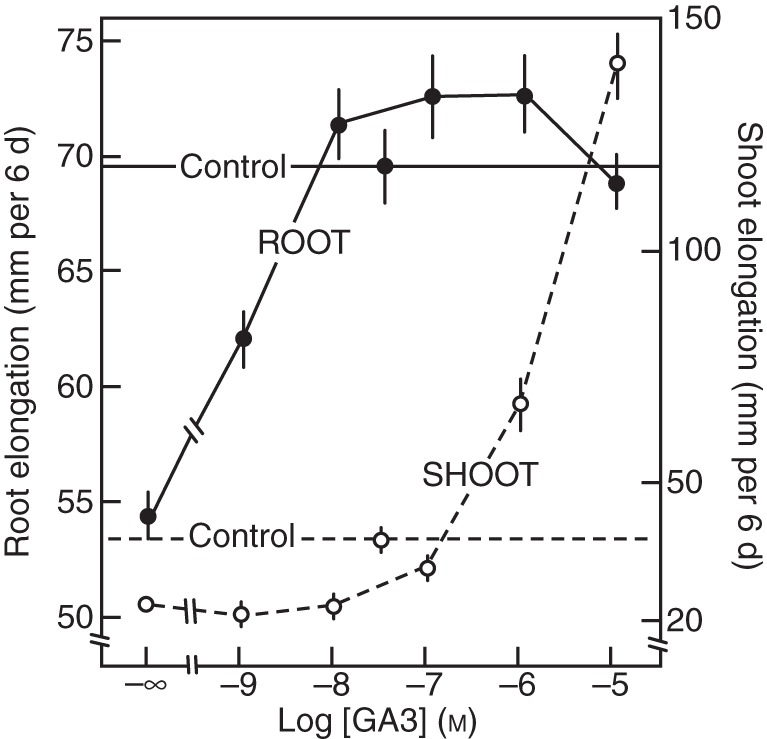
Concentration-dependent stimulation of root and shoot elongation of dwarf pea seedlings by GA3 applied to hydroponics in the presence of ancymidol. Ancymidol-suppressed root elongation was recovered by GA3 at nanomolar concentration and saturated at a 10 nm concentration, whereas shoot elongation was enhanced at the micromolar level. (Adapted from Tanimoto, 1994).
Fig. 5.
Time course of GA-induced root elongation and final hypocotyl length of lettuce seedlings treated with 1 nm (left) and 1 µm (right) GA3 in the presence of ancymidol. Root lengths of 14 seedlings were measured every 30 min in a rhizometer (Tanimoto and Watanabe, 1986). Whole seedlings were dipped for 2 min in hydroponic solution containing growth regulators at 30 min intevals. Root elongation was enhanced by 1 nm GA3, whereas hypocotyl growth was not enhanced. GA3 at 1 µm strongly enhanced both root and hypocotyl elongation. (Adapted from Tanimoto, 2002.)
By these dose–response examinations, the growth-regulating function of GA in roots has been elucidated and the interesting hypothesis has been advanced that root elongation occurs at a lower GA concentration than stem growth, as shown by Fig. 6, in which dose–response curves of GA-regulated root and stem growth are indicated with reference to those of indole-3-acetic acid (IAA)-regulated growth.
Fig. 6.
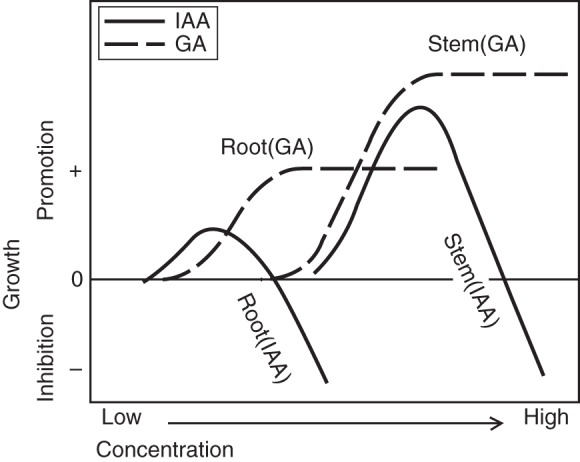
Concentration-dependent promotion or inhibition of root and stem elongation by GA as compared with auxin (IAA). (Adapted from Tanimoto, 2005.)
Since a specific transporter of GA has not been found, there may be no regulatory system to distribute GA molecules to specific tissues in a plant body. How, then, is root elongation enhanced without causing stem elongation in rosette plants? The first strategy may involve a local production of GA in roots at a precisely controlled concentration. The second strategy may be to provide roots with a high sensitivity to GA to enable root elongation at very low GA concentrations that are not sufficient for stem growth. These two methods are compatible with each other, but the first may be hazardous for plants, because overproduction of GA in roots may cause stem elongation under an unfavourable climate. However, it must be noted that the GA1 and GA4 inactivation system may well have been developed to prevent an oversupply of active GAs, and that externally applied GA3, which is often used for dose–response experiments, is relatively more stable than GA1 and GA4. The in planta stability of GA1, GA4 and GA3 remains a question for further quantitative investigation.
