Introduction
Phosphoinositides are essential signaling molecules in eukaryotic cells (Fruman et al., 1998; Strahl and Thorner, 2007). These lipids have both intrinsic signaling capabilities, and also serve as reservoirs for production of other second messengers. As general examples of the former case, phosphoinositides form discerning chemical platforms for spatial and temporal regulation of protein activities, and also serve as co-factors that allosterically regulate the activities of various enzymes and ion channels (McLaughlin and Murray, 2005). In the latter case, phosphatidylinositol-4,5-bisphosphate (PtdIns-4,5-P2) is a precursor for the lipid and soluble second messenger molecules diacylglycerol and inositol polyphosphates, respectively.
The metabolic cycle for regenerating the phosphoinositides consumed during the course of signaling is faced with the problem of navigating the intracellular architecture of eukaryotic cells. Phosphatidylinositol (PtdIns), while a minor cellular phospholipid in many eukaryotes (including mammals), is the metabolic precursor for phosphoinositides. PtdIns biosynthesis is catalyzed by a single PtdIns synthase which utilizes inositol and cytidine-diphospho-diacylglycerol as substrates to produce PtdIns and cytidine-monophosphate. CDP-DAG is itself generated from phosphatidic acid (PtdOH) and cytidine-trisphosphate by the enzyme CDP-DAG synthase. Both PtdIns- and CDP-DAG-synthases are integral membrane proteins of the endoplasmic reticulum – a compartment physically separated from the major compartment of PtdIns-4,5-P2 signaling (i.e. the plasma membrane). In a prescient synthesis of existing data, Robert Michell (Birmingham, UK) posited nearly forty years ago that PtdIns-4,5-P2 hydrolysis at the plasma membrane by phospholipase C generates a soluble inositide (now known to be inositol-1,4,5-trisphosphate or IP3) which sets off a trailing wave of calcium signaling (Michell, 1975). This prediction was verified experimentally (Berridge and Irvine, 1984), and extended to diacylglycerol-stimulated signaling via protein kinases C (Nishizuka, 1984).
Of relevance to this discussion, Michell also recognized that his hypothesis raised the question of how are phosphoinositides replenished at the plasma membrane in the face of robust PLC activity? The crux of the matter lies in the assumption that phosphoinositide resynthesis at the plasma membrane requires PtdIns resupply from the ER – that is, the compartment where PtdIns is synthesized. To this end, a cycle was proposed where, in the first stage, soluble lipid carriers ferry either DAG or PtdOH (produced by plasma membrane DAG kinases) from the plasma membrane back to the ER to fuel PtdIns synthesis. This newly synthesized PtdIns is subsequently transported from the ER to the plasma membrane by a second set of lipid carriers, the PtdIns transfer proteins (PITPs). Indeed, PITPs with the expected biochemical properties (classically with dual activities of PtdIns and phosphatidylcholine binding/transfer) have been identified and are highly conserved proteins (Phillips et al., 2006: Cockcroft and Carvou, 2007). It is a testament to the power of the Michell conjecture linking phosphoinositide signaling with PtdIns synthesis and transport that general interpretations of PITP cellular function still borrow directly from his hypothesis (Cockcroft and Carvou, 2007).
Recent work demonstrates that PITPs are unlikely to be bona fide lipid carriers, however, and that these proteins play unanticipated and interesting roles in regulating PtdIns kinase activities (Schaaf et al., 2008; Bankaitis et al., 2010). Sec14, the major yeast PITP, is the founding member of the Sec14 superfamily, and this protein arguably represents the best understood of the PITPs ( Bankaitis et al., 1989; Bankaitis et al., 1990; Cleves et al., 1991a,b; Bankaitis et al., 2010). Current thought describes Sec14 as a regulated scaffold, or nanoreactor, that chaperones an interfacial presentation of PtdIns to PtdIns-kinases. This presentation function provides an essential level of control that stimulates the biologically inadequate activity of PtdIns-kinases on membrane-incorporated PtdIns substrate. Sec14-mediated PtdIns-presentation is cued by Sec14 binding to a second lipid ligand, and this concept describes how the diverse cohort of Sec14-like proteins integrates diverse channels of lipid metabolism with phosphoinositide signaling (Schaaf et al., 2008; Bankaitis et al., 2010). Herein, we describe our perspective regarding what is known about the mechanism of Sec14 function as a single molecule. We also, identify what we consider to be the key questions for future address. This work is not intended to serve as a comprehensive review of the topic, but describes our own perspective of how we see the problem.
