Abstract
N-Methyl-D-aspartate receptors (NMDARs) are known to be involved in a range of neurological and neurodegenerative disorders and consequently the development of compounds that modulate the function of these receptors has been the subject of intense interest. We have recently reported that 6-bromocoumarin-3-carboxylic acid (UBP608) is a negative allosteric modulator with weak selectivity for GluN2A-containing NMDARs. In the present study, a series of commercially available and newly synthesized coumarin derivatives have been evaluated in a structure-activity relationship (SAR) study as modulators of recombinant NMDAR activity. The main conclusions from this SAR study were that substituents as large as iodo were accommodated at the 6-position and that 6,8-dibromo or 6,8-diiodo substitution of the coumarin ring enhanced the inhibitory activity at NMDARs. These coumarin derivatives are therefore excellent starting points for the development of more potent and GluN2 subunit selective inhibitors, which may have application in the treatment of a range of neurological disorders such as neuropathic pain, epilepsy and depression. Surprisingly, 4-methyl substitution of UBP608 to give UBP714, led to conversion of the inhibitory activity of UBP608 into potentiating activity at recombinant GluN1/GluN2 receptors. UBP714 also enhanced NMDAR mediated field EPSPs in the CA1 region of the hippocampus. UBP714 is therefore a novel template for the development of potent and subunit selective NMDAR potentiators that may have therapeutic applicability in the treatment of patients with cognitive deficits or schizophrenia.
Keywords: NMDA receptors, glutamate, allosteric modulator, antagonist, oocyte, hippocampus
1. Introduction
The primary excitatory neurotransmitter of the vertebrate CNS, L-glutamate, generates fast synaptic responses by the activation of ligand-gated ion channels belonging to three families of receptors, the N-methyl-D-aspartate (NMDA), kainate, and (S)-2-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors (Traynelis et al., 2010; Watkins and Jane, 2006). Considerable interest has focused on NMDA receptors because of their special role underlying synaptic plasticity (Bliss and Collingridge, 1993), memory formation and other aspects of CNS function as well as their central role in various neurpathological and psychiatric conditions. NMDA receptor signaling contributes to the expression of epilepsy, schizophrenia, drug addiction, mood disorders, post-traumatic stress disorder and neuropathic pain (Lindsley et al., 2006; Sanacora et al., 2008; Wasterlain et al., 2008). Excessive NMDA receptor activation may also be a common mechanism causing neuronal cell death in stroke, traumatic brain injury and various neurodegenerative diseases such as Alzheimer’s, Parkinson’s, Huntington’s, amyotrophic lateral sclerosis (ALS), and Creutzfeldt-Jakob disease (Kalia et al., 2008; Khosravani et al., 2008; Villmann et al., 2007).
NMDA receptors are heterotetrameric complexes composed of two glycine-activated GluN1 subunits, of which there are 8 alternatively spliced forms, and two L-glutamate-activated GluN2 subunits of which there are 4 distinct gene products (GluN2A-GluN2D). In limited cases, NMDA receptors may also contain a GluN3 subunit. The functional heterogeneity of NMDA receptors is largely due to the presence of different GluN2 subunits. These have different spatial and temporal expression patterns in the brain and have distinct physiological properties. They differ significantly in their decay times, desensitization, Mg++-sensitivity, L-glutamate and glycine affinity, synaptic/extrasynaptic localization, and downstream signaling (Traynelis et al., 2010).
The discovery that NMDA receptors participate in diverse neuropathological and psychiatric conditions led to high expectations for clinical studies of NMDA receptor antagonists. Unfortunately, the results from these studies have been largely disappointing due to adverse effects and limited therapeutic efficacy (Kalia et al., 2008; Villmann et al., 2007). Despite these clinical failures, there remains considerable untapped potential for NMDA receptor therapeutic agents. Overall, there are at least three general approaches that may significantly improve effectiveness, or expand the functional repertoire, of NMDA receptor therapeutic agents. Identify agents that 1) have greater subtype selectivity, 2) are allosteric modulators, or 3) can potentiate NMDA receptor responses.
Considerable improvements could be made in terms of subtype selectivity. To date, the NMDA receptor pharmacological agents that have been tested in the clinic are either non-selective compounds that cannot distinguish among NMDA receptor subtypes or compounds that have only one pattern of selectivity (GluN2B-selective). Thus it has not been possible to selectively block the most therapeutically relevant subpopulation of NMDA receptors while minimizing adverse effects of blocking other NMDA receptor populations.
Development of allosteric modulators represents another approach for potentially improving NMDA receptor therapeutics. NMDA receptor compounds that have been clinically tested have been NMDA receptor channel blockers, competitive antagonists at either the L-glutamate or glycine binding site or ifenprodil-like ligands that bind at the N-terminal regulatory domain selectively inhibiting GluN2B-containing receptors (Jane et al., 2000). By analogy to G-protein coupled receptors, as well as to AMPA and GABA-A receptors, allosteric modulators can offer therapeutic advantages in having noncompetitive or uncompetitive activity (Arai et al., 2007). Negative allosteric modulators (NAMs) have no intrinsic activity and bind to a different site to L-glutamate to inhibit receptor function (Conn et al., 2009). There can be advantages to modulating the gain of receptor signaling rather than indiscriminately turning the receptor on or off. Based upon the structural homology to AMPA receptors, NMDA receptors are likely to be amenable to allosteric modulators that bind in sites homologous to those by which inhibitors such as GYKI53655 and potentiators such as cyclothiazide bind in AMPA receptors (Sobolevsky et al., 2009; Sun et al., 2002).
A third area of significant potential for new drug discovery is in the identification of compounds that potentiate NMDA receptor responses. Positive allosteric modulators have no intrinsic activity and bind to a different site to L-glutamate to increase its potency (Conn et al., 2009). To date, no NMDA receptor positive allosteric modulators (PAMs) have been developed for clinical study, and yet some clinical indications are predicted to benefit from an NMDA receptor potentiator rather than an inhibitor (e.g. schizophrenia or cognitive enhancement).
Recently, we and others have identified allosteric modulators that include both inhibitors and potentiators with improved subtype-selectivity (Bettini et al., 2010; Costa et al., 2010; Mosley et al., 2010; Mullasseril et al., 2010). In our study, we identified a series of naphthoic and phenanthroic acids that displayed positive and negative allosteric modulatory activity at NMDA receptors (Costa et al., 2010). Intriguingly, these compounds displayed varied patterns of subunit selectivity. For example, one related structure we reported, the coumarin derivative UBP608 displayed a moderate degree of subtype selectivity for GluN2A-containing NMDA receptors. The purpose of the present study was to evaluate a series of structurally-related coumarins for their activity at NMDA receptors. In addition to defining structural features underlying inhibitory activity at NMDA receptors, we report a modification to the coumarin structure that confers potentiating activity.
