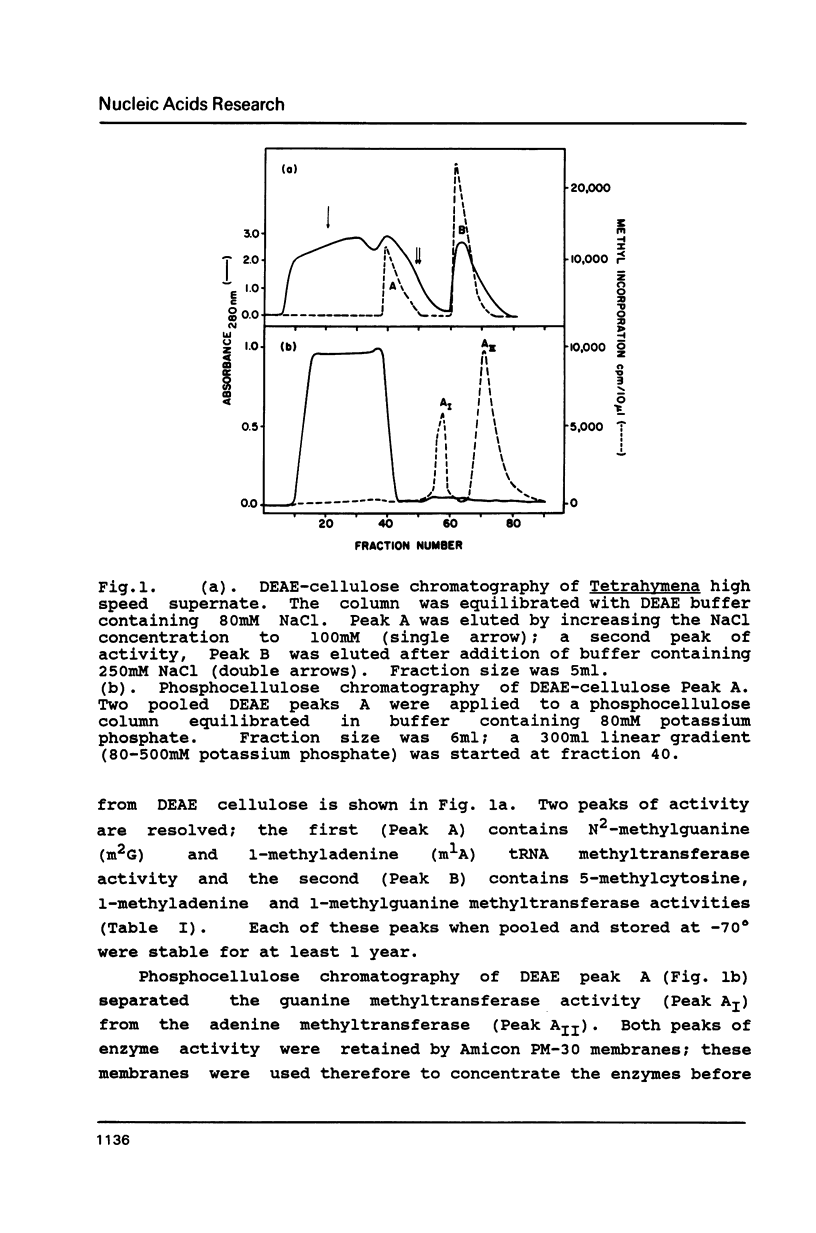
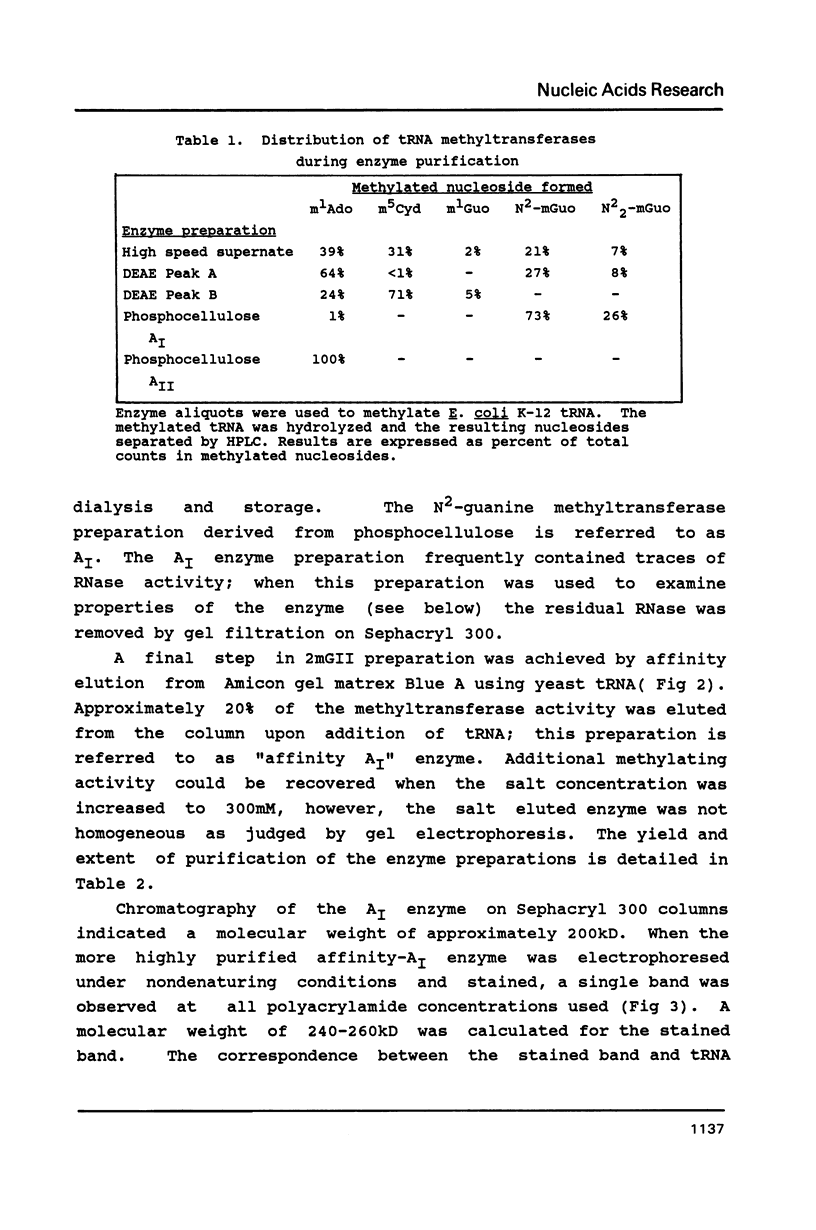
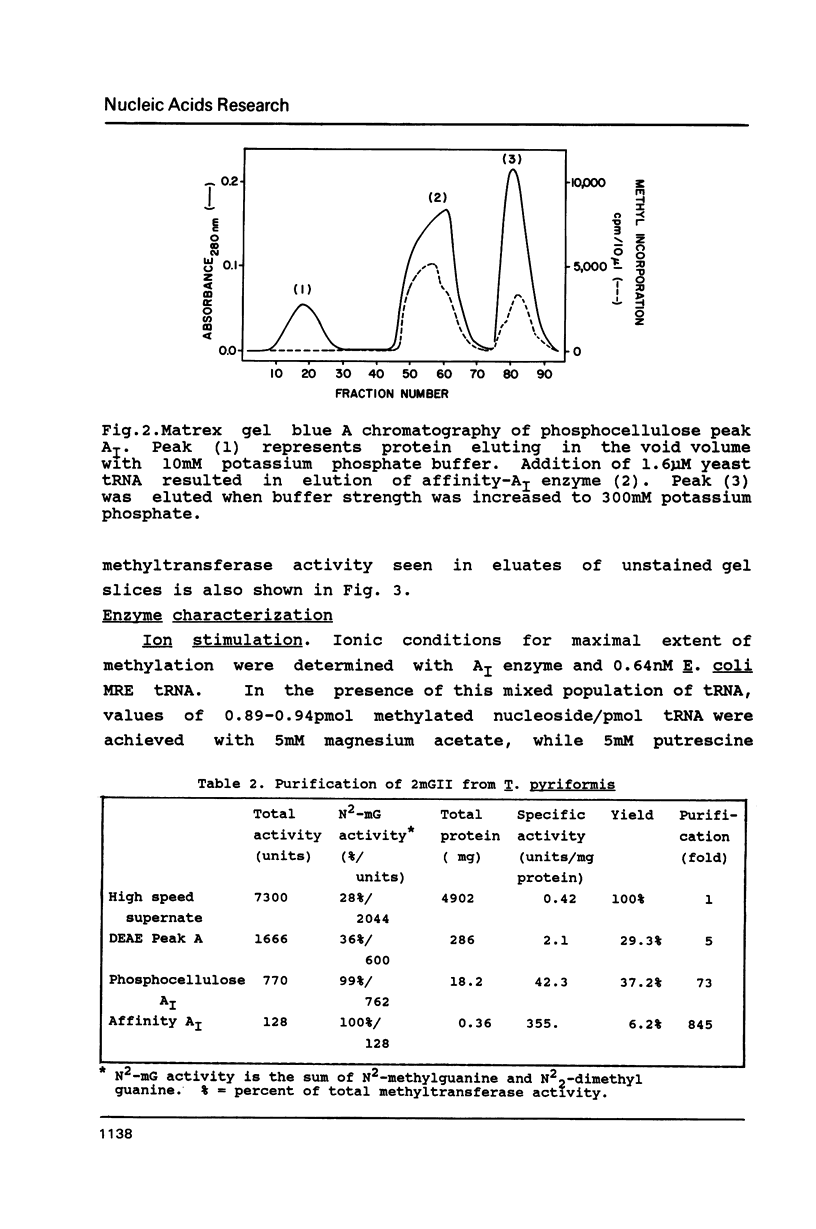
Abstract
A tRNA (guanine-2) methyltransferase has been purified to homogeneity from the protozoan Tetrahymena pyriformis. The enzyme methylates purified E. coli tRNAs which have a guanine residue at position 26 from the 5' end; it also methylates tRNA prepared from the m22G- yeast mutant trm 1. This methyltransferase is therefore equivalent to the guanine methyltransferase 2mGII found in mammalian extracts. The purified 2mGII from Tetrahymena is capable of forming both N2-methylguanine and N22-dimethylguanine on a single tRNA isoaccepting species; under conditions of limiting tRNA or long reaction times the predominant product is dimethylguanine. Analysis of the products formed under varying reaction conditions suggests that dimethylguanine formation is a two step process requiring dissociation of the enzyme-monomethylated tRNA intermediate.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agris P. F., Spremulli L. L., Brown G. M. tRNA methylases from HeLa cells: purification and properties of an adenine-1-methylase and a guanine-N2-methylase. Arch Biochem Biophys. 1974 May;162(1):38–47. doi: 10.1016/0003-9861(74)90102-7. [DOI] [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunke K. J., Leboy P. S. An unusual transfer RNA (guanine-2-)-methyltransferase from transplantable rat mammary tumors. Cancer Res. 1982 Dec;42(12):4979–4984. [PubMed] [Google Scholar]