Biological Function of Sec14
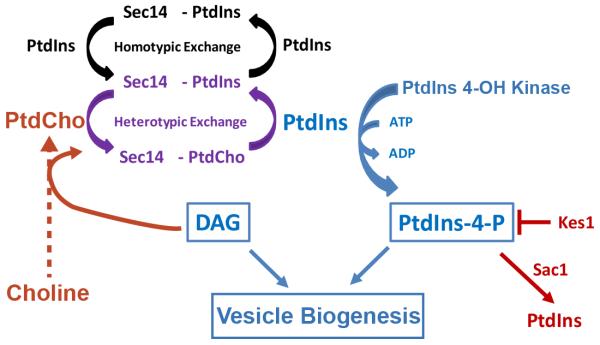
The bulk membrane PtdIns content of Saccharomyces cerevisiae is high, ca. 20-25 mol% of total glycerophospholipid (Strahl and Thorner, 2007). This value is in contrast to PtdIns representing a considerably more minor constituent of mammalian cells (ca. 5% of total glycerophospholipid). Given this natural surfeit of PtdIns, it is not at all clear why yeast would require a PITP-driven plasma membrane PtdIns supply pathway. Yet, Sec14 is essential for cell viability, even under conditions where PtdIns constitutes a remarkable 40 mol% of total membrane phospholipid (Cleves et al., 1991b). The available data, culled largely from studies of ‘bypass Sec14’mutants, argue that the essential biological function for Sec14 is to coordinate lipid metabolism with membrane trafficking in trans-Golgi network (TGN) and endosomal compartments (Cleves et al., 1989; Bankaitis et al., 1990; Cleves et al., 1991a,b; Fang et al., 1996; Li et al., 2002; Mousley et al., 2008). The key lipids involved are PtdCho, PtdIns-4-phosphate (the product of PtdIns 4-OH kinase-mediated activity) and diacylglycerol (Kearns et al., 1997; Hama et al., 1999; Rivas et al., 1999; Bankaitits et al., 2010; Figure 1). Current thought identifies the key regulatory axis as the coordination of PtdCho biosynthesis via the CDP-choline pathway with Sec14-mediated stimulation of PtdIns 4-OH kinase activity (Figure 1).
Figure 1.
Sec14 coordinates lipid metabolism with membrane trafficking. A lipid-regulated vesicle formation pathway is shown. This pathway features diacylglycerol (DAG) and PtdIns-4-P as pro-exocytotic lipids, and its activity is sensitive to flux through the DAG-consuming cytidine-diphosphate (CDP)-choline pathway. Sec14 senses flux by binding newly synthesized PtdCho and thereby becomes activated for initiating heterotypic exchange reactions at the expense of futile PtdIns homotypic exchange reactions. Sec14-mediated heterotypic exchange reactions stimulate PtdIns-4-phosphate production by PtdIns 4-OH kinase. Negative regulators of Sec14p-dependent vesicle budding pathways are highlighted in red -- including the proteins identified by loss-of-function ‘bypass Sec14p’ mutations (enzymes of the CDP-choline PtdCho biosynthetic pathway, the Sac1 PtdIns-4-phosphatase, the sterol and PtdIns-4-P binding protein Kes1p). Positive regulators of Sec14p-dependent vesicle biogenesis pathways are highlighted in blue. The two opposing pathways are regulated by Sec14-mediated heterotypic PtdCho/PtdIns exchange (purple).