2. Materials and methods
2.1 Synthesis of coumarin derivatives
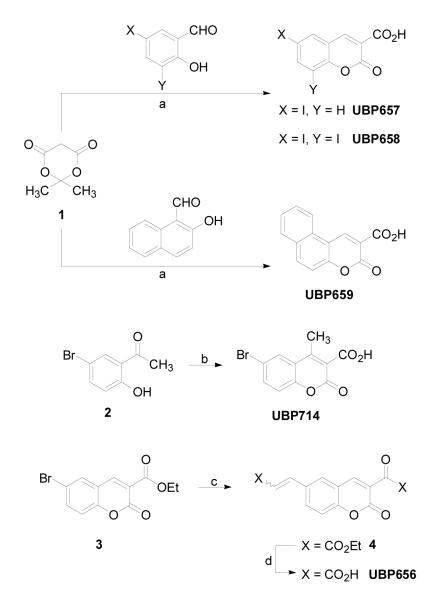
Structures of compounds synthesized and tested for this report are presented in Figure 1. 6-Iodocoumarin-3-carboxylic acid (UBP657), 6,8-diiodocoumarin-3-carboxylic acid (UBP658), benzo[5,6]coumarin-3-carboxylic acid (UBP659), 6-bromo-4-methylcoumarin-3-carboxylic acid (UBP714), and 6-(2-carboxyethen-1-yl)coumarin-3-carboxylic acid (UBP656) were synthesized as shown in Figure 2. With the exception of UBP656, the coumarin derivatives were synthesized via a Knoevenagel condensation reaction between Meldrum’s acid (1) and the appropriate aldehyde or ketone (Song et al., 2003). Due to its lower reactivity toward nucleophilic attack, 5-bromo-2-hydroxyacetophenone (2) was first reacted with ammonia to form a ketimine intermediate which was then reacted with 1 to afford UBP714 (Song et al., 2003). UBP656 was synthesized from ethyl-6-bromocoumarin-3-carboxylate (3) in two steps. Firstly, Heck coupling of 3 with ethyl acrylate afforded di-ester 4 which was then hydrolyzed using base to yield UBP656. After synthesis and purification, compound structure was verified by 1H NMR and mass spectroscopy. All novel compounds had elemental analyses in which the determined percentages for C, H, and N were less than 0.4% different from theoretical values. 7-Bromo-3-hydroxy-2-naphthoic acid (UBP574) and 4,7-dibromo-3-hydroxy-2-naphthoic acid (UBP552) were synthesized according to literature procedure (Murphy et al., 1990). The remaining compounds were purchased from Sigma-Aldrich (coumarin-3-carboxylic acid (UBP649), 6-bromocoumarin-3-carboxylic acid (UBP608), 7-hydroxycoumarin-3-carboxylic acid (UBP652), 7-methoxycoumarin-3-carboxylic acid (UBP653), 7-(diethylamino)coumarin-3-carboxylic acid (UBP654)) and Alfa Aesar (6,8-dibromocoumarin-3-carboxylic acid (UBP651)).
Figure 1.
Structures of recently reported NMDAR allosteric modulators and coumarin derivatives.
Figure 2.
Schemes for the synthesis of coumarin derivatives.
Reagents and conditions: (a) Piperidinium acetate, EtOH, reflux, 2 h then 0 °C, 1 h; (b) (i) MeOH/NH3, 18 h, rt, (ii) 1, EtOH, reflux, 5 h; (c) ethyl acrylate, P(o-tolyl)3, TEA, Pd(OAc)2, DMF, 100 °C, 18 h; (d) (i) 10% NaOH (aq), EtOH, reflux, 2 h, (ii) 2 M HCl (aq).
General Condensation Procedure
A mixture of the appropriate aldehyde (8.06 mmol), Meldrum’s acid (1) (1.16 g, 8.06 mmol) and piperidinium acetate (29 mg, 0.2 mmol) in ethanol (25 mL) was stirred at room temperature for 20 mins and then heated under reflux for 2 h. The reaction mixture was allowed to cool to room temperature before being stirred at 0 °C for another hour. The solid which precipitated out of solution was filtered off, washed thoroughly with ethanol and dried in vacuo to afford the desired product.
6-Iodocoumarin-3-carboxylic acid (UBP657)
Following the general procedure, 2-hydroxy-5-iodo-benzaldehyde (2.00 g, 8.06 mmol) afforded UBP657 as a yellow solid (1.97 g, 77%); mp: 204-206°C; 1H NMR (400 MHz, DMSO-d6) δ 7.25 (d, J = 8.8 Hz, 1H), 7.99 (d, J = 8.8 & 2.0 Hz, 1H), 8.29 (d, J = 2.0 Hz, 1H), 8.65 (s, 1H), 13.34 (br s, 1H); 13C NMR (100 MHz, DMSO-d6) δ 88.2, 118.3, 119.2, 120.1, 137.8, 141.9, 146.8, 154.0, 156.0, 163.6; HRMS-CI calcd for C10H5IO4 [M + H]+ 316.9311; found 316.9303; Analysis (calcd., found for C10H5IO4): C (38.00, 38.16), H (1.59, 1.94).
6,8-Diiodocoumarin-3-carboxylic acid (UBP658)
Following the general procedure, 3,5-diiodo-2-hydroxybenzaldehyde (3.01 g, 8.06 mmol) afforded UBP658 as a light yellow solid (1.85 g, 52%); mp: >250 °C (lit. 273-274 °C, Bonsignore et al., 2003); 1H NMR (400 MHz, DMSO-d6) δ 8.26 (d, J = 2.0 Hz, 1H), 8.40 (d, J = 2.0 Hz, 1H), 8.56 (s, 1H), 13.39 (br s, 1H); 13C NMR (100 MHz, DMSO-d6) δ 86.2, 89.2, 119.7, 120.5, 138.0, 146.8, 149.1, 153.5, 155.6, 163.4.
Benzo[5,6]coumarin-3-carboxylic acid (UBP659)
Following the general procedure, 2-hydroxy-1-naphthaldehyde (1.39 g, 8.06 mmol) afforded UBP659 as a yellow solid (1.76 g, 91%); mp: 234-237 °C (lit. 236-237 °C, Song et al., 2003); 1H NMR (400 MHz, DMSO-d6) δ 7.56 (d, J = 8.8 Hz, 1H), 7.63 (t, J = 8.0 Hz, 1H), 7.75 (t, J = 8.0 Hz, 1H), 8.05 (d, J = 8.0 Hz, 1H), 8.27 (d, J = 8.8 Hz, 1H), 8.55 (d, J = 8.0 Hz, 1H), 9.32 (s, 1H), 13.31 (br s, 1H); 13C NMR (100 MHz, DMSO-d6) δ 112.0, 116.4, 117.1, 122.2, 126.3, 128.9, 128.9, 128.9, 129.7, 135.7, 143.6, 154.9, 156.7, 164.2.
