Abstract
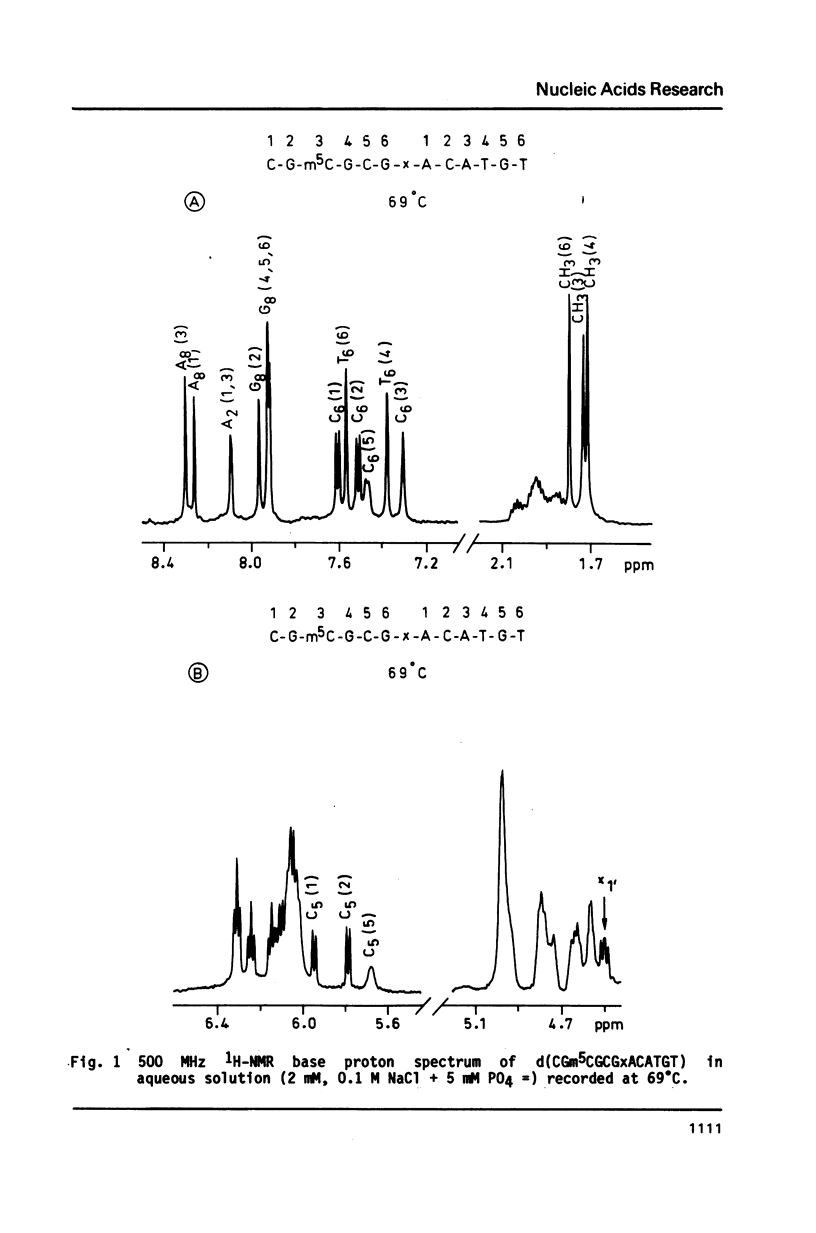
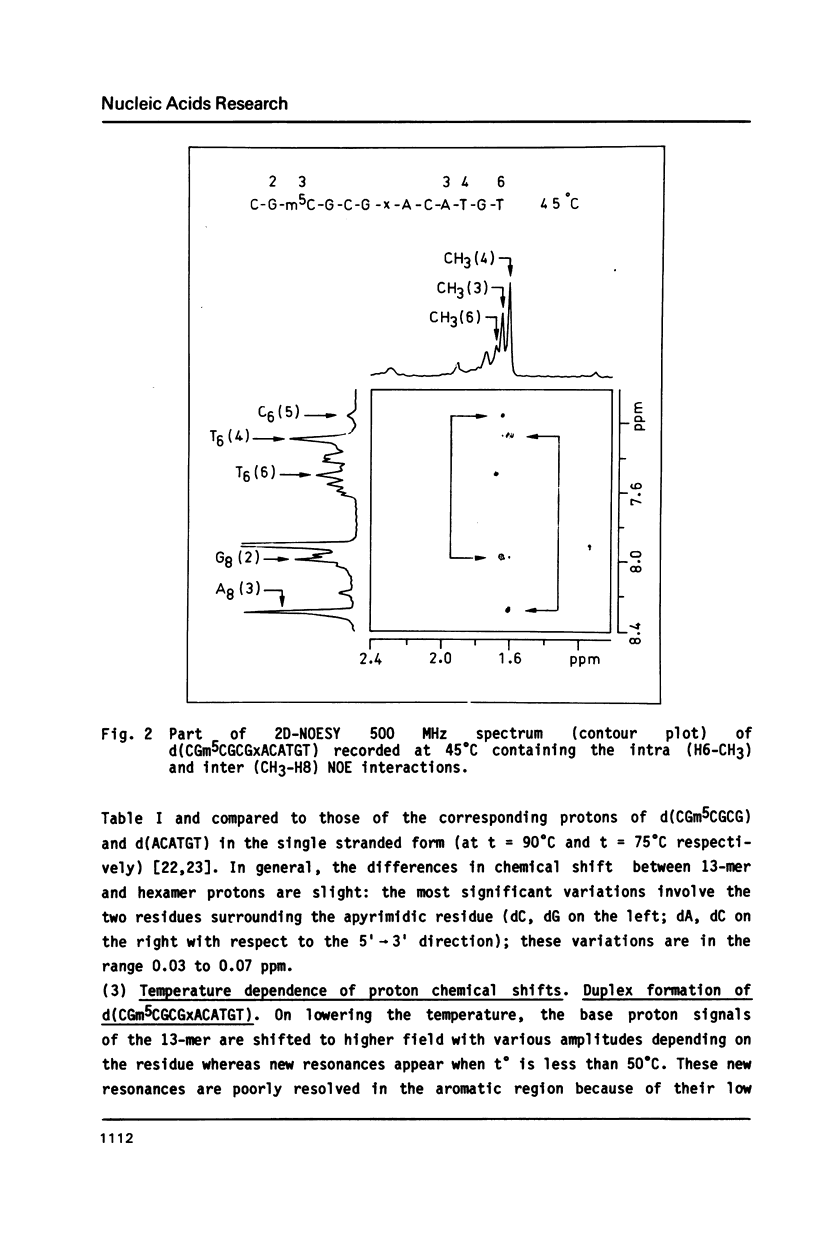
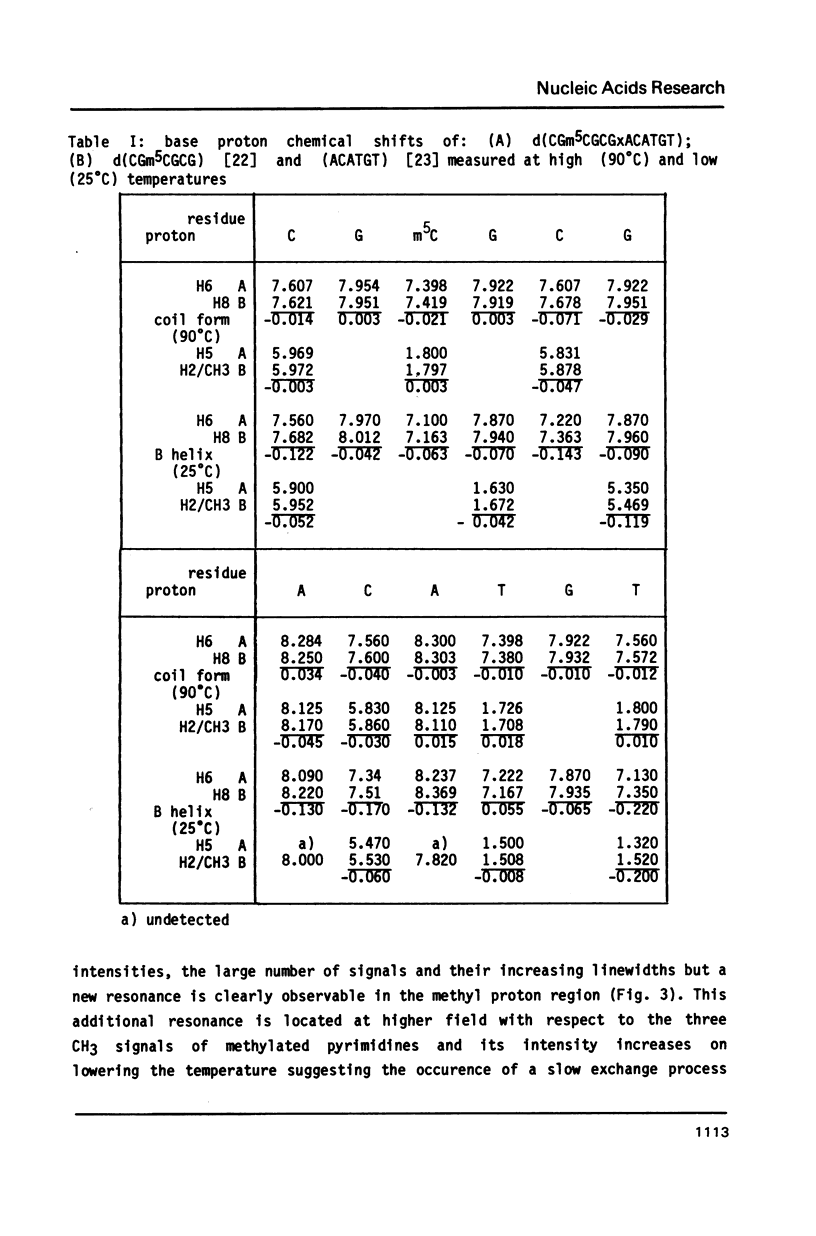
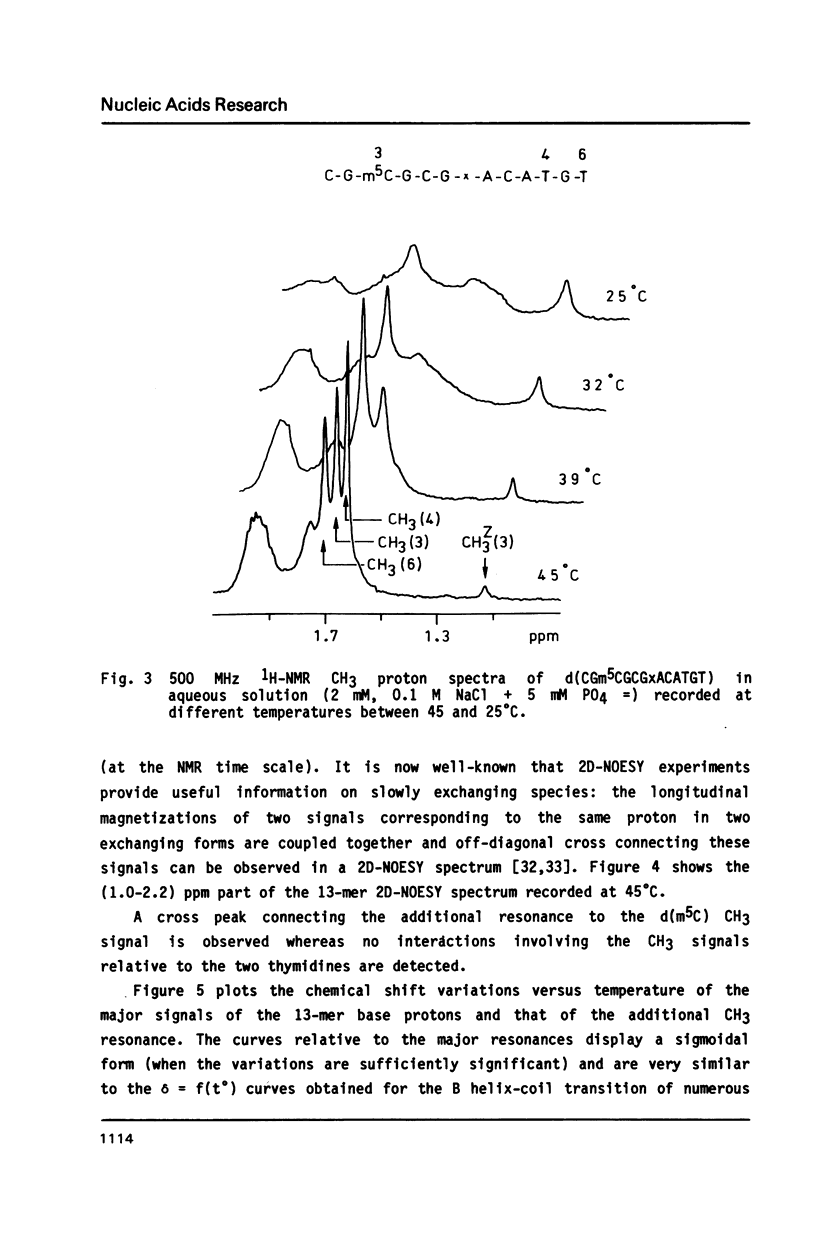
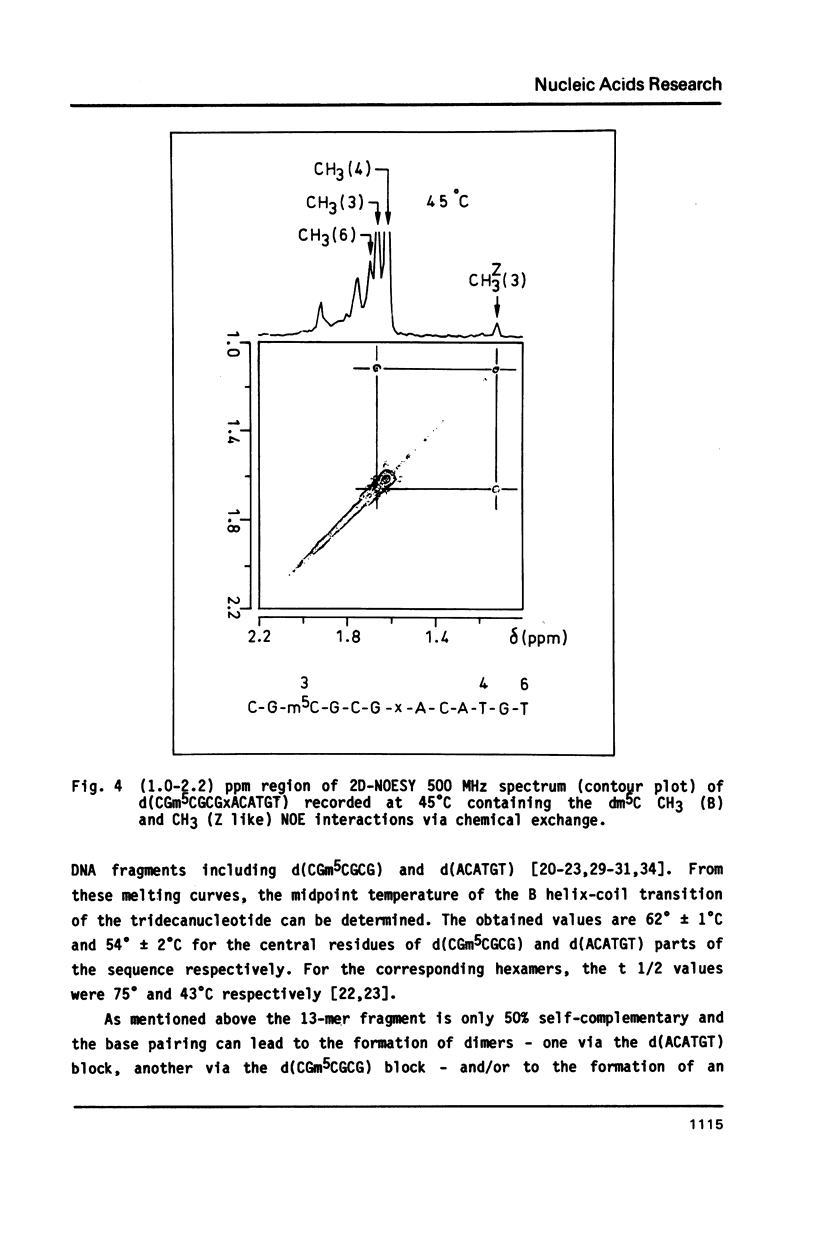
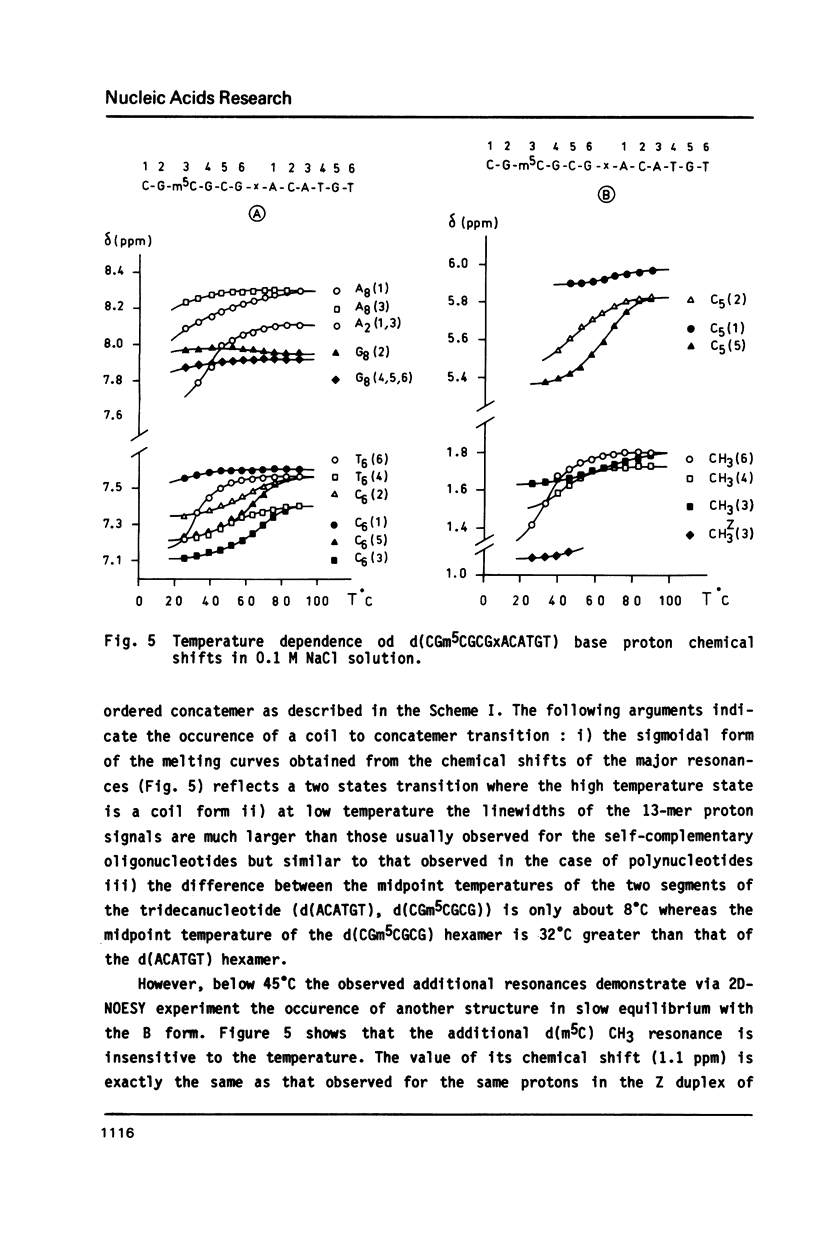
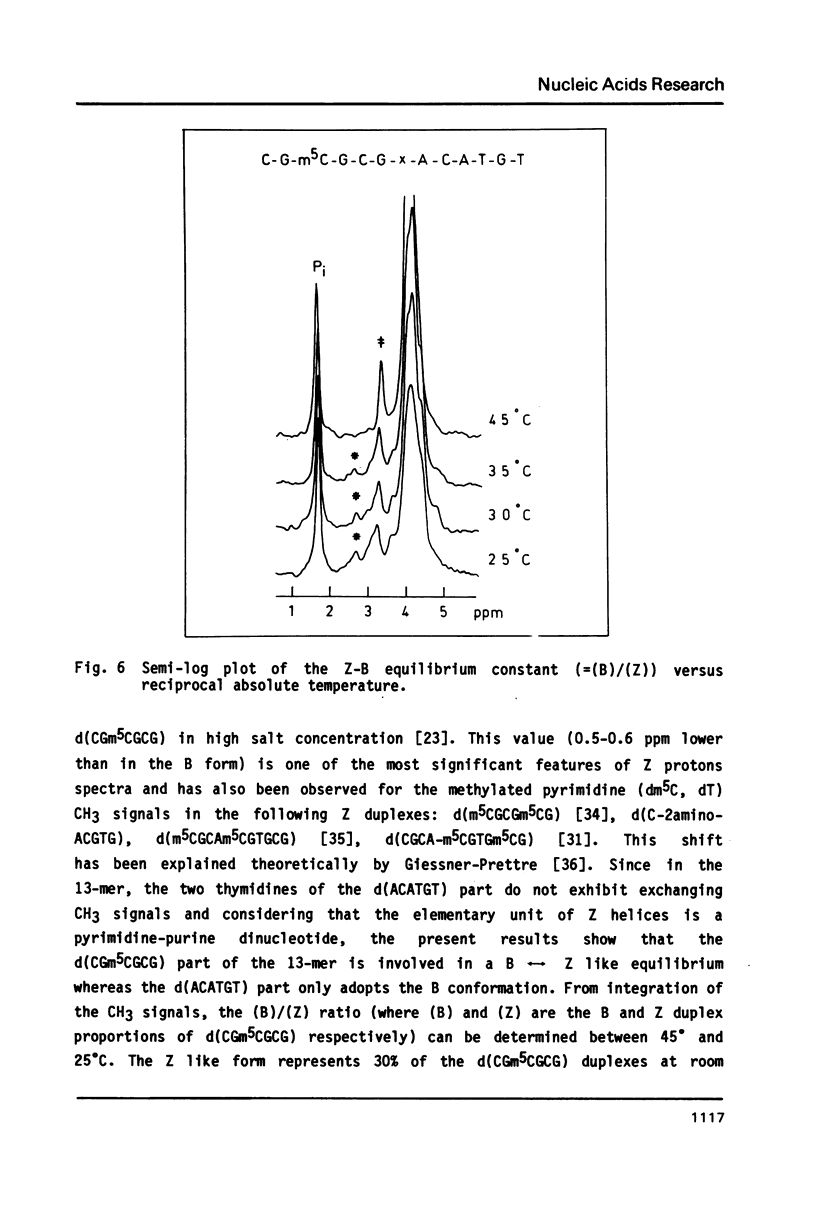
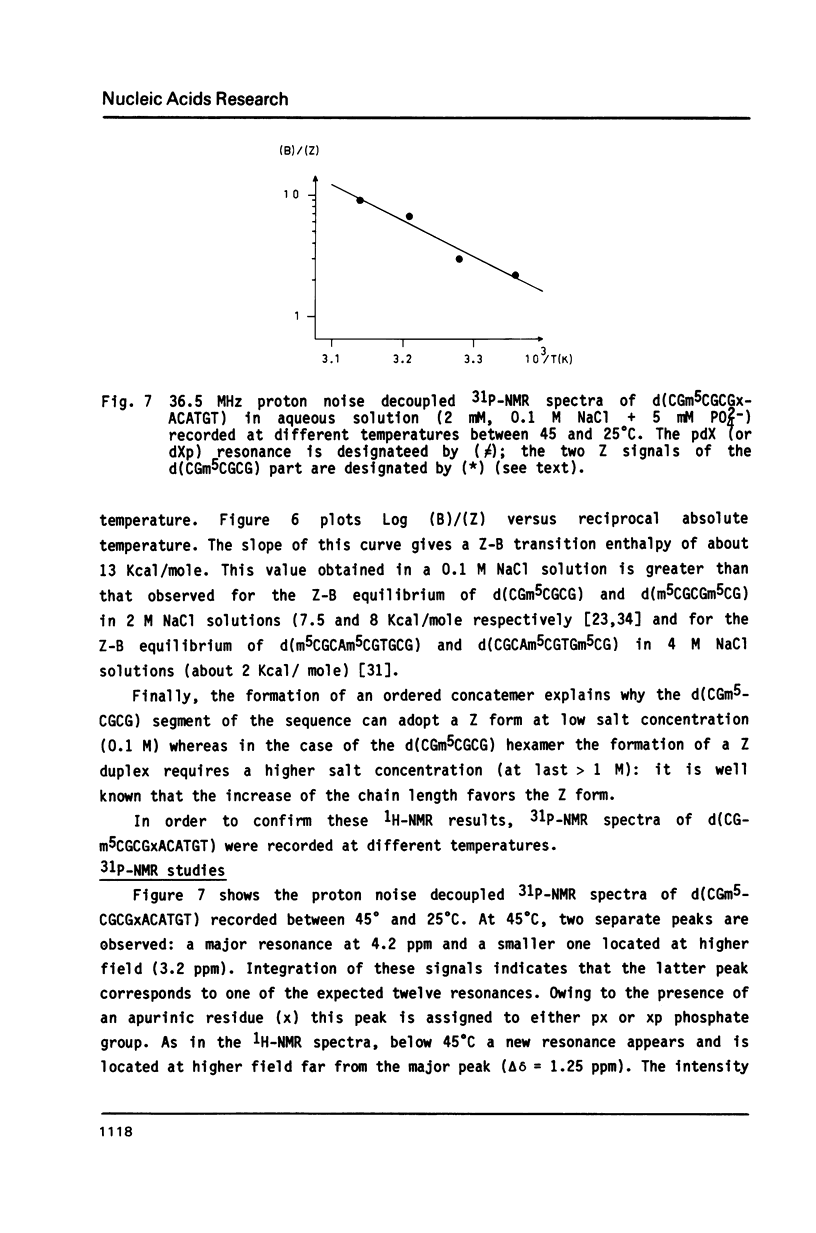
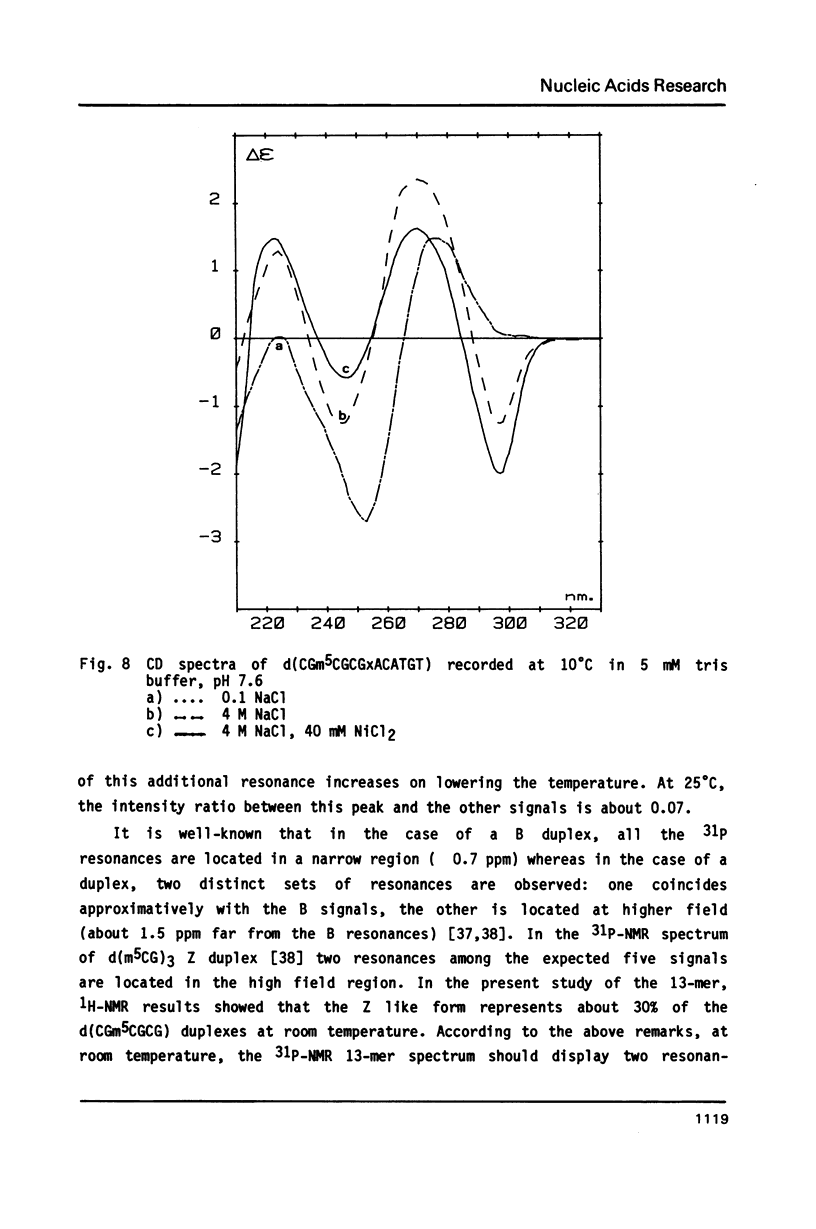
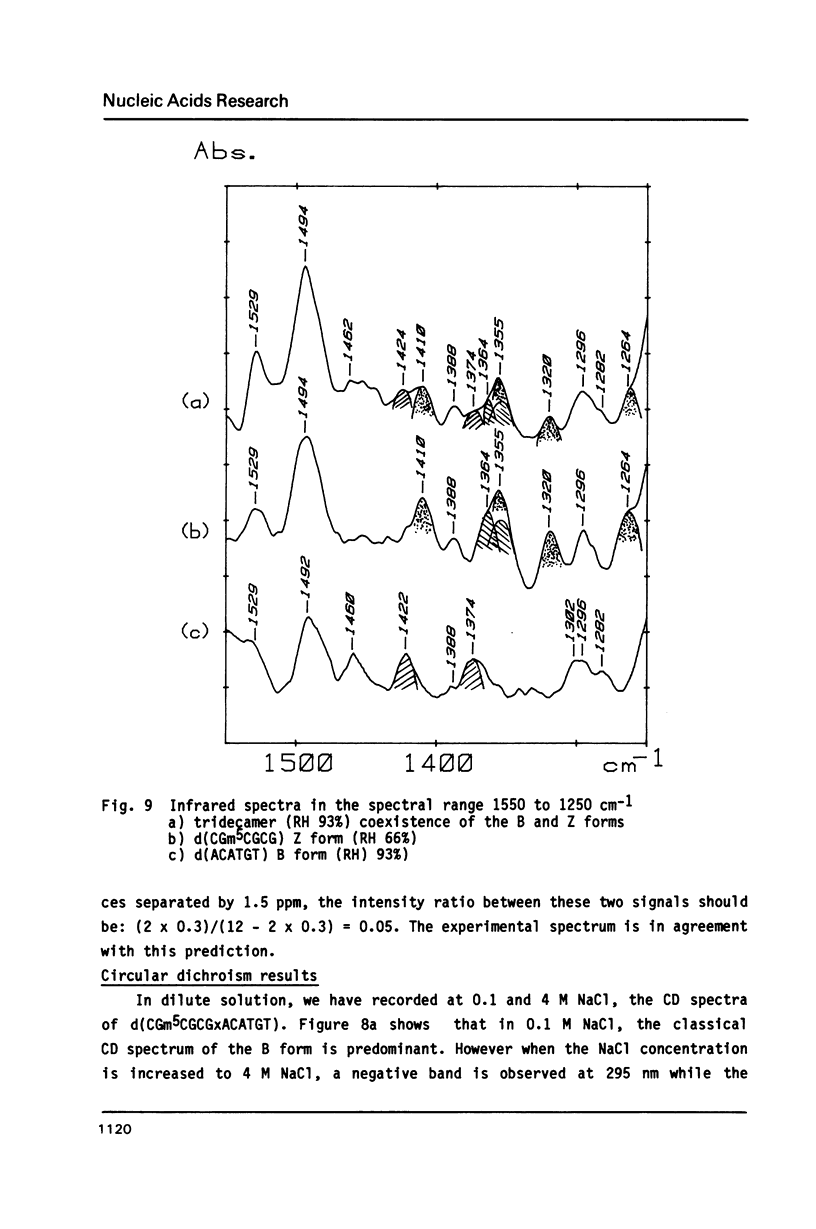
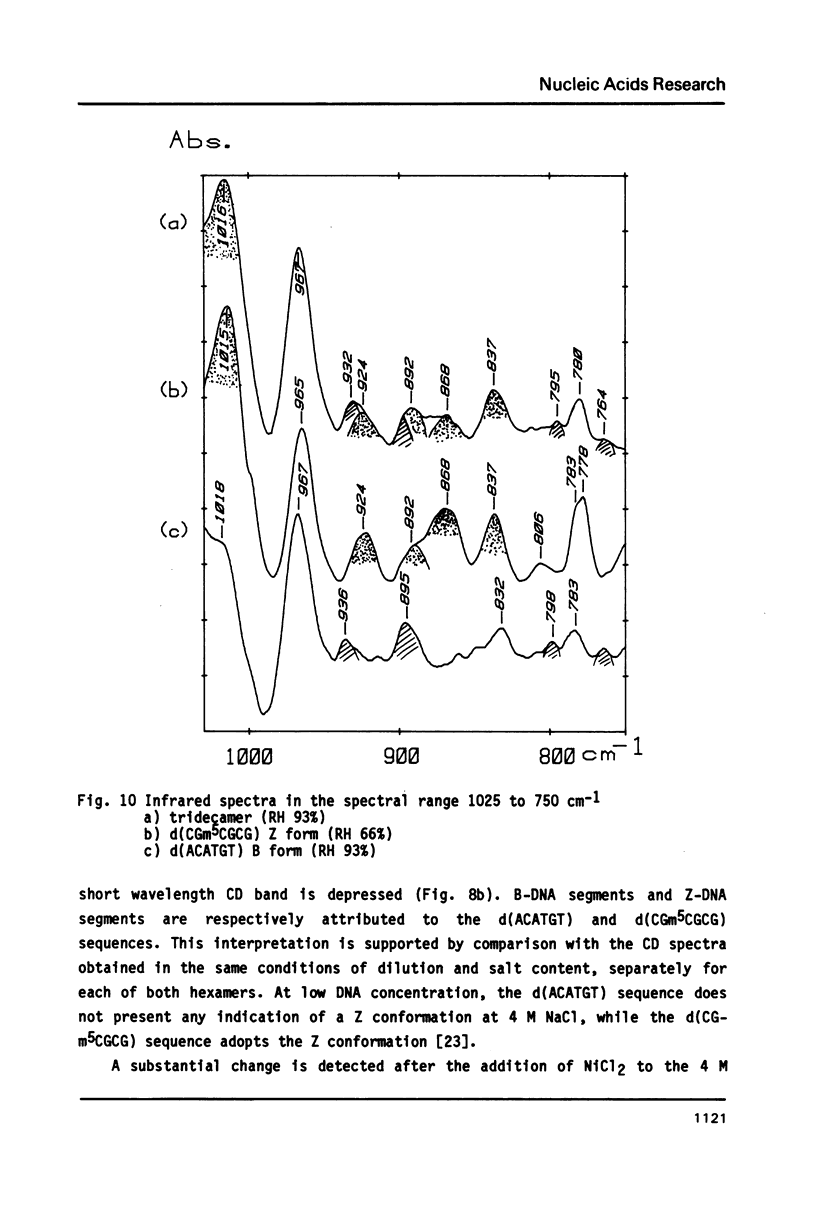
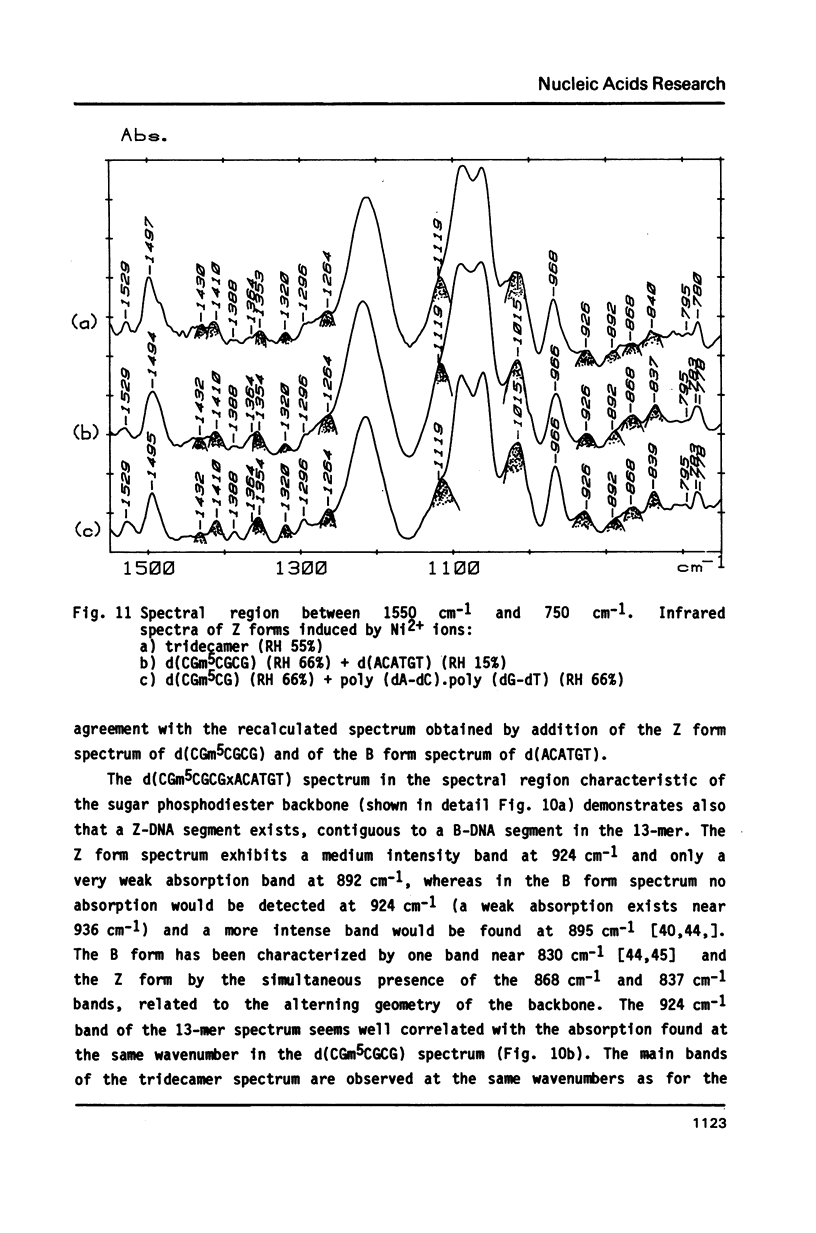
The synthesis of the tridecadeoxynucleotide d(CGm5CGCGxACATGT), where x is the 1-cyano-2-deoxy-beta-D-erythropentofuranose, is described. The NMR, IR, CD studies at various salt concentrations and temperatures of this oligomer show that the B and Z conformations are simultaneously present in the same short DNA fragment. A single apurinic residue is sufficient for the coexistence of the B and Z helices on this oligomer.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arndt-Jovin D. J., Robert-Nicoud M., Zarling D. A., Greider C., Weimer E., Jovin T. M. Left-handed Z-DNA in bands of acid-fixed polytene chromosomes. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4344–4348. doi: 10.1073/pnas.80.14.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azorin F., Hahn R., Rich A. Restriction endonucleases can be used to study B-Z junctions in supercoiled DNA. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5714–5718. doi: 10.1073/pnas.81.18.5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behe M., Felsenfeld G. Effects of methylation on a synthetic polynucleotide: the B--Z transition in poly(dG-m5dC).poly(dG-m5dC). Proc Natl Acad Sci U S A. 1981 Mar;78(3):1619–1623. doi: 10.1073/pnas.78.3.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavailles J. A., Neumann J. M., Taboury J., Langlois d'Estaintot B., Huynh-Dinh T., Igolen J., Tran-Dinh S. B,Z conformations and mechanism of the Z-B-coil transitions of the self-complementary deoxy-hexanucleotide d(C-G-m5C-G-C-G) by 1H-NMR and CD spectroscopy. J Biomol Struct Dyn. 1984 Jun;1(6):1347–1371. doi: 10.1080/07391102.1984.10507525. [DOI] [PubMed] [Google Scholar]
- Cavaillès J. A., Neumann J. M., Tran-Dinh S., Huynh-Dinh T., d'Estaintot B. L., Igolen J. Influence of dA X dT and d(2aminoA) X dT base pairs on the B in equilibrium Z transition of DNA fragments. 1H-NMR study of d(C-G-C-A-m5C-G-T-G-m5C-G), d(m5C-G-C-A-m5C-G-T-G-C-G) and d(C-2aminoA-C-G-T-G). Eur J Biochem. 1985 Feb 15;147(1):183–190. doi: 10.1111/j.1432-1033.1985.tb08735.x. [DOI] [PubMed] [Google Scholar]
- Chinsky L., Jolles B., Laigle A., Turpin P. Y., Taboury J., Taillandier E. Identification of a new electronic transition in the Z form of poly(dG-dC).poly(dG-dC) by infrared absorption and resonance Raman spectroscopy. Biopolymers. 1984 Oct;23(10):1931–1942. doi: 10.1002/bip.360231009. [DOI] [PubMed] [Google Scholar]