How Sec14 binds phospholipids
The question of how Sec14 binds PtdCho and PtdIns was solved by structural studies of the yeast Sec14 homolog Sfh1 in complex with various phospholipid species – including PtdIns and PtdCho (Schaaf et al., 2008). The Sfh1 structural information translates directly to Sec14, and permits formulation of several important conclusions. First, and perhaps most remarkably, Sec14 binds the PtdCho and PtdIns headgroups at physically distinct sites. While the PtdCho headgroup and glycerol backbone moieties are buried deep within the protein’s interior, the corresponding regions of bound PtdIns are positioned much closer to the protein’s surface. Second, rational mutagenesis studies demonstrate PtdCho-binding and PtdIns-binding are both individually required for the biological function of Sec14. A Sec14 molecule must harbor the capacity to bind/exchange both PtdIns- and PtdCho in order to be a biologically active protein that stimulates PtdIns 4-OH kinase activity. What these data demonstrate is that stimulation of lipid kinase activity by Sec14 requires the PITP to undergo heterotypic exchange reactions (e.g. PtdIns for PtdCho or vice versa), and that homotypic exchange reactions (e.g. PtdIns for PtdIns) do not effect a suitable presentation of PtdIns to the PtdIns 4-OH kinase (Figure 1). Shaaf et al (2008) propose a kinetic trap model to account for these results, and an essential component of this hypothesis is that PtdIns and PtdCho traverse different trajectories during lipid exchange into and from the Sec14 hydrophobic pocket. The model also predicts slower kinetics for PtdCho exchange relative to the kinetics of PtdIns exchange.
Another remarkable feature of Sec14/Sfh1 is that minimal conformational adjustments are required for these proteins to bind PtdCho vs PtdIns. Internal H2O flux is the foundation of this particular structural property, and internal H2O also plays an essential role in the conformational dynamics and the energetics associated with lipid exchange (see below). For example, five ordered H2O molecules occupy the ‘empty’ phosphoinositol binding cleft when Sec14/Sfh1 is bound to PtdCho. Reciprocally, two ordered H2O molecules fill the vacant phosphocholine-binding space in the PtdIns-bound protein. The selectivity for PtdIns vs PtdCho is estimated to be small in energetic terms, and H2O rearrangements are sufficient to negotiate the energy barriers that confront heterotypic phospholipid exchange reactions.
Phospholipid-binding bar codes in Sec14 proteins
The structural and functional studies of Sec14/Sfh1 identify PtdIns- and PtdCho-binding signatures at the primary sequence level. These signatures, or bar codes, translate 3-dimensional information into a primary sequence read-out. From a broader perspective, these bar codes drive interesting inferences about the larger Sec14 superfamily. Primary sequence comparisons reveal that the PtdIns-binding bar code is generally conserved among Sec14 superfamily proteins whereas the PtdCho-binding bar code is not (Schaaf et al., 2008; Bankaitis et al., 2010; Nile et al., 2010). This divergence suggests the superfamily has retained PtdIns-binding capability while diversifying the nature of alternate binding ligands. It is therefore proposed that Sec14 superfamily proteins link the metabolism of diverse lipids/lipophilic molecules (i.e. of alternate Sec14-protein ligands such as α-tocopherol, retinaldehyde, PtdCho, etc) with stimulated phosphoinositide synthesis. In this manner, Sec14-like proteins integrate far-flung arms of the lipid metabolome with phosphoinositide signaling.
This concept of primed PtdIns-presentation by Sec14-like proteins/domains offers new insights into the mechanisms of inherited mammalian diseases of with Sec14-like proteins as several naturally occurring disease alleles involve residues of presumptive PtdIns binding bar-codes. Examples include the most common inherited alleles associated with retinal degeneration and acute vitamin E deficiency (reviewed by Nile et al., 2010). We posit that PtdIns binding is the primary defect associated with those specific disease cases, and project that PtdIns is a physiological ligand for the affected Sec14-like proteins (cellular retinadehyde binding protein and α-tocopherol transfer protein, respectively; Schaaf et al., 2008; Bankaitis et al., 2010). Examples of caytaxin and neurofibromin loss-of-function mutations being positioned adjacent to their respective PtdIns-binding bar-codes suggests PtdIns-binding is a biologically important activity for these proteins as well (Nile et al., 2010).
Conformational dynamics of Sec14
The two major conformations of Sec14 are the open form which approximates the conformer(s) involved in lipid exchange on membrane surfaces, and the ‘closed’ form which is stably lipid bound and represents the solution structure of the protein (Sha et al., 1998; Schaaf et al., 2008). The primary difference between the ‘open’ and ‘closed’ conformers is a large (ca. 18 Å) repositioning of a helical gate so that it closes the lipid binding pocket. Cross-linking experiments demonstrate that opening of the helical gate is essential for lipid exchange activity (Ryan et al., 2007).
