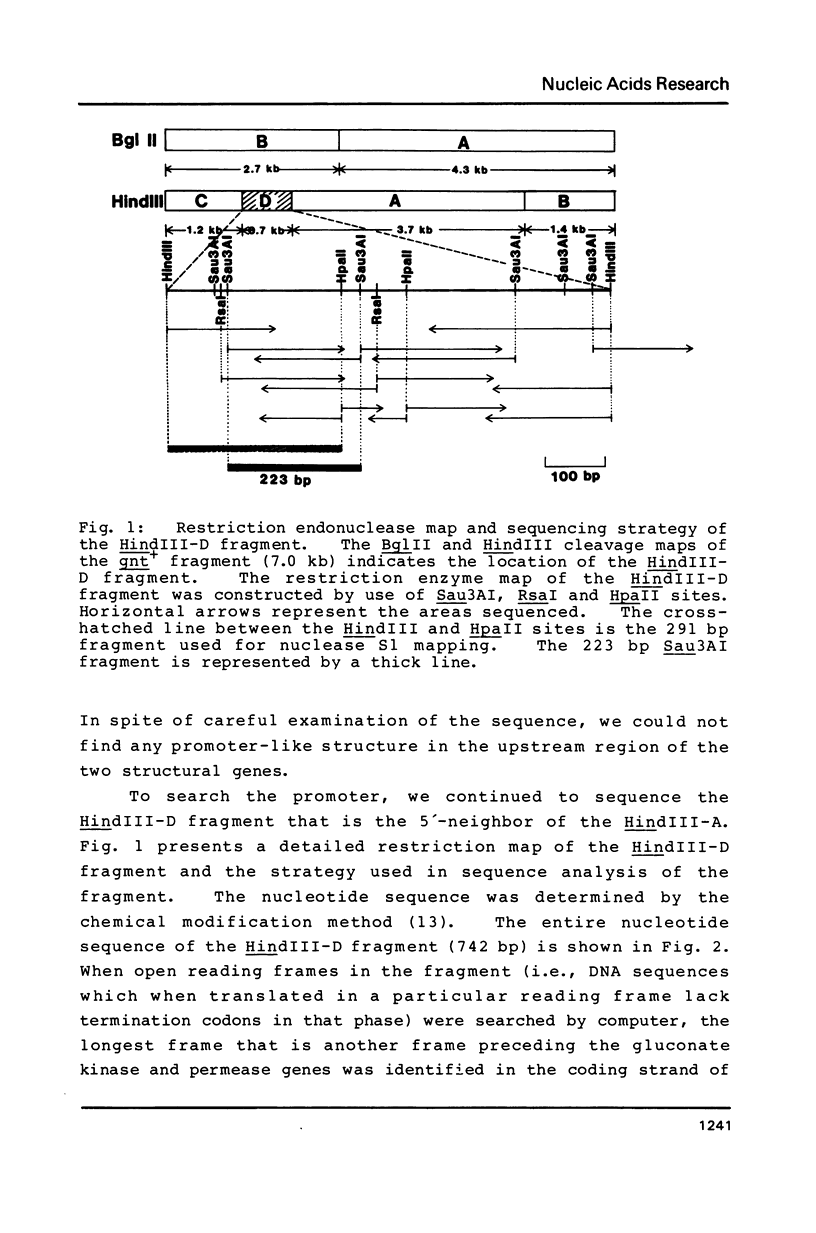
Abstract
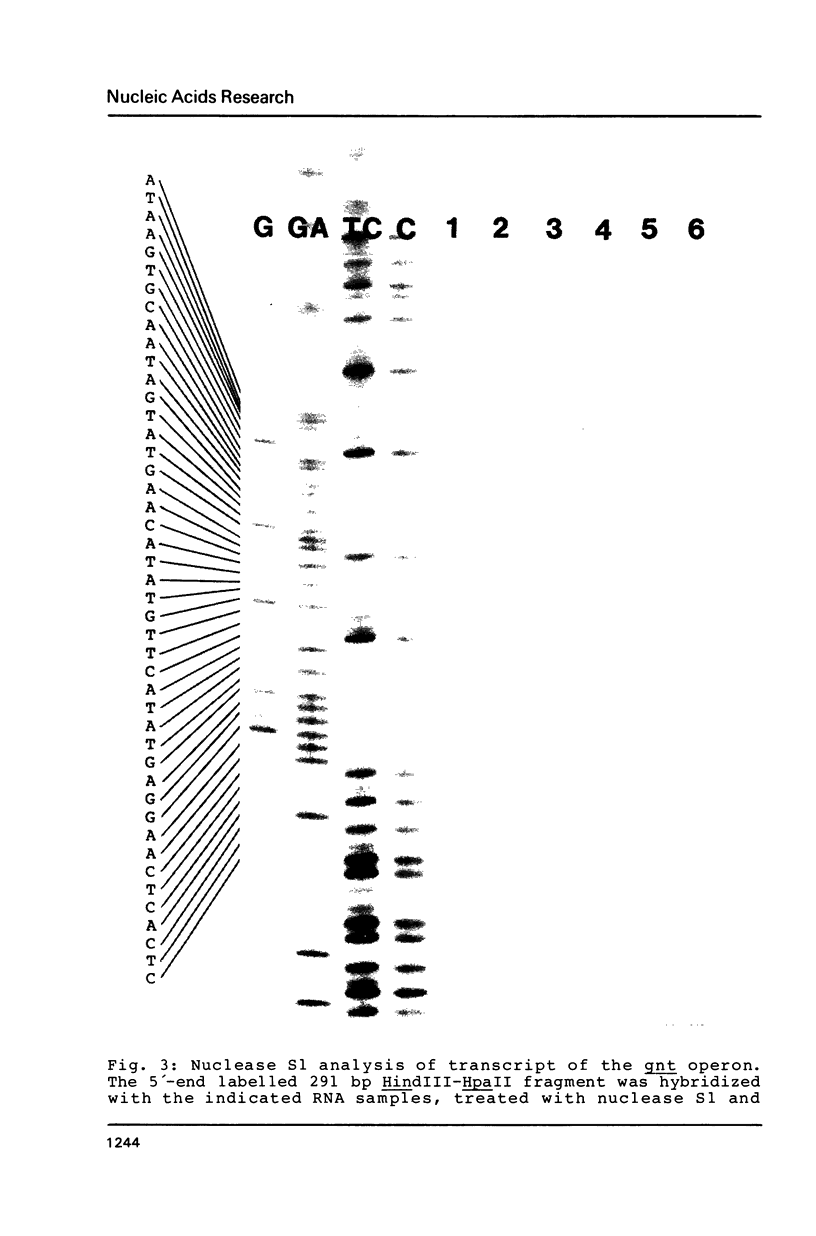
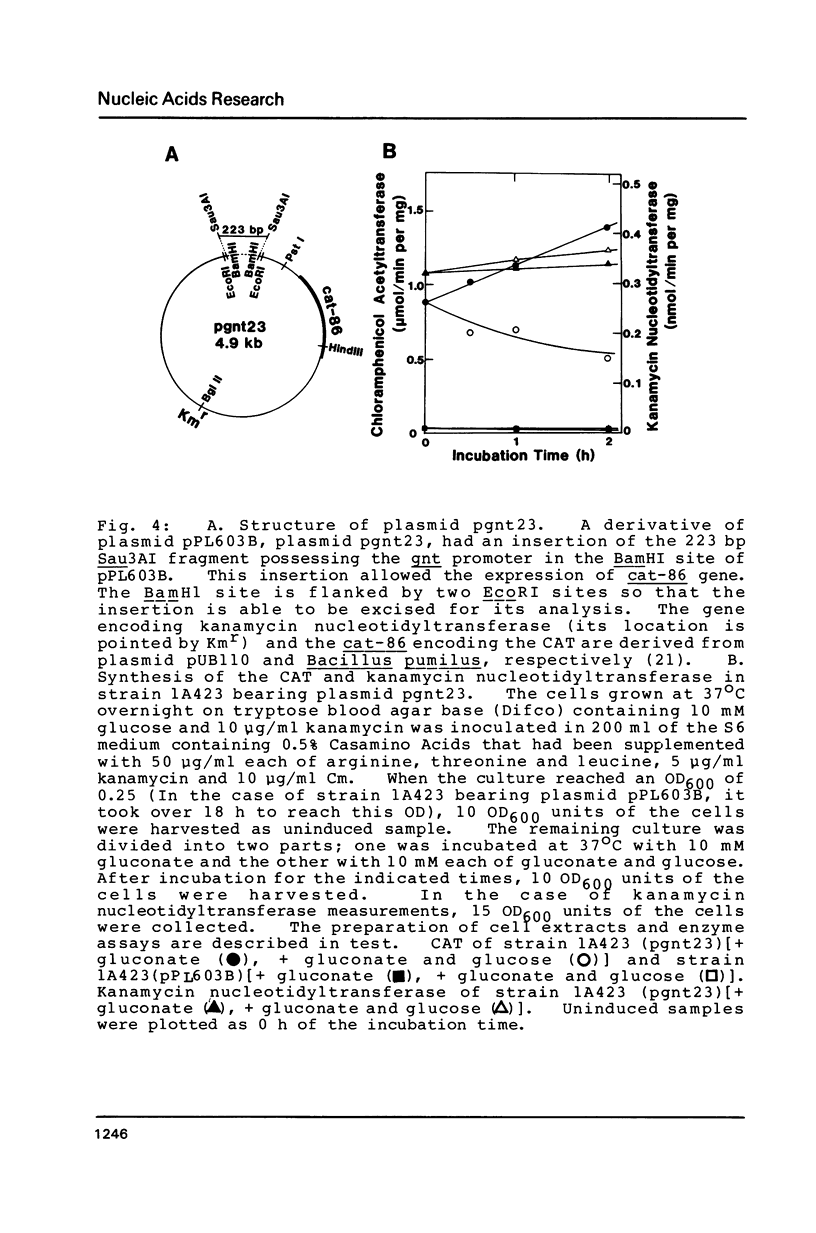
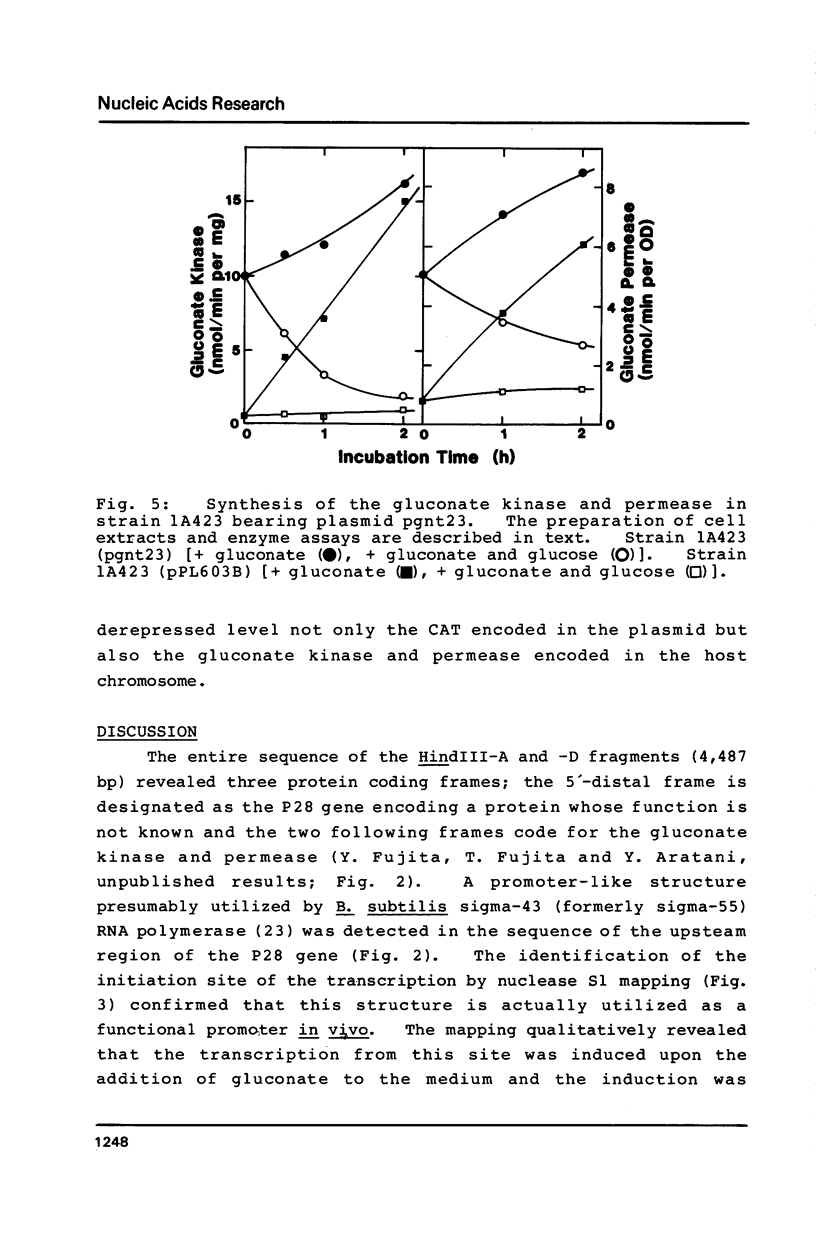
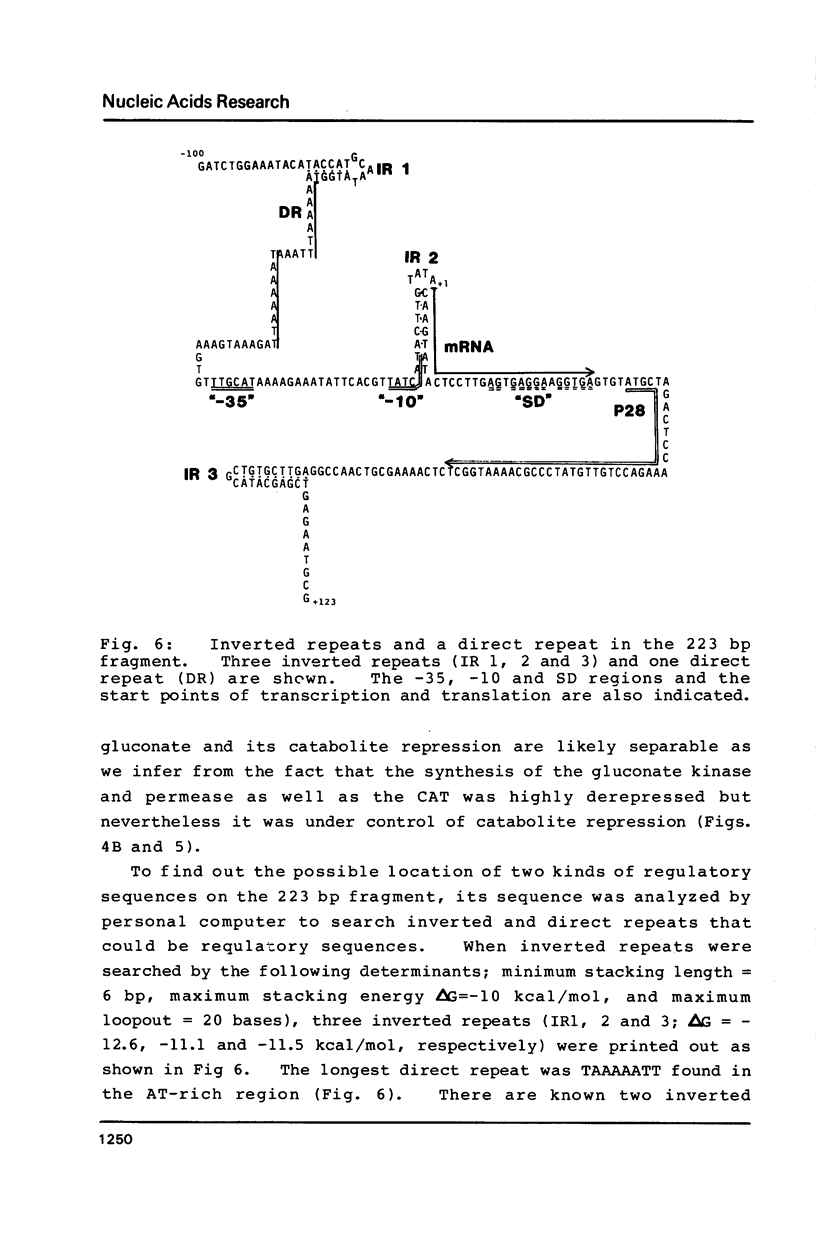
The nucleotide sequence (742 bp) of the promoter region of the Bacillus subtilis gluconate (gnt) operon is presented. Nuclease Sl mapping revealed the start point of the transcription and suggested that the expression of this operon is probably regulated at the transcriptional level. The sequences of the -35 and -10 regions suggested that RNA polymerase possessing sigma-43 may recognize this structure. The 223 bp fragment containing 100 bp upstream from the transcription start site actually exhibited a promoter activity when cloned in a promoter probe vector of pPL603B. This promoter activity was highly derepressed and although still under catabolite repression. The fragment on a high copy plasmid could titrate a regulator of the gnt operon so that the expression of the operon on the host chromosome also became derepressed.