- Clore G. M., Gronenborn A. M. A nuclear-Overhauser-enhancement study of the solution structure of a double-stranded DNA undecamer comprising a portion of the specific target site for the cyclic-AMP-receptor protein in the gal operon. Sequential resonance assignment. Eur J Biochem. 1984 May 15;141(1):119–129. doi: 10.1111/j.1432-1033.1984.tb08166.x. [DOI] [PubMed] [Google Scholar]
- Feigon J., Wang A. H., van der Marel G. A., Van Boom J. H., Rich A. A one- and two-dimensional NMR study of the B to Z transition of (m5dC-dG)3 in methanolic solution. Nucleic Acids Res. 1984 Jan 25;12(2):1243–1263. doi: 10.1093/nar/12.2.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S., Wang A. H., van der Marel G., van Boom J. H., Rich A. Molecular structure of (m5 dC-dG)3: the role of the methyl group on 5-methyl cytosine in stabilizing Z-DNA. Nucleic Acids Res. 1982 Dec 11;10(23):7879–7892. doi: 10.1093/nar/10.23.7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghomi M., Taboury J. A., Taillandier E. Experimental and calculated study of the vibrational modes of poly(dG-dC).poly(dG-dC) in B and Z conformations. Biochimie. 1984 Feb;66(2):87–92. doi: 10.1016/0300-9084(84)90184-6. [DOI] [PubMed] [Google Scholar]