6-Bromo-4-methylcoumarin-3-carboxylic acid (UBP714)
A stirring solution of 5-bromo-2-hydroxyacetophenone (2) (9.95 g, 46 mmol) in methanol (80 mL) was cooled to 0 °C and ammonia gas bubbled through the solution until it was saturated. The resultant mixture was allowed to warm to room temperature, stirred for 18 h, and then concentrated in vacuo. Meldrum’s acid (7.95 g, 55.2 mmol) and ethanol (150 mL) were added to the residue and the resultant mixture heated under reflux for 5 h. The solution was then allowed to cool to room temperature during which time a solid precipitated out of solution. This was filtered off, washed thoroughly with ethanol, and dried in vacuo to afford UBP714 as a light yellow solid (7.55 g, 58%); mp: 201-203 °C; 1H NMR (400 MHz, DMSO-d6) δ 2.30 (s, 3H), 7.30 (d, J = 8.8 Hz, 1H), 7.67 (d, J = 8.8 & 2.4 Hz, 1H), 7.85 (d, J = 2.4 Hz, 1H), 13.32 (br s, 1H); 13C NMR (100 MHz, DMSO-d6) δ 15.5, 116.0, 118.2, 122.5, 127.4, 130.8, 132.7, 139.5, 150.6, 158.0, 166.4.
Ethyl 6-(2-Ethoxycarbonylethen-1-yl)coumarin-3-carboxylate (4)
A flame dried flask was charged with ethyl-6-bromocoumarin-3-carboxylate (3) (1.50 g, 5.05 mmol), palladium acetate (11.5 mg, 1 mol%), and tri-o-tolylphosphine (62.1 mg, 4 mol%). The flask was then briefly evacuated and backfilled with argon three times. Degassed anhydrous dimethylformamide (25 mL) was then added followed by ethyl acrylate (0.69 mL, 6.31 mmol) and triethylamine (1.76 mL, 12.62 mmol). The resultant mixture was heated at 100 °C overnight. After being allowed to cool to room temperature the reaction mixture was filtered through a celite pad to remove any precipitated Pd(0) and then poured into a stirred solution of ethyl acetate (100 mL), water (100 mL) and aqueous 1 M HCl (10 mL). The organic layer was subsequently isolated and the aqueous phase further extracted with ethyl acetate (2 × 30 mL). The organic extracts were pooled, washed with water (5 × 100 mL), brine (100 mL) and dried over MgSO4. Concentration in vacuo afforded a light orange solid which was re-crystallized from ethanol to afford 4 as an off-white solid (935 mg, 58%); mp: 168-170 °C; 1H NMR (400 MHz, CDCl3) δ 1.35 (t, J = 7.2 Hz, 3H), 1.42 (t, J = 7.2 Hz, 3H), 4.29 (q, J = 7.2 Hz, 2H), 4.43 (q, J = 7.2 Hz, 2H), 6.46 (d, J = 16.0 Hz, 1H), 7.38 (d, J = 8.4 Hz, 1H), 7.69 (d, J = 16.0 Hz, 1H), 7.73 (d, J = 1.6 Hz, 1H), 7.81 (dd, J = 8.4 & 1.6 Hz, 1H), 8.52 (s, 1H); 13C NMR (100 MHz, CDCl3) δ 14.2, 14.3, 60.8, 62.2, 117.6, 118.2, 119.2, 119.8, 129.1, 131.5, 133.0, 141.9, 148.0, 155.9, 156.1, 162.8, 166.4; HRMS-CI calcd for C17H16O6 [M + H]+ 317.1025; found 317.1030; Analysis (calcd., found for C17H16O6): C (64.55, 64.60), H (5.10, 5.35).
6-(2-Carboxyethen-1-yl)coumarin-3-carboxylic acid (UBP656)
To a stirring suspension of 4 (450 mg, 1.42 mmol) in aqueous 10% NaOH (30 mL) was added ethanol (30 mL) to aid dissolution. The resultant solution was refluxed for 2 h before being allowed to cool to room temperature. Acidification to pH 1 using aqueous 2M HCl led to precipitation of a light yellow solid. This suspension was stirred at 0 °C for 45 mins and then filtered to afford UBP656 as a light yellow solid which was dried over P2O5 (362.5 mg, 98%); mp: >250 °C; 1H NMR (400 MHz, D2O/NaOD, pH 11) δ 6.03 (d, J = 16.0 Hz, 1H), 6.49 (d, J = 8.4 Hz, 1H), 7.13 (d, J = 16.0 Hz, 1H), 7.25 (dd, J = 8.4 & 2.4 Hz, 1H), 7.28 (s, 1H), 7.57 (d, J = 2.4 Hz, 1H); 13C NMR (100 MHz, D2O/NaOD, pH 11) δ 117.7, 120.5, 120.8, 124.8, 128.8, 129.1, 130.1, 135.2, 142.4, 169.1, 174.0, 176.9, 178.2; MS (ESI−) m/z: 259 (M-H, 100); Analysis (calcd., found for C13H8O6·1.0H2O): C (56.12, 56.05), H (3.62, 3.24).
2.2 NMDA receptor constructs
GRIN1a cDNA encoding the NMDAR1a subunit (GluN1a) was a generous gift of Dr. Shigetada Nakanishi (Kyoto, Japan) (Moriyoshi et al., 1991). cDNA encoding the GluN2A, GluN2C and GluN2D subunits (GRIN2A, GRIN2C, and GRIN2D) were kindly provided by Dr. Peter Seeburg (Heidelburg, Germany) (Monyer et al., 1992) and the GRIN2B cDNA was the generous gift of Drs. Dolan Pritchett and David Lynch (Philadelphia, USA) (Lynch et al., 1995). Plasmids were linearized with Not I (GRIN1a, GRIN2C, and GRIN2D), EcoR I (GRIN2A) or Sal I (GRIN2B) and transcribed in vitro with T7 (GRIN1a, GRIN2A, GRIN2C, and GRIN2D) and SP6 (GRIN2B) RNA polymerase using the mMessage mMachine transcription kits (Ambion, Austin, TX, USA).