How are the conformational dynamics that govern the transitions between open and closed conformers controlled? Unrestrained MD simulations derived from a starting structure of the Sec14 ‘open’ conformer have been informative in this regard (Ryan et al., 2007). These simulations model partial closing and opening of the helical gate – that is, the structural element which regulates access to the Sec14 phospholipid binding cavity. These transitions are projected to involve large rigid body motions subject to control by a hinge unit that interfaces with the N- and C-terminal ends of the helical gate. Of particular interest in this regard is the involvement of a gating module (G-module) which transduces conformational information to the hinge and, ultimately, to the helical gate.
Again, from a broader perspective, the importance of the G-module in conformational dynamics of Sec14-like proteins is on display in distant members of the Sec14 protein superfamily such as human vitamin E binding proteins αTTP and supernatant protein factor, the neurofibromin ras GTPase activating protein, and cellular retinaldehyde binding protein (Stocker et al., 2002; Meier et al., 2003; Min et al., 2003; Saari and Crabb, 2005; D’Angelo et al., 2006). α-tocopherol transfer protein, neurofibromin 1, supernatant protein factor, and cellular retinaldehyde binding protein defects result in human disease and a number of these disease alleles are missense mutations within the hinge regions that either flank the helical gate, or within the G-module itself (Ryan et al., 2007). These alleles emphasize the point that the mechanisms and structural elements that underly the conformational dynamics of Sec14-like proteins/domains are likely conserved. Clearly, a major unresolved question remains: how is the activity of the G-module itself regulated so that helical gate dynamics are properly coordinated with phospholipid exchange?
Conformational dynamics as reported by ‘resurrection-of-function’
The molecular dynamics simulations of Ryan et al (2007) are incomplete because there is no consideration for how a phospholipid ligand influences Sec14 conformational transitions. Although this question remains unanswered, some progress comes from an unexpected direction – the isolation and characterization of Sfh1 resurrection mutants that restore Sec14-like activities to a ‘dead’ Sec14-like protein (Schaaf et al., 2011). The directed evolution approach yielded gain-of-function missense substitutions that endow Sfh1 with enhanced Sec14-like capabilities for stimulating PtdIns-4OH kinase activities in vivo. Remarkably, these missense substitutions involve amino acids conserved between Sec14 and Sfh1, with a strong bias for clustering in a hydrophilic microenvironment buried deep within the hydrophobic pocket. This microenvironment resides adjacent to bound phospholipid and to critical elements of the G-module discussed above. Biochemical and biophysical analyses show that these resurrection mutations increase the kinetics of PtdIns and PtdCho exchange in Sfh1. These do so by enhancing the conformational transitions that control helical gate dynamics.
The resurrection mutants further emphasize the importance of internal H2O in the functional dynamics of Sec14 and other Sec14-like proteins. Ordered H2O is evident in the hydrophobic pocket of the closed ligand-bound state of Sfh1, and this is an interesting result given the lack of ordered H2O in the ‘open’ Sec14p conformer (Sha et al., 1998; Phillips et al., 1999; Schaaf et al., 2008). Ordered H2O in the ligand-binding cavity is also recorded in EPR spectra of spin-labeled PtdCho bound to Sec14 that report domains of the sn-2 acyl chain of Sec14-bound PtdCho resides in an unexpectedly hydrophilic, yet solvent-inaccessible, environment (Smirnova et al., 2006; Smirnova et al., 2007). Current ideas propose that bound H2O facilitates the Sec14 conformational transitions required for lipid exchange. It remains to be determined how H2O penetrates into the hydrophobic cavity during lipid exchange, and this question is likely to be an important part of the mechanism for how Sec14 presents PtdIns to lipid kinases.