Gibberellin and root thickness
Ancymidol-enhanced and GA-suppressed thickening of roots is usually observed in the elongation zone of the root (Fig. 7) and is mainly caused by the expansion of cortical cells (Fig. 8) (Tanimoto, 1987, 1988, 1994). Ancymidol-induced thickening of root is also observed in woody roots of tea, Camellia sinensis (Fig. 7, right). As reported for onion bulbs (Mita and Shibaoka, 1984; Shibaoka, 1994), GA has been shown to limit cell expansion by controlling microtubule orientation and to facilitate the cellulose–microfibril orientation perpendicular to the longitudinal axis of the root (Hogetsu and Oshima, 1986), resulting in the elongation of slender roots. Expansion of cortical cells in the elongation zone of the root may be caused by a disorder of GA-mediated microtubule and microfibril orientation due to GA depletion and/or lack of GA signalling. A similar phenomenon was also reported in maize roots (Baluška et al., 1993). Thickening of root cells by GA depletion is suggested to occur in radish and other rosette plants.
Fig. 7.
Thickening of roots caused by ancymidol and its recovery promoted by GA3. (A) Pea seedlings treated with ancymidol (Ancy.) and/or GA3 (adapted from Tanimoto, 1988). (B) Tea roots treated by ancymidol with or without GA3, grown in hydroponics.
Fig. 8.
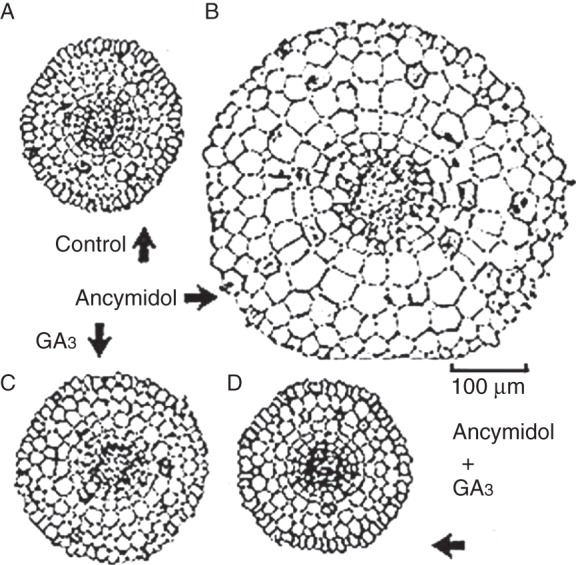
Control of root thickness by ancymidol and GA. Cross-sections of the elongation zone of lettuce roots treated with ancymidol and/or GA3 for 2 d. (Adapted from Tanimoto, 1987.)
REGULATION OF THE ACTIVE GA LEVEL AND GA BIOSYNTHESIS PATHWAY
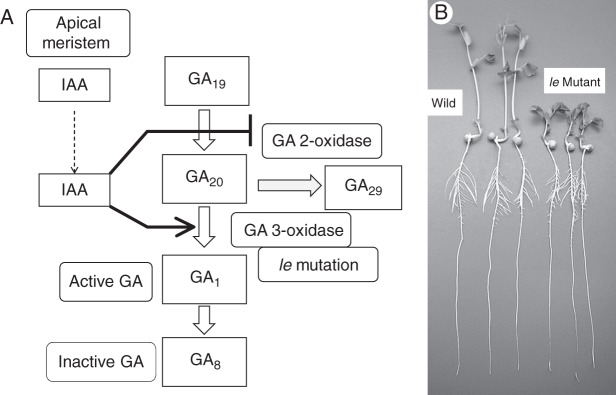
Gibberellin-sensitive dwarf plants have been investigated in order to elucidate the biosynthetic pathway and metabolism of GA. Many dwarfing genes were identified as controlling the enzyme activity of each biochemical step of the pathway, some of which were also suppressed by growth retardants (Hedden and Kamiya, 1997; Tanimoto, 2002; Yamaguchi, 2008). The morphological appearance of dwarf plants affected by these genes is somewhat complicated, particularly in roots, where GA is saturated at a low level. The reason for a variable degree of dwarfism may be ascribed to the redundancy and/or leaky functions of these genes. For example, the le mutant of dwarf pea has considerably stunted stems but their roots grow normally, as shown in Fig. 9. This mutant produces one-tenth of the normal GA content in the shoot, but the amount of GA in the roots was comparable with that of the wild type (Yaxley et al., 2001). However, when the dwarf pea bearing the mutant na gene (cultivar ‘Little Marvel’) was treated with ancymidol, roots were severely stunted and thickened, and stems were also further shortened (Tanimoto, 1994). Ancymidol-suppressed root growth was recovered at a lower concentration of GA3 than the stem growth (Fig. 4). Thus, these dwarf genes controlling GA biosynthesis may be leaky, and a combination of these genes with the GA biosynthesis inhibitor might cause a more severe syndrome of GA depletion, i.e. short stems, dark-green leaves and short and thickened roots.