2.3 GluN subunit expression and electrophysiology in Xenopus oocytes
Oocytes from mature female Xenopus laevis (Xenopus One, Ann Arbor, MI, USA) were removed and isolated using procedures approved by the University of Nebraska Medical Center’s Institutional Animal Care and Use Committee in compliance with the National Institutes of Health guidelines. NMDA receptor subunit RNAs were dissolved in sterile distilled H2O. GluN1a and GluN2 RNAs were mixed in a molar ratio of 1:1-3. 50 nl of the final RNA mixture was microinjected (15-30 ng total) into the oocyte cytoplasm. Oocytes were incubated in ND-96 solution for 1-5 days at 17°C prior to electrophysiological assay. Electrophysiological responses were measured using a standard two-microelectrode voltage clamp model OC-725B (Warner Instruments, Hamden, Connecticut,) designed to provide fast clamp of large cells. The recording buffer contained 116 mM NaCl, 2 mM KCl, 0.3 mM BaCl2 and 5 mM HEPES, pH 7.4. Response magnitude was determined by the steady plateau response elicited by bath application of 10 μM L-glutamate plus 10 μM glycine and held at a membrane potential of −60 mV. Response amplitudes for the four heteromeric complexes were generally between 0.1 to 3 μA. After obtaining a steady-state response to agonist application, test compounds were bath applied (Automate Scientific 16-channel perfusion system) and the responses were digitized for analysis (Digidata 1440A and pClamp-10, Molecular Devices). Dose-response relationships were fit to a single-site with variable slope (GraphPad Prism, ISI Software), using a nonlinear regression to calculate IC50 and % maximal inhibition. All experiments were performed at least 4 times. Inhibition values were compared between drugs using ANOVA followed by a Bonferroni test.
2.4 Electophysiological studies on NMDAR- and AMPAR mediated EPSPs in the CA1 region of the hippocampus
Experiments were performed according to national and EU guidelines for animal care on hippocampal slices from adult male Wistar rats (272 ± 20 g, mean ± SD), as described previously (Volianskis and Jensen, 2003). Briefly, transverse hippocampal slices (400 μm) were prepared using a McIllwain tissue chopper. The slices were pre-incubated submerged at room temperature (≈ 20 °C) for at least 2 h before starting the experiments. During the experiments the slices were kept submerged at ≈ 31 °C and superfused at a rate of 3 ml/min with saline solution (in mM: 124 NaCl, 3.5 KCl, 1.25 NaH2PO4, 26 NaHCO3, 2 CaCl2, 2 MgSO4 and 10 glucose), which was saturated with 95% O2 and 5% CO2.
AMPAR-mediated field excitatory postsynaptic potentials (f-EPSPs) were recorded in the CA1-B area of stratum radiatum using glass electrodes filled with saline solution in response to electrical stimulation (100 μs duration) of the Schaffer collaterals. f-EPSPs were evoked at a frequency of 0.067 Hz and the stimulation current was fixed to three times the threshold for evoking the f-EPSPs. After a stable period of at least 30 min (baseline) NMDAR antagonist L-689,560 (5 μM) was applied for 30 min followed by 100 μM UBP714 to examine its effects on AMPAR-mediated responses. To quantify the effects of the compounds on AMPAR-mediated synaptic transmission the initial (≈ 0.8 ms) slopes of AMPAR f-EPSPs were measured and normalised to baseline.
NMDAR-mediated f-EPSPs were recorded after a baseline period of AMPAR responses, which were then blocked with 10 μM NBQX. To evoke NMDAR f-EPSPs the divalent cation ratio in the saline solution was increased (3 CaCl2, 1 MgSO4) and so was the stimulation intensity (2-5 times). After a further stable period of NMDAR mediated f-EPSPs UBP714 was applied, followed by an application of 30 μM D-AP5 at the end of the experiments. The efficacy of NMDAR-mediated synaptic transmission was assessed by subtracting the mean waveform after application of 30 μM D-AP5 from each preceding waveform in the individual experiments, measuring the amplitudes of NMDAR f-EPSPs and normalising them to baseline. Data are presented both as single experiments and as mean values of experimental groups (± S.E.M., n=10 for effect of UBP714 on NMDAR mediated f-EPSPs and n=4 for effect on AMPAR mediated f-EPSPs).
3. Results
3.1 Characterization of coumarin derivatives on recombinant GluN1/GluN2A-D receptors
A series of coumarin-3-carboxylic acid derivatives were evaluated for their ability to inhibit the activation of GluN1/GluN2A-D NMDA receptor responses expressed in Xenopus oocytes using two-electrode voltage clamp at −60 mV. After obtaining a steady-state response to 10 μM L-glutamate and 10 μM glycine, the individual test compounds were then co-applied with the agonists.
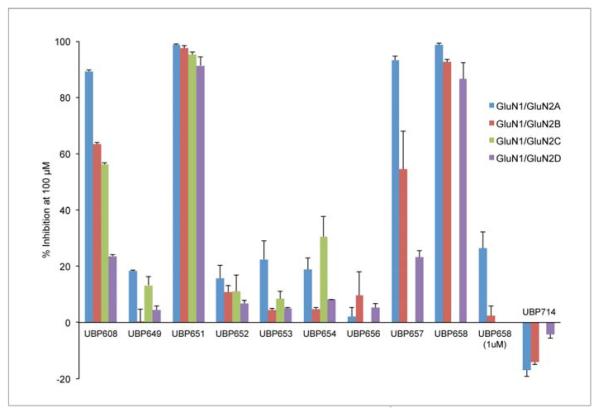
The structures of the compounds tested are shown in Figure 1. In initial studies, the ability of a 100 μM concentration of each compound to inhibit NMDA receptor responses was evaluated (Table 1, Figures 3 and 4). The parent compound of the series, coumarin-3-carboxylic acid (UBP649) displayed very weak antagonist activity at GluN2A and GluN2C containing receptors (approximately 15 – 20% inhibition) and was effectively inactive at GluN2B and GluN2D containing receptors (0 – 5% inhibition). The introduction of either a bromo or iodo substituent at the 6-position of UBP649 (UBP608 and UBP657, respectively) enhanced inhibitory activity, especially at GluN2A-containing receptors (Figure 3). At a concentration of 100 μM, the reference compound UBP608 (data taken from Costa et al., 2010) inhibited approximately 89, 63, 56, and 24% of the GluN2A-D responses. The 6-iodo derivative, UBP657, had similar antagonist activity, inhibiting 93, 55 and 23% of the GluN2A (Figure 4), GluN2B and GluN2D receptor responses, respectively (Figure 3). The difference in inhibitory activity at GluN2A versus GluN2B and GluN2D observed for UBP608 and UBP657 was statistically significant (Table 1). Replacement of the 6-bromo substituent in UBP608 with a more polar 2-carboxyvinyl moiety to give UBP656 reduced activity at GluN2A-D-containing receptors (0 – 10% inhibition). Linking the 5- and 6-positions of UBP649 through a phenyl ring to give UBP659, did not lead to enhanced antagonist activity at GluN2 subunits (Figure 3).
Table 1.