- Bryan J. K. Molecular weights of protein multimers from polyacrylamide gel electrophoresis. Anal Biochem. 1977 Apr;78(2):513–519. doi: 10.1016/0003-2697(77)90111-7. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- FERGUSON K. A. STARCH-GEL ELECTROPHORESIS--APPLICATION TO THE CLASSIFICATION OF PITUITARY PROTEINS AND POLYPEPTIDES. Metabolism. 1964 Oct;13:SUPPL–SUPPL1002. doi: 10.1016/s0026-0495(64)80018-4. [DOI] [PubMed] [Google Scholar]
- Ginell S. L., Parthasarathy R. Conformation of N2-methylguanosine, a modified nucleoside of tRNA. Biochem Biophys Res Commun. 1978 Oct 30;84(4):886–894. doi: 10.1016/0006-291x(78)91666-2. [DOI] [PubMed] [Google Scholar]
- Glick J. M., Averyhart V. M., Leboy P. S. Purification and characterization of two tRNA-(guanine)-methyltransferases from rat liver. Biochim Biophys Acta. 1978 Mar 29;518(1):158–171. doi: 10.1016/0005-2787(78)90125-9. [DOI] [PubMed] [Google Scholar]
- Glick J. M., Leboy P. S. Purification and properties of tRNA(adenine-1)-methyltransferase from rat liver. J Biol Chem. 1977 Jul 25;252(14):4790–4795. [PubMed] [Google Scholar]
- Greenberg R., Dudock B. Isolation and chracterization of m5U-methyltransferase from Escherichia coli. J Biol Chem. 1980 Sep 10;255(17):8296–8302. [PubMed] [Google Scholar]