Fig. 9.
(A) Final steps of GA1 biosynthesis and its inactivation. IAA supplied by the apical meristem controls the active GA1 level through the regulation of enzyme-encoding genes participating in the production of GA1. (B) Pea seedlings of the le mutant (the three seedlings on the right) which has a defective gene for GA 3-oxidase, as indicated in A, and its wild-type counterpart (the three seedlings on the left). (Adapted from Tanimoto, 2002.)
Gibberellin biosynthesis is controlled by feedback and feedforward systems (Hedden and Kamiya, 1997; Hedden, 1999; Olszewski et al., 2002; Yamaguchi, 2008), and is also regulated by another plant hormone, auxin (Ross et al., 2000, 2002). The major native auxin, IAA, which is transported from the apical meristem, increases the active GA1 level in stems by two mechanisms (Fig. 9). IAA stimulates the expression of GA 3-oxidase which converts GA20 to bioactive GA1, and suppresses the GA 2-oxidase which decreases the GA20 level. Although IAA regulation of the GA1 level has not been demonstrated in roots, it may be highly possible that it also participates in the regulation of low GA production in roots. The role of IAA and GA in root growth was reviewed by Tanimoto (2005), and cross-talk between IAA and GA has been demonstrated in the signalling system (Fu and Harberd, 2003; Gou et al., 2010; Heo et al., 2011). The IAA–GA cross-talk for lateral root formation has also been briefly reviewed (Farquharson, 2010).
Local production and translocation of GA are also important factors for the regulation of shoot and root growth. Unlike IAA transport, GA has been shown to move relatively freely from roots to shoots, and vice versa (Prochazka, 1981; Matthysse and Scott, 1984), although no specific transporter of GA has been found. For example, shoot- or cotyledon-applied GA3 was found to enhance root elongation in pea plants (Tanimoto, 1994), and shoot-applied GA4 enhanced root growth in arabidopsis (Bidadi et al., 2010). Using grafting experiments between the wild type and either the le mutant or the nana mutant of pea, the ability of a wild-type rootstock to restore stem elongation of a GA-deficient scion was shown to be dependent on the position of the lesion in the GA biosynthesis pathway (Dodd, 2005), i.e. wild-type root stocks restored stem elongation of nana scions, which are deficient in an early step of the pathway (the conversion of ent-kaurenoic acid to GA12 aldehyde) (Proebsting et al., 1992). In contrast, wild-type rootstocks did not restore stem elongation of le scions, which are deficient in the conversion of inactive GA20 to active GA1 (Reid et al., 1983).
Gibberellin also regulates auxin transport by modulating the turnover of the auxin efflux facilitator, PIN, and thus affects root gravitropism (Willige et al., 2011). In GA-deficient plants, auxin transport is reduced in Arabidopsis thaliana, and this is correlated with a reduction of PIN abundance in GA-deficient plants, which is restored to the wild-type level by external GA application. However, since the endogenous level of auxin is thought to be supraoptimal for many roots, GA may inhibit root elongation when GA increases the endogenous auxin level (Kuraishi and Muir, 1962) and/or when GA increases the tissue sensitivity to auxin, as previously suggested for stems (Ockerse and Galston, 1967; Tanimoto et al., 1967).
GA SIGNALLING PATHWAY AND GA RECEPTORS
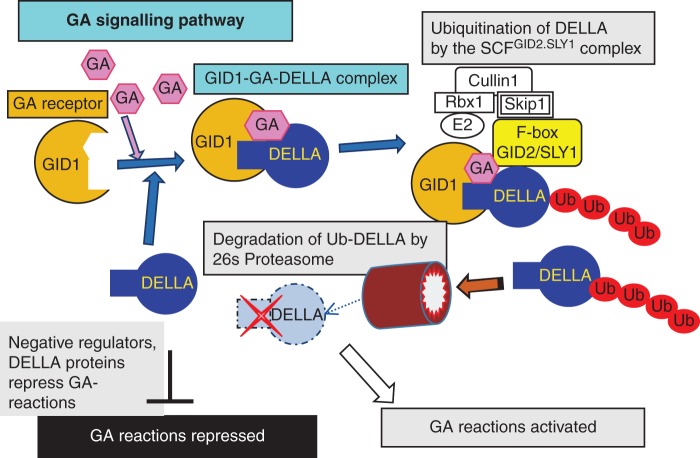
The signalling pathway of GA action has been uncovered in the last decade (Ueguchi-Tanaka et al., 2005, 2007; Hirano et al., 2008; Murase et al., 2008; Sun, 2010). The pathway is initiated by the interaction of GA and the GA receptor protein, GID1 (Fig. 10). The GA–GID1 conjugate binds to the DELLA and TVHYNP motifs of the DELLA proteins, a repressor of GA reactions in the absence of GA. The GA–GID1–DELLA protein complex is recognized by a polyubiquitination system, and polyubiquitinated DELLA protein is degraded by the 26S proteasome machinery. The disappearance of DELLA protein results in the expression of GA-related genes, leading to the activation of GA reactions. One of the GA reactions is the regulation of shoot growth through the functional regulation of DELLA protein, as demonstrated in the ‘green revolution’ in which semi-dwarf cereals were used (Sasaki et al., 2002; Hedden, 2003). Participation of DELLA proteins in the growth regulation of roots was also elucidated (Fu and Harberd, 2003; Ubeda-Tomas et al., 2008). Although it is not yet clear to what extent such DELLA regulation is working in root growth, a quantitative regulation of the GA signalling process might be a possible site of artificial control of root and shoot growth in addition to the regulation of active GA content in situ, as described above.