Effect of coumarin derivatives on NMDAR subtypes expressed in Xenopus oocytesa
| Compound | GluN1/GluN2A | GluN1/GluN2B | GluN1/GluN2C | GluN1/GluN2D |
|---|---|---|---|---|
| UBP649 | 18.3 ± 0.4 BD | 0.2 ± 4.7 C | 13.2 ± 3.2 D | 4.4 ± 1.5 |
| UBP608e | 89.3 ± 0.4 BCD | 63.5 ± 0.5 CD | 56.1 ± 0.8 D | 23.6 ± 0.6 |
| UBP657 | 93.3 ± 1.5 BD | 54.5 ± 13.5 D | NDf | 23.1 ± 2.4 |
| UBP659 | 2.9 ± 3.7 B | 14.3 ± 7.9 | ND | 6.9 ± 1.4 |
| UBP651 | 98.6 ± 0.5d | 97.5 ± 0.9 d | 95.2 ± 0.9 | 91.3 ± 3.1 |
| UBP658 | 98.7 ± 0.5 D | 92.6 ± 0.8 | ND | 86.6 ± 5.9 |
| UBP714 | −17.0 ± 2.2 D g | −14.1 ± 1.0 d g | ND | − 4.4 ± 1.2g |
| UBP656 | 2.1 ± 3.1 | 9.6 ± 8.5 | ND | 5.3 ± 1.5 |
| UBP652 | 15.6 ± 4.9 D | 10.7 ± 2.3 | 11.1 ± 6.0 | 6.8 ± 1.1 |
| UBP653 | 22.4 ± 6.7 BCD | 4.3 ± 0.8 | 8.5 ± 2.5 | 5.0 ± 0.2 |
| UBP654 | 18.9 ± 3.9 BCD | 4.8 ± 0.7 C | 30.5 ± 7.1 D | 8.1 ± 0.1 |
% antagonism or potentiation of 100 μM of the compound of responses induced by 10 μM concentrations of both L-glutamate and glycine on the NMDAR subtype indicated. Data represents mean values ± s.e.m., n ≥ 4.
Statistically different from activity on GluN1/GluN2B responses, b, p < 0.05; B, p < 0.01; B, p < 0.001.
Statistically different from activity on GluN1/GluN2C responses, c, p < 0.05; C, p < 0.01; C, p < 0.001.
Statistically different from activity on GluN1/GluN2D responses, d, p < 0.05; D, p < 0.01; D, p < 0.001.
UBP608 included as a reference compound. Data taken from Costa et al., 2010.
ND = not determined.
A negative value indicates that the compound potentiated the NMDAR response.
Figure 3.
Activity of coumarin derivatives on NMDA receptor responses. After obtaining a steady-state response to 10 μM L-glutamate plus 10 μM glycine, each compound was tested at 100 μM for the inhibition or potentiation of responses by GluN1/GluN2A (2A), GluN1/GluN2B (2B), GluN1/GluN2D (2D) and in some cases GluN1/GluN2C (2C) receptors. At a concentration of 1 μM UBP658 inhibited agonist stimulated responses on GluN1/GluN2A and GluN1/GluN2B by 26.3 ± 5.9 and 2.3 ± 3.5 %, respectively. Values represent mean % inhibition ± s.e.m., n ≥ 4.
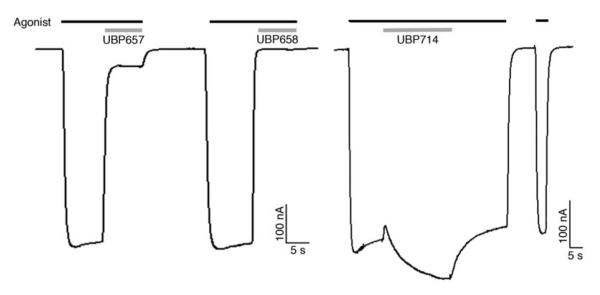
Figure 4.
Representative recordings of inhibition or potentiation of GluN1/GluN2A NMDA receptor responses in Xenopus laevis oocytes. Responses were evoked by 10 μM L-glutamate plus 10 μM glycine (Agonist, black bar) and then 100 μM UBP657, UBP658, or UBP714 was applied as indicated (gray bar).
The introduction of an 8-bromo substituent to UBP608 or an 8-iodo group to UBP657 enhanced antagonist activity at GluN2A-, GluN2B- and GluN2D-containing receptors with UBP651 and UBP658 inhibiting > 85% of the receptor responses (Figures 3 and 4). Introduction of either a 7-hydroxy, 7-methoxy, or 7-diethylamino substituent to UBP649 (UBP652, UBP653, and UBP654, respectively) had little effect on compound activity (Figure 3). At a concentration of 100 μM UBP652 inhibited approximately 16, 11, 11, and 7% of GluN2A-D receptor responses. UBP653 and UBP654 displayed activities that were generally similar to UBP652 and the parent compound UBP649.
Interestingly, the introduction of a methyl substituent to the 4-position of UBP608 altered activity from inhibition to weak potentiation. UBP714 (100 μM) displayed approximately 17, 14, and 4% potentiation of GluN2A (Figure 4), GluN2B and GluN2D responses, respectively (Figure 3). The difference between the potentiating effect of UBP714 on GluN2A and GluN2B versus GluN2D was statistically significant (Table 1).
3.2 Characterization of UBP714 on NMDAR mediated field EPSPs in the CA1 region of the hippocampus
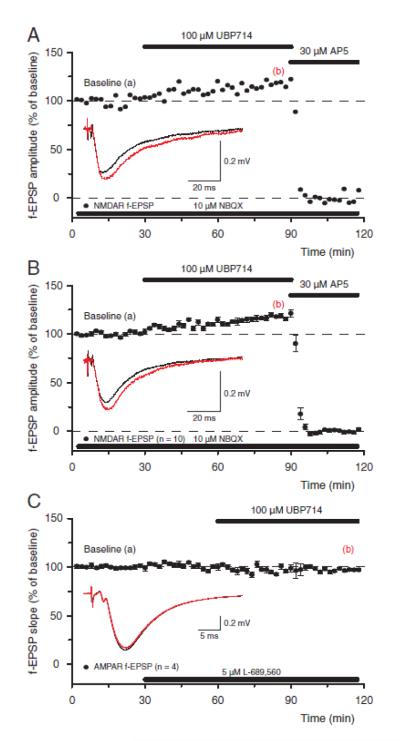
Application of UBP714 in acute hippocampal slices resulted in a potentiation of NMDAR-mediated f-EPSPs, which were evoked in the presence of the AMPA and kainate receptor antagonist NBQX (Figure 5a). More specifically, UBP714 increased the peak of the NMDA response and slowed down its decay without much of an effect on the slope of the response (see inset Figure 5a). Such potentiation of NMDAR-mediated f-EPSPs was very reliable in that it was seen in 10 out of 10 slices and, on average, the amplitudes of NMDA f-EPSPs increased to 118.6 ± 1.5 % of their initial values after 1 h period of UBP714 application (Figure 5b). In contrast, UBP714 did not potentiate the amplitude and did not change the decay of AMPAR-mediated f-EPSPs, which were recorded in the presence of NMDAR antagonist L-689,560 (Figure 5c). Thus, after 1 h period of UBP714 application the slope of AMPAR mediated f-EPSPs was 97.2 ± 3.4 % (n=4) of baseline values.