- Giessner-Prettre C., Pullman B., Tran-Dinh S., Neumann J. M., Huynh-Dinh T., Igolen J. Proton NMR study of the B----Z transition of d(CGm5CG)2 and d(CGm5CGCG)2: theory and experiment. Nucleic Acids Res. 1984 Apr 11;12(7):3271–3281. doi: 10.1093/nar/12.7.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann B., Thuong N. T., Pouyet J., Ptak M., Leng M. Spectroscopic studies of (m5dC-dG)3: thermal stability of B- and Z-forms. Nucleic Acids Res. 1983 Jul 11;11(13):4453–4466. doi: 10.1093/nar/11.13.4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick M. W., Wei C. F., Gray H. B., Jr, Wells R. D. BAL 31 nuclease as a probe in concentrated salt for the B-Z DNA junction. Nucleic Acids Res. 1983 Jun 11;11(11):3811–3822. doi: 10.1093/nar/11.11.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kłysik J., Stirdivant S. M., Larson J. E., Hart P. A., Wells R. D. Left-handed DNA in restriction fragments and a recombinant plasmid. Nature. 1981 Apr 23;290(5808):672–677. doi: 10.1038/290672a0. [DOI] [PubMed] [Google Scholar]
- Lawley P. D., Warren W. Removal of minor methylation products 7-methyladenine and 3-methylguanine from DNA of Escherichia coli treated with dimethyl sulphate. Chem Biol Interact. 1976 Feb;12(2):211–220. doi: 10.1016/0009-2797(76)90100-9. [DOI] [PubMed] [Google Scholar]