Fig. 10.
Model for the activation of GA reactions. GA signalling is initiated by the interaction between the GA molecule and the GA receptor protein GID1 to form a complex with DELLA proteins, negative regulators of GA reactions. The GA–GID1–DELLA complex is recognized by the SCFGID2/SLY1 complex and DELLA proteins are polyubiquitinated, resulting in the degradation of DELLA proteins by the 26S proteasome machinery. By GA-initiated degradation of DELLA proteins, through the ubiquitin–proteasome system, the GA-related genes are expressed and GA reactions are activated.
Evidence for the participation of GA signalling in root growth and development is accumulating; GA regulates meristem size and growth of roots (Ubeda-Tomás et al., 2008, 2009) and lateral root development (Zimmermann et al., 2010).
In addition to the control of DELLA protein itself, receptor molecules of GA are also candidates for the regulation of GA reactions. The GID1 receptor molecule was discovered in a study of a GA-insensitive extreme dwarf of rice (Fig. 11; Ueguchi-Tanaka et al., 2005). The receptor molecule was named after gibberellin-insensitive dwarf rice 1. The mutant rice shows the typical GA-deficient symptoms of dark-green leaves and short and thick stems. Roots look slightly thickened but not very stunted. The reason for this is not still clear.
Fig. 11.
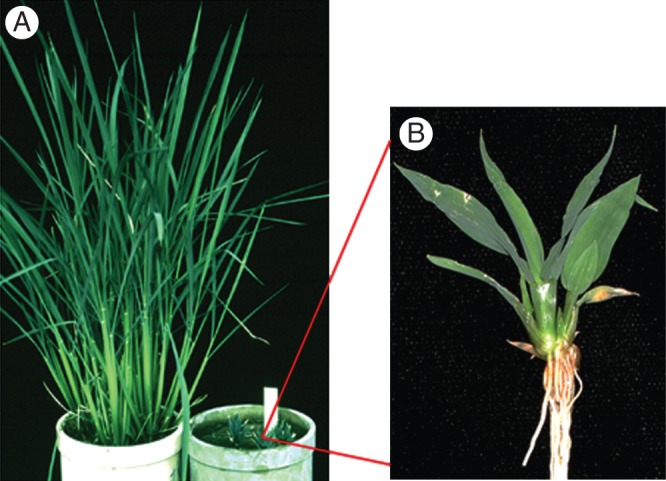
(A) GA-insensitive dwarf1 mutant of rice in the right-hand pot (magnified in B) and its wild-type counterpart (left-hand pot). The GA receptor GID1 was discovered in the study of the gibberellin-insensitive dwarf1 (gid1) mutant, GIBBERELLIN INSENSITIVE DWARF1, which encodes a nuclear-localized soluble receptor for GA. (Courtesy of Dr M. Ueguchi-Tanaka.)
Fig. 12.
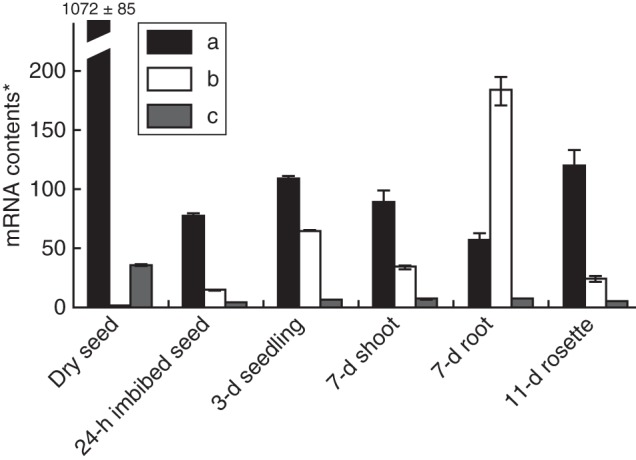
Different mRNA contents of GID1a, b and c in seed, seedling, shoot, root and rosette leaves of Arabidopsis thaliana. *The mRNA contents are shown as relative values to that of GID1b in dry seed, which is set at 1. The root contains the highest amount of GID1b mRNA. (Adapted from Griffiths et al., 2006.)