Figure 5.
UBP714 potentiates native NMDAR responses and has no effect on AMPARmediated f-EPSPs. (a) A single experiment showing that application of 100 μM UBP714 increased the amplitude of NMDAR response (filled circles) to 117.4 % of baseline value whereas subsequent application of 30 μM D-AP5 blocked it. Thick lines indicate the time of compound application. Inset shows f-EPSPs before (black) and 1h after application of UBP714 (red) together with the calibration bar. Please note that UBP714 changes the amplitude of NMDAR f-EPSPs but not their slope. (b) Pooled data from ten experiments showing potentiation of NMDAR f-EPSPs by UBP714 (n=10, mean ± S.E.M.). Inset shows f-EPSPs from another experiment than that shown in panel “a”. (c) A group of experiments, which show that application of 100 μM UBP714 has no effect on AMPAR-mediated f-EPSPs (n=4, mean ± S.E.M.). Inset shows AMPAR f-EPSPs before (black) and 1 h after (red) application of UBP714.
In summary, UBP714 at a concentration of 100 μM is selective between native NMDA and AMPA receptors; it potentiates NMDAR responses and has no effect on AMPARs.
4. Discussion
The development of competitive NMDAR antagonists such as D-AP5 (Evans et al., 1986) and CPP (Aebischer et al., 1989) allowed studies into the functions of these receptors in the CNS and heralded an explosion of interest in the development of such compounds for the treatment of neurological (Lindsley et al., 2006; Sanacora et al., 2008; Wasterlain et al., 2008) and neurodegenerative disorders (Kalia et al., 2008; Villmann et al., 2007). Other sites on the NMDAR were later targeted for drug development leading to the discovery of uncompetitive channel blockers (Lodge and Johnson, 1990), antagonists binding to the glycine site (Leeson and Iversen, 1994), and antagonists that selectively bind to the N-terminal domain of GluN2B such as ifenprodil (Williams, 1993). Few drugs that target these sites have made it into the clinic, though the low affinity ion channel blocker memantine has been licensed for the treatment of moderate-severe Alzheimer’s disease (Kalia et al., 2008). Competitive NMDAR antagonists based on the structure of D-AP5 and CPP were later shown to have the following rank order of affinity when tested on NMDAR subtypes: GluN2A>GluN2B>GluN2C>GluN2D (Buller et al., 1994; Feng et al., 2005). Later, NVP-AAM007 was discovered which was initially reported to show 100-fold selectivity for human GluN2A versus GluN2B (Auberson et al., 2002) but shows 6-10-fold selectivity for rat GluN2A versus GluN2B (Bartlett et al., 2007; Feng et al., 2004). Two competitive antagonists, UBP141 and UBP145, were shown to be 10-17-fold selective for GluN2C/2D versus GluN2A and GluN2B (Costa et al., 2009; Morley et al., 2005).
While developing the competitive GluN2C/GluN2D selective antagonists such as UBP145 we noticed that some of the phenanthrene-3-carboxylic acids used in their synthesis also had NMDAR antagonist activity (Costa et al., 2010). This led to a new class of compounds that were either negative allosteric modulators (NAMs) or positive allosteric modulators (PAMs) of recombinant NMDARs, with different sites of action to previous generations of antagonists (Costa et al., 2010). Structure-activity relationship (SAR) studies revealed that 9-iodophenanthrene-3-carboxylic acid (UBP512) (Figure 1) was a GluN2C/GluN2D antagonist with no activity on GluN2B and weak potentiating activity on GluN2A (Costa et al., 2010). Further SAR studies identified the 6-bromo substituted coumarin derivative, UBP608, as a NAM with weak selectivity for GluN2A versus GluN2B (Costa et al., 2010). Thus, UBP608 had an IC50 value of 19 μM for blocking GluN2A-containing receptors and IC50 values of 90, 68 and 426 μM for blocking NMDARs containing GluN2B, GluN2C or GluN2D, respectively (Costa et al., 2010).
We therefore decided to conduct SAR studies around the coumarin nucleus, using commercially available and newly synthesized derivatives, to identify the structural requirements for antagonism of NMDARs. In the present study we have shown that hydrophobic substituents as large as iodo (UBP657) are well tolerated at the 6-position of the coumarin ring, though a 6-substituent bearing a negatively charged carboxylate group (UBP656) was detrimental to NMDAR antagonist activity. This is consistent with the observation that bromo substitution at the equivalent position on the naphthalene ring of derivatives UBP574 and UBP552 (Figure 1) leads to enhanced NMDAR antagonist activity, though no GluN2 subunit selectivity (Costa et al., 2010). One possible explanation for the weak GluN2A versus GluN2B and GluN2D selectivity of UBP608 and UBP657 is that a hydrogen bond donor such as the 2-hydroxy group of UBP552 is required for optimal GluN2B and GluN2D antagonist activity and replacement with a hydrogen bond acceptor, such as the carbonyl group at the 2-position of the coumarin ring is detrimental to binding to these two subunits but is accommodated by the binding site on GluN2A.
We have determined that the 8-position on the coumarin ring can be substituted with groups as large as iodo to enhance NMDAR antagonist activity, as 6,8-dibromo- (UBP651) and 6,8-diiodo- (UBP658) substituted coumarins have greater NMDAR antagonist potency than the mono substituted 6-bromo (UBP608) or 6-iodo (UBP657) derivatives. However, though addition of a bromo group at the 8-position of UBP608 increases NMDAR antagonist potency, it reduces selectivity for GluN2A. Addition of polar substituents such as hydroxyl (UBP652), methoxy (UBP653) or diethylamino (UBP654) groups at the 7-position of the coumarin ring did not improve NMDAR antagonist activity beyond that observed with the parent compound UBP649. We have reported previously that UBP608 is a negative allosteric modulator (Costa et al., 2010) and it is likely that the UBP608 derivatives described here have the same mode of action, though further experiments will be required to confirm this. Fusing a phenyl ring to the 5- and 6-positions of the coumarin ring to give UBP659 led to weaker NMDAR antagonist activity than that observed for UBP608, perhaps suggesting that substituents at the 5-position are not accommodated by the binding site of the NMDAR. This will need to be confirmed in further SAR studies on 5-substituted coumarin derivatives.