- Lindahl T. DNA repair enzymes. Annu Rev Biochem. 1982;51:61–87. doi: 10.1146/annurev.bi.51.070182.000425. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Nyberg B. Rate of depurination of native deoxyribonucleic acid. Biochemistry. 1972 Sep 12;11(19):3610–3618. doi: 10.1021/bi00769a018. [DOI] [PubMed] [Google Scholar]
- Millican T. A., Mock G. A., Chauncey M. A., Patel T. P., Eaton M. A., Gunning J., Cutbush S. D., Neidle S., Mann J. Synthesis and biophysical studies of short oligodeoxynucleotides with novel modifications: a possible approach to the problem of mixed base oligodeoxynucleotide synthesis. Nucleic Acids Res. 1984 Oct 11;12(19):7435–7453. doi: 10.1093/nar/12.19.7435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller A., Nordheim A., Nichols S. R., Rich A. 7-Methylguanine in poly(dG-dC).poly(dG-dC) facilitates z-DNA formation. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4777–4781. doi: 10.1073/pnas.78.8.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordheim A., Pardue M. L., Lafer E. M., Möller A., Stollar B. D., Rich A. Antibodies to left-handed Z-DNA bind to interband regions of Drosophila polytene chromosomes. Nature. 1981 Dec 3;294(5840):417–422. doi: 10.1038/294417a0. [DOI] [PubMed] [Google Scholar]
- Nordheim A., Tesser P., Azorin F., Kwon Y. H., Möller A., Rich A. Isolation of Drosophila proteins that bind selectively to left-handed Z-DNA. