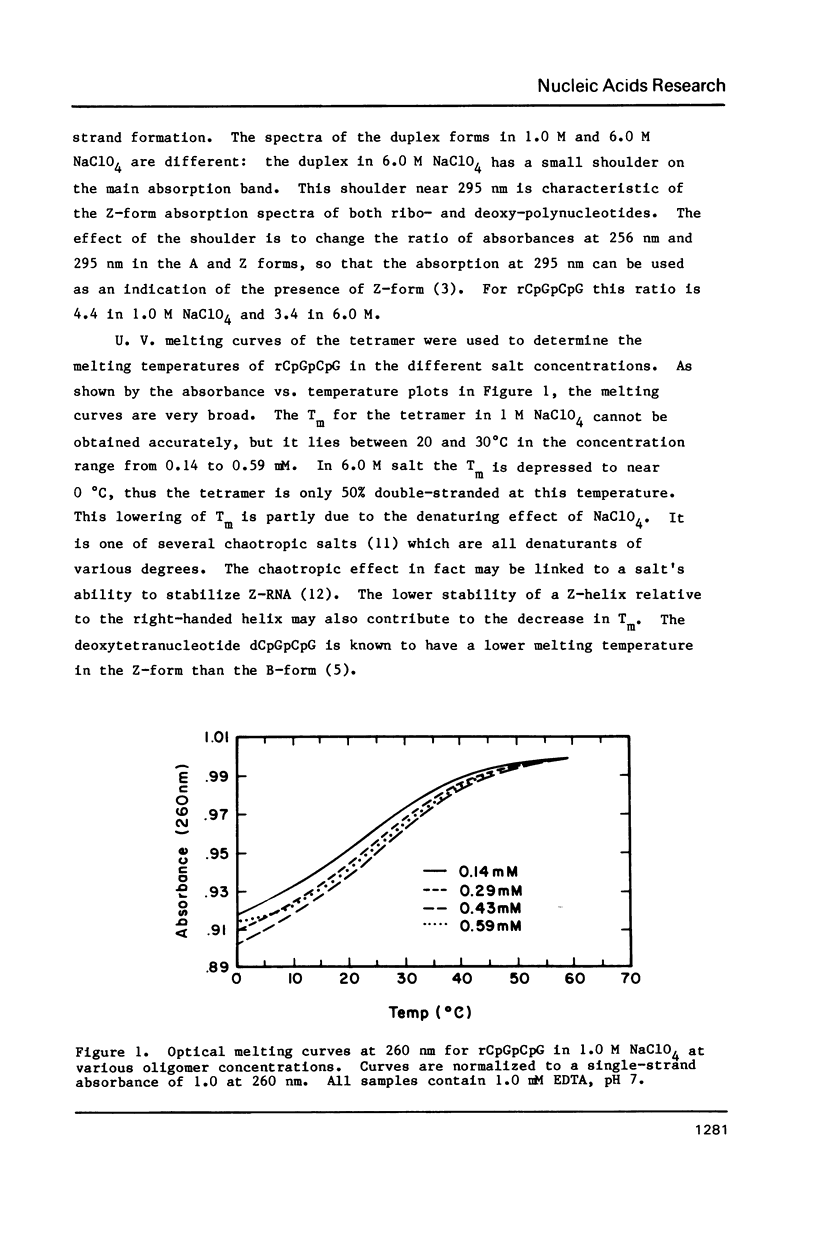
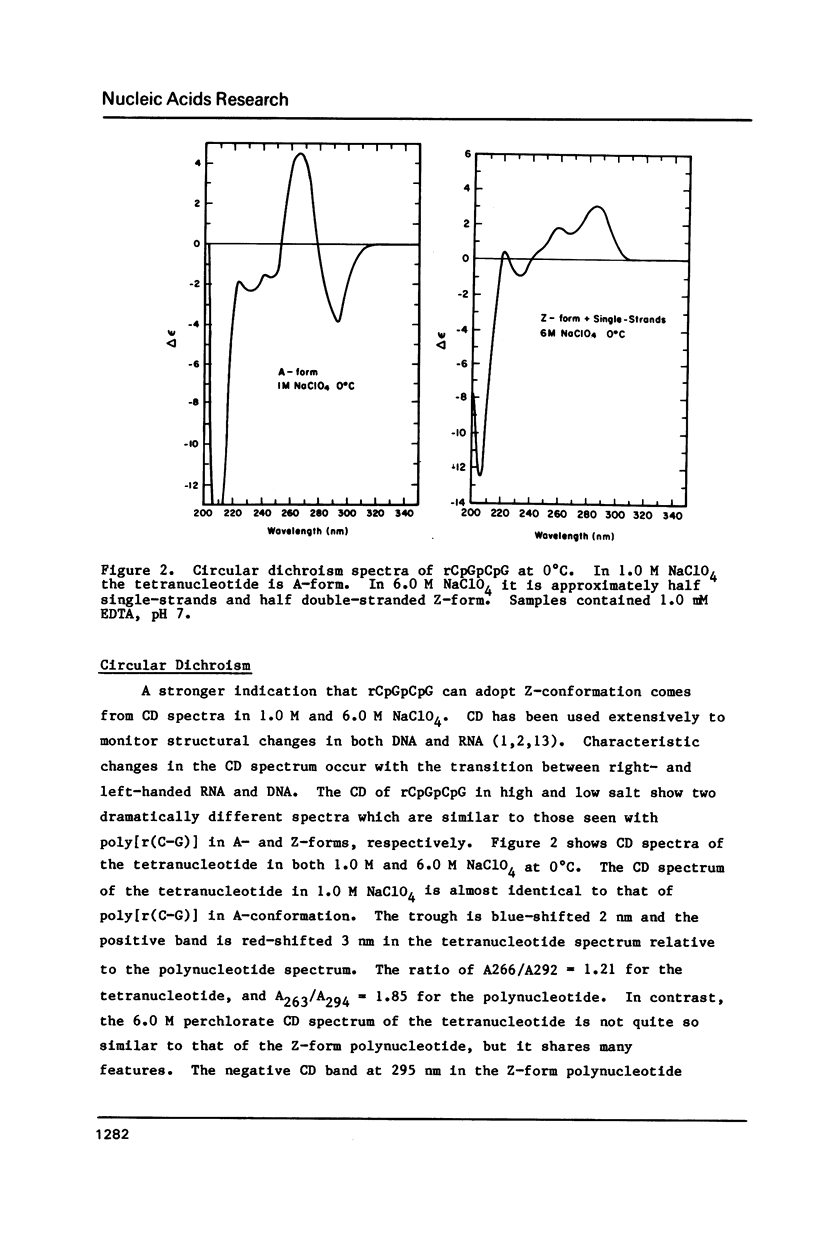
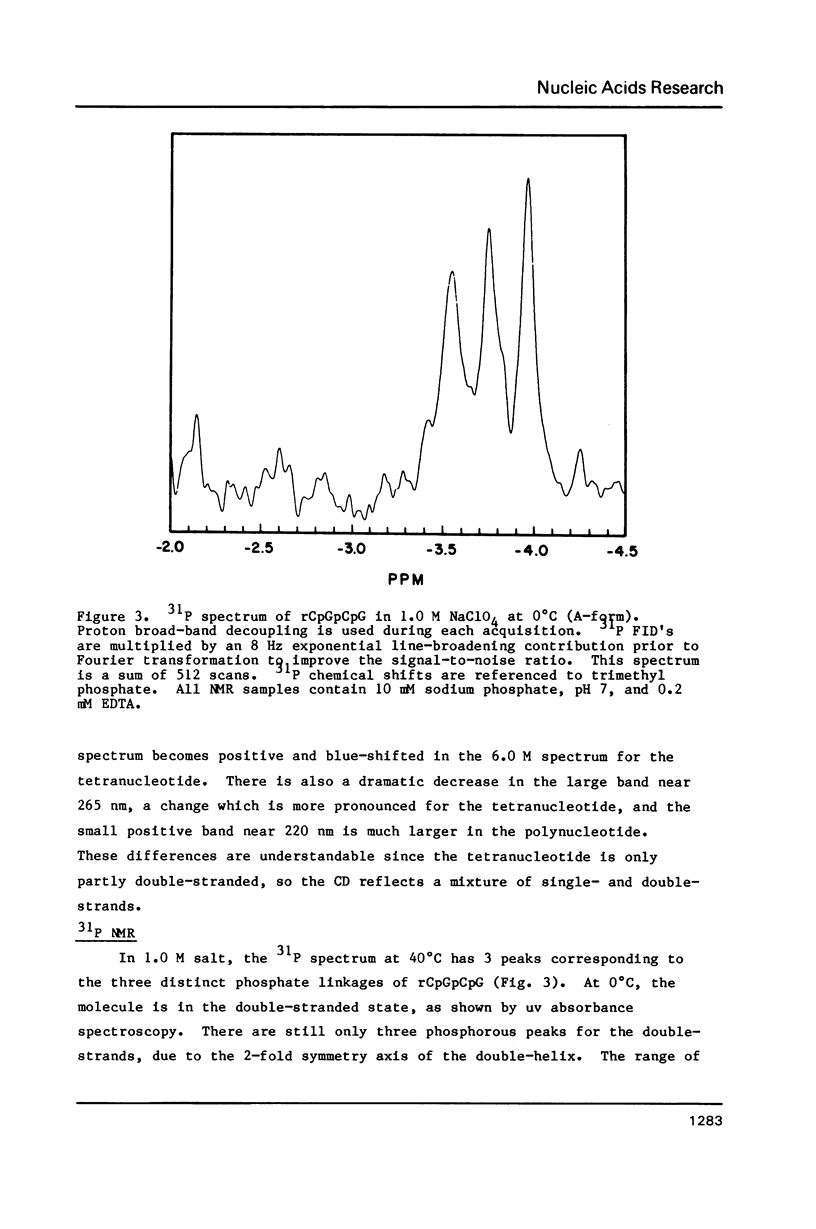
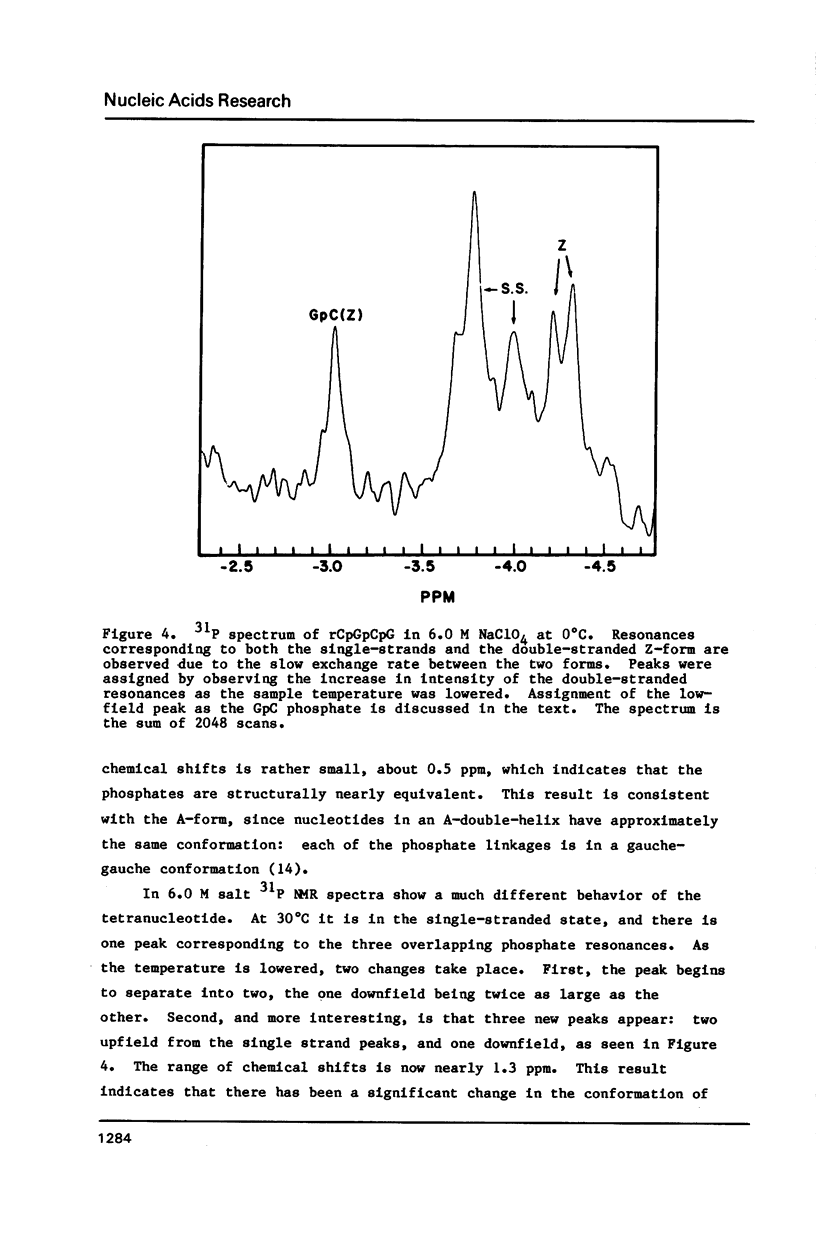
Abstract
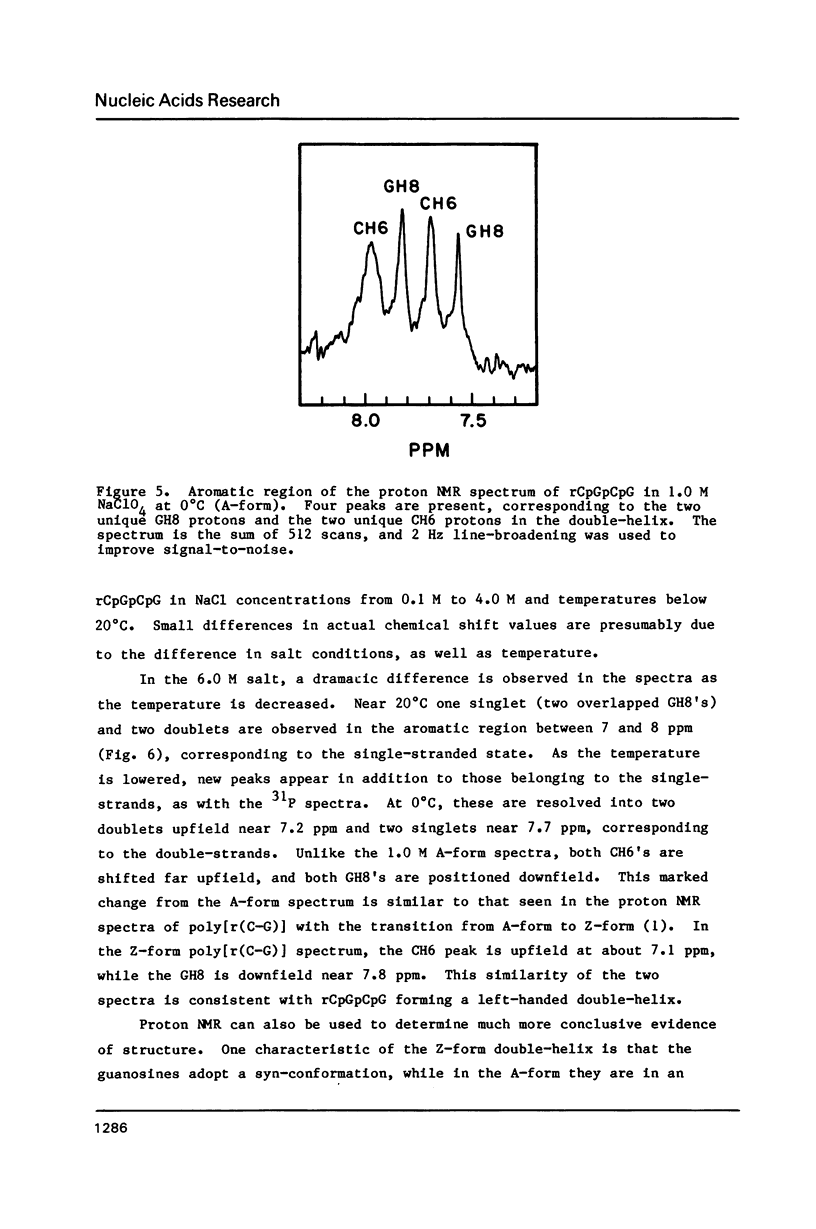
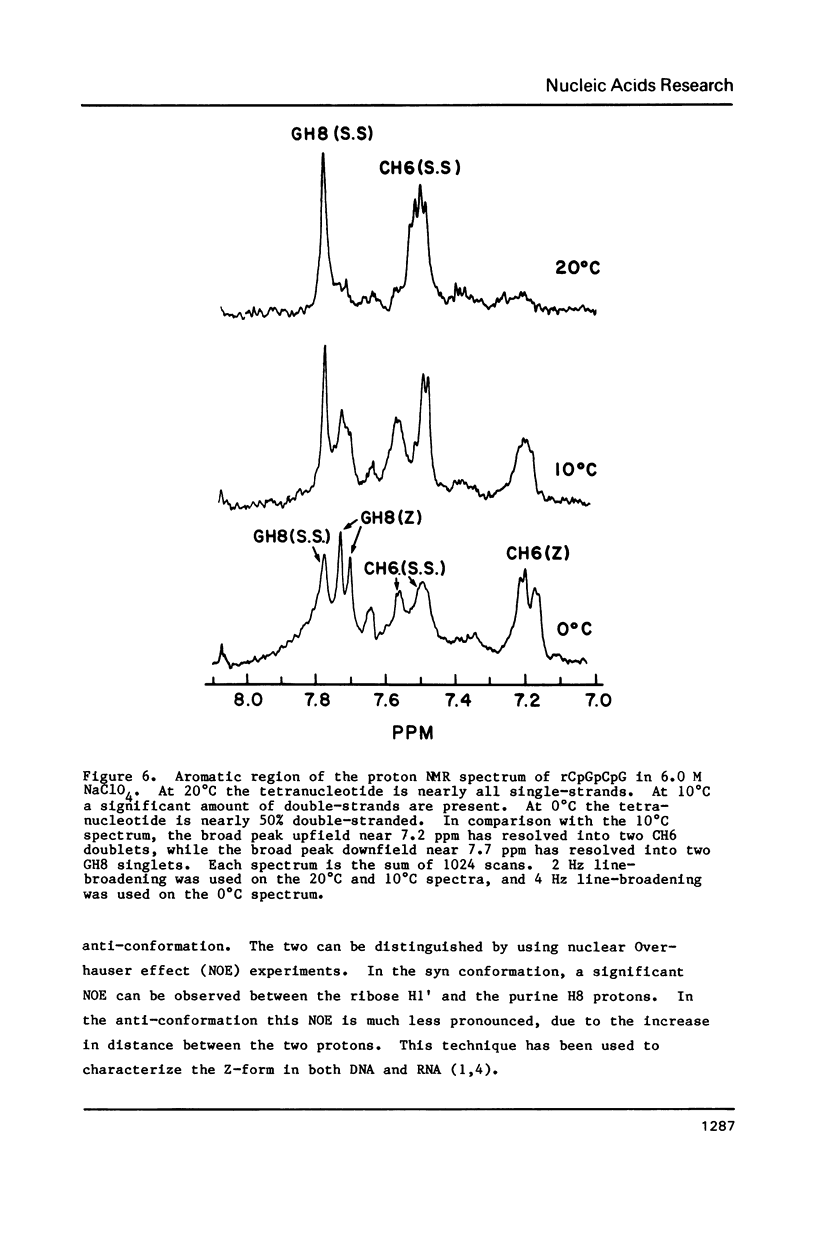
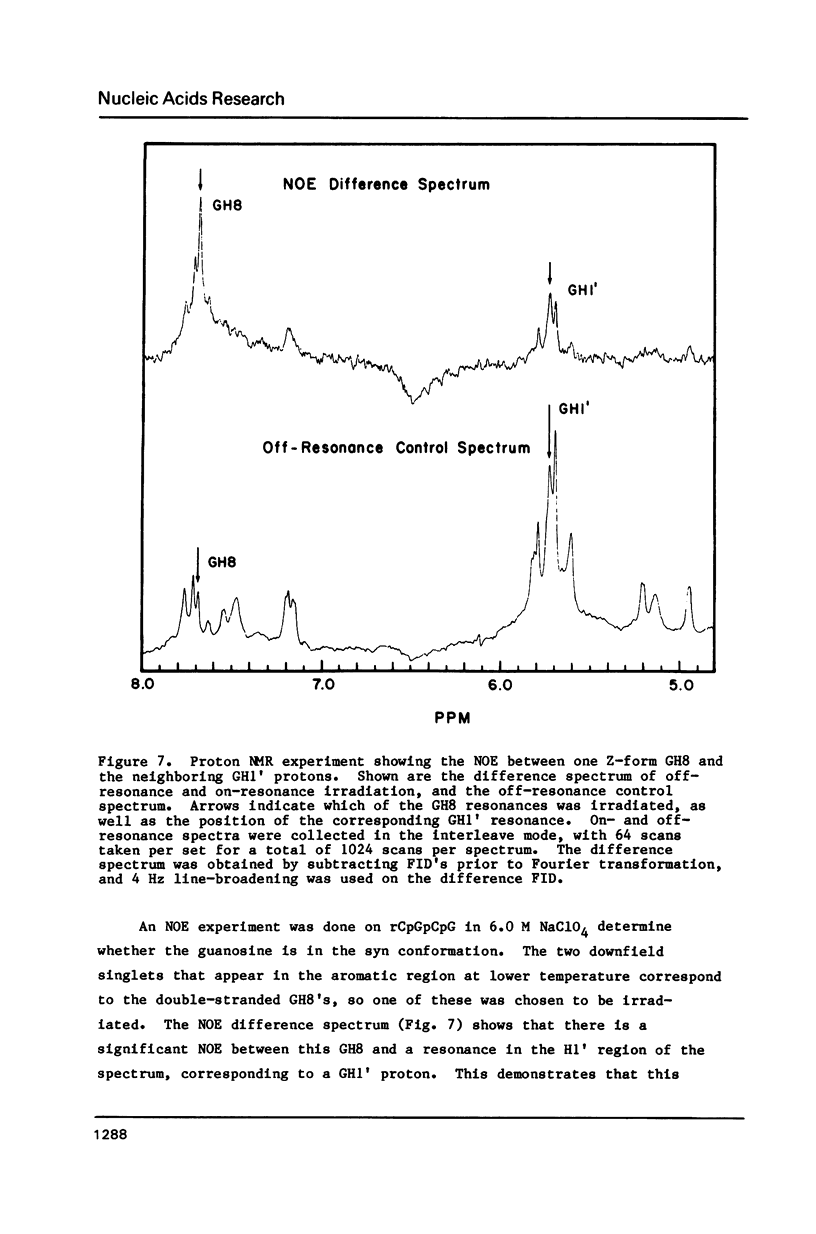
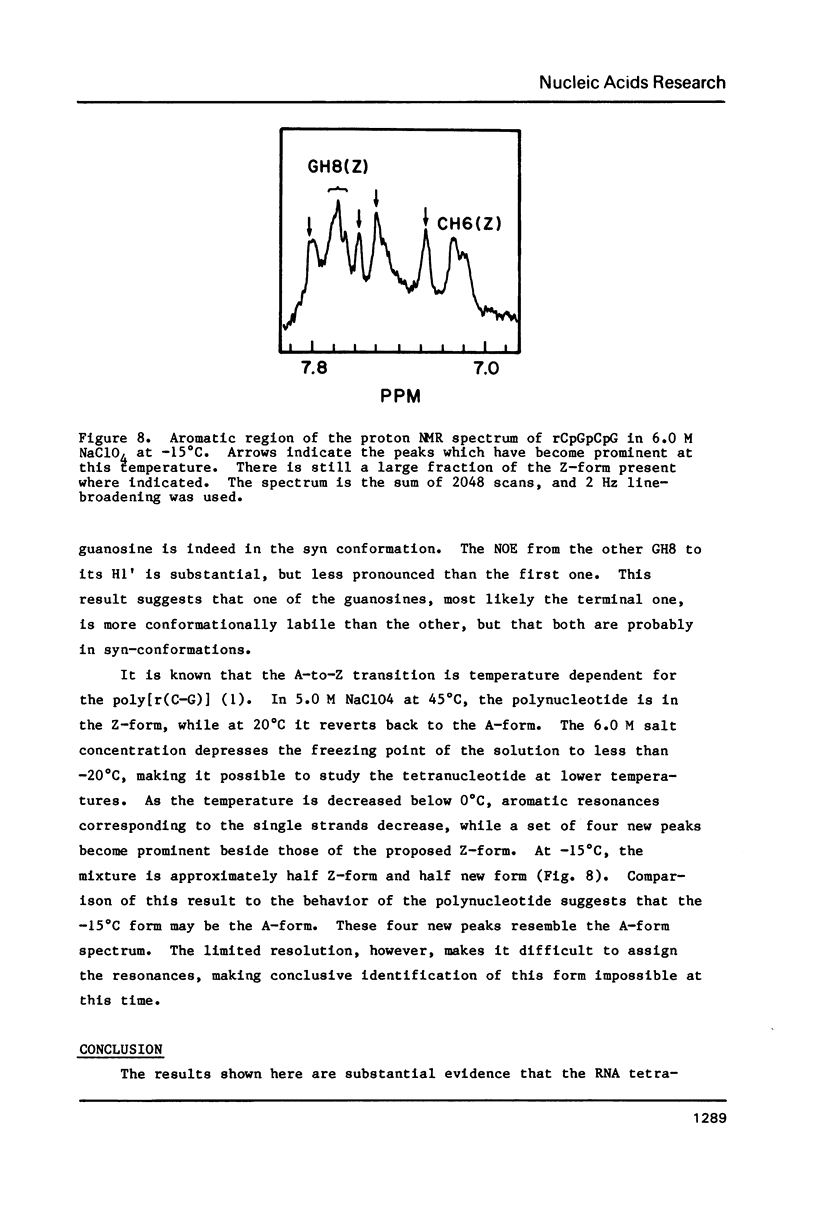
NMR and circular dichroism studies show that the RNA tetranucleotide rCpGpCpG can form a Z-RNA left-handed double-helix. In 1.0 M NaClO4, circular dichroism measurements indicate that the tetramer is in the A-form. In 6.0 M NaClO4, there is a characteristic change in the circular dichroism, indicating