One surprising observation was that UBP714, the 4-methyl-substituted derivative of UBP608, potentiated activity at recombinant NMDARs containing GluN2A or GluN2B and less so at receptors containing GluN2D subunits. We have reported that replacement of the 9-iodo group of UBP512 (Figure 1) with a cyclopropyl or long chain alkyl group leads to compounds with NMDAR potentiating activity (Costa et al., 2010). However, the 4-methyl group on the coumarin ring does not correspond to the 9-position of the phenanthrene ring and so it appears to be a novel site for addition of substituents to produce NMDAR potentiators. The potentiating effect of UBP714 is likely to be due to binding to an allosteric site on the NMDAR; however, further studies will be required to confirm this.
We have shown in previous studies using chimeric GluN2 subunits that the S1 domain rather than the S2 domain of the glutamate binding site has the most influence on the negative allosteric modulatory activity of UBP608 (Costa et al., 2010) In contrast, the GluN2A potentiating activity of UBP512 is influenced mostly by the S2 domain. These studies do not distinguish between the possibilities that the NAMs and PAMs are binding directly to the S1 or S2 domains, respectively, or that these domains are involved in the transduction of their effects. Nonetheless, it is possible that there may be separate binding sites for these NMDAR NAMs and PAMs and this may be the basis of an explanation of the difference in activity of UBP608 and its 4-methyl derivative. Thus, the binding site responsible for the potentiating effect of UBP714 appears to require a hydrophobic substituent at the 4-position of the coumarin ring, whereas this substituent seems not to be accommodated by the binding site, that when occupied leads to inhibitory activity.
It is important to show that UBP714 will not only have a potentiating effect on recombinant NMDARs but also those expressed in neurones. We have shown in this study that application of UBP714 can potentiate field EPSPs generated by NMDARs expressed in the CA1 region of the hippocampus but has little effect on AMPAR-mediated EPSPs in the same brain region. This is an important observation given the role of NMDARs in the hippocampus in mechanisms that are thought to underlie learning and memory (Bliss and Collingridge, 1993; Hrabetova et al., 2000). Enhancement of NMDAR activity in brain areas such as the hippocampus that are involved in learning and memory may be a way of treating cognitive deficits observed in schizophrenia and Alzheimer’s disease.
5. Conclusions
We have shown that coumarins with substituents in the 6- or 6,8-positions are moderately potent NMDAR antagonists, which like UBP608 are likely to be NAMs. Further elaboration of these coumarin derivatives is likely to lead to more potent and GluN2 subunit selective NAMs that may have applicability for the treatment of a range of neurological conditions such as neuropathic pain, epilepsy and depression. Surprisingly, 4-methyl substitution of UBP608 converted this NAM into a NMDAR potentiator (UBP714), showing some selectivity for GluN2A/GluN2B versus GluN2D. UBP714 also potentiated NMDAR mediated EPSPs in the CA1 region of the hippocampus. Thus, UBP714 is a novel template for the development of potent and subunit selective NMDAR potentiators that may have therapeutic applicability in the treatment of schizophrenia and cognitive deficits in neurodegenerative disorders such as Alzheimer’s disease.
Acknowledgements
The authors wish to thank Drs. David Lynch, Shigetada Nakanishi, Pierre Paoletti, Dolan Pritchett, and Peter Seeburg for providing NMDA receptor cDNA constructs. This work was supported by grants from the National Institutes of Health (MH60252) and the UK Medical Research Council (MRC grant number G0601812).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aebischer B, Frey P, Haerter HP, Herrling PL, Mueller WA, Olverman HJ, Watkins JC. Synthesis and NMDA antagonist properties of the enantiomers of 4-(3-phosphonopropyl)piperazine-2-carboxylic acid (CPP) and of the unsaturated analogue (E)-4-(3-phosphonoprop-2-enyl)piperazine-2-carboxylic acid (CPP-ene) Helvetica Chimica Acta. 1989;72:1043–1051. [Google Scholar]
- Arai AC, Kessler M. Pharmacology of ampakine modulators: from AMPA receptors to synapses and behavior. Current drug targets. 2007;8:583–602. doi: 10.2174/138945007780618490. [DOI] [PubMed] [Google Scholar]
- Auberson YP, Allgeier H, Bischoff S, Lingenhoehl K, Moretti R, Schmutz M. 5-Phosphonomethylquinoxalinediones as competitive NMDA receptor antagonists with a preference for the human 1A/2A, rather than 1A/2B receptor composition. Bioorg. Med. Chem. Lett. 2002;12:1099–1102. doi: 10.1016/s0960-894x(02)00074-4. [DOI] [PubMed] [Google Scholar]
- Bartlett TE, Bannister NJ, Collett VJ, Dargan SL, Massey PV, Bortolotto ZA, Fitzjohn SM, Bashir ZI, Collingridge GL, Lodge D. Differential roles of NR2A and NR2B-containing NMDA receptors in LTP and LTD in the CA1 region of two-week old rat hippocampus. Neuropharmacology. 2007;52:60–70. doi: 10.1016/j.neuropharm.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Bettini E, Sava A, Griffante C, Carignani C, Buson A, Capelli AM, et al. Identification and characterisation of novel NMDA receptor antagonists selective for NR2A-over NR2B-containing receptors. J. Pharmacol. Exp. Ther. 2010;335:636–644. doi: 10.1124/jpet.110.172544. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL. A synaptic model of memory - long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bonsignore L, Cottiglia F, Lavagna SM, Loy G, Secci D. Synthesis of coumarin-3-O-acylisoureas by different carbodiimides. Heterocycles. 1999;50:469–478. [Google Scholar]