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7729–7733. doi: 10.1073/pnas.79.24.7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D. J., Kozlowski S. A., Nordheim A., Rich A. Right-handed and left-handed DNA: studies of B- and Z-DNA by using proton nuclear Overhauser effect and P NMR. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1413–1417. doi: 10.1073/pnas.79.5.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadrifoglio F., Manzini G., Dinkelspiel K., Crea R. Simultaneous stability of short alternating Z and B helices in synthetic DNA concatamers. Nucleic Acids Res. 1982 Jun 25;10(12):3759–3768. doi: 10.1093/nar/10.12.3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich A., Nordheim A., Wang A. H. The chemistry and biology of left-handed Z-DNA. Annu Rev Biochem. 1984;53:791–846. doi: 10.1146/annurev.bi.53.070184.004043. [DOI] [PubMed] [Google Scholar]
- Robert-Nicoud M., Arndt-Jovin D. J., Zarling D. A., Jovin T. M. Immunological detection of left-handed Z DNA in isolated polytene chromosomes. Effects of ionic strength, pH, temperature and topological stress. EMBO J. 1984 Apr;3(4):721–731. doi: 10.1002/j.1460-2075.1984.tb01875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheek R. M., Boelens R., Russo N., van Boom J. H., Kaptein R. Sequential resonance assignments in 1H NMR spectra of oligonucleotides by two-dimensional NMR spectroscopy. Biochemistry. 1984 Mar 27;23(7):1371–1376. doi: 10.1021/bi00302a006. [DOI] [PubMed] [Google Scholar]