The PtdIns kinase activation mechanism
The Sec14 nanoreactor mechanism for instructive stimulation of PtdIns 4-OH kinases describes a new paradigm for control of lipid signaling. The human disease alleles in Sec14-like proteins described above portend the broad relevance of this mechanism throughout the Sec14 superfamily, and we find direct functional evidence to this end in plant Sec14-like proteins (Vincent et al., 2005; R. Ghosh and V. Bankaitis, unpublished data). A current challenge is to identify non-PtdCho ligands that prime the PtdIns-presentation functions of other Sec14-likeproteins, and recent progress in crystallization of experimentally tractable yeast Sec14-like proteins forecasts progress in this area (Ren et al., 2011). So precisely how does Sec14 effect a PtdCho-primed presentation of PtdIns to a lipid kinase? This is now the key problem for understanding instructive regulation of PtdIns 4-OH kinase activities. The solution requires a detailed understanding of how Sec14 docks onto membrane surfaces and what are the precise trajectories taken by PtdIns and PtdCho as these enter into, and egress from, the hydrophobic pocket. The rate at which each phospholipid cycles through the hydrophobic pocket is also a critical question. These are challenging questions to address experimentally, and will require application of sophisticated biophysical and computational approaches.
We do know some of the rules, however. For instance, electron spin resonance experiments demonstrate phospholipid cycling through the Sec14 protein interior is a partitioning between two nearly equivalent chemical environments – the Sec14 hydrophobic pocket and the cytoplasmic leaflet of the involved membrane system (Smirnova et al., 2007). The mechanics for how the phospholipids are enticed to choose between the two environments is unknown.
The physical relationship between the PtdIns 4-OH kinase and Sec14 is also a central issue and, while it is most straightforward to envision a direct physical interaction between the enzyme and Sec14, this does not seem to be the simple case. Two lines of evidence argue in this direction. First, no direct interaction between Sec14 and PtdIns 4-OH kinases has been demonstrated. This is negative evidence, however. The second line of evidence is that mammalian/vertebrate PITPs, which have no sequence homology or structural similarity to Sec14, are functional surrogates when expressed in yeast (Skinner et al., 1993; Ile et al., 2010). This powerful demonstration argues there is no dedicated interaction between the PITP and the kinase, and perhaps all the kinase registers is the ‘presented’ headgroup. Such a model again places a premium on understanding the orientation of PITP and bound phospholipids to the membrane surface during the exchange reaction.
Acknowledgments
This work was supported by National Institutes of Health grant RO1 GM44530 and RO1 GM081774 to VAB. JR is supported by a Postdoctoral Fellowship of the American Heart Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bankaitis VA, Malehorn DE, Emr SD, Greene R. The Saccharomyces cerevisiae SEC14 gene encodes a cytosolic factor that is required for transport of secretory proteins from the yeast Golgi complex. J. Cell Biol. 1989;108:1271–1281. doi: 10.1083/jcb.108.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankaitis VA, Aitken JR, Cleves AE, Dowhan W. An essential role for a phospholipid transfer protein in yeast Golgi function. Nature. 1990;347:561–562. doi: 10.1038/347561a0. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Irvine RF. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984;312:315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Cleves AE, McGee TP, Bankaitis VA. Phospholipid transfer proteins: a biological debut. Trends Cell Biol. 1991a;1:30–34. doi: 10.1016/0962-8924(91)90067-j. [DOI] [PubMed] [Google Scholar]