- Buller AL, Larson HC, Schneider BE, Beaton JA, Morrisett RA, Monaghan DT. The molecular basis of NMDA receptor subtypes: native receptor diversity is predicted by subunit composition. J. Neurosci. 1994;14:5471–5484. doi: 10.1523/JNEUROSCI.14-09-05471.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn JP, Christopoulos A, Lindsley CW. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat. Rev. Drug Discov. 2009;8:41–54. doi: 10.1038/nrd2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa BM, Feng B, Tsintsadze TS, Morley RM, Irvine MW, Tsintsadze V, Lozovaya NA, Jane DE, Monaghan DT. N-methyl-D-aspartate (NMDA) receptor NR2 subunit selectivity of a series of novel piperazine-2,3-dicarboxylate derivatives: preferential blockade of extrasynaptic NMDA receptors in the rat hippocampal CA3-CA1 synapse. J. Pharmacol. Exp. Ther. 2009;331:618–626. doi: 10.1124/jpet.109.156752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa BM, Irvine MW, Fang G, Eaves RJ, Mayo-Martin MB, Skifter DA, Jane DE, Monaghan DT. A novel family of negative and positive allosteric modulators of NMDA receptors. J. Pharmacol. Exp. Ther. 2010;335:614–621. doi: 10.1124/jpet.110.174144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RH, Francis AA, Jones AW, Smith DAS, Watkins JC. The effects of a series of ω-phosphonic α-carboxylic amino acids on electrically evoked and amino acid induced responses in isolated spinal cord preparations. Br. J. Pharmacol. 1982;75:65–75. doi: 10.1111/j.1476-5381.1982.tb08758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B, Morley RM, Jane DE, Monaghan DT. The effect of competitive antagonist chain length on NMDA receptor subunit selectivity. Neuropharmacology. 2005;48:354–359. doi: 10.1016/j.neuropharm.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Feng B, Tse HW, Skifter DA, Morley R, Jane DE, Monaghan DT. Structure-activity analysis of a novel NR2C/NR2D-preferring NMDA receptor antagonist: 1-(phenanthrene-2-carbonyl) piperazine-2,3-dicarboxylic acid. Br. J. Pharmacol. 2004;141:508–516. doi: 10.1038/sj.bjp.0705644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabetova S, Serrano P, Blace N, Tse H-W, Jane DE, Monaghan DT, Sacktor TC. Distinct N-methyl-D-aspartate receptor subpopulations contribute to LTP and LTD induction. J. Neurosci. 2000;20:RC81. doi: 10.1523/JNEUROSCI.20-12-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jane DE, Tse HW, Skifter DA, Christie JM, Monaghan DT. Glutamate receptor ion channels: Activators and inhibitors. In: Endo M, Kurachi Y, Mishina M, editors. Handbook of Experimental Pharmacology: Pharmacology of Ionic Channel Function: Activators and Inhibitors. Springer; Berlin: 2000. pp. 415–478. [Google Scholar]
- Kalia LV, Kalia SK, Salter MW. NMDA receptors in clinical neurology: excitatory times ahead. Lancet Neurol. 2008;7:742–755. doi: 10.1016/S1474-4422(08)70165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosravani H, Zhang Y, Tsutsui S, Hameed S, Altier C, Hamid J, et al. Prion protein attenuates excitotoxicity by inhibiting NMDA receptors. J. Gen. Physiol. 2008;131:i5. doi: 10.1085/JGP1316OIA5. [DOI] [PubMed] [Google Scholar]
- Leeson PD, Iversen LL. The glycine site on the NMDA receptor: structure-activity relationships and therapeutic potential. J. Med. Chem. 1994;37:4053–4067. doi: 10.1021/jm00050a001. [DOI] [PubMed] [Google Scholar]
- Lindsley CW, Shipe WD, Wolkenberg SE, Theberge CR, Williams DL, Jr., Sur C, et al. Progress towards validating the NMDA receptor hypofunction hypothesis of schizophrenia. Curr. Top. Med. Chem. 2006;6:771–785. doi: 10.2174/156802606777057599. [DOI] [PubMed] [Google Scholar]
- Lodge D, Johnson KM. Noncompetitive excitatory amino acid receptor antagonists. Trends Pharmacol. Sci. 1990;11:81–86. doi: 10.1016/0165-6147(90)90323-z. [DOI] [PubMed] [Google Scholar]
- Lynch DR, Lawrence JJ, Lenz S, Anegawa NJ, Dichter M, Pritchett DB. Pharmacological characterization of heterodimeric NMDA receptors composed of NR1a and 2B subunits: differences with receptors formed from NR1a and 2A. J. Neurochem. 1995;64:1462–1468. doi: 10.1046/j.1471-4159.1995.64041462.x. [DOI] [PubMed] [Google Scholar]
- Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, et al. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- Moriyoshi K, Masu M, Ishii T, Shigemoto R, Mizuno N, Nakanishi S. Molecular cloning and characterization of the rat NMDA receptor. Nature. 1991;354:31–37. doi: 10.1038/354031a0. [DOI] [PubMed] [Google Scholar]
- Morley RM, Tse HW, Feng B, Miller JC, Monaghan DT, Jane DE. Synthesis and pharmacology of N1-substituted piperazine-2,3-dicarboxylic acid derivatives acting as NMDA receptor antagonists. J Med Chem. 2005;48:2627–2637. doi: 10.1021/jm0492498. [DOI] [PubMed] [Google Scholar]
- Mosley CA, Acker TM, Hansen KB, Mullasseril P, Andersen KT, Le P, et al. Quinazolin-4-one derivatives: A novel class of noncompetitive NR2C/D subunit-selective N-methyl-D-aspartate receptor antagonists. J. Med. Chem. 2010;53:5476–5490. doi: 10.1021/jm100027p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullasseril P, Hansen KB, Vance KM, Ogden KK, Yuan H, Kurtkaya NL, et al. A subunit-selective potentiator of NR2C- and NR2D-containing NMDA receptors. Nat. Commun. 2010;1:1–8. doi: 10.1038/ncomms1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy RA, Kung HF, Kung MP, Billings J. Synthesis and characterization of iodobenzamide analogues: potential D-2 dopamine receptor imaging agents. J. Med. Chem. 1990;33:171–178. doi: 10.1021/jm00163a029. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Zarate CA, Krystal JH, Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nature Reviews Drug Discovery. 2008;7:426–437. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobolevsky AI, Rosconi MP, Gouaux E. X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature. 2009;462:745–758. doi: 10.1038/nature08624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song A, Wang X, Lam KS. A convenient synthesis of coumarin-3-carboxylic acids via Knoevenagel condensation of Meldrum’s acid with ortho-hydroxyaryl aldehydes or ketones. Tet. Lett. 2003;44:1755–1758. [Google Scholar]
- Sun Y, Olson R, Horning M, Armstrong N, Mayer M, Gouaux E. Mechanism of glutamate receptor desensitization. Nature. 2002;417:245–253. doi: 10.1038/417245a. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, et al. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol. Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villmann C, Becker CM. On the hypes and falls in neuroprotection: targeting the NMDA receptor. Neuroscientist. 2007;13:594–615. doi: 10.1177/1073858406296259. [DOI] [PubMed] [Google Scholar]
- Volianskis A, Jensen MS. Transient and sustained types of long-term potentiation in the CA1 area of the rat hippocampus. J Physiol. 2003;550:459–492. doi: 10.1113/jphysiol.2003.044214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasterlain CG, Chen JW. Mechanistic and pharmacologic aspects of status epilepticus and its treatment with new antiepileptic drugs. Epilepsia. 2008;49:63–73. doi: 10.1111/j.1528-1167.2008.01928.x. [DOI] [PubMed] [Google Scholar]
- Watkins JC, Jane DE. The glutamate story. Br. J. Pharmacol. 2006;147:S100–S108. doi: 10.1038/sj.bjp.0706444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol. Pharmacol. 1993;44:851–859. [PubMed] [Google Scholar]