- Taboury J. A., Adam S., Taillandier E., Neumann J. M., Tran-Dinh S., Huynh-Dinh T., Langlois d'Estaintot B., Conti M., Igolen J. The B----Z transition in two synthetic oligonucleotides: d(C-2-amino-ACGTG) and d(m5CGCAm5CGTGCG) studied by IR, NMR and CD spectroscopies. Nucleic Acids Res. 1984 Aug 10;12(15):6291–6305. doi: 10.1093/nar/12.15.6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taboury J. A., Bourtayre P., Liquier J., Taillandier E. Interaction of Z form poly(dG-dC).poly(dG-dC) with divalent metal ions: localization of the binding sites by I.R. spectroscopy. Nucleic Acids Res. 1984 May 25;12(10):4247–4258. doi: 10.1093/nar/12.10.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taillandier E., Taboury J. A., Adam S., Liquier J. Left-handed helical structure of poly[d(A-C)].poly[d(G-T)] studied by infrared spectroscopy. Biochemistry. 1984 Nov 20;23(24):5703–5706. doi: 10.1021/bi00319a007. [DOI] [PubMed] [Google Scholar]
- Taillandier E., Taboury J., Liquier J., Sautière P., Couppez M. Structural transitions in DNAs and nucleohistones studied by I.R. spectroscopy. Biochimie. 1981 Nov-Dec;63(11-12):895–898. doi: 10.1016/s0300-9084(82)80282-4. [DOI] [PubMed] [Google Scholar]
- Tran-Dinh S., Neumann J. M., Huynh-Dinh T., Genissel B., Igolen J., Simonnot G. DNA fragment conformations. 1H-NMR studies of helix-coil transition, conformations and dynamic structures of the self-complementary deoxyhexanucleotide d(A-C-A-T-G-T) in aqueous solution. Eur J Biochem. 1982 Jun;124(3):415–425. [PubMed] [Google Scholar]