- Keith J. M., Winters E. M., Moss B. Purification and characterization of a HeLa cell transfer RNA(cytosine-5-)-methyltransferase. J Biol Chem. 1980 May 25;255(10):4636–4644. [PubMed] [Google Scholar]
- Kersten H. Alteration of tRNA modification in eukaryotes: causes and consequences. Recent Results Cancer Res. 1983;84:255–263. doi: 10.1007/978-3-642-81947-6_19. [DOI] [PubMed] [Google Scholar]
- Kraus J., Staehelin M. N2-guanine specific transfer RNA methyltransferase II from rat liver. Nucleic Acids Res. 1974 Nov;1(11):1479–1496. doi: 10.1093/nar/1.11.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchino Y., Nishimura S. Nucleotide sequence specificities of guanylate residue-specific tRNA methylases from rat liver. Biochem Biophys Res Commun. 1970 Jul 27;40(2):306–313. doi: 10.1016/0006-291x(70)91010-7. [DOI] [PubMed] [Google Scholar]
- Marcu K. B., Dudock B. S. Effect of ribothymidine in specific eukaryotic tRNAs on their efficiency in in vitro protein synthesis. Nature. 1976 May 13;261(5556):159–162. doi: 10.1038/261159a0. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Sites of methylation of purified transfer ribonucleic acid preparations by enzymes from normal tissues and from tumours induced by dimethylnitrosamine and 1,2-dimethylhydrazine. Biochem J. 1974 Feb;137(2):239–248. doi: 