- Cleves AE, McGee TP, Whitters EA, Champion KM, Aitken JR, Dowhan W, Goebl M, Bankaitis VA. Mutations in the CDP-choline pathway for phospholipid biosynthesis bypass the requirement for an essential phospholipid transfer protein. Cell. 1991b;64:789–800. doi: 10.1016/0092-8674(91)90508-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S, Carvou N. Biochemical and biological functions of class I phosphatidylinositol transfer proteins. Biochim Biophys Acta. 2007;1771:677–691. doi: 10.1016/j.bbalip.2007.03.009. [DOI] [PubMed] [Google Scholar]
- D’Angelo I, Welti S, Bonneau F, Scheffzek K. A novel bipartite PL-binding module in the neurofibromatosis type 1 protein. EMBO Rep. 2006;7:174–179. doi: 10.1038/sj.embor.7400602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M, Kearns BG, Gedvilaite A, Kagiwada S, Kearns M, Fung MKY, Bankaitis VA. Kes1p shares homology with human oxysterol binding protein and participates in a novel regulatory pathway for yeast Golgi-derived transport vesicle biogenesis. EMBO J. 1996;15:6447–6459. [PMC free article] [PubMed] [Google Scholar]
- Fruman DA, Meyers RE, Cantley LC. Phosphoinositide kinases. Annu. Rev. Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- Hama H, Schnieders EA, Thorner J, Takemoto JY, DeWald D. Direct involvement of phosphatidylinositol-4-phosphate in secretion in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 1999;274:34294–34301. doi: 10.1074/jbc.274.48.34294. [DOI] [PubMed] [Google Scholar]
- Ile KE, Kassen S, Cao C, Vihtehlic T, Shah SD, Huijbregts RPH, Alb JG, Jr., Stearns GW, Brockerhoff SE, Hyde DR, Bankaitis VA. The zebrafish class 1 phosphatidylinositol transfer protein family: PITPβ isoforms and double cone cell outer segment integrity in retina. Traffic. 2010;11:1151–1167. doi: 10.1111/j.1600-0854.2010.01085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ile KE, Schaaf G, Bankaitis VA. Phosphatidylinositol transfer proteins and cellular nanoreactors for lipid signaling. Nature Chem. Biol. 2006;2:576–583. doi: 10.1038/nchembio835. [DOI] [PubMed] [Google Scholar]
- Kearns BG, McGee TP, Mayinger P, Gedvilaite A, Phillips SE, Kagiwada S, Bankaitis VA. Essential role for diacylglycerol in protein transport from the yeast Golgi complex. Nature. 1997;387:101–105. doi: 10.1038/387101a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Rivas MP, Fang M, Marchena J, Mehotra B, Chaudhary A, Feng L, Prestwich GD, Bankaitis VA. Analysis of oxysterol binding protein homologue Kes1p function in regulation of Sec14p-dependent protein transport from the yeast Golgi complex. J Cell Biol. 2002;157:63–77. doi: 10.1083/jcb.200201037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S, Murray D. Plasma membrane phosphoinositide organization by protein electrostatics. Nature. 2005;438:605–611. doi: 10.1038/nature04398. [DOI] [PubMed] [Google Scholar]
- Meier R, Tomizaki T, Schulze-Briese C, Baumann U, Stocker A. The molecular basis of vitamin E retention: structure of human tocopherol transfer protein. J. Mol. Biol. 2003;331:725–734. doi: 10.1016/s0022-2836(03)00724-1. [DOI] [PubMed] [Google Scholar]
- Michell RH. Inositol phospholipids and cell surface receptor function. Biochim. Biophys. Acta. 1975;415:81–147. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Min KC, Kovall RA, Hendrickson WA. Crystal structure of α-tocopherol transfer protein bound to its ligand: Implications for ataxia with vitamin E deficiency. Proc. Natl. Acad. Sci. 2003;100:14713–14718. doi: 10.1073/pnas.2136684100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousley CJ, Tyeryar K, Ile KE, Schaaf G, Brost RL, Boone C, Guan X, Wenk MR, Bankaitis VA. Trans-Golgi network and endosome dynamics connect ceramide homeostasis with regulation of the unfolded protein response and TOR signaling in yeast. Mol. Biol. Cell. 2008;19:4785–4803. doi: 10.1091/mbc.E08-04-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nile AH, Bankaitis VA, Grabon A. Mammalian diseases of phosphatidylinositol transfer proteins and their homologs. Clinical Lipidology. 2010;5:867–897. doi: 10.2217/clp.10.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984;308:693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Phillips SE, Sha B, Topalof L, Xie Z, Alb JG, Klenchin VA, Swigart P, Cockcroft S, Martin TFJ, Luo M, Bankaitis VA. Yeast Sec14p deficient in phosphatidylinositol transfer activity is functional in vivo. Mol. Cell. 1999;4:187–197. doi: 10.1016/s1097-2765(00)80366-4. [DOI] [PubMed] [Google Scholar]