that the tetramer adopts a left-handed Z-form. This conformation is verified by phosphorus and proton NMR studies. The 31P spectrum shows a large downfield shift in one of the resonances upon an increase in salt concentration. Proton nuclear Overhauser effect (NOE) experiments indicate that the guanosines are in the syn conformation. These results are consistent with the formation of a Z-form double-helix.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cavailles J. A., Neumann J. M., Taboury J., Langlois d'Estaintot B., Huynh-Dinh T., Igolen J., Tran-Dinh S. B,Z conformations and mechanism of the Z-B-coil transitions of the self-complementary deoxy-hexanucleotide d(C-G-m5C-G-C-G) by 1H-NMR and CD spectroscopy. J Biomol Struct Dyn. 1984 Jun;1(6):1347–1371. doi: 10.1080/07391102.1984.10507525. [DOI] [PubMed] [Google Scholar]
- Conner B. N., Yoon C., Dickerson J. L., Dickerson R. E. Helix geometry and hydration in an A-DNA tetramer: IC-C-G-G. J Mol Biol. 1984 Apr 25;174(4):663–695. doi: 10.1016/0022-2836(84)90089-5. [DOI] [PubMed] [Google Scholar]
- Drew H., Takano T., Tanaka S., Itakura K., Dickerson R. E. High-salt d(CpGpCpG), a left-handed Z' DNA double helix. Nature. 1980 Aug 7;286(5773):567–573. doi: 10.1038/286567a0. [DOI] [PubMed] [Google Scholar]