- Tran-Dinh S., Taboury J., Neumann J. M., Huynh-Dinh T., Genissel B., Langlois d'Estaintot B., Igolen J. 1H NMR and circular dichroism studies of the B and Z conformations of the self-complementary deoxyhexanucleotide d(m5C-G-C-G-m5-C-G): mechanism of the Z-B-coil transitions. Biochemistry. 1984 Mar 27;23(7):1362–1371. doi: 10.1021/bi00302a005. [DOI] [PubMed] [Google Scholar]
- Viegas-Péquignot E., Derbin C., Malfoy B., Taillandier E., Leng M., Dutrillaux B. Z-DNA immunoreactivity in fixed metaphase chromosomes of primates. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5890–5894. doi: 10.1073/pnas.80.19.5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]
- Wang J. C., Peck L. J., Becherer K. DNA supercoiling and its effects on DNA structure and function. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):85–91. doi: 10.1101/sqb.1983.047.01.011. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Brennan R., Chapman K. A., Goodman T. C., Hart P. A., Hillen W., Kellogg D. R., Kilpatrick M. W., Klein R. D., Klysik J. Left-handed DNA helices, supercoiling, and the B-Z junction. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):77–84. doi: 10.1101/sqb.1983.047.01.010. [DOI] [PubMed] [Google Scholar]