The molecular mechanism of high GA sensitivity of roots is not clear, but an interesting mechanism has been suggested recently in the author's research group with Drs Miyako Ueguchi-Tanaka and Makoto Matsuoka (Nagoya University). There are three GA receptors in A. thaliana: AtGID1a, AtGID1b and AtGID1c (Ueguchi-Tanaka et al., 2007). One of them, GID1b, has a higher GA affinity, i.e. a lower dissociation constant for, the GA molecule (Fig. 13; Nakajima et al., 2006). The knockout strain of AtGID1b became more sensitive to the GA inhibitor biosynthesis than the other knockout strains, i.e. the root was stunted more significantly at a low level of growth retardant application. Furthermore, another laboratory found that AtGID1b is rather strongly expressed in roots (Griffiths et al., 2006), and AtGID1b overproduction resulted in a slight promotion of root growth (Ariizumi et al., 2008).
Fig. 13.
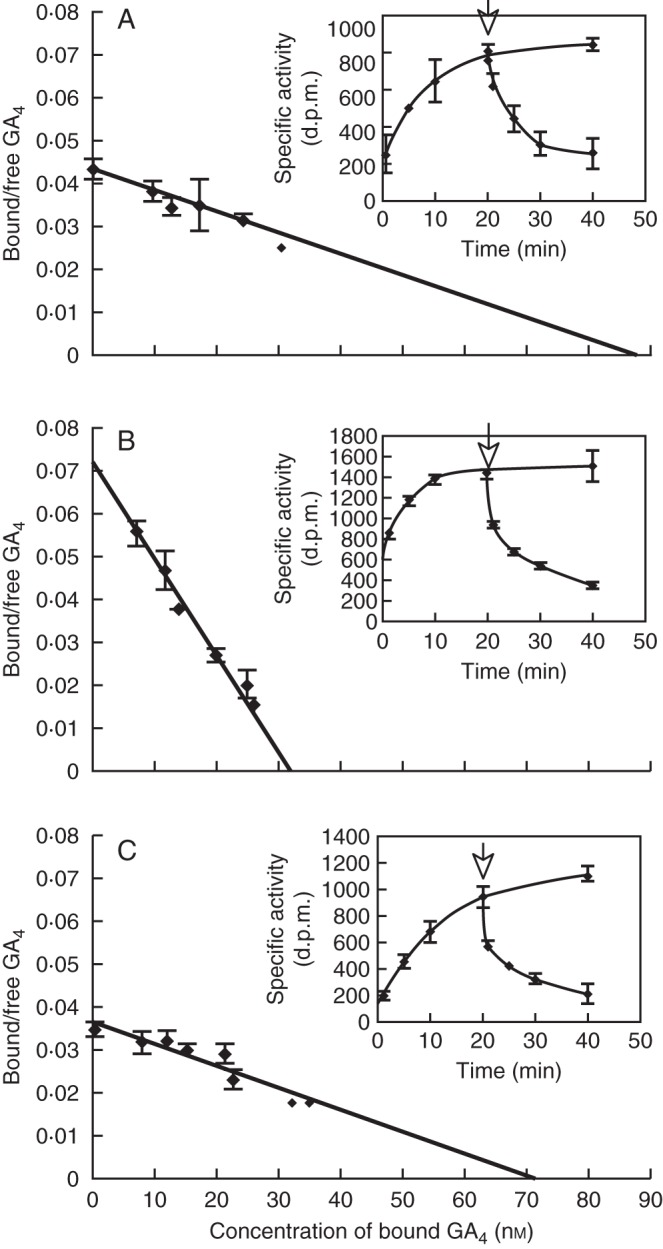
Association and dissociation kinetics of (A) AtGID1a, (B) AtGID1b and (C) AtGID1c. Dissociation constants of GID1a, b and c were calculated from GA binding kinetics of each AtGID1. Kd values are: (A) Kd(H2-GA4):AtGID1a = 2·0 × 10−6 m, (B) Kd(H2-GA4):AtGID1b = 4·8 × 10−7 m, (C) Kd(H2-GA4):AtGID1c = 1·9 × 10−6 m. AtGID1b shows a much lower Kd value than AtGID1a or AtGID1c. Insets show the time course characteristics for GA binding and replacement of AtGID1s with an excess amount of unlabelled GA4 (0·125 mm) (arrow). (Adapted from Nakajima et al., 2006.)
Genetic manipulation of GID1 molecules has been carried out and its biochemical and physiological function can be changed by a partial displacement of the peptide sequence in the GID1 molecule (Yamamoto et al., 2010). At some point in the displacement manipulation, the GID1 molecule became active in GA signalling even in the absence of a GA molecule. Such a molecular manipulation technique provides a strategy for GA-mediated growth regulation of roots and shoots.
CONCLUSIONS
By reviewing research on GA function in roots during the last three decades, the role of GA in root elongation and thickening has become clear, as follows. (1) Root elongation is regulated by a significantly lower concentration of GA than stem elongation. (2) Higher GA sensitivity of roots in comparison with shoots may provide an advantage for rosette plants to have long roots and short stems when there is a limited production of GA, in addition to the local regulation of GA production. Although the molecular mechanism of higher GA sensitivity is not fully understood, this mechanism may endow plants with diverse shapes, tall or short, and slender or thick. (3) Knowledge of the molecular mechanism of higher sensitivity of roots to GA may also provide a new strategy for regulating the shoot/root ratio for agricultural purposes.