- Eckstein F. Nucleoside phosphorothioates. Annu Rev Biochem. 1985;54:367–402. doi: 10.1146/annurev.bi.54.070185.002055. [DOI] [PubMed] [Google Scholar]
- Gorenstein D. G., Findlay J. B., Momii R. K., Luxon B. A., Kar D. Temperature dependence of the 31P chemical shifts of nucleic acids. A prode of phosphate ester torsional conformations. Biochemistry. 1976 Aug 24;15(17):3796–3803. doi: 10.1021/bi00662a023. [DOI] [PubMed] [Google Scholar]
- Hall K., Cruz P., Tinoco I., Jr, Jovin T. M., van de Sande J. H. 'Z-RNA'--a left-handed RNA double helix. Nature. 1984 Oct 11;311(5986):584–586. doi: 10.1038/311584a0. [DOI] [PubMed] [Google Scholar]
- Noller H. F. Structure of ribosomal RNA. Annu Rev Biochem. 1984;53:119–162. doi: 10.1146/annurev.bi.53.070184.001003. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Kozlowski S. A., Nordheim A., Rich A. Right-handed and left-handed DNA: studies of B- and Z-DNA by using proton nuclear Overhauser effect and P NMR. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1413–1417. doi: 10.1073/pnas.79.5.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Riazance J. H., Baase W. A., Johnson W. C., Jr, Hall K., Cruz P., Tinoco I., Jr Evidence for Z-form RNA by vacuum UV circular dichroism. Nucleic Acids Res. 1985 Jul 11;13(13):4983–4989. doi: 10.1093/nar/13.13.