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H., Adhya S., de Crombrugghe B. Evidence for two functional gal promoters in intact Escherichia coli cells. J Biol Chem. 1981 Nov 25;256(22):11905–11910. [PubMed] [Google Scholar]
- Banner C. D., Moran C. P., Jr, Losick R. Deletion analysis of a complex promoter for a developmentally regulated gene from Bacillus subtilis. J Mol Biol. 1983 Aug 5;168(2):351–365. doi: 10.1016/s0022-2836(83)80023-0. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Dickson R. C., Abelson J., Barnes W. M., Reznikoff W. S. Genetic regulation: the Lac control region. Science. 1975 Jan 10;187(4171):27–35. doi: 10.1126/science.1088926. [DOI] [PubMed] [Google Scholar]
- Fujita Y., Freese E. Isolation and properties of a Bacillus subtilis mutant unable to produce fructose-bisphosphatase. J Bacteriol. 1981 Feb;145(2):760–767. doi: 10.1128/jb.145.2.760-767.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y., Fujita T. Genetic analysis of a pleiotropic deletion mutation (delta igf) in Bacillus subtilis. J Bacteriol. 1983 May;154(2):864–869. doi: 10.1128/jb.154.2.864-869.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitt M. A., Wang L. F., Doi R. H. A strong sequence homology exists between the major RNA polymerase sigma factors of Bacillus subtilis and Escherichia coli. J Biol Chem. 1985 Jun 25;260(12):7178–7185. [PubMed] [Google Scholar]
- Goldfarb D. S., Doi R. H., Rodriguez R. L. Expression of Tn9-derived chloramphenicol resistance in Bacillus subtilis. Nature. 1981 Sep 24;293(5830):309–311. doi: 10.1038/293309a0. [DOI] [PubMed] [Google Scholar]
- Gryczan T. J., Contente S., Dubnau D. Characterization of Staphylococcus aureus plasmids introduced by transformation into Bacillus subtilis. J Bacteriol. 1978 Apr;134(1):318–329. doi: 10.1128/jb.134.1.318-329.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroyer J., Chang S. The promoter-proximal region of the Bacillus licheniformis penicillinase gene: Nucleotide sequence and predicted leader peptide sequence. Gene. 1981 Dec;15(4):343–347. doi: 10.1016/0378-1119(81)90177-3. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Moran C. P., Jr, Lang N., LeGrice S. F., Lee G., Stephens M., Sonenshein A. L., Pero J., Losick R. Nucleotide sequences that signal the initiation of transcription and translation in Bacillus subtilis. Mol Gen Genet. 1982;186(3):339–346. doi: 10.1007/BF00729452. [DOI] [PubMed] [Google Scholar]
- Nihashi J., Fujita Y. Catabolite repression of inositol dehydrogenase and gluconate kinase syntheses in Bacillus subtilis. Biochim Biophys Acta. 1984 Mar 22;798(1):88–95. doi: 10.1016/0304-4165(84)90014-x. [DOI] [PubMed] [Google Scholar]
- Pastan I., Adhya S. Cyclic adenosine 5'-monophosphate in Escherichia coli. Bacteriol Rev. 1976 Sep;40(3):527–551. doi: 10.1128/br.40.3.527-551.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaie Y., Burtis K. C., Doi R. H. Purification and characterization of a kanamycin nucleotidyltransferase from plasmid pUB110-carrying cells of Bacillus subtilis. J Bacteriol. 1980 Mar;141(3):1178–1182. doi: 10.1128/jb.141.3.1178-1182.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P. Inability of detect cyclic AMP in vegetative or sporulating cells or dormant spores of Bacillus megaterium. Biochem Biophys Res Commun. 1973 May 15;52(2):365–372. doi: 10.1016/0006-291x(73)90720-1. [DOI] [PubMed] [Google Scholar]
- Shaw W. V. Chloramphenicol acetyltransferase from chloramphenicol-resistant bacteria. Methods Enzymol. 1975;43:737–755. doi: 10.1016/0076-6879(75)43141-x. [DOI] [PubMed] [Google Scholar]
- Shibata T., Saito H. Repair of ultraviolet-induced DNA damage in the subcellular systems of Bacillus subtilis. Mutat Res. 1973 Nov;20(2):159–173. doi: 10.1016/0027-5107(73)90186-3. [DOI] [PubMed] [Google Scholar]
- Shimotsu H., Henner D. J. Characterization of the Bacillus subtilis tryptophan promoter region. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6315–6319. doi: 10.1073/pnas.81.20.6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Williams D. M., Duvall E. J., Lovett P. S. Cloning restriction fragments that promote expression of a gene in Bacillus subtilis. J Bacteriol. 1981 Jun;146(3):1162–1165. doi: 10.1128/jb.146.3.1162-1165.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]