Possible strategies for selective control of shoot and root growth are expected to emerge from more precise understandings of the molecular mechanism of GA signal transduction including the GA receptor and DELLA proteins in roots.
ACKNOWLEDGEMENTS
I thank the following colleagues for their help throughout this study and during the preparation of the manuscript: Dr Masayuki Katsumi and Dr James B. Reid for useful suggestions on the initial steps of GA and growth retardant experiments; Drs Miyako Ueguchi-Tanaka, Ko Hirano and Makoto Matsuoka for updated information and discussion on GA receptors; Dr Alexander Lux, Dr Shigenori Morita, Dr Jun Abe and Dr Tomoo Homma for kind advice and help in the study of root structure and morphology; and Dr Peter W. Barlow for critical reading of and suggestions on the manuscript.
APPENDIX: PLANT MATERIALS AND METHODS FOR ROOT GROWTH EXPERIMENTS
Pea seedlings
Pea seeds were sterilized by dipping in diluted sodium hypochlorite solution for 30 min, and germinated on wet filter paper or a cotton bed in an air-tight box. After selecting germinated seedlings by root length, seedlings were put on a stainless steel mesh fixed on a 100–200 mL cup containing hydroponic solution and growth regulators. Hydroponically cultured seedlings, ten seedlings per cup, are convenient for measuring root growth over a period of a few weeks.
Lettuce seedlings
Lettuce seeds are layed in rows on a double layer of wet filter paper after sterilization (dipped in diluted sodium hypochlorite solution for a few minutes after rinsing with ethanol). The doubled filter paper was vertically attached inside a 100–200 mL cup containing hydroponic solution. Roots grow vertically downwards in the hydroponic solution containing growth regulators.
Arabidopsis seedlings
Sterilized seeds were layed on a 1 % agar plate in a square Petri dish (100 × 140 × 12 mm) and the plate was placed vertically to allow vertical root elongation on the agar surface. The Petri dish must be taped with surgical mending tape to maintain the required level of humidity and aeration.
Growth quantification of roots.
The length of the main root was measured either using a ruler or by image analysis of photographs (Qui et al., 2007). For arabidopsis roots, the lengths of the longest roots, usually the main roots, were measured.
Continuous recording.
For continuous recording of root elongation, image analysis of photographs, or a rhizometer (TRZ-16) with minimum mechanical contact with the root tip, are both useful (Tanimoto and Watanabe, 1986).
LITERATURE CITED
- Ariizumi T, Murase K, Sun TP, Steber CM. Proteolysis-independent down regulation of DELLA repression in Arabidopsis by the gibberellin receptor GIBBERELLIN INSENSITIVE DWARF1. The Plant Cell. 2008;20:2447–2459. doi: 10.1105/tpc.108.058487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluška F, Parker JS, Barlow PW. A role for gibberellic acid in orienting microtubules and regulating cell growth polarity in maize root cortex. Planta. 1993;191:149–157. [Google Scholar]
- Bidadi H, Yamaguchi S, Asahina M, Satoh S. Effects of shoot-applied gibberellin/gibberellin-biosynthesis inhibitors on root growth and expression of gibberellin biosynthesis genes in Arabidopsis thaliana. Plant Root. 2010;4:4–11. [Google Scholar]
- Coolbaugh RC, Hamilton R. Inhibition of ent-kaurene oxidation and growth by α- cyclopropyl-α-(p-methoxyphenyl)-5-pyrimidine methyl alcohol. Plant Physiology. 1976;57:245–248. doi: 10.1104/pp.57.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coolbaugh RC, Hirano SS, West CA. Studies on the specificity and site of action of ancymidol, a plant growth regulator. Plant Physiology. 1978;62:571–576. doi: 10.1104/pp.62.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd IC. Root-to-shoot signalling: assessing the roles of ‘up’ in the up and down world of long-distance signalling in planta. Plant and Soil. 2005;274:251–270. [Google Scholar]
- Farquharson KL. Gibberellin–auxin crosstalk modulates lateral root formation. The Plant Cell. 2010;22:540. doi: 10.1105/tpc.110.220313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Harberd NP. Auxin promotes Arabidopsis root growth by modulating gibberellin response. Nature. 2003;421:740–743. doi: 10.1038/nature01387. [DOI] [PubMed] [Google Scholar]