- Phillips SE, Vincent P, Rizzieri K, Schaaf G, Gaucher EA, Bankaitis VA. The diverse biological functions of phosphatidylinositol transfer proteins in eukaryotes. Crit. Rev. in Bioch. & Mol. Biol. 2006;41:1–28. doi: 10.1080/10409230500519573. [DOI] [PubMed] [Google Scholar]
- Ren J, Ortlund E, Schaaf G, Bankaitis VA, Pathak M. Crystallization and preliminary X-ray diffraction analysis of the nonclassical yeast Sec14-like phosphatidylinositol transfer protein Sfh3. Acta Crystallographica F. 2011;F67:1239–1243. doi: 10.1107/S1744309111027096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas MP, Kearns BG, Xie Z, Guo S, Sekar MC, Hosaka K, Kagiwada S, York JD, Bankaitis VA. Relationship between altered phospholipid metabolism, diacylglycerol, ‘bypass Sec14’, and the inositol auxotrophy of yeast sac1 mutants. Mol. Biol. Cell. 1999;10:2235–2250. doi: 10.1091/mbc.10.7.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MM, Temple BRS, Phillips SE, Bankaitis VA. Conformational dynamics of the major yeast phosphatidylinositol transfer protein Sec14p: Insights into the mechanisms of phospholipid exchange and diseases of Sec14p-like protein deficiencies. Mol. Biol. Cell. 2007;18:1928–1942. doi: 10.1091/mbc.E06-11-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saari JC, Crabb JW. Focus on molecules: cellular retinaldehyde-binding protein (CRALBP) Exp. Eye Res. 2005;81:245–246. doi: 10.1016/j.exer.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Schaaf G, Dynowski M, Mousley CJ, Shah SD, Yuan P, Winklbauer E, de Campos MKF, Trettin K, Quinones M-C, Smirnova T, Yanagisawa LL, Ortlund E, Bankaitis VA. Resurrection of a functional phosphatidylinositol transfer protein from a pseudo-Sec14 scaffold by directed evolution. Mol. Biol. Cell. 2011;22:892–905. doi: 10.1091/mbc.E10-11-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaf G, Ortlund E, Tyeryar K, Mousley C, Ile K, Woolls M, Garrett T, Raetz CRH, Redinbo M, Bankaitis VA. The functional anatomy of phospholipid binding and regulation of phosphoinositide homeostasis by proteins of the Sec14-superfamily. Molecular Cell. 2008;29:191–206. doi: 10.1016/j.molcel.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha B, Phillips SE, Bankaitis VA, Luo M. Crystal structure of the Saccharomyces cerevisiae phosphatidylinositol transfer protein Sec14p. Nature. 1998;391:506–510. doi: 10.1038/35179. [DOI] [PubMed] [Google Scholar]
- Skinner HB, Alb JG, Whitters EA, Helmkamp GM, Jr., Bankaitis VA. Phospholipid transfer activity is relevant to but not sufficient for the essential function of the yeast SEC14 gene product. EMBO J. 1993;I:4775–4784. doi: 10.1002/j.1460-2075.1993.tb06166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova T, Chadwick TG, MacArthur R, Poluekov O, Song L, Ryan M, Schaaf G, Bankaitis VA. The chemistry of phospholipid binding by the Saccharomyces cerevisiae phosphatidylinositol transfer protein Sec14p as determined by electron paramagnetic resonance spectroscopy. J. Biol. Chem. 2006;281:34897–34908. doi: 10.1074/jbc.M603054200. [DOI] [PubMed] [Google Scholar]
- Smirnova T, Chadwick TG, van Tol J, Ozarowski A, Poluektov O, Schaaf G, Ryan MM, Bankaitis VA. Local polarity and hydrogen bonding inside the Sec14p phospholipid-binding cavity: High-field multifrequency electron paramagnetic studies. Biophys. J. 2007;92:3686–3695. doi: 10.1529/biophysj.106.097899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker A, Tomizaki T, Schulze-Briese C, Baumann U. Crystal structure of the human supernatant protein factor. Structure. 2002;10:1533–1540. doi: 10.1016/s0969-2126(02)00884-5. [DOI] [PubMed] [Google Scholar]
- Strahl T, Thorner J. Synthesis and function of membrane phosphoinositides in budding yeast, Saccharomyces cerevisiae. Biochim. Biophys. Acta. 2007;1771:353–404. doi: 10.1016/j.bbalip.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]