10.1042/bj1370239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. H., Kjellin-Stråby K. Studies on microbial ribonucleic acid. IV. Two mutants of Saccharomyces cerevisiae lacking N-2-dimethylguanine in soluble ribonucleic acid. J Mol Biol. 1967 Jun 28;26(3):509–518. doi: 10.1016/0022-2836(67)90318-x. [DOI] [PubMed] [Google Scholar]
- Roe B. A., Tsen H. Y. Role of ribothymidine in mammalian tRNAPhe. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3696–3700. doi: 10.1073/pnas.74.9.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe B., Michael M., Dudock B. Function of N2 methylguanine in phenylalanine transfer RNA. Nat New Biol. 1973 Dec 5;246(153):135–138. doi: 10.1038/newbio246135a0. [DOI] [PubMed] [Google Scholar]
- Salas C. E., Dirheimer G. In vitro methylation of yeast tRNAAsp by rat brain cortical tRNA-(adenine-1) methyltransferase. Nucleic Acids Res. 1979 Mar;6(3):1123–1133. doi: 10.1093/nar/6.3.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Spremulli L. L., Agris P. F., Brown G. M., Rajbhandary U. L. Escherichia coli formylmethionine tRNA: methylation of specific guanine and adenine residues catalyzed by HeLa cells tRNA methylases and the effect of these methylations on its biological properties. Arch Biochem Biophys. 1974 May;162(1):22–37. doi: 10.1016/0003-9861(74)90101-5. [DOI] [PubMed] [Google Scholar]