- Gou J, Strauss SH, Tsai CJ, et al. Gibberellins regulate lateral root formation in Populus through interaction with auxin and other hormones. The Plant Cell. 2010;22:623–639. doi: 10.1105/tpc.109.073239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths J, Murase K, Rieu I, et al. Genetic characterization and functional analysis of the GID1 gibberellin receptors in Arabidopsis. The Plant Cell. 2006;18:3399–3414. doi: 10.1105/tpc.106.047415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden P. Recent advances in gibberellin biosynthesis. Journal of Experimental Botany. 1999;50:553–563. [Google Scholar]
- Hedden P. The genes of the green revolution. Trends in Genetics. 2003;19:5–9. doi: 10.1016/s0168-9525(02)00009-4. [DOI] [PubMed] [Google Scholar]
- Hedden P, Kamiya Y. Gibberellin biosynthesis: enzymes, genes and their regulation. Annual Review of Plant Biology. 1997;48:431–460. doi: 10.1146/annurev.arplant.48.1.431. [DOI] [PubMed] [Google Scholar]
- Heo JO, Chang KS, Kim IA, et al. Funneling of gibberellin signaling by the GRAS transcription regulator scarecrow-like 3 in the Arabidopsis root. Proceedings of the National Academy of Sciences, USA. 2011;108:2166–2171. doi: 10.1073/pnas.1012215108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano K, Ueguchi-Tanaka M, Matsuoka M. GID1-mediated gibberellin signaling in plants. Trends in Plant Science. 2008;13:192–199. doi: 10.1016/j.tplants.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Hogetsu T, Oshima Y. Immunofluorescence microscopy of microtubule arrangement in root cells of Pisum sativum L. var. Alaska. Plant and Cell Physiology. 1986;27:939–945. [Google Scholar]
- Kuraishi S, Muir RM. Increase in diffusible auxin after treatment with gibberellin. Science. 1962;137:760–761. doi: 10.1126/science.137.3532.760. [DOI] [PubMed] [Google Scholar]
- Matthysse AG, Scott TK. Function of hormones at the whole plant level of organization. In: Scott TK, editor. Hormonal regulation of development II, Encyclopedia of Plant Physiology, New Series. Berlin: Springer-Verlag; 1984. pp. 219–243. Vol 10. [Google Scholar]
- Mita T, Shibaoka H. Effects of S-3307, an inhibitor of gibberellin biosynthesis, on swelling of leaf sheath cells and on the arrangement of cortical microtubules in onion seedlings. Plant and Cell Physiology. 1984;25:1531–1539. [Google Scholar]
- Murase K, Hirano Y, Sun TP, Hakoshima T. Gibberellin-induced DELLA recognition by the gibberellin receptor GID1. Nature. 2008;456:459–463. doi: 10.1038/nature07519. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Shimada A, Takashi Y, et al. Identification and characterization of Arabidopsis gibberellin receptors. The Plant Journal. 2006;46:880–889. doi: 10.1111/j.1365-313X.2006.02748.x. [DOI] [PubMed] [Google Scholar]
- Ockerse R, Galston AW. Gibberellin–auxin interaction in pea stem elongation. Plant Physiology. 1967;42:47–54. doi: 10.1104/pp.42.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski N, Sun TP, Gubler F. Gibberellin signaling: biosynthesis, catabolism, and response pathways. The Plant Cell. 2002;14:S61–S80. doi: 10.1105/tpc.010476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazka S. Translocation of growth regulators from roots relation to the stem apical dominance in pea (Pisum sativum L.) seedlings. In: Brouwer R, Gasparikova I, Kolek J, Loughman BC, editors. Structure and function of plant roots. The Hague: Martinus Nijhoff/Dr W. Junk; 1981. pp. 407–409. [Google Scholar]
- Proebsting WM, Hedden P, Lewis MJ, Croker SJ, Proebsting LN. Gibberellin concentration and transport in genetic lines of pea: effects of grafting. Plant Physiology. 1992;100:1354–1360. doi: 10.1104/pp.100.3.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X, Qi J, Wu Y. RootLM: a simple color image analysis program for length measurement of primary roots in Arabidopsis. Plant Root. 2007;1:10–16. [Google Scholar]
- Rademacher W. Inhibitors of gibberellin biosynthesis: application in agriculture and horticulture. In: Takahashi N, Phinney BO, MacMillan J, editors. Gibberellins. New York: Springer-Verlag; 1991. pp. 296–310. [Google Scholar]
- Reid JB, Murfet IC, Potts WC. Internode length in Pisum. 2. Additional information on the relationship and action of loci le, la, cry, na and lm. Journal of Experimental Botany. 1983;34:349–364. [Google Scholar]
- Ross JJ, O'Neill DP, Smith JJ, Kerckhoffs LHJ, Elliot RC. Evidence that auxin promotes gibberellin A1 biosynthesis in pea. The Plant Journal. 2000;21:547–552. doi: 10.1046/j.1365-313x.2000.00702.x. [DOI] [PubMed] [Google Scholar]
- Ross JJ, O'Neill DP, Wolbang CM, Symons GM, Reid JB. Auxin–gibberellin interactions and their role in plant growth. Journal of Plant Growth Regulation. 2002;20:346–353. doi: 10.1007/s003440010034. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Ashikari M, Ueguchi-Tanaka M, et al. Green revolution: a mutant gibberellin-synthesis gene in rice. Nature. 2002;416:701–702. doi: 10.1038/416701a. [DOI] [PubMed] [Google Scholar]