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich A., Nordheim A., Wang A. H. The chemistry and biology of left-handed Z-DNA. Annu Rev Biochem. 1984;53:791–846. doi: 10.1146/annurev.bi.53.070184.004043. [DOI] [PubMed] [Google Scholar]
- Sinclair A., Alkema D., Bell R. A., Coddington J. M., Hughes D. W., Neilson T., Romaniuk P. J. Relative stability of guanosine-cytidine diribonucleotide cores: a 1H NMR assessment. Biochemistry. 1984 Jun 5;23(12):2656–2662. doi: 10.1021/bi00307a018. [DOI] [PubMed] [Google Scholar]
- Thomas G. A., Peticolas W. L. Sequence dependence of conformations of self-complementary duplex tetradeoxynucleotides containing cytosine and guanine. Biochemistry. 1984 Jul 3;23(14):3202–3207. doi: 10.1021/bi00309a014. [DOI] [PubMed] [Google Scholar]
- Tran-Dinh S., Taboury J., Neumann J. M., Huynh-Dinh T., Genissel B., Langlois d'Estaintot B., Igolen J. 1H NMR and circular dichroism studies of the B and Z conformations of the self-complementary deoxyhexanucleotide d(m5C-G-C-G-m5-C-G): mechanism of the Z-B-coil transitions. Biochemistry. 1984 Mar 27;23(7):1362–1371. doi: 10.1021/bi00302a005. [DOI] [PubMed] [Google Scholar]
- Uesugi S., Ohkubo M., Ohtsuka E., Ikehara M., Kobayashi Y., Kyogoku Y., Westerink H. P., van der Marel G. A., van Boom J. H., Haasnoot C. A. Conformation of ribooligonucleotide duplexes containing an alternating C-G sequence which show an unusual circular dichroism spectrum. J Biol Chem. 1984 Feb 10;259(3):1390–1393. [PubMed] [Google Scholar]
- Westerink H. P., van der Marel G. A., van Boom J. H., Haasnoot C. A. Conformational analysis of r(CGCGCG) in aqueous solution: an A-type double helical conformation studied by two-dimensional nuclear Overhauser effect spectroscopy. Nucleic Acids Res. 1984 May 25;12(10):4323–4338. doi: 10.1093/nar/12.10.4323. [DOI] [PMC free article] [PubMed] [Google Scholar]