- Shibaoka H. Plant hormone-induced changes in the orientation of cortical microtubules: alterations in the cross-linking between microtubules and the plasma membrane. Annual Review of Plant Biology. 1994;45:527–544. [Google Scholar]
- Sun TP. Gibberellin–GID1–DELLA: a pivotal regulatory module for plant growth and development. Plant Physiology. 2010;154:567–570. doi: 10.1104/pp.110.161554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Kitamura H, Kawarada A, et al. Biochemical studies on ‘Bakanae’ fungus. XXXIV. Isolation of gibberellins and their properties. Bulletin of the Agricultural Chemical Society of Japan. 1955;19:267. [Google Scholar]
- Takahashi N, Seta Y, Kitamura H, Sumiki Y. Biochemical studies on ‘Bakanae’ fungus. XLII. Bulletin of the Agricultural Chemical Society of Japan. 1957;21:396. [Google Scholar]
- Takahashi N, Phinney BO, MacMillan J. Gibberellins. New York: Springer-Verlag; 1991. [Google Scholar]
- Tanimoto E. Gibberellin-dependent root elongation in Lactuca sativa: recovery from growth retardant-suppressed elongation with thickening by low concentration of GA3. Plant and Cell Physiology. 1987;28:963–973. [Google Scholar]
- Tanimoto E. Gibberellin regulation of root growth with changes in galactose contents of the cell walls in Pisum sativum. Plant and Cell Physiology. 1988;29:269–280. [Google Scholar]
- Tanimoto E. Interaction of gibberellin A3 and ancymidol in the growth and cell-wall extensibility of dwarf pea roots. Plant and Cell Physiology. 1994;35:1019–1028. [Google Scholar]
- Tanimoto E. Gibberellins. In: Waisel Y, Eshel A, Beeckman T, Kafkafi U, editors. Plant roots: the hidden half. 3rd edn. New York: Marcel Dekker; 2002. pp. 405–416. [Google Scholar]
- Tanimoto E. Regulation of root growth by plant hormones. Roles for auxin and gibberellin. Critical Reviews in Plant Sciences. 2005;24:249–265. [Google Scholar]
- Tanimoto E, Watanabe J. Automated recording of lettuce root elongation as affected by indole-3-acetic acid and acid pH by a new rhizometer with minimum mechanical contact to root. Plant and Cell Physiology. 1986;27:1475–1487. [Google Scholar]
- Tanimoto E, Yanagishima N, Masuda Y. Effect of gibberellic acid on dwarf and normal pea plants. Physiologia Plantarum. 1967;20:291–298. [Google Scholar]
- Ubeda-Tomás S, Swarup R, Coates J, et al. Root growth in Arabidopsis requires gibberellin/DELLA signaling in the endodermis. Nature Cell Biology. 2008;10:625–628. doi: 10.1038/ncb1726. [DOI] [PubMed] [Google Scholar]
- Ubeda-Tomás S, Federici F, Casimiro I, et al. Gibberellin signaling in the endodermis controls Arabidopsis root meristem size. Current Biology. 2009;19:1194–1199. doi: 10.1016/j.cub.2009.06.023. [DOI] [PubMed] [Google Scholar]
- Ueguchi-Tanaka M, Ashikari M, Nakajima M, et al. GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature. 2005;437:693–698. doi: 10.1038/nature04028. [DOI] [PubMed] [Google Scholar]
- Ueguchi-Tanaka M, Nakajima M, Ashikari M, Matsuoka M. Gibberellin receptor and its role in gibberellin signaling in plants. Annual Review of Plant Biology. 2007;58:183–198. doi: 10.1146/annurev.arplant.58.032806.103830. [DOI] [PubMed] [Google Scholar]
- Willige BC, Isono E, Richter R, Zourelidou M, Schwechheimer C. Gibberellin regulates PIN-FORMED abundance and is required for auxin transport-dependent growth and development in Arabidopsis thaliana. The Plant Cell. 2011;23:2184–2195. doi: 10.1105/tpc.111.086355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S. Gibberellin metabolism and its regulation. Annual Review of Plant Biology. 2008;59:225–251. doi: 10.1146/annurev.arplant.59.032607.092804. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Hirai T, Yamamoto E, et al. A rice gid1 suppressor mutant reveals that gibberellin is not always required for interaction between its receptor, GID1, and DELLA proteins. The Plant Cell. 2010;22:3589–3602. doi: 10.1105/tpc.110.074542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaxley JR, Ross JJ, Sherriff LJ, Reid JB. Gibberellin biosynthesis mutations and root development in pea. Plant Physiology. 2001;125:627–633. doi: 10.1104/pp.125.2.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann R, Sakai H, Hochholdinger F. The gibberellic acid stimulated-like gene family in maize and its role in lateral root development. Plant Physiology. 2010;152:356–365. doi: 10.1104/pp.109.149054. [DOI] [PMC free article] [PubMed] [Google Scholar]